Abstract
Study Design Case report.
Objective The purpose of this report is to discuss the surgical management of lumbar vertebral osteomyelitis with a spinal epidural abscess (SEA) and present a single-stage, posterior-only circumferential decompression and reconstruction with instrumentation using an expandable titanium cage and without segmental nerve root sacrifice as an option in the treatment of this disease process.
Methods We report a 42-year-old man who presented with 3 days of low back pain and chills who rapidly decompensated with severe sepsis following admission. Magnetic resonance imaging of his lumbosacral spine revealed intramuscular abscesses of the left paraspinal musculature and iliopsoas with SEA and L4 vertebral body involvement. The patient failed maximal medical treatment, which necessitated surgical treatment as a last resort for infectious source control. He underwent a previously undescribed procedure in the setting of SEA: a single-stage, posterior-only approach for circumferential decompression and reconstruction of the L4 vertebral body with posterior segmental instrumented fixation.
Results After the surgery, the patient's condition gradually improved; however, he suffered a wound dehiscence necessitating a surgical exploration and deep wound debridement. Six months after the surgery, the patient underwent a revision surgery for adjacent-level pseudarthrosis. At 1-year follow-up, the patient was pain-free and off narcotic pain medication and had returned to full activity.
Conclusion This patient is the first reported case of lumbar osteomyelitis with SEA treated surgically with a single-stage, posterior-only circumferential decompression and reconstruction with posterior instrumentation. Although this approach is more technically challenging, it presents another viable option for the treatment of lumbar vertebral osteomyelitis that may reduce the morbidity associated with an anterior approach.
Keywords: lumbar vertebral osteomyelitis, spinal epidural abscess, posterior-only approach, circumferential decompression and reconstruction
Introduction
Vertebral osteomyelitis with concomitant spinal epidural abscess (SEA) is an uncommon medical condition with potentially serious consequences. The mortality rates associated with SEA are high and range from 5 to 20%.1 A diagnosis of SEA is suspected based on clinical findings, laboratory data, and radiographic studies and can only be confirmed by drainage.2 It is estimated that ∼50% of patients are misdiagnosed at the time of initial presentation.3 The most common location for vertebral osteomyelitis associated with SEA is the lumbar spine, with one major case series reporting 54.7% of patients having lumbar involvement.4 The optimal treatment of SEA remains controversial, but the growing evidence seems to suggest that surgical decompression together with systemic antibiotics is the treatment of choice.2 The surgical approaches for vertebral osteomyelitis with SEA vary and depend on the location of neural compression and degree of bony involvement. Historically, lumbar vertebral body resection and reconstruction have been performed via the anterior transcavitary approach followed by posterior instrumented fixation. Multiple reports exist describing posterior-only debridement and anterior stabilization of thoracolumbar osteomyelitis in patients with tuberculosis-related and other nontuberculosis-associated pyogenic infections.5 6 In this report, we present a single-stage, posterior-only approach to achieve L4 vertebrectomy in the surgical treatment of lumbar vertebral osteomyelitis with SEA and describe the management and postoperative complications. To our knowledge, this patient is the first reported case of a posterior-only approach for lumbar circumferential decompression and reconstruction in the treatment of pyogenic osteomyelitis with SEA.
Case Report
Presentation
The patient was a 42-year-old man with past medical history significant for human immunodeficiency virus and hepatitis C. He presented with 3 days of low back pain and chills with worsening shortness of breath. On admission, his vital signs revealed a temperature of 37.8°C, blood pressure of 92/54 mm Hg, pulse of 124 beats per minute, and respiratory rate of 22 breaths per minute. The laboratory results revealed a white blood count of 12.1 × 103/µL (40% neutrophils), creatinine phosphokinase level of 2,400 IU/L (normal range, 10 to 120 IU/L), and lactic acid of 4.3 mEq/L (normal range, 0.7 to 2.1 mEq/L). On physical examination, the patient was neurologically intact. A chest X-ray revealed bilateral patchy opacities likely representing an acute infectious or inflammatory process. A computed tomography (CT) scan of the chest, abdomen, and pelvis revealed edema within the musculature of the left flank, left paravertebral region, and left iliacus muscle. The patient was admitted to the medical intensive care unit with a suspected diagnosis of bacteremia with concern of endocarditis with septic embolization to the lungs. Following admission, he rapidly decompensated with severe sepsis necessitating intubation and heavy sedation due to respiratory failure. Magnetic resonance imaging of the lumbar spine revealed intramuscular abscesses of the left paraspinal musculature and iliopsoas with extension into the epidural space and extensive L4 vertebral body osteomyelitis (Fig. 1). Blood cultures grew vancomycin-sensitive, methicillin-resistant Staphylococcus aureus (MRSA), and he was started on intravenous vancomycin (1,000 mg every 12 hours). On the fourth day, he underwent an image-guided percutaneous drainage of the left psoas abscess, yielding ∼10 mL of purulent fluid, which subsequently grew MRSA. On the fifth day, he developed septic emboli to the lungs and his condition continued to deteriorate despite maximal medical management. At this point, it was decided that surgical treatment was necessary for infectious source control for failure of antibiotic treatment in this patient.
Fig. 1.
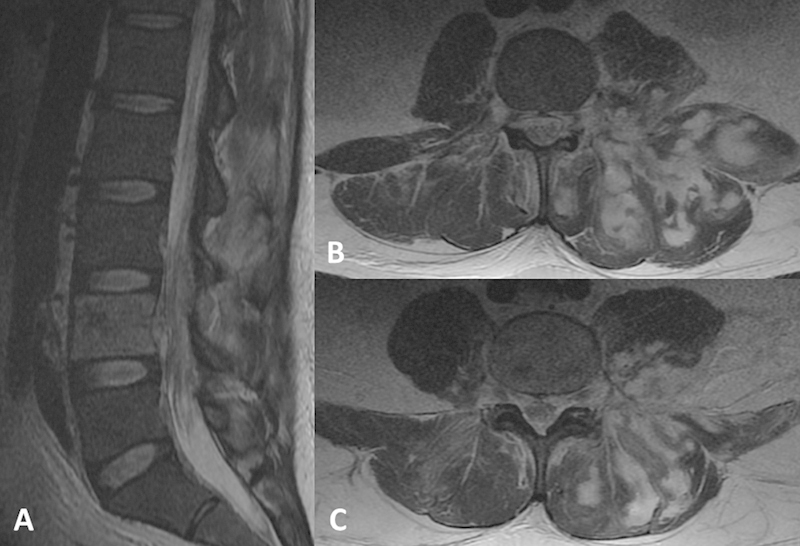
Preoperative T2-weighted noncontrast magnetic resonance imaging. (A) Midsagittal cut demonstrating T2 hyperintensity with limited diffusion of L4 vertebral body and epidural space. (B, C) Axial cuts demonstrating marked inflammatory changes around the left iliopsoas, quadratus lumborum, and left paracentral musculature with multiple ill-defined fluid collections.
Operation
The patient was taken to the operating room 8 days after admission. He was deemed medically stable but with a high risk for complications including mortality. Intraoperatively, several large pockets of purulent material were encountered in the deep paraspinal and iliopsoas musculatures, which were cultured and thoroughly debrided and later grew MRSA. Following subperiosteal dissection, pedicle screw-based posterior segmental instrumentation was performed from L2 to S1. Laminectomy followed by the resection of the superior and inferior facet joints, bilateral transverse processes, and pedicles of L4 was then performed. Finally, vertebrectomy of L4 including diskectomies at L3–L4 and L4–L5 with placement of an expandable titanium cage filled with iliac crest autograft mixed with vancomycin powder was performed using a combination of curettes, rongeurs, and disk shavers. Placement was achieved without sacrificing the L4 nerve root. Two cross-links, placed at L2–L3 and L5–S1, were used for additional axial stabilization. Posterolateral fusion was attempted from L2–S1 with iliac crest autograft (Fig. 2).
Fig. 2.
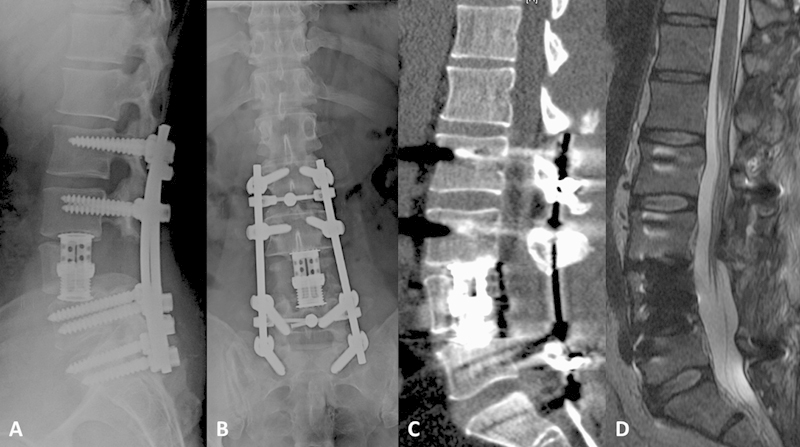
Postoperative sagittal (A) and anteroposterior (B) X-ray films, and sagittal noncontrast computed tomography (C) images demonstrating L4 vertebral body resection with expandable cage reconstruction and pedicle instrumentation from L2 to S1 with cross-link placement at L2–3 and L5–S1. (D) Sagittal T2-weighted noncontrast magnetic resonance image demonstrating resection of the L4 vertebral body with decompressive laminectomy and evacuation of epidural abscess.
Postoperative Course
Postoperatively, the patient's overall medical status gradually improved. However, on postoperative day 29 his wound dehisced necessitating deep wound irrigation and debridement, and the radiographic studies revealed a subfascial collection. The wound was copiously irrigated and all the bone graft placed in the posterolateral gutters removed. Intraoperative cultures grew Enterobacter species and the patient was placed on intravenous doxycycline for 6 weeks based on antibiotic susceptibility results and recommendations from the infectious disease team. The wound subsequently healed without further problems. The patient was discharged to an acute rehabilitation facility because of severe deconditioning on postoperative day 40 and eventually was discharged home on postoperative day 67.
At the 6-month follow-up, the patient was functioning remarkably well but had some low back pain. Plain radiographs and CT of the lumbosacral spine showed pedicle screw loosening in the sacrum (Fig. 3). There was no bone graft left in the posterolateral space, and impending pseudarthrosis and failure of the fusion construct were likely if nothing was done. Therefore, the patient successfully underwent a lateral interbody fusion at L2–L3 and an anterior interbody fusion at L5–S1 (Fig. 4). At the 1-year follow-up, the patient was neurologically intact, pain-free, off narcotic pain medications, and back to full physical activity.
Fig. 3.
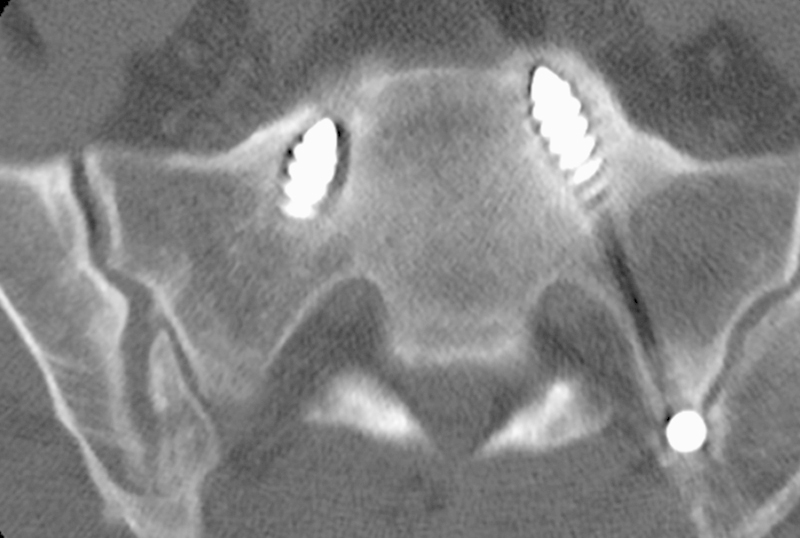
Noncontrast computed tomography scan axial cut image demonstrating lucency around the sacral pedicle screws.
Fig. 4.
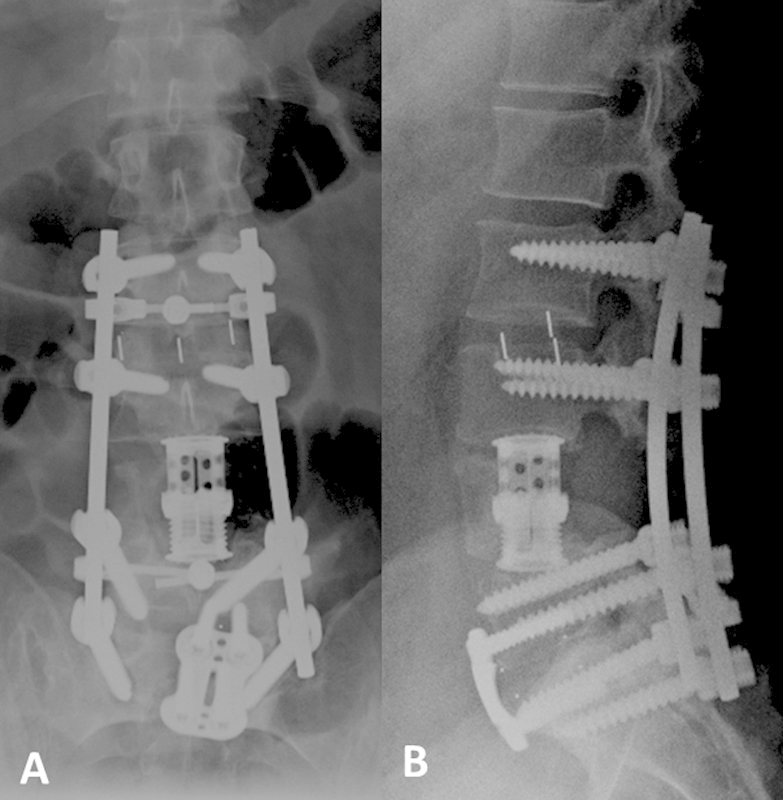
Anteroposterior (A) and lateral (B) X-rays following reoperation for pseudarthrosis demonstrating additional interbody cage placement at L2–L3 and L5–S1 with anterior plate and screw fixation at L5–S1.
Discussion
SEA most often occurs from bacterial infection, with S. aureus identified as the causative pathogen in two-thirds of all cases.1 Our patient had MRSA bacteremia and grew the same pathogen from his wound. The portion of SEA cases due to MRSA has been increasing over the past decade, and there is evidence that MRSA has been associated with poorer outcomes.7
The posterior transpedicular approach with vertebral body reconstruction technique, feasibility, and success have been previously described in patients with spinal tumors, trauma, and deformity.8 9 10 11 12 13 14 15 In spinal tumors, this procedure has been shown to reduce blood loss, operative time, overall morbidity, and hospital stay when compared with the combined anterior and posterior approach.16 Kim et al showed that the morbidity of the anterior thoracolumbar approach was associated with high rates of postoperative pain (32.3%), disk bulging (43.5%), and functional disturbances (24.2%) in a cohort of 62 adult patients who underwent deformity surgery.17 The posterior transpedicular approach is a less invasive treatment option that provides access for a safe and effective circumferential decompression and stabilization through a single posterior midline incision.16
Gorensek et al reported on a series of 23 patients with bacterial osteomyelitis and SEA treated operatively, 7 of whom had lumbar involvement only and underwent all-posterior debridement and instrumented fusion including interbody fusion.18 The authors concluded that the posterior-only approach led to a faster postoperative recovery, shorter surgical times, and less operative blood loss compared with the combined single-stage procedure and double-stage combined approach. Sundararaj et al investigated the use of titanium cages in 70 patients with tuberculosis or bacterial vertebral osteomyelitis who underwent single-stage anterior debridement, reconstruction of the anterior column with titanium mesh cage, and posterior instrumentation and concluded that despite the theoretical risks, titanium cages are a suitable alternative to autologous tricortical iliac crest bone graft in patients with active spinal infections.19 Bydon et al compared outcomes between patients undergoing decompression-only versus decompression and instrumented fusion for primary spinal infection and concluded that spinal instrumentation in patients with primary spinal infection did not lead to greater recurrent infection rates.20 Gonzalvo et al retrospectively reviewed nine patients with spontaneous pyogenic osteomyelitis/diskitis who underwent posterior decompression and instrumented fusion and found that the long-term outcomes (Oswestry Disability Index, EQ-5D) showed that most patients had a quality of life equivalent to that of the general population and most of them were able to return to work or resume their usual activities.21
Our patient presented with advanced sepsis in the setting of lumbar vertebral osteomyelitis and SEA, which presented a formidable challenge, as successful surgery would entail thorough circumferential decompression and subsequent three-column reconstruction. Our choice of surgical approach in this patient was influenced by the patient's rapidly declining medical condition and location of infection. The posterior-only approach offered several advantages to the care of this patient. The infection spanned from the paraspinal musculature, through the epidural space, into the L4 vertebral body and psoas muscles. By choosing a posterior-only approach, we were able to access and debride all the infected sites with a single surgery, thus minimizing the operative morbidity associated with an additional anterior approach. Furthermore, bypassing the need for an anterior approach decreased the risk of introducing infection into a sterile anterior abdominal compartment and potentially worsening this patient's condition.
The surgical reconstruction of the vertebral column in the lumbar spine can present significant challenges using this approach. Given the small and relatively deep operative field with limited space, insertion of a static cage that can span the entire lumbar vertebral body including the disk space above and below is not possible without sacrificing one of the nerve roots. The use of an expandable cage filled with iliac crest autograft allowed us to insert the cage around the L4 nerve root and span the entire vertebrectomy defect while preserving the nerve root. Studies from the spinal tumor literature found the use of expandable cages to be safe and durable with a very low incidence of cage-related construct failures and no significant problems with subsidence.22 23
We mixed vancomycin powder with iliac crest autograft in the expandable titanium cage in hopes of minimizing bacterial colonization and biofilm formation around the cage construct and maximizing infection control. Several recent studies described the use of vancomycin powder within the surgical wound to decrease surgical wound infection and adverse events in patients undergoing spinal surgery.24 25 26 27 The latest meta-analysis evaluating the risk of postoperative infection and/or pseudarthrosis in 3,379 patients undergoing spinal surgery in which vancomycin powder was applied within the surgical wound found a significantly reduced risk of surgical site infections without an increase in pseudarthrosis or adverse events.10
Due to the instability created by the circumferential resection of the L4 vertebra, we chose to use two cross-links to provide increased rotational stiffness to our construct. A biomechanical study on the mechanical stability of thoracolumbar pedicle screw fixation found that rotation stiffness values of the two-cross-link construct were significantly higher than those of the zero-cross-link system, at 2.5 degrees and 3.5 degrees of rotation.28 Furthermore, lateral bending stiffness of the two-cross-link system was found to be higher than that of the zero-cross-link system at all levels of displacement.26
We chose to leave the anterior wall of the vertebral body of L4 intact, as it constituted a barrier to protect the great vessels and also served as a fusion bed with vascular supply from segmental vessels needed to facilitate future fusion.
Our index fusion spanned four lumbar levels with end fixation into the sacrum. Previous studies have shown that in lumbar fusion surgery of three or more levels, the incidence of pseudarthrosis at the L5–S1 level is significantly higher than those in one-level or two-level fusion surgery.26 Pelvic fixation is a viable option of augmenting biomechanical fixation of the distal fusion segment and decreasing the stresses on the sacral instrumentation, which often predisposes them to failure. Spinopelvic fixation via placement of iliac screws has been shown to be a biomechanically sound method of stabilizing multilevel lumbosacral constructs.27 In our patient, we opted against extending the construct to the pelvis for several reasons. Pelvic fixation would require significantly more exposure to noninfected tissues and increased surgical time and operative blood loss in this patient who was critically sick at the time of surgery. We also wanted to minimize the amount of instrumentation implanted into this patient due to the setting of infection.
This patient needed deep wound irrigation and debridement with the removal of the posterolateral bone graft following the index surgery, which predisposed him to fusion failure as evidenced by the loosening of the sacral screws 6 months later. Furthermore, CT imaging of the lumbar spine did not reveal evidence of fusion at the anterior interbody cage. Without the anterior biomechanical load sharing from a fused interbody cage, the posterior instrumentation was placed at increased stresses, which predisposed the construct to failure. At that time, we wanted to avoid another posterior approach because of potential residual subclinical infection and bacterial biofilm formation around the instrumentation. The patient also had a large bony defect with all posterior elements removed from L3 to L5, and an attempt to achieve posterolateral fusion would have been challenging and potentially unsafe. For this reason, we chose to perform interbody fusions from the lateral transpsoas approach at L2–L3 and anterior retroperitoneal approach at L5–S1.
We present the first reported case of lumbar osteomyelitis with SEA treated surgically with a single-stage, posterior-only circumferential decompression and reconstruction with posterior instrumentation. In this case report, a posterior-only vertebral body reconstruction using an expandable titanium cage without the sacrifice of a lumbar nerve root was achieved. Although this approach is more technically challenging, it presents another viable option for the treatment of lumbar vertebral osteomyelitis that may reduce the morbidity associated with an anterior approach.
Footnotes
Disclosures Branko Skovrlj, none Javier Z. Guzman, none John Caridi, Consultancy: Zimmer, Stryker Samuel K. Cho, Consultancy: Stryker
References
- 1.Reihsaus E Waldbaur H Seeling W Spinal epidural abscess: a meta-analysis of 915 patients Neurosurg Rev 2000234175–204., discussion 205 [DOI] [PubMed] [Google Scholar]
- 2.Pradilla G, Nagahama Y, Spivak A M, Bydon A, Rigamonti D. Spinal epidural abscess: current diagnosis and management. Curr Infect Dis Rep. 2010;12(6):484–491. doi: 10.1007/s11908-010-0140-1. [DOI] [PubMed] [Google Scholar]
- 3.Darouiche R O. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012–2020. doi: 10.1056/NEJMra055111. [DOI] [PubMed] [Google Scholar]
- 4.Davis D P, Wold R M, Patel R J. et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26(3):285–291. doi: 10.1016/j.jemermed.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Patel A R, Alton T B, Bransford R J, Lee M J, Bellabarba C B, Chapman J R. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. 2014;14(2):326–330. doi: 10.1016/j.spinee.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 6.Gellin B G, Weingarten K, Gamache F W. , Jr. Philadelphia, PA: Lippincott-Raven Publishers; 1997. Epidural abscess; p. 507. [Google Scholar]
- 7.Rigamonti D Liem L Sampath P et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management Surg Neurol 1999522189–196., discussion 197 [DOI] [PubMed] [Google Scholar]
- 8.Akeyson E W, McCutcheon I E. Single-stage posterior vertebrectomy and replacement combined with posterior instrumentation for spinal metastasis. J Neurosurg. 1996;85(2):211–220. doi: 10.3171/jns.1996.85.2.0211. [DOI] [PubMed] [Google Scholar]
- 9.Bilsky M H Boland P Lis E Raizer J J Healey J H Single-stage posterolateral transpedicle approach for spondylectomy, epidural decompression, and circumferential fusion of spinal metastases Spine (Phila Pa 1976) 200025172240–2249., discussion 250 [DOI] [PubMed] [Google Scholar]
- 10.Kamat A, Gilkes C, Barua N U, Patel N R. Single-stage posterior transpedicular approach for circumferential epidural decompression and three-column stabilization using a titanium cage for upper thoracic spine neoplastic disease: a case series and technical note. Br J Neurosurg. 2008;22(1):92–98. doi: 10.1080/02688690701671029. [DOI] [PubMed] [Google Scholar]
- 11.Mouchaty H Conti R Conti P Desogus N Maleci A Di Lorenzo N A one-session circumferential reconstruction in thoracic and lumbar spine fractures using a small expandable cage J Neurosurg Sci 2008524101–106., discussion 106 [PubMed] [Google Scholar]
- 12.Sasani M, Ozer A F. Single-stage posterior corpectomy and expandable cage placement for treatment of thoracic or lumbar burst fractures. Spine (Phila Pa 1976) 2009;34(1):E33–E40. doi: 10.1097/BRS.0b013e318189fcfd. [DOI] [PubMed] [Google Scholar]
- 13.Suk S I, Chung E R, Lee S M, Lee J H, Kim S S, Kim J H. Posterior vertebral column resection in fixed lumbosacral deformity. Spine (Phila Pa 1976) 2005;30(23):E703–E710. doi: 10.1097/01.brs.0000188190.90034.be. [DOI] [PubMed] [Google Scholar]
- 14.Suk S I, Kim J H, Kim W J, Lee S M, Chung E R, Nah K H. Posterior vertebral column resection for severe spinal deformities. Spine (Phila Pa 1976) 2002;27(21):2374–2382. doi: 10.1097/00007632-200211010-00012. [DOI] [PubMed] [Google Scholar]
- 15.Wang J C, Boland P, Mitra N. et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(3):287–298. doi: 10.3171/spi.2004.1.3.0287. [DOI] [PubMed] [Google Scholar]
- 16.Metcalfe S, Gbejuade H, Patel N R. The posterior transpedicular approach for circumferential decompression and instrumented stabilization with titanium cage vertebrectomy reconstruction for spinal tumors: consecutive case series of 50 patients. Spine (Phila Pa 1976) 2012;37(16):1375–1383. doi: 10.1097/BRS.0b013e318250a172. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y B, Lenke L G, Kim Y J. et al. The morbidity of an anterior thoracolumbar approach: adult spinal deformity patients with greater than five-year follow-up. Spine (Phila Pa 1976) 2009;34(8):822–826. doi: 10.1097/BRS.0b013e31818e3157. [DOI] [PubMed] [Google Scholar]
- 18.Gorensek M, Kosak R, Travnik L, Vengust R. Posterior instrumentation, anterior column reconstruction with single posterior approach for the treatment of pyogenic osteomyelitis of thoracic and lumbar spine. Eur Spine J. 2013;22(3):633–641. doi: 10.1007/s00586-012-2487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundararaj G D, Amritanand R, Venkatesh K, Arockiaraj J. The use of titanium mesh cages in the reconstruction of anterior column defects in active spinal infections: can we rest the crest? Asian Spine J. 2011;5(3):155–161. doi: 10.4184/asj.2011.5.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bydon M, De la Garza-Ramos R, Macki M. et al. Spinal instrumentation in patients with primary spinal infections does not lead to greater recurrent infection rates: an analysis of 118 cases. World Neurosurg. 2014;82(6):e807–e814. doi: 10.1016/j.wneu.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalvo A, Abdulla I, Riazi A, De La Harpe D. Single-level/single-stage debridement and posterior instrumented fusion in the treatment of spontaneous pyogenic osteomyelitis/discitis: long-term functional outcome and health-related quality of life. J Spinal Disord Tech. 2011;24(2):110–115. doi: 10.1097/BSD.0b013e3181dd8115. [DOI] [PubMed] [Google Scholar]
- 22.Thongtrangan I, Balabhadra R S, Le H, Park J, Kim D H. Vertebral body replacement with an expandable cage for reconstruction after spinal tumor resection. Neurosurg Focus. 2003;15(5):E8. doi: 10.3171/foc.2003.15.5.8. [DOI] [PubMed] [Google Scholar]
- 23.Viswanathan A, Abd-El-Barr M M, Doppenberg E. et al. Initial experience with the use of an expandable titanium cage as a vertebral body replacement in patients with tumors of the spinal column: a report of 95 patients. Eur Spine J. 2012;21(1):84–92. doi: 10.1007/s00586-011-1882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcalá-Cerra G, Paternina-Caicedo A J, Moscote-Salazar L R, Gutiérrez-Paternina J J, Niño-Hernández L M. [Application of vancomycin powder into the wound during spine surgery: systematic review and meta-analysis] Rev Esp Cir Ortop Traumatol. 2014;58(3):182–191. doi: 10.1016/j.recot.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Heller A, McIff T E, Lai S M, Burton D C. Intrawound vancomycin powder decreases staphylococcal surgical site infections following posterior instrumented spinal arthrodesis. J Spinal Disord Tech. 2013 doi: 10.1097/BSD.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill B W, Emohare O, Song B, Davis R, Kang M M. The use of vancomycin powder reduces surgical reoperation in posterior instrumented and noninstrumented spinal surgery. Acta Neurochir (Wien) 2014;156(4):749–754. doi: 10.1007/s00701-014-2022-z. [DOI] [PubMed] [Google Scholar]
- 27.Martin J R, Adogwa O, Brown C R. et al. Experience with intrawound vancomycin powder for spinal deformity surgery. Spine (Phila Pa 1976) 2014;39(2):177–184. doi: 10.1097/BRS.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 28.Lynn G Mukherjee D P Kruse R N Sadasivan K K Albright J A Mechanical stability of thoracolumbar pedicle screw fixation. The effect of crosslinks Spine (Phila Pa 1976) 199722141568–1572., discussion 1573 [DOI] [PubMed] [Google Scholar]


