Abstract
Self-control refers to the capacity to override or alter a predominant response tendency. The current experiment tested the hypothesis that exercising self-control temporarily increases approach motivation, as revealed by patterns of electrical activity in the prefrontal cortex. Participants completed a writing task that did vs did not require them to exercise self-control. Then they viewed pictures known to evoke positive, negative or neutral affect. We assessed electroencephalographic (EEG) activity while participants viewed the pictures, and participants reported their trait levels of behavioral inhibition system (BIS) and behavioral activation system (BAS) sensitivity at the end of the study. We found that exercising (vs not exercising) self-control increased relative left frontal cortical activity during picture viewing, particularly among individuals with relatively higher BAS than BIS, and particularly during positive picture viewing. A similar but weaker pattern emerged during negative picture viewing. The results suggest that exercising self-control temporarily increases approach motivation, which may help to explain the aftereffects of self-control (i.e. ego depletion).
Keywords: approach motivation, ego depletion, frontal asymmetry, self-control, relative left frontal cortical activity
Introduction
Self-control facilitates goal pursuits such as losing weight, breaking habits and persisting at tedious chores. Despite increasing the likelihood of long-term benefits, self-control may also carry short-term costs. Numerous experiments have observed that overriding or altering a predominant response tendency temporarily reduces success at subsequent self-control attempts (Hagger et al., 2010). The leading account for this behavioral pattern, known as the ego depletion effect, is that exercising self-control consumes a limited inner resource or strength, and in the interim period before the resource is replenished further efforts at self-control are prone to failure (Muraven and Baumeister, 2000).
An alternative, non-resource-based account for the ego depletion effect has been proposed. According to the process model of ego depletion, self-control is an aversive act that causes shifts in motivational orientation away from self-control and toward more immediately gratifying behaviors (Inzlicht and Schmeichel, 2012; Inzlicht et al., 2014). In this view, the aftereffects of self-control stem in part from increased approach motivation (which can be defined as physical or psychological orienting toward reward and incentive; see Elliot et al., 2013). The current experiment tested the hypothesis that exercising self-control increases approach motivation as revealed by patterns of electrical activity in the prefrontal cortex.
Shifts in motivational orientation after exercising self-control
The process model of ego depletion received initial support in an experiment testing the aftereffects of self-control on self-reported approach motivation (Schmeichel et al., 2010). Participants either did or did not suppress their emotions while viewing aversive images and then completed Carver and White’s (1994) behavioral inhibition system and behavioral activation system (BIS/BAS) scales. Participants who had suppressed their emotions reported higher BAS and non-significantly lower BIS compared to other participants. In a second experiment, participants who inhibited (vs did not inhibit) the use of two common letters on a writing task went on to take more risks on a low-stakes gambling game, a risk-taking tendency that had been positively correlated with BAS in a separate study. In a third experiment, the same self-controlled writing task increased performance on a subsequent perceptual acuity test involving the detection of a ubiquitous reward-related symbol—dollar signs.
The results from Schmeichel et al. (2010) were consistent with the prediction that exercising self-control increases approach motivation. However, one could question the extent to which the dependent measures they used were sensitive mainly to variations in approach motivation or whether other variables (e.g. BIS) may also have played a role. Further, their findings did not address potential individual difference moderators underlying the observed effects. This is important because individual differences in approach motivation have been found to moderate the aftereffects of self-control (Crowell et al., 2014). The current experiment took individual differences in BIS and BAS into account and tested the hypothesis that exercising self-control causes a shift toward increased approach-related patterns of electrical activity in the prefrontal cortex.
Motivational orientation and asymmetric frontal cortical activity
Asymmetric electrical activity in the prefrontal cortex covaries with motivational orientation. BAS sensitivity and approach motivation are positively associated with relatively more activity in the left vs right side of the prefrontal cortex (Harmon-Jones and Allen, 1997; Sutton and Davidson, 1997; Coan and Allen, 2003; Barrós-Loscertales et al., 2010; see review by Harmon-Jones et al., 2010), and experimental manipulations to increase relative left frontal cortical activity have been found to increase approach-motivated responding (Harmon-Jones et al., 2008; Kelley et al., in press). Conversely, BIS sensitivity, withdrawal motivation and anxiety have been positively associated with relatively more activity in the right side of the prefrontal cortex (Davidson et al., 2000; Shackman et al., 2009; Sutton and Davidson, 1997; cf. Coan and Allen, 2003; Harmon-Jones and Allen, 1997; Hewig et al., 2006).
To further illuminate the motivational consequences of exercising self-control, in the current experiment we manipulated the exercise of self-control and then assessed electroencephalographic (EEG) activity in the prefrontal cortex while participants viewed pictures. We also measured individual differences in BIS/BAS. Based on the process model of ego depletion, we predicted that exercising self-control would cause a subsequent increase in relative left prefrontal cortical activity, suggesting a shift toward increased approach motivation. As described below, we further expected that the aftereffects of self-control may be moderated by the type of picture being viewed and by individual differences in BIS and BAS, respectively.
Role of individual differences in BIS and BAS
One method for elucidating mechanisms underlying the effect of an experimental manipulation is to examine the contribution of individual differences that influence the tendency to engage the proposed mechanisms (Gohm and Clore, 2000; see Underwood, 1975). The current study concerned the effect of exercising self-control on asymmetric frontal cortical activity, and the mechanism presumed to underlie this effect is a shift in motivational orientation toward increased approach motivation. Individual differences in BAS have been linked to variations in relative left frontal cortical activity (Harmon-Jones and Allen, 1997). Evidence that BAS moderates the aftereffects of exercising self-control on relative left frontal cortical activity would thus lend support to the idea that a shift toward increased approach motivation may be one mechanism by which exercising self-control influences subsequent behavior.
A similar line of reasoning applies to BIS. BIS is thought to underlie inhibition, anxiety and sensitivity to response conflict (Shackman et al., 2009). Individual differences in BIS have been linked to variations in asymmetrical frontal cortical activity (Sutton and Davidson, 1997) and to neural signals of response monitoring and conflict detection (Amodio et al., 2008). Further, some evidence suggests that BIS-related neural activity is reduced under ego depletion (Inzlicht and Gutsell, 2007; see also Friese et al., 2014). Evidence that BIS moderates the effects of exercising self-control on relative left frontal cortical activity would thus suggest that shifts away from behavioral inhibition or response monitoring may help to account for the aftereffects of self-control.
It is also plausible that individuals who are both lower in BIS and higher in BAS are particularly prone to exhibit increased approach motivation after exercising self-control. According to the joint subsystems hypothesis (Corr, 2001), BIS and BAS interact to influence approach-related behavior, such that approach-related states may be more pronounced among individuals both higher in BAS sensitivity and lower in BIS sensitivity (Gomez et al., 2004; Mortensen et al., 2015). Indeed, it may be precisely those individuals with higher BAS than BIS who exhibit the greatest relative left prefrontal cortical activity (Sutton and Davidson, 1997).
Role of affective context
Because approach motivation is thought to influence responding to positive or appetitive stimuli, we reasoned that shifts toward increased approach motivation would be most likely to occur when participants viewed positive (as opposed to negative or neutral) picture stimuli. To be sure, an increase in relative left frontal cortical activity during neutral or negative picture viewing would also be consistent with the hypothesis that exercising self-control increases approach motivation. However, we favored the more modest hypothesis that the effects of prior self-control would emerge mainly in affective contexts relevant to approach motivation (i.e. during positive picture viewing) and mainly among individuals prone to approach motivation (i.e. those with higher BAS and lower BIS sensitivity). These predictions are compatible with evidence that even relatively brief exposure to pictures can elicit changes in relative left prefrontal cortical activity in some individuals (Harmon-Jones et al., 2006; Gable and Poole, 2014; see also Costumero et al., 2013).
The current experiment
Participants wrote a story that did vs did not require them to exercise self-control. Then they viewed pictures known to evoke positive, negative or neutral affect. We assessed EEG activity while participants viewed the pictures, and at the end of the study participants reported their trait levels of BIS and BAS sensitivity. We predicted that exercising (vs not exercising) self-control would increase relative left prefrontal cortical activity during picture viewing, particularly among individuals predisposed to greater relative left frontal cortical activation and particularly during positive picture viewing.
Method
Participants
Seventy-eight undergraduate students (39 women and 39 men; age M = 18.94, SD = 1.17) completed the experiment in exchange for credit toward a course requirement. Twenty additional participants completed the study but were excluded from analyses for the following reasons: 14 had missing EEG data due to computer errors, 5 had excessive muscle artifacts in their EEG data and 1 did not complete the writing task.1
Materials and procedure
After participants provided informed consent, an experimenter attached sensors to participants’ heads using 59 tin electrodes in a stretch-lycra electrode cap. Electrodes were also placed on participants’ earlobes for offline re-referencing. EEG electrode impedances were below 5000 Kohms, and differences in impedance at homologous sites were below 1000 Kohms.2
After cap placement and a 4-min period for recording electrical activity in the brain at rest, participants completed a modified flanker task adapted from Eriksen and Eriksen (1974). The flanker task measured individual differences in neural responses to errors; results associated with this task will not be presented here.
Participants then completed a writing task that served as the manipulation of self-control. Participants in the free writing condition were instructed to write a story about a recent trip they had taken. Participants in the controlled writing condition were instructed to write a story about a recent trip they had taken but to refrain from using the letters a or n. Thus, one group had to exercise self-control over the use of two commonly-used letters and the other group wrote without restrictions. This manipulation has previously been used to induce a state of ego depletion (Schmeichel, 2007; Lewandowski et al., 2012).
Following the self-control manipulation participants viewed a series of images on a computer screen. The first four images were neutral practice trials and were not analyzed. Participants then viewed 19 positive, 19 neutral and 19 negative pictures from the International Affective Picture System (IAPS; Lang et al., 2008). Positive pictures featured individuals involved in exciting or fun activities. Neutral pictures mainly featured individuals in mundane activities. Negative pictures mainly featured acts of violence and mutilations. Pictures were presented in randomized order provided that pictures of the same type (e.g. negative) never appeared in succession.
Picture viewing trials proceeded as follows. A fixation cross appeared onscreen for 3 s, followed by a picture for 6 s, and an inter-trial interval of 8–12 s. Then another fixation cross and picture appeared onscreen. The image viewing task lasted 18 min.
Following the image viewing task participants sat quietly for 4 min to permit another recording of resting brain activity. Then participants completed questionnaires including the BIS/BAS scales (Carver and White, 1994) using a response scale from 1 (strongly disagree) to 4 (strongly agree). The mean total score on the 13-item BAS scale (e.g. ‘If I see a chance to get something I want I move right away’) in the present sample was 39.67 (SD = 5.25; α = 0.82). The mean total score on the seven-item BIS scale (e.g. ‘I worry about making mistakes’) was 20.19 (SD = 3.79; α = 0.80).3 Last, participants were debriefed about the purpose of the experiment and dismissed.
Psychophysiological recording and quantification
EEG signals were amplified with Neuroscan SynAmps2 (El Paso, TX), bandpass filtered (0.05–100 Hz), notch filtered (60 Hz) and digitized at 500 Hz. Eye movements were recorded from an electrode at FP2 (10–20 placement system). Artifacts (e.g. horizontal eye movements, muscle movements) were first removed by hand. Then a regression-based eye movement correction was applied to correct vertical eye movements (Semlitsch et al., 1986), after which the data were again visually inspected to ensure proper correction.
All 1.024-s epochs were extracted through a Hamming window. A fast Fourier transformation extracted power within the alpha (8–13 Hz) frequency range. For the picture viewing task, an average frontal index (AF3/4, F1/2, F3/4, F5/6, F7/8) was created for the first 3 s of each picture, and positive, negative and neutral picture indices of frontal asymmetry were calculated (natural log right minus natural log left). Alpha power is inversely related to cortical activity, so higher scores indicate greater left than right frontal cortical activity.4
Results
Asymmetric frontal activity during picture viewing as a function of experimental condition
A 2 (Writing Condition) × 3 (Picture Type) mixed-model analysis of variance (ANOVA) on relative left frontal activity during picture viewing yielded a non-significant within-subjects effect of picture type, F (2, 152) < 1, P = 0.41. This null effect is consistent with past research and suggests that viewing emotional vs neutral pictures does not alter asymmetric frontal cortical activity (Harmon-Jones, 2007; Gable and Harmon-Jones, 2008; Uusberg et al., 2014). In addition, no other main effects or interactions occurred, Fs < 1, ps > 0.70.
Asymmetric frontal activity during positive picture viewing as a function of condition and trait BIS/BAS
Our primary prediction was that performing the controlled writing task (vs the free writing task) would increase relative left frontal cortical activation to positive pictures particularly among individuals prone to increased approach motivation. We regressed relative left frontal cortical activity during positive picture viewing on writing condition (0 = free writing; 1 = controlled writing), trait BIS (centered), trait BAS (centered) and their interactions. See Table 1. The predicted two-way interactions were significant and in line with predictions, but the three-way interaction among writing condition, BIS, and BAS was non-significant.5
Table 1.
Summary of multiple regression predicting relative left frontal cortical activity during positive picture viewing
| Variable | B | SE B | β |
|---|---|---|---|
| Step 1 | |||
| Writing condition | −0.01 | 0.06 | −0.03 |
| BIS | 0.00 | 0.01 | −0.00 |
| BAS | 0.00 | 0.01 | 0.05 |
| Step 2 | |||
| Writing condition × BIS | −0.04 | 0.02 | −0.45* |
| Writing condition × BAS | 0.02 | 0.01 | 0.35* |
| BIS × BAS | 0.00 | 0.00 | −0.02 |
| Step 3 | |||
| Writing condition × BIS × BAS | −0.00 | 0.00 | −0.20 |
Notes: BIS and BAS were centered at their means. Writing condition coded 0 = free writing condition, 1 = controlled writing condition; BIS = behavioral inhibition sensitivity; BAS = behavioral activation sensitivity.
*P < 0.05.
Because this study may have lacked statistical power to detect a three-way interaction among writing condition, BAS and BIS, we reduced the number of predictor variables by calculating a relative BAS-BIS score to capture individual differences in the relative strength of the BAS over the BIS.6 Based on the joint subsystem hypothesis (Corr, 2001) and following the approach used by Sutton and Davidson (1997), we subtracted the z-transformed BIS score from the z-transformed BAS score for each participant (M = 0.00, SD = 1.29) to arrive at a BAS-BIS difference score; higher scores reflect greater relative BAS. Then we regressed relative left frontal cortical activity during positive picture viewing on writing condition, the BAS-BIS difference score and their interaction term.
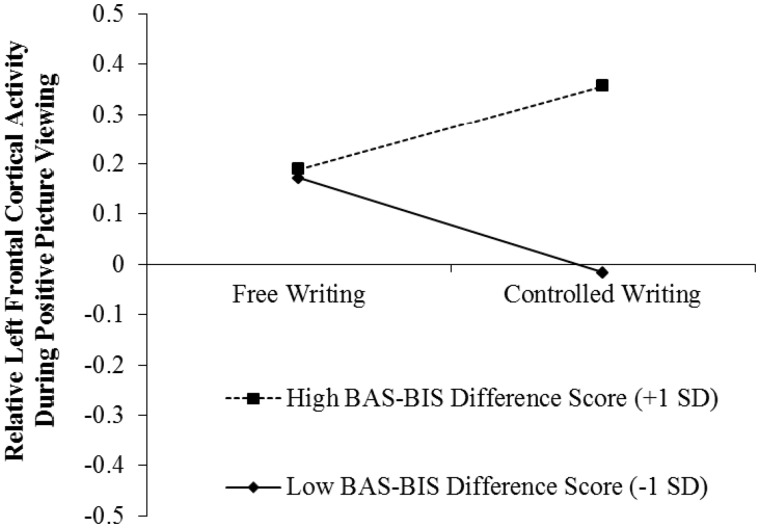
Consistent with expectations we found a significant interaction between writing condition and the BAS-BIS difference score, B = 0.14, t (74) = 3.15, P = 0.002.7 Simple slopes analysis revealed an increase in relative left frontal cortical activity among participants with a higher BAS-BIS difference score in the controlled writing vs free writing condition, B = 0.17, t (74) = 2.08, P = 0.041. Among those with a lower BAS-BIS difference score, the simple slope was significant in the opposite direction, B = −0.19, t (74) = 2.32, P = 0.023. These results are displayed in Figure 1.
Fig. 1.
Relative left frontal cortical activity during positive picture viewing as a function of experimental condition and trait BAS-BIS difference score.
Asymmetric frontal activity during negative picture viewing as a function of condition and trait BIS/BAS
We repeated the regression analysis, this time with frontal asymmetry during negative pictures as the criterion variable and the same predictors as above. The only significant predictor in this analysis was an unexpected interaction between writing condition and BIS, b = −0.05, t (74) = 2.57, P = 0.012. No other effects were significant, ps > 0.17.8
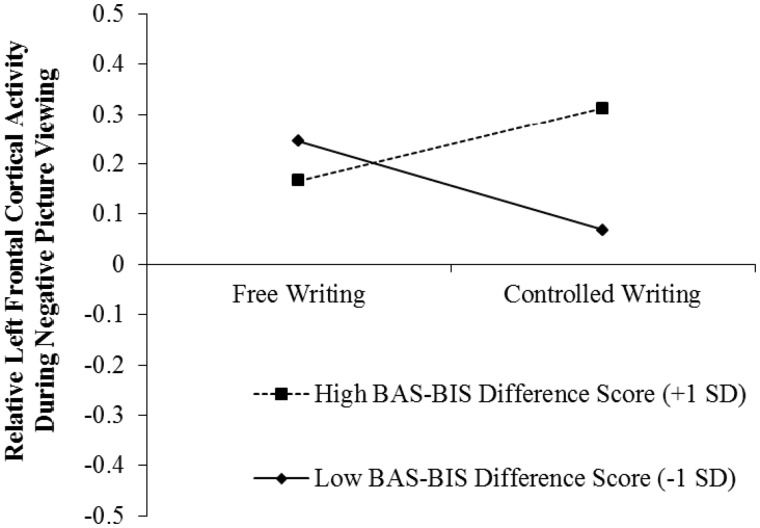
We repeated the analysis, this time including the BAS-BIS difference score as the individual difference variable to reduce the number of predictor variables and increase statistical power. Here again we observed a significant interaction between writing condition and the BAS-BIS difference score, B = 0.13, t (74) = 2.40, P = 0.019. This interaction is displayed in Figure 2. Simple slopes analyses found no significant changes in relative left frontal cortical activity among participants with a higher BAS-BIS difference score in the controlled writing vs free writing condition, B = 0.15, t (74) = 1.54, P = 0.128, or among those with a lower BAS-BIS difference score, B = −0.18, t (74) = 1.85, P = 0.069. Although neither of these simple slopes was significant, the overall patterns were similar to those observed during positive picture viewing.9
Fig. 2.
Relative left frontal cortical activity during negative picture viewing as a function of experimental condition and trait BAS-BIS difference score.
Asymmetric frontal activity during neutral picture viewing as a function of condition and trait BIS/BAS
We repeated the regression analysis a third time, this time with relative left frontal cortical activity during neutral pictures as the criterion variable. None of the predictors were significant in this model, ps > 0.09. We also found null results during neutral picture viewing using the BAS-BIS difference score as the individual difference variable, ps > 0.14.
Discussion
The current experiment found that exercising self-control causes a subsequent increase in relative left frontal cortical activity among individuals prone to higher levels of approach motivation. More specifically, among participants who inhibited (vs did not inhibit) the use of two commonly-used letters on a writing task, higher BAS-BIS difference scores (which reflect individual differences in the relative strength of the BAS over the BIS) predicted increases in relative left frontal cortical activity while viewing positive pictures. Conversely, lower BAS-BIS difference scores predicted a reduction in relative left frontal cortical activity among participants who inhibited (vs did not inhibit) responding on the writing task.
The aftereffects of the self-control manipulation and approach-proneness on relative left frontal cortical activity did not emerge in response to neutral pictures. However, an unexpected effect emerged in response to negative pictures. Among participants who inhibited (vs did not inhibit) the use of specific letters on a writing task, BAS-BIS difference scores predicted changes in relative left frontal cortical activity while viewing negative pictures in a manner similar to but somewhat weaker than the changes observed during positive picture viewing. Although this pattern of results is consistent with the hypothesis that exercising self-control increases approach-related brain activity, we did not predict increased relative left frontal cortical activity during negative picture viewing.
Evidence for increased relative left frontal activity during both positive and negative picture viewing may be consistent with a capability model of frontal asymmetry (Coan et al., 2006; Stewart et al., 2014). According to this view, emotional challenges may more evoke more approach or withdrawal motivation depending on the predisposition or personality of a particular individual. Thus, when encountering emotional stimuli high BAS/low BIS individuals may respond in a more approach-oriented way (greater left than right frontal activity) to both pleasant and unpleasant stimuli relative to someone who is low BAS/high BIS. We prefer to refrain from drawing strong conclusions from the negative picture viewing findings observed in the current study until the pattern can be replicated in a separate study.
According to the process model of ego depletion (Inzlicht and Schmeichel, 2012; Inzlicht et al., 2014), exercising self-control causes lingering shifts in motivation and attention that should be evident in a wide variety of responses, including responses that entail little or no self-control. The current study found that exercising self-control caused subsequent shifts in relative left prefrontal cortical activity while participants simply looked at emotional pictures. This result suggests that the aftereffects of self-control are not limited to behavioral measures of self-control and can be glimpsed in neural activity during a passive viewing task.
The shifts toward increased relative left prefrontal cortical activity in the controlled writing condition occurred specifically among persons with higher BAS than BIS sensitivity. The writing manipulation did not exert a main effect on prefrontal cortical activity. This pattern of findings suggests that acts of self-control do not influence prefrontal cortical activity in a uniform fashion. The evidence that approach-prone persons were particularly likely to show increased relative left frontal cortical activity after exercising self-control is congruent with the joint subsystem hypothesis, which proposes that approach-motivated responding is likely to be strongest among persons both higher in BAS and lower in BIS (Corr, 2001). The current results are also consistent with the view that approach motivation is one mechanism by which exercising self-control may influence subsequent behavior. One approach to identifying mechanisms for experimental effects is to test whether those effects are moderated by relevant individual differences (Underwood, 1975). Following the process model of self-control (Inzlicht and Schmeichel, 2012), we have proposed that a shift toward increased approach motivation is one mechanism by which exercising self-control influences subsequent behavior. If that is correct, then participants who are prone to higher levels of approach motivation should exhibit more pronounced effects of prior self-control exertions, particularly on dependent measures that are relevant to approach motivation. The current findings strongly support this view (see also Crowell et al., 2014).
The observed shifts in relative left prefrontal cortical activity also fit well with the results of one of the only other studies of ego depletion to include EEG recordings. Inzlicht and Gutsell (2007) found that a prior exercise of self-control reduces the magnitude of the error-related negativity (ERN). The ERN is a characteristic spike in activity following the commission of an error that has been linked to the action-monitoring function of the anterior cingulate cortex (Van Veen and Carter, 2002; Botvinick et al., 2004; Herrmann et al., 2004). Exercising self-control thus appears to reduce electro-cortical activity associated with action monitoring and to increase activity associated with approach motivation. Not coincidentally, these patterns of brain activity have been associated in previous research (Nash et al., 2012).
Evidence of increased approach motivation and reduced action monitoring following the exertion of self-control lends support to the process model of ego depletion, which emphasizes the attentional and motivational consequences self-control exertion (Inzlicht and Schmeichel, 2012). According to this view, the behavioral decrements in self-control associated with the ego depletion effect stem from characteristic shifts in motivation and attention. The current study provided novel support for the idea that exercising self-control causes shifts in motivation as revealed by asymmetric electrical activity in the prefrontal cortex.
Conflict of interest. None declared.
Footnotes
1 Fifteen more students received credit for participating but did not complete the study due to time constraints.
2 To measure startle eyeblink responses two 9-mm tin electrodes were placed over participants' left inferior orbicularis oculi below the inner and outer canthi. During the picture viewing task (described below), some images were accompanied by loud bursts of noise occurring 3.5 s after picture onset. Data pertaining to startle eyeblink responses will not be presented here.
3 Neither BIS nor BAS scores differed as a function of writing condition: BAS, t (76) = 1.13, P = 0.26; BIS, t (76) = 1.45, P = 0.15.
4 Resting frontal asymmetry did not correlate with self-reported BAS, r (76) = −0.01, P = 0.923, BIS, r = 0.07, P = 0.574, or BAS-BIS, r (76) = −0.06, P = 0.610 (see also Wacker et al., 2010). Further, resting asymmetry did not differ as a function of experimental condition, t (76) = −0.13, P = 0.894, suggesting that random assignment to condition was successful in distributing these traits equally across the two conditions.
5 We repeated this analysis and added baseline resting relative left frontal cortical activity as a first-level predictor. Greater baseline resting relative left frontal cortical activity predicted greater left frontal cortical activity during positive picture viewing, b = 0.49, P < 0.001. More importantly, both the interaction between writing condition and BAS, b = 0.02, P = 0.029, and the interaction between writing condition and BIS, b = −0.03, P = 0.038, remained significant when baseline resting relative left frontal activity was included as a predictor. The three-way interaction among writing condition, BAS, and BIS was non-significant, b = −0.00, P = 0.122.
6 A meta-analysis of published studies (Hagger et al., 2010) found that ego depletion manipulations have an average effect size of d = 0.62. Our study had Power = 0.75 to detect a main effect of this size.
7 We repeated this analysis including baseline resting relative left frontal cortical activity as a first-level predictor. Greater baseline resting relative left frontal cortical activity predicted greater left frontal cortical activity during positive picture viewing, b = 0.49, P < 0.001. More importantly, the interaction between writing condition and the BAS-BIS difference score, b = 0.11, P = 0.005, remained significant.
8 We repeated this analysis and added baseline resting relative left frontal cortical activity as a first-level predictor. Greater baseline resting relative left frontal cortical activity predicted greater left frontal cortical activity during negative picture viewing, b = 0.65, P < 0.001. The interaction between writing condition and BIS, b = −0.03, P = 0.044, remained significant when baseline resting relative left frontal activity was included as a predictor. The interaction between writing condition and BAS, b = 0.00, P = 0.288, and the three-way interaction among writing condition, BAS, and BIS, b = −0.00, P = 0.122, were non-significant.
9 We repeated this analysis including baseline resting relative left frontal cortical activity as a first-level predictor. Greater baseline resting relative left frontal cortical activity predicted greater left frontal cortical activity during negative picture viewing, b = 0.65, P < 0.001. The interaction between writing condition and the BAS-BIS difference score, b = 0.09, P = 0.041, remained significant.
References
- Amodio D. M., Master S. L., Yee C. M., Taylor S. E. (2008). Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology, 45, 11–19. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A., Ventura-Campos N., Sanjuán-Tomás A., Belloch V., Parcet M. A., Avila C. (2010). Behavioral activation system modulation on brain activation during appetitive and aversive stimulus processing. Social Cognitive and Affective Neuroscience , 5, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M. M., Cohen J. D., Carter C. S. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences , 8, 539–46. [DOI] [PubMed] [Google Scholar]
- Carver C. S., White T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology , 67, 319–33. [Google Scholar]
- Coan J. A., Allen J. J. B. (2003). Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology , 40, 106–14. [DOI] [PubMed] [Google Scholar]
- Coan J. A., Allen J. J. B., McKnight P. E. (2006). A capability model of individual differences in frontal EEG asymmetry. Biological Psychology , 72, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr P. J. (2001). Testing problem’s in J. A. Gray’s personality theory: A commentary on Matthews and Gilliland (1999). Personality and Individual Differences , 30, 333–52. [Google Scholar]
- Costumero V., Barrós-Loscertales A., Bustamante J. C., Ventura-Campos N., Fuentes P., Rosell-Negre P., et al. (2013). Reward sensitivity is associated with brain activity during erotic stimulus processing. PloS One , 8, e66940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell A., Kelley N. J., Schmeichel B. J. (2014). Trait approach motivation moderates the aftereffects of self-control. Frontiers in Psychology , 5, 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. J., Marshall J. R., Tomarken A. J., Henriques J. B. (2000). While a phobic waits: Regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry , 47, 85–95. [DOI] [PubMed] [Google Scholar]
- Elliot A. J., Eder A. B., Harmon-Jones E. (2013). Approach and avoidance motivation and emotion: Convergence and divergence. Emotion Review , 5, 308–11. [Google Scholar]
- Eriksen B. A., Eriksen C. W. (1974). Effects of noise letters upon identification of a target letter in a non-search task. Perception and Psychophysics , 16, 143–9. [Google Scholar]
- Friese M., Binder J., Luechinger R., Boesiger P., Rasch B. (2013). Suppressing emotions impairs subsequent Stroop performance and reduces prefrontal brain activation. PLoS One, 8, e60385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable P. A., Harmon-Jones E. (2008). Relative left frontal activation to appetitive stimuli: Considering the role of individual differences. Psychophysiology , 45, 275–8. [DOI] [PubMed] [Google Scholar]
- Gable P. A., Poole B. D. (2014). Influence of trait behavioral inhibition and behavioral approach motivation systems on the LPP and frontal asymmetry to anger pictures. Social Cognitive and Affective Neuroscience , 9, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohm C. L., Clore G. L. (2000). Individual differences in emotional experience: Mapping available scales to processes. Personality and Social Psychology Bulletin , 26, 679–97. [Google Scholar]
- Gomez R., Cooper A., McOrmond R., Tatlow S. (2004). Gray’s reinforcement sensitivity theory: Comparing the separable and joint systems.: Hypotheses in the predictions of pleasant and unpleasant emotional information processing. Personality and Individual Differences , 37, 289–305. [Google Scholar]
- Hagger M., Wood C., Stiff C., Chatzisarantis N. (2010). Ego depletion and the strength model of self-control: A meta-analysis. Psychological Bulletin , 136, 495–525. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. (2007). Trait anger predicts relative left frontal cortical activation to anger-inducing stimuli. International Journal of Psychophysiology , 66, 154–60. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Allen J. J. (1997). Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology , 106, 159–63. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Gable P. A., Peterson C. K. (2010). The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology , 84, 451–62. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Harmon-Jones C., Fearn M., Sigelman J. D., Johnson P. (2008). Action orientation, relative left frontal cortical activation, and spreading of alternatives: A test of the action-based model of dissonance. Journal of Personality and Social Psychology , 94, 1–15. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Lueck L., Fearn M., Harmon-Jones C. (2006). The effect of personal relevance and approach-related action expectation on relative left frontal cortical activity. Psychological Science , 17, 434–40. [DOI] [PubMed] [Google Scholar]
- Herrmann M. J., Römmler J., Ehlis A. C., Heidrich A., Fallgatter A. J. (2004). Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe). Cognitive Brain Research , 20, 294–99. [DOI] [PubMed] [Google Scholar]
- Hewig J., Hagemann D., Seifert J., Naumann E., Bartussek D. (2006). The relation of cortical activity and BIS/BAS on the trait level. Biological Psychology , 71, 42–53. [DOI] [PubMed] [Google Scholar]
- Inzlicht M., Gutsell J. N. (2007). Running on empty: Neural signals for self-control failure. Psychological Science , 18, 933–7. [DOI] [PubMed] [Google Scholar]
- Inzlicht M., Schmeichel B. J. (2012). What is ego depletion? Toward a mechanistic revision of the resource model of self-control. Perspectives on Psychological Science , 7, 450–63. [DOI] [PubMed] [Google Scholar]
- Inzlicht M., Schmeichel B. J., Macrae C. N. (2014). Why self-control seems (but may not be) limited. Trends in Cognitive Sciences , 18, 127–33. [DOI] [PubMed] [Google Scholar]
- Kelley N. J., Eastwick P. W., Harmon-Jones E., Schmeichel B. J. (in press). Jealousy increased by induced relative left frontal cortical activity. Emotion. [DOI] [PubMed] [Google Scholar]
- Lang P. J., Bradley M. M., Cuthbert B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. [Google Scholar]
- Lewandowski G. W., Ciarocco N. J., Pettanato M. (2012). Pick me up: Ego depletion and receptivity to relationship initiation. Journal of Social and Personal Relationships , 29, 1071–84. [Google Scholar]
- Mortensen J. A., Lehn H., Evensmoen H. R., Håberg A. K. (2015). Evidence for an antagonistic interaction between reward and punishment sensitivity on striatal activity: A verification of the joint subsystems hypothesis. Personality and Individual Differences , 74, 214–9. [Google Scholar]
- Muraven M., Baumeister R. F. (2000). Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin , 126, 247–59. [DOI] [PubMed] [Google Scholar]
- Nash K., Inzlicht M., McGregor I. (2012). Approach-related left prefrontal EEG asymmetry predicts muted error-related negativity. Biological Psychology , 91, 96–102. [DOI] [PubMed] [Google Scholar]
- Schmeichel B. J. (2007). Attention control, memory updating, and emotion regulation temporarily reduce the capacity for executive control. Journal of Experimental Psychology: General , 136, 241–55. [DOI] [PubMed] [Google Scholar]
- Schmeichel B. J., Harmon-Jones C., Harmon-Jones E. (2010). Exercising self-control increases approach motivation. Journal of Personality and Social Psychology , 99, 162–73. [DOI] [PubMed] [Google Scholar]
- Semlitsch H. V., Anderer P., Schuster P., Presslich O. (1986). A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology , 23, 695–703. [DOI] [PubMed] [Google Scholar]
- Shackman A. J., McMenamin B. W., Maxwell J. S., Greischar L. L., Davidson R. J. (2009). Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychological Science , 20, 1500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. L., Coan J. A., Towers D. N., Allen J. J. B. (2014). Resting and task-elicited prefrontal EEG alpha asymmetry in depression: Support for the capability model. Psychophysiology , 51, 446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S. K., Davidson R. J. (1997). Prefrontal brain asymmetry: a biological substrate of the behavioral approach and inhibition systems. Psychological Science , 8, 204–10. [Google Scholar]
- Underwood B. J. (1975). Individual differences as a crucible in theory construction. American Psychologist , 30, 128–34. [Google Scholar]
- Uusberg A., Uibo H., Tiimus R., Sarapuu H., Kreegipuu K., Allik J. (2014). Approach-avoidance activation without anterior asymmetry. Frontiers in Psychology , 5, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen V., Carter C. S. (2002). The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior , 77, 477–82. [DOI] [PubMed] [Google Scholar]
- Wacker J., Chavanon M.-L., Stemmler G. (2010). Resting EEG signatures of agentic extraversion: New results and meta-analytic integration. Journal of Research in Personality , 44, 167–79. [Google Scholar]