Abstract
Squamous cell carcinoma is the second most common cutaneous malignancy after basal cell carcinoma. Although the gold standard of diagnosis for squamous cell carcinoma is biopsy followed by histopathology evaluation, optical non-invasive diagnostic tools have obtained increased attention. Dermoscopy has become one of the basic diagnostic methods in clinical practice. The most common dermoscopic features of squamous cell carcinoma include clustered vascular pattern, glomerular vessels and hyperkeratosis. Under reflectance confocal microscopy, squamous cell carcinoma shows an atypical honeycomb or disarranged pattern of the spinous-granular layer of the epidermis, round nucleated bright cells in the epidermis and round vessels in the dermis. High frequency ultrasound and optical coherence tomography may be helpful in predominantly in pre-surgical evaluation of tumor size. Emerging non-invasive or minimal invasive techniques with possible application in the diagnosis of squamous cell carcinoma of the skin, lip, oral mucosa, vulva or other tissues include high-definition optical coherence tomography, in vivo multiphoton tomography, direct oral microscopy, electrical impedance spectroscopy, fluorescence spectroscopy, Raman spectroscopy, elastic scattering spectroscopy, differential path-length spectroscopy, nuclear magnetic resonance spectroscopy, and angle-resolved low coherence interferometry.
Keywords: actinic keratosis, dermoscopy, diagnosis, skin cancer, oral mucous membrane, RCM, spectroscopy, squamous cell carcinoma, ultrasonography, vulva
Introduction
The term non-melanoma skin cancers includes cutaneous lymphomas, adenexal tumors, Merkel-cell carcinoma, and other rare primary cutaneous neoplasms, but is mainly used in clinical practice to define basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).[1] Squamous cell carcinoma is the second most common cutaneous malignancy after basal cell carcinoma with an increasing incidence worldwide.[2,3] The most frequent presentation of SCC in situ is an erythematous scaly patch or slightly elevated plaque. Invasive SCC is often papulonodular, but can be plaque-like, papilomatous or exophytic. SCC usually arises within a background of sun-exposed skin, most commonly on the bald scalp, face, neck, forearms and dorsal hands. Actinic keratosis (AK) is a common sun-induced erythematous lesion covered with scale. Historically AK has been described as a precursor of cancer or a precancerous lesion that have potential to develop into SCC. Some authors have postulated that both AK and SCC in situ share the same clinical, histological and molecular features and that they represent a continuum of disease.[4] Clinical difference of SCC from actinic keratosis, superficial basal cell carcinoma, clear cell acanthoma, amelanotic melanoma, psoriasis, warts or eczema may be difficult. In case of diagnostic difficulties, a confirmatory diagnosis can be achieved by histopathological examination. Although the gold standard of diagnosis for SCC is an invasive biopsy followed by histopathological evaluation, non-invasive and minimally invasive diagnostic tools have obtained increased attention.
Dermoscopy and videodermoscopy
Dermoscopy (dermatoscopy) is a non-invasive diagnostic technique that allows in vivo evaluation of colors and microstructures of the epidermis, the dermaepidermal junction and the papillary dermis at 10-fold magnification. The term "dermatoscopy" was used in 1920 for the first time by Saphier, who detailed description of possible applications of skin surface microscopy.[5] Many different terms have been used in the literature for this technique: dermatoscopy, dermoscopy,[6] surface microscopy,[7] incident light microscopy, epiluminescence light microscopy.[8] Videodermoscopy is a digitalized form of dermoscopy, which allows dermoscopic visualization of structures in high magnification (70-fold and higher).
The dynamic development of dermoscopy and the development of numerous dermoscopic algorithms for distinguishing benign from malignant skin lesions began in the late 80’s and 90’s of the twentieth century. Several studies comparing dermoscopy examination with conventional clinical examination have documented that the diagnostic accuracy of pigmented lesions, especially for malignant melanoma, is significantly better with dermoscopy when performed by a trained and experienced user.[9,10] In addition, dermoscopy has been shown to improve diagnostic accuracy of nonpigmented skin malignancies.[11,12]
Dermoscopic evaluation can be performed with non-polarized light contact dermoscopy, polarized light contact dermoscopy or polarized light non-contact dermoscopy. It is imperative that the users of these techniques should be aware of the subtle differences in color and structures between polarized and non-polarized images to avoid misdiagnosis. In non-polarized light dermoscopy reduction in skin surface reflection is achieved by using an immersion interface between the skin and the lens. Polarized light is usually created with the use of filters. These filters allow the dermoscope to selectively capture backscattered light from deeper levels of the skin and avoid the superficially reflected light. Unlike non-polarized light dermoscopy, polarized light dermoscopy allows visualization of deep skin structures without the necessity of a liquid interface or direct skin contact with the instrument.[12] One of the differences between polarized dermoscopy and non-polarized dermoscopy is the ability to visualize white shiny structures using polarized light contact dermoscopy or polarized light non-contact dermoscopy. Three defined morphologies may be present by white shiny structures and include white shiny lines, white shiny areas and rosettes.White shiny areas are characterized as white shiny clods or larger structureless areas with a shiny, bright white color. Rosettes are defined as four bright white points grouped together akin to a four-leaf clover.[13] Liebman et al. showed that the presence of white shiny lines of any length accompanied by white shiny areas is most suggestive of a diagnosis of BCC, and white rosettes speak in favor of the diagnosis of AK or SCC.[14]
Dermoscopic criteria have been described for two superficial forms of squamous cell carcinoma (pigmented and non-pigmented Bowens disease and interepidermal carcinoma (IEC) and for invasive cutaneous squamous cell carcinoma.
Bowen disease and interepidermal carcinoma are characterized by two types of vascular patterns: classic small dotted vessels and glomerular vessels. Both patterns often appear within the same lesion and are distributed in small, densely packed clusters or groups.[15-19] Pan et al.[20] reported a clustered vascular pattern, glomerular vessels and hyperkeratosis as the most frequent features of interepidermal carcinoma. The authors showed that the concurrent presence all three features in one lesion allows the siagnosis of interepidermal carcinoma with a probability of 98%.[20] In pigmented Bowen’s disease, additional dermoscopic features are represented by small brown globules, which are regularly packed in a patchy distribution and by a grey to brown homogeneous pigmentation.[16] In contrast to the dermoscopic patterns of Bowen’s disease and interepidermal carcinoma invasive SCC presents a greater polymorphism of vascular structures. One can observe a vascular polymorphism comprised of linear irregular, hairpin and grouped glomerular/dotted vessels over a whitish background with a central mass of keratin or ulceration.[21-23]
Lallas et al.[24] revealed a significant difference in the dermoscopy pattern between poorly differentiated SCC compared to well- and moderately differentiated tumors. Poorly differentiated SCCs were dermoscopically typified by a predominantly red color, resulting from the presence of bleeding and/or dense vascularity, in the absence of scaling and keratin or other white-colored criteria. In contrast, white-colored criteria, including scales/keratin, white circles, white halos and structureless whitish areas were shown to be associated with well- or moderately differentiated variants. In a study, which included 143 SCCs, Lallas et al.[24] showed that dermoscopically, the presence of a predominantly red color is associated with a 13-fold increased possibility of poor differentiation, whereas a predominantly white and white-yellow color decreased the odds of poorly differentiated SCC by 97% each. Furthermore, presence of vessels in more than 50% of the lesion's surface, a diffuse distribution of vessels and bleeding are markers of poor differentiation, while scaling is indicative of well- or moderately differentiated tumors.[24]
Keratoacanthoma is a common low-grade tumor that originates in the pilosebaceous glands and microscopically closely resembles SCC. It is characterized by rapid growth over a few weeks to months, followed by spontaneous resolution over 4-6 months in most cases, but rarely it may progress to invasive carcinoma. Rosendahl et al.[25] identified several dermoscopic criteria that help differential keratoacanthoma from SCC and other nonpigmented lesions. The most important dermoscopic clues to keratoacanthoma and SCC are white circles, keratin crust/scale, blood spots, and white structurless zones.White circles are observed in both types of lesions but more frequent in SCCs than in keratoacanthomas (60% vs. 25.6%). Keratin crust/scale is present in 79.1% of keratoacanthomas and in 70.0% of SCCs and the presence of central keratin masses is more common in keratoacanthoma compared to SCC (51.2% vs 30.0%). Keratin crust/scale had the highest sensitivity for keratoacanthoma and SCC (79%) and with circles had the highest specificity (87%).[25] Lin et al.[26] have observed white circles in 32% of SCCs and 38% of keratoacanthomas, keratin crust/scale in 90% of SCCs and all of keratoacanthomas and central keratin masses in 32% of SCCs and 88% keratoacanthomas.
Based on dermoscopic findings, Zalaudek et al. proposed a progression model of facial actinic keratosis developing into IEC and invasive SCC. Those AK that progress to increasing atypia tend to display vessels around follicles that become dotted or coiled on higher magnification, then as the lesion develops into SCC in situ the dotted/coiled vessels appear to enlarge, become more convoluted and clustered, and the follicles in this area appear to miniaturize and disappear. In concert with these changes in neovascularization, in other areas of AK the whitish keratotic follicles appear to coalesce, eventually forming the discrete whitish, opaque scaly areas typically seen in IEC. With progression of IEC to invasive SCC, the lesion thickens clinically while dermoscopically hairpin and/or linear-irregular vessels will appear. Along with these vascular changes, a central mass of keratin forms and ulceration may occur.[27]
Figure 1 and Table 1 present typical dermoscopy features of SCC.
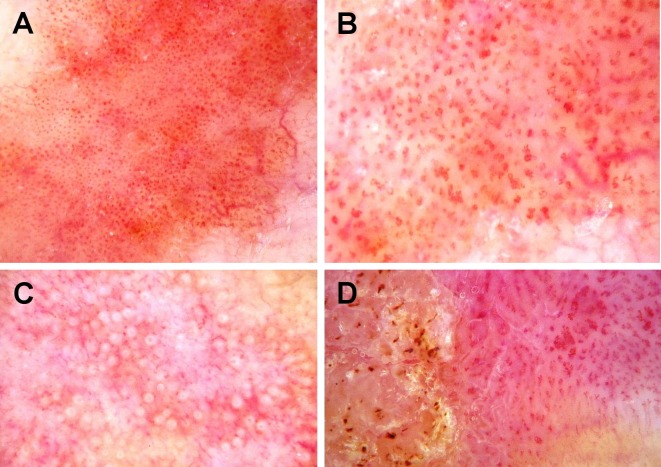
Figure 1.
Videodermoscopy of squamous cell carcinoma in situ. 70-fold magnification. (A) SCC in situ with small dotted vessels distributed in packed clusters; (B) SCC in situ with glomerular vessels; (C) SCC in situ with white circles; (D) SCC in situ with dotted and glomerular vessels, and hyperkeratosis.
Table 1. Dermoscopy features of squamous cell carcinoma.
| DERMOSCOPIC FEATURES OF SCC | |
|---|---|
| Dotted vessels | 3.3-86.6% 25,27,69,70 |
| Coiled/glomerular vessels | 42-90% 16,25,69 |
| Herpin vessels | 15-38.5% 25,27 |
| Keratin crust/scale | 42.3-90% 16,25,27 |
| White circle | 60% 25 |
| Ulceration | 17.9-28.6% 16,25,27 |
Reflectance confocal microscopy
Reflectance confocal microscopy (RCM) is a non-invasive optical imaging technique that allows high-resolution visualization images of skin of the epidermis and the superficial level of the dermis in real-time at nearly histologic resolution.[28] The technique was first described for skin imaging by Rajadhyaksha et al. in 1995.[29] RCM relies on a low-power laser that emits a near-infrared light (830 nm), coherent laser beam by witch the skin is illuminated. As the laser beam passes through the upper skin layers, it is partially backscattered due to the natural refractive index of microanatomical structures. This backscattered light has to pass through a narrow pinhole, which guarantees that only light reflected from structures "in focus" is detected; light from elsewhere is blocked. After passing the pinhole, the beam is diverted by a semireflective mirror system and, finally, directed to a detector. The obtained data are processed and visualized by special software on a computer screen.[30] The basic principles of RCM are based on the different refractive indexes of distinctive intracellular structures. Highly reflective structures appear bright/white, while non-reflective structures appear dark.[31] In contrast to the colored, vertical conventional histopathology sections, RCM optical slices are horizontal and black-and-white. RCM was reported as a useful tool for in vivo assessment of nevi, melanoma, basal cell carcinoma, squamous cell carcinoma, inflammatory diseases, autoimmune bullous disease, bacterial, fungal and viral skin infection.[32-39]
Under RCM squamous cell carcinoma revealed the presence of scale at the stratum corneum level. The presence of the scale may be limited diagnostic value because it can be present in many benign lesions. However, within the constellation of other RCM features of SCC, it may be helpful for diagnosis. Under RCM, SCC presents an atypical honeycomb and/or a disarranged pattern of the spinous-granular layer of the epidermis. Round nucleated bright cells in a pagetoid pattern are observed at the spinous-granular layer.[40] There are two types of targetiod cells. The first type is a large cell with a bright center and a dark peripheral halo. The second type is a cell with a dark center and a bright rim surrounded by a dark halo. The first one correspond on histologic examination to large dyskeratotic keratinocytes separated from adjacent cell by a clear retraction halo, and the second type correspond histologically to dyskeratotic keratinocytes containing a pycnotic nucleus.[41] RCM images of SCC reveled in the center of dermal papillae a round blood vessels.[40] While there is some overlap in the RCM features of SCC and AK, architectural disarray in the stratum granulosum and the spinous layer and the presence of nest-like structures in the dermis are highly suggestive of SCC.[42]
Figure 2 and Table 2 present typical RCM features of SCC.
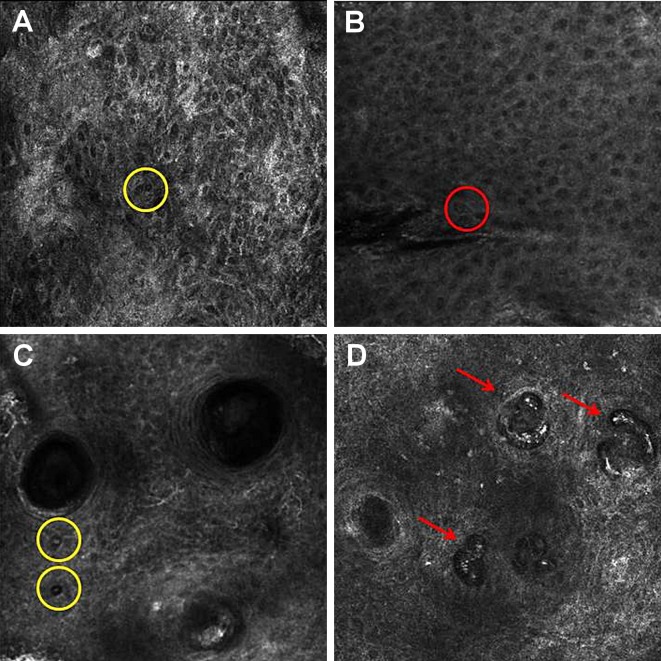
Figure 2.
Reflectance confocal microscopy of squamous cell carcinoma. (A) SCC with a disarranged pattern of the spinous-granular layer of the epidermis and round cell with a bright center and a dark peripheral halo (yellow circle); (B) SCC with an atypical honeycomb and round cell with a dark center and a bright rim surrounded by a dark halo (red circle); (C) SCC with an atypical honeycomb and round cell with a bright center and a dark peripheral halo (yellow circle); (D) SCC with round blood vessels in the dermis.
Table 2. Reflectance confocal microscopy features of squamous cell carcinoma.
| RCM FEATURES OF SCC | |
|---|---|
| Scale at the stratum corneum level | 95% 40 |
| Atypical honeycomb/ a disarranged pattern | 100% 40-42 |
| Round nucleated cells at the spinous-Granular Layer | 50-65% 40,41 |
| Round vessels in the dermis | 39-100% 40,41 |
High frequency ultrasonography
Ultrasonography is a valuable diagnostic tool widely used in medicine. This is attributed to its versatility, unlimited repeatability with high diagnostic value and the lack of risk to the patients. It provides real-time visual information about benign and malignant process in the skin and subcutis. Since in 1979 Alexander and Miller first time used ultrasonography for measuring skin thickness, great progress in the development of high-frequency scanners occurred. Good correlation between ultrasonographic and histological measurements of melanomas thickness was obtained using transductors of 20-100MH frequency, which allow a resolution of 80-200 μm and achieve a penetration depth from 1.5 to 8 mm.[43]
The value of high frequency ultrasonography (HFUS) in clinical dermatology is still being debated. All nonmelanoma skin cancer lesions appeared hypoechogenic in HFUS, suggesting that this method alone is not suitable from differential diagnosis.[44-46] However, it gives a clear picture of the size and depth of the tumor. The method should be used as a complementary method in preoperative evolution of the tumor.[47-49] Wortsman et al., in a large retrospective study of 4338 ultrasonographic skin examinations, showed that the addition of HF ultrasonography increased the correctness of clinical diagnosis from 73% to 97%.[50]
Figure 3 shows a HFUS image of SCC.
Figure 3.
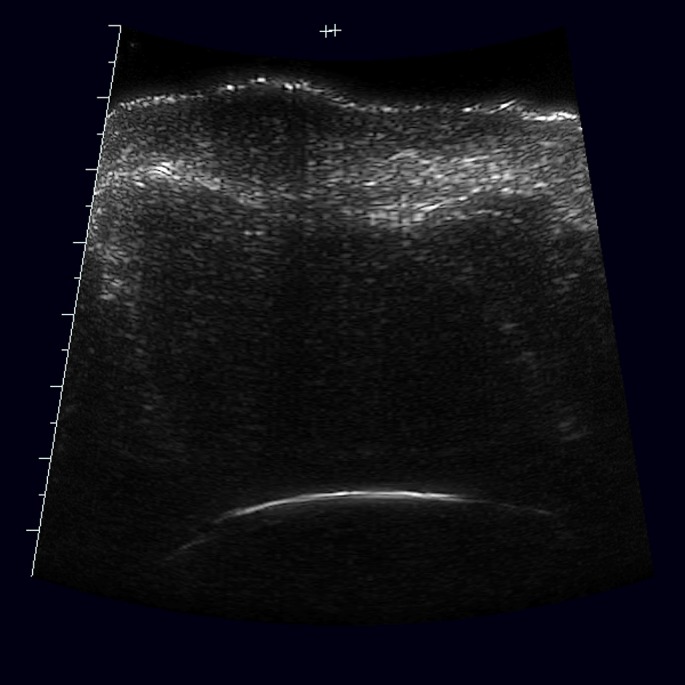
Ultrasound scan of squamous cell carcinoma: hypoechogenic lesion with irregular, but quiet well defined border.
Optical coherent tomography
Optical coherence tomography (OCT) is an emerging technology, which uses infrared light for performing high-resolution cross-sectional imaging. OCT is analogous to ultrasound imaging, except that it uses light instead of sound. OCT systems can generate real-time images of tissue to a depth of about 1.5 mm at a resolution of 10 μm over a field-of-view up to about 5 mm diameter.[51] In dermatology, OCT was introduced in 1995 and is now increasingly used in clinical skin research. It can be used for the investigation of the skin morphology epidermis, dermis, dermo-epidermal junction, hair follicle unit, blood vessels and sweat glands.[52] The method allows preoperative evaluation of the tumor size in patients with SCC.
Recently, a new non-invasive technique, High-Definition Optical Coherence Tomography (HD-OCT) was introduced permitting the combination of horizontal and vertical images, and therefore a real time three-dimensional (3-D) imaging of nonmelanoma skin cancer. This technique provides an exploration depth up to 570 μm and a lateral and axial resolution of 3 μm, thus allowing the visualization of cytological aspects in their micro-architectural context.[53-55]
The presence or absence of an outlined dermo-epidermal junction appeared to be the most powerful criterion to distinguish SCC from AK and normal skin. The absence of the dermo-epidermal junction outline in HD-OCT in SCC lesion appears to be related to irregular budding of the epidermis outstanding into the upper dermis and/or presence of periadenexal collars penetrating through the dermo-epidermal junction. Other features SCC in HD-OCT include hyperkeratosis, parakeratosis, disarranged epidermal architecture on cross-sectional imaging and variability in shape, size and reflective cell in one or more epidermal layers. Moreover, HD-OCT enables the identification of adnexal involvement, based on the presence of the "cocarde image" around the hair follicles.[56]
Table 3 shows typical OCT features of SCC.
Table 3. Optical coherence tomography features of squamous cell carcinoma.56.
| OCT FEATURES OF SCC | |
|---|---|
| absence of an outlined the dermo-epidermal junction | 100% |
| hyperkeratosis, parakeratosis, | 100% |
| disarranged epidermal architecture | 100% |
| the "cocarde image" around the hair follicles | 87% |
Multiphoton tomography
Multiphoton tomography (MPT) employs near-infrared light for multiphoton absorption. There are several endogenous fluorophores in the skin tissue, which can be excited by this process, and their autofluorescence signals can be recorded. The optical resolution of MPT is typically about 0.5 μm in lateral and 1-2 μm in axial direction with a field of view (FOV) of 350 x 350 μm and an imaging depth of 200 μm.[57,58] Klemp et al.[59] in their study proved that MPT can be a valuable non-invasive imaging methode for in vivo detection and discrimination of AK and SCC from health skin. They showed that the cell nuclei of AK and SCC were significantly larger compared to healthy skin cells in all cell layers, and cell density in AK and SCC was significantly lower than in the basal and spinous cell layers of healthy skin. In SCC, the cell density was significantly lower than in AK. The nucleus-cytoplasm ratio was significantly higher for AK and SCC than for the healthy skin cells.[59]
Squamous cell carcinoma of the lips and oral mucosa
Among tumors of the oral cavity, oral squamous cell carcinoma (OSCC) comprises 90% of cases. OSCC is seen typically on the lip or lateral part of the tongue usually as a nodule or ulcer, which is white, red, or whitish-red. The five year survival rate of OSCC has not improved significantly in the last three decades and early detection is still a challenge in clinical practice. The literature consists of few articles about dermoscopy of pigmented lesions of the mucosa and the mucocutaneous junction but none of them refer squamous cell carcinoma.[60,61]
Few emerging techniques may play a role in early detection of SCC of the oral mucosa in future clinical practice.[62,63] Light-based detection systems are chemiluminescence and tissue fluorescence imaging. Tissue fluorescence spectroscopy is being developed on the basis of the observation that changes in the physical and chemical characteristics of malignant tissues caused by sub-cellular architectural changes make it possible to distinguish normal tissues from malignant tissues. Studies have shown that when an ultraviolet or near ultraviolet light source is used a normal oral mucosa emits more green fluorescence than malignant lesions. The sensitivity of fluorescence spectroscopy technologies was estimated to be up to 81% and the specificity up to 100%.[64] Raman spectroscopy is based on the Raman effect that occurs when light impinges on a molecule and interacts with the electron cloud and bonds of that molecule. Raman is being investigated as a diagnostic tool for characterizing cancer cells and early malignant changes and distinguishing these cells from normal cells.[65]
In elastic scattering spectroscopy photons hit tissue and are backscattered without changes in wavelength. The relative intensity of this backscattering is influenced by the composition of the interrogated tissue, specifically the relative concentration of scatterers and absorbers. These method detects changes at the subcellular level and may find application in future differential diagnosis of SCC. The sensitivity of this technique was estimated to be 92% and specificity 60%.[66] Differential path-length spectroscopy is a form of elastic scattering spectroscopy that has fixed photon path length, fixed photon visitation depth and absolute measurement of absorbers. This method gives indirect information about cell biochemistry, intracellular morphology and microvascular properties such as oxygen saturation and average vessel diameter. The sensitivity of this method is 69% and specificity 85%.[67] Nuclear magnetic resonance spectroscopy uses the magnetic properties of certain atomic nuclei to determine the physical and chemical properties of atoms or the molecules in which they are contained. This technique has been used to identify metabolic signatures of oral squamous cell carcinoma compared with normal tissues. Reflectance confocal microscopy has significant potential to non-invasively diagnose SCC of the lip and reachable OSCC. The confocal images of OSCC reveled markedly disorganized epithelium with haphazardly distributed nuclei.[68] Optical tomography uses light scattering to construct an image (optical coherence tomography) or to measures the average size of different cell structures (angle-resolved low coherence interferometry).[62,63] These techniques are being investigated and their potential future value in clinical practice remains unknown.
Squamous cell carcinoma of the vulva
Vulvar cancer accounts for over 5% of gynecological malignancies and for approximately 1% of malignancies in women. The most common vulvar malignancy is squamous cell carcinoma (over 90% of malignant tumors).[71] The literature about application of non-invasive techniques in differential diagnosis of intraepithelial neoplasia and squamous cell carcinoma is sparse and based mainly on case reports. Dermoscopy has been shown to be a helpful diagnostic aid in a case of Bowen's disease of the vulva that progressed to invasive SCC.[72] Optical coherence tomography was used to differentiate epidermal thickness of normal vulva tissue and epidermal thickness of vulvar intraepithelial neoplasia.[73] Literature data show that optical coherent tomography may be applied for determining appropriate surgical margins in patients operated for vulvar squamous cell carcinoma.[74]
Conclusion
Dermoscopy has become a state of the art technique in early diagnosis of squamous cell carcinoma. Other techniques, such as reflectance confocal microscopy, optical coherence tomography or high frequency ultrasonography are being used with increased frequency as a diagnostic tool or for evaluation of tumor size prior to surgery. Other techniques are under pre-clinical development. The rapid progress in noninvasive skin imaging indicates that pre-surgery histopathological evaluation may become history in near future.
Acknowledgments
This publication was supported by the research grant number N N404 517838 from the National Science Centre in Poland.
References
- Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375:673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R. Consensus for Nonmelanoma Skin Cancer Treatment, Part II: Squamous Cell Carcinoma, Including a Cost Analysis of Treatment Methods. Dermatol Surg. 2015;41:1214–1240. doi: 10.1097/DSS.0000000000000478. [DOI] [PubMed] [Google Scholar]
- Röwert-Huber J, Patel MJ, Forschner T, Ulrich C, Eberle J, Kerl H, Sterry W, Stockfleth E. Actinic keratosis is an early in situ squamous cell carcinoma: a proposal for reclassification. Br J Dermatol. 2007;156 Suppl 3:8–12. doi: 10.1111/j.1365-2133.2007.07860.x. [DOI] [PubMed] [Google Scholar]
- Saphier J. Die Dermatoskopie.I. Mitteilung. Arch Dermatolo Syphiol. 1920;128:1–19. [Google Scholar]
- Friedman RJ, Rigel DS, Silverman MK, Kopf AW, Vossaert KA. Malignant melanoma in the 1990s: the continued importance of early detection and the role of physician examination and self-examination of the skin. CA Cancer J Clin. 1991;41:201–226. doi: 10.3322/canjclin.41.4.201. [DOI] [PubMed] [Google Scholar]
- Soyer HP, Smolle J, Hödl S, Pachernegg H, Kerl H. Surface microscopy. A new approach to the diagnosis of cutaneous pigmented tumors. Am J Dermatopathol. 1989;11:1–10. [PubMed] [Google Scholar]
- Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571–583. doi: 10.1016/s0190-9622(87)70239-4. [DOI] [PubMed] [Google Scholar]
- Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669–676. doi: 10.1111/j.1365-2133.2008.08713.x. [DOI] [PubMed] [Google Scholar]
- Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343–1350. doi: 10.1001/archderm.137.10.1343. [DOI] [PubMed] [Google Scholar]
- Warshaw EM, Lederle FA, Grill JP, Gravely AA, Bangerter AK, Fortier LA, Bohjanen KA, Chen K, Lee PK, Rabinovitz HS, Johr RH, Kaye VN, Bowers S, Wenner R, Askari SK, Kedrowski DA, Nelson DB. Accuracy of teledermatology for pigmented neoplasms. J Am Acad Dermatol. 2009;61:753–765. doi: 10.1016/j.jaad.2009.04.032. [DOI] [PubMed] [Google Scholar]
- Benvenuto-Andrade C, Dusza SW, Agero AL, Scope A, Rajadhyaksha M, Halpern AC, Marghoob AA. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329–338. doi: 10.1001/archderm.143.3.329. [DOI] [PubMed] [Google Scholar]
- Cuellar F, Vilalta A, Puig S, Palou J, Salerni G, Malvehy J. New dermoscopic pattern in actinic keratosis and related conditions. Arch Dermatol. 2009;145:732. doi: 10.1001/archdermatol.2009.86. [DOI] [PubMed] [Google Scholar]
- Liebman TN, Rabinovitz HS, Dusza SW, Marghoob AA. White shiny structures: dermoscopic features revealed under polarized light. J Eur Acad Dermatol Venereol. 2012;26:1493–1497. doi: 10.1111/j.1468-3083.2011.04317.x. [DOI] [PubMed] [Google Scholar]
- Zalaudek I, Argenziano G. Dermoscopy of actinic keratosis, intraepidermal carcinoma and squamous cell carcinoma. Curr Probl Dermatol. 2015;46:70–76. doi: 10.1159/000366539. [DOI] [PubMed] [Google Scholar]
- Zalaudek I, Argenziano G, Leinweber B, Citarella L, Hofmann-Wellenhof R, Malvehy J, Puig S, Pizzichetta MA, Thomas L, Soyer HP, Kerl H. Dermoscopy of Bowen's disease. Br J Dermatol. 2004;150:1112–1116. doi: 10.1111/j.1365-2133.2004.05924.x. [DOI] [PubMed] [Google Scholar]
- Zalaudek I, Di Stefani A, Argenziano G. The specific dermoscopic criteria of Bowen's disease. J Eur Acad Dermatol Venereol. 2006;20:361–362. doi: 10.1111/j.1468-3083.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- Marghoob AA, Braun RP, Kopf AW. Re: Differentiating vessels from globules on dermoscopy. Dermatol Surg. 2005;31:120. [PubMed] [Google Scholar]
- Bugatti L, Filosa G, De Angelis R. The specific dermoscopical criteria of Bowen's disease. J Eur Acad Dermatol Venereol. 2007;21:700–701. doi: 10.1111/j.1468-3083.2006.01995.x. [DOI] [PubMed] [Google Scholar]
- Pan Y, Chamberlain AJ, Bailey M, Chong AH, Haskett M, Kelly JW. Dermatoscopy aids in the diagnosis of the solitary red scaly patch or plaque-features distinguishing superficial basal cell carcinoma, intraepidermal carcinoma, and psoriasis. J Am Acad Dermatol. 2008;59:268–274. doi: 10.1016/j.jaad.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Argenziano G, Zalaudek I, Corona R, Sera F, Cicale L, Petrillo G, Ruocco E, Hofmann-Wellenhof R, Soyer HP. Vascular structures in skin tumors: a dermoscopy study. Arch Dermatol. 2004;140:1485–1489. doi: 10.1001/archderm.140.12.1485. [DOI] [PubMed] [Google Scholar]
- Kreusch JF. Vascular patterns in skin tumors. Clin Dermatol. 2002;20:248–254. doi: 10.1016/s0738-081x(02)00227-4. [DOI] [PubMed] [Google Scholar]
- Felder S, Rabinovitz H, Oliviero M, Kopf A. Dermoscopic differentiation of a superficial basal cell carcinoma and squamous cell carcinoma in situ. Dermatol Surg. 2006;32:423–425. doi: 10.1111/j.1524-4725.2006.32085.x. [DOI] [PubMed] [Google Scholar]
- Lallas A, Pyne J, Kyrgidis A, Andreani S, Argenziano G, Cavaller A, Giacomel J, Longo C, Malvestiti A, Moscarella E, Piana S, Specchio F, Hofmann-Wellenhof R, Zalaudek I. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br J Dermatol. 2015;172:1308–1315. doi: 10.1111/bjd.13510. [DOI] [PubMed] [Google Scholar]
- Rosendahl C, Cameron A, Argenziano G, Zalaudek I, Tschandl P, Kittler H. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Arch Dermatol. 2012;148:1386–1392. doi: 10.1001/archdermatol.2012.2974. [DOI] [PubMed] [Google Scholar]
- Lin MJ, Pan Y, Jalilian C, Kelly JW. Dermoscopic characteristics of nodular squamous cell carcinoma and keratoacanthoma. Dermatol Pract Concept. 2014;4:9–15. doi: 10.5826/dpc.0402a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalaudek I, Giacomel J, Schmid K, Bondino S, Rosendahl C, Cavicchini S, Tourlaki A, Gasparini S, Bourne P, Keir J, Kittler H, Eibenschutz L, Catricalà C, Argenziano G. Dermatoscopy of facial actinic keratosis, intraepidermal carcinoma, and invasive squamous cell carcinoma: a progression model. J Am Acad Dermatol. 2012;66:589–597. doi: 10.1016/j.jaad.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Ulrich M, Lange-Asschenfeldt S. In vivo confocal microscopy in dermatology: from research to clinical application. J Biomed Opt. 2013;18:061212. doi: 10.1117/1.JBO.18.6.061212. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946–952. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- Hofmann-Wellenhof R, Wurm EM, Ahlgrimm-Siess V, Richtig E, Koller S, Smolle J, Gerger A. Reflectance confocal microscopy -- state-of-art and research overview. Semin Cutan Med Surg. 2009;28:172–179. doi: 10.1016/j.sder.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Que SK, Fraga-Braghiroli N, Grant-Kels JM, Rabinovitz HS, Oliviero M, Scope A. Through the looking glass: Basics and principles of reflectance confocal microscopy. J Am Acad Dermatol. 2015;73:276–284. doi: 10.1016/j.jaad.2015.04.047. [DOI] [PubMed] [Google Scholar]
- Carrera C, Puig S, Malvehy J. In vivo confocal reflectance microscopy in melanoma. Dermatol Ther. 2012;25:410–422. doi: 10.1111/j.1529-8019.2012.01495.x. [DOI] [PubMed] [Google Scholar]
- Carrera C, Palou J, Malvehy J, Segura S, Aguilera P, Salerni G, Lovatto L, Puig-Butillé J, Alós L, Puig S. Early stages of melanoma on the limbs of high-risk patients: clinical, dermoscopic, reflectance confocal microscopy and histopathological characterization for improved recognition. Acta Derm Venereol. 2011;91:137–146. doi: 10.2340/00015555-1021. [DOI] [PubMed] [Google Scholar]
- Guitera P, Menzies SW, Longo C, Cesinaro AM, Scolyer RA, Pellacani G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J Invest Dermatol. 2012;132:2386–2394. doi: 10.1038/jid.2012.172. [DOI] [PubMed] [Google Scholar]
- Astner S, González S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182–191. doi: 10.2310/6620.2006.05052. [DOI] [PubMed] [Google Scholar]
- Ardigo M, Donadio C, Franceschini C, Catricalà C, Agozzino M. Interest of reflectance confocal microscopy for inflammatory oral mucosal diseases. J Eur Acad Dermatol Venereol. 2015;29:1850–1853. doi: 10.1111/jdv.12540. [DOI] [PubMed] [Google Scholar]
- Kurzeja M, Czuwara J, Rakowska A, Sicińska J, Maj M, Nasierowska-Guttmejer A, Rudnicka L, Olszewska M. Reflectance confocal microscopy as a non-invasive diagnostic tool for Hailey-Hailey disease. Skin Res Technol. 2014;20:503–509. doi: 10.1111/srt.12146. [DOI] [PubMed] [Google Scholar]
- Kurzeja M, Rakowska A, Rudnicka L, Olszewska M. Criteria for diagnosing pemphigus vulgaris and pemphigus foliaceus by reflectance confocal microscopy. Skin Res Technol. 2012;18:339–346. doi: 10.1111/j.1600-0846.2011.00574.x. [DOI] [PubMed] [Google Scholar]
- Venturini M, Sala R, Semenza D, Santoro A, Facchetti F, Calzavara-Pinton P. Reflectance confocal microscopy for the in vivo detection of Treponema pallidum in skin lesions of secondary syphilis. J Am Acad Dermatol. 2009;60:639–642. doi: 10.1016/j.jaad.2008.11.901. [DOI] [PubMed] [Google Scholar]
- Rishpon A, Kim N, Scope A, Porges L, Oliviero MC, Braun RP, Marghoob AA, Fox CA, Rabinovitz HS. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol. 2009;145:766–772. doi: 10.1001/archdermatol.2009.134. [DOI] [PubMed] [Google Scholar]
- Ulrich M, Kanitakis J, González S, Lange-Asschenfeldt S, Stockfleth E, Roewert-Huber J. Evaluation of Bowen disease by in vivo reflectance confocal microscopy. Br J Dermatol. 2012;166:451–453. doi: 10.1111/j.1365-2133.2011.10563.x. [DOI] [PubMed] [Google Scholar]
- Peppelman M, Nguyen KP, Hoogedoorn L, van Erp PE, Gerritsen MJ. Reflectance confocal microscopy: non-invasive distinction between actinic keratosis and squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2015;29:1302–1309. doi: 10.1111/jdv.12806. [DOI] [PubMed] [Google Scholar]
- Jasaitiene D, Valiukeviciene S, Linkeviciute G, Raisutis R, Jasiuniene E, Kazys R. Principles of high-frequency ultrasonography for investigation of skin pathology. J Eur Acad Dermatol Venereol. 2011;25:375–382. doi: 10.1111/j.1468-3083.2010.03837.x. [DOI] [PubMed] [Google Scholar]
- Desai TD, Desai AD, Horowitz DC, Kartono F, Wahl T. The use of high-frequency ultrasound in the evaluation of superficial and nodular basal cell carcinomas. Dermatol Surg. 2007;33:1220-1227; discussion 1226-1227. [DOI] [PubMed] [Google Scholar]
- Gambichler T, Moussa G, Bahrenberg K, Vogt M, Ermert H, Weyhe D, Altmeyer P, Hoffmann K. Preoperative ultrasonic assessment of thin melanocytic skin lesions using a 100-MHz ultrasound transducer: a comparative study. Dermatol Surg. 2007;33:818–824. doi: 10.1111/j.1524-4725.2007.33175.x. [DOI] [PubMed] [Google Scholar]
- Mogensen M, Nürnberg BM, Forman JL, Thomsen JB, Thrane L, Jemec GB. In vivo thickness measurement of basal cell carcinoma and actinic keratosis with optical coherence tomography and 20-MHz ultrasound. Br J Dermatol. 2009;160:1026–1033. doi: 10.1111/j.1365-2133.2008.09003.x. [DOI] [PubMed] [Google Scholar]
- Maj M, Warszawik-Hendzel O, Szymanska E, Walecka I, Rakowska A, Antczak-Marczak M, Kuna P, Kruszewski J, Nasierowska-Guttmejer A, Litniewski J, Nowicki A, Olszewska M, Rudnicka L. High frequency ultrasonography: a complementary diagnostic method in evaluation of primary cutaneous melanoma. G Ital Dermatol Venereol. 2015;150:595–601. [PubMed] [Google Scholar]
- Crisan M, Crisan D, Sannino G, Lupsor M, Badea R, Amzica F. Ultrasonographic staging of cutaneous malignant tumors: an ultrasonographic depth index. Arch Dermatol Res. 2013;305:305–313. doi: 10.1007/s00403-013-1321-1. [DOI] [PubMed] [Google Scholar]
- Guitera P, Li LX, Crotty K, Fitzgerald P, Mellenbergh R, Pellacani G, Menzies SW. Melanoma histological Breslow thickness predicted by 75-MHz ultrasonography. Br J Dermatol. 2008;159:364–369. doi: 10.1111/j.1365-2133.2008.08681.x. [DOI] [PubMed] [Google Scholar]
- Wortsman X, Wortsman J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J Am Acad Dermatol. 2010;62:247–256. doi: 10.1016/j.jaad.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Fujimoto JG, Pitris C, Boppart SA, Brezinski ME. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia. 2000;2:9–25. doi: 10.1038/sj.neo.7900071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457–473. doi: 10.1007/s00403-011-1152-x. [DOI] [PubMed] [Google Scholar]
- Boone MA, Norrenberg S, Jemec GB, Del Marmol V. Imaging actinic keratosis by high-definition optical coherence tomography. Histomorphologic correlation: a pilot study. Exp Dermatol. 2013;22:93–97. doi: 10.1111/exd.12074. [DOI] [PubMed] [Google Scholar]
- Boone M, Jemec GB, Del Marmol V. High-definition optical coherence tomography enables visualization of individual cells in healthy skin: comparison to reflectance confocal microscopy. Exp Dermatol. 2012;21:740–744. doi: 10.1111/j.1600-0625.2012.01569.x. [DOI] [PubMed] [Google Scholar]
- Boone MA, Norrenberg S, Jemec GB, Del Marmol V. Imaging of basal cell carcinoma by high-definition optical coherence tomography: histomorphological correlation. A pilot study. Br J Dermatol. 2012;167:856–864. doi: 10.1111/j.1365-2133.2012.11194.x. [DOI] [PubMed] [Google Scholar]
- Boone MA, Marneffe A, Suppa M, Miyamoto M, Alarcon I, Hofmann-Wellenhof R, Malvehy J, Pellacani G, Del Marmol V. High-definition optical coherence tomography algorithm for the discrimination of actinic keratosis from normal skin and from squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2015;29:1606–1615. doi: 10.1111/jdv.12954. [DOI] [PubMed] [Google Scholar]
- König K. Clinical multiphoton tomography. J Biophotonics. 2008;1:13–23. doi: 10.1002/jbio.200710022. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Choe CS, Ahlberg S, Meinke MC, Alexiev U, Lademann J, Darvin ME. Penetration of silver nanoparticles into porcine skin ex vivo using fluorescence lifetime imaging microscopy, Raman microscopy, and surface-enhanced Raman scattering microscopy. J Biomed Opt. 2015;20:051006. doi: 10.1117/1.JBO.20.5.051006. [DOI] [PubMed] [Google Scholar]
- Klemp M, Meinke MC, Weinigel M, Röwert-Huber J, König K, Ulrich M, Lademann J, Darvin ME. Comparison of morphologic criteria for actinic keratosis and squamous cell carcinoma using in vivo multiphoton tomography. Exp Dermatol. 2015 Dec 14. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Blum A, Simionescu O, Argenziano G, Braun R, Cabo H, Eichhorn A, Kirchesch H, Malvehy J, Marghoob AA, Puig S, Ozdemir F, Stolz W, Tromme I, Weigert U, Wolf IH, Zalaudek I, Kittler H. Dermoscopy of pigmented lesions of the mucosa and the mucocutaneous junction: results of a multicenter study by the International Dermoscopy Society (IDS) Arch Dermatol. 2011;147:1181–1187. doi: 10.1001/archdermatol.2011.155. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Buccini P, Covello R, De Simone P, Silipo V, Mariani G, Eibenschutz L, Mariani L, Catricalà C. The ringlike pattern in vulvar melanosis: a new dermoscopic clue for diagnosis. Arch Dermatol. 2008;144:1030–1034. doi: 10.1001/archderm.144.8.1030. [DOI] [PubMed] [Google Scholar]
- Omar E. Future Imaging Alternatives: The Clinical Non-invasive Modalities in Diagnosis of Oral Squamous Cell Carcinoma (OSCC) Open Dent J. 2015;9:311–318. doi: 10.2174/1874210601509010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K, Connolly JM, Dockery P, Wheatley AM, Olivo M, Keogh I. Point of care optical diagnostic technologies for the detection of oral and oropharyngeal squamous cell carcinoma. Surgeon. 2015;13:321–329. doi: 10.1016/j.surge.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Kulbersh BD, Duncan RD, Magnuson JS, Skipper JB, Zinn K, Rosenthal EL. Sensitivity and specificity of fluorescent immunoguided neoplasm detection in head and neck cancer xenografts. Arch Otolaryngol Head Neck Surg. 2007;133:511–515. doi: 10.1001/archotol.133.5.511. [DOI] [PubMed] [Google Scholar]
- Duraipandian S, Sylvest Bergholt M, Zheng W, Yu Ho K, Teh M, Guan Yeoh K, Bok Yan So J, Shabbir A, Huang Z. Real-time Raman spectroscopy for in vivo, online gastric cancer diagnosis during clinical endoscopic examination. J Biomed Opt. 2012;17:081418. doi: 10.1117/1.JBO.17.8.081418. [DOI] [PubMed] [Google Scholar]
- Lovat LB, Johnson K, Mackenzie GD, Clark BR, Novelli MR, Davies S, O'Donovan M, Selvasekar C, Thorpe SM, Pickard D, Fitzgerald R, Fearn T, Bigio I, Bown SG. Elastic scattering spectroscopy accurately detects high grade dysplasia and cancer in Barrett's oesophagus. Gut. 2006;55:1078–1083. doi: 10.1136/gut.2005.081497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax A, Terry NG, Dellon ES, Shaheen NJ. Angle-resolved low coherence interferometry for detection of dysplasia in Barrett's esophagus. Gastroenterology. 2011;141:443-447, 447.e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland KC, Gillenwater AM, Williams MD, El-Naggar AK, Descour MR, Richards-Kortum RR. In vivo imaging of oral neoplasia using a miniaturized fiber optic confocal reflectance microscope. Oral Oncol. 2008;44:1059–1066. doi: 10.1016/j.oraloncology.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A, Rosendahl C, Tschandl P, Riedl E, Kittler H. Dermatoscopy of pigmented Bowen's disease. J Am Acad Dermatol. 2010;62:597–604. doi: 10.1016/j.jaad.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Bugatti L, Filosa G, De Angelis R. Dermoscopic observation of Bowen's disease. J Eur Acad Dermatol Venereol. 2004;18:572–574. doi: 10.1111/j.1468-3083.2004.01008.x. [DOI] [PubMed] [Google Scholar]
- Alkatout I, Schubert M, Garbrecht N, Weigel MT, Jonat W, Mundhenke C, Günther V. Vulvar cancer: epidemiology, clinical presentation, and management options. Int J Womens Health. 2015;7:305-313. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridou E, Fotiadou C, Giannopoulou C, Ioannides D. Persistent erythematous lesion of the vulva: a diagnostic and treatment challenge. J Drugs Dermatol. 2012;11:110–112. [PubMed] [Google Scholar]
- Wessels R, de Bruin DM, Faber DJ, van Boven HH, Vincent AD, van Leeuwen TG, van Beurden M, Ruers TJ. Optical coherence tomography in vulvar intraepithelial neoplasia. J Biomed Opt. 2012;17:116022. doi: 10.1117/1.jbo.17.11.116022. [DOI] [PubMed] [Google Scholar]
- Wessels R, van Beurden M, de Bruin DM, Faber DJ, Vincent AD, Sanders J, van Leeuwen TG, Ruers TJ. The value of optical coherence tomography in determining surgical margins in squamous cell carcinoma of the vulva: a single-center prospective study. Int J Gynecol Cancer. 2015;25:112–118. doi: 10.1097/IGC.0000000000000310. [DOI] [PubMed] [Google Scholar]