To the Editor
Niemann-Pick-A/B disease is a lysosomal storage disease caused by deficiency of acid sphingomyelinase (1). Efforts are underway to develop novel therapies for this disease, and some newborn screening centers have started to test for acid sphingomyelinase enzymatic activity in dried blood spots on newborn screening cards. Available assays for newborn screening include tandem mass spectrometry (MS/MS)1 (2) and fluorometry (3). Diagnostic laboratories have typically used a radiometric assay with radiolabeled sphingomyelin or the fluorometric assay (4). However, in a detailed study of 24 patients confirmed as having Niemann-Pick-A/B disease, 4 had the Q292K missense allele. Fibroblasts from these 4 patients showed a normal to increased acid sphingomyelinase activity when assayed with the fluorometric substrate but displayed activity in the radiometric assay with natural sphingomyelin substrate in the low end of the range of the affected samples (4). The age of onset of symptoms in these 4 patients was <1, 1, <2, and <3 years, suggesting that the disease can be quite severe in patients harboring this allele (4). This led to the conclusion of a “diagnostic pitfall” in the use of the fluorometric substrate for analysis of Niemann-Pick-A/B disease. This issue was also reported in the original paper describing the fluorometric reagent (3), and it was shown that this mutant displayed a decreased affinity for sphingomyelin, which presumably explained the low activity on the natural substrate (3).
Fig. 1 shows the natural substrate and the substrates used in the MS/MS and fluorometric assays. The MS/MS substrate is a close structural analog of the natural substrate sphingomyelin, the only difference being the length of the fatty acyl chain linked to the sphingosine amino group. The fluorometric substrate has some structural resemblance to sphingomyelin but is substantially different owing to the need to incorporate a fluorogenic group in the substrate. Apparently the Q292K mutation causes a deformation in the active site of acid sphingomyelinase that reduces the affinity of the natural substrate but not the unnatural substrate.
Fig.1. The left panel shows the fluorimetric assay results for acid sphingomyelinase using HMU-PC substrate (upper right) and the cell culture medium indicated below each bar.
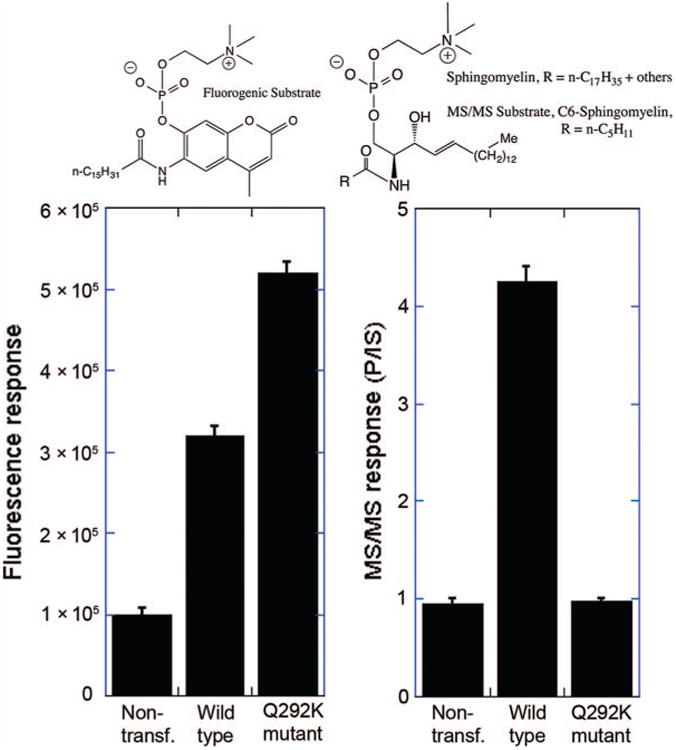
The right panel shows MS/MS results using C6-sphingomyelin substrate (upper right along with natural sphingomyelin) together with C4-sphingomyelin internal standard (IS) (not shown). Error bars are the SDs from triplicate assays. P/IS is the mass spectrometry ion response of the product divided by that of the internal standard. Nontransf., nontransfected.
To explore the assay behavior of wild-type human acid sphingomyelinase and the Q292K mutant, we transfected HEK293 cells with a plasmid expressing either wild-type or mutant protein. Twenty-four hours posttransfection, the culture medium was submitted to both the fluorometric assay with 6-hexadecanoylamino-4-methylumbelliferylphosphorylcholine (HMU-PC) (3) and to the MS/MS assay with C6-sphingomyelin (2). As shown in Fig. 1 (left panel), the fluorescence response was approximately 3-fold higher in culture medium from cells expressing wild-type acid sphingomyelinase than in medium from nontransfected cells (treated with transfection reagent but no plasmid DNA added). With medium from cells expressing the Q292K mutant, the fluorescence response was approximately 5-fold higher than the response with nontransfected cells. This higher activity of the Q292K mutant is consistent with the earlier study using fibroblast lysates from patients harboring the Q292K mutation (4). The results show that the Q292K shows high activity on HMU-PC.
Fig. 1 (right panel) shows the assay results using C6-sphingomyelin and the MS/MS assay. Because these were transfected cells, we did not convert the observed MS/MS response to the standard activity (i.e., μmol product per hour per mg cell protein). Rather, we show the ratio of acid sphingomyelinase product MS/MS ion counts to internal standard MS/MS ion counts. Assays contained a known amount of internal standard, a close analog of the product, but with a shorter fatty acyl chain (C4-sphingomyelin). The product/internal standard ratio was approximately 4.5-fold higher when culture medium from cells transfected with wild-type acid sphingomyelinase was used compared to culture medium from nontransfected cells. In contrast, the Q292K mutant displayed essentially no activity in the MS/MS assay. Thus, the MS/MS assay with C6-sphingomyelin gave an activity with the Q292K mutant similar to that reported using the radiometric assay with natural sphingomyelin (approximately 2% of normal activity) (4).
In conclusion, the MS/MS assay for acid sphingomyelinase should provide more reliable newborn screening and diagnosis for Niemann-Pick-A/B than that provided by the fluorometric assay. The use of the fluorometric assay may lead to a substantial number of missed cases (false negatives) due to the Q292K mutation. The MS/MS assay will be useful in laboratories not able to use radiometric assays. At the very least, if the fluorometric assay is used, 2 independent assays for each sample need to be carried out in the presence and absence of the natural substrate sphingomyelin as noted previously (3).
Footnotes
Nonstandard abbreviations: MS/MS, tandem mass spectrometry; HMU-PC, 6-hexadecanoylamino-4-methylumbelliferylphosphorylcholine.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors' Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: C.R. Scott, Genzyme Corp.; M.H. Gelb, Perkin Elmer.
Stock Ownership: None declared.
Honoraria: C.R. Scott, Genzyme Corp.
Research Funding: Genzyme Corp./Shire Pharma; grant from the National Institutes of Health (DK067859) to M.H. Gelb, F. Turecek, and C.R. Scott, PIs.
Expert Testimony: None declared.
Patents: None declared.
References
- 1.Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited disease. 8th. New York: McGraw-Hill; 2001. [Google Scholar]
- 2.Li Y, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Turecek F, Gelb MH. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004;50:1785–96. doi: 10.1373/clinchem.2004.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Diggelen OP, Voznyi YV, Keulemans JL, Schooderwoerd K, Ledvinova J, Mengel E, et al. A new fluorimetric enzyme assay for the diagnosis of Niemann-Pick A/B, with specificity of natural sphingomyelinase substrate. J Inherit Metab Dis. 2005;28:733–41. doi: 10.1007/s10545-005-0105-y. [DOI] [PubMed] [Google Scholar]
- 4.Harzer K, Rolfs A, Bauer P, Zschiesche M, Mengel E, Backes J, et al. Niemann-Pick disease type A and Bare clinically but also enzymatically heterogenous: pitfall in the laboratory diagnosis of sphingomyelinase deficiency associated with the mutation q292k. Neuropediatrics. 2003;34:301–6. doi: 10.1055/s-2003-44668. [DOI] [PubMed] [Google Scholar]


