Abstract
Objective
The purpose of this study was to test the efficacy of a tailored motivational interviewing (MI) intervention versus usual care for improving HF self-care behaviors, physical HF symptoms and quality of life.
Methods
This is a single-center, randomized controlled trial. Participants were enrolled in the hospital. Immediately after discharge, those in the intervention group received a single home visit and 3–4 follow-up phone calls by a nurse over 90 days.
Results
A total of 67 participants completed the study (mean age 62 ± 12.8 years), of which 54% were African American, 30% were female, 84% had class III/IV symptoms, and 63% were educated at a high school level or less. There were no differences between the groups in self-care maintenance, self-care confidence, physical HF symptoms, or quality of life at 90 days.
Conclusion
Patients who received the MI intervention had significant and clinically meaningful improvements in HF self-care maintenance over 90 days that exceeded that of usual care.
Practice Implications
These data support the use of a nurse-led MI intervention for improving HF self-care. Identifying methods to improve HF self-care may lead to improved clinical outcomes.
Keywords: motivational interviewing, behavior, heart failure, self care, self efficacy, quality of life, diet sodium-restricted
Heart failure (HF) affects more than 5.7 million Americans [1] and costs the United States $39.2 billion annually [2]. HF is currently the most common reason for the hospitalization of Medicare recipients [3–6]. The costs for preventable readmissions are estimated to be about $17 billion or 20% of Medicare’s hospital payments [7]. Patients with HF are frequently admitted to the hospital because they experience exacerbations in symptoms with fluid retention, shortness of breath, and fatigue on exertion [8]. Considering the increasing prevalence, cost and social burden to patients and their families, interventions that incorporate self-care with effective medical therapy are critical to optimize patient health and improve patient outcomes [9–11].
The Situation-Specific Theory of Heart Failure Self-Care specifies three unique aspects of HF self-care: maintenance (routine behaviors associated with treatment adherence), symptom perception (body listening, monitoring, recognition, interpretation and labeling of symptoms) and management (response to symptoms) [12–14]. Confidence or self-efficacy has been shown to be an important element contributing to success in the performance of self-care [15]. Effective self-care behaviors have the potential to reduce hospitalizations and improve quality of life. Several studies have examined the impact of self-care interventions on patient-oriented and clinical outcomes including self-care behaviors [16], self-efficacy [17], quality of life [17–20], physical activity [21], health status [22], hospitalizations [23, 24], mortality [20], myocardial stress [25] and systemic inflammation [26]. Overall, there has been an apparent lack of effectiveness of education alone on outcomes related to HF self-care. One reason is that patients face a number of prohibitive barriers to mastering HF self-care skills and knowledge, including cognitive impairment, excessive daytime sleepiness, low-health literacy [27–29], and poor motivation [30]. One suggested approach is to use motivational interviewing (MI) [16, 31], a counseling approach grounded in client-centered counseling, cognitive-behavioral therapy, and social cognitive therapy [32]. MI assesses a patient’s readiness to change behavior and develops strategies to move toward taking action to change behavior [33].
The purpose of this randomized controlled trial (Motivational Interviewing Tailored Intervention for Heart Failure [MITI-HF]) was to test a tailored MI intervention designed to improve self-care compared with usual care. The main hypothesis was that HF patients enrolled in the group receiving the tailored MI intervention would improve in self-care maintenance after 90 days. Secondary outcomes included physical HF symptoms and quality of life.
Methods
Study design
MITI-HF was a prospective, single-blinded, randomized controlled trial. The University’s Institutional Review Board approved the study. Participants were actively enrolled from January 2012 to December 2013. Detailed description of study methods including participant eligibility, recruitment procedures and data collection have been registered (Clinicaltrials.gov ID: NCT02177656), reported in a study design paper [34] and are summarized here. The target recruitment size was 66 participants, a sample size calculated to provide 90% power (5% alpha) to detect a difference of 80% versus 50% (intervention and control group) in the likelihood of scoring over 70 on the self-care maintenance scale in the Self-Care of Heart Failure Index (SCHFI) v.6.2 at 90 days. The estimated attrition was 35%, based on previous studies in this population [16], so participants were overenrolled to account for anticipated attrition. The power analysis was performed using G*Power [35] and confirmed with PASS [36].
Procedure
Potential participants were approached during an inpatient HF-related hospitalization at a University affiliated urban hospital. The study inclusion and exclusion criteria are shown in Table 1. All eligible patients were screened for health literacy [37], cognitive impairment (using a six-item screener derived from the Mini Mental Status Exam) [38], baseline self-care (using the SCHFI v.6.2) [39], and symptomatic status (using a standardized interview to assess New York Heart Association (NYHA) functional class) [40]. Health literacy was measured with three screening questions (e.g., “How often do you have someone help you read hospital materials?”) [37]. Responses of never, occasionally, sometimes, often, always are scaled 0 to 4. These questions have been shown to be sensitive to poor health literacy in multiple patient populations (receiver operating curves (0.87, 0.80, 0.76)) [37, 41].
Table 1.
Eligibility Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Abbreviations: HF: heart failure, NYHA: New York Heart Association, VAD: ventricular assist device
Participant characteristics of age, gender, co-morbid conditions, prescribed medications, diagnostic lab tests, and echocardiogram results were obtained from the medical record. During baseline interviews research assistants obtained information from the participant on race/ethnicity, insurance status, years of education, perceived and self-rated health compared to one-year ago. Participants were also asked about the quality of their social support and responses included fair, satisfactory, good or very good. Traditional questions about income characteristics (sources, amounts received) have been wrought with a wide range of bias [42] and random error [43], so income was measured with the question, “Financially, would you say you are: comfortable; have more than enough to make ends meet; have enough to make ends meet; or do not have enough to make ends meet?”
Those who met the inclusion criteria and agreed to participate provided written informed consent. To standardize care across the groups, all participants received a set of educational fliers described further below. Participants were then randomized by minimization [44] with stratification by NYHA functional class and gender to the intervention or control group in a 2:1 randomization ratio [45].
Baseline data were collected approximately two weeks after hospital discharge. Two research assistants (blinded to study group allocation) called participants to obtain socio-demographic information and to administer the baseline questionnaires. If participants did not complete the baseline data collection they were not enrolled in the study. Approximately 90 days after the baseline call, participants in both groups were called to complete all of the follow-up questionnaires. If the first follow-up call was unsuccessful, the research assistant would try every 3 to 5 days for up to 60 days. If there was no contact with the participant after 60 days from the expected follow-up date, the participant was considered lost to follow-up.
Usual Care
Individuals randomized to the usual care group received care as usual from their respective care providers. To standardize care across the study groups all participants received patient education materials designed by Krames StayWell. These materials were designed to assist patients to identify and address self-care barriers, maintain a lower sodium diet, and lead an active lifestyle. All of the educational sheets targeted goal behavior changes through participant interaction, such as writing down the names of support people who would help them see habits that might block their progress toward change.
Intervention Description
As described in detail elsewhere [46], participants assigned to the intervention group received an MI tailored intervention that included a home-based MI intervention and 3–4 follow-up phone calls over the course of 90 days. During the home visit the nurse worked with the participant using an MI approach to identify at least two specific client-centered goals related to HF self-care. After establishing the client-directed plan for accomplishing the goals it was reinforced in the follow-up phone calls. For example, if a participant said that one of his goals was to be able to attend his grandson’s football games in the fall, the nurse tailored the intervention around smaller daily goals focused on improving physical activity. The day-to-day self-care goals were considered relevant to the participant because they were contextualized as part of his self-defined goal of attending his grandson’s football games.
Study Outcome Measures
Self-care
Self-care was measured using the SCHFI v. 6.2, a 22-item instrument that quantifies HF self-care maintenance, self-care management, and self-care confidence (self-efficacy) [14, 39]. The SCHFI was written for a sixth grade reading level and takes less than 10 minutes to complete. Scores on each scale are standardized to range from 0 to 100—higher scores indicate better self-care. A score of 70 or greater on each scale is considered adequate and an improvement of 8 points is considered clinically meaningful [39]. The reliability coefficient for the self-care maintenance scale is 0.78 [47] and construct validity scores are 0.92 for self-care maintenance and 0.99 for self-care confidence [48].
Acute Physical Heart Failure Symptoms
Acute physical HF symptoms were measured with the heart failure somatic perception scale (HFSPS), which asks about distress associated with 18 common symptoms of HF during the previous week. Responses range from 0 (I did not have this symptom) to 5 (extremely bothersome) [49]. The total HFSPS score ranges from 0 to 90 with higher scores indicating worse physical symptom distress [50]. In addition to the total score, which has good reliability (Cronbach’s α 0.90) [51], the scale also has two separate domains, dyspnea (6-items, range 0–30 points) and early/non-specific symptoms of congestion (7-items, range 0–35 points), both of which are associated with survival at 180 and 365-days [50].
Quality of life
Quality of life was measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ), which has 23 items that can be quantified into five subscales: physical limitations, symptoms, quality of life, social interference, and self-efficacy. Each domain-specific subscale and the overall clinical summary score range from 0 to 100 (higher scores indicate better outcomes) [52]. In a comparable sample of patients with heart failure, the internal consistency of the KCCQ was high (Cronbach’s α 0.92) [53]. Construct validity has been established with NYHA class, Medical Outcomes Study Short Form-36 Health Survey and the six minute-walk-test [52].
Data Analysis
Standard descriptive statistics were used to describe all study covariates at baseline. Chi square and student’s t-tests were used to examine differences based on group assignment. Baseline demographic and clinical characteristics between the two study groups were assessed to determine adequacy of randomization. Response bias was assessed by comparing participants who completed the study to those who were lost to follow-up. Students t-tests were used to assess for change in self-care maintenance and self-care confidence, physical HF symptoms and quality of life between baseline and 90 days between groups. Cohen’s d was calculated as a standardized index of effect sizes.
For the primary outcome (self-care maintenance) a model comparison approach [54] was applied throughout the model building process including a priori factors (gender, age, NYHA class, race, marital status, left ventricular ejection fraction (LVEF) and having a home care nurse) and covariates associated in bi-variate analyses with the outcome variable (p<0.05). Factors that were considered in the models but were not significant and did not contribute to the robustness of the model were removed systematically using a manual backwards elimination process. The final multiple linear regression model for predictors of change in self-care maintenance included the following variables: intervention group, LVEF, sleep apnea, gender, hypertension, perceived general health and quality of social support. These seven variables were adjusted in the main analyses of group differences over time. Statistical interactions by group and gender were also evaluated. Data analyses were conducted using StataSE 13.1 (College Station, Texas).
Results
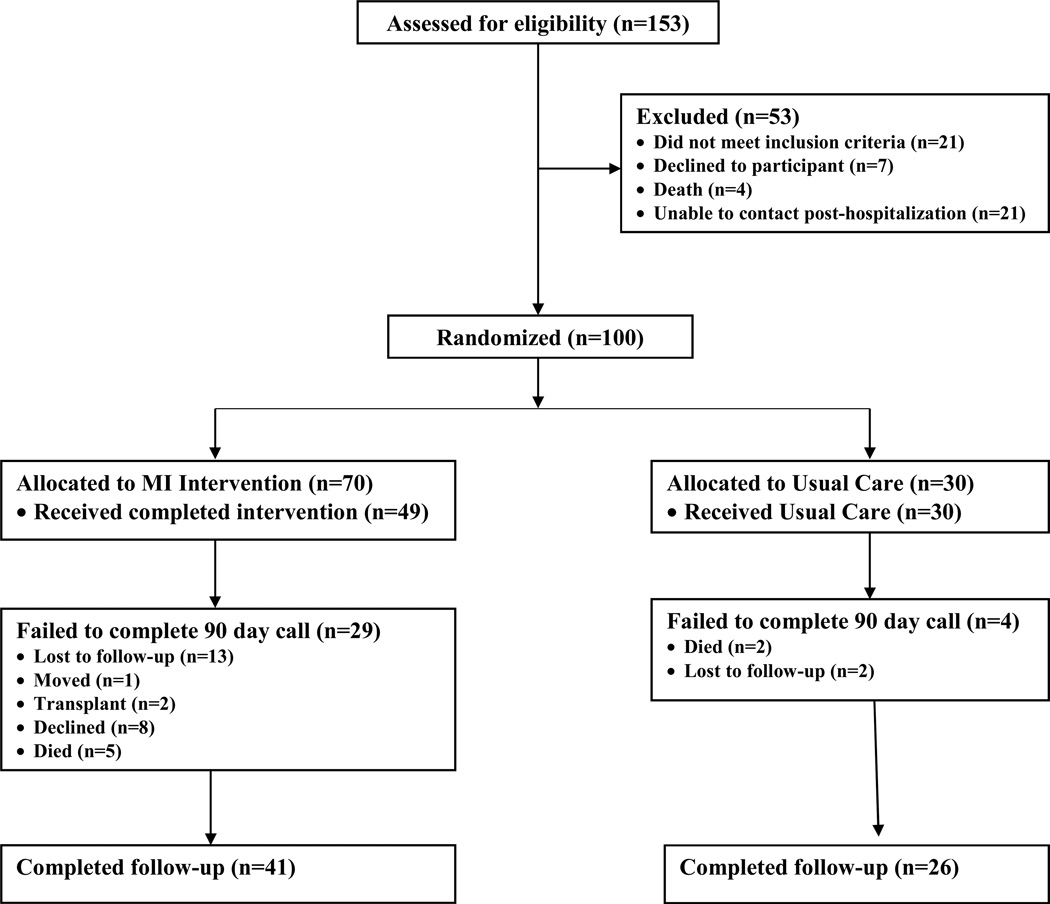
The CONSORT diagram (Figure 1) reflects participants who were screened, enrolled, randomized and included in the analyses for self-reported outcomes between the two groups. A total of 100 participants were enrolled and 67 completed the study of self-reported outcomes. The overall attrition rate was 33% (n=33), (13% in the usual care group and 42% in the intervention group) consistent with other studies of patients with HF [55]. There were no statistically significant differences in the socio-demographic or clinical characteristics of participants who completed versus did not complete the study. There were also no statistically significant differences in the self-care, physical HF symptom or quality of life at baseline between those who completed and did not complete the study. Thus, there was no evidence to suggest a violation of the missing at random assumption.
Figure 1.
CONSORT diagram.
Participants who completed the study were predominantly male (60%) and African American (>50%) with a mean age of 62 years. The majority was functionally compromised (83.6% NYHA Class III/IV) with a mean left ventricular ejection fraction (LVEF) < 36 percent (Tables 2a & 2b). Most participants had no more than a high school education and reported poor or fair health. Participants were on an average of 12 daily medications and had an average of 5.5 comorbid conditions.
Table 2.
| a. Baseline socio-demographic characteristics by randomization group | ||||
|---|---|---|---|---|
| Randomization group (mean +/− SD or %) | ||||
| Variables | Overall Total (N=67) |
Control Total (N=26) |
Intervention Total (N=41) |
p-value |
| Age | 62 (13.4) | 63 (12.6) | 60 (13.9) | 0.397 |
| Gender | 0.130 | |||
| Female | 20 (29.9) | 5 (19.3) | 15 (36.6) | |
| Male | 47 (70.2) | 21 (80.8) | 26 (63.4) | |
| Married/Partnered | 31 (46.3) | 15 (57.7) | 16 (39.0) | 0.135 |
| Race | 0.128 | |||
| Black | 36 (53.7) | 17 (65.4) | 19 (46.3) | |
| White | 31 (46.3) | 9 (34.6) | 22 (53.7) | |
| Education | 0.233 | |||
| ≤High School | 42 (62.7) | 14 (53.9) | 28 (68.3) | |
| College/Grad School | 25 (37.3) | 12 (46.2) | 13 (31.7) | |
| Total years education | 13 (2.3) | 13 (2.2) | 13 (2.4) | |
| Employment Status | 0.834 | |||
| Employed/Retired | 32 (47.8) | 12 (46.2) | 20 (48.8) | |
| Unemployed/Disabled | 35 (52.2) | 14 (53.9) | 21 (51.2) | |
| Financial Status | 0.435 | |||
| Comfortable/Enough | 45 (67.2) | 16 (61.5) | 29 (70.7) | |
| Not enough | 22 (32.8) | 10 (38.5) | 12 (29.3) | |
| Insurance Type | 0.419 | |||
| Government | 50 (74.6) | 18 (69.2) | 32 (78.1) | |
| Commercial | 17 (25.4) | 8 (30.8) | 9 (22.0) | |
| Health Perception | 0.088 | |||
| Poor/Fair | 49 (73.1) | 16 (61.5) | 33 (80.5) | |
| Good/Very Good/Excellent | 18 (26.9) | 10 (38.5) | 8 (19.5) | |
| Health in General | 0.746 | |||
| Worse/Same | 37 (55.2) | 15 (57.7) | 22 (53.7) | |
| Better/Much Better | 30 (44.8) | 11 (42.3) | 19 (46.3) | |
| Home Health Nurse | 49 (73.1) | 18 (69.2) | 31 (75.6) | 0.566 |
| Provider Specialty | 0.292 | |||
| Medicine/Cardiology | 16 (23.9) | 8 (30.8) | 8 (19.5) | |
| HF Specialist | 51 (76.1) | 18 (69.2) | 33 (80.5) | |
| Lives with another | 51 (76.1) | 21 (80.8) | 30 (73.2) | 0.477 |
| Support Quality | 0.241 | |||
| Fair/Satisfactory | 11 (16.4) | 6 (23.1) | 5 (12.2) | |
| Good/Very Good | 56 (83.6) | 20 (76.9) | 36 (87.8) | |
| Nurse Interventionist | 0.902 | |||
| Nurse 1 | 51 (76.1) | 20 (76.9) | 31 (75.6) | |
| Nurse 2 | 16 (23.9) | 6 (23.1) | 10 (24.4) | |
| b. Baseline clinical factors by randomization group | ||||
|---|---|---|---|---|
| Randomization group (mean +/− SD or %) | ||||
| Variables | Overall Total (N=67) |
Control Total (N=26) |
Intervention Total (N=41) |
p-value |
| NYHA Functional Class | 0.125 | |||
| Class I/II | 11 (16.4) | 2 (7.7) | 9 (22.0) | |
| Class III/IV | 56 (83.6) | 24 (92.3) | 32 (78.1) | |
| HF Etiology | 0.743 | |||
| Ischemic | 23 (36.5) | 9 (39.1) | 14 (35.0) | |
| Non-ischemic | 40 (63.5) | 14 (60.9) | 26 (65.0) | |
| HF Type | 0.419 | |||
| Systolic | 50 (74.6) | 18 (69.2) | 32 (78.1) | |
| Diastolic | 17 (25.4) | 8 (30.8) | 9 (22.0) | |
| Ejection Fraction (%) | 36 (18.14) | 39 (17.9) | 35 (18.3) | 0.393 |
| Charlson Comorbidity Index | 0.420 | |||
| Low (1–2) | 15 (22.4) | 8 (30.8) | 7 (17.1) | |
| Medium (3–4) | 34 (50.8) | 12 (46.2) | 22 (53.7) | |
| High (5–11) | 18 (26.9) | 6 (23.1) | 12 (29.3) | |
| Pacemaker (any type) | 21 (31.3) | 8 (30.8) | 13 (31.7) | 0.936 |
| Medications (total) | 12 (5.5) | 12 (5.6) | 12 (5.6) | 0.782 |
| Beta Blocker | 57 (85.1) | 24 (92.3) | 33 (80.5) | 0.186 |
| Ace Inhibitor/ARB | 39 (58.2) | 15 (57.7) | 24 (58.5) | 0.725 |
| Statin | 40 (59.7) | 16 (61.5) | 24 (58.5) | 0.807 |
| Diuretic | 59 (88.1) | 23 (88.5) | 36 (87.8) | 0.936 |
| Baseline Lab Values | ||||
| Sodium | 135.7 (15.6) | 137.6 (2.8) | 134.5 (19.9) | 0.434 |
| Hemoglobin | 11.6 (1.9) | 11.8 (1.8) | 11.4 (1.9) | 0.370 |
| BUN/Creatinine ratio | 20.6 (10.4) | 17.5 (10.0) | 22.5 (10.3) | 0.056 |
| Comorbid conditions | ||||
| Hypertension | 47 (70.2) | 20 (76.9) | 27 (65.9) | 0.335 |
| Atrial Fibrillation | 21 (31.3) | 9 (34.6) | 12 (29.3) | 0.646 |
| Diabetes | 33 (49.2) | 12 (46.1) | 21 (51.2) | 0.686 |
| Renal Disease | 43 (64.2) | 15 (57.7) | 28 (68.3) | 0.378 |
| COPD | 10 (14.9) | 2 (7.7) | 8 (19.5) | 0.186 |
| Depression | 3 (4.9) | 0 | 3 (7.3) | 0.158 |
| Chronic pain | 7 (10.5) | 4 (15.4) | 3 (7.3) | 0.293 |
Abbreviations HF: heart failure, NYHA: New York Heart Association, ARB: Angiotensin II Receptor Blockers,
BUN:blood urea nitrogen,
COPD: chronic obstructive pulmonary disease
Self-care Maintenance and Confidence
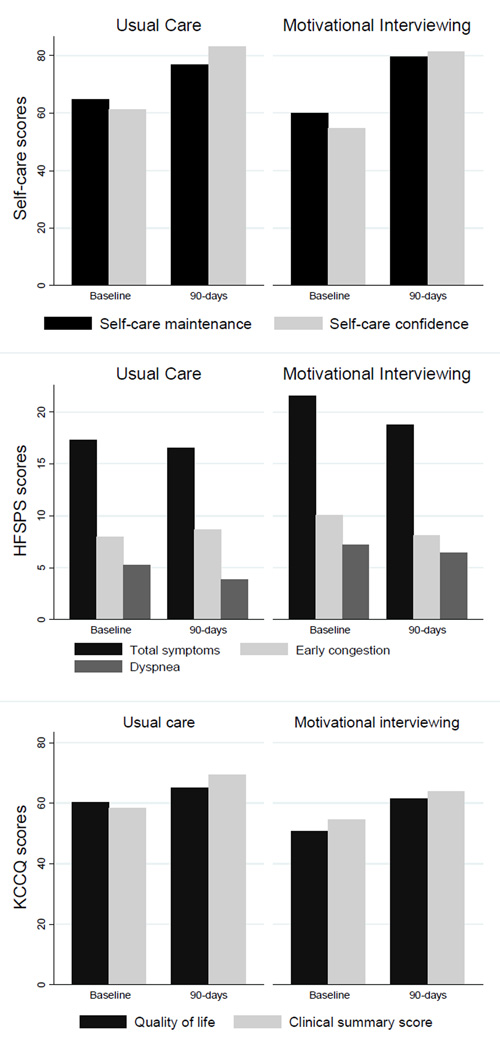
There was overall improvement in self-care maintenance in both groups over 90 days (intervention: 19.7 ± 16.0, usual care: 12.1 ± 18.3) (Table 3). The improvement in self-care maintenance was numerically greater in the intervention group compared with usual care (Figure 2). Though the effect size was moderate (Cohen’s d= 0.44), the difference between groups was not initially statistically significant. After adjusting for intervention group, LVEF, sleep apnea, gender, hypertension, perceived general health and quality of social support, there was a statistically significant 8.7-point increase (95% CI: 1.1 – 16.3, p=0.026) in the MI group compared to the usual care group at 90 days (Table 4). Patients with sleep apnea (β=17.9, p=0.020), worse perceived general health (β=13.5, p=0.002), or worse social support (β=10.6, p=0.042) also improved in self-care maintenance over 90-days after adjusting for other factors. In addition, for each unit increase in LVEF, the change in self-care maintenance decreased 0.32 points. There was no evidence of interactions by study group or gender.
Table 3.
Mean changes in outcomes from baseline to 90-days in the intervention and usual care groups (n=67).
| Variable | Intervention mean (SD) |
Usual care mean (SD) |
t-value (p) |
|---|---|---|---|
| Self-care maintenance | 19.7 (16.0) | 12.1 (18.3) | −1.8 (0.08) |
| Self-care confidence | 26.6 (20.8) | 21.6 (16.8) | −1.0 (0.31) |
| HFSPS Total Score | −2.8 (16.8) | −0.73 (17.1) | 0.5 (0.63) |
| KCCQ QOL | 10.8 (28.2) | 4.81 (21.4) | 0.9 (0.36) |
| KCCQ CSS | 9.3 (23.9) | 11.86 (20.9) | 0.4 (0.67) |
Abbreviations: SD: standard deviation; HFSPS: heart failure somatic perception scale; KCCQ: Kansas City Cardiomyopathy Questionnaire, QOL: quality of life, CSS: clinical summary score.
Figure 2.
Absolute change in outcomes at baseline and 90-days in the intervention and usual care groups.
Table 4.
Multiple linear regression model for predictors of change in self-care maintenance from baseline to 90-days (n=67).
| Independent Variables | β- coefficient |
SE | 95% CI | p-value |
|---|---|---|---|---|
| Intervention (ref control) | 8.69 | 3.80 | (1.09 to 16.30) | 0.026 |
| Left ventricular ejection fraction | −0.32 | 0.11 | (−0.53 to −0.11) | 0.004 |
| Sleep apnea (ref no sleep apnea) | 17.85 | 5.58 | (6.67 to 29.02) | 0.002 |
| Gender (ref male) | −1.80 | 4.22 | (−10.25 to 6.64) | 0.671 |
| Perceived general health (ref better health) | 13.49 | 3.80 | (5.86 to 20.77) | 0.002 |
| Hypertension (ref no hypertension) | 7.80 | 4.15 | (−0.50 to 16.11) | 0.065 |
| Quality of social support (ref good/very good) | 10.55 | 5.01 | (0.43 to 20.78) | 0.042 |
In both groups, self-care confidence improved more than 20 points, although the absolute change in self-care confidence was numerically higher in the intervention group compared with usual care (Cohen’s d=0.26) (Table 3). There were no statistically significant differences in improvement in self-care confidence between the two study groups (p=0.31).
Physical HF symptoms and quality of life
At 90 days, the sample mean HFSPS was 17.9 ± 18.1 with no differences between groups (p=0.63). For the early and non-specific symptoms of congestion scale and dyspnea sub-scales there were also no differences between groups. The difference in quality of life between groups was not significantly different between the groups (p=0.36) (Figure 2).
Discussion
The results of this randomized controlled trial designed to test the efficacy of a tailored MI intervention show that although there were no differences in the univariate analysis, there was a trend towards improved self-care maintenance for patients who received the MI intervention. These results support our hypothesis that motivating people with HF to take more control over their health using MI can help them achieve improved self-care [56].
Our results are similar to those of Ogedegbe and colleagues who tested whether hypertensive African American patients randomized to patient education alone or MI would have improved adherence to prescribed anti-hypertensive medication, one element of self-care maintenance [57, 58]. Their results demonstrated more improvement in medication adherence assessed with an electronic event-monitoring device over 12 months in the MI intervention compared to the control group [58], consistent with our results. The consistency in results between these two studies may be attributed to some similarities in study design, including the same number of MI sessions, similar racial demographics and a focus on a cardiovascular disease with a shared pathophysiology. Together, these studies suggest a benefit of using MI as a behavioral intervention for African American patients with hypertension and heart failure.
The MITI-HF results differ from those of the Osteoporosis Telephonic Intervention to Improve Medication Adherence (OPTIMA) trial. OPTIMA investigators examined the effectiveness of an MI based telephone-based counseling program to improve adherence to the medication regimen for osteoporosis [59]. These investigators found no significant improvement in medication adherence measured electronically. In addition to different patient populations, there are a few other critical differences between MITI-HF and OPTIMA that may explain the differences in results. As suggested by Lavoie in a letter to the editor regarding OPTIMA, one key tenet of behavioral trial design is targeting participants with evidence of poor behavior at the beginning of the trial [60]. As a pragmatic trial, OPTIMA enrolled any patient who received a new prescription regardless of baseline adherence. Some patients without problems with adherence were enrolled and this could have diluted the treatment effect. Participants enrolled in MITI-HF were all patients who had been hospitalized and reported “never/rarely” or only “sometimes” performing at least one or two self-care maintenance behaviors. Secondly, each of the 10 counseling sessions in the OPTIMA study had pre-specified educational topics which included a series of open-ended questions to elicit subjects’ attitudes and barriers [59]. In contrast, consistent with the MI approach, each of the counseling sessions in MITI-HF was driven by participant preference.
In MITI-HF, the MI intervention did not improve participants’ self-care confidence (self-efficacy) over time compared with usual care. In contrast, there is early evidence from a study by Paradis and colleagues that reports improvement in self-efficacy using an MI approach in patients with HF [61]. In the Paradis study, patients received a similar dose of MI from a nurse (one face-to-face MI intervention followed up by two telephone conversations). They reported no improvement in self-care maintenance but an improvement in self-care confidence after one month. Differences in study design between these two studies may explain the differences in self-care outcomes, including length of patient follow-up (30 versus 90 days) and at least one or two more follow-up MI phone calls in MITI-HF.
In MITI-HF, quality of life improved in both study groups over 90 days; however, there were no statistically significant differences between groups. This improvement, regardless of group, may reflect the known improvement in quality of life after discharge from an acute hospitalization. Consistent with MITI-HF, a study by Chair and colleagues tested MI in patients diagnosed with coronary heart disease in Hong Kong. They reported improvement in health-related quality of life across all subscales of the Medical Outcomes short-form-36 (SF-36) in both the MI and usual care groups with no differences between them [62]. The Chair study also reported no changes in clinical outcomes between groups (systolic or diastolic blood pressure, body mass index, multiple measures of cholesterol and triglycerides); however unlike MITI-HF, self-care was not measured except for medication adherence [62]. In another study of patients with HF randomized to a MI physical activity intervention or usual care, the results were mixed for changes in quality of life measured with both the SF-36 and Minnesota Living with Heart Failure questionnaire [17]. Overall, the results from all three studies of patients with cardiovascular diseases report similar findings. MI alone is most likely not enough to improve the quality of life of patients who are severely ill with chronic cardiovascular disease. This is not surprising given that these interventions were focused on specific aspects of self-care and a wide array of complex factors influence quality of life, which were not addressed in these studies. It is also possible that in a functionally compromised population of patients with severe HF that there is a ceiling effect of how much quality of life can improve over time due to the impact of worsening disease severity.
Limitations
A major limitation of the MITI-HF, a nurse-led intervention that included one inhome visit and 3–4 follow-up calls over a 90-day period, was the loss of participants to follow-up and specifically the difference in attrition for the self-reported outcomes between the usual care and MI group. One of the proposed reasons for differential dropout was that the MI group had at least 60% more points of contact than the usual care group, thus increasing opportunities for dropout. A consideration for evaluating a similar intervention in a future study would be to alter the number of, or duration between, points of contact in order to address the issue of dropout. In future studies, we will also collect feedback from participants who decline to participate to gain insight into reasons for declining participation. Another limitation was that objective measures of self-care behaviors (e.g. pedometer for exercise) were not used in this study. All of the comorbid conditions were abstracted from the medical record so the prevalence of depression and anxiety may also be underestimated in the sample.
Strengths of MITI-HF include high minority participation (over 50%), with women well represented. Future research is needed to determine if a similarly designed nurse-led MI intervention can be effective and cost-effective if implemented in a clinical practice setting rather than in the home.
Conclusion
This study reports a novel nurse-led behavioral intervention that uses MI to help patients with HF improve their self-care. Although there was no statistically significant difference in the primary outcome over 90-days, there was a clinically significant difference after adjusting for confounding factors.
Practice Implications
More work is still needed to identify which behavioral interventions improve clinical and patient-oriented outcomes for patients with HF. However, MI does appear to be a promising approach. Healthcare providers should consider incorporating MI into consultations with patients.
Highlights.
Clinically significant difference in self-care maintenance in the MI group
MI is a promising approach for improving self-care maintenance
No difference between groups in physical HF symptoms or quality of life
Acknowledgments
This study was supported in full by the Edna G Kynett Memorial Foundation. We thank P.J. Bell, Wendy Hiller Gee and Stephanie Manning of Krames StayWell for designing the patient education materials for the study participants. We also thank Dr. Linda Hoke for assisting us to recruit patients from the Hospital of the University of Pennsylvania and Thomas A. Gillespie, MD, FACC for scoring the NYHA interviews. We gratefully acknowledge the pre-doctoral funding for Ruth Masterson Creber provided by the National Hartford Centers of Geriatric Nursing Excellence Patricia G. Archbold Scholarship program (2012–2014) and NIH/NINR (F31NR014086-01). We also acknowledge the post-doctoral funding for Ruth Masterson Creber by NIH/NINR (T32 NR007969) at Columbia University School of Nursing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2015 Update: A Report From the American Heart Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jencks S, Williams M, Coleman E. Rehospitalizations among Patients in the Medicare Fee-for-Service Program. NEJM. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 8.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, et al. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 10.Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF, Jr, et al. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10:460–466. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Havranek EP, Masoudi FA, Rumsfeld JS, Steiner JF. A broader paradigm for understanding and treating heart failure. J Card Fail. 2003;9:147–152. doi: 10.1054/jcaf.2003.21. [DOI] [PubMed] [Google Scholar]
- 12.Riegel B, Dickson VV, Faulkner KM. The Situation-Specific Theory of Heart Failure Self-Care: Revised and Updated. J Cardiovasc Nurs. 2015 doi: 10.1097/JCN.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 13.Riegel B, Dickson VV. A situation-specific theory of heart failure self-care. J Cardiovasc Nurs. 2008;23:190–196. doi: 10.1097/01.JCN.0000305091.35259.85. [DOI] [PubMed] [Google Scholar]
- 14.Riegel B, Carlson B, Moser DK, Sebern M, Hicks FD, Roland V. Psychometric testing of the self-care of heart failure index. J Card Fail. 2004;10:350–360. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Riegel B, Lee CS, Dickson VV. Self care in patients with chronic heart failure. Nat Rev Cardiol. 2011;19:644–654. doi: 10.1038/nrcardio.2011.95. [DOI] [PubMed] [Google Scholar]
- 16.Riegel B, Dickson VV, Hoke L, McMahon JP, Reis BF, Sayers S. A motivational counseling approach to improving heart failure self-care: mechanisms of effectiveness. J Cardiovasc Nurs. 2006;21:232–241. doi: 10.1097/00005082-200605000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Brodie DA, Inoue A, Shaw DG. Motivational interviewing to change quality of life for people with chronic heart failure: a randomised controlled trial. Int J Nurs Stud. 2008;45:489–500. doi: 10.1016/j.ijnurstu.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grady KL. Self-care and quality of life outcomes in heart failure patients. J Cardiovasc Nurs. 2008;23:285–292. doi: 10.1097/01.JCN.0000305092.42882.ad. [DOI] [PubMed] [Google Scholar]
- 20.Ditewig JB, Blok H, Havers J, van Veenendaal H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: a systematic review. Patient Educ Couns. 2010;78:297–315. doi: 10.1016/j.pec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CS, Suwanno J, Riegel B. The relationship between self-care and health status domains in Thai patients with heart failure. Eur J Cardiovasc Nurs. 2009;8:259–266. doi: 10.1016/j.ejcnurse.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 24.Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. 2006;6:43. doi: 10.1186/1471-2261-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner RS, Ozalp F, Murday AJ, Robb SD, McDonagh TA. N-terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003;24:1735–1743. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Lee CS, Moser DK, Lennie TA, Tkacs NC, Margulies KB, Riegel B. Biomarkers of Myocardial Stress and Systemic Inflammation in Patients Who Engage in Heart Failure Self-care Management. J Cardiovasc Nurs. 2011;26:321–328. doi: 10.1097/JCN.0b013e31820344be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams MV, Parker RM, Baker DW, Parikh NS, Pitkin K, Coates WC, et al. Inadequate functional health literacy among patients at two public hospitals. Jama. 1995;274:1677–1682. [PubMed] [Google Scholar]
- 28.DeWalt DA, Pignone M, Malone R, Rawls C, Kosnar MC, George G, et al. Development and pilot testing of a disease management program for low literacy patients with heart failure. Patient Educ Couns. 2004;55:78–86. doi: 10.1016/j.pec.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Dewalt DA, Berkman ND, Sheridan S, Lohr KN, Pignone MP. Literacy and health outcomes: a systematic review of the literature. J Gen Intern Med. 2004;19:1228–1239. doi: 10.1111/j.1525-1497.2004.40153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker DW, DeWalt DA, Schillinger D, Hawk V, Ruo B, Bibbins-Domingo K, et al. The Effect of Progressive, Reinforcing Telephone Education and Counseling Versus Brief Educational Intervention on Knowledge, Self-Care Behaviors and Heart Failure Symptoms. Journal of Cardiac Failure. 2011;17:789–796. doi: 10.1016/j.cardfail.2011.06.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherwood A, O’Connor CM, Routledge FS, Hinderliter AL, Watkins LL, Babyak MA, et al. Coping Effectively With Heart Failure (COPE-HF): Design and Rationale of a Telephone-Based Coping Skills Intervention. Journal of Cardiac Failure. 2011;17:201–207. doi: 10.1016/j.cardfail.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller WR, Rollnick S. Motivational Interviewing: Preparing people to change addictive behavior. New York: Guilford Press; 2002. [Google Scholar]
- 33.Prochaska JO, DiClemente CC, Norcross JC In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47:1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 34.Masterson Creber R, Patey M, DeCesaris M, Gee WH, Dickson V, Riegel B. Motivational Interviewing Tailored Interventions for Heart Failure (MITI-HF): Study Design and Methods. Contempory Clinical Trials. 2015 doi: 10.1016/j.cct.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faul F, Erdfelder E, Buchner A, Lang A. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 36.Fleiss JL. Statistical methods for rates and proportions. 2nd ed. Toronto: John Wiley & Sons; 1981. [Google Scholar]
- 37.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Family medicine. 2004;36:588–594. [PubMed] [Google Scholar]
- 38.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Riegel B, Lee CS, Dickson VV, Carlson B. An update on the self-care of heart failure index. J Cardiovasc Nurs. 2009;24:485–497. doi: 10.1097/JCN.0b013e3181b4baa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and blood vessels. Boston: Little Brown; 1964. [Google Scholar]
- 41.Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874–877. doi: 10.1111/j.1525-1497.2006.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horowitz J, Manski C. Identification and Robustness with Contaminated and Corrupted Data. Econometrica. 1995;63:281–302. [Google Scholar]
- 43.Moore J, Stinson L, Welniak JE. Income Measurement Error in Surveys: A Review. Journal of official statistics. 2000;16:331–365. [Google Scholar]
- 44.Evans S, Day S, Royston P. Minim: allocation by minimisation in clinical trials. 2013 [Google Scholar]
- 45.Altman DG, Bland JM. Treatment allocation by minimisation. Bmj. 2005;330:843. doi: 10.1136/bmj.330.7495.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masterson Creber R, Patey M, DeCesaris M, Dickson V, Riegel B. Motivational Interviewing Tailored Interventions for Heart Failure (MITI-HF): Study Design and Methods. Contempory Clinical Trials. 2015;41:62–68. doi: 10.1016/j.cct.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbaranelli C, Lee CS, Vellone E, Riegel B. Dimensionality and reliability of the self-care of heart failure index scales: further evidence from confirmatory factor analysis. Res Nurs Health. 2014;37:524–537. doi: 10.1002/nur.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vellone E, Riegel B, Cocchieri A, Barbaranelli C, D'Agostino F, Antonetti G, et al. Psychometric testing of the self-care of heart failure index version 6.2. Res Nurs Health. 2013;36:500–511. doi: 10.1002/nur.21554. [DOI] [PubMed] [Google Scholar]
- 49.Jurgens CY, Fain JA, Riegel B. Psychometric testing of the heart failure somatic awareness scale. J Cardiovasc Nurs. 2006;21:95–102. doi: 10.1097/00005082-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Jurgens CY, Lee CS, Riegel B. Patient Perception of Heart Failure Symptoms Predicts One-Year Survival. Dallas, TX: American Heart Association; 2013. [Google Scholar]
- 51.Jurgens CY, Lee CS, Reitano JM, Riegel B. Heart failure symptom monitoring and response training. Heart Lung. 2013;42:273–280. doi: 10.1016/j.hrtlng.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 53.Masterson Creber RM, Polomono R, Ferrar J, Riegel B. Psychometric Properties of the Kansas City Cardiomyopathy Questionnaire (KCCQ) European Journal of Cardiovascular Nursing. 2012;11:197–206. doi: 10.1177/1474515111435605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maxwell SE, Delancey H. Designing experiments and analyzing data: A model comparison perspective. 2nd ed. Mahwah, NJ: Erlbaum; 2004. [Google Scholar]
- 55.Dunbar SB, Clark PC, Reilly CM, Gary RA, Smith A, McCarty F, et al. A Trial of Family Partnership and Education Interventions in Heart Failure. Journal of Cardiac Failure. 2013;19:829–841. doi: 10.1016/j.cardfail.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 57.Ogedegbe G, Schoenthaler A, Richardson T, Lewis L, Belue R, Espinosa E, et al. An RCT of the effect of motivational interviewing on medication adherence in hypertensive African Americans: rationale and design. Contemp Clin Trials. 2007;28:169–181. doi: 10.1016/j.cct.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Ogedegbe GO, Boutin-Foster C, Wells MT, Allegrante JP, Isen AM, Jobe JB, et al. A randomized controlled trial of positive-affect intervention and medication adherence in hypertensive African Americans. Arch Intern Med. 2012;172:322–326. doi: 10.1001/archinternmed.2011.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solomon DH, Iversen MD, Avorn J, Gleeson T, Brookhart MA, Patrick AR, et al. Osteoporosis telephonic intervention to improve medication regimen adherence: a large, pragmatic, randomized controlled trial. Arch Intern Med. 2012;172:477–483. doi: 10.1001/archinternmed.2011.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lavoie KL, Campbell TS, Bacon SL. Does motivational interviewing improve medication adherence? Arch Intern Med. 2012;172:1351–1352. doi: 10.1001/archinternmed.2012.2575. author reply-2. [DOI] [PubMed] [Google Scholar]
- 61.Paradis V, Cossette S, Frasure-Smith N, Heppell S, Guertin MC. The efficacy of a motivational nursing intervention based on the stages of change on self-care in heart failure patients. J Cardiovasc Nurs. 2010;25:130–141. doi: 10.1097/JCN.0b013e3181c52497. [DOI] [PubMed] [Google Scholar]
- 62.Chair SY, Chan SW, Thompson DR, Leung KP, Ng SK, Choi KC. Short-term effect of motivational interviewing on clinical and psychological outcomes and health-related quality of life in cardiac rehabilitation patients with poor motivation in Hong Kong: a randomized controlled trial. European journal of preventive cardiology. 2012;19:1383–1392. doi: 10.1177/1741826711425428. [DOI] [PubMed] [Google Scholar]