Abstract
Growing evidence indicates that targeting nociceptin receptor (NOP) signaling may have therapeutic efficacy in treating alcohol and opioid addiction. However, little is known about the therapeutic value of selective NOP agonists for the treatment of cocaine dependence. Recently, we identified a highly selective, brain-penetrant NOP small molecule agonist (SR-8993), and using this compound, we previously showed that nociceptin receptor activation attenuated consolidation of fear-related memories. Here, we sought to determine whether SR-8993 also affects the rewarding properties of cocaine. Using a conditioned place preference (CPP) procedure, we show that SR-8993 (3 or 10 mg/kg) failed to disrupt acquisition or expression of cocaine CPP (7.5 or 15 mg/kg) in C57BL/6 mice. Additionally, SR-8993 did not affect rate of extinction or reinstatement (yohimbine- and cocaine-induced) of cocaine CPP. These studies indicate that selective activation of NOP may not be sufficient in reducing behavioral responses to cocaine.
Introduction
Nociceptin (orphanin FQ, N/OFQ) is an endogenous 17 amino acid peptide that has high affinity for the nociceptin receptor (NOP; sometimes called opioid-like receptor 1) (Lachowicz et al., 1995; Meunier et al., 1995; Reinscheid et al., 1995). NOP has similar sequence homology to classical opioid receptors (mu, delta and kappa) and is classified as the fourth member of the opioid receptor family (Witkin et al., 2014), but classical opioid peptides do not bind to NOP (Reinscheid et al., 1995; Wollemann and Benyhe, 2004). Although originally studied in pain, nociceptin and NOP are anatomically located in an ideal position for reward processing, as they are distributed throughout reward- and stress-related brain regions such as medial prefrontal cortex, ventral tegmental area, nucleus accumbens, lateral hypothalamus, central amygdala and bed nucleus of stria terminalis (Neal et al., 1999b; Neal et al., 1999a). Recent animal studies have confirmed an important role for nociceptin and NOP in multiple aspects of addiction-related processes, making nociceptin an attractive target for the development of therapeutics for the management of drug dependence (Lambert, 2008; Zaveri, 2011; Witkin et al., 2014).
Several lines of evidence support a role for nociceptin in addiction-related processes. For example, when injected intracranially, nociceptin reduces drug-induced dopamine release and c-fos activation in the nucleus accumbens, key mechanisms involved in reward and motivation (Ciccocioppo et al., 2000; Di Giannuario and Pieretti, 2000; Lutfy et al., 2001). In behavioral studies, intracranial administration of nociceptin attenuates addiction-related responses to opioids (Murphy et al., 1999; Ciccocioppo et al., 2000), psychostimulants (Lutfy et al., 2002; Kotlinska et al., 2003; Zhao et al., 2003; Bebawy et al., 2010) and ethanol (Ciccocioppo et al., 1999; Martin-Fardon et al., 2000), and withdrawal symptoms induced by morphine (Kotlinska et al., 2000) and ethanol (Economidou et al., 2011; Aujla et al., 2013). More recently, using small molecule compounds, preclinical studies have revealed that NOP agonists attenuate addiction-related behaviors similarly to nociceptin (Shoblock et al., 2005; Economidou et al., 2006; Kuzmin et al., 2007; Toll et al., 2009), although some discordant result have been reported in cocaine studies (Kotlinska et al., 2002). The discrepancy between nociceptin and small molecule agonist data may be explained by some of the NOP agonists having affinity for other opioid receptors (Wichmann et al., 2000; Toll et al., 2009), thus making it difficult to rule out interaction with multiple receptors. Therefore, development of highly selective NOP ligands is needed to identify the clinical significance of NOP in the treatment of addiction.
Recently, SR-8993 was identified as a highly selective, brain-penetrable NOP agonist, and using this compound, Andero and colleagues showed that nociceptin regulates consolidation of fear-related memories (Andero et al., 2013). Here, we sought to determine whether the same systemically active compound, also affects cocaine conditioned behaviors. Using a conditioned place preference (CPP) procedure, we show that SR-8993 failed to disrupt acquisition, expression, extinction and reinstatement of cocaine CPP in mice. Thus, these studies indicate that selective activation of NOP with SR-8993 is not sufficient in reducing place conditioning effects of cocaine.
Results
Effects of SR-8993 on acquisition of cocaine CPP
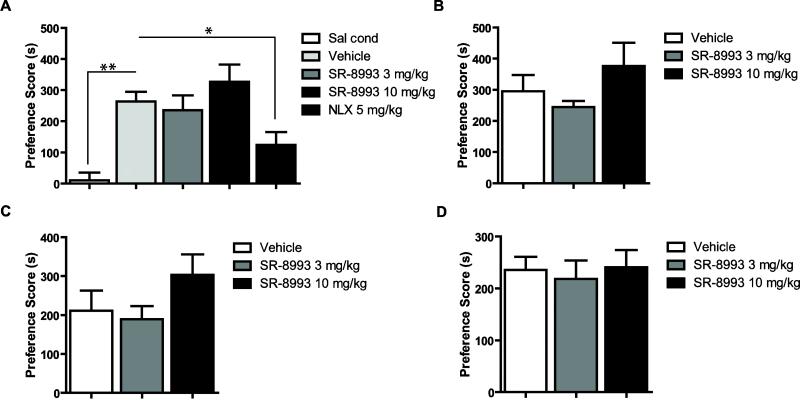
SR-8993 (3 or 10 mg/kg) was injected 30 min or 2 h before each cocaine (7.5 or 15 mg/kg) conditioning session. For additional controls, some mice were conditioned with saline, and in other mice naloxone (NLX), which has been previously shown to attenuate acquisition of cocaine CPP (Kim et al., 1997), was used as a positive control. In mice conditioned with 15 mg/kg of cocaine and treated 30 min before conditioning (Figure 1A), a one-way ANOVA revealed a significant main effect of group (F4, 33 = 8.6, p < 0.0001). Cocaine conditioned mice treated with vehicle during acquisition showed robust CPP compared to saline condition animals (Newman-Keuls test, p < 0.001 for saline cond. vs. vehicle), indicating that cocaine effectively elicited a CPP. Compared to vehicle, naloxone significantly reduced CPP, but SR-8993 had no effect (Newman-Keuls test, p < 0.05 for vehicle vs. NLX, p > 0.05 for vehicle vs. SR-8993 at 3 and 10 mg/kg). In mice conditioned with 15 mg/kg of cocaine and treated with SR-8993 (3 or 10 mg/kg) 2 h before conditioning (Figure 1B), no significant main effect was observed (F2, 20 = 1.4, p > 0.05). Similarly, in mice conditioned with 7.5 mg/kg of cocaine and treated with SR-8993 30 min (Figure 1C) or 2 h (Figure 1D) before conditioning, no significant main effect was observed (F2, 16 = 1.7, p > 0.05 at 30 min and F2, 20 = 0.1, p > 0.05 at 2 h).
Figure 1. Effects of SR-8993 on acquistion of cocaine CPP.
(A) Cocaine conditioned mice that received vehicle injections prior to each conditioning session showed robust CPP compared to saline conditioned mice. Naloxone significantly reduce cocaine CPP compared to vehicle. SR-8993 (3 mg/kg or 10 mg/kg) injections 30 min (A) or 2 h (B) before conditioning did not alter acquisition of CPP in mice conditioned with 15 mg/kg of cocaine. SR-8993 when injected (C) 30 min or (D) 2 h before conditioning did not change acquisition of CPP in mice conditioned with 7.5 mg/kg of cocaine. *p < 0.05, **p < 0.001 indicate a significant difference from vehicle via Newman-Keuls post hoc test (n = 6-9 per group). Data are mean ±SEM.
Effects of SR-8993 on expression of cocaine CPP
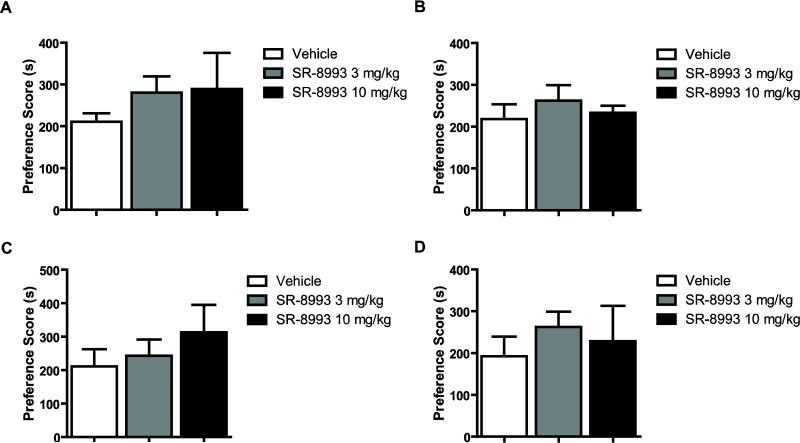
To examine the effects of NOP activation on the expression of cocaine CPP, mice were conditioned with 7.5 or 15 mg/kg of cocaine, and SR-8993 (3 mg/kg or 10 mg/kg) or vehicle was administered 30 min or 2 h prior to the CPP expression test. At both time points, SR-8993 failed to alter preference scores in mice conditioned with 15 mg/kg (F2, 28 = 0.5, p > 0.05 for 30 min and F2, 21 = 0.5, p > 0.05 for 2 h, Figures 2A-B) or 7.5 mg/kg of cocaine (F2 17 = 0.8 p > 0.05 for 30 min and F2, 18 = 0.5, p > 0.05 for 2 h, Figures 2C-D). In a different group of animals, we found that SR-8993 (10 mg/kg) had no effect on locomotor activity compared to vehicle-treated animals (distance traveled in cm: Veh = 1556 ±242; SR-8993 = 1716 ±261; t9 = 0.4, p > 0.05, n = 5-6).
Figure 2. Effects of SR-8993 on expression of cocaine CPP.
SR-8993 (3 mg/kg or 10 mg/kg) injection 30 min (A) or 2 h (B) before the expression test did not alter expression of CPP in mice conditioned with 15 mg/kg of cocaine, nor did SR-8993 injection (C) 30 min or (D) 2 h before the expression test change CPP scores in mice conditioned with 7.5 mg/kg of cocaine (n = 6-11 per group). Data are mean ±SEM.
Effects of SR-8993 on cocaine CPP extinction
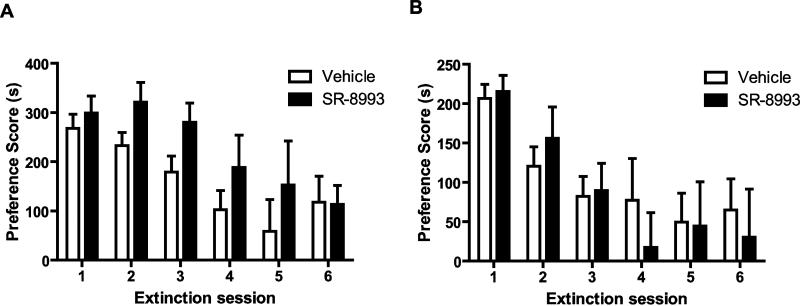
Next, we sought to determine whether SR-8993 (10 mg/kg) affects the rate of extinction when injected 30 min or 2 h before each extinction session. A two-way repeated measures ANOVA found a significant effect of time (F5, 65 = 8.0, p < 0.0001 at 30 min, Figure 3A and F5, 55 = 9.1, p < 0.0001 at 2 h, Figure 3B) but no interaction or group effect (p > 0.05), indicating that SR-8993 has no overall effect on the rate of extinction compared to vehicle.
Figure 3. Effects of SR-8993 on extinction of cocaine CPP.
Cocaine conditioned animals from both groups had similar preference scores in the initial, (drug-free) preference test (session 1). SR-8993 injections 30 min (A) or 2 h (B) prior to subsequent extinction tests (sessions 2-6) did not significantly alter CPP scores compared to vehicle-treated mice (n = 6-7). Data are mean ±SEM.
Effects of SR-8993 on cocaine- and yohimbine-induced reinstatement of CPP
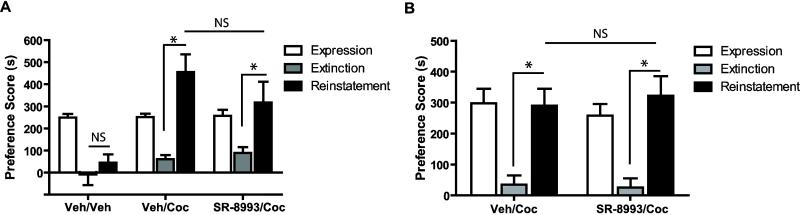
In subsequent experiments, we injected SR-8993 (10 mg/kg) 30 min or 2 h before cocaine- (10 mg/kg) or yohimbine-induced (2 mg/kg) reinstatement test. As a control, additional mice received a vehicle injection prior to reinstatement test. When mice were treated with SR-8993 or vehicle 2 hr before cocaine-induced reinstatement test (Figure 4A), a two-way ANOVA revealed a significant main effect of group (F2, 36 = 6.9, p < 0.01), test (F2, 36 = 19.9, p < 0.0001) and interaction (F4, 36 = 5.2, p < 0.01). Bonferroni post hoc test, however, found no difference in cocaine-induced reinstatement between vehicle and SR-8993 treated mice (p > 0.05). In mice injected with SR-8993 or vehicle 30 min before cocaine-induced reinstatement test (Figure 4B), a two-way ANOVA revealed a significant effect of test (F2, 26 = 34.7, p < 0.0001), but no significant group or interaction effect was observed (p > 0.05). In mice injected with SR-8993 or vehicle 30 min or 2 h before yohimbine-induced reinstatement, a two-way ANOVA revealed a significant effect of test (F2, 24 = 18.5, p < 0.0001 at 2 h, Figure 5A and F2, 22 = 21.0, p < 0.0001 at 30 min, Figure 5B), but no significant group or interaction effect was observed (p > 0.05).
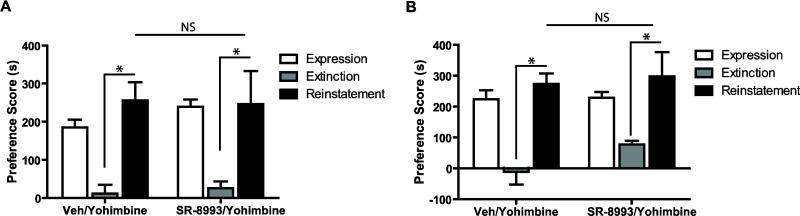
Figure 4. Effects of SR-8993 on cocaine-induced reinstatement of CPP.
Conditioned mice showed similar preference during the expression test, and repeated exintction testing atteuated the place preference (A-B). A cocaine priming injection significantly reinstated the extinguished CPP, while mice treated with vehicle before testing did not show reinstatement (A). Injection of SR-8993 2 h (A) or 30 min (B) before reinstatement did not alter CPP scores compared to vehicle treated mice (n = 6-8). *p < 0.01 via Bonferroni post hoc test. Data are mean ±SEM.
Figure 5. Effects of SR-8993 on yohimbine-induced reinstatement of CPP.
Conditioned mice showed similar preference during the expression test, and repeated exintction testing atteuated the place preference (A-B). Yohimbine significantly reinstated the extinguished CPP, but SR-8993 injections 2 h (A) or 30 min (B) before the yohimbined-induced reinstatement test did not alter CPP scores compared to vehicle (n = 6-7). *p < 0.05 via Bonferroni post hoc test. Data are mean ±SEM.
Discussion
Recently, we demonstrated that administration of the selective NOP receptor agonist SR-8993 impaired consolidation of fear memories in animal models of post-traumatic stress disorder (Andero et al., 2013). Given that previous studies identified a role for nociceptin in the rewarding properties of cocaine (Kotlinska et al., 2002; Marquez et al., 2008; Rutten et al., 2010), and that similar neural mechanisms are known to be involved in stress- and reward-related behaviors (Koob and Kreek, 2007), we sought to determine whether SR-8993 would also affect behaviors related to cocaine reward and reinstatement. Using a conditioned place preference (CPP) procedure, we found that SR-8993 when injected 30 min or 2 h before testing did not significantly affect acquisition and expression of cocaine CPP when mice were conditioned 7.5 or 15 mg/kg of cocaine, nor did SR-8993 affect extinction, stress- or cocaine-induced reinstatement of CPP. These results indicate that selectively targeting the nociceptin receptor may not be sufficient for reducing cocaine reward-related behaviors.
Previous studies using nociceptin receptor agonists in cocaine-related behaviors have obtained mixed results. For example, Kotlinska and colleagues reported that intracranial delivery of nociceptin attenuated the expression of cocaine CPP, but intraperitoneal administration of the NOP agonist, Ro65-6570, had no significant effect under the same conditions (Kotlinska et al., 2002). Similarly, we also found that SR-8993 had no effect of expression of cocaine CPP. In a more recent study testing the effects of Ro65-6570 on the acquisition of cocaine CPP in rats, intraperitoneal delivery of Ro65-6570 increased the minimal effective dose of cocaine (but not dexamphetamine) required to induce a place preference (Rutten et al., 2010). However, Ro65-6570's affinity for NOP, mu- and delta-opioid receptors (Hashiba et al., 2001) prevents extrapolation that the effects of the drug are only due to nociceptin receptor agonism. Indeed, other mixed-action opioid/NOP compounds, such as buprenorphine, also attenuate the rewarding properties of cocaine (Kosten et al., 1991; Carroll and Lac, 1992; Sorge et al., 2005; Sorge and Stewart, 2006; Placenza et al., 2008). Here, we used SR-8993, which is known to have minimal affinity at mu and delta receptors, and at the concentrations used in the present study, SR-8993 is not likely to engage the other opioid receptors (Andero et al., 2013). Yet, it still did not affect acquisition of cocaine CPP. Thus, our current data indicates that selective activation of NOP with SR-8993 is not sufficient for altering behavioral responses to cocaine and that compounds with mixed NOP/opioid affinity are likely required to attenuate cocaine CPP.
NOP in reinstatement of drug-seeking behaviors
The effects of nociceptin receptor agonists on reinstatement and extinction of drug-seeking behaviors have also varied depending on the type of drug used. Shoblock and colleagues revealed that Ro64-6189 attenuated drug-primed reinstatement of morphine CPP but had no affect on extinction (Shoblock et al., 2005). In alcohol studies, Ro64-6198 reduced alcohol-induced reinstatement of CPP (Kuzmin et al., 2003), while the NOP receptor agonist MT-7716 prevented context- and stress-induced reinstatement of alcohol self-administration (Ciccocioppo et al., 2014; de Guglielmo et al., 2014). In another set of studies, intracranial delivery of nociceptin inhibited footshock- and cue-induced reinstatement of alcohol but not cocaine self-administration (Martin-Fardon et al., 2000; Ciccocioppo et al., 2004). Although SR-8993 did not affect extinction or reinstatement of cocaine CPP in our current study, our preliminary unpublished findings indicate that SR-8993 blocks stress- (yohimbine) and cue-induced reinstatement of alcohol self-administration (Aziz et al., 2014). Together, these data signify that selectively targeting the nociceptin system may attenuate relapse behaviors associated with morphine and alcohol but not cocaine.
Mixed-action NOP/opioid ligands as a potential treatment for cocaine dependence
Stimulation of the nociceptin receptor along with other opioid receptors may be necessary for therapeutic efficacy in treating cocaine addiction. For example, in animal studies, buprenorphine, a partial agonist at the mu-opioid and NOP receptors and antagonist at kappa- and delta opioid receptors (Cowan et al., 1977; Sadee et al., 1982), has been shown to attenuate cocaine self-administration, CPP and sensitization (Kosten et al., 1991; Carroll and Lac, 1992; Sorge et al., 2005; Sorge and Stewart, 2006; Placenza et al., 2008). In clinical studies, high doses of buprenorphine, such as those that would be required to activate NOP, showed efficacy in treating cocaine use in addicted individuals (Schottenfeld et al., 1993). Buprenorphine in combination with naltrexone (to block buprenorphine's activity at the mu opioid receptor) also reduced cocaine self-administration under short and extended access conditions and reinstatement of cocaine CPP in animals (Mello et al., 1993; Wee et al., 2012; Cordery et al., 2014), suggesting that buprenorphine's affinity for receptors other than mu (potentially kappa, delta and/or NOP) contribute to its anti-addiction effects. Therefore, treatment of cocaine addiction may be more effectively achieved by mixed agonist-antagonist opioid receptor modulators.
Methods and materials
Animals
Male c57bl/6 mice (8-10 weeks old, Charles River Laboratories) were housed 2-4 animals per cage under a regular 12h/12h light/dark cycle and had ad libitum access to food and water. Mice were housed in a humidity and temperature-controlled, AAALAC-accredited, animal facility at the University of Miami Miller School of Medicine. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) and conducted according to specifications of the NIH as outlined in the Guide for the Care and Use of Laboratory Animals.
Drug Treatments
Cocaine HCl (NIDA, Research Triangle Park, NC) was dissolved in 0.9% sterile saline. Animals received one intraperitoneal (i.p.) injection of saline or cocaine (7.5, 10 or 15 mg/kg) during behavioral testing. The nocicpetin receptor agonist SR-8993 and naloxone (Tocris) was dissolved in saline; 3-10 mg/kg of SR-8993 and 5 mg/kg of naloxone was given in a volume of 0.08 - 0.1 ml in behavioral tests described below. Vehicle (saline) was delivered at the same volume as the drug solution. SR-8993 is highly selective when tested in a panel of GPCRs, ion channels and transporters and showed >50-fold selectivity over all receptors tested (Andero et al., 2013). The doses for all drugs were based on their salt form.
Conditioned place preference
In conditioned place preference experiments, we utilized previously published methods (Sartor and Aston-Jones, 2012; Sartor et al., 2015). Briefly, the CPP apparatus consisted of 2 distinct environments that were separated by a removable divider. In a pre-test, mice freely explored both environments for 15 minutes via an opening in the divider. EthoVision tracking software was used to measure the time spent on each side of the CPP chamber. Groups were then organized such that mean baseline pre-test scores were not different between treatments. On the next 3 days of conditioning, mice were injected with cocaine (7.5 or 15 mg/kg, i.p.) and confined to one side of the chamber by a solid divider for 30 minutes, or injected with saline and restricted to the other side of the chamber for 30 minutes. As a control, additional mice received a saline injection on both sides of the chamber. Injections during conditioning were administered in a balanced fashion in morning and afternoon sessions (at least 4 h apart). Conditioned animals were then given a 15-minute post-test. To test the role of SR-8993 in the acquisition of cocaine CPP, vehicle or SR-8993 (3 or 10 mg/kg, i.p.) was injected 30 min or 2 h before each cocaine conditioning session. To test CPP expression, SR-8993 (3 or 10 mg/kg, i.p.) or vehicle was injected 30 min or 2 h before the CPP test. Injecting 30 min or 2 h before testing was based on SR-8993's half-life and previous behavioral data showing that this regimen reduced fear conditioning and ethanol-seeking in rodents (Andero et al., 2013; Aziz et al., 2014). To demonstrate that we could attenuate cocaine CPP, naloxone (NLX, 5 mg/kg, i.p.) was given 10 min before cocaine conditioning sessions as it has previously been shown to reduce cocaine place preference (Kim et al., 1997).
For extinction experiments, conditioned animals were first given a drug-free preference test (session 1) in order to confirm CPP and to balance treatment groups based on initial CPP scores. For the next 5 sessions (one session per day, sessions 2-6) SR-8993 (10 mg/kg) or vehicle was administered 30 min or 2 h before each passive extinction test in which each animal had free access to both sides of the chamber. In reinstatement experiments, additional animals were passively extinguished by giving a drug-free CPP test each day until preference scores were reduced by at least 50% for two consecutive days. In cocaine-primed reinstatement tests, SR-8993 (10 mg/kg) or vehicle was administered 30 min or 2 h before the cocaine-primed (10 mg/kg) reinstatement test, and cocaine was injected immediately before testing. As a control, additional mice received vehicle injections (vehicle/vehicle group) prior to the reinstatement test. For stress-induced reinstatement, SR-8993 (10 mg/kg) or vehicle was administered 30 min or 2 h before yohimbine-induced (2 mg/kg, i.p.) reinstatement test. Yohimbine was injected 30 min before testing.
Locomotor test
Distance traveled was measured in a 27 × 27 cm open field chamber using EthoVision tracking software. Baseline locomotor behavior was measured in a 15 minute habituated test. Animals were grouped such that baseline locomotor activity did not differ between groups. Next, animals were injected with SR-8993 (10 mg/kg) or vehicle and were placed back in their home cage. Two hours later (when SR-8993 concentration peaks in the brain), animals received another 15-minute locomotor test.
Data Analysis
Graphpad Prism software was used for graph preparation and statistical analysis. Preference scores were analyzed by calculating the time spent in the cocaine-paired side on post-test minus the time spent in the cocaine-paired side during pre-test. In acquisition and expression experiments, mean values from CPP preference scores were compared between groups using a one-way analysis of variance (ANOVA). Extinction data were analyzed with a two-factor repeated measures ANOVA with group as the between-subjects factor and session number as the repeated factor. A two-factor repeated measures ANOVA with group as the between-subjects factor and test condition (expression, extinction and reinstatement) as the repeated factor was used to compare groups during reinstatement. When a significant F-value was obtained, comparisons were carried out using Newman-Keuls or Bonferroni post hoc analysis. Data are mean ±SEM, and the level of significance was set to 0.05.
Highlights.
We tested the selective NOP agonist, SR8993, in cocaine reward-related behaviors.
SR8993 failed to attenuate cocaine CPP acquisition, expression and reinstatement.
Selective activation of NOP may not be sufficient in reducing cocaine reward.
Acknowledgements
This research was supported by National Institute on Drug Abuse Grant R01DA035055. The cocaine used in these studies was obtained from the NIDA Drug Supply Program. S.K.P. was supported by Howard Hughes Medical Institute (HHMI) Undergraduate Research Program. G.C.S., C.W. and S.P.B. designed the experiments. G.C.S., S.K.P., H.J.W. and S.P.B. conducted the experiments. G.C.S., S.K.P., and S.P.B. wrote the paper. We thank Juliette Pozzuoli for her assistance in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M, Bannister TD, Almli L, Stevens JS, Bradley B, Binder EB, Wahlestedt C, Ressler KJ. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci Transl Med. 2013;5:188ra173. doi: 10.1126/scitranslmed.3005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla H, Cannarsa R, Romualdi P, Ciccocioppo R, Martin-Fardon R, Weiss F. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol. 2013;18:467–479. doi: 10.1111/j.1369-1600.2012.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz AM, Brothers SP, Holm L, Heilig M, Wahlestedt C, Thorsell A. A novel, peripherally available Nociceptin/Orphanin FQ receptor agonist SR-8993 reverses hangover anxiety, attenuates alcohol intake, and prevents reinstatement in Wistar rats. SfN meeting planner. 2014;428.04 [Google Scholar]
- Bebawy D, Marquez P, Samboul S, Parikh D, Hamid A, Lutfy K. Orphanin FQ/nociceptin not only blocks but also reverses behavioral adaptive changes induced by repeated cocaine in mice. Biol Psychiatry. 2010;68:223–230. doi: 10.1016/j.biopsych.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Effects of buprenorphine on self-administration of cocaine and a nondrug reinforcer in rats. Psychopharmacology (Berl) 1992;106:439–446. doi: 10.1007/BF02244812. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Stopponi S, Economidou D, Kuriyama M, Kinoshita H, Heilig M, Roberto M, Weiss F, Teshima K. Chronic Treatment with Novel Brain-Penetrating Selective NOP Receptor Agonist MT-7716 Reduces Alcohol Drinking and Seeking in the Rat. Neuropsychopharmacology. 2014;39:2601–2610. doi: 10.1038/npp.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordery SF, Taverner A, Ridzwan IE, Guy RH, Delgado-Charro MB, Husbands SM, Bailey CP. A non-rewarding, non-aversive buprenorphine/naltrexone combination attenuates drug-primed reinstatement to cocaine and morphine in rats in a conditioned place preference paradigm. Addict Biol. 2014;19:575–586. doi: 10.1111/adb.12020. [DOI] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, Macfarlane IR. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977;60:537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Martin-Fardon R, Teshima K, Ciccocioppo R, Weiss F. MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict Biol. 2014 doi: 10.1111/adb.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S. Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–1130. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- Economidou D, Fedeli A, Fardon RM, Weiss F, Massi M, Ciccocioppo R. Effect of novel nociceptin/orphanin FQ-NOP receptor ligands on ethanol drinking in alcohol-preferring msP rats. Peptides. 2006;27:3299–3306. doi: 10.1016/j.peptides.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, Martin-Fardon R, Weiss F, Massi M, Ciccocioppo R. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res. 2011;35:747–755. doi: 10.1111/j.1530-0277.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiba E, Harrison C, Galo G, Guerrini R, Rowbotham DJ, Smith G, Lambert DG. Characterisation and comparison of novel ligands for the nociceptin/orphanin FQ receptor. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:28–33. doi: 10.1007/s002100000327. [DOI] [PubMed] [Google Scholar]
- Kim HS, Park WK, Jang CG, Oh KW, Kong JY, Oh S, Rheu HM, Cho DH, Kang SY. Blockade by naloxone of cocaine-induced hyperactivity, reverse tolerance and conditioned place preference in mice. Behav Brain Res. 1997;85:37–46. doi: 10.1016/s0166-4328(96)00162-3. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Marby DW, Nestler EJ. Cocaine conditioned place preference is attenuated by chronic buprenorphine treatment. Life Sci. 1991;49:PL201–206. doi: 10.1016/0024-3205(91)90490-3. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Suder P, Legowska A, Rolka K, Silberring J. Orphanin FQ/nociceptin inhibits morphine withdrawal. Life Sci. 2000;66:PL119–123. doi: 10.1016/s0024-3205(99)00648-7. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Wichmann J, Legowska A, Rolka K, Silberring J. Orphanin FQ/nociceptin but not Ro 65-6570 inhibits the expression of cocaine-induced conditioned place preference. Behav Pharmacol. 2002;13:229–235. doi: 10.1097/00008877-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Rafalski P, Biala G, Dylag T, Rolka K, Silberring J. Nociceptin inhibits acquisition of amphetamine-induced place preference and sensitization to stereotypy in rats. Eur J Pharmacol. 2003;474:233–239. doi: 10.1016/s0014-2999(03)02081-8. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. The nociceptin/orphanin FQ receptor agonist Ro 64-6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology. 2007;32:902–910. doi: 10.1038/sj.npp.1301169. [DOI] [PubMed] [Google Scholar]
- Lachowicz JE, Shen Y, Monsma FJ, Jr., Sibley DR. Molecular cloning of a novel G protein-coupled receptor related to the opiate receptor family. J Neurochem. 1995;64:34–40. doi: 10.1046/j.1471-4159.1995.64010034.x. [DOI] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Khaliq I, Carroll FI, Maidment NT. Orphanin FQ/nociceptin blocks cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2002;164:168–176. doi: 10.1007/s00213-002-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Nguyen AT, Hamid A, Lutfy K. The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology. 2008;54:564–568. doi: 10.1016/j.neuropharm.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol-but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH, Drieze J. Naltrexone-buprenorphine interactions: effects on cocaine self-administration. Neuropsychopharmacology. 1993;9:211–224. doi: 10.1038/npp.1993.57. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr., Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999a;406:503–547. [PubMed] [Google Scholar]
- Neal CR, Jr., Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999b;412:563–605. [PubMed] [Google Scholar]
- Placenza FM, Rajabi H, Stewart J. Effects of chronic buprenorphine treatment on levels of nucleus accumbens glutamate and on the expression of cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2008;200:347–355. doi: 10.1007/s00213-008-1210-z. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr., Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Rutten K, De Vry J, Bruckmann W, Tzschentke TM. Effects of the NOP receptor agonist Ro65-6570 on the acquisition of opiate- and psychostimulant-induced conditioned place preference in rats. Eur J Pharmacol. 2010;645:119–126. doi: 10.1016/j.ejphar.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Sadee W, Rosenbaum JS, Herz A. Buprenorphine: differential interaction with opiate receptor subtypes in vivo. J Pharmacol Exp Ther. 1982;223:157–162. [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci. 2012;32:4623–4631. doi: 10.1523/JNEUROSCI.4561-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor GC, Powell SK, Brothers SP, Wahlestedt C. Epigenetic Readers of Lysine Acetylation Regulate Cocaine-Induced Plasticity. J Neurosci. 2015;35:15062–15072. doi: 10.1523/JNEUROSCI.0826-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes J, Ziedonis D, Kosten TR. Buprenorphine: dose-related effects on cocaine and opioid use in cocaine-abusing opioid-dependent humans. Biol Psychiatry. 1993;34:66–74. doi: 10.1016/0006-3223(93)90258-f. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Wichmann J, Maidment NT. The effect of a systemically active ORL-1 agonist, Ro 64-6198, on the acquisition, expression, extinction, and reinstatement of morphine conditioned place preference. Neuropharmacology. 2005;49:439–446. doi: 10.1016/j.neuropharm.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The effects of chronic buprenorphine on intake of heroin and cocaine in rats and its effects on nucleus accumbens dopamine levels during self-administration. Psychopharmacology (Berl) 2006;188:28–41. doi: 10.1007/s00213-006-0485-1. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Rajabi H, Stewart J. Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology. 2005;30:1681–1692. doi: 10.1038/sj.npp.1300712. [DOI] [PubMed] [Google Scholar]
- Toll L, Khroyan TV, Polgar WE, Jiang F, Olsen C, Zaveri NT. Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu opioid receptor ligands: implications for therapeutic applications. J Pharmacol Exp Ther. 2009;331:954–964. doi: 10.1124/jpet.109.157446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Vendruscolo LF, Misra KK, Schlosburg JE, Koob GF. A combination of buprenorphine and naltrexone blocks compulsive cocaine intake in rodents without producing dependence. Sci Transl Med. 2012;4:146ra110. doi: 10.1126/scitranslmed.3003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann J, Adam G, Rover S, Hennig M, Scalone M, Cesura AM, Dautzenberg FM, Jenck F. Synthesis of (1S,3aS)-8-(2,3,3a,4,5, 6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4. 5]decan-4-one, a potent and selective orphanin FQ (OFQ) receptor agonist with anxiolytic-like properties. Eur J Med Chem. 2000;35:839–851. doi: 10.1016/s0223-5234(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, Tucker RC, Ciccocioppo R. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther. 2014;141:283–299. doi: 10.1016/j.pharmthera.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollemann M, Benyhe S. Non-opioid actions of opioid peptides. Life Sci. 2004;75:257–270. doi: 10.1016/j.lfs.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Zaveri NT. The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications. Curr Top Med Chem. 2011;11:1151–1156. doi: 10.2174/156802611795371341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RJ, Woo RS, Jeong MS, Shin BS, Kim DG, Kim KW. Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport. 2003;14:2383–2385. doi: 10.1097/00001756-200312190-00019. [DOI] [PubMed] [Google Scholar]