Abstract
Study Design Case report.
Objective There is a paucity of literature describing the use of bone graft substitutes to achieve fusion in the pediatric cervical spine. The outcomes and complications involving the off-label use of bone morphogenetic protein (BMP)-2 in the pediatric cervical spine are not clearly defined. The purpose of this article is to report successful fusion without complications in two pediatric patients who had instrumented occipitocervical fusion using low-dose BMP-2.
Methods A retrospective review of the medical records was performed, and the patients were followed for 5 years. Two patients under 10 years of age with upper cervical instability were treated with occipitocervical instrumented fusion using rigid occipitocervical fixation techniques along with conventionally available low-dose BMP-2. A Medline and PubMed literature search was conducted using the terms “bone morphogenetic protein,” “BMP,” “rh-BMP2,” “bone graft substitutes,” and “pediatric cervical spine.”
Results Solid occipitocervical fusion was achieved in both pediatric patients. There were no reported perioperative or follow-up complications. At 5-year follow-up, radiographs in both patients showed successful occipital cervical fusion without evidence of instrumentation failure or changes in the occipitocervical alignment. To date, there are few published reports on this topic. Complications and the appropriate dosage application in the pediatric posterior cervical spine remain unknown.
Conclusions We describe two pediatric patients with upper cervical instability who achieved successful occipital cervical fusion without complication using off-label BMP-2. This report underscores the potential for BMP-2 to achieve successful arthrodesis of the posterior occipitocervical junction in pediatric patients. Use should be judicious as complications and long-term outcomes of pediatric BMP-2 use remain undefined in the existing literature.
Keywords: BMP, bone morphogenic protein, rh-BMP-2, bone graft substitutes, pediatric cervical spine
Introduction
The use of bone morphogenetic protein (BMP)-2 in pediatric patients is not approved by the U.S. Food and Drug Administration (FDA) and is considered off-label use of the product. Use of BMP-2 in children is controversial, and there are very few published reports involving BMP use in the pediatric cervical spine.1 2 3 4 5 This article describes off-label BMP-2 use in the pediatric posterior cervical spine and reviews the existing literature on this topic.
Case Reports
Case 1
A 9-year-old boy with developmental delay and trisomy 16 presented to the emergency room with severe neck pain and a 4-week history of progressive weakness in all four extremities and difficulty ambulating. The radiographs demonstrated severe C1–C2 instability with a widened atlanto-dens interval and a narrowed posterior atlanto-dens interval (Fig. 1). Computed tomography (CT) scan showed hypoplasia of the atlas condyles along with an intact posterior arch. The ossicle terminal portion of the nonfused pediatric odontoid was noted to be superiorly located through the region of the foramen magnum (Fig. 2). Magnetic resonance imaging (MRI) revealed severe canal stenosis and myelomalacia in the C1–C2 region (Fig. 3).
Fig. 1.
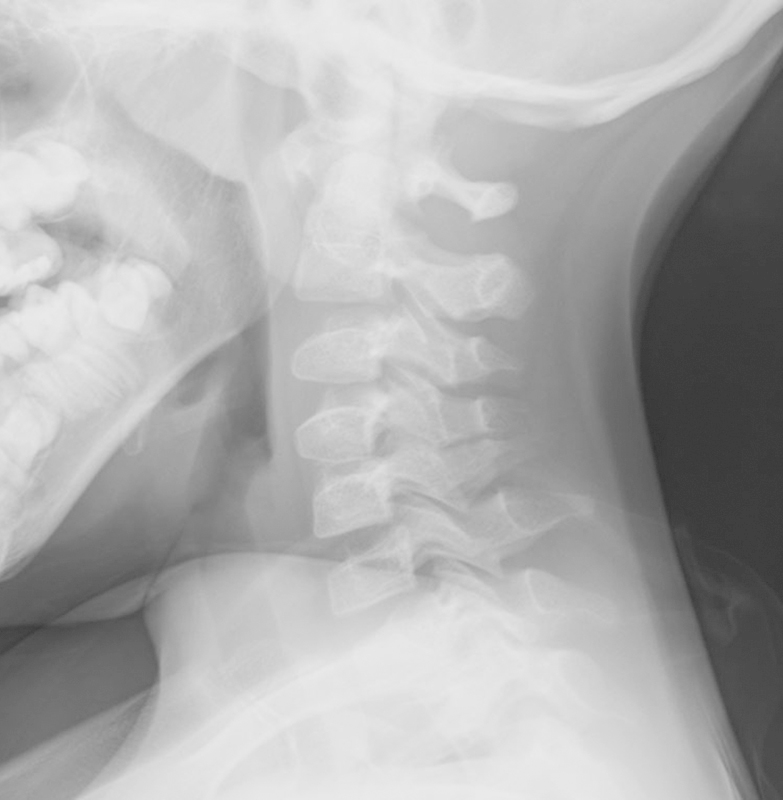
Lateral radiograph demonstrating severe C1–C2 instability with a widened atlanto-dens interval and narrowed posterior atlanto-dens interval.
Fig. 2.
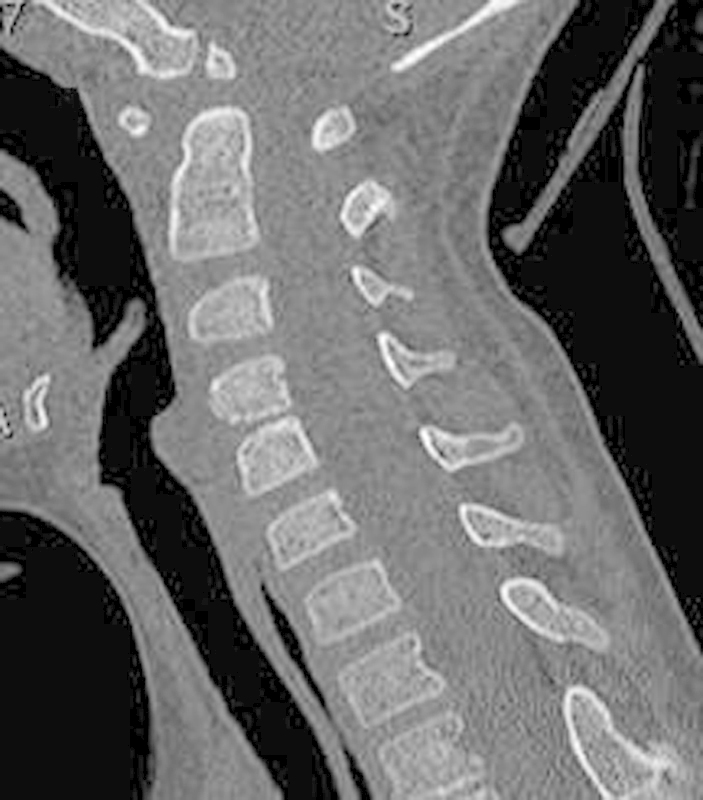
Computed tomography scan showing abnormal widening at C1–C2. The ossicle terminal portion of the nonfused pediatric odontoid was noted to be superiorly located through the region of the foramen magnum.
Fig. 3.
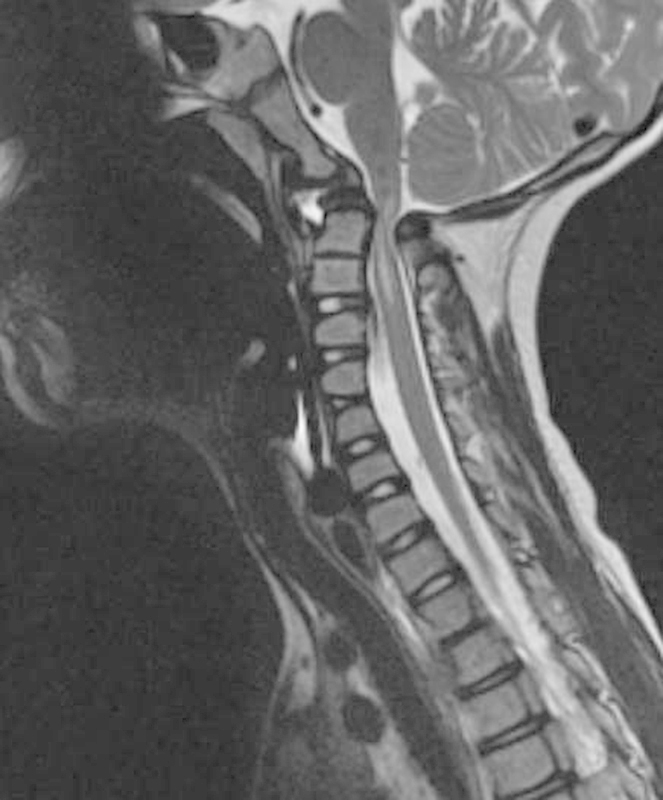
Magnetic resonance imaging with severe canal stenosis and myelomalacia in the region of C1–C2.
Occipitocervical stabilization and fusion surgery was recommended. There was concern regarding a paucity of available autogenous bone graft due to the patient's relatively younger age and small body stature. Other concerns included the potential for poor iliac crest wound healing and increased graft site pain. The potential risks and benefits of off-label BMP use were discussed with the parents preoperatively. Informed consent was obtained from the parents after a thorough discussion of the existing BMP potential complication literature and the concept of off-label use. The surgery was performed using occipital plating and subaxial fixation including bilateral C2 translaminar screws and C3 lateral mass screws. One large kit of Infuse recombinant human (rh)-BMP-2 (12 mg) (Medtronic, Minneapolis, MN, United States) was placed on a 7.5 × 10-cm collagen sponge and combined with 20 mL Mastergraft extender product (Medtronic), which is purified bovine collagen combined with biphasic calcium phosphate ceramic. No other bone grafting materials were used. The surgery was performed without intraoperative complications. The postoperative MRI demonstrated excellent decompression of the spinal canal (Fig. 4).
Fig. 4.
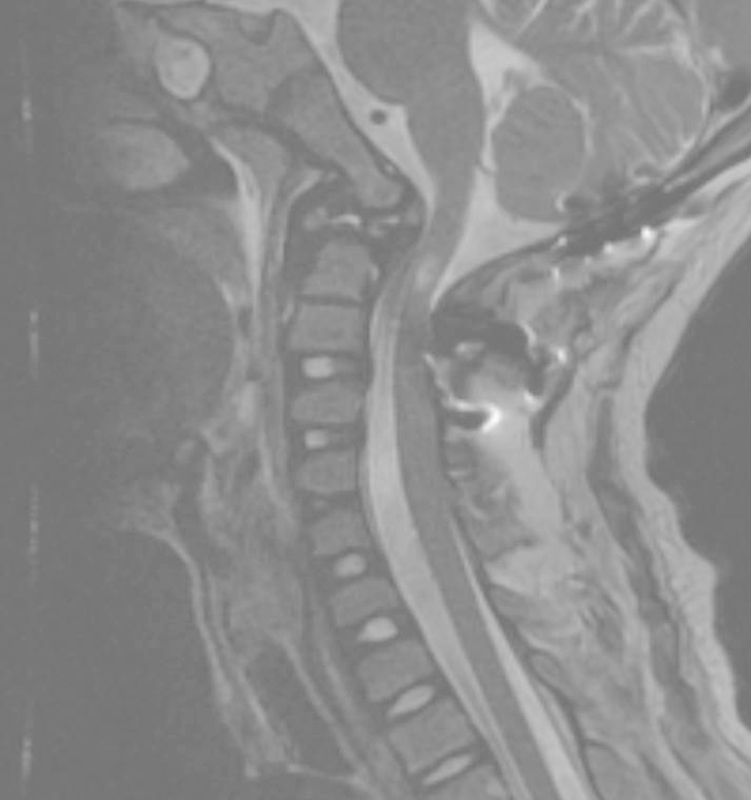
Postoperative magnetic resonance imaging demonstrated excellent decompression of the spinal canal.
The patient's postoperative course was uneventful, and he was discharged to home on postoperative day 5. His neurologic motor function improved significantly, and his wound healed primarily without any reported problems during the weeks following the surgery. He was followed regularly as an outpatient for 62 months postoperatively. Solid radiographic union was achieved without loss of occipitocervical alignment. The lateral radiograph demonstrated extension of the fusion to include two levels below the instrumentation levels, which may be associated with either surgical exposure or the BMP-2 use. There was no evidence of implant breakage or loosening (Fig. 5a, b).
Fig. 5.
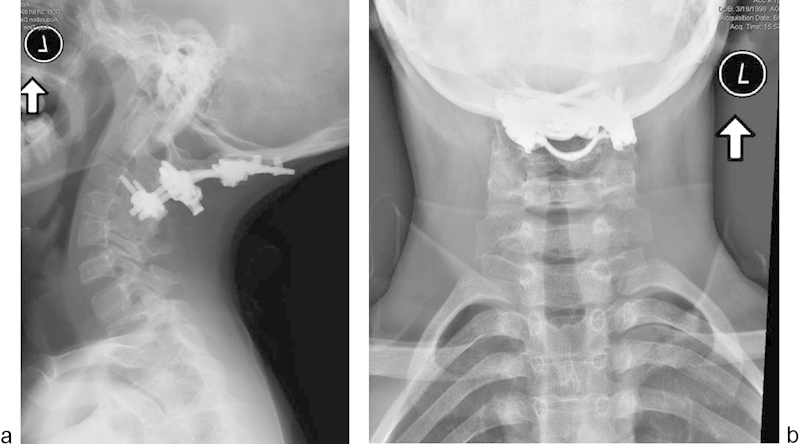
Follow-up lateral (a) and anteroposterior (b) radiographs showing solid radiographic union and maintained occipitocervical alignment.
Case 2
A 9-year-old girl with Down syndrome fell off a chair in her home and presented to the emergency room with incomplete quadriplegia (American Spinal Injury Association classification B) and severe neck pain.6 Radiographs revealed severe C1–C2 instability with a widened atlanto-dens interval and a severely narrowed posterior atlanto-dens interval. The CT showed hypoplasia of the C1 lateral masses with an intact posterior C1 arch. The MRI demonstrated cord compression with myelomalacia in the C1–C2 region (Figs. 6, 7, 8).
Fig. 6.
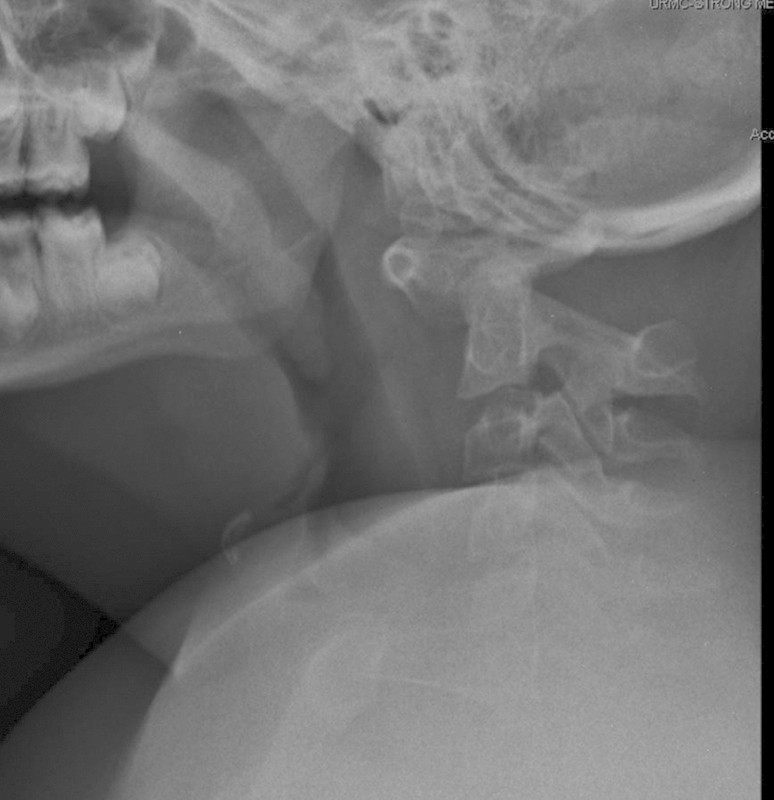
Lateral radiographs demonstrating severe C1–C2 instability with a widened atlanto-dens interval and severely narrowed posterior atlanto-dens interval.
Fig. 7.
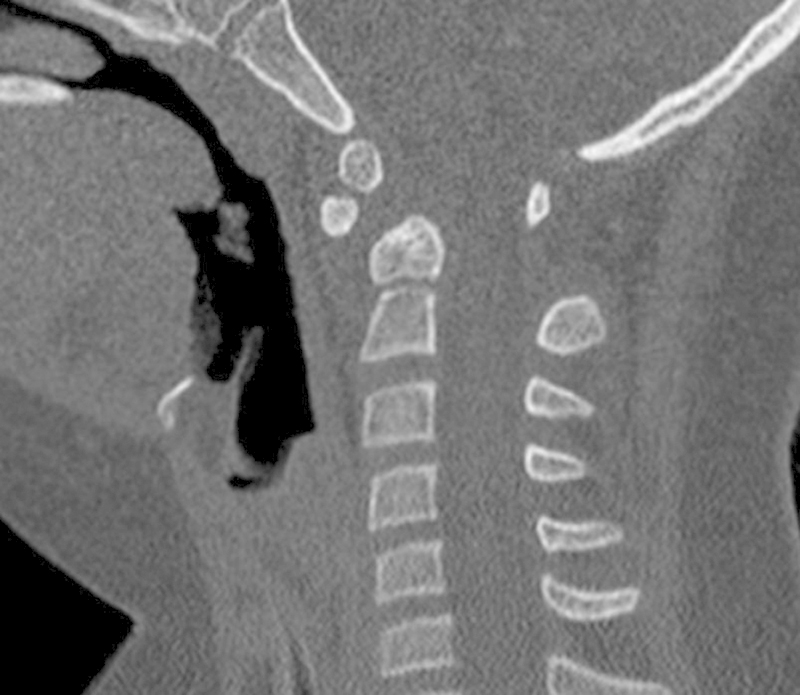
Computed tomography scan showing abnormal widening at C1–C2. The ossicle terminal portion of the nonfused pediatric odontoid is displaced anteriorly.
Fig. 8.
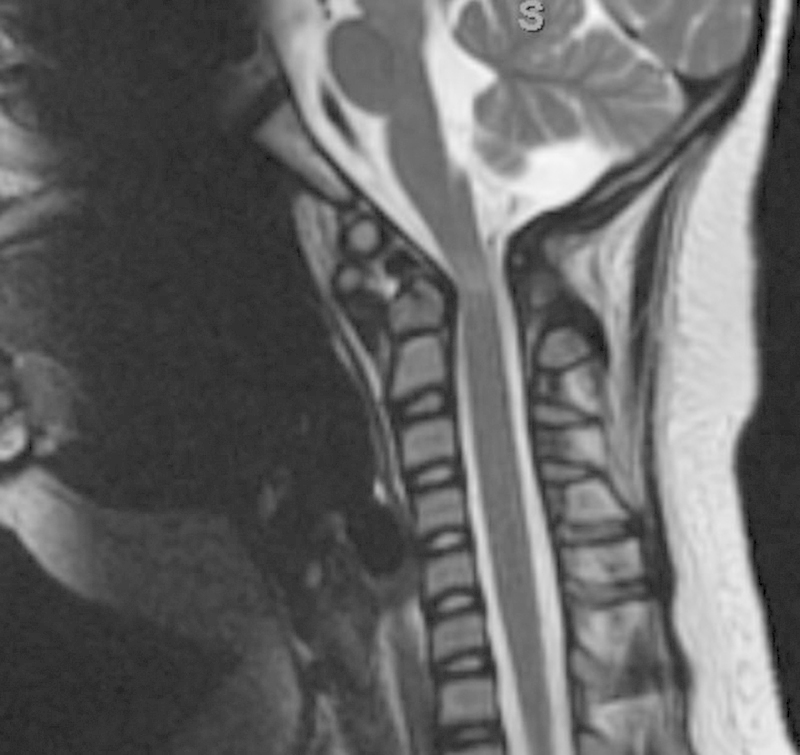
Magnetic resonance imaging showing cord compression with myelomalacia in the region of C1–C2.
Occipitocervical fusion surgery was recommended. Informed consent was obtained from the patient's parents after a thorough discussion of the existing BMP potential complication literature and the concept of off-label use, including the risks and potential benefits. The patient had occipitocervical fusion using an occipital plate connected to bilateral C2 translaminar screws. One large kit of Infuse rh-BMP-2 (12 mg) was placed on a 7.5 × 10-cm collagen sponge and combined with 20 mL Mastergraft extender product. No other bone grafting materials were used. There were no intraoperative complications.
The patient's postoperative course was uneventful, and her neurologic function improved during the postoperative hospitalization. Primary wound healing without complication was noted. At the time of ultimate follow-up (61 months), the patient remained neurologically normal with maintained occipitocervical alignment. Solid union was noted on follow-up flexion and extension radiographs without evidence of broken instrumentation (Fig. 9a, b). The lateral radiograph demonstrates extension of the fusion to include one level below the instrumentation levels, which may be associated with either surgical exposure or the BMP-2 use.
Fig. 9.
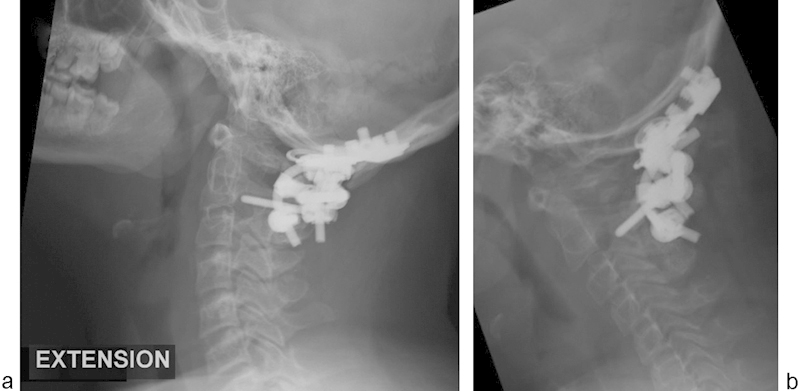
Follow-up extension (a) and flexion (b) radiographs demonstrating maintained occipitocervical alignment and solid union.
Discussion
Off-label use of BMP-2 has increased since its approval by the FDA for use in anterior lumbar interbody fusion in 2002.3 BMP-2 affords many advantages compared with autologous bone grafting, especially in younger children where there is paucity of autologous bone quantity available for harvest. Advantages associated with BMP-2 use include decreased operating time, decreased blood loss, decreased donor site morbidity, ease of use, and efficacy.7 We report successful occipitocervical fusion in two pediatric patients under the age of 10 years using off-label BMP-2. Both patients were followed more than 5 years after surgery and neither demonstrated significant adverse events related to BMP-2 use.
This article highlights the paucity of literature involving BMP use in the pediatric cervical spine. There are no published reports describing BMP use in the anterior cervical spine and very few describing off-label use in the posterior pediatric cervical spine.
Off-label use of BMP-2 remains controversial in the pediatric spine. The FDA has not approved the use of bone morphogenic protein in children. Age less than 18 years or lack of epiphyseal closure are considered by the manufacturer to be contraindications to BMP use. There are also serious drawbacks related to the use of BMP-2, including high cost and complications.8 9 10 Postoperative complications including seroma, infection, heterotopic bone growth, new radiculopathy, and bone resorption around fixation devices have been reported.8 10 11 Additionally, it is unknown whether the long-term effects in children may include the possibility of increasing future risk of cancer. Although we do not report any adverse events related to BMP-2 use in our two patients, the risk of complications remains undefined. A thorough discussion with the parents of pediatric patients must take place during the informed consent process and must include all of the aforementioned topics concerning BMP use in children.
There is scant literature reporting the increasing BMP-2 use and efficacy in pediatric thoracic and lumbar spinal surgery. Jain et al demonstrated that surgeons in the United States are more frequently using BMP in pediatric thoracolumbar spine fusions.3 In 2009, nearly 10% of pediatric spine fusions involved use of BMP-2. Use increased from 2.7% in 2003 to 9.3% in 2009. The highest rate of pediatric BMP-2 use was in patients with lumbar spondylolisthesis. The authors also reported increasing BMP-2 use in patients with scoliosis and thoracolumbar fractures.
Rocque et al reported BMP-2-associated complications in the early postoperative period in pediatric patients undergoing thoracolumbar spinal fusion.12 A total of 4,650 patients underwent spinal fusion. BMP was used in 1,752 patients (37.61%). There was no difference in the rate of BMP-2 use when comparing male and female patients or in older versus younger than 10 years of age. No significant difference in complication rates was apparent when comparing the patients who had BMP-2 with the patients who had autologous bone graft. The reoperation rate was nearly identical between the two groups. The authors concluded that BMP use is higher than expected in the percentage of pediatric spinal fusions performed in the United States. However, the rate of complications was not initially higher.
There are few reports of BMP-2 use in the posterior pediatric spine. Mladenov et al described three pediatric patients who had BMP-2 used in the posterior cervical spine as a salvage fusion procedure.4 Solid fusion was achieved in all three patients despite underlying bone dysplasia conditions in each of the patients. No complications were observed. Lu and Sun reported successful C1–C2 fusion in a 4-year-old patient with Down syndrome, and Oluigbo and Solanki in a 2-year-old with C1–C2 instablity.13 14
Mazur et al reported outcomes and complications in pediatric patients who had occipital cervical fusion with average 43-month follow-up.15 Revision surgery was required in 16% of patients. Ultimately bony union was achieved in 44 of 57 patients. BMP-2 was used in 7 patients (5.5%) without reported complications. However, the authors clearly stated that they do not routinely use BMP-2 for pediatric occipitocervical fusion because, in their opinion, the documented risks outweigh the benefits. BMP-2 is only used for salvage procedures.
We are unaware of any published reports involving BMP-2 use in the pediatric anterior cervical spine, and we do not recommend BMP-2 use in anterior approaches to the cervical spine due to the high reported complication rates. The existing literature also remains unclear on the appropriate BMP-2 dosage when used in the posterior pediatric spine. We have demonstrated that using a single packet of the large “low-dose” (12 mg) commercially available BMP-2 formulation and combining it with an appropriate graft extender led to successful occipitocervical fusion in two young children.
We also must emphasize that the long-term adverse effects of BMP-2 use in children remain unknown. In a systematic review of the published studies on complications associated with BMP-2 use, Carragee et al described the risks of adverse events related to both FDA-approved and off-label BMP-2 use. BMP-2 use in pediatric spine surgery should still be considered experimental.9 With few published pediatric spine outcome studies involving BMP-2, success and complication rates remain unknown.1 We recommend exercising judicious caution when considering whether or not to use BMP-2 in pediatric spine surgery, especially in the posterior cervical spine where relatively fewer studies exist. Families and patients should be thoroughly informed of the risks and potential benefits of such use, and this discussion should be carefully documented in the medical record.
We have demonstrated in two pediatric patients with complex upper cervical instability that the surgical benefits of conventionally available low-dose BMP-2 may outweigh the risk. There remains a paucity of literature on this topic and further study is encouraged.
Conclusions
We describe two pediatric patients with upper cervical instability who achieved successful occipitocervical fusion without complications using low-dose off-label BMP-2. This report underscores the potential for BMP-2 to achieve successful arthrodesis of the posterior occipitocervical junction in pediatric patients. Use should be judicious as complications and long-term outcomes of pediatric BMP use remain undefined in the existing literature.
Footnotes
Disclosures Robert W. Molinari, none Christine Molinari, none
References
- 1.Haft G F Is off-label use of BMP in pediatric spine surgery now a standard of care? Commentary on an article by Amit Jain, MD, et al.: “Factors associated with use of bone morphogenetic protein during pediatric spinal fusion surgery. an analysis of 4817 patients.” J Bone Joint Surg Am 20139514e103, 1–2 [DOI] [PubMed] [Google Scholar]
- 2.Hwang S W, Gressot L V, Rangel-Castilla L. et al. Outcomes of instrumented fusion in the pediatric cervical spine. J Neurosurg Spine. 2012;17(5):397–409. doi: 10.3171/2012.8.SPINE12770. [DOI] [PubMed] [Google Scholar]
- 3.Jain A, Kebaish K M, Sponseller P D. Factors associated with use of bone morphogenetic protein during pediatric spinal fusion surgery: an analysis of 4817 patients. J Bone Joint Surg Am. 2013;95(14):1265–1270. doi: 10.2106/JBJS.L.01118. [DOI] [PubMed] [Google Scholar]
- 4.Mladenov K V, Kunkel P, Stuecker R. The use of recombinant human BMP-2 as a salvage procedure in the pediatric spine: a report on 3 cases. Eur Spine J. 2010;19 02:S135–S139. doi: 10.1007/s00586-009-1179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pakkasjärvi N, Mattila M, Remes V, Helenius I. Upper cervical spine fusion in children with skeletal dysplasia. Scand J Surg. 2013;102(3):189–196. doi: 10.1177/1457496913486742. [DOI] [PubMed] [Google Scholar]
- 6.Maynard F M Jr, Bracken M B, Creasey G. et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. Spinal Cord. 1997;35(5):266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- 7.Savage J G, Fulkerson D H, Sen A N, Thomas J G, Jea A. Fixation with C-2 laminar screws in occipitocervical or C1–2 constructs in children 5 years of age or younger: a series of 18 patients. J Neurosurg Pediatr. 2014;14(1):87–93. doi: 10.3171/2014.3.PEDS13626. [DOI] [PubMed] [Google Scholar]
- 8.Aspenberg P. Under-reported complications related to BMP use in spine surgery. Acta Orthop. 2011;82(5):511–512. doi: 10.3109/17453674.2011.623576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carragee E J, Chu G, Rohatgi R. et al. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am. 2013;95(17):1537–1545. doi: 10.2106/JBJS.L.01483. [DOI] [PubMed] [Google Scholar]
- 10.Epstein N E. Complications due to the use of BMP/INFUSE in spine surgery: the evidence continues to mount. Surg Neurol Int. 2013;4 05:S343–S352. doi: 10.4103/2152-7806.114813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tannoury C A, An H S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014;14(3):552–559. doi: 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 12.Rocque B G, Kelly M P, Miller J H, Li Y, Anderson P A. Bone morphogenetic protein-associated complications in pediatric spinal fusion in the early postoperative period: an analysis of 4658 patients and review of the literature. J Neurosurg Pediatr. 2014;14(6):635–643. doi: 10.3171/2014.8.PEDS13665. [DOI] [PubMed] [Google Scholar]
- 13.Lu D C Sun P P Bone morphogenetic protein for salvage fusion in an infant with Down syndrome and craniovertebral instability. Case report J Neurosurg 2007106(6, Suppl):480–483. [DOI] [PubMed] [Google Scholar]
- 14.Oluigbo C O, Solanki G A. Use of recombinant human bone morphogenetic protein-2 to enhance posterior cervical spine fusion at 2 years of age: technical note. Pediatr Neurosurg. 2008;44(5):393–396. doi: 10.1159/000149907. [DOI] [PubMed] [Google Scholar]
- 15.Mazur M D, Sivakumar W, Riva-Cambrin J, Jones J, Brockmeyer D L. Avoiding early complications and reoperation during occipitocervical fusion in pediatric patients. J Neurosurg Pediatr. 2014;14(5):465–475. doi: 10.3171/2014.7.PEDS1432. [DOI] [PubMed] [Google Scholar]


