Abstract
Study Design Retrospective review.
Objective To describe the surgical outcomes in patients with high preoperative Spinal Instability Neoplastic Score (SINS) secondary to spinal giant cell tumors (GCT) and evaluate the impact of en bloc versus intralesional resection and preoperative embolization on postoperative outcomes.
Methods A retrospective analysis was performed on 14 patients with GCTs of the spine who underwent surgical treatment prior to the use of denosumab. A univariate analysis was performed comparing the patient demographics, perioperative characteristics, and surgical outcomes between patients who underwent en bloc marginal (n = 6) compared with those who had intralesional (n = 8) resection.
Results Six patients underwent en bloc resections and eight underwent intralesional resection. Preoperative embolization was performed in eight patients. All patients were alive at last follow-up, with a mean follow-up length of 43 months. Patients who underwent en bloc resection had longer average operative times (p = 0.0251), higher rates of early (p = 0.0182) and late (p = 0.0389) complications, and a higher rate of surgical revision (p = 0.0120). There was a 25% (2/8 patients) local recurrence rate for intralesional resection and a 0% (0/6 patients) local recurrence rate for en bloc resection (p = 0.0929).
Conclusions Surgical excision of spinal GCTs causing significant instability, assessed by SINS, is associated with high intraoperative blood loss despite embolization and independent of resection method. En bloc resection requires a longer operative duration and is associated with a higher risk of complications when compared with intralesional resection. However, the increased morbidity associated with en bloc resection may be justified as it may minimize the risk of local recurrence.
Keywords: embolization, en bloc, Enneking class, giant cell tumor, intralesional, SINS score, spine
Introduction
Giant cell tumors (GCT) are relatively uncommon benign, osseous lesions that represent ∼5% of all adult primary bone tumors and most often arise near the articulations of long bones.1 GCTs are rarely found in the spine. Although there is a predilection for the sacrum,2 3 spinal GCTs of the mobile spine are much less common, representing only 1.9 to 9.4% of cases.4 The peak incidence of GCT occurs between 30 to 40 years of age,3 with lesions of the mobile spine occurring at a slightly younger age than GCTs of long bones.4 Patients with spinal GCT typically present with back pain, but the symptoms of spinal cord and nerve root compression may also occur with large lesions.1
Spinal instability may result from significant bony destruction due to the osteolytic nature of GCTs leading to pathologic fracture, subsequently increasing a patients' risk for debilitating pain, neurologic compromise, and progressive deformity.5 6 The Spinal Instability Neoplastic Score (SINS) has been increasingly utilized to assist spine surgeons in making decisions regarding the most appropriate management for metastatic spinal lesions; yet, no study to date has assessed its utility in patients with spinal GCT. Nevertheless, use of the SINS may provide further rationale for surgical resection and stabilization in patients with instability secondary to spinal GCT.
Despite their benign pathology, GCTs are locally aggressive osteolytic tumors that have a high propensity for recurrence.1 As such, extensive surgical resection is the treatment of choice for almost all cases, with many authors supporting the use of en bloc resection to decrease the risk of recurrence.1 2 4 7 However, the benefits of en bloc resection must be weighed against the associated risk of extensive intraoperative bleeding and postoperative morbidity.2 4 8 In cases that are not amenable to en bloc resection, radiation therapy, and more recently the anti-RANKL monoclonal antibody, denosumab, are used as adjunctive therapies to surgery.1 2 5 9
In addition, preoperative embolization is a safe and effective technique that can be utilized to reduce the significant blood loss associated with excision of these highly vascular tumors.2 10 Embolization also can help reduce operative times by decreasing the amount of time spent managing intraoperative hemorrhage.1 Likewise, preoperative embolization can improve visualization, potentially increasing the likelihood of maximal tumor resection.10 However, despite its use, intraoperative blood loss can remain significant.
In the present study, we describe the surgical outcomes in patients with high preoperative SINS secondary to spinal GCT and evaluate the impact of en bloc versus intralesional resection and use of preoperative embolization on the postoperative outcomes.
Methods
Patient Characteristics
Under an active Institutional Review Board–approved protocol (NA_00067508), a single-institution, retrospective cohort review of 14 patients who underwent surgery between 2005 and 2011 for spinal GCT was performed. Demographic information was obtained, including gender, age at diagnosis, history of previous spine tumor surgery, history of chemoradiation, presenting symptoms/signs, symptom duration, and method of diagnosis. The Frankel classification was used to determine patients' preoperative functional status, and the SINS was used to evaluate the degree of spinal instability. Additional information regarding the GCT was determined such as the tumor location, preoperative tumor volume, Enneking classification, and evidence of pathologic vertebral body fracture. Perioperative data was collected concerning the operative duration, use of preoperative embolization, intraoperative blood loss, transfusion requirements, surgical approach, procedures performed, intraoperative pathology findings, and intraoperative complications. Postoperative information was also reviewed, including length of stay, postoperative neurologic status, postoperative complications, local recurrence, use of adjuvant chemoradiation, need for revision, clinical status at last follow-up, follow-up duration, and mortality.
Choice of Surgical Approach, Procedures, and Staging
The approach used for resection, decompression, and/or stabilization was determined at the discretion of the treating neurosurgeon. Each surgeon's surgical approach and choice of procedures were based on the patients' overall medical and neurologic condition; the size and location of the primary lesion; evidence of ventral, paraspinal, and/or lateral tumor extension; evidence of spinal cord and/or nerve root compression; and spinal instability resulting from pathologic vertebral body collapse. Staged procedures were performed in patients who required a separate anterior and posterior approach for adequate correction of their disease. En bloc resection was performed when anatomically feasible. Intralesional resection was performed if the patient had a prior surgical decompression and/or biopsy at an outside institution, or if it was not anatomically feasible.
Statistical Analysis
Quantitative data is described as frequency (percentage) for categorical data, and mean (standard error) was used for continuous data. The Student unpaired t test was used to compare groups for nonparametric, continuous data, and the chi-square test was used to compare categorical data. GraphPad Prism (La Jolla, California, United States) software was used to perform all statistical analysis. A two-tailed p value was used to compare preoperative characteristics, and a one-tailed test was used for comparison of outcomes. Statistical significance was determined by a p value of <0.05.
Results
Demographics and Clinical Presentation
Over a 6-year period, 8 (57%) female and 6 (43%) male patients with a primary spinal GCT underwent surgical resection at our institution. The mean age at the time of diagnosis was 40 (±4) years. The majority of patients (n = 13, 93%) presented with back pain, at a mean of 10 (±3) months after symptom onset. In addition, 3 (21%) patients displayed signs of myelopathy and 4 (29%) patients had evidence of cauda equina syndrome prior to surgery. Several patients had mild neurologic impairment preoperatively, with Frankel classification of either D (n = 7, 50%) or E (n = 7, 50%) prior to surgery. Each patient's clinical synopsis is outlined in Table 1.
Table 1. Individual patient treatment summaries.
| Patient no. | Age (y) | Sex | Tumor location | SINS | Prior treatment | Surgical approach | Instrumented levels | Complications | Follow-up duration (mo) | Recurrence | Mortality by LFU | Sarcoma transformation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | M | T12 | 11 | Embo | En bloc marginal | T10–L2 | Large (2L) pleural effusion, rod fracture | 84 | No | No | No |
| 2 | 31 | F | L4 | 9 | None | En bloc marginal | L4 cage, L2–pelvis | None | 30 | No | No | No |
| 3 | 58 | F | L4–L5 | 14 | Embo | En bloc marginal | L4–L5 cage/plate, L2–pelvis | Wound dehiscence (×2), CSF leak, delayed hardware failure | 51 | No | No | No |
| 4 | 35 | F | L5 | 11 | Embo | En bloc marginal | L5 cage/plate, L3–pelvis | Wound dehiscence | 103 | No | No | No |
| 5 | 42 | M | S1–S3 | 10 | None | En bloc marginal | L3–pelvis | Small bowel perforation, peritonitis, hypotension requiring vasopressors | 51 | No | No | No |
| 6 | 45 | F | S1–S3 | 10 | Embo | En bloc marginal | L4–pelvis | None | 0.5 | No | No | No |
| 7 | 62 | F | C4 | 15 | None | Intralesional | C4 cage, C3–C5 plate, C3–C6 posterior cervical fusion | None | 3 | Yes, 4 mo after surgery | No | No |
| 8 | 31 | F | C6 | 9 | Embo | Intralesional | C5–C7 plate with structural allograft | None | 21 | No | No | No |
| 9 | 64 | F | T11 | 11 | T10–T12 hemilaminectomy with biopsy | Intralesional | T11 cage, T8–L1 | Durotomy, pleural effusion, PE/DVT, superficial wound dehiscence | 1.5 | No | No | No |
| 10 | 47 | M | L5 | 12 | Open biopsy | Intralesional | L5 PMMA VBR, L4–S1 | None | 79 | No | No | No |
| 11 | 29 | M | S1–S2 | 10 | Embo | Intralesional | L3–pelvis | None | 0.5 | No | No | No |
| 12 | 21 | F | S1–S3 | 8 | None | Intralesional | L3–pelvis | None | 7 | No | No | No |
| 13 | 26 | M | S1–S3 | 10 | Embo | Intralesional | L3–pelvis | None | 85 | No | No | No |
| 14 | 39 | M | S2–S3 | 6 | Embo | Intralesional | None | None | 81 | Yes, 6 and 9 mo after surgery | No | No |
Abbreviations: CSF, cerebrospinal fluid; DVT, deep venous thrombosis; Embo, embolization; LFU, last follow-up; PE, pulmonary embolism; PMMA, polymethyl methacrylate; SINS, Spinal Instability Neoplastic Score; VBR, vertebral body replacement.
Tumor Characteristics and Tumor Burden
Baseline demographic information and preoperative tumor characteristics are presented in Table 2. All patients had a histologic diagnosis of giant cell tumor, determined via computed tomography–guided core biopsy in 12 (86%) patients and open biopsy in 2 (14%) patients. Mean preoperative tumor volume was 81.7 (45.2) cm3. Five (36%) patients had an Enneking stage II tumor and 9 (64%) patients had an Enneking stage III tumor. The lesions were located in the cervical (n = 2), thoracic (n = 2), lumbar (n = 4), and sacral (n = 6) spine. Osteolytic destruction secondary to GCT resulted in pathologic vertebral body fracture in 6 (43%) patients. Mean SINS score was 10 (±1), classified as potentially unstable (n = 12) or unstable (n = 2) warranting surgical management. Patients with Enneking stage III tumors had a higher chance of preoperative instability (p = 0.0198), assessed by the SINS, and greater length of stay (p = 0.0330) than patients with Enneking stage II tumors.
Table 2. Baseline demographic information and preoperative characteristics.
| Characteristics | All patients (n = 14) | En bloc (n = 6) | Intralesional (n = 8) | p Value |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age at diagnosis, y (SEM) | 40 (4) | 40 (4) | 40 (6) | 0.9382 |
| Sex, female, n (%) | 8 (57) | 4 (67) | 4 (50) | 0.5329 |
| Symptoms/signs at presentation, n (%) | ||||
| Back pain | 13 (93) | 6 (100) | 7 (88) | 0.3688 |
| Myelopathy | 3 (21) | 0 (0) | 3 (38) | 0.0906 |
| Cauda equina | 4 (29) | 2 (33) | 2 (25) | 0.7327 |
| Duration of preoperative symptoms, mo (SEM) | 10 (3) | 8 (3) | 13 (5) | 0.4315 |
| Preoperative Frankel class | 0.2801 | |||
| D | 7 (50) | 2 (33) | 5 (63) | |
| E | 7 (50) | 4 (67) | 3 (38) | |
| History of spine tumor surgery, n (%) | 1 (7) | 0 (0) | 1 (13) | 0.3688 |
| History of chemotherapy, n (%) | 1 (7) | 1 (17) | 0 (0) | 0.2308 |
| History of radiotherapy, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Preoperative embolization, n (%) | 8 (57) | 4 (67) | 4 (50) | 0.5329 |
| Tumor characteristics | ||||
| Spine level, n (%) | ||||
| Cervical | 2 (14) | 0 (0) | 2 (25) | 0.1859 |
| Thoracic | 2 (14) | 1 (17) | 1 (13) | 0.8255 |
| Lumbar | 4 (29) | 3 (50) | 1 (13) | 0.1243 |
| Sacral | 6 (43) | 2 (33) | 4 (50) | 0.5329 |
| Pathologic fracture, n (%) | 6 (43) | 2 (33) | 4 (50) | 0.5329 |
| Preoperative SINS score, mean (SEM) | 10 (1) | 11 (1) | 10 (1) | 0.5855 |
| Enneking classification stage | 0.8721 | |||
| II | 5 (36) | 2 (33) | 3 (38) | |
| III | 9 (64) | 4 (67) | 5 (63) | |
| Tumor volume, cm3 (SEM) | 81.7 (45.2) | 142.1 (104.2) | 36.3 (11.8) | 0.2626 |
Abbreviations: SINS, Spinal Instability Neoplastic Score; SEM, standard error of the mean.
Adjuvant Therapies
One (7%) patient had a history of previous T10–T12 hemilaminectomy with biopsy for GCT. Another (7%) patient had a history of chemotherapy, and no (0%) patient received neoadjuvant radiation to the spine. Eight (57%) patients were successfully treated with preoperative embolization in an effort to reduce the risk of bleeding during surgery. Embolization was attempted in four additional patients, but was unsuccessful due to the absence of an available vessel, or direct supply to the anterior spinal artery. Two (14%) patients received postoperative radiation to the spine.
Operative Information
A summary of the operative characteristics is presented in Table 3. GCTs were resected via an anterior approach in 1 (7%) case, posterior approach in 7 (50%) cases, combined anterior-posterior approach in 2 (14%) cases, and combined posterior-anterior approach in 4 (29%) cases. Surgery was staged into two separate operations in all posterior-anterior approach cases. En bloc marginal resection was performed in 6 (43%) cases. Intralesional resection was performed in 8 (57%) cases, with subtotal resection achieved in 1 (7%) case and gross total resection achieved in 7 (50%) cases. The risk of operative morbidity associated with en bloc resection was considered too high in patients 7 and 8, due to the proximity of the lesion to important neurovascular structures in the cervical spine and tumor encasement of the vertebral artery in patient 8. Likewise, patients 9 and 10 underwent intralesional resection due to a history of prior decompression and/or biopsy at an outside institution, precluding en bloc resection. Patients 11, 12, and 14 underwent intralesional resection due to an epidural extension of the tumor with compression of the cauda equina and a desire to maintain neurologic function. En bloc resection was attempted in patient 13; however, a conversion to posterior intralesional resection was made intraoperatively after discovering that the tumor did not have a favorable capsule to allow for complete removal without tumor disintegration.
Table 3. Operative characteristics.
| Perioperative characteristics | All patients (n = 14) | En bloc (n = 6) | Intralesional (n = 8) | p Value |
|---|---|---|---|---|
| Surgical approach, n (%) | ||||
| Anterior | 1 (7) | 0 (0) | 1 (13) | – |
| Posterior | 7 (50) | 2 (33) | 5 (63) | – |
| Anterior-posterior | 2 (14) | 1 (17) | 1 (13) | – |
| Posterior-anterior | 4 (29) | 3 (50) | 1 (13) | – |
| Staged operation, n (%) | 4 (29) | 3 (50)) | 1 (13) | – |
| Instrumented reconstruction, n (%) | 13 (93) | 6 (100) | 7 (88) | – |
| Nerve root sacrifice, n (%) | 2 (14) | 2 (33) | 0 (0) | – |
| Intraoperative blood loss, cm3 (SEM) | 2,885 (756) | 3,663 (1516) | 2,367 (817) | 0.2692 |
| Required blood transfusion, n (%) | 12 (86) | 6 (100) | 6 (75) | 0.0929 |
| Total amount of transfusion, U (SEM) | 4 (1) | 5 (1) | 2 (1) | 0.0587 |
| Operative duration, h (SEM) | 9.9 (0.8) | 11.6 (1.1) | 8.5 (0.9) | 0.0251 |
Abbreviation: SEM, standard error of the mean.
The diagnosis of GCT was confirmed via intraoperative pathology in all cases. Instrumental spinal reconstruction was performed in 13 (93%) cases. Bilateral nerve roots were sacrificed in 2 (14%) cases. Cordectomy or sacrifice of the cauda equina was not performed in any case. The mean intraoperative blood loss was 2,885 (756) mL, requiring a mean of 4 (±1) U of blood for repletion, and the mean operative time was 9.9 (±1) hours. Intraoperative durotomies were encountered in 2 patients.
Surgical Outcomes
A summary of the surgical outcomes is presented in Table 4. The mean length of hospital stay was 13 (±3) days after surgery. Early (<30 days) complications were encountered in 5 (31%) patients, and late (>30 days) complications secondary to hardware failure occurred in 2 (14%) patients. Early (<30 days) complications included wound dehiscence (n = 4), cerebrospinal fluid leak (n = 2), large pleural effusion (n = 2), small bowl perforation, peritonitis, venothromboembolism, and hypotension requiring vasopressor therapy. Three (21%) patients required surgical revision after en bloc resection. One patient underwent two subsequent surgeries, the first for wound dehiscence and cerebrospinal fluid leak repair and the second for hardware failure. Another patient underwent a wound revision 3 weeks postoperatively due to a wound dehiscence. The third patient had a large sacral tumor (Fig. 1), and an anterior approach with a planned colostomy and rectus cutaneous flap was performed first. Next, an en bloc marginal excision with L3–pelvis reconstruction was performed (Fig. 1). However, the patient developed a significant small bowel obstruction by postoperative day 5 with hemodynamic instability and was taken back to the operating room for an abdominal exploration by general surgery. A small bowel perforation with a transition zone was noted and repaired. Two (14%) patients experienced local tumor recurrence, with one patient undergoing additional embolization, bisphosphonate treatment, and radiation, and the other undergoing additional intralesional tumor resection.
Table 4. Surgical outcomes.
| Outcome variable | All patients (n = 14) | En bloc (n = 6) | Intralesional (n = 8) | p Value |
|---|---|---|---|---|
| Operative complications, n (%)a | ||||
| Early complication, n (%)a | 5 (31) | 4 (67) | 1 (13) | 0.0182 |
| Late complication, n (%)a | 2 (14) | 2 (33) | 0 (0) | 0.0389 |
| Length of hospital stay, d (SEM) | 13 (3) | 16 (5) | 11 (3) | 0.1981 |
| Surgical revision, n (%) | 3 (21) | 3 (50) | 0 (0) | 0.0120 |
| Local recurrence | 2 (14) | 0 (0) | 2 (25) | 0.0929 |
| Adjuvant therapies, n (%) | ||||
| Postoperative chemotherapy | 0 (0) | 0 (0) | 0 (0) | – |
| Postoperative radiation | 2 (14) | 0 (0) | 2 (25) | 0.0929 |
| Neurologic status at LFU, n (%) | 0.0929 | |||
| Intact | 9 (64) | 5 (83) | 4 (50) | |
| Symptomatic | 5 (36) | 1 (17) | 4 (50) | |
| Residual tumor at LFU, n (%) | 2 (14) | 0 (0) | 2 (25) | 0.0929 |
| Duration of follow-up, mo (SEM) | 43 (10) | 53 (15) | 35 (14) | 0.1945 |
Abbreviations: LFU, last follow-up; SEM, standard error of the mean.
Early versus late complications were stratified by complications that occurred <30 days perioperatively or >30 days postoperatively, respectively.
Fig. 1.
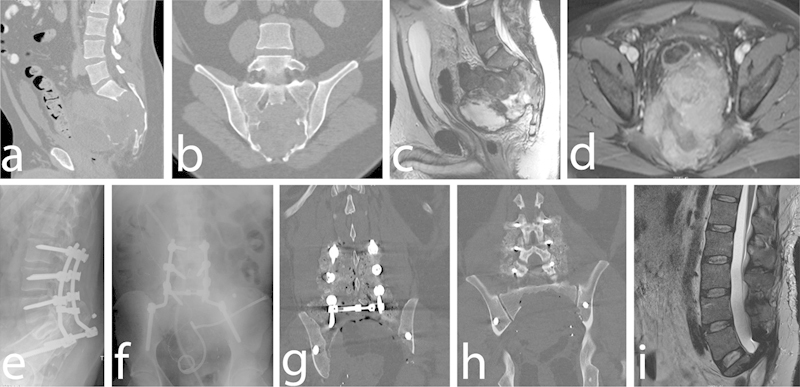
(a) Preoperative sagittal and (b) coronal nonenhanced computed tomography (CT) scans demonstrating 13.3 × 8.5-cm osteolytic S2–S4 sacral mass with invasion of the sacral foramina and anterior displacement of the sigmoid colon and bladder. (c) Preoperative sagittal T2-weighted magnetic resonance imaging (MRI) demonstrating heterogeneous-appearing mass arising from the sacrum. (d) Preoperative axial gadolinium-enhanced T1-weighted MRI demonstrating sacral mass extending into the pelvis and abutting the rectum. Postoperative (e) lateral and (f) anteroposterior radiographs demonstrating L3–pelvis instrumentation. Postoperative coronal nonenhanced CT scan demonstrating (g) instrumentation and (h) extent of en bloc sacral resection. (i) Postoperative sagittal T2-weighted MRI demonstrating extent of tumor resection.
Patients received follow-up for a mean of 43 (10) months. All patients were alive at last follow-up; 12 (86%) patients had no evidence of local or systemic disease and 2 (14%) patients had evidence of local residual disease. No patient experienced distant metastasis to the lungs or sarcoma transformation. Furthermore, 9 (64%) patients were neurologically intact, 3 (21%) had stable bowel/bladder incontinence, 1 (7%) had stable bilateral L5 weakness, and 1 (7%) had stable bilateral upper extremity weakness at last follow-up.
En Bloc versus Intralesional Resection
Patients who underwent an en bloc (n = 6) versus intralesional (n = 8) resection were similar with respect to all preoperative characteristics (Table 2). The mean operative duration was significantly longer during en bloc cases (p = 0.0251; Table 3). Likewise, the occurrence of both early (p = 0.0182) and late (p = 0.0389) complications was significantly higher following en bloc resection, requiring revision after 50% of cases (p = 0.0120; Table 4). There was also a trend toward an increased amount of blood transfusion required after en bloc resection (p = 0.0587; Table 3). However, there was a trend (p = 0.0929) toward a lower local recurrence rate for en bloc (0%) compared with intralesional resection (25%; Table 4).
Discussion
The results of this study suggest that surgical excision of spinal GCTs causing significant instability, assessed by SINS, is associated with high intraoperative blood loss despite embolization and independent of the resection method. Additionally, en bloc resection for spinal GCT is associated with a longer operative duration and a higher rate of complications compared with intralesional resection, and surgical revision due to wound dehiscence and delayed hardware failure is more often needed after en bloc resection. However, the increased morbidity of en bloc resection may be justified as there may be a decreased risk of local tumor recurrence following en bloc resection. Although previous reports focused on the separate outcomes of en bloc and/or intralesional resection with limited follow-up, our data is unique in that the long-term outcomes after each resection type are directly compared among an otherwise homogenous cohort of patients treated at a single, high-volume institution with multidisciplinary expertise. Likewise, our study is the first to date to apply the SINS for patients with spinal GCT to provide further rationale for surgery with resection and stabilization. This study provides surgeons with important outcomes data regarding the risks and benefits associated with two commonly employed surgical approaches for the treatment of spinal GCT and introduces the SINS as a potentially useful measure in patients with spinal GCT.
The instability associated with spinal GCT is not explicitly considered in the majority of studies. However, the bony destruction of these tumors, particularly of the vertebral body,4 can result in pathologic fracture leading to significant instability. Using the SINS, we found that all of the patients in this study were classified as potentially unstable (12) or unstable (2), warranting surgical management for tumor control as well as stabilization. Not surprisingly, a higher chance of preoperative instability as assessed by the SINS and greater length of stay were associated with patients with Enneking stage III tumors compared with Enneking stage II tumors. Thus, the SINS score can be useful in patients with higher Enneking classification.
GCTs are unpredictable, osteolytic neoplasms that rarely affect the spine.2 3 11 When affecting the mobile spine, GCTs result in a substantially worse prognosis than GCTs of the appendicular skeleton due to osteolytic destruction causing compression of the adjacent neurologic structures.11 As a result, several studies advocate aggressive treatment with the goal of gross total resection. En bloc resection of the tumor is often recommended, with the goal of achieving total resection of the tumor within its compartment, allowing wide histologic margins, resulting in lower rates of recurrence and improved long-term survival relative to intralesional resection.2 11 12 13 However, en bloc resection, particularly in the mobile spine, is a complex and technically challenging procedure that requires stabilization via instrumentation and meticulous avoidance of neurologic injury.13 Ultimately, the overall objective of spinal surgical oncology is to maximize the extent of resection to reduce the risk of neurologic compromise and tumor recurrence while limiting the operative morbidity and postoperative complications.
In many cases, en bloc resection may still be warranted, despite longer operative times and higher rates of surgical complications, due to the lower risk of tumor recurrence and potentially extended functional survival compared with intralesional resection. As all cases of tumor recurrence in this study occurred in patients who underwent intralesional resection, en bloc resection may be associated with less local recurrences. For example, Balke et al reported a high rate of local recurrence associated with intralesional resection for GCT of the axial skeleton, with recurrence occurring in 4 of 6 patients.3 In their retrospective analysis of 102 patients with GCT of the mobile spine, Xu et al found that long-term use of bisphosphonate along with en bloc or piecemeal removal of the entire bony compartment may significantly reduce the rate of tumor recurrence.14 Moreover, Boriani et al found that en bloc resection allowed better control of Enneking stage III GCT of the mobile spine (p = 0.01), and intralesional resection was sufficient for Enneking stage II tumors.4 Tumor recurrence was also lower after en bloc resection in Martin and McCarthy's series of 23 cases, with no sacral and 2 of 11 spinal GCTs developing recurrence after en bloc resection compared with recurrence in 2 of 6 sacral GCTs and both spinal GCTs treated with intralesional resection.2 Junming et al in a series of 22 patients with GCT of the cervical spine concluded that total spondylectomy, including intralesional resection, with adjuvant radiation may be utilized to significantly lower local recurrence rate in cases not amenable to en bloc resection due to close proximity to critical neurovascular structures within the cervical spine.15 Therefore, careful evaluation of the potential risks and benefits must be employed during surgical planning, and postoperative quality of life must be carefully considered in light of the patients' age and functional capacity.
It should be noted that five patients did not have follow-up beyond 1 year, which is required to truly assess for tumor recurrence. However, four of these patients with only short-term follow-up had intralesional resections. Therefore, it is likely that longer follow-up of these patients may lead to an increased difference in the rate of tumor recurrence between patients with en bloc and intralesional resections.
Another important factor to consider is the high degree of intraoperative blood loss and requirement for long hospital stays associated with resection of this highly vascular tumor. Prior studies have shown that the use of preoperative embolization is a safe and effective technique that can be utilized to reduce blood loss and operative morbidity associated with GCT.2 10 Despite the use of preoperative embolization in the majority of the patients in the present study, intraoperative blood loss remained high, irrespective of treatment type. Yet, it is important to note that the amount of blood transfusion required after en bloc resection also approached significance (p = 0.0587) in this study. Recently, the use of denosumab, a novel monoclonal antibody to RANKL, with or without concomitant radiation, has gained popularity due to its ability to shrink the tumor prior to treatment and reduce the morbidity associated with subsequent resection. In 2013, Chawla et al showed that denosumab was able to decrease tumor progression and reduced the need for morbid surgery in patients with actively growing GCT in a phase II trial.16 Later, Mattei et al were the first to report a sustained long-term complete regression in a patient with GCT of the spine.5 Thus, it is likely that the blood loss experienced by our cohort could have been substantially decreased with the preoperative use of denosumab and/or radiation. Likewise, the use of these adjuvant therapies may justify less invasive surgical techniques, such as intralesional resection, in appropriately selected cases to reduce the extent of postoperative morbidity without the associated risk of local recurrence and/or decreased survival.13 Therefore, these adjuvant therapies should be considered along with preoperative embolization prior to surgical intervention of spinal GCT.
Although the findings of this study suggest that surgical management of spinal GCT causing instability, particularly en bloc resection, is a highly morbid procedure associated with significant blood loss, long operative duration and hospitalization, and a relatively high rate of complications despite the use of preoperative embolization, several limitations should be carefully considered. Important limitations of this study include the limited sample size, absence of preoperative denosumab use, and retrospective data collection. One factor limiting the quality of the evidence typically attained in studies regarding primary spine tumors, including GCT, is the low prevalence of the disease. Thus, the clinical utility of the study findings is typically given priority over the quality of the evidence.13 As such, the results of this study support the need for larger multicenter trials, with the use of preoperative denosumab and/or radiation, to fully evaluate the outcomes achieved after surgical treatment of spinal GCTs.
Conclusions
Surgical excision of spinal GCTs causing significant instability, assessed by SINS, is associated with high intraoperative blood loss despite embolization and independent of resection method. En bloc resection requires a longer operative duration and is associated with a higher risk of complications when compared with intralesional resection. However, the increased morbidity associated with en bloc resection may be justified as it may be associated with less local recurrences. To reduce the risk of blood loss during GCT resection, neoadjuvant denosumab and/or radiation could be considered prior to surgical intervention.
Disclosures Benjamin D. Elder, none Eric W. Sankey, none C. Rory Goodwin, Research grants: UNCF Merck Postdoctoral Fellow, Burroughs Wellcome Fund, NREF Thomas A. Kosztowski, none Sheng-Fu L. Lo, none Ali Bydon, Research grant: DePuy Spine; Board membership: MedImmune, LLC Jean-Paul Wolinsky, none Ziya L. Gokaslan, Research grant: DePuy, NREF, AOSpine, AO North America; Stock ownership: US Spine and Spinal Kinetics; Consulting: AO Foundation Timothy F. Witham, Research grant: Eli Lilly and Company; Funding: Gordon and Marilyn Macklin Foundation Daniel M. Sciubba, Consulting: Medtronic, Nuvasive, DePuy, Stryker
Note
This article reflects the views of the authors (B.E. and S-F.L.) and should not be construed to represent the U.S. Food and Drug Administration's views or policies. The authors report no external source of funding. The corresponding author had full access to all data in this study and has final responsibility for the decision to submit for publication.
References
- 1.Luther N, Bilsky M H, Härtl R. Giant cell tumor of the spine. Neurosurg Clin N Am. 2008;19(1):49–55. doi: 10.1016/j.nec.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Martin C, McCarthy E F. Giant cell tumor of the sacrum and spine: series of 23 cases and a review of the literature. Iowa Orthop J. 2010;30:69–75. [PMC free article] [PubMed] [Google Scholar]
- 3.Balke M, Henrichs M P, Gosheger G. et al. Giant cell tumors of the axial skeleton. Sarcoma. 2012;2012:410973. doi: 10.1155/2012/410973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boriani S, Bandiera S, Casadei R. et al. Giant cell tumor of the mobile spine: a review of 49 cases. Spine (Phila Pa 1976) 2012;37(1):E37–E45. doi: 10.1097/BRS.0b013e3182233ccd. [DOI] [PubMed] [Google Scholar]
- 5.Mattei T A, Ramos E, Rehman A A, Shaw A, Patel S R, Mendel E. Sustained long-term complete regression of a giant cell tumor of the spine after treatment with denosumab. Spine J. 2014;14(7):e15–e21. doi: 10.1016/j.spinee.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Fourney D R, Frangou E M, Ryken T C. et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29(22):3072–3077. doi: 10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh P C Li K W Sciubba D M Suk I Wolinsky J P Gokaslan Z L Posterior-only approach for total en bloc spondylectomy for malignant primary spinal neoplasms: anatomic considerations and operative nuances Neurosurgery 200965(6, Suppl):173–181., discussion 181 [DOI] [PubMed] [Google Scholar]
- 8.Shen C C, Li H, Shi Z L, Tao H M, Yang Z M. Current treatment of sacral giant cell tumour of bone: a review. J Int Med Res. 2012;40(2):415–425. doi: 10.1177/147323001204000203. [DOI] [PubMed] [Google Scholar]
- 9.Balke M. Denosumab treatment of giant cell tumour of bone. Lancet Oncol. 2013;14(9):801–802. doi: 10.1016/S1470-2045(13)70291-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhou M, Yang H, Chen K. et al. Surgical treatment of giant cell tumors of the sacrum and spine combined with pre-operative transarterial embolization. Oncol Lett. 2013;6(1):185–190. doi: 10.3892/ol.2013.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santiago-Dieppa D R, Hwang L S, Bydon A, Gokaslan Z L, McCarthy E F, Witham T F. L4 and L5 spondylectomy for en bloc resection of giant cell tumor and review of the literature. Evid Based Spine Care J. 2014;5(2):151–157. doi: 10.1055/s-0034-1387804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawahara N, Tomita K, Murakami H, Demura S, Yoshioka K, Kato S. Total en bloc spondylectomy of the lower lumbar spine: a surgical techniques of combined posterior-anterior approach. Spine (Phila Pa 1976) 2011;36(1):74–82. doi: 10.1097/BRS.0b013e3181cded6c. [DOI] [PubMed] [Google Scholar]
- 13.Fisher C G Keynan O Ondra S Gokaslan Z Introduction to focus issue in spine oncology: the synthesis of evidence and expert opinion for best practice recommendation Spine (Phila Pa 1976) 200934(22, Suppl):S21–S25. [DOI] [PubMed] [Google Scholar]
- 14.Xu W, Li X, Huang W. et al. Factors affecting prognosis of patients with giant cell tumors of the mobile spine: retrospective analysis of 102 patients in a single center. Ann Surg Oncol. 2013;20(3):804–810. doi: 10.1245/s10434-012-2707-6. [DOI] [PubMed] [Google Scholar]
- 15.Junming M, Cheng Y, Dong C. et al. Giant cell tumor of the cervical spine: a series of 22 cases and outcomes. Spine (Phila Pa 1976) 2008;33(3):280–288. doi: 10.1097/BRS.0b013e318162454f. [DOI] [PubMed] [Google Scholar]
- 16.Chawla S, Henshaw R, Seeger L. et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901–908. doi: 10.1016/S1470-2045(13)70277-8. [DOI] [PubMed] [Google Scholar]


