Abstract
Converging evidence identifies trait optimism and the orbitofrontal cortex (OFC) as personality and brain factors influencing anxiety, but the nature of their relationships remains unclear. Here, the mechanisms underlying the protective role of trait optimism and of increased OFC volume against symptoms of anxiety were investigated in 61 healthy subjects, who completed measures of trait optimism and anxiety, and underwent structural scanning using magnetic resonance imaging. First, the OFC gray matter volume (GMV) was associated with increased optimism, which in turn was associated with reduced anxiety. Second, trait optimism mediated the relation between the left OFC volume and anxiety, thus demonstrating that increased GMV in this brain region protects against symptoms of anxiety through increased optimism. These results provide novel evidence about the brain–personality mechanisms protecting against anxiety symptoms in healthy functioning, and identify potential targets for preventive and therapeutic interventions aimed at reducing susceptibility and increasing resilience against emotional disturbances.
Keywords: personality, brain volume, resilience, vulnerability, emotional dysregulation
Introduction
With an estimated 40 million adults affected each year (Kessler et al., 2005b), and almost 30% of the adult population meeting the criteria for a diagnosis at some point in life (Kessler et al., 2005a), anxiety disorders are the most prevalent class of mental illness in the USA (US Burden of Disease Collaborators, 2013). Given their heavy personal and societal burden, there is an increased need to identify new biological and psychological markers influencing susceptibility or resilience to anxiety disorders. Here, we adopted a brain–personality–symptom approach to investigate the relations among specific brain and personality factors that provide protection against symptoms of anxiety in healthy participants. Based on previous evidence linking trait optimism and the orbitofrontal cortex (OFC) to symptoms of anxiety (Zenger et al., 2010; Talati et al., 2013), and identifying associations between OFC and optimism (Kringelbach, 2005), the present study specifically focused on investigating the role of individual differences in trait optimism and the OFC volume as personality and brain factors influencing vulnerability or resilience to anxiety symptoms in healthy participants. In addition, it examined the potential mediating role of optimism in the relation between the OFC gray matter volume (GMV) and anxiety. The multidimensional approach employed in the present study has the potential to advance the understanding of the psychological and neural factors providing protection against anxiety symptoms, and to inform the development of evidence-based preventive and therapeutic interventions targeting enhanced resilience to emotional dysregulation characterizing affective disorders.
Despite a limited understanding of risk and resilience factors of anxiety, recent investigations have identified a number of personality factors and brain regions linked to anxiety. Among the personality factors, trait optimism has been consistently linked to resilience against symptoms of affective dysregulation, in general, and against anxiety, in particular (Wu et al., 2013). Defined as ‘the dispositional tendency for people to hold generalized favorable expectancies about their future’ (Carver et al., 2010), trait optimism has been acknowledged to promote general psychological well-being, and to be particularly beneficial in times of adversity (Andersson, 1996; Carver et al., 2010). Optimism has been shown to motivate active and persistent coping behavior (Nes and Segerstrom, 2006), and as a result, it has been linked to reduced anxiety symptoms in both healthy (Scheier et al., 1994) and clinical (Zenger et al., 2010) participants.
Regarding the brain regions, structural and functional neuroimaging evidence indicates volume reductions (Talati et al., 2013) and reduced responses (Porcelli et al., 2012; Grupe and Nitschke, 2013) in the OFC, in patients with anxiety disorders. In healthy individuals, available results largely echo the findings from clinical groups, linking the OFC volume to decreased negative affect and less stressful life experiences (Ansell et al., 2012; Holmes et al., 2012; Sekiguchi et al., 2013). The volumetric approach is seemingly advantageous in the study of personality, as stable individual differences may be more clearly manifested in structural changes of relevant regions than in task-related activations identified in functional neuroimaging research (DeYoung et al., 2010). Extending the personality and structural imaging evidence, functional neuroimaging evidence in healthy individuals also suggests a link between the OFC and trait optimism. Specifically, OFC has been previously implicated in reward-related (Kringelbach and Berridge, 2009; Grabenhorst and Rolls, 2011; Phelps et al., 2014) and approach-oriented (Eddington et al., 2007) processing, which might underlie the positive cognitive bias of optimistic individuals. Such an orientation is likely to motivate adaptive emotion regulation and has been linked to reduced anxiety symptoms (Nes and Segerstrom, 2006; Llewellyn et al., 2013).
Interestingly, previous evidence indicates valence- and approach-related hemispheric lateralization in the prefrontal cortex (PFC: Davidson and Irwin, 1999; Harmon-Jones and Gable, 2009), with the left hemisphere being associated with positive valence and approach tendencies, and the right hemisphere with negative valence and avoidance tendencies (Dolcos et al., 2004; Eddington et al., 2007). However, recent evidence points to a more complex nature of frontal activations and lateralizations (reviewed in Miller et al., 2013). Even within a hemisphere, frontal areas can show contrasting relationships with psychological variables, some of which are consistent with the traditional valence hypothesis, whereas others are not (Miller et al., 2013). For instance, contrary to the traditional lateralization prediction, Spielberg et al. (2011) identified two partially overlapping left hemisphere regions that showed positive relations with approach and avoidance temperaments, and whose overlapping activations were positively related with both approach and avoidance temperaments. Moreover, evidence from the anxiety literature suggests that different types of anxiety modulate frontal activity in distinct ways (Nitschke et al., 1999, 2001). For instance, worry/anxious apprehension activates a left PFC brain region, in contrasts with anxious arousal, which activates a right-hemisphere region (Engels et al., 2007). Overall, this evidence suggests that a left-lateralization pattern might exist at the structural level in the OFC, with respect to anxiety.
Together, the evidence reviewed above suggests a three-way brain–personality–behavior interaction among the OFC, trait optimism and anxiety, with OFC volume and trait optimism possibly being positively related with one another and both of them being negatively associated with anxiety. Both trait optimism and anxiety index individual differences, but they tap into different aspects of personality. Trait optimism, which primarily involves a cognitive orientation toward more positive expectancies of future outcomes (Carver and Scheier, 2014), has been credited with contributing to successful emotion regulation, which builds resilience to anxiety (Carver et al., 2010; Wu et al., 2013). Trait anxiety, on the other hand, indexes consequences of poor emotion regulation (D'Avanzato et al., 2013; Grupe and Nitschke, 2013).
Investigating the relation between OFC and trait anxiety in healthy populations may provide insight about predispositions to psychopathology (Giuliani et al., 2011). Investigation of non-clinical participants is becoming increasingly important, as it allows examination of what would be regarded as subthreshold psychopathology (Insel et al., 2010). Thus, our understanding of anxiety disorders can greatly benefit from first understanding the normal anxiety responses in healthy individuals (Bateson et al., 2011; Giuliani et al., 2011; Montag et al., 2013). Moreover, recent interventions targeting the OFC (Scheinost et al., 2013, 2014) identified a shared neurobiological mechanism for improved control in healthy and clinical populations. These findings support the predictive value of targeting this region in both populations, and suggest that interventions developed in the healthy population are likely to translate to the clinical population.
Although it is difficult to determine the direction of influences in these relations, there is evidence supporting the idea of a directional link from brain structures to perceptual, cognitive and personality-level variables (Kanai and Rees, 2011; Montag et al., 2013; Gilaie-Dotan et al., 2014). Therefore, in formulating the mediation hypothesis, the present study adopted the view that optimistic individuals would have lower anxiety levels due to a positive bias in their general cognitive expectancy. Based on these arguments, the present study constructed mediation models to predict personality-level and behavioral/symptom-level variables from the brain volumes (i.e. OFC GMV). We had the following two predictions: (i) Trait optimism would be positively associated with the GMV in the OFC and negatively associated with anxiety and (ii) trait optimism would mediate the relationship between the OFC volume and anxiety. However, the lack of longitudinal evidence leaves unclear the direction of influences in these relations, and thus we also tested different configurations of the brain, personality and symptom variables in the mediation models.
Methods
Subjects
Structural MR images and personality assessments were collected from a sample of 61 healthy young adults (18–34 years of age, average = 23.23, s.d. = 4.00; 37 females). Power calculations indicate that a sample size of 59 is needed to reach a power of 0.8 (Fritz and Mackinnon, 2007; Faul et al., 2007, 2009). To account for potential data attrition, two additional datasets were included. This sample size is consistent with previous studies using a similar volumetric approach (e.g. Kuhn et al., 2011; Giuliani et al., 2011). None of the subjects had previously been diagnosed with neurological, psychiatric or personality disorders, and there were no significant age differences between the female and male participants [t(59) = −0.16, P > 0.05, two-tailed]. All participants provided written consent, and were compensated with either course credit or money.
Imaging protocol and MRI data processing
Structural scanning was conducted on a 1.5-T Siemens Sonata scanner. After the sagittal localizer, 3-D MPRAGE anatomical images were obtained using the following parameters: TR = 1600 ms; TE = 3.82 ms; FOV = 256 × 256 mm2. This resulted in anatomical volumes with 112 axial slices and voxel size of 1 × 1 × 1 mm3. Cortical reconstruction was performed with the Freesurfer image analysis suite, Version 5.3.0 (Fischl, 2012), which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). A semi-automatic workflow was adopted to ensure quality control at the following stages: Talairach registration, skull stripping, white matter surface reconstruction and pial surface reconstruction. The outputs at each stage were manually inspected and corrected if necessary before the next stage was implemented. Anatomical regions of interest (ROIs) of the bilateral medial OFC (mOFC) and lateral OFC (lOFC) were defined based on the Desikan–Killiany atlas (Desikan et al., 2006). According to this atlas, the mOFC and lOFC are defined relative to the midpoint of the medial orbital sulcus (mMOS). The mOFC, the portion of the OFC medial to the mMOS, is described as a region within the rostral and caudal extent of the medial orbitofrontal gyrus/gyrus rectus, bordering the cingulate cortex and the medial bank of the superior frontal gyrus at the medial aspect. The mOFC ROI identified with the Desikan atlas in Freesurfer partially overlaps with the ventromedial PFC (Kringelbach, 2005). The lOFC, the portion of the OFC lateral to the mMOS, is described as a region within the rostral and caudal extent of the lateral orbitofrontal gyrus, bordering the lateral bank of the lateral orbital sulcus and/or the circular insular sulcus at the lateral aspect.
For each subject, the GMVs of these anatomical ROIs and the intracranial volume (ICV) were extracted from the parcellation results, and an index of the adjusted volume was obtained for each ROI by dividing the raw volumes by the ICV, and then multiplying them by 100. The resulting adjusted volumetric indices were used for group-level statistical analyses. To test the specificity of the hypothesized effects to OFC, we also conducted exploratory analyses targeting other putative brain regions involved in reward processing and linked to optimism (Liu et al., 2011; Bartra et al., 2013; Sescousse et al., 2013). These regions included basal ganglia nuclei (accumbens area, caudate, putamen and pallidum), cingulate cortex (rACC) and inferior frontal gyrus (IFG; pars orbitalis, pars triangularis and pars opercularis).
Personality measures
Subjects completed personality measures that included scales assessing trait optimism and trait anxiety. Also, to test the specificity of the relations between OFC, trait optimism and anxiety, other related personality traits, including positive affect, negative affect and depression were also assessed.
Trait optimism
Trait optimism was assessed using the revised Life Orientation Test (LOT; Scheier et al., 1994), in which subjects are asked to indicate their agreement with each of the 10 statements on the test using a 0–4 Likert scale (0 = strongly disagree, 4 = strongly agree). Examples of the LOT key statements are ‘In uncertain times, I usually expect the best’ or ‘If something can go wrong for me, it will.’ A total score is calculated for each subject based on six statements, with three of them being reversed coded. The largest possible range of this scale is from 0 to 24. Higher scores on this scale would indicate higher level of optimism at the trait level. The LOT has shown high test–retest reliability and good discriminant validity to distinguish optimism from other conceptually related constructs (Scheier et al., 1994). The Cronbach’s alpha for LOT in our sample was 0.708.
Trait anxiety
Trait anxiety was assessed using the State-Trait Anxiety Inventory-Trait (STAI-T; Spielberger et al., 1970). STAI is the most typically used measure to investigate anxious characteristics in non-clinical samples, due to its known sensitivity as a marker of risk for anxiety disorders (Grupe and Nitschke, 2013). It allows identification of relative risk or vulnerability to anxiety symptoms, reflected in higher STAI scores that can be identified within the range of normative behavior. Participants are asked to indicate how they generally feel about 20 statements, such as ‘I worry too much over something that really doesn’t matter’, using a 1–4 Likert scale (1 = not at all, 4 = very much so). Ratings of individual statements are summed to obtain a total score for each subject (ranging from 20 to 80). Higher total scores are considered to reflect a general vulnerability factor for anxiety disorders, and lower total scores as potentially indexing lower vulnerability, and hence potential markers of resilience against anxiety. The STAI-T has shown high internal consistency, test–retest reliability and good construct and concurrent validity (Spielberger et al., 1983). The Cronbach’s alpha for STAI-T in our sample was 0.875.
Depression symptoms
Depression symptoms were assessed using the Beck Depression Inventory (BDI; Beck et al., 1961). It consists of 21 questions, each having four possible answers ranging in intensity from 0 to 3 (e.g. 0 = ‘I do not feel sad’, 1 = ‘I feel sad’, 2 = ‘I am sad all the time and I can’t snap out of it’ and 3 = ‘I am so sad or unhappy that I can’t stand it’). Subjects were instructed to answer each question using one of the four choices. The corresponding numbers of the choices were summed to obtain a total score for each subject, which would reflect the severity of depression symptoms. The total score on this scale ranges from 0 to 63. Considerable evidence has attested to the reliability and validity of the BDI (Beck et al., 1988). The Cronbach’s alpha for BDI in our sample was 0.778.
Positive and negative affect
The Positive and Negative Affect Schedule-Trait (PANAS; Watson et al., 1988) was used to measure trait positive and negative affect. It includes a list of 20 emotion words concerning positive affect (e.g. ‘interested’, ‘enthusiastic’) and negative affect (e.g. ‘irritable’, ‘upset’). Items are rated on a 5-point scale from 1 (very slightly or not at all) to 5 (extremely) based on the extent to which the respondent feels this way during a longer period of time. Ratings on the positive descriptors and negative descriptors were summed up separately to get scores for trait positive affect and trait negative affect, respectively. The range of each subscale is 10–50. PANAS is widely used as a measure for trait affect, and has demonstrated good reliability and validity (Watson et al., 1988). The Cronbach’s alpha for PANAS in our sample was 0.682.
Statistical analyses
Zero-order correlations were first used to assess the two-by-two relations between the OFC volumes, trait optimism and trait anxiety scores; partial correlations were also performed to account for the potential effects of age and sex. Standardized scores were calculated for the personality and volumetric measures to detect outliers, using a criterion of 2.5 standard deviations (Stevens, 2009). In total, three subjects were identified as outliers on four of the variables except for the STAI-T and the left mOFC (Table 1); one of the outlying subjects had extreme values on more than one variable. Outliers were excluded list-wise. The same outlier criterion was also applied to scores on other personality scales and volumetric measures of the exploratory ROIs, and identified outliers were excluded accordingly. To test the specificity of the relations between OFC, trait optimism and anxiety, we further explored the relationships between the OFC volumes and PANAS and BDI scores.
Table 1.
Averages, standard deviations (SDs) and correlations between personality and volumetric measures
| Variables | Mean | s.d. | Range | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|---|
| Personality measures | |||||||||
| 1. Optimism/LOT | 16.63 | 3.92 | [6, 23] | − | |||||
| N | 60 | ||||||||
| 2. Anxiety/STAI-T | 36.25 | 8.30 | [23, 55] | −0.460** | – | ||||
| N | 61 | 60 | |||||||
| CI | [−1.434, −0.468] | ||||||||
| Volume statistics | |||||||||
| 3. Left mOFC | 0.42 | 0.08 | [0.24, 0.60] | 0.310* | −0.250*** | – | |||
| N | 61 | 60 | 61 | ||||||
| CI | [2.950, 27.613] | [−52.752, 0.249] | |||||||
| 4. Left lOFC | 0.60 | 0.09 | [0.36, 0.79] | 0.301* | −0.269* | 0.714** | – | ||
| N | 60 | 59 | 60 | 60 | |||||
| CI | [2.084, 24.243] | [−48.824, −1.446] | [0.609, 1.033] | ||||||
| 5. Right mOFC | 0.41 | 0.07 | [0.23, 0.57] | 0.196 | −0.146 | 0.745** | 0.726** | – | |
| N | 60 | 59 | 60 | 60 | 60 | ||||
| CI | [−3.493, 24.649] | [−46.744, 13.099] | [0.609, 0.984] | [0.670, 1.115] | |||||
| 6. Right lOFC | 0.61 | 0.09 | [0.39, 0.83] | 0.218 | −0.275* | 0.751** | 0.859** | 0.739** | – |
| N | 59 | 58 | 59 | 59 | 59 | 59 | |||
| CI | [−1.816, 20.123] | [−50.933, −1.956] | [0.492, 0.791] | [0.691, 0.950] | [0.441, 0.721] | ||||
Notes: N, number of participants; CI, confidence interval. All correlations remained significant after controlling for age and sex, except for the trending relation between left mOFC and STAI-T, noted as an exception. LOT, Life Orientation Test (Scheier et al., 1994); STAI-T, State-Trait Anxiety Inventory-Trait (Spielberger et al., 1970); mOFC, medial orbitofrontal cortex; lOFC, lateral orbitofrontal cortex.
*significant at P < 0.05 (two-tailed), **significant at P < 0.001 (two-tailed), ***marginally significant at P = 0.052 (two-tailed).
To test our mediation hypothesis, suggesting a potential mediating role of trait optimism in the relation between the OFC volumes and anxiety scores, we conducted mediation analyses, with trait optimism as the mediator (M), the volumes of the mOFC and the lOFC as the predictor (X) and anxiety scores as the outcome variable (Y). Using standard conventions (Preacher and Hayes, 2004), the mediation analysis focused on testing the regression coefficients in the following relations: (i) Path a, representing the X to M relation, (ii) path b, representing the M to Y relation controlling for X, (iii) path c, representing the regression coefficient of the total effect from X to Y and (iv) path c′, representing the regression coefficient of the direct effect from X to Y controlling for M. The magnitude of the mediation was measured by the indirect effect a × b, representing the X to Y relation while taking M into account. This measure of the indirect effect was submitted to a bootstrapping procedure (number of samplings = 5000) to obtain 95% confidence intervals (CIs), which is recommended for the present sample size (Preacher and Hayes, 2004). The term a × b has been shown to be equivalent to c−c′ in most cases (Preacher and Hayes, 2004), and thus a CI that does not contain zero would indicate that the total effect from X to Y has been significantly reduced upon the addition of the mediator to the model. The index of mediation (standardized a × b) was calculated as the effect size measure (Preacher and Kelley, 2011). The same mediation analysis was performed with the OFC volumes as the mediator, trait optimism as the predictor and anxiety symptoms as the outcome as a test of the alternative hypothesis that brain volumes mediated the relation from personality to symptoms. To test for the specificity of the mediation effect to the OFC, the same mediation analysis was repeated for the other ROIs extracted (i.e. basal ganglia, rACC and IFG). All statistical analyses were performed using SPSS for Windows, Version 20.0 (IBM Corp., 2011).
Results
Increased trait optimism linked to increased OFC volume and decreased anxiety
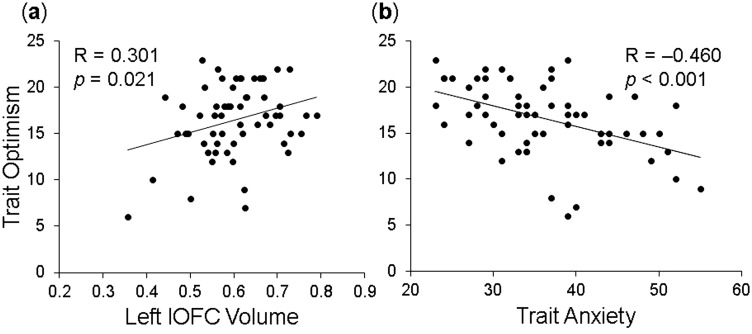
Confirming our first prediction, trait optimism was positively associated with the OFC volumes (for the left lOFC, R = 0.301, P = 0.021, shown in Figure 1; for the left mOFC, R = 0.310, P = 0.016; both surviving Bonferroni correction for laterality), and negatively associated with anxiety (R = −0.460, P < 0.001).
Fig. 1.
Increased trait optimism linked to increased GMVs in the left lateral orbitofrontal cortex (lOFC) and decreased anxiety scores. Presented here are scatterplots showing a significant positive correlation between trait optimism and the GMV of the left lOFC (a), and a significant negative correlation between trait optimism and anxiety symptoms (b).
Descriptive statistics and zero-order correlation coefficients among the major targeted personality and volumetric variables are reported in Table 1. Descriptive statistics for the exploratory variables are reported in Supplementary Table S1. There were no significant relations between depression, positive affect or negative affect and any of the OFC volumes (all P > 0.1), suggesting a selective relationship between our target personality traits (i.e. trait optimism and anxiety) and OFC volumes (Supplementary Table S2).
Trait optimism mediates the relationship between the OFC volume and anxiety
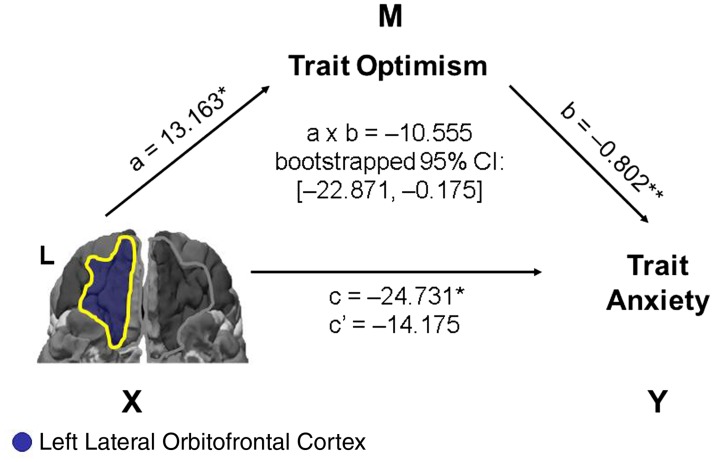
Confirming our second prediction, mediation analyses identified significant indirect negative effects of trait optimism on the relation between the left OFC volumes and anxiety. Two independent mediation models were constructed, with the left lOFC and mOFC as the predictors, trait optimism as the mediator and anxiety as the outcome variable. For the left lOFC model (Figure 2), the CI of indirect effect a × b did not contain zero, indicating that the indirect effect was significant (a = 13.163, P = 0.021; b = −0.802, P = 0.003; c = −24.731, P = 0.037; c′ = −14.175, P > 0.2; a × b = −10.555, bootstrapped 95% CI = [−22.871, −0.175]; N = 59, index of mediation = −0.117). A similar pattern was also identified for the left mOFC (a = 15.281, P = 0.016; b = −0.881, P = 0.001; c = −24.601, P = 0.064; c′ = −11.138, P > 0.3; a × b = −13.463, bootstrapped 95% CI = [−26.085, −0.741]; N = 60; index of mediation = −0.132).
Fig. 2.
Trait optimism mediates the relationship between the OFC volume and anxiety. Presented here is the mediation model showing the significant negative indirect effect of trait optimism on the relation between the GMV in the left lOFC and anxiety. Path a refers to the relation from the predictor variable, X, to the mediator variable, M, and path b refers to the relation from M to the outcome variable, Y, while controlling for X. Path c refers to the total effect from X to Y, and path c′ refers to the direct effect from X to Y controlling for M. The indirect effects were represented by the interaction term a × b, and the significance of these effects was tested using bootstrapped 95% confidence intervals (CIs). Unstandardized regression coefficients are displayed. *P < 0.05; **P < 0.01.
The two mediation models survived Bonferroni correction for laterality (for left lOFC, a × b = −10.555, bootstrapped 97.5% CI = [−29.037, −0.776]; for left mOFC a × b = −13.463, bootstrapped 97.5% CI = [−32.239, −1.578]).
To further probe the laterality effect, mediation analyses were repeated for the OFC ROIs in the right hemisphere, but none of the mediation models were significant. To explore the directionality of the two significant mediation models (i.e. left lOFC and mOFC), different model configurations were tested. The results showed that only the models with the OFC volumes entered as predictor variables were significant, and that anxiety mediated the relationship between the left lOFC (a = −24.731, P = 0.037; b = −0.180, P = 0.003; c = 13.163, P = 0.021; c′ = 8.612, P = 0.114; a × b = 4.551, bootstrapped 95% CI = [0.823, 9.861]; N = 60; index of mediation = 0.099) and the left mOFC (a = −24.601, P = 0.064; b = −0.198, P = 0.001; c = 15.281, P = 0.016; c′ = 10.417, P = 0.079; a × b = 4.864, bootstrapped 95% CI = [0.009, 9.834]; N = 60; index of mediation = 0.099) and optimism.
Similar analyses performed on the exploratory ROIs supported the specificity of the identified effects to the OFC (Supplementary Table S3). Despite significant positive correlations between the basal ganglia nuclei (left accumbens area, bilateral caudate and left pallidum) and optimism, and negative correlations between the basal ganglia nuclei (left accumbens area, bilateral caudate and left pallidum) and anxiety, mediation analyses did not identify significant mediating effects of optimism in the relation between any of these regions and anxiety. Similarly, the left rACC was positively correlated with optimism, but its correlation with anxiety was not significant. Finally, the IFG subregions (pars orbitalis, pars triangularis and pars opercularis) failed to show significant correlations with optimism; only the left pars opercularis was negatively correlated with anxiety, but there were no significant mediation effects.
Discussion
The present investigation yielded two main findings. First, higher OFC GMV was associated with increased optimism, which in turn was associated with reduced anxiety. Second, trait optimism mediated the relationship between the left OFC volume and anxiety. These findings provide initial evidence identifying the OFC as a neural marker of trait optimism, and demonstrate that its protective role against anxiety symptoms in healthy functioning is mediated by trait optimism. These findings will be discussed in turn below.
Increased trait optimism linked to increased OFC volume and decreased anxiety
The present volumetric investigation identifies the OFC GMV as a structural neural marker of trait optimism in healthy functioning. The association between optimism and the OFC volume considerably advances previous structural neuroimaging evidence, linking greater OFC volume to decreased negative affect and fewer stressful life experiences (Ansell et al., 2012), and functional neuroimaging evidence linking transient activation of the OFC with positive, reward-related and approach-oriented processing. Specifically, activation of the OFC has been consistently associated with processing of positive affect, such as the encoding of reward, value and pleasure (Kringelbach and Berridge, 2009; Grabenhorst and Rolls, 2011), approach-oriented coping strategies and processing (Eddington et al., 2007), and sensitivity to environmental changes (Kringelbach, 2005), which suggests that this region might contribute to the maintenance of positive self-evaluations when threatened (Flagan and Beer, 2013), and to the flexibility in coping strategies observed in optimistic individuals when facing adversity.
The current findings showing a negative association between trait optimism and anxiety symptoms in healthy young adults confirm previous evidence identifying trait optimism as a resilience factor in healthy (Scheier et al., 1994) and clinical (Zenger et al., 2010) adults. Optimism has been consistently associated with positive outcomes, such as improved subjective well-being and physical health, and has been shown to motivate active and persistent coping behavior, which are particularly beneficial in times of adversity (Nes and Segerstrom, 2006). Unrealistic optimism, however, has been associated with negative outcomes, such as imprudent financial decisions (Gibson and Sanbonmatsu, 2004; Puri and Robinson, 2007), poor goal achievement (Kappes and Oettingen, 2011) and impaired self-regulation of health-related behaviors (Oettingen and Wadden, 1991; Davidson and Prkachin, 1997). Overall, by identifying the OFC volume as an important neural marker of this personality trait, the present findings add to the extant evidence linking optimism with positive behavioral outcomes.
Trait optimism mediates the relationship between the OFC volumes and anxiety
The present study also showed a mediating role of trait optimism in the protective effect of the OFC GMV against anxiety in healthy participants. The finding that brain- but not the personality-level factors predicted anxiety in our mediation models provides support to the idea of a directional link from brain structures to personality-level variables (Montag et al., 2013), and is consistent with previous evidence linking structural neuroanatomical features of the human brain with individual cognitive and personality traits (Kanai and Rees, 2011; Gilaie-Dotan et al., 2014). However, because the mediation models tested in the present study did not distinguish the mediation effects between optimism and anxiety, further longitudinal studies are needed to clarify this issue. It is also possible that frequent engagement of processes tapping into the OFC function may result in increased volume in this region, but this alternative interpretation seems less likely given the lack of significance for the mediation models testing optimism as the predictor and the OFC as the mediating variable.
Despite the common finding in the functional literature regarding a valence-related dissociation between the medial and lateral OFC (e.g. Kringelbach, 2005), linked to processing of reward and punishment, the present study did not find such dissociations between these OFC regions and optimism or anxiety. This lack of dissociation may be due to potential (slight) differences between the aspects captured by functional and anatomical approaches (Buhle et al., 2014; Giuliani et al., 2011), or the ones captured by different measures. Specifically, optimism is a higher level trait that, beyond reflecting reward-related processing, also reflects individual differences in self-regulation and goal-directed behavior (Nes and Segerstrom, 2006; Carver et al., 2010). Consistent with this idea, there is evidence in the functional literature pointing to different brain regions sensitive to basic manipulation of valence (processing positive and negative pictures; Dolcos et al., 2004) and those linked to higher level of integration of valence-related information reflected by traits indexing individual differences in goal regulation (promotion vs prevention, Higgins et al., 2001; Eddington et al., 2007). Therefore, volumetric differences between the medial and lateral OFC might not be sensitive enough to capture the individual differences on trait optimism.
Consistent with the view that the left hemisphere is more sensitive to the processing of positive information (Davidson and Irwin, 1999; Hecht, 2013), our mediation effects were identified only in the left hemisphere. Previous evidence has shown that optimistic estimations of negative events activated the left IFG (Sharot et al., 2011), and temporary disruption to the activity in the left (but not right) IFG reduced individuals’ tendency to maintain positive expectancies (Sharot et al., 2012). In addition, emotion regulation strategies aimed to enhance positive emotional states (e.g. reappraisal), which are likely to underlie trait optimism, were linked to the activity in the left hemisphere, specifically the left medial OFC (Ochsner et al., 2002, 2004). Therefore, the left lateralization of the mediating effect of optimism in the present investigation may reflect the correspondence between the preference for positive information in optimistic individuals and the left-lateralized tendency in processing positive information in these cortical regions (Davidson and Irwin, 1999; Eddington et al., 2007).
However, the present study did not find a positive relationship between the right OFC and anxiety trait, as one may expect based on traditional functional evidence linking the right PFC with negative affect and avoidance motivation (Davidson and Irwin, 1999; Dolcos et al., 2004; Eddington et al., 2007; Harmon-Jones and Gable, 2009). Instead, our left-lateralization findings are consistent with more recent evidence acknowledging the problematic nature of frontal lateralizations involving distinctions among dimensions of anxiety (reviewed in Miller et al., 2013). This evidence suggests that anxiety is not a ‘monolithic phenomenon’ involving a single brain region or a single, consistent pattern of lateralization (Miller et al., 2013), and that different types of anxiety modulate PFC activity in distinct ways (Nitschke et al., 1999, 2001). For instance, worry/anxious apprehension activates a left PFC brain region, in contrast to anxious arousal, which activates a right-hemisphere region (Engels et al., 2007). Moreover, there is also evidence that hemispheric dissociations are found across groups, linked to the clinical status. Specifically, Eddington et al. (2009) showed that the left OFC was sensitive to promotion-related processing in healthy participants, whereas the right OFC was sensitive to prevention-related processing in depressed participants. The findings from the healthy group from that study suggest that our null results in the right hemisphere may reflect relatively weaker activation of avoidance system in healthy subjects. However, it is difficult to conclude whether the optimism and anxiety traits tested in the present study are generally mediated by different systems, or are modulated by different directions of one system.
Overall, the present mediation findings are important because they show promise that, by modifying brain- and/or personality-level factors, it is possible to change behavioral-level outcomes reflected in symptoms of anxiety, even in healthy functioning. The OFC volume has been shown to respond to significant changes in life (Sekiguchi et al., 2013), and cognitive therapies designed to impart an optimistic attitude hold promise in alleviating symptoms of emotional dysregulation and disturbances (Meevissen et al., 2011). The malleability of brain structures and trait-level resilience factors reflects the dynamic interaction between the brain and behavior, and suggests the possibility that resilience and well-being can indeed be ‘learned’ through training (Davidson and McEwen, 2012). Hence, by identifying concrete brain (OFC volume) and personality (trait optimism) factors influencing resilience against anxiety symptoms, the present investigation provides specific targets for future therapeutic and preventive interventions. Indeed, recent intervention studies have provided initial evidence supporting the effectiveness of targeting OFC in alleviating symptoms of anxiety. For instance, OFC was found to be responsive to a single session of cognitive-behavioral therapy in phobic patients, compared with a patient control group (Schienle et al., 2007). Two recent studies (Scheinost et al., 2013, 2014) have shown that modulating OFC activity through real-time neurofeedback significantly improved anxiety symptoms in both healthy subjects and clinical patients, and that baseline global connectivity between the OFC and the rest of the brain predicted greater improvement as the result of the intervention. Importantly, the beneficial effects of OFC training were seen days after the last training session, thus possibly reflecting more persistent neural changes in the OFC. Overall, these complementary lines of evidence emphasize that knowledge about the OFC can be used to identify individuals more likely to benefit from training, and that OFC interventions can enable enhanced control over anxiety, possibly leading to persistent anatomical reorganization of the OFC and its networks. Clearly, longitudinal studies are needed to establish the speculated long-term impact of interventions targeting OFC.
In summary, the present study responds to the increasing need to identify new bio-psychological markers of resilience against emotional dysregulation, in general, and anxiety symptoms, in particular. Overall, the present findings shed light on the OFC GMV as a neural marker for trait optimism, and demonstrate the role of this personality trait in mediating the protective role of the OFC volume against anxiety. These results provide initial evidence about the brain–personality mechanisms protecting against anxiety, and inform the development of therapeutic and preventive interventions aimed at reducing susceptibility to and increasing resilience against symptoms of affective dysregulation and emotional disturbances, to promote overall psychological well-being.
Author contributions
S.D. and F.D. conceived the research project, S.D. contributed to data collection, S.D. and A.I. provided analytical tools, S.D., Y.H. and M.M. analyzed data, Y.H., S.D., and F.D. wrote the manuscript, A.I. and M.M. provided comments on the manuscript and all authors approved the content of the manuscript.
Supplementary Material
Acknowledgment
The authors thank members of the Dolcos Laboratory for assisting with data collection.
Funding
This work was supported by the National Alliance for Research on Schizophrenia and Depression (currently, the Brain & Behavior Research Foundation to F.D.), the Canadian Psychiatric Research Foundation (currently, Healthy Minds Canada), the Canadian Institutes of Health Research, the University Hospital Foundation, the University of Alberta and the University of Illinois.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Andersson G. (1996). The benefits of optimism: A meta-analytic review of the life orientation test. Personality and Individual Differences , 21(5), 719–25. [Google Scholar]
- Ansell E.B., Rando K., Tuit K., Guarnaccia J., Sinha R. (2012). Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biological Psychiatry , 72(1), 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. (2013). The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage , 76, 412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson M., Brilot B., Nettle D. (2011). Anxiety: An evolutionary approach. Canadian Journal of Psychiatry , 56(12), 707–15. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Garbin G.M. (1988). Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review , 88, 77–100. [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. (1961). An inventory for measuring depression. Archives of General Psychiatry , 4, 561–71. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex , 24(11), 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver C.S., Scheier M.F. (2014). Dispositional optimism. Trends in Cognitive Sciences , 18(6), 293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver C.S., Scheier M.F., Segerstrom S.C. (2010). Optimism. Clinical Psychology Review , 30(7), 879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Avanzato C., Joormann J., Siemer M., Gotlib I.H. (2013). Emotion regulation in depression and anxiety: Examining diagnostic specificity and stability of strategy use. Cognitive Therapy and Research , 37(5), 968–80. [Google Scholar]
- Davidson K., Prkachin K. (1997). Optimism and unrelistic optimism have an interacting impact on health-promoting behavior and knowledge changes. Personality and Social Psychology Bulletin , 23(6), 617–25. [Google Scholar]
- Davidson R.J., Irwin W. (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences , 3(1), 11–21. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., McEwen B.S. (2012). Social influences on neuroplasticity: Stress and interventions to promote well-being. Nature Neuroscience , 15(5), 689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage , 31(3), 968–80. [DOI] [PubMed] [Google Scholar]
- DeYoung C.G., Hirsh J.B., Shane M.S., Papademetris X., Rajeevan N., Gray J.R. (2010). Testing predictions from personality neuroscience: Brain structure and the big five. Psychological Science , 21(6), 820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., LaBar K.S., Cabeza R. (2004). Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. NeuroImage , 23(1), 64–74. [DOI] [PubMed] [Google Scholar]
- Eddington K.M., Dolcos F., Cabeza R., Krishnan K.R.R., Strauman T.J. (2007). Neural correlates of promotion and prevention goal activation: An fMRI study using an idiographic approach. Journal of Cognitive Neuroscience , 19(7), 1152–62. [DOI] [PubMed] [Google Scholar]
- Eddington K.M., Dolcos F., McLean A.N., Krishnan K.R., Cabeza R., Strauman T.J. (2009). Neural correlates of idiographic goal priming in depression: Goal-specific dysfunctions in the orbitofrontal cortex. Social Cognitive and Affective Neuroscience , 4(3), 238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels A.S., Heller W., Mohanty A., et al. (2007). Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology , 44(3), 352–63. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Buchner A., Lang A.G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods , 41(4), 1149–60. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods , 39(2), 175–91. [DOI] [PubMed] [Google Scholar]
- Fischl B. (2012). FreeSurfer. NeuroImage , 62(2), 774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagan T., Beer J.S. (2013). Three ways in which midline regions contribute to self-evaluation. Frontiers in Human Neuroscience , 7, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M.S., Mackinnon D.P. (2007). Required sample size to detect the mediated effect. Psychological Science , 18(3), 233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson B., Sanbonmatsu D.M. (2004). Optimism, pessimism, and gambling: The downside of optimism. Personality and Social Psychology Bulletin , 30(2), 149–60. [DOI] [PubMed] [Google Scholar]
- Gilaie-Dotan S., Tymula A., Cooper N., Kable J.W., Glimcher P.W., Levy I. (2014). Neuroanatomy predicts individual risk attitudes. Journal of Neuroscience , 34(37), 12394—401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Drabant E.M., Gross J.J. (2011). Anterior cingulate cortex volume and emotion regulation: Is bigger better? Biological Psychology, 86(3), 379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. (2011). Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences , 15(2), 56–67. [DOI] [PubMed] [Google Scholar]
- Grupe D.W., Nitschke J.B. (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience , 14(7), 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E., Gable P.A. (2009). Neural activity underlying the effect of approach-motivated positive affect on narrowed attention. Psychological Science , 20(4), 406–9. [DOI] [PubMed] [Google Scholar]
- Hecht D. (2013). The neural basis of optimism and pessimism. Experimental Neurobiology , 22(3), 173–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins E.T., Friedman R.S., Harlow R.E., Ayduk O.N., Tylor A. (2001). Achievement orientations from subjective histories of success: Promotion pride versus prevention pride. European Journal of Social Psychology, 31(1), 3–23. [Google Scholar]
- Holmes A., Lee P., Hollinshead M., et al. (2012). Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. Journal of Neuroscience , 32(50), 18087–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (2011). IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. [Google Scholar]
- Insel T., Cuthbert B., Garvey M., et al. (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. The American Journal of Psychiatry , 167(7), 748–51. [DOI] [PubMed] [Google Scholar]
- Kanai R., Rees G. (2011). The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience , 12(4), 231–42. [DOI] [PubMed] [Google Scholar]
- Kappes H.B., Oettingen G. (2011). Positive fantasies about idealized futures sap energy. Journal of Experimental Social Psychology , 47(4), 719–29. [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. (2005a). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry , 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Walters E.E. (2005b). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry , 62(6), 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L. (2005). The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience , 6(9), 691–702. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L., Berridge K.C. (2009). Towards a functional neuroanatomy of pleasure and happiness. Trends in Cognitive Sciences , 13(11), 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S., Gallinat J., Brass M. (2011). “Keep calm and carry on”: Structural correlates of expressive suppression of emotions. PLoS One , 6(1), 216569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. (2011). Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews , 35(5), 1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn N., Dolcos S., Iordan A.D., Rudolph K.D., Dolcos F. (2013). Reappraisal and suppression mediate the contribution of regulatory focus to anxiety in healthy adults. Emotion , 13(4), 610–5. [DOI] [PubMed] [Google Scholar]
- Meevissen Y.M.C., Peters M.L., Alberts H.J.E.M. (2011). Become more optimistic by imagining a best possible self: Effects of a two week intervention. Journal of Behavior Therapy and Experimental Psychiatry , 42(3), 371–8. [DOI] [PubMed] [Google Scholar]
- Miller G.A., Crocker L.D., Spielberg J.M., Infantolino Z.P., Heller W. (2013). Issues in localization of brain function: The case of lateralized frontal cortex in cognition, emotion, and psychopathology. Frontiers in Integrative Neuroscience , 7, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C., Reuter M., Jurkiewicz M., Markett S., Panksepp J. (2013). Imaging the structure of the human anxious brain: A review of findings from neuroscientific personality psychology. Reviews in the Neurosciences , 24(2), 167–90. [DOI] [PubMed] [Google Scholar]
- Nes L.S., Segerstrom S.C. (2006). Dispositional optimism and coping: A meta-analytic review. Personality and Social Psychology Review , 10(3), 235–51. [DOI] [PubMed] [Google Scholar]
- Nitschke J.B., Heller W., Imig J.C., McDonald R.P., Miller G.A. (2001). Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research , 25(1), 1–22. [Google Scholar]
- Nitschke J.B., Heller W., Palmieri P.A., Miller G.A. (1999). Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology , 36(5), 628–37. [PubMed] [Google Scholar]
- Oettingen G., Wadden T.A. (1991). Expectation, fantasy, and weight loss: Is the impact of positive thinking always positive?. Cognitive Therapy and Research , 15(2), 167–75. [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D.E. (2002). Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience , 14(8), 1215–29. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage , 23(2), 483–99. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Lempert K.M., Sokol-Hessner P. (2014). Emotion and decision making: Multiple modulatory neural circuits. Annual Review of Neuroscience , 37, 263–87. [DOI] [PubMed] [Google Scholar]
- Porcelli A.J., Lewis A.H., Delgado M.R. (2012). Acute stress influences neural circuits of reward processing. Frontiers in Neuroscience , 6, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers , 36(4), 717–31. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Kelley K. (2011). Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychological Methods , 16(2), 93–115. [DOI] [PubMed] [Google Scholar]
- Puri M., Robinson D. (2007). Optimism and economic choice. Journal of Financial Economics , 86(1), 71–99. [Google Scholar]
- Scheier M.F., Carver C.S., Bridges M.W. (1994). Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology , 67(6), 1063–78. [DOI] [PubMed] [Google Scholar]
- Scheinost D., Stoica T., Saksa J., et al. (2013). Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting-state connectivity. Translational Psychiatry , 3, e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D., Stoica T., Wasylink S., et al. (2014). Resting state functional connectivity predicts neurofeedback response. Frontiers in Behavioral Neuroscience , 8, 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A., Schafer A., Hermann A., Rohrmann S., Vaitl D. (2007). Symptom provocation and reduction in patients suffering from spider phobia: An fMRI study on exposure therapy. European Archives of Psychiatry and Clinical Neuroscience , 257(8), 486–93. [DOI] [PubMed] [Google Scholar]
- Sekiguchi A., Sugiura M., Taki Y., et al. (2013). Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Molecular Psychiatry , 18(5), 618–23. [DOI] [PubMed] [Google Scholar]
- Sescousse G., Caldu X., Segura B., Dreher J.C. (2013). Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews , 37(4), 681–96. [DOI] [PubMed] [Google Scholar]
- Sharot T., Kanai R., Marston D., Korn C.W., Rees G., Dolan R.J. (2012). Selectively altering belief formation in the human brain. Proceedings of the National Academy of Sciences of the United States of America , 109(42), 17058–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T., Korn C.W., Dolan R. (2011). How unrealistic optimism is maintained in the face of reality. Nature Neuroscience , 14(11), 1475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J.M., Miller G.A., Engels A.S., et al. (2011). Trait approach and avoidance motivation: Lateralized neural activity associated with executive function. NeuroImage , 54(1), 661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. (1970). Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E., Vagg P.R., Jacobs G.A. (1983). Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stevens J.P. (2009). Applied Multivariate Statistics for the Social Sciences, 5 edn New York, NY: Routledge Academic. [Google Scholar]
- Talati A., Pantazatos S.P., Schneier F.R., Weissman M.M., Hirsch J. (2013). Gray matter abnormalities in social anxiety disorder: Primary, replication, and specificity studies. Biological Psychiatry , 73(1), 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Burden of Disease Collaborators. (2013). The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. The Journal of American Medial Association , 310(6), 591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology , 54(6), 1063–70. [DOI] [PubMed] [Google Scholar]
- Wu G., Feder A., Cohen H., et al. (2013). Understanding resilience. Frontiers in Behavioral Neuroscience , 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenger M., Brix C., Borowski J., Stolzenburg J.U., Hinz A. (2010). The impact of optimism on anxiety, depression and quality of life in urogenital cancer patients. Psycho-Oncology , 19(8), 879–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.