Abstract
Post-traumatic stress disorder (PTSD) is presumably the result of life threats and conditioned fear. However, the neurobiology of fear fails to explain the impact of traumas that do not entail threats. Neuronal function, assessed as glucose metabolism with 18fluoro-deoxyglucose positron emission tomography, was contrasted in active duty, treatment-seeking US Army Soldiers with PTSD endorsing either danger- (n = 19) or non-danger-based (n = 26) traumas, and was compared with soldiers without PTSD (Combat Controls, n = 26) and Civilian Controls (n = 24). Prior meta-analyses of regions associated with fear or trauma script imagery in PTSD were used to compare glucose metabolism across groups. Danger-based traumas were associated with higher metabolism in the right amygdala than the control groups, while non-danger-based traumas associated with heightened precuneus metabolism relative to the danger group. In the danger group, PTSD severity was associated with higher metabolism in precuneus and dorsal anterior cingulate and lower metabolism in left amygdala (R2 = 0.61). In the non-danger group, PTSD symptom severity was associated with higher precuneus metabolism and lower right amygdala metabolism (R2 = 0.64). These findings suggest a biological basis to consider subtyping PTSD according to the nature of the traumatic context.
Keywords: post-traumatic stress disorder, FDG PET, glucose metabolism, fear
Introduction
Post-traumatic stress disorder (PTSD) is putatively linked to peritraumatic fear arising from life-threatening trauma (APA, 2000). As a result, a good deal of PTSD research has focused on elucidating parameters of fear reactivity. However, in many traumatic contexts peritraumatic fear is not present, nor is life threat the most distressing or haunting experience, even in danger contexts (Stein et al., 2012). Indeed many stressors, especially those involving human maliciousness, are traumatizing not because of life threat or danger, but because the experience is an insult to personal and shared morality or entails violent loss of life (Green, 1996; Cloitre et al., 2009; Litz et al., 2009, Neria and Litz, 2004). In these contexts, individuals do not report intense peritraumatic fear, and there may be no personal harm or threat. Rather, individuals who develop PTSD following non-danger-based harms report intense feelings of disgust, anguish, guilt, shame or sadness. Currently, studies of the pathophysiology of PTSD aggregate these danger- and non-danger-based traumatic events, which is problematic in brain studies of post-traumatic adaptation because of evidence that sadness, grief, guilt or shame engage different neural systems than threat-based, conditioned reactions (Freed et al., 2009; Basile et al., 2011;).
Neuroimaging in PTSD has largely focused on in-scanner tasks designed to identify brain responses to fearful stimuli. Some tasks are structured to elicit passive emotion identification/recognition (e.g. brief exposure to fearful faces), while others entail longer periods of processing traumatic stimuli (e.g. script imagery). Studies that employ brief fearful triggers yield findings that center in fear circuitry, namely the amygdalae, hippocampi, insula, anterior cingulate and medial prefrontal cortex (Pitman et al., 1989; Felmingham et al., 2009, 2010; Fonzo et al., 2010; Simmons et al., 2011; Cisler et al., 2013; Killgore et al., 2014). In contrast, coordinate-based meta-analyses of studies employing personalized trauma scripts or symptom provocation implicate hyperactivity of precuneus and cingulate regions (Etkin and Wager, 2007; Patel et al., 2012; Ramage et al., 2012; Zhang and Li, 2012). These regions are associated with autobiographical memory (Spreng et al., 2009), guilt (Basile et al., 2011) and moral cognition (Bzdok et al., 2012) and are not within fear circuitry but are functionally interconnected with it (Ramage et al., 2012; Brown et al., 2014a). This disparity in task-related brain activity has led some to hypothesize that activity in fear circuitry may be a non-specific common pathway in anxiety disorders. By contrast, it is thought that activity in medial frontal, cingulate and parietal cortex reflects deficits in emotion regulation or modulation in PTSD (Etkin and Wager, 2007; Duval et al., 2015).
The meta-analytic findings suggest that fear circuitry activation is minimal or absent when individuals with PTSD process trauma memories. However, it is difficult to draw inferences from these meta-analyses because highly divergent trauma types were evaluated between studies (Shin et al., 1999; Lanius et al., 2004; Morey et al., 2008). It could be that the studies failed to show activity in fear circuits because the events did not reflect fear-based harms. Disaggregating trauma type and principal harms may show that fear circuitry is differentially engaged by danger-based relative to non-danger-based traumatic events.
The evaluation of task-based brain activity in PTSD may limit external validity because results are constrained to specific experimental parameters, which have most often been fear-based. While informative, these paradigmatic biases are considerably reduced when resting-state is examined because it is task and stimulus independent. 18Fluoro-deoxyglucose positron emission tomography (18FDG PET) indexes the brain’s consumption of glucose (CMRglu) as a proxy of neuronal activity. A handful of 18FDG PET studies in PTSD report conflicting findings in CMRglu across parietal, occipital, temporal, cingulate, hippocampus and amygdalae (Bremner, 1997; Shin et al., 2009; Molina et al., 2010; Kim et al., 2012; Petrie et al., 2014) relative to trauma-exposed or non-trauma-exposed controls; likely because they were conducted with small sample sizes, varied widely in time since onset of PTSD, and did not consider the heterogeneity of trauma exposures.
In this study, we explored whether trauma type results in differing effects on brain function, using treatment-seeking US Army Soldiers with PTSD. Participants were sorted into danger- and non-danger groups based on the content of self-reported scripts of the worst and most distressing trauma using a modification of the Stein (Stein et al., 2012) categorization (Table 1). 18FDG PET images were analyzed to identify group differences in neuronal activity. We also examined the association between the severity of PTSD, comorbid depression and anxiety symptoms, and neuronal activity in each group. We hypothesized that soldiers with danger-based traumas would manifest greater resting neuronal activity in brain regions involved in heightened fear or hyperarousal (amygdalae), possibly indicating nascent defensive states, whereas non-danger-based traumas would be associated with brain regions involved in emotion regulation (rostral or dorsal anterior cingulate cortex—dACC; cf. Admon et al., 2013). We tested our hypotheses using a priori region of interest (ROI) analyses of brain regions most commonly active under fear-based conditions or uniquely implicated in PTSD during trauma script imagery.
Table 1.
Modified coding system for classification of trauma script
| Category | Description | |
|---|---|---|
| 1 | Danger | Life Threat to Self—Personal: Exposure to the threat of death or actual threatened serious injury |
| Life Threat to Other—Personal: Exposure to the actual or threatened death of others | ||
| 2 | Non-Danger | Aftermath of Violence—Personal: Exposure to grotesque or haunting images, sounds, or smells of dead or severely injured humans or animals |
| Traumatic Loss—Witnessed or Learned About: e.g. death of a family member, friend, or unit member | ||
| Moral Injury by Self: Committing an act that is perceived to be a gross violation of moral or ethical standards (e.g. killing or injuring others, rape, atrocities). A service member who nearly committed these acts could also experience moral injury | ||
| Moral Injury by Others: Witnessing or being the victim of an act that is perceived to be a gross violation of moral or ethical standards (e.g. killing or injuring others, rape, atrocities, betrayal). Events can also be indirectly experienced (i.e. learned about) if they are directly relevant to the individual |
Methods
This study was conducted at the Carl R. Darnall Army Medical Center at Fort Hood, Texas, and at The University of Texas Health Science Center at San Antonio (UTHSCSA) as part of the South Texas Research Organizational Network Guiding Studies on Trauma and Resilience (STRONG STAR) consortium. The study was approved by the Institutional Review Boards at Brooke Army Medical Center, UTHSCSA and the VA Boston Healthcare System and by the Human Research Protection Office at Fort Detrick, Maryland. Participants were recruited from a larger study of active duty service members seeking PTSD treatment after deployments in support of Operations Enduring Freedom, Iraqi Freedom and New Dawn (PI: Resick). Treatment study participants were invited to participate in the neuroimaging study, which was optional and did not affect treatment participation. Two control groups were also recruited: Combat Controls recruited from Fort Hood and Civilian Controls without prior military service. After potential participants heard complete study description, written informed consent was obtained.
Participants
Participants were male, 18+ years and English speakers. They were screened for presence of metal in the body, previous penetrating head injuries, prior neurosurgical procedures or history of neurological disorders. Combat control participants were not undergoing a military Medical Evaluation Board. Civilian control participants were not taking psychoactive medications and did not meet DSM-IV-TR criteria for any Axis 1 disorder.
Participants were diagnosed with the PTSD Symptom Scale – Interview Version (Foa et al., 1993). The Mini International Neuropsychiatric Interview (Lecrubier et al., 1997) was administered to assess comorbidities in PTSD and Combat Control participants and to rule out Axis I disorders in the Civilian Controls. PTSD symptoms and severity were assessed using the PTSD Checklist – Stressor Specific (PCL-S, Weathers et al., 1996) in all of the groups. PTSD subjects completed the Cognitive Emotion Regulation Questionnaire (CERQ, Garnefski and Kraaij 2006) as well as war-zone and life-span stressor exposure questionnaires (DRRI, King et al., 2006) and the Life Events Checklist (Gray et al., 2004). The Beck Depression Inventory-II (BDI-II, Beck et al., 1996) and Beck Anxiety Inventory (BAI, Epstein et al., 1988) were used to evaluate comorbid depression and anxiety symptoms, respectively.
Trauma script-based trauma-type acquisition
To disaggregate the nature of principal war-zone Criterion-A events, detailed self-reports of each participant’s ‘worst’ and most currently distressing trauma were acquired as trauma scripts. Participants were instructed to imagine being in the traumatic situation and to identify physical sensations or feelings experienced, people present, activities and to describe their surroundings (Pitman et al., 1989). Study staff verified script details with the participant.
Trauma script coding procedures
Coding categories from Litz and colleagues (Stein et al., 2012) were used to classify each script (Table 1). An independent sample of cases was used to train coders (inter-rater reliability 0.755–0.847). Consensus was established when discrepancies arose such that coders had 100% agreement on the Danger and Non-Danger categories.
Image acquisition
The PET session included: a 10-min transmission scan using a 68Ge/68Ga rod source for attenuation correction of the emission scan, and a 20-min emission scan following intravenous administration of 185–370 mBq (∼5 mCi) of 18FDG with a 30-min uptake period during which participants rested with eyes closed in a darkened room. Images were reconstructed by filtered back projection resulting in a single PET scan with average emission per voxel. Images were filtered again with a Gaussian kernel to a full width at half maximum of 7 mm isotropic and value normalized to a whole-brain mean value of 1000 PET counts, thus correcting for global differences in glucose metabolism across participants. PET counts and 18FDG uptake are linearly correlated (Reivich et al., 1977); therefore, images were not converted to standard uptake values.
A high-resolution T1-weighted whole-brain MRI scan was obtained for each subject. Image parameters were: time to recovery (TR) 2200 ms; echo time (TE) 2.83 ms; flip angle 13° for 0.8 mm3 voxels. T1-weighted images were used to visually assess brain structure integrity and for spatial normalization of the 18FDG PET images.
Image spatial normalization
PET images were coregistered to the corresponding MRI with the anterior commissure as the origin, the midsagittal plane as the y–z plane, and in the dimension of the MNI atlas using Statistical Parametric Mapping 8 (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm). Normalization was estimated using normalized mutual information between the PET images and the MNI template. The images were resliced using trilinear interpolation, preserving concentrations of intensity from the original images and finally smoothed with an 8-mm Gaussian filter.
ROI analysis
A coordinate-based meta-analysis was conducted to identify brain regions most commonly activated under a condition of fear. Details of the experiments included in the analysis are given in Supplemental Materials. The analysis identified the bilateral amygdalae and right rostral anterior cingulate (Brodmann Area 32) as most probable to activate given fearful stimuli (Table 2). Also included in the analyses were ROIs found previously (Etkin and Wager, 2007; Ramage et al., 2012) to be activated in PTSD subjects, relative to controls, during traumatic script imagery—the dorsal anterior cingulate cortex, left posterior cingulate cortex and left precuneus.
Table 2.
Fear and script imagery meta-analyses coordinates
| Brain region | Volume (mm3) | x | Y | z | ALE (×10−2) | Meta-analysis |
|---|---|---|---|---|---|---|
| Right rostral anterior cingulate cortex | 7616 | 4 | 50 | −4 | 5.03 | Fear |
| Right amygdala | 2368 | 26 | −6 | −18 | 3.19 | Fear |
| Left amygdala | 19 072 | −26 | −2 | −20 | 5.14 | Fear |
| Right dACC | 2112 | 4 | 18 | 32 | 3.49 | Trauma Script |
| lPcun | 128 | −3 | −60 | 28 | 1.89 | Trauma Script |
| lPCC | 448 | −1 | −46 | 9 | 2.27 | Trauma Script |
PET data were sampled by extracting the peak-level voxel within an 8-mm sphere around the coordinates derived from the above meta-analyses for each subject (Table 2). CMRglu for these ROIs were contrasted in SPSS (version 21.0) using the general linear model function for group effects with post-hoc pairwise comparisons, Bonferroni corrected and linear regression was used to determine variables predictive of PCL-S scores.
Analytic plan
Analyses identified CMRglu differences between PTSD and Controls, between all groups and specifically between danger- and non-danger-based PTSD. To validate that regional CMRglu in each group was associated with PTSD symptom severity, within-group analyses regressed PCL-S by CMRglu in the ROIs, controlling for comorbid symptoms, namely, BDI-II and BAI scores.
Results
Participant and group demographics
Male service members seeking PTSD treatment (N = 45) were sorted into Danger (n = 19) and Non-Danger (n = 26) groups. Control groups included 26 previously deployed service members without PTSD (Combat Control) and 24 Civilian Controls without PTSD (Table 3).
Table 3.
Group demographics
| Danger | Non-Danger | Combat control | Civilian control | |
|---|---|---|---|---|
| N or Mean | N or Mean | N or Mean | N or Mean | |
| N | 19 | 26 | 26 | 24 |
| Age | 37 ± 8 | 31 ± 7 | 36 ± 9 | 34 ± 11 |
| Handedness (right:left:ambidextrous) | 19:0:1 | 26:0:0 | 24:1:1 | |
| Number of comorbidities per participant | 3.1 ± 2 | 3.4 ± 2 | 0.2 ± 0.5 | 0 |
| Agoraphobia | 14 | 24 | 0 | 0 |
| Major depressive disorder | 14 | 21 | 0 | 0 |
| Panic disorder | 13 | 16 | 0 | 0 |
| Alcohol dependence | 7 | 8 | 5 | 0 |
| Generalized anxiety disorder | 1 | 6 | 1 | 0 |
| Obsessive compulsive disorder | 4 | 9 | 0 | 0 |
| Bipolar disorder | 5 | 6 | 0 | 0 |
| Other substance dependence (lifetime, not current) | 3 | 0 | 0 | 0 |
| Social phobia | 2 | 7 | 0 | 0 |
| Pain disorder | 2 | 2 | 0 | 0 |
| Specific phobia | 1 | 0 | 0 | 0 |
| Delusional disorder | 1 | 0 | 0 | 0 |
| Eating disorder | 0 | 0 | 0 | 0 |
| Medications | ||||
| Antidepressants | 12 | 10 | 0 | 0 |
| Anxiolytics | 4 | 4 | 0 | 0 |
| Antipsychotics | 7 | 1 | 0 | 0 |
| Anticonvulsantsb | 4 | 3 | 0 | 0 |
| Sympatholytics | 2 | 3 | 0 | 0 |
| Opioids | 4 | 6 | 1 | 0 |
| Benzodiazepine | 0 | 0 | 0 | 0 |
| Sedatives | 9 | 7 | 3 | 0 |
| Stimulants | 0 | 2 | 0 | 0 |
| BDI-IIa | 28 ± 12 | 26.3 ± 12 | 2.3 ± 4 | 1.6 ± 2 |
| BAIa | 23 ± 12 | 25 ± 14 | 1.8 ± 2 | 1.9 ± 2 |
| PCL-Sa | 54 ± 17 | 56 ± 11 | 20.2 ± 4 | 19.5 ± 4 |
aP < 0.05, PTSD > Controls.
bThe anticonvulsant/neurepileptic medications Topiramate and Gabapentin were prescribed for treatment of headaches in all cases.
PTSD participants reported higher PTSD symptom severity (PCL-S,F1,92 = 490, P < 0.0001), depression (F1,92 = 195, P < 0.0001) and anxiety (F1,93 = 131, P < 0.0001) than those without PTSD. The Danger group was older than the Non-Danger group (F1,43 = 8.6, P = 0.005) but otherwise well matched on demographic variables. The Non-Danger group reported higher scores on the DRRI Aftermath-of-Battle (F1,42 = 5.5, P = 0.024) and the CERQ Self-Blame sub-scale (F1,43 = 9, P = 0.004) than the Danger group.
CMRglu: PTSD groups vs controls groups
The collapsed event-type groups (all PTSD participants) had higher CMRglu in the right amygdala (F1,92 = 4.5, P = 0.036), relative to each control group. There were no differences in the other ROIs.
CMRglu: trauma type groups
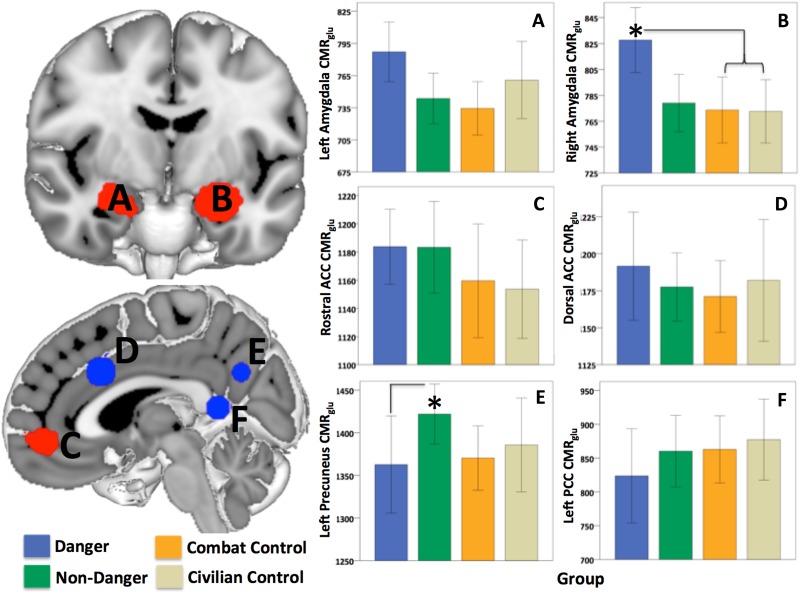
Right amygdala CMRglu was higher in the Danger group, relative to the Combat and Civilian Control groups (F3,90 = 3.9, P = 0.012). The Combat and Civilian control groups did not differ significantly from each other in any of the ROIs. Right amygdala CMRglu was not different between the two PTSD groups (F1,42 = 3.2, P = 0.08), however, the Danger group had significantly lower CMRglu in the left precuneus (F1,42 = 4.9, P = 0.033; Figure 1) than the Non-Danger group.
Fig. 1.
Group differences in CMRglu within the ROI. ROIs involved in fear processing (red) included the left (A) and right (B) amygdala and the rostral anterior cingulate cortex (E). ROIs involved in trauma script imagery (blue) included the dorsal anterior cingulate cortex (C), precuneus (D) and posterior cingulate cortex (F). CMRglu differed significantly between the Danger and both control groups in the right amygdala (B) and the Non-Danger and Danger groups in the left precuneus (D). *P < 0.05. Bars represent ±2 standard error.
CMRglu associations with PTSD symptom severity
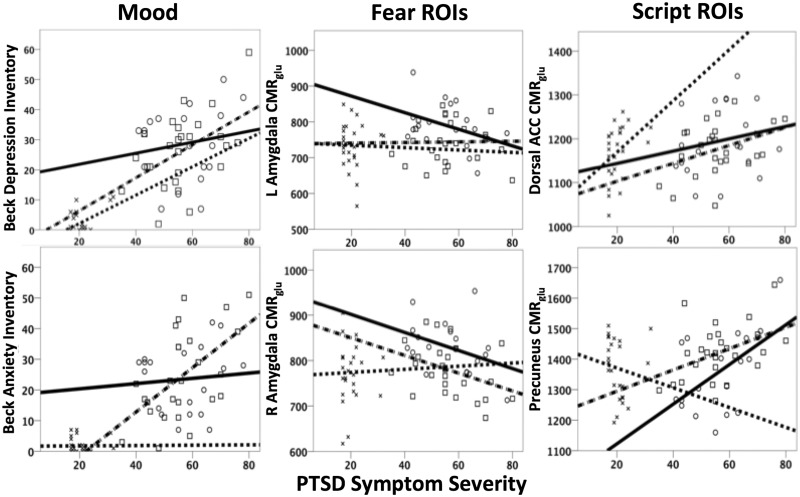
In the Danger group, lower CMRglu in the left amygdala (β = −0.38, P = 0.033), and higher CMRglu in the left precuneus (β = 0.67, P = 0.001) and dorsal anterior cingulate cortex (β = 0.42, P = 0.025) predicted PCL-S scores (overall model fit R2 = 0.61), controlling for comorbid symptoms. For the Non-Danger group, lower CMRglu in the right amygdala (β = −0.44, P = 0.003), higher CMRglu in the precuneus (β = 0.43, P = 0.003) and BAI scores (β = 0.52, P = 0.001) predicted PCL-S scores (overall model fit R2 = 0.64). Finally, although the range of PCL-S scores was low in the Combat Control group, in this group PTSD symptom severity was predicted by BDI-II (β = 0.53, P = 0.003) and CMRglu in the dorsal anterior cingulate (β = 0.38, P = 0.025) with an overall model fit of R2 = 0.42 (Figure 2).
Fig. 2.
Variables predicting PTSD severity in Danger-Based and Non-Danger-Based PTSD or Combat Controls.  , Danger; □, Non-Danger; ×, Combat Control.
, Danger; □, Non-Danger; ×, Combat Control.
Conclusions
Individuals with PTSD are haunted by memories of traumatic events, but the nature of the events and context differ considerably between individuals. This is especially true for complex traumatic stressors occurring in sustained malicious environments such as war-zones. In these contexts, many PTSD patients do not endorse threat-based events as their primary trauma (67% in this study) and many do not report peritraumatic fear. Contrary to the prevailing model in PTSD, life-threatening danger is not necessarily the worst or most currently distressing experience predominating the thoughts and feelings of patients with PTSD.
Our 18FDG PET data identified brain regions that differ in service members with PTSD resulting from danger vs non-danger-based harms. Those reporting danger-based traumas had higher resting neuronal activity in the right amygdala relative to the Combat and Civilian Control groups. Elevated CMRglu in the amygdalae may represent a trait marker of susceptibility to develop PTSD (Admon et al., 2013), or it may be a state marker resulting from danger-based trauma, stressful life events, current life stressors or it may reflect a pre-potent vigilance. It is possible that the methods employed in this study, namely lying in a dark room with eyes closed for the 18FDG uptake period, may have elicited a fear-based defensive state that is particularly evident in the Danger group.
The relationships between amygdalae CMRglu and PTSD severity differed between groups. This difference was most particularly evident in regard to laterality. Specifically, although the Danger group had higher CMRglu amygdalae activity than the Control groups, PTSD symptom severity was associated with lower left amygdala CMRglu. By contrast, in the Non-Danger group, lower right amygdala CMRglu was associated with higher PCL-S scores. Unfortunately, it is difficult to contextualize these findings because laterality of findings in amygdalae structure and function in PTSD is highly variable across studies (Rogers et al., 2009; Woon and Hedges 2009; Kuo et al., 2012). Further, findings in the amygdalae do not always relate to PTSD severity (Koren et al., 2005) and may be attributable to early life stress (Corbo et al., 2014), although this finding has not been replicated (Kuo et al., 2012). The PTSD symptom association results may indicate that variation in the amygdalae is a marker of vulnerability to PTSD and possibly more generally to anxiety disorders (Admon et al., 2013; Duval et al., 2015). In healthy subjects, the left and right amygdalae have slightly different structural and functional connections with other brain regions (Robinson et al., 2009), particularly with medial prefrontal and cingulate cortex. Our findings suggest that PTSD symptoms are differentially linked with divergent amygdalae anomalies in Danger and Non-Danger-based traumas.
The left precuneus also differed by trauma type, with lower CMRglu in the Danger relative to the Non-Danger group, and resting activity in this region was associated with PTSD symptom severity in both trauma types. Precuneus metabolism at rest is associated with the default mode network (Tomasi and Volkow, 2011; Jann et al., 2015), a network that demonstrates heightened cohesive brain activity during rest. The precuneus is also known to be involved in task conditions using self-referential information (Sajonz et al., 2010). It may be that non-danger-based war-zone harms lead to higher resting neuronal activity in this region because of greater introspection and moral cognition (Bzdok et al., 2012). CMRglu in the lPcun was also associated with symptoms of comorbid anxiety, reports of the cognitions related to blaming others, and with the intensity of the combat stressor exposure in the Danger group (Supplementary Table 1). The latter suggests that the Danger group’s neuronal activity in the left precuneus, while on average being significantly lower than that seen in the Non-Danger group, may be as relevant to the nature of comorbid anxiety, intrusive thoughts about blame and the extent of exposure to varied war-zone events.
Dorsal anterior cingulate CMRglu did not differ between the groups, but was predictive of PTSD symptom severity in the Danger and Combat Control groups. Previous findings in the dorsal anterior cingulate cortex have demonstrated alterations in function and structure in PTSD (Shin et al., 2007; Woodward et al., 2009), particularly regarding its functional connectivity with the amygdala (Cisler et al., 2013; Brown et al., 2014b). Function and structure in this region may relay familial risk (Shin et al., 2011) or pre-disposition for PTSD (Admon et al., 2013; Duval et al., 2015). Dorsal anterior cingulate cortex appears to be selectively vulnerable to the effects of stress as well as to chronic pain states (Vogt et al., 2003), suggesting it may play a role in allostasis. Specific to stress, higher resting metabolic activity in this region is a candidate familial risk factor for future development of PTSD (Shin et al., 2009). Our data do not clarify the role of the dorsal anterior cingulate, but its relevance to symptoms only in the Danger and Combat Control groups may highlight its importance in sensitivity to stress that is non-specific to PTSD.
It is important to point out that the Non-Danger group was comprised of service members with PTSD who endorsed exposure to two types of non-danger-based war-zone trauma, namely traumatic loss and various moral transgressions. This could explain the fact that the Danger group had altered fear-related brain activity, and in turn, the Non-Danger group had altered brain activity in regions not specific to fear and possibly also not specific to PTSD, as neuronal activity in the precuneus, for example, is also aberrant in psychopathology and neurodegenerative disorder (Menon 2011; Roffman et al., 2014). Moreover, anxiety was a strong predictor of PTSD symptoms in the Non-Danger group. The reason for this unexpected finding is uncertain. One possibility is that anxiety symptoms were present prior to deployment (six Non-Danger participants, but only one Danger participant, met criteria for current or lifetime generalized anxiety disorder), suggesting it may be a pre-disposing risk factor. Alternatively, because survivor guilt from war-zone loss and shame from war-related moral transgressions are distinguished from life-threat dangers in part because they entail real or internalized threats to social esteem and acceptance (Litz et al., 2009), although speculative, reports of anxiety may be a proxy for these fears in non-danger-based traumas.
In summary, our findings implicate neural markers for “subtypes” of PTSD. Although it was not surprising to find elevated neuronal activity in fear-related brain regions in PTSD, it is critical to note that it was only seen in the group reporting danger-based traumas, which made up less than half of this sample. And, although the Non-Danger group demonstrated higher neuronal activity in the left precuneus, the finding common in both PTSD groups was that precuneus activity positively predicted symptom severity. The mechanisms for how these differences arise and whether or not they provide insight into resilience to, or recovery from, distinct war-zone harms and PTSD are unclear and warrant further investigation.
Limitations
The external validity of these findings may be limited to service members seeking treatment for PTSD. Some participants were taking psychotropic medications or had comorbid disorders, factors that could alter glucose metabolism in various brain regions. Most of these confounds were equally distributed across the Danger- and Non-Danger-based PTSD groups and, if anything, may have increased the statistical error, limiting our ability to detect significant effects. However, considerably more participants in the Danger group (n = 7) were taking antipsychotic medications than Non-Danger participants (n = 1). Consequently, as a post-hoc analysis, we removed all eight cases from the analyses to explore whether this altered the results. The findings pertaining to the Danger and Non-Danger groups remained statistically equivalent in terms of PTSD, depression and anxiety scores. However, the right amygdala CMRglu in the Danger group was only marginally higher relative to the control groups (F3,82 = 2.5, P = 0.06), which may be the result of reduced power (observed power was 0.81 in the full sample, 0.61 with those eight subjects removed). However, the finding of reduced left precuneus CMRglu in the Danger relative to the Non-Danger group was no longer significant (F1,34 = 2.7, P = 0.11). This suggests that use of antipsychotic medications may have reduced neuronal activity in this region for participants in the Danger group. Future research is needed to determine the role of anti-psychotic medications, and the comorbidities for which they are prescribed, in the neurobiology of PTSD among individuals exposed to danger-based traumas.
Supplementary Material
Acknowledgements
The authors thank a large team of investigators and research staff for their contributions to this study. Jim Mintz, PhD, Raymond Aguilar, BS, and Kevin Muenzler, BS (University of Texas Health Science Center at San Antonio [UTHSCSA]), supported this study as part of the STRONG STAR Data and Biostatistics Core and assisted in the coordination of data sharing amongst the STRONG STAR studies, thereby facilitating this research. Jamie Anderson, BS, Sonia Holleman, BS, Nicholas D. Holder, BS, Paul Fowler, BA, Michelle Barrera, BA, Katina Campbell, MA, Michelle Hall-Graham, BA, Kelly Leaming, MA, and Sharon Primeaux, MS, from UTHSCSA served as project coordinators and research assistants and coordinated the recruitment of service members at Carl R. Darnall Army Medical Center (CRDAMC). Tamara Britt, MA, Lucas Brilliott, MA, Brooke Fina, LCSW, Monica Gauna, Ph,D, Brittany Hall-Clark, PhD, Steffany Malach, PhD, Crystal Mendoza, MA, Susan Paschall, MS, Veronica Vargas, LCSW, and Edward Wright, PhD, from UTHSCSA provided support as independent evaluators. Major Mitchell Kok, MD, and Major Paul C. Robinson, DO, served as CRDAMC Site-PIs for this study. Ashley Acheson, PhD, of UTHSCSA facilitated the development of regulatory documents and protocol development for the STRONG STAR Imaging Core. David Martinez, B.A., Olga Chavez, B.A., Paolo Medrano, B.A., and Alan Vela, B.A. at UTHSCSA assisted in recruitment, coordination, and assessment during imaging sessions for service members and civilians at the Research Imaging Institute. Elizabeth A. Hembree, PhD (University of Pennsylvania), provided expert training and certification of the study independent evaluators in the PTSD diagnostic evaluations using the PSS-I. John D. Roache, PhD (UTHSCSA), trained all research staff in the STRONG STAR Adverse Event Monitoring Program and assisted with the management of Consortium research operations. Antoinette Brundige, MA, Susan Deason, BA, and Gary Burk, MA, MBA, at UTHSCSA supported this study as part of the STRONG STAR Administrative Core. Christopher Harte, PhD, and Nathan Stein, PhD (VA Boston Healthcare System) supported this study as part of the STRONG STAR Assessment Core. Kimberly Del Carmen, PhD, and Holly Campbell-Rosen, PhD, from the Department of Defense, Office of Congressionally Directed Medical Research Programs, supported this study as grant officer representatives. Julie R. Collins, BA, of UTHSCSA provided editorial support for the manuscript. Finally, we thank Lisa M. Shin, PhD, Tufts University, for her comments on a previous draft of this manuscript.
Funding
The data collection for this study was conducted with support to the STRONG STAR Multidisciplinary PTSD Research Consortium from the U.S. Department of Defense through the U.S. Army Medical Research and Materiel Command, Congressionally Directed Medical Research Programs, Psychological Health and Traumatic Brain Injury Research Program awards W81XWH-08-02-109 (Alan Peterson), W81XWH-08-02-0112 (Peter Fox), W81XWH-08-02-0114 (Brett Litz), and W81XWH-08-02-0116 (Patricia Resick). The analysis and interpretation of data for this study was supported by the U.S. Department of Defense, Defense Health Program and the U.S. Department of Veterans Affairs, Office of Research & Development through the Psychological Health and Traumatic Brain Injury Research Program (PH/TBI RP), Consortium to Alleviate PTSD (CAP) award W81XWH-13-2-0065.
Disclaimer
The views expressed in this article are solely those of the authors and do not reflect an endorsement by or the official policy or position of the U.S. Army, the Department of Defense, the Department of Veterans Affairs, or the United States Government.
Conflict of interest. Ramage, Litz, Resick, Woolsey, Dondanville, Young-McCaughan, A. M. Borah, E. V. Borah, and Peterson report no financial relationships with commercial interests. Dr Fox has received funding from the following sources over the past two years: equity interest in Cerebral Magnetics, LLC and msMRI, LLC. In addition, he receives royalties from Wiley-Blackwell for serving as the Editor-in-Chief of Human Brain Mapping.
Supplementary data
Supplementary data are available at SCAN online.
References
- Admon R., Milad M.R., Hendler T. (2013). A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends in Cognitive Sciences , 17, 337–47. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders, 4th edn Washington, DC: APA. [Google Scholar]
- Basile B., Mancini F., Macaluso E., Caltagirone C., Frackowiak R.S.J., Bozzali M. (2011). Deontological and altruistic guilt: evidence for distinct neurobiological substrates. Human Brain Mapping , 32, 229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. (1996). Manual for the BDI-II. San Antonio: The Psychological Corporation. [Google Scholar]
- Bremner J.D. (1997). Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Archives of General Psychiatry , 54, 246–54. [DOI] [PubMed] [Google Scholar]
- Brown V.M., Labar K.S., Haswell C.C., et al. (2014a). Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology , 39, 351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V.M., Strauss J.L., Labar K.S., Gold A.L., McCarthy G., Morey. R.A. (2014b). Acute effects of trauma-focused research procedures on participant safety and distress. Psychiatry Research , 215(1), 154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D., Schilbach L., Vogeley K., et al. (2012). Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure and Function , 217, 783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., James G.A., Tripathi S., et al. (2013). Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychological Medicine , 43, 507–18. [DOI] [PubMed] [Google Scholar]
- Cloitre M., Stolbach B.C., Herman J.L., et al. (2009). A developmental approach to complex PTSD: childhood and adult cumulative trauma as predictors of symptom complexity. Journal of Traumatic Stress , 22, 399–408. [DOI] [PubMed] [Google Scholar]
- Corbo V., Salat D.H., Amick M.M., Leritz E.C., Milberg W.P., McGlinchey R.E. (2014). Reduced cortical thickness in veterans exposed to early life trauma. Psychiatry Research , 223, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval E.R., Javanbakht A., Liberzon I. (2015). Neural circuits in anxiety and stress disorders: a focused review. Therapy Clinical Risk Management , 11, 115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein N., Brown G., Steer R.A. (1988). An inventory for measuring clinical anxiety: psychometric properties. Journal of consulting and Clinical Psychology , 56(6), 893–7. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry , 164, 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K., Williams L.M., Kemp A.H., et al. (2010). Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. Journal of Abnormal Psychology , 119, 241–7. [DOI] [PubMed] [Google Scholar]
- Felmingham K., Williams L.M., Whitford T.J., et al. (2009). Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport , 20, 1402–6. [DOI] [PubMed] [Google Scholar]
- Foa E.B., Riggs D.S., Dancu C.V. (1993). Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress , 6(4), 459–73. [Google Scholar]
- Fonzo G.A., Simmons A.N., Thorp S.R., Norman S.B., Paulus M.P., Stein M.B. (2010). Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological Psychiatry , 68, 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed P.J., Yanagihara T.K., Hirsch J., Mann J.J. (2009). Neural mechanisms of grief regulation. Biological Psychiatry , 66, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnefski N., Kraaij V. (2006). Cognitive emotion regulation questionnaire—development of a short 18-item version (CERQ-short). Personality and Individual Differences , 41(6), 1045–53. [Google Scholar]
- Gray M.J., Litz B.T., Hsu J.L., Lombardo T.W. (2004). Psychometric properties of the life events checklist. Assessmentment , 11, 330–41. [DOI] [PubMed] [Google Scholar]
- Green B.L. (1996). Traumatic Stress and Disaster: Mental Health Effects and Factors Influencing Adaptation. International Review of Psychiatry, Vol. 2, Washington, DC: American Psychiatric Press, 177–210. [Google Scholar]
- IBM Corporation (2012). IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp. [Google Scholar]
- Jann K., Gee D.G., Kilroy E., et al. (2015). Functional connectivity in BOLD and CBF data: similarity and reliability of resting brain networks. NeuroImage , 106, 111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W.D.S., Britton J.C., Schwab Z.J., et al. (2014). Cortico-limbic responses to masked affective faces across PTSD, panic disorder, and specific phobia. Depression & Anxiety , 31, 150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-Y., Chung Y.-K., Kim B.S., Lee S.J., Yoon J.-K., An Y.-S. (2012). Psychiatry research: neuroimaging. Psychiatry Research: Neuroimaging , 201, 214–7.22464826 [Google Scholar]
- King L.A., King D.W., Vogt D.S., Knight J. (2006). Deployment risk and resilience inventory: a collection of measures for studying deployment-related experiences of military personnel and veterans. Military Psychology , 18(2), 89–120. [Google Scholar]
- Koren D., Norman D., Cohen A., Berman J., Klein E.M. (2005). Increased PTSD risk with combat-related injury: a matched comparison study of injured and uninjured soldiers experiencing the same combat events. American Journal Psychiatry , 162, 276–82. [DOI] [PubMed] [Google Scholar]
- Kuo J.R., Kaloupek D.G., Woodward S.H. (2012). Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Archives of General Psychiatry , 69, 1080–6. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Williamson P.C., Densmore M., et al. (2004). The nature of traumatic memories: a 4-T FMRI functional connectivity analysis. American Journal of Psychiatry , 161, 36–44. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y., Sheehan D.V., Weiller E., Amorim P. (1997). The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European Psychiatry , 12(5), 224–31. [Google Scholar]
- Litz B.T., Stein N., Delaney E., et al. (2009). Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clinical Psychology Review , 29, 695–706. [DOI] [PubMed] [Google Scholar]
- Menon V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences , 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Molina M.E., Isoardi R., Prado M.N., Bentolila S. (2010). Basal cerebral glucose distribution in long-term post-traumatic stress disorder. World Journal of Biological Psychiatry , 11(2 Pt. 2), 493–501. [DOI] [PubMed] [Google Scholar]
- Morey R.A., Petty C.M., Cooper D.A., Labar K.S., McCarthy G. (2008). Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Research , 162, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neria Y., Litz B.T. (2004). Bereavement by traumatic means: the complex synergy of trauma and grief. Journal of Loss & Trauma , 9, 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Spreng R.N., Shin L.M., Girard T.A. (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neuroscience Biobehavioral Review , 36, 2130–42. [DOI] [PubMed] [Google Scholar]
- Petrie E.C., Cross D.J., Yarnykh V.L., et al. (2014). Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan War veterans. Journal of Neurotrauma , 31, 425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman R.K., Orr S.P., Steketee G.S. (1989). Psychophysiological investigations of posttraumatic stress disorder imagery. Psychopharmacology Bulletin , 25, 426–31. [PubMed] [Google Scholar]
- Ramage A.E., Laird A.R., Eickhoff S.B., et al. (2012). A coordinate-based meta-analytic model of trauma processing in posttraumatic stress disorder. Human Brain Mapping , 34, 3392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reivich M., Kuhl D., Wolf A., et al. (1977). Measurement of local cerebral glucose metabolism in man with 18F-2-fluoro-2-deoxy-d-glucose. Acta Neurologica Scandinavica Supplementum , 64, 190–1. [PubMed] [Google Scholar]
- Robinson J.L., Laird A.R., Glahn D.C., Lovallo W.R., Fox P.T. (2009). Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Human Brain Mapping , 31(2), 173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman J.L., Witte J.M., Tanner A.S., et al. (2014). Neural predictors of successful brief psychodynamic psychotherapy for persistent depression. Psychotherapy and Psychosomatics , 83, 364–70. [DOI] [PubMed] [Google Scholar]
- Rogers M.A., Yamasue H., Abe O., et al. (2009). Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Research , 174, 210–6. [DOI] [PubMed] [Google Scholar]
- Sajonz B., Kahnt T., Margulies D.S., et al. (2010). Delineating self-referential processing from episodic memory retrieval: common and dissociable networks. NeuroImage , 50, 1606–17. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Bush G., Milad M.R., et al. (2011). Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. American Journal of Psychiatry , 168, 979–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Bush G., Whalen P.J., et al. (2007). Dorsal anterior cingulate function in posttraumatic stress disorder. Journal of Traumatic Stress , 20, 701–12. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Lasko N.B., Macklin M.L., et al. (2009). Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Archives of General Psychiatry , 66, 1099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., McNally R.J., Kosslyn S.M., et al. (1999). Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. American Journal of Psychiatry , 156, 575–84. [DOI] [PubMed] [Google Scholar]
- Simmons A.N., Matthews S.C., Strigo I.A., et al. (2011). Altered amygdala activation during face processing in Iraqi and Afghanistani war veterans. Biology of Mood & Anxiety Disorders , 1(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S.N. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience , 21, 489–510. [DOI] [PubMed] [Google Scholar]
- Stein N.R., Mills M.A., Arditte K., et al. (2012). A scheme for categorizing traumatic military events. Behavior Modification , 36, 787–807. [DOI] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. (2011). Association between functional connectivity hubs and brain networks. Cerebral Cortex , 21, 2003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B.A., Berger G.R., Derbyshire S.W.G. (2003). Structural and functional dichotomy of human midcingulate cortex. European Journal Neuroscience , 18, 3134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F.W., Litz B.T., Keane T.M., et al. (1996). The utility of the SCL-90-R for the diagnosis of war-zone related posttraumatic stress disorder. Journal of Traumatic Stress , 9, 111–28. [DOI] [PubMed] [Google Scholar]
- Woodward S.H., Schaer M., Kaloupek D.G., Cediel L., Eliez S. (2009). Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Archives of General Psychiatry , 66, 1373–82. [DOI] [PubMed] [Google Scholar]
- Woon F.L., Hedges D.W. (2009). Amygdala volume in adults with posttraumatic stress disorder: a meta-analysis. Journal of Neuropsychiatry and Clinical Neuroscience , 21, 5–12. [DOI] [PubMed] [Google Scholar]
- Zhang S., Li C.-S.R. (2012). Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage , 59, 3548–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.