Abstract
What determines how close you choose to stand to someone? Why do some people prefer farther distances than others? We hypothesized that an important factor is one’s sensory sensitivity level, i.e. how sensitive one is to nearby visual stimulation, noise, touch or smell. This study characterizes the behavioral, hormonal and electrophysiological metrics of interpersonal distance (IPD) preferences in relation to levels of sensory sensitivity. Using both an ecologically realistic task and electroencephalogram (EEG), we found that sensory sensitivity levels predicted IPD preferences, such that the more sensitive one is the farther distance they prefer. Furthermore, electrophysiological evidence revealed that individuals with higher sensory sensitivity show more alpha suppression for approaching stimuli, strengthening the notion that early sensory cortical excitability is involved in one’s social decision of how close to stand to another. The results provide evidence that a core human metric of social interaction is influenced by individual levels of sensory sensitivity.
Keywords: interpersonal distance, personal space, sensory sensitivity, stress, alpha rhythms
Introduction
What determines how close you choose to stand to someone? Why do some people prefer farther distances than others? Interpersonal distance (IPD), the space between two people, plays an important role in communication and social interactions. Although different among cultures, IPD is always experienced, especially if one stands or sits nearer or farther than what is the social norm. At a proper IPD, people may signal responsiveness and feelings of comfort and safety to one another (Meisels and Guardo, 1969; Birtchnell, 1996; Roberts, 1997; Feeney, 1999; Kaitz et al., 2004); however, a violation of expected IPD can be construed as a threat and induce a state of anxiety (Lloyd, 2009; Perry et al., 2013). Apart from cultural and situational differences (e.g. encountering a friend or a stranger, Hayduk, 1983), there is evidence for individual trait-like IPD preferences, i.e. preferences that remain more or less stable through life (Patterson, 1995; Andersen and Sull, 1985). These have been related to social attachment styles (Bar-Haim et al., 2002; Kaitz et al., 2004), early child abuse (Vranic, 2003), post-traumatic stress disorder (Bogović et al., 2013) and trait levels of social anxiety (Scheele et al., 2012; Perry et al., 2013). Two independent studies report that individuals with autistic spectrum disorder (ASD) show greater variance than controls in their IPD preferences—some prefer distances farther than the norm, while others walk right into the experimenter without experiencing any discomfort (Kennedy and Adolphs, 2014; Perry et al., 2015a). This suggests that there are multiple factors contributing to IPD variance among both healthy and ASD individuals.
However, an important neglected factor that may contribute to this variance is one’s level of sensory sensitivity, i.e. how sensitive one is to nearby visual stimulation, noise, touch or smell (Dunn, 2001). The idea behind this hypothesis is that if one is more sensitive, for example, to touch or smell, s/he would prefer further distances from others, to avoid being over stimulated and uncomfortable. Numerous studies report that individuals with ASD experience abnormal sensory sensitivity (Kientz and Dunn, 1997; Ermer and Dunn, 1998; Watling et al., 2001; Kern et al., 2007; Tomchek and Dunn, 2007). In fact, some researchers have argued that atypical sensory processing should be one of the diagnostic criteria of ASD (Chamak et al., 2008). Robertson and Simmons (2013) measured variance in sensory sensitivity in a healthy population of 212 individuals. Their results showed that atypical sensory responsiveness in healthy individuals (including both hyper- and hyposensitivity), as measured by a sensory questionnaire, was more common in individuals with higher levels of autistic traits, as measured by the zero (AQ; Baron-Cohen et al., 2001). This result was not confined to a specific sensory modality and did not seem to favor a particular subscale of the AQ. Just as those with ASD exist at the extreme end of a continuum of autistic traits which are evident in different degrees in the normal population, sensory sensitivity is also a continuum in which hyper and hyposensitivity are at the extremes.
The Sensory Profile is a model for sensory processing that incorporates the nervous system’s threshold levels for acting as well as a person’s pattern of responses in accordance with those levels (Dunn, 2001). Sensory sensitivity, a dimension within the Sensory Profile, is characterized by low sensory thresholds and a passive self-regulation strategy (Dunn, 1997). Those who demonstrate behavior consistent with the sensory sensitivity dimension are said to be ‘hypersensitive’. Hypersensitivity occurs when one experiences an ‘overload’ of stimuli—e.g. gentle touch feels painful, noises seem exceptionally loud or lights unbearably bright (Dunn, 1997). The opposite of this experience is ‘hyposensitivity’, which occurs when an individual under-reacts to the presentation of sensory stimuli (or actively seeks them out—sometimes called ‘sensory seeking behavior’, Bogdashina, 2003). In such instances, one may not respond to stimuli that most would find painful, or seem unaware of loud noise, bright light or extreme temperature changes.
This study sought to characterize IPD preferences in terms of their behavioral, hormonal and electrophysiological basis, specifically in relation to levels of sensory sensitivity. The first experiment investigated whether sensory sensitivity and stress levels (as measured by salivary cortisol) predicted one’s preferred IPD. Salivary cortisol levels were measured as an indication of baseline stress in general and social stress in particular. Cortisol is considered a reliable marker of hypothalamic–pituitary–adrenal axis function and is routinely used as an indirect measurement of psychophysiological stress in response to a particular stimulus (Kirschbaum et al., 1995; McEwen, 2000; Takahashi et al., 2005; Hellhammer et al., 2009). Central to this study, social stressors (public speaking, interactions with judges) have been shown to reliably affect cortisol levels (Kirschbaum et al., 1993). Moreover, cortisol levels have been shown to be affected by physical proximity. For example, increased salivary cortisol was measured when anticipating a meeting with a member of an outgroup (e.g. a patient with schizophrenia, Norman et al., 2010), and commuters on public transport showed elevated self-report stress levels, increased salivary cortisol and performance after effects when sitting in close proximity to other commuters (Evans and Wener, 2007). Social anxiety was also measured as it was shown to correlate with IPD (Scheele et al., 2012; Perry et al., 2013), and we hypothesized that it would correlate with levels of sensory sensitivity as well. The rationale behind this hypothesis is that sensitivity to touch, sound, smell and other sensory stimuli would elevate one’s anxiety as a function of close social interactions.
To measure IPD preferences in an ecological manner, we used the stop-distance paradigm, a realistic behavioral measure of IPD in which participants choose their preferred distance from a stranger in a live interaction in the laboratory. Cortisol levels were measured before and after the behavioral IPD task, which enabled us to measure whether the task itself served as a stressor.
Following the behavioral task, the same participants participated in an EEG study, in which preferred IPD was assessed using a modified version of the comfortable IPD paradigm (CID; Duke and Nowicki, 1972; Duke and Kiebach, 1974). In this paradigm, participants were instructed to imagine themselves in the center of a room visualized on a computer screen with a virtual target (friend, stranger or computer screen) approaching them (see Methods section). We hypothesized that alpha suppression, an EEG correlate of cognitive (mainly visual) attention (Klimesch, 1999), would be greater for the first frame of the target entering the room, and greater for human figures than for the non-biological screen (and perhaps greater for the stranger than for the friend). Most importantly, we hypothesized that alpha suppression would be greater for participants with higher sensory sensitivity, supporting the notion that early cortical excitability affects later social behavior.
Methods
Participants
Participants were 42 undergraduate English speaking students from the University of California, Berkeley, who received course credit or payment for participating in the experiment. We focused on female participants, ranging in age from 18 to 35 (mean 22.53, s.d. = 4.29) to assure a more homogenous group in terms of hormonal level and distance preferences. All were born in USA, except for four, who arrived between the ages of 4–10. One participant was left-handed. All participants reported normal or corrected to normal visual acuity and had no history of psychiatric or neurological disorders as confirmed by a screening interview. To account for menstrual cycle stage, the participants were asked to state the first day of their last period. Eight participants reported using oral contraceptives, and four additional participants did not remember the exact date of their cycle. Hence, we had menstrual cycle information from 30 participants.
Saliva collection and analysis
Testing was scheduled in the afternoon (12:00 p.m.–4:00 p.m.) to control for diurnal rhythms in hormones. Saliva samples were taken 30 min after arriving in the lab (Mean: 29.57 min, s.d. 2.26 min; Time 1) and again 30 min after the social distance manipulation (Mean: 30.98 min, s.d. 2.27 min; Time 2).
Standard salivary-hormone collection procedures were used (Dickerson and Kemeny, 2004; Schultheiss and Stanton, 2009). Before providing saliva samples, participants did not eat, drink or brush their teeth for at least 1 h. While waiting for 30 min prior to saliva collection #1, the participants filled out the consent form and, if they finished early, were instructed to sit calmly and relax until the next phase of the study. Participants provided a saliva sample using a simple absorption method, where the participant was instructed to swish around a piece of sterile cotton in their mouth for either 3 min or until the cotton was fully saturated with saliva, whichever came first. Samples were immediately frozen to avoid hormone degradation and to precipitate mucins. After completion of data collection (4 months), samples were shipped for analysis to the laboratory of Professor D.C. Kirschbaum (Dresden, Germany). Saliva samples were frozen and stored at −20 °C until analysis. After thawing, salivettes were centrifuged at 3000 rpm for 5 min, which resulted in a clear supernatant of low viscosity. Salivary concentrations were measured using commercially available chemiluminescence immunoassay with high sensitivity (IBL International, Hamburg, Germany). The intra and interassay coefficients for cortisol were below 8%.
Social distance—behavioral
A modified version of the stop-distance paradigm was used to assess IPD. This procedure is a frequently used paradigm for assessing preferred or tolerated IPD under varied conditions, with high reliability measures (see Hayduk, 1983; Aiello, 1987 for reviews). Testing began with the participant positioned at one end of the room with her toes against a drawn line, and a male experimenter (not familiar to any of the participants) facing the participant from a distance of 2.8 m (3.28 feet). The participant was told that several measurements of distance between the participant and the experimenter will be recorded, and that there was no wrong answer. For Distance 1, the participant was instructed to walk toward the experiment and stop at a comfortable distance where they would normally interact with a person. After the participant stopped and the distance between participant and experimenter was recorded, the participant was instructed to keep walking toward the experimenter until she felt uncomfortable and then stop again (Distance 2). After the two distances were recorded, the participant and experimenter switched places on the two ends of the measurement tape. The participant was told that the experimenter would now walk toward her and was instructed to tell him when to stop. First, the participant stopped the experimenter at a comfortable distance away from her, where she would normally interact with someone (Distance 3). Then the experimenter kept walking toward the participant until she stopped him at a distance that made her feel uncomfortable (Distance 4). In all conditions the experimenter kept his eyes open, gazing down at the participant’s knees, with a neutral facial expression. A second experimenter recorded the distances.
Social distance—EEG
The participants completed a modified computerized version of the comfortable interpersonal task (CID; Duke and Nowicki, 1972) while having their electroencephalogram recorded.
Stimuli task and design
In the original version of the paper and pen CID, a circle was presented where participants were instructed to imagine themselves in the center of the room and to respond to an imaginary protagonist approaching them along one of eight radii by making a mark on the radius indicating where they would want the person to stop. In the current, modified computerized version of the CID, we transformed the test (using E-Prime), such that instead of eight entrances the participant observed the protagonist coming from one of four entrances, corresponding to 0, 90, 180 and 270 °. We further defined the protagonist entering the room by having the participant imagine the protagonist as a male friend (not boyfriend), stranger or computer screen. Selecting from a collection of 10 electronic arts figures, the participants selected (i) a figure to represent themselves, (ii) a figure to represent a male friend and (iii) a figure to represent a male stranger. An identical figure of a computer screen was used for all participants. After choosing the figures, participants were told they would have to imagine how they would feel as each of the figures approached them in an imaginary room shown on the screen. The participant saw a fixation point for 0.5 s followed by a circular room on the screen, with the figure the participant chose to represent herself in the middle of the circle and either the friend, stranger or computer screen at one of the four entrances. To avoid eye movements, the stimuli size was reduced such that the radius of the circle was 45 mm, and the participant’s figure length was 17 mm. The experiment was presented on a monitor, 70 cm away from the participant’s eyes, with the circle’s diameter creating a visual angle of 7.36 °. E-Prime (Psychological Software Tools) was used for stimulus presentation.
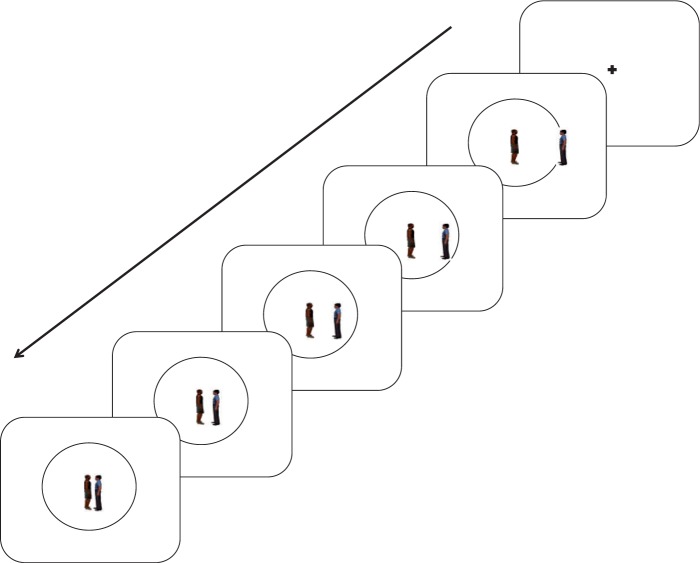
The stranger, friend or screen approached the center of the room in increments of four intervals, giving a total of five still pictures, each appearing for 800 ms (Figure 1). The participant was instructed to imagine how they would feel as that figure approached themselves, and where they would want that figure to stop. There were 60 trials of each approaching figure, and the 180 trials appeared in random order. Every 3–6 trials, following the trial, the participant was asked to mark with the computer mouse using their right hand where she last wanted the friend, stranger or computer screen to stop along the line radius. This was done to keep the participants focused and on task. For the EEG analysis, three still pictures of distances were chosen: the room with the protagonist ready to approach, the room with the protagonist in the middle of the radius and the room with the protagonist right near the self-figure at the center of the room. There were two breaks during the experiment, enabling the participants to rest.
Fig. 1.
An example of a trial. A fixation point appeared for 800 ms, followed by an image of a circular room with a figure representing one’s self in the center and one of three figures entering the room: a friends, stranger or computer screen. The figure would come closer for the next four images. Each image appeared for 800 ms. The first, middle and last images were analyzed for alpha suppression.
Data acquisition and analysis
EEG was recorded continuously (from DC with a low-pass filter set at 100 Hz) from 64 Ag-AgCl pin-type active electrodes mounted on an elastic cap (Biosemi, http://www.biosemi.com/headcap.htm), according to the extended 10–20 system, and from two additional electrodes placed at the right and left mastoids. All electrodes were referenced during recording to the common-mode signal electrode between POz and PO3 and were subsequently rereferenced digitally (see data processing later). Eye movements, as well as blinks, were monitored using bipolar horizontal and vertical Electrooculography (EOG) derivations via two pairs of electrodes, with one pair attached to the external canthi and the other to the infraorbital and supraorbital regions of the right eye. Both EEG and EOG were digitally amplified and sampled at 1024 Hz using a Biosemi Active II system (www.biosemi.com).
Data processing
Data were analyzed using Brain Vision Analyzer software (Brain Products). Raw EEG data were initially 0.5 Hz high-pass filtered, 30 Hz low-pass filtered (24 dB) and notch filtered at 60 Hz; then the data was rereferenced off-line to the average of all electrodes1. EEG deflections resulting from eye movements and blinks were corrected using an Interdependent component analysis (ICA) procedure (Jung et al., 2000). Remaining artifacts exceeding plus minus 100 microvolts in amplitude were rejected.
EEG analysis
For each of the 800 ms segments, the integrated power in the 8–13 Hz range was computed using a fast Fourier transform performed at 0.5 Hz intervals (using a Hanning window). The segments were then averaged for each condition. A suppression index was calculated as the logarithm of the ratio of the power during each condition relative to the power during 60 randomly chosen fixation points (baseline), and used as dependent variable. The ratio, as opposed to a simple subtraction, was used to control for the variability in absolute EEG power as a result of individual differences such as scalp thickness and electrode impedance. The log transform was applied to the ratio before statistical analyses because ratio data are inherently not normally distributed as a result of lower bounding. A log ratio of less than zero indicates suppression in the EEG amplitude, whereas a value of zero indicates no change and values greater than zero indicate enhancement. Although we expected alpha suppression to occur mainly in occipital sites (Klimesch, 1999), suppression was computed at six sites (F3, C3, O1, F4, C4 and O2) to compare the signal between hemispheres and locations.
Questionnaires
Immediately after the experiment, each participant was sent via email an online version of the Sensory Profile (Dunn, 2001). This profile divides a person’s responses into four quadrants: Low Registration, Sensation Seeking, Sensation Sensitivity and Sensation Avoidance. We were interested in the level of sensation sensitivity, as we predicted that this would have the largest effect on one’s preferred IPD. To examine whether sensory sensitivity correlated with social anxiety, participants also received the Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987), one of the most commonly used and validated scales for the assessment of social anxiety. The LSAS provides a total score and six subscale scores: total fear, fear of social interaction, fear of performance, total avoidance, avoidance of social interaction and avoidance of performance.
Results
Behavioral
Sensory sensitivity and interactions with Cortisol predict preferred distance
One participant had a ‘Distance 1’ that was more than 4 s.d.s away from the mean and was removed from the analysis. Since all four distances were highly correlated with each other (Distance 1: M = 57.18, s.d. = 13.99; Distance 2: M = 21.0, s.d. = 11.16; Distance 3: M = 52.90, s.d. = 14.78; Distance 4: M = 23.47, s.d. = 9.54; all Pearson r > 0.33, P < 0.05), they were averaged into one variable of Preferred Distance. A multiple regression analysis was run to predict the preferred distance (centered) from sensory sensitivity and baseline cortisol levels, including interactions between the two variables (all variables were centered). The multiple regression model produced R2 = 0.249, F(3,38) = 3.862, P = 0.017.
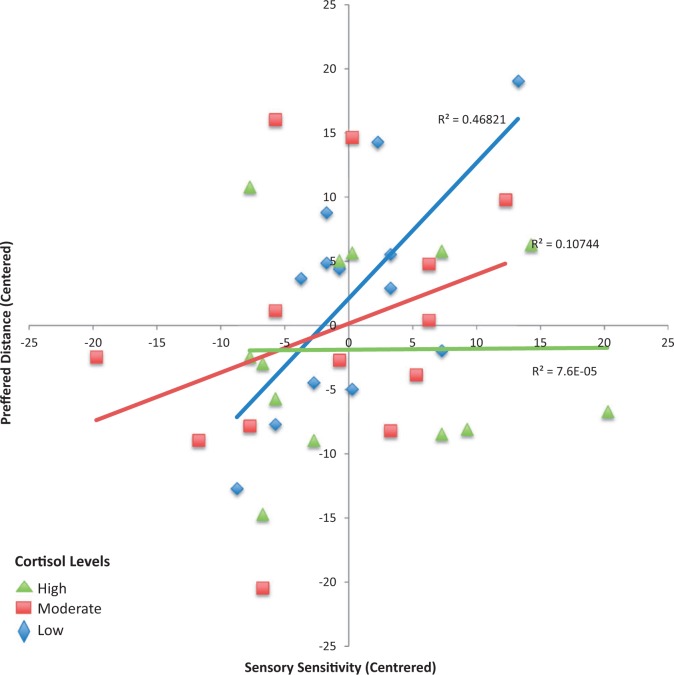
Sensory sensitivity had significant positive regression weights, indicating, as predicted, that individuals with higher sensory sensitivity scores preferred farther distances (Table 1). Significant interactions between cortisol levels and sensory sensitivity were observed, indicating that different levels of baseline cortisol moderated the effect of sensory sensitivity on preferred distances. To investigate this further, we divided the results into three equal-sized groups by baseline cortisol level [low (<10), moderate (10–16) and high (>16)], and then computed and plotted the relationship between sensory sensitivity and preferred distance for each of the three groups. As can be seen in Figure 2, the correlation between sensory sensitivity and preferred distance was strongest for the low cortisol group (R2 = 0.468), and weaker for the two other groups (R2 = 0.107 for moderate cortisol level, and R2 = ∼0 for the high cortisol group). Both sensory sensitivity and social anxiety were comparable between the three groups (Mean sensory sensitivity in low cortisol group 37.9, medium 36.5, high 38.4, F < 1, P = 0.79; Mean social anxiety in low cortisol group 37.9, medium 36.8, high 36.1, F < 1, P = 0.96). Lastly, as hormonal levels differ in different stages of the menstrual cycle and may affect IPD preferences, a correlation between IPD preferences and day of the menstrual cycle was analyzed, but was not significant (N = 30, P = 0.278); neither was an analysis of variance (ANOVA) comparing IPD preference between early stages of the cycle (days 1–14, N = 12) and the late luteal stage (>14 days, N = 18; F < 1, P = 0.473; further comparisons between sub stages could not be done due to the small number of participants).
Table 1.
Multiple regression coefficients
| Model | Unstandardized coefficients |
Standardized coefficients | t | Sig | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| (Constant) | −0.062 | 1.292 | −0.048 | 0.962 | |
| Sensory sensitivity (centered) | 0.482 | 0.175 | 0.431 | 2.754 | 0.009 |
| Cortisol (centered) | −0.206 | 0.255 | −0.119 | −0.808 | 0.424 |
| Sensory sensitivity X cortisol | −0.097 | 0.035 | −0.437 | −2.775 | 0.009 |
There was a significant effect of sensory sensitivity on preferred IPD, such that the more sensitive one is the farther the distance they choose. This was modulated by an interaction of sensory sensitivity with cortisol levels.
Fig. 2.
The correlation between sensory sensitivity (centered) and preferred distance (centered), modified by different baseline cortisol levels. The correlation between sensory sensitivity is strongest for the low cortisol group (blue), lower for the moderate cortisol level group (red) and absent for the high cortisol level group (green).
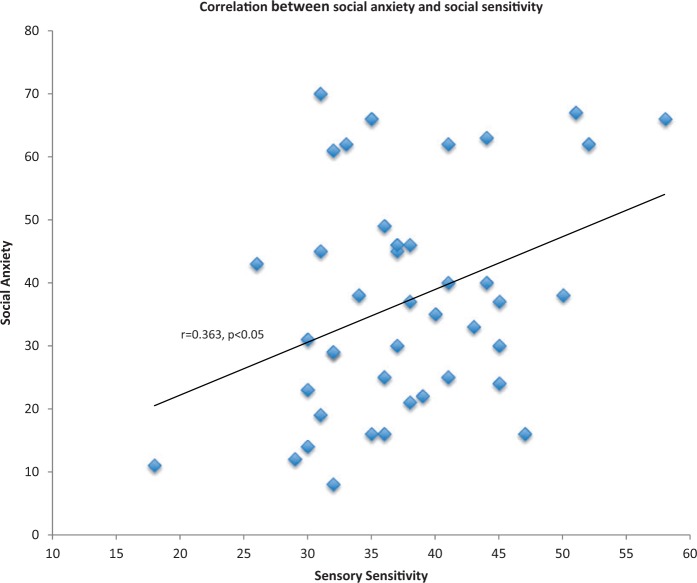
Next we analyzed whether the task itself served as a stressor, by comparing between cortisol levels 30 min after the task and baseline cortisol levels. One participant was excluded from this analysis, as we had no measurement for her second Cortisol level. This difference was not significant (Baseline Cortisol Mean = 12.82, s.d. = 5.22; Task Cortisol Mean = 12.07, s.d. = 6.68, P = 0.33), neither was the correlation between the difference of the two measures (Task Cortisol—Baseline Cortisol) and sensory sensitivity (r = 0.14, P = 0.368). Lastly, as predicted, sensory sensitivity correlated with the total score of the social anxiety scale (n = 42; Pearson r = 0.363, P < 0.05; Figure 3)2. Breaking the social anxiety scale into its subscales revealed that the correlation was significant between sensory sensitivity and the two avoidance scales [i.e. avoidance of social interactions, (r = 0.387, P < 0.05), and avoidance of performance, (r = 0.406, P < 0.005)] but not with the fear subscales (P > 0.1).
Fig. 3.
A significant correlation was found between sensory sensitivity as measured by Dunn’s Sensory Profile (Dunn, 2001) and social anxiety levels as measured by the LSAS (Liebowitz, 1987).
EEG
Alpha suppression and sensory sensitivity
The statistical analysis of alpha suppression was based on a mixed ANOVA design, with high and low sensory sensitivity as a between subjects variable, and hemisphere (Left, Right), Site (Frontal, Central and Occipital) figure approaching (Friend, Stranger and Screen) and distance (close, middle and far) as within subjects variables. One participant did not complete the EEG task, and four participants had noisy recordings in one of the six sites of interest (1 in O2, 2 in C4 and 1 in F4) and the reported results are based on 37 participants (18 low in sensory sensitivity).
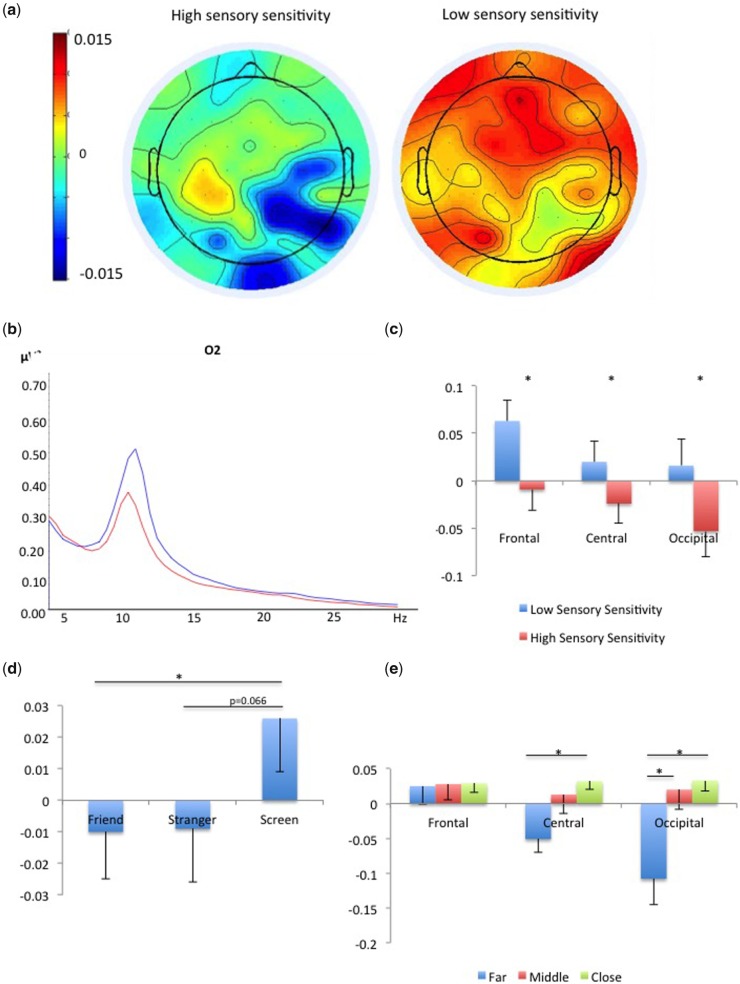
Most relevant to our hypothesis, there was a significant between-group effect, indicating that those with higher sensory sensitivity show greater alpha suppression to the IPD stimuli than those with lower sensory sensitivity [High mean: −0.029, Low mean = 0.033; F(1,35) = 4.496, P ≤ 0.05, η2p = 0.114; Figure 4a–c] In addition, there was a significant effect for site, revealing that suppression was most prominent at occipital sites, less so in central sites and least in the frontal sites [Occipital mean: −0.018, Central Mean: −0.002, Frontal mean: 0.027; F(2,70) = 4.976, P < 0.05, η2p = 0.124, Figure 4a and 4c]. There was also a significant main effect for figure, revealing that suppression for human figures was greater than for the computer screen [Friend: −0.01, Stranger: −0.01 Screen: 0.03; F(2,70) = 4.779, P < 0.05, η2p = 0.120; Figure 4d]. Bonferroni corrected pairwise comparisons indicated that the difference between Friend and Screen was significant (P < 0.05), while the distance between Stranger and Screen approached significance (P = 0.066). Lastly, there was a significant main effect of distance [F(2,70) = 5.01, P < 0.01, η2p = 0.125], modified by a Site X Distance interaction [F(4,140) = 9.797, P < 0.001, η2p = 0.219; Figure 4e] and a second order Site X Distance X Hemisphere interaction [F(4,140) = 4.640, P < 0.005, η2p = 0.117]. Further analyses of these interactions revealed that in both hemisphere the distance effect was significant in the occipital sites, approaching significance in the central sites and non-existent in frontal sites (since this was not the main focus of the article, these results are not detailed further).
Fig. 4.
EEG results: (a) Alpha suppression was greater for the high sensory sensitivity group than for the low sensory sensitivity group; (b) Power in 5–30 Hz averaged across all conditions for low (red) and high (blue) sensory sensitivity groups in electrode O2; (c) Alpha suppression compared with fixation (baseline) in high and low sensory sensitivity groups, in frontal, central and occipital sites. Error bars depict standard error and asterisks depict significant results (P < 0.05). (d) Alpha suppression was greater for the human figures compared with the computer screen; (e) Alpha suppression was greater for the farthest distance, which was the first appearance of the figure approaching, significant in the occipital and central sites. In both figures error bars depict standard error and asterisks depict significant results (P < 0.05).
Discussion
IPD preferences are typically explained by cultural and social differences (Hayduk, 1983; Remland et al., 1995; Bar-Haim et al., 2002; Vranic, 2003; Kaitz et al., 2004; Scheele et al., 2012; Bogović et al., 2013; Perry et al., 2013). We hypothesized that an additional ‘low-level’ dimension should be taken into account, specifically the level of individual sensory sensitivity. Several findings relevant to the initial hypotheses emerged. Behaviorally, levels of sensory sensitivity predicted IPD preferences. There is substantial heterogeneity among adults in relation to sensory profiles, and sensory sensitivity can be useful for identifying patterns of preferred activities and responses among different people (Brown et al., 2002). Here, we show that within a healthy population, differences in sensory sensitivity affect social behavior—they affect IPD preferences and correlate with social anxiety measures. Note that levels of baseline cortisol behaved as moderators of IPD preferences, interacting with sensory sensitivity levels such that the higher one’s initial cortisol level, the less effect sensory sensitivity has on preferred IPD. A possible interpretation of this result is that when one is less stressed, more importance is placed on bodily sensations so preferred IPD is driven more by sensory sensitivity. This interaction highlights the importance of both trait (sensory sensitivity) and state (hormonal levels, stress) conditions on social behavior. The task itself did not serve as a significant social stressor, as indicated by similar cortisol levels before and after the task, with no correlation between the difference in Cortisol level and sensory sensitivity. Perhaps this is due to the fact that participants had full control over the distances they chose. Future studies using more stressful situations may find additional interactions between stress and sensory sensitivity and their effects on social behavior.
The importance of sensory sensitivity levels in IPD decisions was supported by neural differences in alpha suppression, which has been extensively related to sensory (mostly visual) attention (Klimesch, 1999), between high and low sensory sensitivity groups performing the IPD task. Although not relevant to our main focus (as no interactions were found between these effects and sensory sensitivity), we also found that alpha suppression was stronger for the human figures compared with the computer screen and for the far distances than for the closer ones. The first finding may relate to the significance of human interaction, especially for an approaching figure (see also 2010). Note, however, that the greater suppression for human figures might also be the result of more attention needed to differentiate between the friend and stranger figures then between human figures and the computer screen. The second finding was predicted as attention should be greatest when a new person/figure comes into the room, and generally for a first instance of a stimulus (i.e. repetition inhibition, for a review see Grill-Spector et al., 2006). These results also relate to previous findings linking IPD preferences with attention and with the N1 Event related potential (ERP) component (Perry et al., 2013). The N1 has been shown to correlate with alpha suppression (Ergenoglu et al., 2004; Sauseng et al., 2005) and is thought to be the result of phase synchronization between alpha and other frequencies (Gruber et al., 2005).
These results have important implications for social cognition, stressing how early ‘low level’ physiological mechanisms, such as sensory sensitivity, affect ‘high level’ social decisions such as where one feels comfortable when talking to another. A possible candidate for mediating these effects is Oxytocin, a neuropeptide that has been suggested to play important roles in social salience (Shamay-Tsoory, 2010; Bartz et al., 2011), IPD and touch (Scheele et al., 2012; Scheele et al., 2014; Perry et al., 2015b), and has also been linked to modulation of alpha rhythms in response to social stimuli (Perry et al., 2010a). In fact, it has recently been suggested that Oxytocin may play a role in interoception, and that impairment in this neuromodulation may give rise to autistic phenotypes, including sensory and social deficits.
These results also fit in well with growing evidence suggesting that social chemosignalling plays a significant role in human behavior. Frumin et al. (2015) recently suggested that human handshakes are used to sample conspecific social chemosignals, and so act as active yet subliminal social chemosignalling. Choosing one’s distance from another may play an additional (or perhaps substitute) role in social chemosignalling. Even without shaking hands, the closer one stands to another, the better they can smell them. Although speculative, there may be a balance between the need to smell the other and the desire not to convey too much chemical information about oneself. If true, it is clear why anyone breaking the distance ‘norm’, in either direction, would make others feel uncomfortable
There are limitations to this study. First, we focused only on female participants who were university students born in USA or immigrated as young children. Furthermore, the experimenter approaching the participants in the behavioral study was always male. Since there are known cultural (Lomranz, 1976) and sex differences (Fisher and Byrne, 1975) affecting IPD preferences, future studies should test the effect of sensory sensitivity on IPD in male participants as well as in different cultures and should examine the effect of within/between sex or within/between culture interactions. Although we made sure the male experimenter would always be a stranger with no prior acquaintance with the participants, asking the participants about their feelings of trust or liking toward the male figure might be able to explain some of the variance in IPD preferences. In addition, sensory sensitivity was measured using a questionnaire. It would be useful in future studies to include laboratory testing for different sensory sensitivities and to measure their effects on social behavior. Lastly, the image of the circle depicting the circular room in the EEG study was identical for all participants. Future studies could vary the size if the room and the distances between the figures as a function of the individuals’ preferred distances, and measure the effect of these manipulations on attention and stress.
To conclude, this study used psychophysiological and neural methods in an ecological and an EEG setting, to reveal a relationship between sensory sensitivity and preferred IPD. Behaviorally, sensory sensitivity levels predicted IPD preferences, such that the more sensitive one is the farther distance they prefer. Furthermore, this study reveals that individuals with higher sensory sensitivity show more alpha suppression for approaching stimuli, strengthening the notion that early cortical excitability is involved in one’s later social decisions. The findings reveal that sensory sensitivity influences the core human behavior of preferred social distance.
Acknowledgements
The authors thank Daniela Cohen for her help in creating the animated figures.
Footnotes
1 An identical analysis was run with combined-mastoid reference, yielding similar results.
2 Since social anxiety was not our main focus, and since it correlated with sensory sensitivity, we did not add it into the original regression model, to avoid multicollinearity. However, we would like to note that when adding social anxiety to the model, sensory sensitivity still predicted IPD, R2 = 0.354, F(6,35) = 3.195, P = 0.013; with significant weights for sensory sensitivity (P < 0.05) but not for social anxiety.
Funding
R.T.K. was supported by National Institute of Neurological Disorders and Stroke (NINDS) R37231135 and the Nielsen Corporation.
Conflict of interest. None declared.
References
- Aiello J.R. (1987). Human spatial behavior. Handbook of Environmental Psychology , 1, 389–504. [Google Scholar]
- Andersen P. A.,, Sull K. K. (1985). Out of touch, out of reach: Tactile predispositions as predictors of interpersonal distance. Western Journal of Speech Communication, 49, 57–72. [Google Scholar]
- Bar-Haim Y., Aviezer O., Berson Y., Sagi A. (2002). Attachment in infancy and personal space regulation in early adolescence. Attachment & Human Development , 4(1), 68–83. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. (2001). The autism-spectrum quotient (AQ): evidence from asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. Journal of Autism and Developmental disorders , 31(1), 5–17. [DOI] [PubMed] [Google Scholar]
- Bartz J.A., Zaki J., Bolger N., Ochsner K.N. (2011). Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences , 15(7), 301–9. [DOI] [PubMed] [Google Scholar]
- Birtchnell J. (1993). How Humans Relate: A New Interpersonal Theory. Hove, UK: Psychology Press. [Google Scholar]
- Bogdashina O. (2003). Sensory Perceptual Issues in Autism and Asperger Syndrome: Different Sensory Experiences, Different Perceptual Worlds. London, UK: Jessica Kingsley Publishers. [Google Scholar]
- Bogović A., Mihanović M., Jokić-Begić N., Švagelj A. (2013). Personal space of male war veterans with posttraumatic stress disorder. Environment and Behavior , 13, 9165. [Google Scholar]
- Brown C., Cromwell R.L., Filion D., Dunn W., Tollefson N. (2002). Sensory processing in schizophrenia: missing and avoiding information. Schizophrenia Research , 55(1), 187–95. [DOI] [PubMed] [Google Scholar]
- Chamak B., Bonniau B., Jaunay E., Cohen D. (2008). What can we learn about autism from autistic persons? Psychotherapy and Psychosomatics , 77(5), 271–9. [DOI] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin , 130(3), 355. [DOI] [PubMed] [Google Scholar]
- Duke M.P., Kiebach C. (1974). A brief note on the validity of the Comfortable Interpersonal Distance Scale. The Journal of Social Psychology , 94(2), 297–8. [Google Scholar]
- Duke M.P., Nowicki S. (1972). A new measure and social-learning model for interpersonal distance. Journal of Experimental Research in Personality, 6, 119–32. [Google Scholar]
- Dunn W. (1997). The impact of sensory processing abilities on the daily lives of young children and their families: a conceptual model. Infants & Young Children , 9(4), 23–35. [Google Scholar]
- Dunn W. (2001). The sensations of everyday life: empirical, theoretical, and pragmatic considerations. American Journal of Occupational Therapy , 55(6), 608–20. [DOI] [PubMed] [Google Scholar]
- Ergenoglu T., Demiralp T., Bayraktaroglu Z., Ergen M., Beydagi H., Uresin Y. (2004). Alpha rhythm of the EEG modulates visual detection performance in humans. Cognitive Brain Research , 20(3), 376–83. [DOI] [PubMed] [Google Scholar]
- Ermer J., Dunn W. (1998). The sensory profile: a discriminant analysis of children with and without disabilities. American Journal of Occupational Therapy , 52(4), 283–90. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Wener R.E. (2007). Crowding and personal space invasion on the train: please don’t make me sit in the middle. Journal of Environmental Psychology , 27(1), 90–4. [Google Scholar]
- Feeney J.A. (1999). Adult romantic attachment and couple relationships. In Shaver J.C.P.R., editors. Handbook of Attachment: Theory, Research, and Clinical Applications (pp. 355–77). New York, NY: Guilford Press. [Google Scholar]
- Fisher J.D., Byrne D. (1975). Too close for comfort: sex differences in response to invasions of personal space. Journal of Personality and Social Psychology , 32(1), 15. [Google Scholar]
- Frumin I., Perl O., Endevelt-Shapira Y., et al. (2015). A social chemosignaling function for human handshaking. eLife , 4, e05154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K., Henson R., Martin A. (2006). Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Sciences , 10(1), 14–23. [DOI] [PubMed] [Google Scholar]
- Gruber W. R., Klimesch W., Sauseng P., Doppelmayr M. (2005). Alpha phase synchronization predicts P1 and N1 latency and amplitude size. Cerebral Cortex , 15(4), 371–7. [DOI] [PubMed] [Google Scholar]
- Hayduk L.A. (1983). Personal-space - where we now stand. Psychological Bulletin , 94(2), 293–335. [Google Scholar]
- Hellhammer D.H., Wüst S., Kudielka B.M. (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology , 34(2), 163–71. [DOI] [PubMed] [Google Scholar]
- Kaitz M., Bar-Haim Y., Lehrer M., Grossman E. (2004). Adult attachment style and interpersonal distance. Attachment & Human Development , 6(3), 285–304. [DOI] [PubMed] [Google Scholar]
- Kennedy D. P., Adolphs R. (2014). Violations of personal space by individuals with autism spectrum disorder. PLoS One , 9(8), e103369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J. K., Trivedi M. H., Grannemann B. D., et al. (2007). Sensory correlations in autism. Autism , 11(2), 123–34. [DOI] [PubMed] [Google Scholar]
- Kientz M.A., Dunn W. (1997). A comparison of the performance of children with and without autism on the Sensory Profile. American Journal of Occupational Therapy , 51(7), 530–7. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.-M., Hellhammer D.H. (1993). The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology , 28(1-2), 76–81. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Prussner J.C., Stone A.A., et al. (1995). Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic Medicine , 57(5), 468–74. [DOI] [PubMed] [Google Scholar]
- Klimesch W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews , 29(2), 169–95. [DOI] [PubMed] [Google Scholar]
- Liebowitz M.R. (1987). Social phobia. Modern Problems of Pharmacopsychiatry , 22, 141–73. [DOI] [PubMed] [Google Scholar]
- Lloyd D.M. (2009). The space between us: a neurophilosophical framework for the investigation of human interpersonal space. Neuroscience & Biobehavioral Reviews , 33(3), 297–304. [DOI] [PubMed] [Google Scholar]
- Lomranz J. (1976). Cultural variations in personal space. The Journal of Social Psychology , 99(1), 21–7. [Google Scholar]
- McEwen B.S. (2000). Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology , 22(2), 108–24. [DOI] [PubMed] [Google Scholar]
- Meisels M., Guardo C.J. (1969). Development of Personal Space Schemata. Child Development , 40(4), 1167–78. [PubMed] [Google Scholar]
- Norman R.M.G., Gawronski B., Hampson E., Sorrentino R.M., Szeto A., Ye Y. (2010). Physical proximity in anticipation of meeting someone with schizophrenia: the role of explicit evaluations, implicit evaluations and cortisol levels. Schizophrenia Research , 124(1), 74–80. [DOI] [PubMed] [Google Scholar]
- Patterson M.L. (1995). Invited article: A parallel process model of nonverbal communication. Journal of Nonverbal Behavior, 19, 3–29. [Google Scholar]
- Perry A., Bentin S., Shalev I., et al. (2010a). Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology , 35(10), 1446–53. [DOI] [PubMed] [Google Scholar]
- Perry A., Levy-Gigi E., Richter-Levin G., Shamay-Tsoory S.G. (2015a). Interpersonal distance and social anxiety in autistic spectrum disorders: a behavioral and ERP study. Social Neuroscience, 10, 1–12. [DOI] [PubMed] [Google Scholar]
- Perry A., Mankuta D., Shamay-Tsoory S.G. (2015b). OT promotes closer interpersonal distance among highly empathic individuals. Social Cognitive and Affective Neuroscience , 10(1), 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A., Rubinsten O., Peled L., Shamay-Tsoory S.G. (2013). Don’t stand so close to me: a behavioral and ERP study of preferred interpersonal distance. NeuroImage , 83, 761–9. [DOI] [PubMed] [Google Scholar]
- Remland M.S., Jones T.S., Brinkman H. (1995). Interpersonal distance, body orientation, and touch: effects of culture, gender, and age. The Journal of Social Psychology , 135(3), 281–97. [DOI] [PubMed] [Google Scholar]
- Roberts J.S.W. (1997). Children’s personal distance and their empathy: indices of interpersonal closeness. International Journal of Behavioral Development , 20(3), 385–403. [Google Scholar]
- Robertson A.E., Simmons D.R. (2013). The relationship between sensory sensitivity and autistic traits in the general population. Journal of Autism and Developmental disorders , 43(4), 775–84. [DOI] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Stadler W., et al. (2005). A shift of visual spatial attention is selectively associated with human EEG alpha activity. European Journal of Neuroscience , 22(11), 2917–26. [DOI] [PubMed] [Google Scholar]
- Scheele D., Kendrick K.M., Khouri C., et al. (2014). An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology , 39(9), 2078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D., Striepens N., Güntürkün O., et al. (2012). Oxytocin modulates social distance between males and females. The Journal of Neuroscience , 32(46), 16074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss O.C., Stanton S.J. (2009). Assessment of salivary hormones. In: Harmon-Jones E., Beer J. (eds.), Methods in Social Neuroscience. New York: Guilford Press, 17–44. [Google Scholar]
- Shamay-Tsoory S.G. (2010). Oxytocin, social salience, and social approach. Biological Psychiatry , 67(6), e35. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Ikeda K., Ishikawa M., et al. (2005). Anxiety, reactivity, and social stress-induced cortisol elevation in humans. Neuroendocrinology Letters , 26(4), 351–4. [PubMed] [Google Scholar]
- Tomchek S.D., Dunn W. (2007). Sensory processing in children with and without autism: a comparative study using the short sensory profile. American Journal of Occupational Therapy , 61(2), 190–200. [DOI] [PubMed] [Google Scholar]
- Vranic A. (2003). Personal space in physically abused children. Environment and Behavior , 35(4), 550–65. [Google Scholar]
- Watling R.L., Deitz J., White O. (2001). Comparison of sensory profile scores of young children with and without autism spectrum disorders. American Journal of Occupational Therapy , 55(4), 416–23. [DOI] [PubMed] [Google Scholar]