Abstract
Fast threat detection is crucial for survival. In line with such evolutionary pressure, threat-signaling fear-conditioned faces have been found to rapidly (<80 ms) activate visual brain regions including the fusiform gyrus on the conditioning day. Whether remotely fear conditioned stimuli (CS) evoke similar early processing enhancements is unknown. Here, 16 participants who underwent a differential face fear-conditioning and extinction procedure on day 1 were presented the initial CS 24 h after conditioning (Recent Recall Test) as well as 9-17 months later (Remote Recall Test) while EEG was recorded. Using a data-driven segmentation procedure of CS evoked event-related potentials, five distinct microstates were identified for both the recent and the remote memory test. To probe intracranial activity, EEG activity within each microstate was localized using low resolution electromagnetic tomography analysis (LORETA). In both the recent (41–55 and 150–191 ms) and remote (45–90 ms) recall tests, fear conditioned faces potentiated rapid activation in proximity of fusiform gyrus, even in participants unaware of the contingencies. These findings suggest that rapid processing enhancements of conditioned faces persist over time.
Keywords: fear conditioning, extinction, microstate, face processing
Introduction
The ability to learn social threat signals like danger-related faces and to rapidly process such signals is essential for survival. Consistent with this evolutionary pressure, electroencephalogram (EEG) and magnetoencephalogram (MEG) studies have shown that fear-conditioned faces and non-social threat stimuli evoke increased brain activity within 50–200 ms (Pizzagalli et al., 2003; Stolarova et al., 2006; Keil et al., 2007; Steinberg et al., 2011; Miskovic and Keil, 2012). A brain region that rapidly processes fear-conditioned faces is the fusiform gyrus (FG) (Pizzagalli et al., 2003), which shows enhanced reactions to threatening stimuli as early as 30–60 ms after stimulus onset (Morel et al., 2012). Increased FG activity to threat-related faces has also been observed with functional Magnetic Resonance Imaging (fMRI) studies (Petrovic et al., 2008) and may be particularly relevant for trait anxiety (Etkin et al., 2004) and anxiety disorders (Mueller et al., 2008). As rapid EEG (Rotshtein et al., 2010) and FG (Vuilleumier et al., 2004) enhancements to threat are absent in individuals with amygdala lesions, these early FG responses to threat may be linked to fast projections from the amygdala (Vuilleumier and Pourtois, 2007), a major structure for fear learning and threat processing (LeDoux, 2014).
While imaging studies have been able to consistently demonstrate conditioned stimulus (CS)–associated potentiation in human FG during or shortly after fear conditioning, the long-term stability of FG enhancements to previously fear conditioned stimuli remains unknown. Remote memory of fear is of particular relevance for anxiety disorders, given that prior learning experiences (e.g. trauma, panic attacks) are often causally related to the development and course of anxiety months or years later (Mineka and Oehlberg, 2008). However, despite the plethora of studies on recently or acutely conditioned fear in healthy individuals (Sehlmeyer et al., 2009; Miskovic and Keil, 2012) and in anxiety disorders (Lissek et al., 2005), only few investigations with human subjects have assessed fear recall more than 24 h after conditioning (Kalisch et al., 2009; Schiller et al., 2010) and none of these studies investigated effects on rapid sensory processing. Rapid threat processing may be particularly relevant in anxiety disorders (Schulz et al., 2013) given that initial threat perception and attention to potential threat may be the first processing steps that are biased in the information processing stream of anxious individuals, which may ultimately lead to an elevated sense of imminent danger, high levels of subjective anxiety and maladaptive behavior (Mathews and MacLeod, 2005). Although remotely fear conditioned stimuli (i.e., 10–14 months) may evoke fear reactions as measured with skin conductance (Schiller et al., 2010), modulations of sensory processing could be more labile and decay within days or weeks. In addition to the decay of memory traces, qualitative transitions or re-organizations from recent to remote memory may occur (Moscovitch et al., 2006). In this regard, remote but not recent fear memory storage in rats has been shown to crucially depend on secondary sensory cortices (Sacco and Sacchetti, 2010).
The primary goal of the present study was to investigate the long-term stability of rapid sensory enhancements to fear-conditioned faces by re-inviting subjects of a previously published fear-conditioning study (Mueller et al., 2014) and presenting them with the original fear conditioned faces in a Remote Recall Test 9–17 months after the initial conditioning (average latency: 13 months, SD: 3 months). To probe the temporal dynamics of remote fear memory recall, EEG was recorded during the Remote Recall Test and analyzed with a data-driven microstate segmentation procedure (Koenig and Lehmann, 1996; Khanna et al., 2015) in conjunction with a distributed source localization technique (Low Resolution Electromagnetic Tomography, LORETA; Pascual-Marqui et al., 1999).
In addition to rapid sensory enhancements, we have previously shown that fear conditioned faces evoke increased theta (4–8 Hz) activity within the anterior midcingulate cortex (aMCC; Vogt, 2005; Shackman et al., 2011) one day after conditioning (Mueller et al., 2014). This finding is of high relevance as it brings together rodent research showing altered prelimbic cortex (a putative homologue region of human aMCC) theta activity during fear (Burgos-Robles et al., 2009) and human fMRI studies suggesting aMCC involvement in fear expression (Buchel et al., 1999; Phelps et al., 2004; Milad and Quirk, 2012). Because it is unknown whether the effect of fear conditioning on human aMCC theta activity is stable over extended periods, our secondary goal was to also probe aMCC theta to fear conditioned vs non-conditioned faces during the Remote Recall Test.
Materials and methods
Participants
Participants from an earlier fear conditioning and extinction study (Mueller et al., 2014) were invited 8–16 months after the initial study to take part in another experiment for additional monetary compensation (30 €) or course credit. Out of the 42 participants who took part in the first experiment, N = 16 (7 females, mean age: 22.7 years, SD = 3.7 years) were available for the current study. Participants who did vs did not participate in the follow-up study did not differ with regard to age or gender distribution or fear ratings during the first part of the experiment (all Ps >0.3). Depending on the date of the initial experiment, the interval between the two sessions ranged from 273 to 504 days (mean: 388 days, SD: 92 days) allowing us to test for systematic relationships between the length of the retention interval and remote fear memory recall (see Supplementary material). The study was approved by the local ethics committee of the Marburg University Psychology Department.
Procedure and fear conditioning paradigm
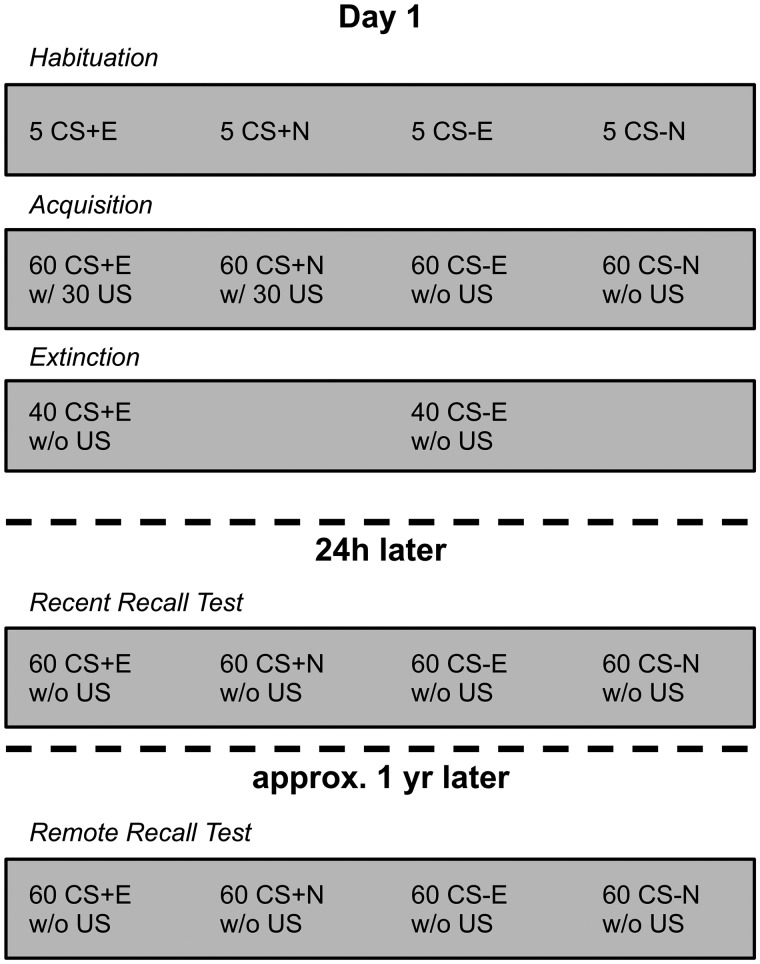
The procedure for days 1 and 2 is reported in detail elsewhere (Mueller et al., 2014) and the timeline of conditioning phases is illustrated in Figure 1. In short, day 1 consisted of three phases: Habituation, Conditioning and Extinction. Four different faces from the Ekman series (Ekman and Friesen, 1976), presented for 4 s, served as CS. During Acquisition, each CS was presented 60 times in random order. Two of the CSs (i.e. CS+) co-terminated with the unconditioned stimulus (US, an aversive 95 dB noise burst) in 50% of the trials whereas the other two CSs (i.e. CS−) were never paired with the US. During extinction, one CS+ (CS+E) and one CS− (CS−E) were presented without the US, 40 times each. The other two CSs (CS+N and CS−N) were not presented during extinction. During the Recent Recall Test on day 2 and the Remote Recall Test approximately 1 year later each CS was presented 60 times in random order without any US presentations. Note that immediately after the Recall Test, one cohort of participants (N = 6) performed another task, in which they imagined different scenarios (e.g. ‘Imagine you are sitting in a pub. There is a group of drunken men who start bullying you and other guests’) before they were presented the previously conditioned faces. Results from this task will be presented elsewhere. However, we included the factor Cohort in a set of control analyses and confirmed that the remote fear memory findings of the current study did not significantly differ between the cohort that performed vs not performed the secondary task (see Supplementary material).
Fig. 1.
Timeline of the five task phases for day 1, 24 h and 1 year later and summary of the number and type of stimuli presented during each phase. CS+E/CS−E, CS presented during Extinction; CS+N/CS−N, CS not presented during Extinction.
Here, we focus on the long-term stability of conditioning rather than extinction. In an initial set of analyses we found no effects of extinction on subjective ratings or EEG activity during the Remote Recall Test (data available upon request). This lack of remote extinction effects at the behavioral and neural level is consistent with the general assumption that extinction memories are relatively unstable (Vervliet et al., 2013) and may be severely attenuated within a few days after extinction (Norrholm et al., 2008; Schiller et al., 2008; Huff et al., 2009). We therefore collapsed extinguished and non-extinguished CS in order to achieve more trials and a better signal to noise ratio for EEG analyses comparing CS+ vs CS-. However, analyses on specific brain activity in response to extinguished vs non-extinguished CS are provided in the Supplementary material.
Stimulus recognition and explicit contingency recall
To assess explicit recognition of the face stimuli, participants were asked to indicate whether they recognized a face from the previous study sessions which had occurred 9–17 months before. This was done for each of the four CS and two additional novel distractor faces from the same stimulus set (Ekman and Friesen, 1976). There were four answer options: ‘certainly not’, ‘probably not’, ‘probably yes’ and ‘certainly yes’. To assess explicit remote contingency recall, participants were asked for each of the four CS how often it had been paired with the loud noise on the first day of the study. Answer options were ‘1 = never’, ‘2 = sometimes’, ‘3 = always’ and ‘4 = don’t know’. Subjects were classified as contingency aware or contingency unaware based on these answers. Specifically, as soon as subjects stated for at least one CS+ that it had been paired with the US more often (i.e. ‘2’ or ‘3’) than at least one of the CS− (i.e. ‘1’ or ‘2’), they were considered as potentially contingency aware. Note that this criterion conservatively classified participants as ‘contingency unaware’.
EEG recording and analysis
EEG was recorded at 512 Hz with a 64 channel BioSemi Active Two system. Offline, EEG was re-referenced to the average reference, filtered using a 0.5–30 Hz (24 dB/octave) band-pass and a 50 Hz notch filter (all Butterworth zero phase filters) and manually screened for non-ocular artifacts using Brain Vision Analyzer 2 (Brain Products, Germany). Eye-blinks and saccades were corrected using independent component analysis (Fast ICA with classic principal component analysis sphering on the whole artifact-free EEG dataset) with manual rejection of clear eye-blink related components as identified by frontopolar topography and blink/saccade like waveforms. The EEG was then segmented (−200 to 2000 ms relative to CS onset) and event-related potentials (ERPs) were computed by averaging the segments by condition.
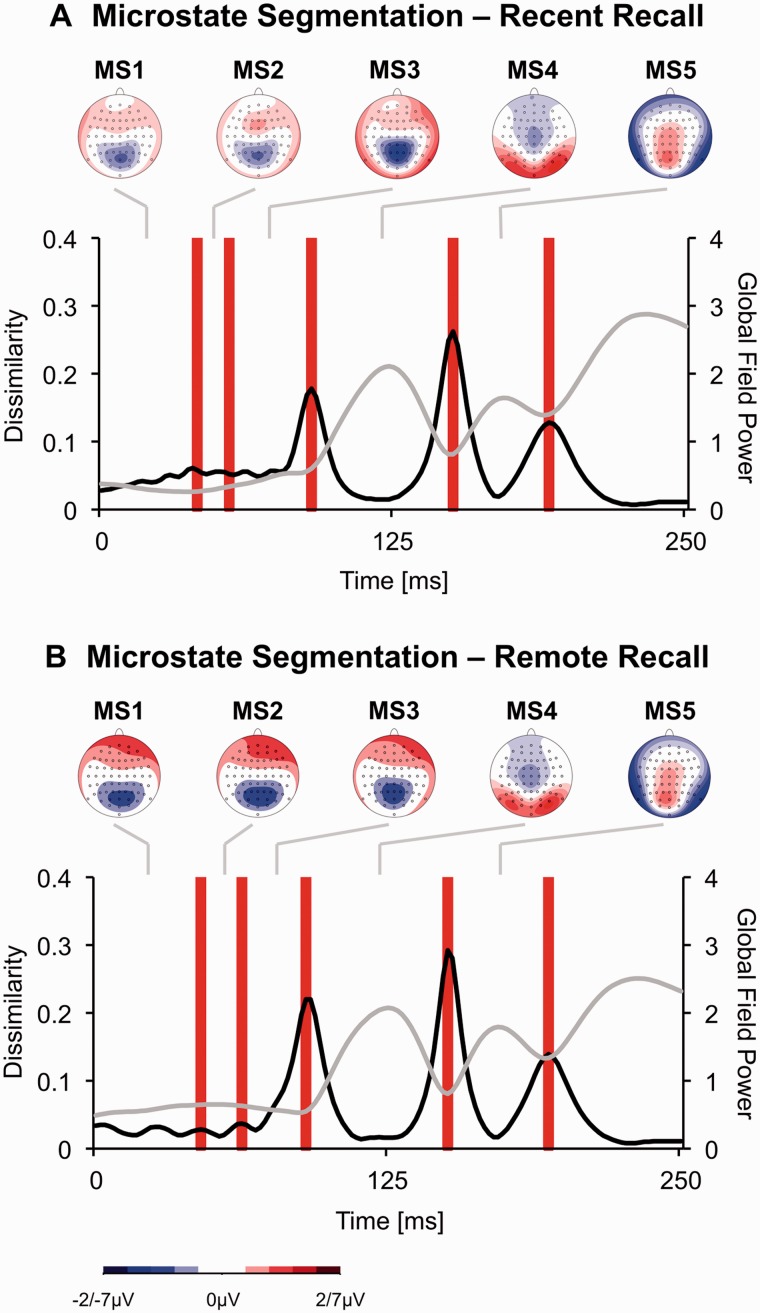
The ERP was segmented into microstates (Koenig and Lehmann, 1996; Khanna et al., 2015). Microstates reflect periods of quasi-stable map configurations, presumably representing discrete brain functions (Koenig and Lehmann, 1996). The approach is based on the observation that the brain electric field configurations change step-wise and discontinuously. To identify microstates, for each time frame, the Global Map Dissimilarity Index (GMDI) of the grand average ERP across participants and conditions was computed (Figure 2). The GMDI indicates the difference of landscape configuration for two successive maps and ranges from 0 to 2, with higher values indicating larger successive topography changes (Lehmann and Skrandies, 1984; Pizzagalli et al., 2003). As shown in Figure 2 (black line), there are periods with relatively little changes (i.e. low GMDI), which are separated by abrupt transitions of topography (GMDI peaks). Whenever GMDI peaks exceeded a certain threshold, the latency of the GMDI peak local maximum was defined as a microstate border. As in the original published work describing this data-driven segmentation technique (Koenig and Lehmann, 1996), the threshold was empirically determined using bootstrap procedures that maximized the product of (i) the length of the resulting microstates (i.e. stability criterion) and (ii) the number of the resulting microstates (i.e. discrimination criterion).
Fig. 2.
(A) Microstate segmentation of the grand average event-related potential evoked by CS (averaged across the 2 × 2 CS types) during the Recent Recall Test. The black line (for values see primary x-axis) represents the global dissimilarity, whereas the gray line represents the global field power (for values see secondary x-axis). Red bars indicate microstate borders as identified with global dissimilarity peaks. The topography of the voltage distribution for each microstate is also shown. Note that in order to avoid saturation, topography plots for microstates 1, 2 and 3 range from −2 to 2 µV whereas topography plots for microstates 4 and 5 range from −7 to 7 µV. (B) Microstate segmentation of the grand average event-related potential evoked by CS (averaged across the 2 × 2 CS types) during the Remote Recall Test.
Next, for each microstate, participant and condition, the intracranial source activation was estimated with Low Resolution Electromagnetic Tomography Analysis (LORETA) (Pascual-Marqui et al., 1999) based on the 64-channel ERP (no over-smoothing). LORETA solves the inverse problem by assuming that neighboring neuronal sources should show similar orientations and strengths at a given time. The source solution space is limited to cortical gray matter and hippocampi and partitioned into 2394 voxels with a size of 7 mm3. At each voxel the current source density (CSD) is computed as the linear, weighted sum of the voltage at all electrodes. CSD values were normalized to unity within subjects and multiplied by 2394 prior to statistical analysis.
In addition to analyses of source-localized EEG amplitudes within particular microstates, we also source-localized oscillatory theta (4–8 Hz) activity within the anterior midcingulate cortex (aMCC) by applying LORETA to the cross-spectra of the 2-s segments for details see Mueller et al., 2014 and averaging normalized CSD maps across all segments of one CS type.
Skin conductance
Skin conductance was measured with the BioSemi system (Amsterdam, Netherlands) using two electrodes applied to the middle phalanges of the non-dominant hand. Skin conductance was manually screened for artifacts, and the peak response in a window from 1 to 5 s relative to the CS onset was automatically determined for each trial. Skin conductance responses were then normalized by dividing by the subject’s maximum overall response across conditions (Lykken and Venables, 1971), and averaged across trials by CS type.
Statistical analyses
Subjective ratings were analyzed with a repeated measures ANOVA with Contingency (CS+ vs CS−), Extinction (E vs N) and Block (1, 2, 3, 4) as factors. To detect brain-regions sensitive for prior fear conditioning, voxel-wise t-tests were performed contrasting LORETA estimated CSD in response to CS+ (CS+E and CS+N collapsed) and CS− (CS−E and CS−N collapsed) for each microstate and for source-localized theta activity. Based on prior LORETA studies (Krusemark and Li, 2011; Mueller et al., 2015), the nominal significance threshold for LORETA analyses was set to P < 0.005 with a minimal cluster threshold of 5 significant contiguous voxels to reduce the likelihood of false positives. In addition, the main finding was confirmed with a non-parametric tmax based, step down randomization test (10 000 randomizations) which yields a whole-brain statistical correction (Holmes et al., 1996; Pizzagalli et al., 2001). Because k = 5 microstates were identified and comparisons were performed for each microstate, we also show in Table 1 that the main findings survive the corresponding Bonferroni correction (P’ = P/k = 0.001). In addition to whole-brain analyses, a region of interest (ROI) analysis was performed for source-localized theta activity in the aMCC. To test whether previously reported recent fear memory effects on aMCC theta (Mueller et al, 2014) persisted in the remote fear memory test, the aMCC ROI from the previous study was used.
Table 1.
Regions with significant CS+ > CS− difference for LORETA estimated current density
| Region | MNI coordinates |
Cluster extent (voxels P < 0.005) | T-Value, df = 15 | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Recent Memory Recall | |||||
| Microstate 2 (41–55 ms) | |||||
| Middle temporal gyrus | −59 | −67 | 8 | 6 | 4.32** |
| Microstate 5 (150–191 ms) | |||||
| Middle temporal gyrus | −59 | −60 | 1 | 10 | 4.31** |
| Postcentral gyrus | −59 | −18 | 22 | 10 | 4.01* |
| Remote Memory Recall | |||||
| Microstate 2 (46–63 ms) | |||||
| Lingual + Fusiform Gyrus | −17 | −46 | −6 | 6 | 5.02*** |
| Microstate 3 (63–90 ms) | |||||
| Lingual + Fusiform Gyrus | −17 | −46 | −6 | 6 | 4.43** |
*P < 0.005 (uncorrected), **P < 0.005 (Bonferroni-corrected for five microstates), ***P < 0.001 (Bonferroni-corrected for five microstates).
Results
Day 1 acquisition and extinction
CS ratings
Consistent with successful conditioning, the subset of participants for whom the 1 year follow-up data was available rated the CS+ as significantly less pleasant (F1,15 = 16.30, P < 0.0015) and marginally more arousing (F1,15 = 3.13, P < 0.1) than the CS− after day 1 Acquisition.
24 h recent recall test
CS ratings
At the end of the day 2 Recent Recall Test, participants still rated the CS+ as more unpleasant than the CS− (F1,14 = 7.15, P < 0.02) consistent with successful recent fear conditioning recall within participants of the present subsample.
Skin conductance
A Contingency × Extinction ANOVA on range-corrected skin conductance reactions revealed no main effects or interactions (Ps > 0.13).
Microstate-ERP
Microstate analyses based on global dissimilarity (Figure 2A) revealed five distinct microstates within the first 200 ms ranging from 0 to 41 ms (‘MS1’), 41 to 55 ms (‘MS2’), 55 to 90 ms (‘MS3’), 90 to 150 ms (‘MS4’) and 150 to 191 ms (‘MS5’).
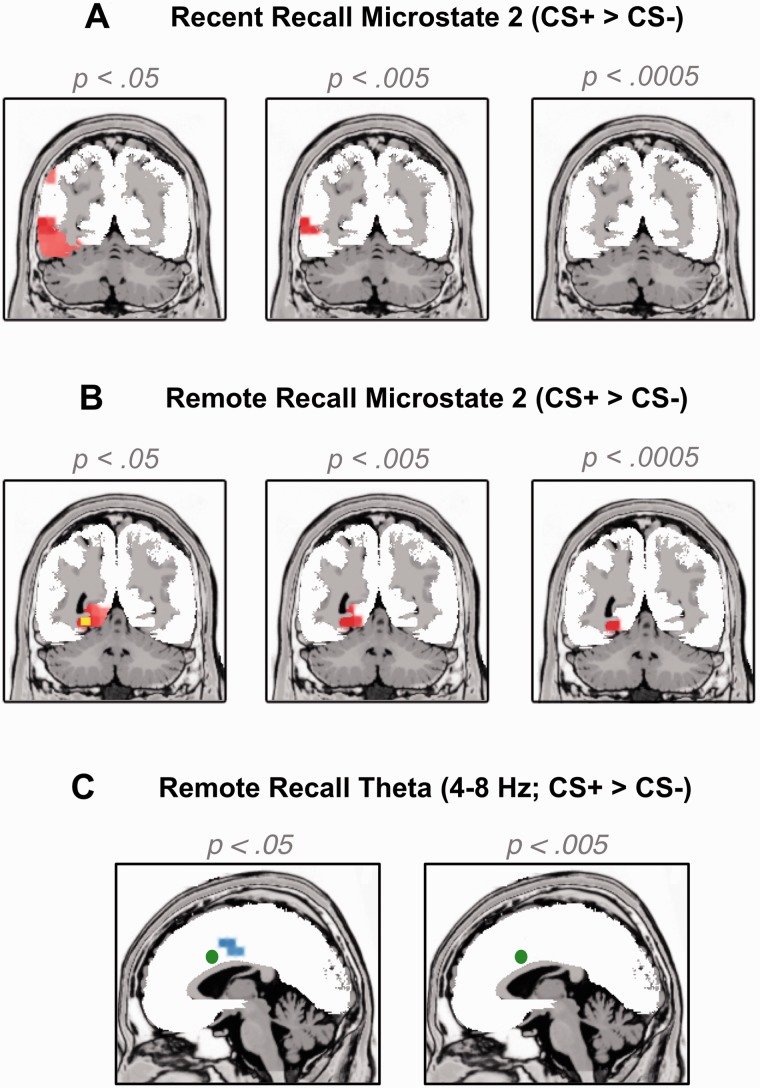
LORETA analyses revealed significant (P < 0.005) differences between CS+ and CS− in MS2 and 5 but not in MS 1, 3 and 4. In MS 2, CS+ evoked significantly more CSD than CS− in a cluster around the left middle temporal gyrus (Table 1), which extended to the left FG (MNI: −40, −53, −21) when the significance threshold was lowered (Figure 3A, t15 = 2.93, P = 0.01). Similarly, CS+ evoked more activity than CS− in MS 5, in a cluster surrounding the left temporal gyrus (Montreal Neurological Institute [MNI] coordinates: −59, −60, 1, P < 0.0007) which extended to the left FG when the significance threshold was lowered to P = 0.01.
Fig. 3.
Voxels with higher current source density to CS+ vs CS− as estimated by LORETA for three different significance thresholds (left: P < 0.05, middle: P < 0.005, right: P < 0.0005, uncorrected) during (A) microstate 2 in the Recent Recall test (MNI coordinate: Y = −60) and (B) in the Remote Recall Test (MNI coordinate: Y = −53). The yellow voxels in panel (B) further indicate where the tmax based whole-brain correction with 10 000 randomizations revealed significantly higher current source density to CS+ vs CS−. Panel (C) shows voxels with higher current source density in the theta band (4–8 Hz) to CS+ vs CS− during the Remote Recall Test. The green circle indicates the voxel with the peak CS+ vs CS− difference in theta activity during the Recent Recall Test as previously published (Mueller et al. 2014).
Theta power
In our previous report on the entire sample we found that day 1 conditioning and extinction modulated theta activity within the aMCC ROI during the 24 h Recall Test (Mueller et al., 2014). Accordingly, we tested whether these effects can be found in the present subsample of N = 16 during the 24 h Recall Test. Consistent with the report on the entire dataset, theta power within the aMCC ROI was also modulated by a Contingency × Extinction interaction in the present subsample (F(1,15) = 13.22, P < 0.003).
1 year remote recall test
Stimulus recognition and contingency awareness
At the beginning of the Remote Recall Test, 15 out of 16 participants (94%) stated for each of the four CS that they were ‘probably’ or ‘certainly’ presented to them before and all participants stated that the two distractor stimuli were ‘probably not’ or ‘certainly not’ presented to them before. This indicates that almost all participants were able to explicitly recognize previously presented stimuli one year after conditioning.
Although almost all participants were able to recognize the stimuli, 8 out of 16 participants (50%) did not correctly recall which of the stimuli had been paired with the aversive US one year before. The remaining 8 participants may have had (partial) contingency awareness as they correctly stated for at least one CS+ that it had been paired with the US more often than at least one CS−.
CS ratings
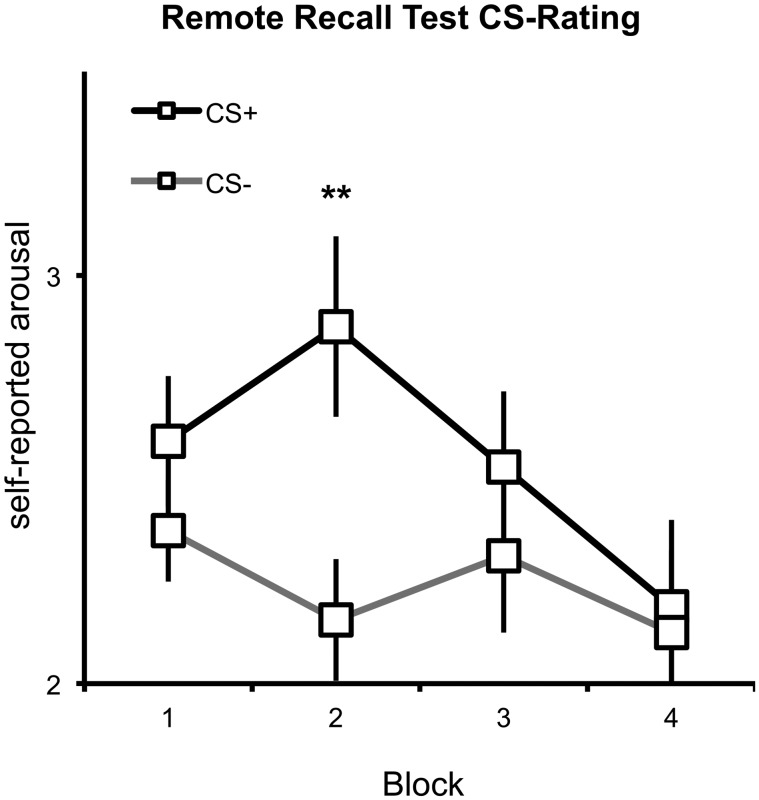
A Contingency × Extinction × Block ANOVA on subjective CS-associated arousal ratings revealed a significant Block × Contingency interaction (F1,15 = 3.36, P < 0.04) and a marginally significant main effect of Contingency (F1,15 = 3.73, P = 0.073). As shown in Figure 4, the CS+ were rated as more arousing than the CS−, particularly in the initial three blocks.
Fig. 4.
Arousal ratings for the CS+ (black line) and CS− (gray line) during the Remote Recall Test.
Skin conductance
A Contingency × Extinction ANOVA on range-corrected skin conductance reactions revealed no main effects or interactions (Ps > 0.3).
Microstate-ERP
Microstate analyses based on global dissimilarity (Figure 2B) revealed five distinct microstates within the first 200 ms after CS presentation (see Supplementary material). Microstates ranged from 0 to 45 ms (‘MS1’), 45 to 63 ms (‘MS2’), 63 to 90 ms (‘MS3’), 90 to 150 ms (‘MS4’) and 150 to 193 ms (‘MS5’).
LORETA analyses revealed significantly increased CSD for CS+ vs CS- in MS 2, 3 and (marginally) 5, but not in MS 1 or 4. In none of the microstates, CS− elicited greater CSD than CS+. Importantly, in MS 2 (46–63 ms), CS+ evoked significant more activity in the left FG (MNI: −24, −53, −6; t15 = 4.71, P < 0.0003, Figure 3B) and adjacent left lingual gyrus (P < 0.0002, for coordinates and cluster extent see Table 1). Moreover, a non-parametric tmax based, whole-brain correction with 10 000 randomizations (Holmes et al., 1996) confirmed that activity to CS+ vs CS− was significantly elevated in the left fusiform and lingual gyri (P < 0.05, see Supplementary material). Finally, because the face-condition assignment was not balanced in the current subsample, we confirmed that the four different faces (rather than the four different CS conditions) were not associated with different means of FG activity in MS 2. As this was not the case (see Supplementary material) this control analysis indicates that the increased FG activations in MS 2 can be attributed to prior conditioning rather than differential face processing of the four different individuals. There were no significant effects in any other region during MS2.
For MS3 (63–90 ms) again the left FG (t15 = 4.37, P < 0.0006) and left lingual gyrus (P < 0.0005) showed significantly larger activation to CS+ as opposed to CS−. As with MS2, there were no significant effects in any other regions (P > 0.005) and we again confirmed that the four different faces (rather than the four different CS conditions) were not associated with different means of FG activity in MS 3 (see Supplementary material). In MS 5, the right FG showed significantly higher CSD for CS+ vs CS- (MNI: 60, −18, −34, P < 0.005), although this effect was restricted to two voxels and therefore did not survive the cluster threshold of 5 contiguous significant voxels. There were no significant voxels in any other regions during MS 5 (P > 0.005).
Theta power
aMCC theta activity was not modulated by day 1 Contingency or Extinction or their interaction when the same ROI as in our previous study (Mueller et al., 2014) was used (all Ps > 0.08). However, an exploratory, liberally thresholded (P < 0.05, uncorrected) whole-brain contrast of CS+ vs CS− confirmed significantly enhanced theta power to CS+ vs CS− in a slightly more posterior section within the MCC (Figure 3C, k = 13 voxels, MNI coordinates of peak voxel: X = −10, Y = 3, Z = 43; P < 0.014).
Exploratory Analyses on Contingency Awareness
As stated above, the CS+ was rated as more arousing than the CS− during the Remote Recall Test. To assess whether this effect also occurred in the absence of explicit contingency awareness (Knight et al., 2003), arousal ratings for CS+ and CS− were compared within the eight (conservatively classified) contingency unaware subjects. A significant difference (t7 = 1.96, P < 0.05, one-sided) indicated that the CS+ was perceived as more arousing than the CS− even in subjects who could not explicitly recall that the CS+ had been paired with the US one year before.
To similarly probe the effect of contingency awareness on CS-related brain activity, first a Contingency ×Microstate (MS 2 vs MS 3) ANOVA was performed on source-localized FG activation (MNI: −24, −53, −6) in response to CS presentation only in contingency unaware subjects. This ANOVA revealed a main effect of Contingency (F1,8 = 47.22, P < 0.00015), suggesting that even subjects who were unable to recall that the CS+ had been paired with the US one year before showed enhanced FG activity to the CS+ vs CS− across MS 2 and 3 (i.e. from 46 to 90 ms). In addition, a Contingency ANOVA on MCC-localized (MNI coordinates X = −10, Y = 3, Z = 43) theta current density also confirmed that the above reported main effect of Contingency on CS-evoked MCC theta power was significant in individuals without contingency awareness (F1,8 = 9.06, P < 0.02).
Discussion
The present study investigated whether remotely fear-conditioned faces modulated early processing in visual cortices. A novel finding was that cortical activation rapidly (<63 ms) discriminated between stimuli that had vs had not been paired with an aversive US about 1 year prior to conditioning. Moreover, the discrimination of CS+ and CS− was localized to (or near) the FG, a structure sensitive for fear conditioned faces in previous ERP (Pizzagalli et al., 2003), MEG (Morel et al., 2012) and fMRI (Petrovic et al., 2008) studies. The present findings therefore demonstrate, we believe for the first time, that human fear memory may affect rapid sensory threat processing even when this fear memory was acquired in the remote past.
Data-driven segmentation procedures revealed five distinct microstates within the first 200 ms for both the Recent and Remote Recall Test. The remarkably similar microstate boundary latencies of the recent (41, 55, 90, 150, 190 ms) and remote (45, 63, 90, 150, 193 ms) recall test can be considered a cross-validation of the identified microstates. Microstates are characterized by quasi-stable scalp topographies and changes in topography occurring during transitions from one microstate to another are thought to reflect different configurations of active cortical cell assemblies (Khanna et al., 2015). The latency of MS 2 (Recent Recall Test: 41–55 ms; Remote Recall Test: 45–63 ms) roughly corresponds to the earliest previously reported time-windows sensitive for visual conditioned fear (i.e. 30–60 ms; Morel et al., 2012) and auditory conditioned fear (i.e. 20–50 ms; Brockelmann et al., 2011) as identified with MEG. The latencies and topographies of MS 3 (55–90 ms), 4 (90–150 ms) and 5 (150–190 ms) correspond with the typical latencies and topographies of the C1 (Jeffreys and Axford, 1972; Rauss et al., 2011), P100 (Clark and Hillyard, 1996) and N170 (Bentin et al., 1996) ERP components.
In the present study we found evidence for remote fear memory effects in the FG from 45 to 90 ms (i.e. MS2 and 3). This latency is very early given that the first brain regions outside of V1 become activated within 40–85 ms after visual stimulus onset in macaques (Lamme and Roelfsema, 2000). Together with similar threat-sensitive time-windows found in previous human fear conditioning studies investigating acute or recent fear memory (Stolarova et al., 2006; Keil et al., 2007; Steinberg et al., 2011; Morel et al., 2012), the current latencies indicate rapid and privileged processing of remotely acquired threat signals in human FG. Rapid FG activation to remotely acquired fear could be driven by quick signals from the amygdala, which modulates FG activation to threat stimuli (Vuilleumier et al., 2004) and is a major site for associative fear learning (LeDoux, 2014) including remote fear memory storage (Poulos et al., 2009). Although amygdala activity cannot be assessed with ERP, the finding that patients with amygdala lesions lack potentiated early ERP and FG responses to threat-related stimuli (Vuilleumier et al., 2004; Rotshtein et al., 2010) supports this hypothesis. The presumably amygdala-driven enhancements of visual cortex activity may serve to amplify the sensory gain of CS-related features (Miskovic and Keil, 2012) and may thus facilitate rapid and accurate detection of threat.
Notably, FG, amygdala and other structures may show increased responses to threat-related stimuli even if individuals lack explicit awareness of threat association (de Gelder et al., 2005; LeDoux, 2014). At least half of our subjects were unable to explicitly recall contingencies approximately one year after conditioning. Nevertheless, even fully contingency-unaware subjects found the CS+ to be more arousing than the CS− and produced a fast FG potentiation to CS+ vs CS−. In addition, we observed no effects of remote fear conditioning on SCR one year after conditioning. Together, these findings support a two-level account of human fear conditioning, whereby fear-conditioned memories can be implicit memories that potentiate sensory processing by a fast route involving thalamus and amygdala that (i) does not require conscious awareness of CS-US contingencies (Moratti et al., 2006) and (ii) does not modulate skin conductance (Hamm and Weike, 2005). In particular, the present findings suggest that implicit fear-conditioned memories quickly activate the FG and are highly stable over time.
In addition to early FG enhancements to CS+ vs CS−, there was preliminary evidence that mid-latency (MS5, 150−190 ms) left and right FG activation was also enhanced to fear conditioned faces in the Recent and Remote Recall Tests, respectively. This time window, centered at 170 ms, is typically associated with face processing in FG (Bentin et al., 1996; Halgren et al., 2000) and increased FG and occipito-temporo-parietal activity in response to fear conditioned faces during that time period has been reported at the day of the conditioning (Pizzagalli et al., 2003; Steinberg et al., 2011). While these studies demonstrated that mid-latency brain activity discriminated between CS+ and CS− during conditioning, the present findings suggest that mid-latency FG activity may discriminate between CS+ and CS− 24 h after conditioning and even one year later.
Remote fear conditioning not only affected rapid responses to the CS in visual regions but there was also preliminary evidence for altered MCC theta to CS+ vs CS− one year after conditioning. In our initial study on the entire N = 42 sample, we had shown that aMCC theta is increased to non-extinguished CS+ vs CS− one day after conditioning but does not differ between previously extinguished CS+ and CS- (Mueller et al., 2014). Consistent with prior animal (Burgos-Robles et al., 2009) and human (Milad and Quirk, 2012) studies, we interpreted this finding to indicate that human aMCC theta relates to fear expression. Within the present subsample of those participants who were also tested 1 year later, the effect of enhanced aMCC theta activity to non-extinguished CS+ vs CS− during the recent recall test was replicated. Moreover, MCC theta was potentiated in response to CS+ vs CS− during the remote recall test. Consistent with the behavioral and microstate/visual ERP data, there was no effect of day-1 extinction on MCC theta activity one year later, consistent with relatively instable extinction memories. While the present findings raise the possibility that the MCC is still sensitive to previously acquired fear one year after conditioning, it should be noted that (i) the effect of remote fear conditioning on MCC theta only emerged in exploratory analyses with a more liberal significance threshold and (ii) the effect was slightly more posterior than the aMCC (Vogt, 2005). Therefore, future studies with shorter Remote Recall Test intervals or larger sample sizes should replicate this finding.
The limitations of the current study should be acknowledged. First, due to a follow-up period of up to one and a half years, which had not been announced to the participants at the beginning of the study, only sixteen of the original 42 participants could be recruited (Mueller et al., 2014). Because small samples increase the likelihood for false positives and for missing true effects, the current findings await replication. Based on our findings, future studies could achieve larger sample sizes by (i) scheduling the Remote Recall Test after a period of 9 rather than 17 months (given that the length of the re-test interval had little impact on the current findings; see Supplementary material), (ii) recruit a larger initial sample and (iii) inform participants about the follow-up measurement at the beginning of the study. Second, all CS had been repeatedly presented without the US during the Recent Recall Test one day after conditioning (Mueller et al., 2014). Accordingly, all stimuli underwent some degree of extinction on day 2. In addition, day 2 fear reactivation caused by CS presentation may have affected the fear memory re-consolidation process. It is likely that both fear extinction and modulated re-consolidation reduce rather than enhance remote fear memory recall (Schiller et al., 2010) and that the presented findings on rapid visual processing in the Remote Recall Test would have been even stronger had we omitted the day 2 Recent Recall Test. Nevertheless, future remote fear memory studies with and without a recall test shortly after conditioning are needed to systematically test the influence of extinction and re-consolidation on rapid FG enhancements to remotely acquired fear.
In conclusion we showed that faces that had been fear conditioned approximately one year before, rapidly (i.e. <80 ms) evoke increased activity in or in proximity of left FG. This effect can occur outside of explicit contingency awareness and may have evolved to facilitate the quick perception of particular conspecifics or other threats that have been dangerous in the past. Future studies should test whether rapid enhancements of FG activity to threats (i) relate to actual threat perception speed and/or accuracy, (ii) are also observed for remotely fear conditioned stimuli other than faces (Dunsmoor et al., 2014) and (iii) are exacerbated in anxiety disorders.
Supplementary data
Supplementary data are available at SCAN online.
Acknowledgements
We thank Christian Panitz for supervising parts of data collection and preparation. We further thank Hannah Münch, Anika Schulze and Wiebke Schröter who collected the data as part of their theses.
Funding
This research was funded by DFG grant DFG MU3535/2-1 (E.M.M.).
Conflict of interest. Over the last two years, Dr. Pizzagalli has received consulting fees from Otsuka America Pharmaceutical and Pfizer for studies unrelated to this project. E.M.M. has no biomedical financial interests or potential conflicts of interest to report.
References
- Bentin S., Allison T., Puce A., Perez E., McCarthy G. (1996). Electrophysiological Studies of Face Perception in Humans. Journal of Cognitive Neuroscience, 8, 551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockelmann A. K., Steinberg C., Elling L., Zwanzger P., Pantev C., Junghofer M. (2011). Emotion-associated tones attract enhanced attention at early auditory processing: magnetoencephalographic correlates. Journal of Neuroscience, 31, 7801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C., Dolan R. J., Armony J. L., Friston K. J. (1999). Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. Journal of Neuroscience, 19, 10869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A., Vidal-Gonzalez I., Quirk G. J. (2009). Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. Journal of Neuroscience, 29, 8474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V. P., Hillyard S. A. (1996). Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. Journal of Cognitive Neuroscience, 8, 387–402. [DOI] [PubMed] [Google Scholar]
- de Gelder B., Morris J. S., Dolan R. J. (2005). Unconscious fear influences emotional awareness of faces and voices. Proceedings of the National Academy of Science of the United States of America, 102, 18682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor J. E., Kragel P. A., Martin A., LaBar K. S. (2014). Aversive learning modulates cortical representations of object categories. Cerebral Cortex, 24, 2859–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P., Friesen W. V. (1976). Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Etkin A., Klemenhagen K. C., Dudman J. T., et al. (2004). Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron, 44, 1043–55. [DOI] [PubMed] [Google Scholar]
- Halgren E., Raij T., Marinkovic K., Jousmaki V., Hari R. (2000). Cognitive response profile of the human fusiform face area as determined by MEG. Cerebral Cortex, 10, 69–81. [DOI] [PubMed] [Google Scholar]
- Hamm A. O., Weike A. I. (2005). The neuropsychology of fear learning and fear regulation. International Journal of Psychophysiology, 57, 5–14. [DOI] [PubMed] [Google Scholar]
- Holmes A. P., Blair R. C., Watson J. D., Ford I. (1996). Nonparametric analysis of statistic images from functional mapping experiments. Journal of Cerebral Blood Flow & Metabolism, 16, 7–22. [DOI] [PubMed] [Google Scholar]
- Huff N. C., Hernandez J. A., Blanding N. Q., LaBar K. S. (2009). Delayed extinction attenuates conditioned fear renewal and spontaneous recovery in humans. Behavioral Neuroscience, 123, 834–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys D. A., Axford J. G. (1972). Source locations of pattern-specific components of human visual evoked potentials. I. Component of striate cortical origin. Experimental Brain Research, 16, 1–21. [DOI] [PubMed] [Google Scholar]
- Kalisch R., Holt B., Petrovic P., et al. (2009). The NMDA agonist D-cycloserine facilitates fear memory consolidation in humans. Cerebral Cortex, 19, 187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A., Stolarova M., Moratti S., Ray W. J. (2007). Adaptation in human visual cortex as a mechanism for rapid discrimination of aversive stimuli. Neuroimage, 36, 472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A., Pascual-Leone A., Michel C. M., Farzan F. (2015). Microstates in resting-state EEG: Current status and future directions. Neuroscience & Biobehavioral Reviews, 49C, 105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. C., Nguyen H. T., Bandettini P. A. (2003). Expression of conditional fear with and without awareness. Proceedings of the National Academy of Science of the United States of America, 100, 15280–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig T., Lehmann D. (1996). Microstates in language-related brain potential maps show noun-verb differences. Brain Language, 53, 169–82. [DOI] [PubMed] [Google Scholar]
- Krusemark E. A., Li W. (2011). Do all threats work the same way? Divergent effects of fear and disgust on sensory perception and attention. Journal of Neuroscience, 31, 3429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme V. A., Roelfsema P. R. (2000). The distinct modes of vision offered by feedforward and recurrent processing. Trends in Neuroscience, 23, 571–9. [DOI] [PubMed] [Google Scholar]
- LeDoux J. E. (2014). Coming to terms with fear. Proceedings of the National Academy of Science of the United States of America, 111, 2871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D., Skrandies W. (1984). Spatial analysis of evoked potentials in man—a review. Progress in Neurobiology, 23, 227–50. [DOI] [PubMed] [Google Scholar]
- Lissek S., Powers A. S., McClure E. B., et al. (2005). Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy, 43, 1391–424. [DOI] [PubMed] [Google Scholar]
- Lykken D. T., Venables P. H. (1971). Direct measurement of skin conductance: a proposal for standardization. Psychophysiology, 8, 656–72. [DOI] [PubMed] [Google Scholar]
- Mathews A., MacLeod C. (2005). Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology, 1, 167–95. [DOI] [PubMed] [Google Scholar]
- Milad M. R., Quirk G. J. (2012). Fear extinction as a model for translational neuroscience: ten years of progress. Annual Review of Psychology, 63, 129–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S., Oehlberg K. (2008). The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychology (Amst), 127, 567–80. [DOI] [PubMed] [Google Scholar]
- Miskovic V., Keil A. (2012). Acquired fears reflected in cortical sensory processing: a review of electrophysiological studies of human classical conditioning. Psychophysiology, 49, 1230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratti S., Keil A., Miller G. A. (2006). Fear but not awareness predicts enhanced sensory processing in fear conditioning. Psychophysiology, 43, 216–26. [DOI] [PubMed] [Google Scholar]
- Morel S., Beaucousin V., Perrin M., George N. (2012). Very early modulation of brain responses to neutral faces by a single prior association with an emotional context: evidence from MEG. Neuroimage, 61, 1461–70. [DOI] [PubMed] [Google Scholar]
- Moscovitch M., Nadel L., Winocur G., Gilboa A., Rosenbaum R. S. (2006). The cognitive neuroscience of remote episodic, semantic and spatial memory. Current Opinion in Neurobiology, 16, 179–90. [DOI] [PubMed] [Google Scholar]
- Mueller E. M., Hofmann S. G., Santesso D. L., Meuret A. E., Bitran S., Pizzagalli D. A. (2008). Electrophysiological evidence of attentional biases in social anxiety disorder. Psychological Medicine, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller E. M., Panitz C., Hermann C., Pizzagalli D. A. (2014). Prefrontal oscillations during recall of conditioned and extinguished fear in humans. Journal of Neuroscience, 34, 7059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller E. M., Pechtel P., Cohen A. L., Douglas S. R., Pizzagalli D. A. (2015). Potentiated processing of negative feedback in depression is attenuated by anhedonia. Depress Anxiety, 32, 296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm S. D., Vervliet B., Jovanovic T., et al. (2008). Timing of extinction relative to acquisition: a parametric analysis of fear extinction in humans. Behavioral Neuroscience, 122, 1016–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui R. D., Lehmann D., Koenig T., et al. (1999). Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Research, 90, 169–79. [DOI] [PubMed] [Google Scholar]
- Petrovic P., Kalisch R., Pessiglione M., Singer T., Dolan R. J. (2008). Learning affective values for faces is expressed in amygdala and fusiform gyrus. Social Cognitive & Affective Neuroscience, 3, 109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E. A., Delgado M. R., Nearing K. I., LeDoux J. E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron, 43, 897–905. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D., Pascual-Marqui R. D., Nitschke J. B., et al. (2001). Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. American Journal of Psychiatry, 158, 405–15. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D. A., Greischar L. L., Davidson R. J. (2003). Spatio-temporal dynamics of brain mechanisms in aversive classical conditioning: high-density event-related potential and brain electrical tomography analyses. Neuropsychologia, 41, 184–94. [DOI] [PubMed] [Google Scholar]
- Poulos A. M., Li V., Sterlace S. S., Tokushige F., Ponnusamy R., Fanselow M. S. (2009). Persistence of fear memory across time requires the basolateral amygdala complex. Proceedings of the National Academy of Science of the United States of America, 106, 11737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauss K., Schwartz S., Pourtois G. (2011). Top-down effects on early visual processing in humans: a predictive coding framework. Neuroscience & Biobehavioral Review, 35, 1237–53. [DOI] [PubMed] [Google Scholar]
- Rotshtein P., Richardson M. P., Winston J. S., et al. (2010). Amygdala damage affects event-related potentials for fearful faces at specific time windows. Human Brain Mapping, 31, 1089–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco T., Sacchetti B. (2010). Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science, 329, 649–56. [DOI] [PubMed] [Google Scholar]
- Schiller D., Cain C. K., Curley N. G., et al. (2008). Evidence for recovery of fear following immediate extinction in rats and humans. Learning & Memory, 15, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D., Monfils M. H., Raio C. M., Johnson D. C., Ledoux J. E., Phelps E. A. (2010). Preventing the return of fear in humans using reconsolidation update mechanisms. Nature, 463, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C., Mothes-Lasch M., Straube T. (2013). Automatic neural processing of disorder-related stimuli in social anxiety disorder: faces and more. Frontiers in Psychology, 4, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C., Schoning S., Zwitserlood P., et al. (2009). Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One, 4, e5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A. J., Salomons T. V., Slagter H. A., Fox A. S., Winter J. J., Davidson R. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12, 154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg C., Dobel C., Schupp H. T., et al. (2011). Rapid and highly resolving: affective evaluation of olfactorily conditioned faces. J Cognitive Neuroscience. [DOI] [PubMed] [Google Scholar]
- Stolarova M., Keil A., Moratti S. (2006). Modulation of the C1 visual event-related component by conditioned stimuli: evidence for sensory plasticity in early affective perception. Cerebral Cortex, 16, 876–87. [DOI] [PubMed] [Google Scholar]
- Vervliet B., Craske M. G., Hermans D. (2013). Fear extinction and relapse: state of the art. Annual Review of Clinical Psychology, 9, 215–48. [DOI] [PubMed] [Google Scholar]
- Vogt B. A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Natural Reviews Neuroscience, 6, 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P., Pourtois G. (2007). Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia, 45, 174–94. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Richardson M. P., Armony J. L., Driver J., Dolan R. J. (2004). Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Natural Neuroscience, 7, 1271–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.