Abstract
Our understanding of the role played by the subthalamic nucleus (STN) in human emotion has recently advanced with STN deep brain stimulation, a neurosurgical treatment for Parkinson’s disease and obsessive-compulsive disorder. However, the potential presence of several confounds related to pathological models raises the question of how much they affect the relevance of observations regarding the physiological function of the STN itself. This underscores the crucial importance of obtaining evidence from healthy participants. In this study, we tested the structural and functional connectivity between the STN and other brain regions related to vocal emotion in a healthy population by combining diffusion tensor imaging and psychophysiological interaction analysis from a high-resolution functional magnetic resonance imaging study. As expected, we showed that the STN is functionally connected to the structures involved in emotional prosody decoding, notably the orbitofrontal cortex, inferior frontal gyrus, auditory cortex, pallidum and amygdala. These functional results were corroborated by probabilistic fiber tracking, which revealed that the left STN is structurally connected to the amygdala and the orbitofrontal cortex. These results confirm, in healthy participants, the role played by the STN in human emotion and its structural and functional connectivity with the brain network involved in vocal emotions.
Keywords: emotion, fMRI, obsessive-compulsive disorder, Parkinson’s disease, subthalamic nucleus
Introduction
Subthalamic nucleus (STN) deep brain stimulation (DBS), a neurosurgical treatment for Parkinson’s disease (PD) and obsessive-compulsive disorder, has recently advanced our understanding of the role played by the STN in human emotion (see Péron et al., 2013 for a review). Recent studies in this field have yielded three main observations (Péron et al., 2013). First, STN DBS (in PD) induces behavioral (e.g. Dujardin et al., 2004) and electrophysiological (e.g. Kühn et al., 2005) modifications in all the components of emotion (i.e. cognitive appraisal, autonomic response, expressive, motivational and subjective feeling), irrespective of stimulus valence and irrespective of the sensory-input modality, suggesting that the involvement of the STN in emotional processing does not depend on type of emotion component, type of emotion or valence (positive or negative). Second, whereas the question of the compartmentalization of the STN into three segregate regions is deservedly still a matter of debate (Mallet et al., 2008; Keuken et al., 2012), the ventral part of the STN appears to be more closely involved in emotion processing. This hypothesis was tested in a clinical trial involving the stimulation of the associative and limbic (ventral) parts of the STN in severe obsessive-compulsive disorder, showing that DBS can dramatically reduce the symptoms related to this pathological condition. This finding underlines the crucial functional role of the STN in affective disorders (Mallet et al., 2008). Third, by exploring the brain networks underlying such emotional modulations, positron emission tomography (PET) studies have shown that the metabolic modifications following surgery are task and sensory-input dependent and that stimulation of the STN has an impact on the distributed neural networks involved in different paradigms and tasks. For example, alterations in the recognition of emotional facial expressions following bilateral STN DBS in PD have been significantly correlated with metabolic modifications in the right orbitofrontal cortex and amygdala (Le Jeune et al., 2008), as well as in the face fusiform area (Geday et al., 2006), all of which are structures known to be related to the recognition of emotional facial expressions (Adolphs, 2002). Using the same methodology, difficulties in the recognition of social emotions after STN DBS have been significantly correlated with metabolic modifications in a different brain network known to be involved in theory of mind (Péron et al., 2010b), that is, the ability to represent one’s own and other people’s mental states (Premack and Woodruff, 1978). In sum, these results suggest that the STN is functionally a constituent part of all the distributed networks that subtend the specific emotional subprocesses involved in these different paradigms. This inference was corroborated by an 18fluoro-deoxyglucose PET study comparing resting-state glucose metabolism before and after STN DBS in PD (Le Jeune et al., 2010a); the results showed that STN DBS modifies metabolic activity in a large and distributed network known for its involvement in the associative and limbic brain circuits.
On the basis of this corpus of literature, we recently put forward an integrative model of the functional specialization and integration of the STN in human emotions (Péron et al., 2013). We hypothesized that the STN forms part of a distributed neural network underlying emotion processing in humans. We hypothesize that the STN, rather than playing a specific function in a given emotional process, acts as a coordinator of neural patterns, together with other basal ganglia, either synchronizing or desynchronizing the activity of the different neuronal populations involved in specific emotion components (Péron et al., 2013). For example, in the context of emotional vocal expression recognition (i.e. emotional prosody), the basal ganglia recruits and synchronizes the activity of the structures involved in the different steps of emotional prosody processing, while competing neuronal patterns are inhibited.
These propositions, along with previous research findings in this field, raise many questions that have yet to be elucidated. Crucially, the potential presence of several confounds related to the use of pathological models (obsessive-compulsive disorder and PD) raises the question of how much these potential confounds affect the relevance of observations on the physiological function of the STN itself, underscoring the importance of obtaining evidence from healthy participants.
Recent structural and resting state functional connectivity studies (Brunenberg et al., 2012; Lambert et al., 2012) have confirmed that the STN occupies a central position within the limbic circuit of the basal ganglia, which is composed of the anterior cingulate and orbitofrontal cortices, the nucleus accumbens, the ventral pallidum, the ventral tegmental area and the basolateral amygdala (Lambert et al., 2012). Within this network, the existence of direct prefrontal-STN projections (the so-called hyperdirect pathway) in humans seems to be conceivable for most researchers in this field. This pathway has been demonstrated in rats (Maurice et al., 1998; Degos et al., 2008) and primates (Haynes and Haber, 2013) but remains to be directly proved in humans. These projections would have a crucial functional significance in human behavior, potentially exerting top-down (inhibiting) control over all (motor and cognitive, but also emotional) programs processed through the basal ganglia. In the emotional domain, the existence of this hyperdirect pathway linking the STN to the human prefrontal cortical structures, notably the orbitofrontal cortex, has been hypothesized to explain not only the emotional disturbances following STN DBS in PD (Le Jeune et al., 2008), but also the postoperative improvement of obsessive-compulsive disorder symptoms (Le Jeune et al., 2010b). It has been proposed that STN DBS would de- (in the case of PD) or re- (in the case of obsessive-compulsive disorder) synchronize the coordinated activity of the STN and the orbitofrontal cortex, leading to a dys- or re-regulation of affective behaviors (Péron et al., 2013).
In this work, we tested our hypothesis at both the structural and the functional connectivity level between the STN and other brain regions related to vocal emotion decoding on the basis of an analysis of a previous high-resolution functional magnetic resonance imaging (fMRI) study in healthy participants (Frühholz et al., 2012). In the previous study, the participants had to perform two tasks in response to emotional (and neutral) prosody stimuli. In the first task, they had to identify the emotions expressed by the voices: this is the emotional task. In the second one, they had to determine the gender of the voices they perceived: this is the gender task. Left STN activity was specifically reported during the processing of emotional voices (in comparison to neutral voices), but only when participants had to focus on the speaker’s gender (i.e. during the gender task) instead of discriminating between the emotions conveyed (anger vs neutral, i.e. the emotional task). In other words, these results indicate that the STN is specifically activated during the processing of non-task-relevant emotional information when attention has to be focused elsewhere (i.e. on gender information).
Here, we performed diffusion tensor imaging (DTI) and psychophysiological interaction (PPI) analysis on functional imaging data (Friston et al., 1997), taking the left STN as the seed region. It is important to note that we performed these additional analyses by focusing only on the significant contrasts previously found, that is, the left STN during the processing of emotional information in the gender task (Frühholz et al., 2012).
From the model by Péron et al. (2013), we hypothesized that the STN is connected to the structures known for their involvement in emotional prosody decoding (for reviews, see Witteman et al., 2012; Frühholz and Grandjean, 2013a,c), notably the orbitofrontal cortex and the auditory cortices (voice-sensitive areas), as well as the amygdala and the other basal ganglia.
Materials and methods
We performed structural and functional connectivity analyses on data previously reported (Frühholz et al., 2012). We refer the reader to these data for specific details of the methods and the original analysis, from which we derived our primary regions of interest for these connectivity analyses.
Participants
We initially included 17 healthy participants but excluded two from the analysis because of signal artifacts in the diffusion-weighted data. The remaining sample consisted of 3 males and 12 females, with a mean age of 25.12 years (s.d. = 4.95, range 20–38 years). All participants were right-handed, native French speakers, and had normal or corrected-to-normal vision and normal hearing. None of them had a history of neurological disease or psychiatric disorder. Participants gave written informed consent for their participation in accordance with the ethical and data security guidelines of the University of Geneva. The study was approved by the local ethics committee.
Stimulus material and trial sequence
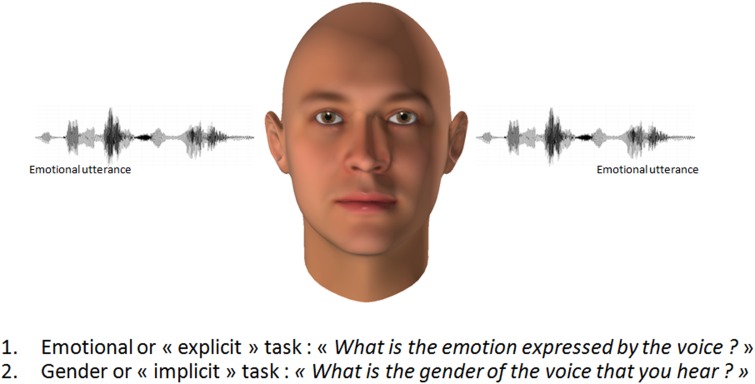
The set of vocal stimuli used in the experiment has been extensively described elsewhere (Frühholz et al., 2012; Figure 1). It consists of meaningless speech (pseudowords: ‘molen’, ‘belam’, ‘nikalibam’, ‘kudsemina’) spoken in either an angry or a neutral tone by two male and two female speakers and taken from an existing database (Bänziger and Scherer, 2010). We used 32 different stimuli [mean duration of 690 ms (s.d. = 185)] which were classified as neutral and angry voices with high accuracy (see Frühholz et al., 2012 for the results of the behavioral evaluation of the stimuli). Auditory stimuli were 16-bit recordings sampled at a 44.1 kHz sampling rate. During scanning, auditory stimuli were presented with MRI-compatible headphones (MR Confon) at a sound pressure level of ∼70 dB, and were preceded by a visual fixation cross (1 × 1°) for 1 ± 0.5 s. The fixation cross remained on the screen for as long as 2 s after the auditory stimulus. During this time window, participants were required to give a response (see below). We presented auditory stimuli in the silent gap between functional volume acquisitions and had an onset of 4 ± 0.75 s prior to the onset of the following volume acquisition using a sparse temporal acquisition protocol.
Fig. 1.
In our previous study (Frühholz et al., 2012), participants were asked to perform two different tasks. 1) The ‘emotional task’: participants were presented with (emotional or neutral) pseudo-words in which they were asked to explicitly focus their attention on the emotional prosody of the stimuli and to categorize the emotional valence of the voices (forced-choice decision if the voice was ‘neutral’ or ‘angry’). 2) The ‘gender task’: participants were asked to categorize the gender of the voices (forced-choice decision if the voice was ‘male’ or ‘female’). During attention to the gender of the voices as a nonemotional stimulus feature, the emotional (angry) prosody was assumed to be non-task-relevant. In our previous study (Frühholz et al., 2012), left STN activity was specifically reported when participants had to perform the task related to nonemotional features of the voice (i.e. when the focus was on the speaker’s sex) instead of performing a task related to emotional vocal features (i.e. discriminating anger/neutral). In other words, these results indicate that the STN is specifically activated during the processing of non-task-relevant emotional information when attention has to be focused elsewhere (i.e. on gender information). In this study, we performed DTI and PPI analysis on functional imaging data (Friston et al., 1997), taking the left STN as the seed region, in order to explore the structural and functional connectivity between the STN and other brain regions related to vocal emotion in a healthy population.
Participants were asked to perform two different tasks (see Figure 1). 1) The ‘emotional task’: participants were presented with two blocks (with the same set of stimuli in pseudo-randomized order) in which they were asked to explicitly focus their attention on the emotional prosody of the stimuli and to categorize the emotional valence of the voices (forced-choice decision if the voice was ‘neutral’ or ‘angry’ with the right index and middle finger). 2) The ‘gender task’: during another two blocks, participants were asked to categorize the gender of the voices (forced-choice decision if the voice was ‘male’ or ‘female’). During attention to the gender of the voices as a nonemotional stimulus feature, the angry prosody was assumed to be non-task-relevant. This means that although participants had to focus on the gender of the voice, the emotional value of voices might still attract some attention and might receive some kind of implicit recognition, as previously reported (Grandjean et al., 2005; Bach et al., 2008; Frühholz et al., 2012). Each of the four experimental blocks consisted of 38 trials, including six silent events with no auditory stimulation. The two tasks were alternated between subsequent blocks of trials, and block order and response buttons were counterbalanced across all participants.
Functional voice localizer scanning
To determine human voice-sensitive regions in the bilateral superior temporal cortex, participants listened passively to 8-s sound clips taken from an existing database (see http://vnl.psy.gla.ac.uk/ and Belin et al., 2000). These clips consisted of 20 sequences of nonemotional human voices and 20 sequences of animal or environmental sounds. Each sound clip was presented once with a fixation cross on the screen and a 4-s gap between each clip. The scanning sequence also contained twenty 8-s silent events.
Image acquisition
High-resolution imaging data were recorded on a 3-T SIEMENS Trio System (Siemens, Erlangen, Germany) using a T2*-weighted gradient echo-planar imaging sequence. Twenty-five axial slices were aligned to the superior temporal sulcus along its anterior-posterior orientation [thickness/gap = 2/0.4 mm, field of view (FoV) = 192 mm, in-plane 1.5 × 1.5 mm]. We used a sparse temporal acquisition protocol with TR = 10 s, which consisted of 1.75 s for volume acquisition and 8.25 s of silent gap. For the voice localizer, continuous whole-head acquisition was used, including 36 slices (thickness/gap = 3.2/0.64 mm, FoV = 205 mm, in-plane 3.2 × 3.2 mm) aligned to the anterior to posterior commissure plane with TR/TE = 2.1/0.03 s. Finally, to obtain structural brain images from each participant, we obtained a high-resolution magnetization prepared rapid acquisition gradient echo T1-weighted sequence (192 contiguous 1-mm slices, TR/TE/TI = 1.9 s/2.27 ms/900 ms, FoV 296 mm, in-plane 1 × 1 mm) in sagittal orientation.
Two repetitions of monopolar diffusion-weighted images (Stejskal-Tanner; TR/TE = 8200/82 ms, vocal size 2 mm3, 65 slices) along 30 independent directions, including a b-value of 1000 s/mm2, were performed. We also obtained a reference image with no diffusion weighting (b = 0 s/mm2) during each diffusion-weighted acquisition.
Image analysis
PPI analysis
Statistical parametric mapping software SPM (Version 8; Welcome Department of Cognitive Neurology, London, UK) was used for preprocessing, for the statistical analysis of functional images and for the voice localizer (see Frühholz et al., 2012 for a full description).
For the PPI analyses, the left STN [sphere of 3-mm radius centered on voxel −12, −16, −6 (Montreal Neurological Institute (MNI) coordinates) for the STN region, as reported in Frühholz et al.’s (2012) paper] was chosen as the seed region for performing a PPI analysis (Friston et al., 1997). As indicated in the Introduction, the left STN was chosen because this region was reported to be significantly activated during the (non-task-relevant) processing of vocal emotions in Frühholz et al.’s (2012) paper; the right STN was not. The PPI analysis models activity in other brain regions from the time course of the functional activity in a seed region. A seed and a target region are assumed to be functionally connected if brain activity in the target region can be explained on the basis of a model, which results from multiplying the time course activity in the seed region with a binary comparison of task conditions (‘1’ and ‘−1’; see below). This time course multiplied by the comparison of task conditions represents the interaction between the physiological and the psychological variables, respectively. We extracted the time course of activation in the left STN in a 2-mm-radius sphere around the peak activity for each participant.
The PPI analysis was set up as a general linear model separately for the general comparison of emotional with neutral voices, as well as for the comparison of emotional and neutral voices within each task, including three regressors for each analysis. For the first regressor, we included the extracted and deconvolved time course of functional activity in a seed region (the physiological variable). The second regressor included the comparison between angry and neutral voices independent of the task (the psychological variable); that is, we created a time course regressor for the task, including as many sampling points as for the physiological variable. The values in this regressor were set to ‘1’ for trials including angry voices and to ‘−1’ for trials including neutral voices during the emotional task. Only those trials were included in the PPI analysis in which participants gave a correct response during both tasks (96.19% of all trials). An additional analysis included a regressor for angry and neutral voices during the gender task. Note that although participants focused on the gender of voices during this task, the comparison of angry with neutral voices during the gender task gives functional activations, which are solely driven by the emotional value of angry voices and not by gender information. The third regressor included the interaction between the first two regressors. This interaction was created by a point-by-point multiplication of the time course for the physiological variable and the time course for the psychological variable. The last regressor was the only regressor of interest, whereas the psychological variable and the deconvolved time course served as regressors of no interest in each PPI analysis. The inclusion of the first two regressors ensures that the resulting functional activation is determined solely by the interaction between the physiological variable and the psychological variable. These data for the emotional and gender tasks were separately entered into a PPI analysis.
Individual results for these PPI analyses were entered into two separate second-level random effects analyses. All resulting statistical maps were thresholded by a combined voxel and cluster threshold of P < 0.005 (uncorrected) and a cluster extent of k = 21 (corresponding to P < 0.05 corrected at the cluster level).
DTI analysis
We used the FSL software package (version 4.1.6; www.fmrib.ox.ac.uk/fsl) for preprocessing and for the analysis of the diffusion-weighted data. The data were corrected for eddy currents and motion, and they were subsequently averaged across the two acquisitions. We estimated the two most likely diffusion directions at each voxel by using Bayesian estimation, as implemented in the FDT toolbox. This estimation included a model that accounts for the possibility of crossing fibers within each voxel (Behrens et al., 2007). The estimation was performed in native DTI space. An exclusion mask was applied that covered the whole nonbrain space. The MNI location of the left STN [−12 −16 −6] group level activations was back-transformed to each individual’s native DTI space in order to initiate fiber tracking from the fMRI-derived activation peak. This back-transformation was done by using the inverse normalization parameters, obtained by the segmentation of the anatomical images using SPM8. A 3-mm-radius sphere was then placed around each peak coordinate and the resulting volumes served as seed and target regions for the probabilistic fiber tracking. From each seed voxel, we sent out 25 000 samples (step length 0.5 mm, curvature threshold 0.2) mapping the probabilistic connectivity pattern. The probabilistic fiber tracking with the left STN as the seed region included the left and right striatum as a waypoint mask, thus allowing only the fiber pathway orientated from the left STN and passing through the striatum. The resulting maps were thresholded at 5% and normalized to MNI standard space by using nonlinear transformation warp fields (provided by the TBSS pipeline; Smith et al., 2006). Group analysis was performed on group connectivity probability maps (binarized and summed) across participants. The resulting group maps were thresholded such that at least 8 of the 15 subjects consistently showed the respective connecting fiber tracks, as done in previous studies (Ethofer et al., 2012; Gschwind et al., 2012).
Results
PPI analysis
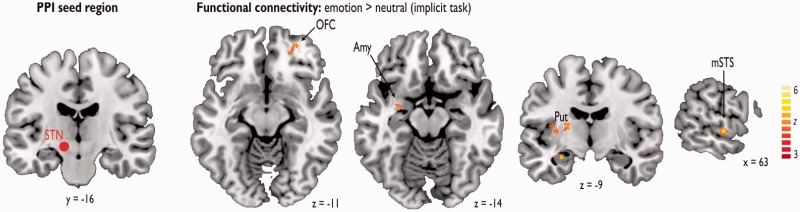
Functional activations for PPI analysis, including the left STN as the seed region, and for emotional compared with neutral voices during the gender task, included the right inferior frontal gyrus, orbital gyrus, superior temporal sulcus and postcentral gyrus, as well as the left postcentral and precentral gyri, pallidum and amygdala (Table 1 and Figure 2).
Table 1.
Functional activations for the PPI analysis, including the left STN as the seed region, and for emotional compared with neutral voices during the implicit task
| Region | Cluster size | Z value | MNI |
||
|---|---|---|---|---|---|
| x | y | Z | |||
| R inferior frontal gyrus | 24 | 3.32 | 27 | 53 | −10 |
| R orbital gyrus | 3.15 | 21 | 46 | −10 | |
| 3.06 | 24 | 50 | −10 | ||
| R superior temporal sulcus | 28 | 3.98 | 65 | −12 | −6 |
| R postcentral gyrus | 23 | 4.24 | 50 | −15 | 30 |
| L postcentral gyrus | 42 | 3.18 | −39 | −10 | 12 |
| L precentral gyrus | 29 | 3.96 | −38 | 1 | −18 |
| L pallidum | 38 | 3.49 | −21 | −10 | 14 |
| 3.09 | −26 | −4 | 4 | ||
| 2.77 | −26 | −9 | 8 | ||
| 3.14 | −32 | −10 | 8 | ||
| 2.98 | −35 | −6 | 12 | ||
| L amygdala | 28 | 3.43 | −27 | −9 | −18 |
| L amygdala | 3.00 | −32 | −1 | −18 | |
Fig. 2.
Functional activations for the PPI analysis, including the left STN as the seed region, and for emotional compared with neutral voices during the gender task. Amy, amygdala; mSTS, middle superior temporal sulcus; OFC, orbitofrontal cortex; Put, putamen; STN, subthalamic nucleus.
DTI data
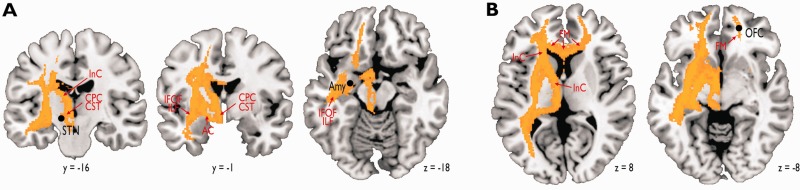
The probabilistic fiber tracking with the left STN as the seed region revealed a structural fiber connectivity via the left striatum and the inferior fronto-occipital fasciculus/inferior longitudinal fasciculus to the left amygdala, and also via the left striatum, the left internal capsule and the forceps minor to the right orbitofrontal cortex. The terminal localization of these fiber tracks closely matched the functional peak activations in the amygdala and the right orbitofrontal cortex that resulted from the (non-task-relevant) processing of vocal emotions (Figure 3).
Fig. 3.
(A) Probabilistic fiber tracking with the left STN as the seed region (left panel) revealed structural connections to the left amygdala (Amy) (middle left panel) via the cortico-ponto-cerebellar (CPC) tract as part of the cortico-spinal tract (CST) that interfaced—probably via the anterior commissure (AC)—with the inferior fronto-occipital fasciculus (ILF) and the inferior fronto-occipital fasciculus (IFOF). (B) The left STN also revealed structural connections to the right orbitofrontal cortex via the CPC, the internal capsule (InC), and the forceps minor (FM).
Discussion
We investigated the structural and functional connectivity between the STN and other brain regions related to vocal emotions (i.e. emotional prosody) by using DTI and PPI analyses (Friston et al., 1997) on high-resolution fMRI data in healthy participants (Frühholz et al., 2012) and taking the STN as the seed region of these analyses. We focused our analyses on the regions that were reported to be significantly activated during conditions in which the STN was activated, namely, the non-task-relevant (gender) condition on emotional vocal emotions. As expected, we showed that the STN is functionally connected to the structures known for their involvement in emotional prosody decoding, notably the orbitofrontal cortex and the auditory cortices, as well as the other basal ganglia and the amygdala (see Witteman et al., 2012 for a review). More specifically, we observed that the left STN showed functional connectivity to the contralateral right orbitofrontal gyrus, the right inferior frontal gyrus and the right temporal sulcus, including the voice-sensitive areas, as well as the ipsilateral left pallidum and the left amygdaloid nuclei during emotional prosody processing. These functional results were corroborated by probabilistic fiber tracking, which revealed that, with the left STN as the seed region, the terminal localization of these fiber tracks closely matched the functional peak activations in the amygdala and the right orbitofrontal cortex that resulted from the (non-task-relevant) processing of vocal emotions. Two important findings thus emerged from the present study. First, the present results confirm, in healthy participants, the role played by the STN in human emotion and its structural and functional connectivity with the brain network involved in vocal emotion decoding. Second, the present results are compatible with STN connectivity with ipsilateral subcortical structures and contralateral cortical structures during the processing of emotional prosody.
STN functional involvement in the neural network underlying vocal emotions
The present results revealed a structural and functional connectivity of the STN to the brain structures involved in the perception and decoding of emotional prosody underlying the functional involvement of the STN in this affective process. Models of emotional prosody processing have long postulated that information is processed in multiple successive stages related to different levels of representations involving a frontotemporal network in addition to subcortical structures such as the amygdaloid nuclei and the basal ganglia (see Witteman et al., 2012; Frühholz and Grandjean, 2013b,c for reviews). More precisely, following the processing of auditory information in the primary and secondary auditory cortices (Wildgruber et al., 2009; Bruck et al., 2011a; Frühholz and Grandjean, 2013b), and with the activation of predominantly right-hemispheric regions (Grandjean et al., 2006) (Stage 1), two successive stages of prosody decoding have been identified. The second stage, related to the representation of meaningful suprasegmental acoustic sequences, is thought to involve projections from the superior temporal gyrus to the anterior superior temporal sulcus. These cortical structures have been identified as forming the so-called temporal voice-sensitive area (Belin and Zatorre, 2000), made up of voice-sensitive neuronal populations. In the third stage, emotional information is made available by the superior temporal sulcus for higher order cognitive processes mediated by the orbitofrontal cortex (Wildgruber et al., 2004; Sander et al., 2005; Ethofer et al., 2006; Grandjean et al., 2008) and the inferior frontal gyrus via its functional connections to the auditory cortex (Leitman et al., 2010; Frühholz and Grandjean, 2012, 2013b). This stage appears to be related to the explicit evaluation of vocally expressed emotions. Our data precisely revealed a functional connectivity of the STN to both the orbitofrontal cortex and the inferior frontal gyrus, as well as to the auditory cortices, including the voice-sensitive area.
In addition to this frontotemporal network, increased activity has also been repeatedly observed within the amygdaloid nuclei and the basal ganglia in response to emotional prosody (Grandjean et al., 2005; Sander et al., 2005; Frühholz et al., 2012; Frühholz and Grandjean, 2013a). Consistent with this, and as predicted, our present results revealed a functional connectivity of the STN to the amygdala and the pallidum. The involvement of the striatum and the pallidum in vocal emotion has been observed in fMRI, patient and electroencephalography studies (Morris et al., 1999; Kotz et al., 2003; Sidtis and Van Lancker Sidtis, 2003; Grandjean et al., 2005; Bach et al., 2008; Paulmann et al., 2008, 2009; Frühholz et al., 2012). More recently, and as revealed in the Introduction, the studies exploring the emotional effects of STN DBS in PD have highlighted the potential involvement of the STN in the brain network subtending emotional prosody processing (Péron et al., 2010a; Bruck et al., 2011b; see also, Péron et al., 2013 for a review). In the study by Péron et al. (2010a), an original emotional prosody paradigm was administered to postoperative PD patients, preoperative PD patients and matched controls. Results showed that, compared with the other two groups, the postoperative group exhibited a systematic emotional bias, with emotions being perceived more strongly. For example, contrasts revealed that, compared with preoperative patients and healthy matched controls, the postoperative group rated ‘happiness’ more intensely when they listened to fearful stimuli. It is worth noting that, while these results seem to confirm the involvement of the basal ganglia, with further supporting evidence coming from numerous sources (for a review, see Gray and Tickle-Degnen, 2010; see also Péron et al., 2012), most models of emotional prosody processing fail to specify the functional role of either the basal ganglia in general or the STN in particular, although some authors have attempted to do so (for an extensive presentation of these propositions, see Péron et al., 2013; see also Péron et al., 2015).
Contralateral functional connectivity
Surprisingly, we did not find a functional coupling between the left STN and the ipsilateral orbitofrontal cortex; the coupling was found between the left STN and the contralateral orbitofrontal cortex, which was corroborated by fiber tracking. Indeed, given the hypothesis of a hyperdirect prefrontal limbic (orbitofrontal cortex)-STN pathway (Péron et al., 2013), we anticipated functional connectivity between the left STN and ipsilateral prefrontal cortical structures. The contralaterality of cortical projections to the basal ganglia, and notably the STN (Parent and Hazrati, 1995), is controversial. However, physiological animal studies have shown that stimulation of the contralateral prefrontal cortex provokes excitatory responses in the STN that are totally suppressed by a parasagittal knife cut of the corpus callosum (Fujimoto and Kita, 1993). More recently, in the cognitive domain, it was shown that animals with disconnected lesions of the medial prefrontal cortex and the STN in contralateral hemispheres presented profound cognitive (attentional) impairments, were highly perseverative, and had slowed response latencies. In contrast, animals with combined medial prefrontal cortex and STN lesions in the ipsilateral side produced behavior that was no different than that of sham controls (Chudasama et al., 2003). In humans, to the best of our knowledge, there is no evidence suggesting contralateral prefrontal-STN projections. However, at the behavioral level, it has been observed that unilateral stimulation of the STN in PD patients induces motor improvement that is predominantly contralateral but also ipsilateral, suggesting crossed projections. Electrophysiological studies have also reported (in PD) modulations in the discharge pattern of STN neurons in response to contralateral STN DBS (Walker et al., 2011), suggesting that unilateral neuronal STN modulation is associated with changes in the activity of the contralateral STN (Brun et al., 2012). Finally, the studies using structural and resting state functional connectivity measures also reported ipsilateral and contralateral coupling between the STN and, notably, cortical structures such as the prefrontal cortices and the cingulate gyrus (Brunenberg et al., 2012). Interestingly, a recent meta-analytic study investigating the overlap between connectivity maps derived from the STN and the speech network consistently revealed that the left STN notably coactivated with the right prefrontal cortices, such as the inferior frontal gyrus, but also (and as reported in the present study) in the left pallidum (Manes et al., 2014).
Conclusion
The connectivity models of our STN seed showed expected coactivation with the neural structures known to be involved in emotional prosody. This finding reinforces the view of the apparently major role played by the STN in human affective behavior, and its structural and functional connectivity with the neural network involved in emotional processing, here in the vocal modality. These results enable us to overcome limitations inherent in the use of pathological models (obsessive-compulsive disorder and PD) and to make inferences regarding the physiological role of the STN itself. Future studies should investigate the functional significance of the contralaterality between prefrontal structures and the basal ganglia during affective behavior.
Funding
The authors declare no competing financial interests. This study was funded by the National Center of Competence in Research (NCCR – 51A240-104897 - DG) and the project No 105314_140622 - DG, financed by the Swiss National Science Foundation and hosted by the University of Geneva. The funders had no role in data collection, discussion of content, preparation of the manuscript, or decision to publish.
Conflict of interest. None declared.
References
- Adolphs R. (2002). Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Review, 1(1), 21–62. [DOI] [PubMed] [Google Scholar]
- Bach D.R., Grandjean D., Sander D., Herdener M., Strik W.K., Seifritz E. (2008). The effect of appraisal level on processing of emotional prosody in meaningless speech. Neuroimage, 42(2), 919–27. [DOI] [PubMed] [Google Scholar]
- Bänziger T., Scherer K.R. (2010). Introducing the Geneva multimodal emotion portrayal (GEMEP) corpus. In: Bänziger T., Scherer K.R., Roesch E.B., editors. Blueprint for Affective Computing: A Sourcebook. pp. 271–94. Oxford, England: Oxford University Press. [Google Scholar]
- Behrens T.E., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W. (2007). Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage, 34(1), 144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin P., Zatorre R.J. (2000). ‘What’, ‘where’ and ‘how’ in auditory cortex. Nature Neuroscience, 3(10), 965–6. [DOI] [PubMed] [Google Scholar]
- Belin P., Zatorre R.J., Lafaille P., Ahad P., Pike B. (2000). Voice-selective areas in human auditory cortex. Nature, 403(6767), 309–12. [DOI] [PubMed] [Google Scholar]
- Bruck C., Kreifelts B., Wildgruber D. (2011a). Emotional voices in context: a neurobiological model of multimodal affective information processing. Physics of Life Reviews, 8(4), 383–403. [DOI] [PubMed] [Google Scholar]
- Bruck C., Wildgruber D., Kreifelts B., Kruger R., Wachter T. (2011b). Effects of subthalamic nucleus stimulation on emotional prosody comprehension in Parkinson's disease. PLoS One, 6(4), e19140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun Y., Karachi C., Fernandez-Vidal S., et al. (2012). Does unilateral basal ganglia activity functionally influence the contralateral side? What we can learn from STN stimulation in patients with Parkinson's disease. Journal of Neurophysiology, 108(6), 1575–83. [DOI] [PubMed] [Google Scholar]
- Brunenberg E.J., Moeskops P., Backes W.H., et al. (2012). Structural and resting state functional connectivity of the subthalamic nucleus: identification of motor STN parts and the hyperdirect pathway. PLoS One, 7(6), e39061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y., Baunez C., Robbins T.W. (2003). Functional disconnection of the medial prefrontal cortex and subthalamic nucleus in attentional performance: evidence for corticosubthalamic interaction. The Journal of Neuroscience, 23(13), 5477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degos B., Deniau J.M., Le Cam J., Mailly P., Maurice N. (2008). Evidence for a direct subthalamo-cortical loop circuit in the rat. The European Journal of Neuroscience, 27(10), 2599–610. [DOI] [PubMed] [Google Scholar]
- Dujardin K., Blairy S., Defebvre L., et al. (2004). Subthalamic nucleus stimulation induces deficits in decoding emotional facial expressions in Parkinson's disease. Journal of Neurology Neurosurgery and Psychiatry, 75(2), 202–8. [PMC free article] [PubMed] [Google Scholar]
- Ethofer T., Anders S., Erb M., et al. (2006). Cerebral pathways in processing of affective prosody: a dynamic causal modeling study. Neuroimage, 30(2), 580–7. [DOI] [PubMed] [Google Scholar]
- Ethofer T., Bretscher J., Gschwind M., Kreifelts B., Wildgruber D., Vuilleumier P. (2012). Emotional voice areas: anatomic location, functional properties, and structural connections revealed by combined fMRI/DTI. Cerebral Cortex, 22(1), 191–200. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. (1997). Psychophysiological and modulatory interactions in neuroimaging. Neuroimage, 6(3), 218–29. [DOI] [PubMed] [Google Scholar]
- Frühholz S., Ceravolo L., Grandjean D. (2012). Specific Brain Networks during Explicit and Implicit Decoding of Emotional Prosody. Cerebral Cortex, 22(5), 1107–17. [DOI] [PubMed] [Google Scholar]
- Frühholz S., Grandjean D. (2012). Towards a fronto-temporal neural network for the decoding of angry vocal expressions. Neuroimage, 62(3), 1658–66. [DOI] [PubMed] [Google Scholar]
- Frühholz S., Grandjean D. (2013a). Amygdala subregions differentially respond and rapidly adapt to threatening voices. Cortex, 49(5), 1394–403. [DOI] [PubMed] [Google Scholar]
- Frühholz S., Grandjean D. (2013b). Multiple subregions in superior temporal cortex are differentially sensitive to vocal expressions: a quantitative meta-analysis. Neuroscience and Biobehavioral Reviews, 37(1), 24–35. [DOI] [PubMed] [Google Scholar]
- Frühholz S., Grandjean D. (2013c). Processing of emotional vocalizations in bilateral inferior frontal cortex. Neuroscience and Biobehavioral Reviews, 37(10 Pt 2), 2847–55. [DOI] [PubMed] [Google Scholar]
- Fujimoto K., Kita H. (1993). Response characteristics of subthalamic neurons to the stimulation of the sensorimotor cortex in the rat. Brain Research, 609(1-2), 185–92. [DOI] [PubMed] [Google Scholar]
- Geday J., Ostergaard K., Gjedde A. (2006). Stimulation of subthalamic nucleus inhibits emotional activation of fusiform gyrus. Neuroimage, 33(2), 706–14. [DOI] [PubMed] [Google Scholar]
- Grandjean D., Banziger T., Scherer K.R. (2006). Intonation as an interface between language and affect. Progress in Brain Research, 156, 235–47. [DOI] [PubMed] [Google Scholar]
- Grandjean D., Sander D., Lucas N., Scherer K.R., Vuilleumier P. (2008). Effects of emotional prosody on auditory extinction for voices in patients with spatial neglect. Neuropsychologia, 46(2), 487–96. [DOI] [PubMed] [Google Scholar]
- Grandjean D., Sander D., Pourtois G., et al. (2005). The voices of wrath: brain responses to angry prosody in meaningless speech. Nature Neuroscience, 8(2), 145–6. [DOI] [PubMed] [Google Scholar]
- Gray H.M., Tickle-Degnen L. (2010). A meta-analysis of performance on emotion recognition tasks in Parkinson's disease. Neuropsychology, 24(2), 176–91. [DOI] [PubMed] [Google Scholar]
- Gschwind M., Pourtois G., Schwartz S., Van De Ville D., Vuilleumier P. (2012). White-matter connectivity between face-responsive regions in the human brain. Cerebral Cortex, 22(7), 1564–76. [DOI] [PubMed] [Google Scholar]
- Haynes W.I., Haber S.N. (2013). The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. The Journal of Neuroscience, 33(11), 4804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuken M.C., Uylings H.B., Geyer S., Schafer A., Turner R., Forstmann B.U. (2012). Are there three subdivisions in the primate subthalamic nucleus? Frontiers in Neuroanatomy, 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz S.A., Meyer M., Alter K., Besson M., von Cramon D.Y., Friederici A.D. (2003). On the lateralization of emotional prosody: an event-related functional MR investigation. Brain and Language, 86(3), 366–76. [DOI] [PubMed] [Google Scholar]
- Kühn A.A., Hariz M.I., Silberstein P., et al. (2005). Activation of the subthalamic region during emotional processing in Parkinson disease. Neurology, 65(5), 707–13. [DOI] [PubMed] [Google Scholar]
- Lambert C., Zrinzo L., Nagy Z., et al. (2012). Confirmation of functional zones within the human subthalamic nucleus: patterns of connectivity and sub-parcellation using diffusion weighted imaging. Neuroimage, 60, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Jeune F., Péron J., Biseul I., et al. (2008). Subthalamic nucleus stimulation affects orbitofrontal cortex in facial emotion recognition: a PET study. Brain, 131(Pt 6), 1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Jeune F., Péron J., Grandjean D., et al. (2010a). Subthalamic nucleus stimulation affects limbic and associative circuits: a PET study. European Journal of Nuclear Medicine and Molecular Imaging, 37(8), 1512–20. [DOI] [PubMed] [Google Scholar]
- Le Jeune F., Vérin M., N'Diaye K., et al. (2010b). Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biological Psychiatry, 68(11), 1016–22. [DOI] [PubMed] [Google Scholar]
- Leitman D.I., Wolf D.H., Ragland J.D., et al. (2010). “It's Not What You Say, But How You Say it”: a reciprocal temporo-frontal network for affective prosody. Frontiers in Human Neuroscience, 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L., Polosan M., Jaafari N., et al. (2008). Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. The New England Journal of Medicine, 359(20), 2121–34. [DOI] [PubMed] [Google Scholar]
- Manes J.L., Parkinson A.L., Larson C.R., et al. (2014). Connectivity of the subthalamic nucleus and globus pallidus pars interna to regions within the speech network: a meta-analytic connectivity study. Human Brain Mapping, 35(7), 3499–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N., Deniau J.M., Menetrey A., Glowinski J., Thierry A.M. (1998). Prefrontal cortex-basal ganglia circuits in the rat: involvement of ventral pallidum and subthalamic nucleus. Synapse, 29(4), 363–70. [DOI] [PubMed] [Google Scholar]
- Morris J.S., Scott S.K., Dolan R.J. (1999). Saying it with feeling: neural responses to emotional vocalizations. Neuropsychologia, 37(10), 1155–63. [DOI] [PubMed] [Google Scholar]
- Parent A., Hazrati L.N. (1995). Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Research Brain Reseach Reviews, 20(1), 128–54. [DOI] [PubMed] [Google Scholar]
- Paulmann S., Pell M.D., Kotz S.A. (2008). Functional contributions of the basal ganglia to emotional prosody: evidence from ERPs. Brain Research, 1217, 171–8. [DOI] [PubMed] [Google Scholar]
- Paulmann S., Pell M.D., Kotz S.A. (2009). Comparative processing of emotional prosody and semantics following basal ganglia infarcts: ERP evidence of selective impairments for disgust and fear. Brain Research, 1295, 159–69. [DOI] [PubMed] [Google Scholar]
- Péron J., Cekic S., Haegelen C., et al. (2015). Sensory contribution to vocal emotion deficit in Parkinson's disease after subthalamic stimulation. Cortex, 63C, 172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péron J., Dondaine T., Le Jeune F., Grandjean D., Verin M. (2012). Emotional processing in Parkinson's disease: a systematic review. Movement Disorders, 27(2), 186–99. [DOI] [PubMed] [Google Scholar]
- Péron J., Frühholz S., Vérin M., Grandjean D. (2013). Subthalamic nucleus: A key structure for emotional component synchronization in humans. Neuroscience and Biobehavioral Reviews, 37(3), 358–73. [DOI] [PubMed] [Google Scholar]
- Péron J., Grandjean D., Le Jeune F., et al. (2010a). Recognition of emotional prosody is altered after subthalamic nucleus deep brain stimulation in Parkinson's disease. Neuropsychologia, 48(4), 1053–62. [DOI] [PubMed] [Google Scholar]
- Péron J., Le Jeune F., Haegelen C., et al. (2010b). Subthalamic nucleus stimulation affects theory of mind network: a PET study in Parkinson's disease. PLoS One, 5(3), e9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D., Woodruff G. (1978). Chimpanzee problem-solving: a test for comprehension. Science, 202(4367), 532–5. [DOI] [PubMed] [Google Scholar]
- Sander D., Grandjean D., Pourtois G., et al. (2005). Emotion and attention interactions in social cognition: brain regions involved in processing anger prosody. Neuroimage, 28(4), 848–58. [DOI] [PubMed] [Google Scholar]
- Sidtis J.J., Van Lancker Sidtis D. (2003). A neurobehavioral approach to dysprosody. Seminars in Speech and Language, 24(2), 93–105. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 31(4), 1487–505. [DOI] [PubMed] [Google Scholar]
- Walker H.C., Watts R.L., Schrandt C.J., et al. (2011). Activation of subthalamic neurons by contralateral subthalamic deep brain stimulation in Parkinson disease. Journal of Neurophysiology, 105(3), 1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildgruber D., Ethofer T., Grandjean D., Kreifelts B. (2009). A cerebral network model of speech prosody comprehension. International Journal of Speech-Language Pathology, 11(4), 277–81. [Google Scholar]
- Wildgruber D., Hertrich I., Riecker A., et al. (2004). Distinct frontal regions subserve evaluation of linguistic and emotional aspects of speech intonation. Cereb Cortex, 14(12), 1384–9. [DOI] [PubMed] [Google Scholar]
- Witteman J., Van Heuven V.J., Schiller N.O. (2012). Hearing feelings: a quantitative meta-analysis on the neuroimaging literature of emotional prosody perception. Neuropsychologia, 50(12), 2752–63. [DOI] [PubMed] [Google Scholar]