Abstract
There is a need for effective, early post-trauma preventive interventions for post-traumatic stress disorder (PTSD). Attenuating amygdala hyperreactivity early post-trauma, a likely PTSD vulnerability factor, may decrease PTSD risk. Since oxytocin modulates amygdala reactivity to emotional stimuli, oxytocin administration early post-trauma may be a promising candidate for PTSD prevention. In a randomized double-blind placebo-controlled fMRI study, we investigated effects of a single intranasal oxytocin administration (40 IU) on amygdala reactivity to happy, neutral and fearful faces in 41 recently trauma-exposed men and women showing moderate to high distress after initial post-trauma screening. We explored treatment interactions with sex. Participants were scanned within 11 days post-trauma. Compared with placebo, oxytocin significantly increased right amygdala reactivity to fearful faces. There was a significant treatment by sex interaction on amygdala reactivity to neutral faces, with women showing increased left amygdala reactivity after oxytocin. These findings indicate that a single oxytocin administration may enhance fearful faces processing in recently trauma-exposed individuals and neutral faces processing in recently trauma-exposed women. These observations may be explained by oxytocin-induced increased salience processing. Clinical implications of these findings for PTSD prevention should be further investigated. Trial register: Netherlands Trial Registry; Boosting Oxytocin after trauma: Neurobiology and the Development of Stress-related psychopathology (BONDS); NTR3190; http://www.trialregister.nl/trialreg/admin/rctview.asp?TC = 3190;
Keywords: oxytocin, amygdala, PTSD, trauma, prevention, sex differences
Introduction
Approximately 80 percent of the general population will experience a traumatic event in their life, of which, ∼10% subsequently develops post-traumatic stress disorder (PTSD) (de Vries and Olff, 2009). Since PTSD is a disabling, costly disorder (Kessler, 2000) and not all patients benefit from currently available treatments (Pull and Pull, 2014), there is a clear need for effective preventive interventions. As a traumatic event is by definition a reference point for potential onset of PTSD symptoms, the first hours or days post-trauma may provide a unique opportunity to prevent PTSD development. Previously studied pharmacological agents (e.g. benzodiazepines and beta-adrenergic receptor blocking agents) have not yet proven to be effective for PTSD prevention (Amos et al., 2014), although there are indications that early post-trauma glucocorticoid administration may prevent PTSD symptom development (Zohar et al., 2011b; Delahanty et al., 2013). A potentially powerful prevention strategy is specifically targeting modifiable biological risk factors for PTSD development (van Zuiden et al., 2013). Here, we focused on oxytocin (OT) administration as a potential preventive intervention by investigating its effects on amygdala reactivity early post-trauma.
The amygdala with its functionally distinct subregions is pivotal in fear detection, learning, expression and extinction (LeDoux, 2000). Altered amygdala function appears to be etiologically involved in PTSD development: in military paramedics it was shown that pre-deployment, higher amygdala reactivity in response to both deployment-related and non-deployment related pictures (Admon et al., 2009), and to risky decision making in a gambling game (Admon et al., 2012) predicted PTSD symptom development post-deployment. Similarly, in adolescents amygdala reactivity to negative emotional stimuli prior to exposure to the Boston Marathon terrorist attacks predicted PTSD symptoms 4–6 weeks after the attack (McLaughlin et al., 2014). Findings in car accident survivors scanned within 2 weeks post-trauma suggest that higher amygdala reactivity in response to traumatic script imagery predicts PTSD symptoms 6 months later (deRoon-Cassini et al., 2014). These findings indicate that attenuating amygdala reactivity early post-trauma might decrease PTSD risk.
OT may be a promising pharmacological agent for PTSD prevention (Ostrowski and Delahanty, 2014), since animal studies demonstrated OT’s fear-mitigating properties in the amygdala (Huber et al., 2005), and in previous functional magnetic resonance imaging (fMRI) studies in humans it was repeatedly observed that a single OT administration may attenuate amygdala reactivity to (negative) emotional facial expressions. In healthy men, OT attenuated right amygdala responses to fearful, neutral and happy facial expressions (Domes et al., 2007), and left amygdala reactivity to fearful (Gamer et al., 2010) and angry (Kirsch et al., 2005) faces, although no effects of OT have also been reported (Montag et al., 2013; Sauer et al., 2013) Additionally, in healthy men participating in a fear-conditioning paradigm it was observed that OT administration decreased right amygdala reactivity to fearful faces previously associated with an electric shock (Petrovic et al., 2008). In addition, OT administration was associated with lower amygdala reactivity during fear extinction in healthy men (Eckstein et al., 2014).
However, a growing body of literature indicates that OT effects on amygdala reactivity may differ depending on interindividual factors (e.g. sex, psychopathology) and context (e.g. stimulus valence) (Bartz et al., 2011). For example, Gamer et al. (2010) found that OT not only decreased left amygdala reactivity to fearful faces but also increased left amygdala reactivity to happy faces in healthy men. Gender may also be an important moderator of OT effects, as in healthy women OT increased right amygdala reactivity to angry (Bertsch et al., 2013), and left amygdala reactivity to fearful facial expression (Domes et al., 2010), in contrast to findings of reduced left (Domes et al., 2007) and right (Kirsch et al., 2005; Gamer et al., 2010) amygdala reactivity to such stimuli in men. As higher amygdala reactivity is generally associated with higher anxiety, this OT-induced increase in amygdala reactivity in healthy women suggests that OT may not only uniformly act as an anxiolytic agent but could also have anxiogenic properties. This is in line with several recent rodent and human studies showing that—under certain circumstances—OT increased anxiety (Grillon et al., 2013; Guzmán et al., 2013; MacDonald et al., 2013).
Regarding psychopathology, findings in healthy men have been replicated in psychiatric patient populations characterized by prominent anxiety. In men with generalized social anxiety disorder (GSAD), OT normalized bilateral amygdala hyperresponsiveness to fearful faces (Labuschagne et al., 2010), and a similar dampening OT effect on right amygdala responses to angry faces was found in women with borderline personality disorder (BPD) (Bertsch et al., 2013). Furthermore, in male GSAD patients, symptom severity was associated with a greater increase of amygdala—medial prefrontal cortex resting-state functional connectivity after OT (Dodhia et al., 2014). In contrast, in males with Asperger’s syndrome—who, under placebo (PL), had lower amygdala reactivity to neutral faces compared with controls—OT administration did not decrease but increased left (Domes et al., 2014) and right (Domes et al., 2013) amygdala responsiveness to neutral facial stimuli. These findings suggest that OT effects on amygdala function may differ between individuals depending on presence, type and severity of psychopathology.
No studies have yet been performed that assessed OT administration effects on amygdala reactivity in recently trauma-exposed individuals. OT’s potential anxiolytic effects, subjectively and at the level of amygdala function to emotional stimuli (Wigton et al., 2015), together with evidence that amygdala reactivity may be etiologically involved in PTSD development (Admon et al., 2013), suggest that OT may be an effective early post-trauma preventive intervention for PTSD (Frijling et al., 2014). Therefore, we investigated acute effects of a single intranasal OT vs PL administration on amygdala reactivity to emotional faces in recently trauma-exposed healthy individuals reporting moderate to high levels of distress, expecting that OT would attenuate amygdala reactivity to fearful faces. Furthermore, as we included both men and women, we explored sex-differential effects. Additionally, we assessed whether baseline PTSD symptom severity was (differentially) associated with amygdala reactivity to emotional faces in PL- and OT-treated participants, to explore whether in recently trauma-exposed individuals amygdala reactivity is related to acute PTSD symptom severity and whether symptom severity may influence intranasal OT effects on amygdala reactivity.
Methods
Participants
Forty-one participants gave verbal and written informed consent, were included in the study, and were analyzed (for recruitment details, see CONSORT Flow Diagram in Supplementary material). The study was approved by the Institutional Review Board of the Academic Medical Center (AMC) and conducted following Good Clinical Practice guidelines. Participants were recruited from three Emergency Departments in Amsterdam, following experiencing a traumatic event—according to the Diagnostic and Statistical Manual of Psychiatric Disorders (4th edition) PTSD A1 criterion (American Psychiatric Association 2000) (see Table 1 for types of trauma experienced). Dutch or English speaking adults (18–65 years) scoring above the cutoff on screening questionnaires indicating acute distress and hence increased PTSD risk (see later) were eligible to participate. Previous studies on PTSD prevention demonstrated that it is ineffective to apply preventive interventions to all trauma-exposed individuals, including resilient individuals (Zohar et al., 2011a). Additionally, as preventive interventions potentially interfere with natural recovery processes (Rose et al., 2002), it is preferable to administer interventions only to a subset of trauma-exposed individuals, i.e. to those at high risk for PTSD who are least likely to recover naturally. Exclusion criteria were MRI contraindications, severe/chronic systemic disease, current PTSD and current psychotic/major depressive/bipolar/substance-related/personality disorder, mental retardation, neurological/endocrinological disorder, ongoing traumatization, medication-use potentially interfering with OT, allergy for OT, impaired consciousness, amnesia or confusion, pregnancy and breastfeeding.
Table 1.
Participant characteristics
| Oxytocin | Placebo | Statistics | |
|---|---|---|---|
| N | 23 | 18 | |
| Males (%) | 9 (39%) | 8 (44%) | χ2 = 0.1, df=1, P = 0.73b |
| Age | 39.7 ± 11.5 | 31.3 ± 11.5 | T(39) = −0.4, P = 0.66a |
| Type of trauma | χ2 = 2.2, df=3, P = 0.52 | ||
| Traffic accident (%) | 12 (52%) | 13 (72%) | |
| Accident at work/home (%) | 5 (22%) | 3 (17%) | |
| Interpersonal trauma (%) | 5 (22%) | 2 (11%) | |
| Other (%) | 1 (4%) | 0 (0%) | |
| Days between trauma and scan session | 8.7 ± 1.3 | 7.8 ± 2.9 | T(39) = 1.4, P = 0.18a |
| CAPS total | 46.8 ± 20.0 | 39.0 ± 17.8 | T(39) = 1.3, P = 0.20a |
| CAPS reexperiencing | 19.9 ± 9.5 | 13.1 ± 8.2 | T(39) = 2.4, P = 0.02a |
| CAPS avoidance | 10.2 ± 7.7 | 10.7 ± 5.6 | T(39) = −0.3, P = 0.80a |
| CAPS hyperarousal | 16.7 ± 7.6 | 15.2 ± 8.2 | T(39) = 0.6, P = 0.54a |
| Baseline anxiety VAS | med: 4.5 (range 0–53) | med: 4.0 (range 0–58) | U(38) = 195, P = 0.93c |
Means ( ± s.d.) or medians (and range) are listed for the oxytocin (OT) and placebo (PL) groups at baseline. CAPS, Clinical Administered PTSD scale; med median; OT, oxytocin; PL, placebo; VAS, visual analog scale; atested with two-sample t-test; btested with chi-square test; ctested with Mann-Whitney U-test.
Design and intranasal treatment
The fMRI experiment was set up as a randomized, double-blind, PL-controlled between-subject study. Participants were randomly assigned to a single intranasal OT [40 IU OT (Defiante Farmaceutica, S.A., Funchal, Portugal); 5 puffs 4 IU OT per nostril] or PL (0.8% NaCl solution) administration, applied 45 min prior to fMRI scanning, which occurred not later than 11 days post-trauma [mean (s.d.): 8.3 (2.2) days]. We selected a 40 IU dose as this fMRI study was part of a larger randomized controlled trial (RCT) investigating the efficacy of multiple OT administrations for PTSD prevention (Frijling et al., 2014). In this RCT, an 8 day OT treatment regimen was administered (40 IU dose twice daily; 15 doses in total, including the one prior to fMRI scanning). As previous multiple administration studies (which were at the start of the RCT (Feifel et al., (2010); and Ohlsson et al., (2005)) used 40 IU to investigate OT administration effects on clinical outcomes in schizophrenia patients and patients with irritable bowel syndrome, we opted for the same dose, instead of the more commonly used 24 IU in fMRI studies investigating effects of a single OT administration on neural functioning. For all 22 participants also participating in an ongoing RCT on the efficacy of multiple OT intranasal doses in PTSD prevention (Frijling et al., 2014) scanning took place following the first intranasal dose.
Procedure
Potentially trauma-exposed emergency department patients were identified and contacted for screening within 1 week post-trauma [mean (s.d.): 3.1 (2.0) days]. Potential eligible participants were assessed for moderate to severe distress indicating increased PTSD risk using the trauma screening questionnaire [TSQ, cutoff score ≥ 5 (Brewin et al., 2002; Mouthaan et al., 2014, Walters et al., 2007)] and peritraumatic distress inventory [PDI, cutoff score ≥ 17, (Nishi et al., 2010), see Frijling et al. (2014) for detailed information]. Patients scoring above cutoff on the TSQ and/or PDI were invited to participate. At baseline [mean (s.d.): 5.9 (2.0) days post-trauma] current and lifetime psychopathology was assessed with the MINI International Neuropsychiatric Interview (Sheehan et al., 1998). Additionally, severity of acute PTSD symptoms was measured with the Clinician-Administered PTSD Scale (CAPS) (Blake et al., 1995) assessing the frequency and intensity of DSM-IV PTSD symptoms in the time period following trauma. The MRI session occurred not later than 11 days post-trauma [mean (s.d.): 8.3 (2.2) days]. Participants abstained from food, beverages (except water), smoking and exercise 3.5 h prior to scanning.
Task
As a hyperresponsive amygdala to (negative) emotional stimuli is thought to play a central role in PTSD and PTSD development, and was previously shown to predict PTSD development (Admon et al., 2013), we used a blocked emotional-face matching task, an easy and relatively low burden task that robustly activates the amygdala (Fusar-Poli et al., 2009). In addition, a face-matching task allows investigating differential amygdala responses to positive, neutral and negative emotions, which enables assessing whether OT generally affects amygdala function, or whether OT’s effects are valence-specific in recently trauma-exposed individuals. Each of the 12 emotional faces blocks (20 s, 4 blocks per emotion) consisted of four trials (5 s) with trios of faces all depicting the same emotional facial expression: neutral, happy or fearful. Participants were instructed to match the upper face to 1 of 2 lower faces based on sex. Frontal camera pictures of 8 men and 8 women were selected from the Radboud Faces Database (Langner et al., 2010). The number of male and female matches was equal between blocks. As a control condition we included 5 blocks (20 s) with 4 trios of horizontally and vertically oriented shapes of scrambled faces (to be matched on shape-orientation). See Supplementary Figure S1 for a schematic overview of block presentation order.
MRI data acquisition
On a 3T Philips Ingenia MR system (Philips Medical Systems, Best, The Netherlands) with a 16-channel head coil located at the AMC, we collected high resolution T2*-weighted echoplanar images with Blood-oxygen-level dependent (BOLD) contrast (repetition time (TR) = 2300 ms, echo time (TE) = 27 ms, matrix size: 96 × 96, voxel size: 2.29 × 2.29 mm, slice thickness = 3.0 mm, no gap, field of view = 220 × 220 × 120, flip angle = 80°, 40 transverse slices). Anatomical images were acquired using a T1-weighted 3D magnetization-prepared rapid acquisition with gradient echo (MPRAGE) sequence (TR = 6.7 ms, TE = 3.1 ms), matrix size: 256 × 256, voxel size: 1.11 × 1.11 mm, flip angle = 9°, 170 sagittal slices).
Data analysis
Functional MRI data were processed and analyzed using Statistical Parametric Mapping 8 (SPM8) (UCL, London, UK) and Matlab 2013b (The Mathworks Inc., Natick, MA). Preprocessing consisted of spatial realignment, slice time correction, segmentation, normalization to montreal neurological institute (MNI) space (resampled to 1 × 1 × 1 mm voxels), and smoothing using a 5 mm Full width at half maximum (FWHM) Gaussian kernel. By applying a data preprocessing approach similar to previous studies (Bertsch et al., 2013; Gamer et al., 2010), the normalization and smoothing procedures were aimed at localizing effects in amygdala subregions.
Participant-specific general linear models were created by modeling all emotional faces blocks as box-car functions convolved with the hemodynamic response function. A high-pass filter (128 s) was applied to remove low-frequency signals and six realignment parameters were included in the model as nuisance regressors. Four individual contrast images were generated: all faces > shapes, neutral > shapes, happy > shapes, fearful > shapes.
Whole-brain task effects were assessed using one-sample t-tests over all task conditions and participants, with a statistical threshold of 0.05 family-wise-error (FWE) corrected for multiple comparisons (for results see Supplementary Table S1). In addition, to test if the task successfully resulted in differential amygdala responses to neutral, happy and fearful faces, the main effect of type of emotion was tested in the amygdala (for methods and results see Supplementary Figure S2). For group inferences, second-level random effects analyses were performed to test OT > PL and PL > OT contrasts for each condition separately using two-sample t-tests. Since we hypothesized that OT affects amygdala reactivity, we conducted region of interest (ROI) analyses for the bilateral amygdala using pre-defined anatomical amygdala masks from the Automated Anatomical Labeling (AAL) atlas in the SPM Wake Forest University Pickatlas toolbox (Tzourio-Mazoyer et al., 2002). Subsequently, we explored treatment by sex interactions for each condition using two-way Analyses of variance (ANOVAs) with treatment and sex as factors. If the F-test reached significance, post hoc two-sample t-tests were conducted to assess the direction of the interaction. Furthermore, we explored whether baseline PTSD symptom severity was (differentially) associated with amygdala reactivity depending on intranasal treatment. To this end, we extracted mean contrast estimates (beta-weights) from a 5 mm sphere around the peak voxel of significant OT effects using MarsBar (Brett et al., 2002). In Statistical Package for the Social Sciences (SPSS), we conducted bivariate (Pearson) correlation analyses between CAPS total scores and the amygdala contrast estimates, in the PL- and OT-treated participants separately. We applied a Fischer r-to-z transformation to test whether the obtained correlation coefficients significantly differed between treatment groups.
For statistically significant treatment effects, we adjusted for participant characteristics that differed significantly at baseline between treatment groups (Table 1). Significant activations [P-value < 0.05, for amygdala ROI analyses small volume corrected (SVC) for multiple comparisons within the ROI] are reported in MNI space. For peak voxel sublocalization within the amygdala, we used the probabilistic atlas of the SPM Anatomy toolbox atlas (Eickhoff et al., 2005).
Results
Participant characteristics
Participant characteristics are listed in Table 1. Treatment groups did not significantly differ in age, type of trauma, time since trauma at scan and PTSD symptom severity scores, apart from significantly higher levels of reexperiencing symptoms in the OT-treated group (mean ± s.d.: 19.9 ± 9.5) relative to the PL-treated group (mean ± s.d.: 13.1 ± 8.2, P = 0.02).
Amygdala ROI analyses
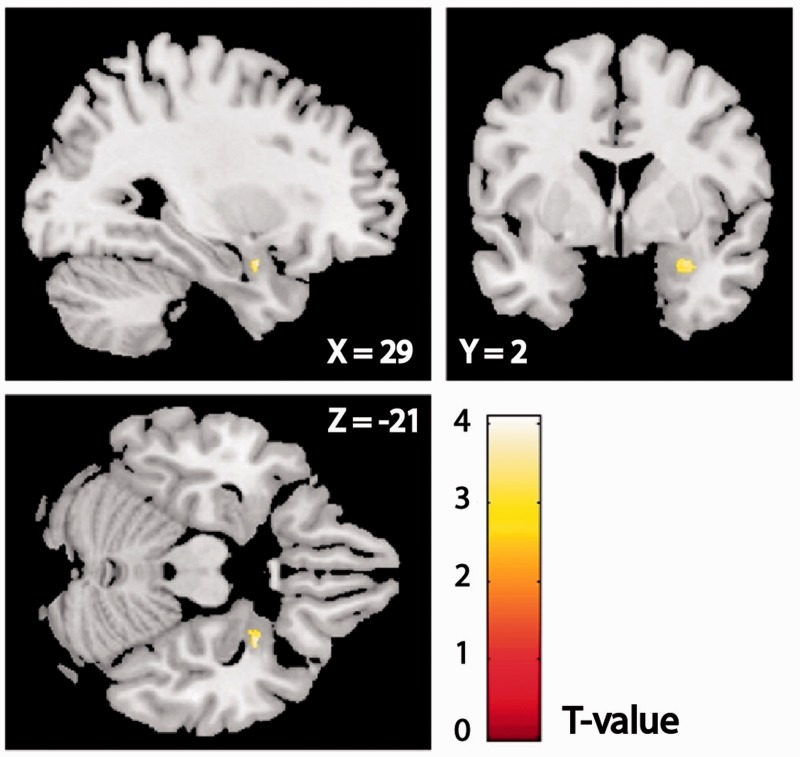
We investigated effects of intranasal OT relative to PL on amygdala reactivity to emotional faces. For happy and neutral faces, there were no significant group differences in amygdala reactivity (see Table 2 for all peak voxels and statistics). For fearful faces, the OT > PL contrast revealed a significant peak in the right (basolateral) amygdala (Figure 1, peak voxel xyz: 29 3 − 21, z = 3.70, PSVC = 0.02), also when adjusting for the baseline difference between the OT and PL groups in severity of reexperiencing symptoms (i.e. CAPS B subscale scores (z=3.61, PSVC=0.03)). For the PL > OT contrast for fearful faces no voxels survived the statistical threshold.
Table 2.
Results of amygdala ROI analyses
| Right amygdala |
Left amygdala |
||||||
|---|---|---|---|---|---|---|---|
| Contrast | Peak coordinate (xyz) | z-score | P-value | Peak coordinate (xyz) | z-score | P-value | |
| Happy | PL > OT | 36 0 −27 | 2.61 | 0.30 | −22 −8 −15 | 2.26 | 0.47 |
| OT > PL | 28 3 −29 | 2.27 | 0.51 | −28 −5 −25 | 1.88 | 0.69 | |
| Treatment × sex | 25 2 −22 | 2.16 | 0.65 | −23 −1 −25 | 2.69 | 0.28 | |
| Neutral | PL > OT | 17 3 −16 | 1.87 | 0.74 | −31 −2 −27 | 2.60 | 0.28 |
| OT > PL | 28 −2 −12 | 1.99 | 0.68 | −22 −3 −13 | 2.40 | 0.39 | |
| Treatment × sex | 30−4 −18 | 3.95 | 0.009 | −25 −8 −16 | 4.21 | 0.003 | |
| Women PL > OT | 25−3 −28 | 1.13 | 0.94 | −30 −1 −26 | 2.10 | 0.58 | |
| Men PL > OT | 30 −4 −18 | 3.16 | 0.09 | −25 −8 −16 | 3.21 | 0.07 | |
| Women OT > PL | 31−3 −18 | 3.26 | 0.07 | −25 −9 −17 | 3.56 | 0.03 | |
| Men OT > PL | 30 4 −19 | 1.78 | 0.78 | −23 −2 −12 | 2.93 | 0.15 | |
| Fearful | PL > OT | 24 −9 −12 | 2.83 | 0.20 | −20 −6 −18 | 2.69 | 0.24 |
| OT > PL | 29 3 −21 | 3.70 | 0.02 | −14 0 −16 | 1.50 | 0.85 | |
| Treatment × sex | 27 −9 −12 | 2.38 | 0.51 | −12 −1 −17 | 1.99 | 0.73 | |
All analyses were conducted in SPM8, testing for the effects of OT vs PL treatment in response to happy, neutral and fearful faces (t-tests). Results of the exploratory sex by treatment interactions (F-tests) are additionally reported, as well as results of post hoc t-tests after significant interaction effects. Peak voxel MNI coordinates, z-scores, and P-values for significantly activated clusters are listed. Statistically significant results (PSVC < 0.05) are printed in bold. MNI, Montreal Neurological Institute; PL, placebo; OT, oxytocin; SVC, small volume corrected.
Fig. 1.
A statistical map of the fearful faces OT > PL contrast for the right amygdala is overlaid on a single-subject template brain image (display threshold Puncorr < 0.01). Peak voxel xyz: 29 3 −21, z = 3.70, Psvc = 0.02. OT, oxytocin; PL, placebo.
We also explored whether baseline PTSD symptom severity was differentially associated with amygdala reactivity to fearful faces depending on intranasal treatment. In both groups, correlations between CAPS total score and amygdala reactivity to fearful faces were not significant (PL: r = −0.33, P = 0.18; OT: r = −0.21, P = 0.34). Fisher r-to-z transformation showed that the correlation coefficients did not significantly differ between groups (z = 0.38, P = 0.70), indicating that baseline acute PTSD symptom severity was not differentially associated with amygdala reactivity to fearful faces in PL- and OT-treated participants.
Amygdala ROI analysis for exploratory treatment by sex interactions in the amygdala
Finally, we explored sex-differential OT effects on amygdala reactivity by testing treatment by sex interactions for each emotion condition. There were no significant main effects of sex, likewise, for happy and fearful faces, treatment by sex interaction effects were not significant. For neutral faces, we found significant treatment by sex interactions in the left and right (basolateral) amygdala [left peak voxel xyz: −25 −8 −16, F(1,37) = 25.35, z = 4.21, PSVC < 0.01; right peak voxel xyz: 30 −4 −18, F(1,37) = 21.76, z = 3.95, PSVC < 0.01]. Post hoc t-tests revealed significantly greater left amygdala reactivity to neutral faces after OT administration in women (peak voxel xyz: −26 −8 −17, z = 3.56, PSVC = 0.03) (see Table 2 for peak voxels and statistics of all sex by treatment interactions and post hoc t-tests). The interaction and posthoc test remained significant when adjusting for the baseline difference between the OT and PL groups in severity of reexperiencing symptoms.
Discussion
We investigated the acute effects of a single OT administration on amygdala reactivity to emotional faces in recently trauma-exposed individuals with moderate to high levels of distress acutely post-trauma. Our results demonstrate that intranasal OT enhanced amygdala reactivity in this population, depending on stimulus valence and sex. OT administration increased right amygdala reactivity to fearful faces. Furthermore, OT increased left amygdala reactivity to neutral faces in women. No effects of OT on amygdala reactivity to happy faces were found. Acute PTSD symptom severity was not (differentially) associated with amygdala reactivity to fearful faces in PL- and OT-treated participants, suggesting that early post-trauma PTSD symptoms were not related to amygdala reactivity to fearful faces, and that OT’s effect on amygdala reactivity to fearful faces did not depend on the participant’s acute PTSD symptom severity.
Our observation that OT increased right amygdala reactivity to fearful faces is in line with previous studies in healthy women (Domes et al., 2010). However, the results are in contrast with findings in healthy men (Kirsch et al., 2005; Domes et al., 2007; Gamer et al., 2010), male GSAD patients (Labuschagne et al., 2010), and women with BPD (Bertsch et al., 2013) and contrary to our expectations for recently trauma-exposed individuals. Our findings further demonstrate that (potentially therapeutic) effects of OT in one population (e.g. GSAD) cannot be directly generalized to other populations (e.g. recently trauma-exposed individuals).
The observed valence-dependent OT effect suggests that OT specifically enhanced fear processing but not face processing in general. The amygdala has a well-established role in fear processing (LeDoux, 2000). In healthy individuals, higher amygdala reactivity has been associated with better fear recognition and higher anxiety (Etkin et al., 2004; Derntl et al., 2009), and in PTSD patients, amygdala reactivity was associated with symptom severity (Shin et al., 2006). Additionally, as higher amygdala reactivity both pre- and early post-trauma has additionally been associated with greater subsequent PTSD symptom severity (Admon et al., 2009; Admon et al., 2012; deRoon-Cassini et al., 2014; McLaughlin et al., 2014), our observation of increased amygdala reactivity to fearful faces after OT does not directly support using OT administration for PTSD prevention, and might translate to behavioral anxiogenic effects. There are several possible explanations for the observed effects of increased processing of fear-related stimuli after OT administration in our study. First, OT administration has previously been suggested to increase (social) salience detection (Bartz et al., 2011). Although it is plausible that processing of threat- and/or safety-related stimuli may become enhanced during a state of increased salience detection, which specific environmental cues are evaluated as increasingly salient in a given context, may depend on interindividual factors. We therefore propose that (OT induced) increased salience processing in individuals who were recently exposed to a threatening event, may result in increased processing of fearful faces (and not happy faces), as threat-related stimuli (e.g. fearful faces) may be specifically more salient to these individuals than safety-related stimuli (e.g. happy faces). However, apart from interindividual differences, context characteristics may also guide which specific environmental cues within the given context are evaluated as salient (Shamay-Tsoory et al., 2009). Within our specific study context—i.e. looking at different emotional faces in an MRI scanner—in PL-treated participants we observed that amygdala reactivity was higher in response to fearful faces compared with happy faces (see Supplementary material, and Fusar-Poli et al., 2009 for a meta-analysis showing similar results). This may indicate that within our task, fearful faces generally captured more attention than happy faces, and that OT may have increased this effect as a consequence of increased salience processing.
Second, recent data from our group’s study on OT administration effects in PTSD patients indicate that OT administration increased amygdala reactivity to emotional faces in the healthy trauma-exposed control group of this study (Koch et al., 2015). As the recently trauma-exposed participants of this study did not have PTSD at time of their recent trauma, the described results here may reflect OT administration effects in trauma-exposed but relatively healthy, individuals (their acute post-traumatic stress reactions, indicating increased PTSD risk, aside).
Of note, in healthy women, OT administration has previously been demonstrated to enhance left (Domes et al., 2010) and right (Bertsch et al., 2013) amygdala reactivity to fearful (Domes et al., 2010) and angry (Bertsch et al., 2013) facial expressions. Our results demonstrate that OT may not only increase left amygdala reactivity to fearful facial expressions in women but also to neutral faces. Sex differences in OT administration effects on amygdala reactivity may be explained by estrogen enhancing central OT-receptor affinity (Caldwell et al., 1994), combined with indications of inverted U-shaped dose-response effects for OT (Chini et al., 2013). Regarding dose-response effects for OT, Cardoso et al. (2012) found that only 24 IU of intranasal OT, and not 48 IU, attenuated cortisol response to physical exercise in healthy men, indicating that in healthy men, relatively lower OT system stimulation may be associated with attenuated neuroendocrine responses to stress. It may be possible that under high OT-levels, OT-binding to arginine vasopressin V1a-receptors in the amygdala results in opposite effects on fear expression (i.e. anxiogenic) than when binding to OT-receptors (i.e. anxiolytic) (Huber et al., 2005).
In addition to potential dose-response effects, it should be noted that outcomes of OT administration may vary between various treatment durations, as differential effects of single and prolonged OT treatments on anxiety have been demonstrated in rodents (Slattery and Neumann, 2010). Therefore, as previously suggested (Macdonald and Feifel, 2014), OT’s acute effects should not be directly translated to its effects after prolonged administration, analogous to selective serotonin reuptake inhibitor (SSRI) use (e.g. Burghardt and Bauer, 2013).
Given that higher amygdala responsiveness has previously been associated with higher anxiety, our observations may indicate an anxiogenic effect of OT administration, which is in line with observations of several other recent studies. In socially defeated rats, endogenous OT release in the lateral septum augmented the intensity of the memory of social defeat, which resulted in potentiated fear behavior upon reexposure to the aggressor (Guzmán et al., 2013). Potential anxiogenic OT effects have also been observed in humans. A single intranasal OT administration increased startle responses to unexpected shocks (Grillon et al., 2013) and OT administration prior to psychotherapy in male patients with major depressive disorder increased anxiety during the session (MacDonald et al., 2013).
However, it should be noted that high amygdala reactivity to fearful faces, and thus also the OT effect in our sample, may not automatically reflect induced anxiety and increased PTSD risk in recently trauma-exposed individuals. Illustrative of this notion, with our PL-treated participants we did not observe a significant association between acute PTSD symptoms and amygdala reactivity to fearful faces, suggesting that in our sample higher amygdala reactivity did not necessarily reflect higher acute symptom levels, and/or increased PTSD risk. In addition, in military personnel who did not develop clinically significant PTSD symptoms in response to deployment, increased amygdala reactivity to threat-related faces was observed post-deployment relative to pre-deployment (van Wingen et al., 2011). Since none of the participants of this study developed PTSD, this finding may suggests that increased amygdala reactivity to negative facial expressions after severe stress does not necessarily predict adverse outcome.
Although the results of this study on effects of a single intranasal OT administration in recently trauma-exposed individuals do not support our hypothesis that OT administration may prevent PTSD by attenuating amygdala reactivity, more research on (repeated) OT administration in this population is needed before definite conclusions about OT’s (lack of) therapeutic potential for PTSD prevention can be drawn. First, how OT-induced increased amygdala reactivity to threat-related stimuli relate to long-term PTSD symptom development needs to be evaluated, since long-term clinical effects of increased processing of fear-related stimuli remain unclear. Of note, in BPD patients, in contrast to findings of beneficial OT effects on stress and fear processing (Simeon et al., 2011; Bertsch et al., 2013), adverse effects from OT administration on social behavior were also observed (Bartz et al., 2010). This illustrates that measures related to both stress/fear processing and behavior should be included in future studies, not only to deepen our understanding of how OT can affect (trauma-exposed) patients neurobiologically, but also clinically. In addition, recruitment of the amygdala is not only necessary for fear conditioning but also for fear extinction (LaBar et al., 1998). Consequently, OT effects on fear memory should be assessed, as PTSD is thought to develop due to overconsolidation of fear memories, and impaired fear extinction (Mahan and Ressler, 2012). Therefore, timing of OT administration in relation to the fear conditioning phase, and associated effects on amygdala reactivity, should be evaluated. In rodents, contrasting effects on fear expression were demonstrated for OT administration, depending on whether OT was administered prior to fear conditioning or extinction (Toth et al., 2012). Moreover, evidence from previous studies in rodents and healthy men suggest that OT administration closely timed with fear extinction improves fear extinction outcomes, and may reinforce adaptive memory reconsolidation (Cohen et al., 2010; Acheson et al., 2013; Zoicas et al., 2014), although this was not always found (Toth et al., 2012). In addition, prolonged administration and dose–response effects of intranasal OT on amygdala reactivity should be investigated. Further, in this study we explored a limited number of potential moderators of OT effects (i.e. CAPS scores and sex). Other interindividual characteristics such as a history of childhood trauma (Grimm et al., 2014), and variations in genes involved in OT secretion (Sauer et al., 2013) also may influence OT effects on amygdala reactivity. These are also PTSD risk factors (Ozer et al., 2003; Feldman et al., 2014). A better understanding on how OT administration interacts with these characteristics is needed. Similarly, since effects of OT-signaling and OT administration on anxiety appear to depend on (social) context (e.g. Guzmán et al., 2014), future studies should provide more insight how potential beneficial or adverse outcomes of OT administration can be reinforced or prevented, respectively.
This study is the first to assess acute effects of OT administration in a recently trauma-exposed population, and thereby provide empirical evidence relevant when considering the use of OT for PTSD prevention. Furthermore, no previous fMRI studies on OT administration included a mixed gender sample. Our study also has several limitations. First, we did not include a recently trauma-exposed control group with low distress. Therefore, we could not assess (OT effects on) amygdala reactivity specifically associated with high, relative to low distress early post-trauma. Such assessment would have allowed us to more accurately interpret the observed OT effect on increasing amygdala reactivity in relation to early post-trauma PTSD risk, as higher acute distress early post-trauma is associated with higher PTSD risk. Second, although our data support, at least in part, the hypothesis that OT effects are sex-dependent, the study was not adequately powered to detect more subtle sex-related effects. Third, since participants were scanned within 11 days post-trauma, we could not plan scanning during specific phases of women menstrual cycles. Moreover, 61% of female participants used hormonal contraceptives. Therefore, we could not account for the influence of gonadal hormone levels on OT effects on amygdala reactivity. However, earlier studies in healthy women have shown similar effects of OT on amygdala reactivity in the luteal (Domes et al., 2010) and follicular phase (Bertsch et al., 2013), indicating that in healthy women menstrual phase may not strongly influence the direction of OT administration effects on amygdala reactivity to negative stimuli. Finally, it should be noted that we specifically aimed to include participants based on moderate to high levels of acute distress according to the PDI and/or TSQ, which resulted in a relative underrepresentation of individuals with low CAPS scores compared with the general population of traumatic injury patients (Mouthaan et al., 2014). This approach could have led to less robust associations between acute PTSD symptom severity and amygdala reactivity to fearful faces in our sample than would be observed in a non–pre-selected recently trauma-exposed population.
In all, our findings suggest that a single OT administration enhances processing of fearful faces in recently trauma-exposed individuals and also of neutral faces in recently trauma-exposed women, which may be explained by OT-induced increased salience processing. Clinical implications of these findings for PTSD prevention should be further investigated.
Supplementary Material
Acknowledgements
The authors thank J.C. Goslings, J.S.K. Luitse, T. Biesheuvel, E. Hofstra, F.C. Bakker, A. Honig, P. Philipse and R. van Nieuwenhuizen as well as all other emergency department and trauma surgery department personnel for their hospitable attitude toward our study at their departments. They thank all emergency department patients that were willing to participate in the initial screening and in the study. We also thank Kim van Dijk, Joanne Will and Annike Bekius for scanning assistance as well as all other students involved for their assistance in recruitment and assessment procedures.
Funding
This study was funded by grants from The Netherlands organization for Health research and Development (ZonMw, grant no. 91210041) and from the AMC Research Council (grant no. 110614), which both had no further role in study design; in the collection, analysis and interpretation of data; in writing the report or in deciding to submit the article for publication.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Acheson D., Feifel D., de Wilde S., Mckinney R., Lohr J., Risbrough V. (2013). The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology , 229(1), 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R., Milad M.R., Hendler T. (2013). A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends in Cognitive Sciences , 17(7), 337–47. [DOI] [PubMed] [Google Scholar]
- Admon R., Lubin G., Rosenblatt J.D., et al. (2012). Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cerebral Cortex , 23(1), 28–35. [DOI] [PubMed] [Google Scholar]
- Admon R., Lubin G., Stern O., et al. (2009). Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences of the United States of America , 106(33), 14120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). DSM-IV-TR: Diagnostic and statistical manual of mental disorders, 4th edition, text revision Washington, DC: American Psychiatric Association. [Google Scholar]
- Amos T., Stein D.J., Ipser J.C. (2014). Pharmacological interventions for preventing post-traumatic stress disorder (PTSD). The Cochrane Database of Systematic Reviews, 7, CD006239. [Google Scholar]
- Bartz J., Simeon D., Hamilton H., et al. (2011). Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognitive and Affective Neuroscience, 6(5), 556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J.A., Zaki J., Bolger N., Ochsner K.N. (2011). Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences , 15(7), 301–9. [DOI] [PubMed] [Google Scholar]
- Bertsch K., Gamer M., Schmidt B., et al. (2013). Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. American Journal of Psychiatry , 170(10), 1169–77. [DOI] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., et al. (1995). The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress , 8(1), 75–90. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J., Valabregue R., Poline J. (2002). Region of interest analysis using the MarsBar toolbox for SPM 99. NeuroImage, 16(2), S497. [Google Scholar]
- Brewin C.R., Rose S., Andrews B., et al. (2002). Brief screening instrument for post-traumatic stress disorder. The British Journal of Psychiatry , 181, 158–62. [DOI] [PubMed] [Google Scholar]
- Burghardt N., Bauer E. (2013). Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits. Neuroscience , 247, 253–72. [DOI] [PubMed] [Google Scholar]
- Caldwell J.D., Walker C.H., Pedersen C.A., Baraka A.S., Mason G.A. (1994). Estrogen increases affinity of oxytocin receptors in the medial preoptic area-anterior hypothalamus. Peptides , 15(6), 1079–84. [DOI] [PubMed] [Google Scholar]
- Cardoso C., Ellenbogen M.A., Orlando M.A., Bacon S.L., Joober R. (2012). Intranasal oxytocin attenuates the cortisol response to physical stress: a dose–response study. Psychoneuroendocrinology , 38(3), 399–407. [DOI] [PubMed] [Google Scholar]
- Chini B., Leonzino M., Braida D., Sala M. (2013). Learning about oxytocin: pharmacologic and behavioral issues. Biological Psychiatry , 76(5), 360–6. [DOI] [PubMed] [Google Scholar]
- Cohen H., Kaplan Z., Kozlovsky N., Gidron Y., Matar M.A., Zohar J. (2010). Hippocampal microinfusion of oxytocin attenuates the behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. Journal of Neuroendocrinology , 22(8), 889–904. [DOI] [PubMed] [Google Scholar]
- de Vries G.J., Olff M. (2009). The lifetime prevalence of traumatic events and posttraumatic stress disorder in the Netherlands. Journal of Traumatic Stress , 22(4), 259–67. [DOI] [PubMed] [Google Scholar]
- Delahanty D.L., Gabert-Quillen C., Ostrowski S.A., et al. (2013). The efficacy of initial hydrocortisone administration at preventing posttraumatic distress in adult trauma patients: a randomized trial. CNS Spectrums , 18(2), 103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Habel U., Windischberger C., et al. (2009). General and specific responsiveness of the amygdala during explicit emotion recognition in females and males. BMC Neuroscience , 10, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deRoon-Cassini T., Taubitz L., Larson C. (2014). Prediction of chronic PTSD using early fMRI evidence of emotion dysregulation in trauma survivors. Biological Psychiatry, 75(9), 169S. [Google Scholar]
- Dodhia S., Hosanagar A., Fitzgerald D.A., et al. (2014). Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology, 39(9), 2061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G., Kumbier E., Heinrichs M., Herpertz S.C. (2014). Oxytocin promotes facial emotion recognition and amygdala reactivity in adults with asperger syndrome. Neuropsychopharmacology , 39(3), 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Kumbier E., Grossmann A., Hauenstein K., Herpertz S.C. (2013). Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biological Psychiatry, 74(3), 164–71. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Glascher J., Buchel C., Braus D.F., Herpertz S.C. (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry , 62(10), 1187–90. [DOI] [PubMed] [Google Scholar]
- Domes G., Lischke A., Berger C., et al. (2010). Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology , 35(1), 83–93. [DOI] [PubMed] [Google Scholar]
- Eckstein M., Becker B., Scheele D., et al. (2014). Oxytocin facilitates the extinction of conditioned fear in humans. Biological Psychiatry, 78(3), 194–202. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage , 25(4), 1325–35. [DOI] [PubMed] [Google Scholar]
- Etkin A., Klemenhagen K.C., Dudman J.T., et al. (2004). Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron , 44(6), 1043–55. [DOI] [PubMed] [Google Scholar]
- Feifel D., Macdonald K., Nguyen A., et al. (2010). Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biological Psychiatry , 68(7), 678–80. [DOI] [PubMed] [Google Scholar]
- Feldman R., Vengrober A., Ebstein R. (2014). Affiliation buffers stress: cumulative genetic risk in oxytocin–vasopressin genes combines with early caregiving to predict PTSD in war-exposed young children. Translational Psychiatry , 4(3), e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling J.L., van Zuiden M., Koch S.B., et al. (2014). Efficacy of oxytocin administration early after psychotrauma in preventing the development of PTSD: study protocol of a randomized controlled trial. BMC Psychiatry , 14(1), 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., et al. (2009). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience , 34(6), 418–32. [PMC free article] [PubMed] [Google Scholar]
- Gamer M., Zurowski B., Buchel C. (2010). Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences of the United States of America , 107(20), 9400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Krimsky M., Charney D.R., Vytal K., Ernst M., Cornwell B. (2013). Oxytocin increases anxiety to unpredictable threat. Molecular Psychiatry, 18(9), 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S., Pestke K., Feeser M., et al. (2014). Early life stress modulates oxytocin effects on limbic system during acute psychosocial stress. Social Cognitive and Affective Neuroscience , 9(11), 1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán Y.F., Tronson N.C., Jovasevic V., et al. , (2013). Fear-enhancing effects of septal oxytocin receptors. Nature Neuroscience, 16, 1185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán Y.F., Tronson N.C., Sato K., et al. (2014). Role of oxytocin receptors in modulation of fear by social memory. Psychopharmacology , 231, 2097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D., Veinante P., Stoop R. (2005). Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science , 308(5719), 245–8. [DOI] [PubMed] [Google Scholar]
- Kessler R.C. (2000). Posttraumatic stress disorder: the burden to the individual and to society. Journal of Clinical Psychiatry , 61(Suppl 5), 4–12. [PubMed] [Google Scholar]
- Koch S. B. J., Van Zuiden M., Nawijn L., Frijling J. L., Veltman D. J. (2015). Intranasal Oxytocin Administration Dampens Amygdala Reactivity Towards Emotional Faces in Male and Female PTSD Patients. Neuropsychopharmacology . doi: 10.1038/npp.2015.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P., Esslinger C., Chen Q., et al. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience , 25(49), 11489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K.S., Gatenby J.C., Gore J.C., LeDoux J.E., Phelps E.A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron , 20(5), 937–45. [DOI] [PubMed] [Google Scholar]
- Labuschagne I., Phan K.L., Wood A., et al. (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology , 5(12), 2403–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner O., Dotsch R., Bijlstra G., Wigboldus D.H., Hawk S.T., van Knippenberg A. (2010). Presentation and validation of the Radboud Faces Database. Cognition and Emotion , 24(8), 1377–88. [Google Scholar]
- LeDoux J.E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience , 23, 155–84. [DOI] [PubMed] [Google Scholar]
- MacDonald K., MacDonald T.M., Brüne M., et al. (2013). Oxytocin and psychotherapy: a pilot study of its physiological, behavioral and subjective effects in males with depression. Psychoneuroendocrinology, 38(12), 2831–43. [DOI] [PubMed] [Google Scholar]
- Macdonald K., Feifel D. (2014). Oxytocins role in anxiety: a critical appraisal. Brain Research , 1580, 22–56. [DOI] [PubMed] [Google Scholar]
- Mahan A.L., Ressler K.J. (2012). Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends in Neurosciences , 35(1), 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Busso D.S., Duys A., et al. (2014). Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depression and Anxiety , 31(10), 834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C., Sauer C., Reuter M., Kirsch P. (2013). An interaction between oxytocin and a genetic variation of the oxytocin receptor modulates amygdala activity toward direct gaze: evidence from a pharmacological imaging genetics study. European Archives of Psychiatry and Clinical Neuroscience , 263(2), 169–75. [DOI] [PubMed] [Google Scholar]
- Mouthaan J., Sijbrandij M., Reitsma J.B., Gersons B.P., Olff M. (2014). Comparing screening instruments to predict posttraumatic stress disorder. PLoS One , 9(5), e97183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi D., Matsuoka Y., Yonemoto N., Noguchi H., Kim Y., Kanba S. (2010). Peritraumatic distress inventory as a predictor of post-traumatic stress disorder after a severe motor vehicle accident. Psychiatry and Clinical Neurosciences , 64(2), 149–56. [DOI] [PubMed] [Google Scholar]
- Ohlsson B., Truedsson M., Bengtsson M., et al. (2005). Effects of long-term treatment with oxytocin in chronic constipation; a double blind, placebo-controlled pilot trial. Neurogastroenterology and Motility , 17(5), 697–704. [DOI] [PubMed] [Google Scholar]
- Ostrowski S.A., Delahanty D.L. (2014). Prospects for the pharmacological prevention of post-traumatic stress in vulnerable individuals. CNS Drugs , 28(3), 195–203. [DOI] [PubMed] [Google Scholar]
- Ozer E.J., Best S.R., Lipsey T.L., Weiss D.S. (2003). Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychological Bulletin , 129(1), 52–73. [DOI] [PubMed] [Google Scholar]
- Petrovic P., Kalisch R., Singer T., Dolan R.J. (2008). Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. The Journal of Neuroscience , 28(26), 6607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull C.N., Pull C.B. (2014). Current status of treatment for posttraumatic stress disorder: focus on treatments combining pharmacotherapy and cognitive-behavioral therapy. International Journal of Cognitive Therapy , 7(2), 149–61. [Google Scholar]
- Rose S., Bisson J., Churchill R., Wessely S. (2002). Psychological debriefing for preventing post-traumatic stress disorder (PTSD). The Cochrane Database of Systematic Reviews, 2, CD000560. [Google Scholar]
- Sauer C., Montag C., Reuter M., Kirsch P. (2013). Imaging oxytocin× dopamine interactions: an epistasis effect of CD38 and COMT gene variants influences the impact of oxytocin on amygdala activation to social stimuli. Frontiers in Neuroscience, 7, 45. [Google Scholar]
- Shamay-Tsoory S.G., Fischer M., Dvash J., Harari H., Perach-Bloom N., Levkovitz Y. (2009). Intranasal administration of oxytocin increases envy and schadenfreude (gloating). Biological Psychiatry, 66(9), 864–70. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry , 59(Suppl), 2022–33. [PubMed] [Google Scholar]
- Shin L.M., Rauch S.L., Pitman R.K. (2006). Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences , 1071, 67–79. [DOI] [PubMed] [Google Scholar]
- Simeon D., Bartz J., Hamilton H., et al. (2011). Oxytocin administration attenuates stress reactivity in borderline personality disorder: a pilot study. Psychoneuroendocrinology , 3(9), 1418–21. [DOI] [PubMed] [Google Scholar]
- Slattery D.A., Neumann I.D. (2010). Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology , 58(1), 56–61. [DOI] [PubMed] [Google Scholar]
- Toth I., Neumann I.D., Slattery D.A. (2012). Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology , 223(2), 149–58. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage , 15(1), 273–89. [DOI] [PubMed] [Google Scholar]
- van Wingen G.A., Geuze E., Vermetten E., Fernandez G. (2011). Perceived threat predicts the neural sequelae of combat stress. Molecular Psychiatry , 16(6), 664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zuiden M., Kavelaars A., Geuze E., Olff M., Heijnen C.J. (2013). Predicting PTSD: pre-existing vulnerabilities in glucocorticoid-signaling and implications for preventive interventions. Brain, Behavior, and Immunity , 30, 12–21. [DOI] [PubMed] [Google Scholar]
- Walters J.T., Bisson J.I., Shepherd J.P. (2007). Predicting post-traumatic stress disorder: validation of the trauma screening questionnaire in victims of assault. Psychological Medicine , 37(1), 143–50. [DOI] [PubMed] [Google Scholar]
- Wigton R., Radua J., Allen P., et al. (2015). Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. Journal of Psychiatry & Neuroscience , 40(1), E1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar J., Juven-Wetzler A., Sonnino R., Cwikel-Hamzany S., Balaban E., Cohen H. (2011a). New insights into secondary prevention in post-traumatic stress disorder. Dialogues in Clinical Neuroscience , 13(3), 301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar J., Yahalom H., Kozlovsky N., et al. (2011b). High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. European Neuropsychopharmacology , 21(11), 796–809. [DOI] [PubMed] [Google Scholar]
- Zoicas I., Slattery D.A., Neumann I.D. (2014). Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology , 39(13), 3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.