Abstract
Background
Intensive dietary intervention programs may lead to benefits in vitality and other components of health quality. The Women’s Health Initiative (WHI) Dietary Modification (DM) intervention includes a large randomized controlled trial of an intensive intervention.
Objective
To evaluate whether the intervention is associated with improved health-related quality of life (HRQoL) subscales, overall self-reported health, depressive symptoms, cognitive functioning, and sleep quality.
Design
Randomized controlled trial was analyzed as intent to treat.
Participants
Between 1993 and 1998, 48,835 women aged 50 to 79 years were recruited by 40 clinical centers across the United States. Eligibility included having fat intake at baseline ≥32% of total calories, and excluded women with any prior colorectal or breast cancer, recent other cancers, type-1 diabetes, medical conditions with predicted survival less than three years.
Intervention
Goals were to reduce calories from fat to 20%, increase vegetables and fruit to 5+ servings and increase grain servings to 6+ servings a day. During the first year, 18 group sessions were held, with quarterly sessions thereafter.
Main Outcome Measures
RAND 36-Item Health Survey was used to assess HRQoL at baseline, year 1, and close-out (about 8 years post randomization), and estimate differential HRQoL subscale change scores.
Statistical analysis performed
Mean change in HRQoL scores (year 1 minus baseline) were compared by randomization group using linear models.
Results
At one year, there was a differential change between intervention and comparison group of 1.7 units (1.5, 2.0) in general health associated with the intervention. DM intervention improved physical functioning by 2.0 units (1.7, 2.3), vitality by 1.9 units (1.6, 2.2), and global QOL by 0.09 units (0.07, 0.12). With the exception of global QOL, these effects were significantly modified by BMI at baseline.
Conclusions
DM intervention was associated with small, but significant improvements in three health related quality of life subscales: general health, physical functioning, and vitality at one year follow-up, with the largest improvements seen in the women with the greatest baseline BMI.
Keywords: Health-related-quality of life, Dietary Intervention, Postmenopausal, Body Mass Index, Randomized Controlled Trial
INTRODUCTION
Cross-sectional literature has shown that lifestyle factors such as higher physical activity1,2 as well as normal weight status compared with overweight or obesity3 are associated with indicators of higher health related quality of life (HRQoL). Additionally, changes in diet 4-11 and weight status are associated with changes in quality of life: weight gain is associated with lower measures of quality of life while weight loss is associated with improvements.4,12-20 Because of its focus on dietary change and its size, the dietary modification (DM) trial of the Women’s Health Initiative (WHI) may be able to contribute to the question of whether or not making healthy diet changes is associated with improved HRQoL, both overall and within subgroups of women. The DM trial in the WHI was a randomized controlled trial designed to test the hypothesis that a reduction of fat intake to 20 percent of the total daily calories and an increase in the intake of fruit, vegetables, and whole grains would reduce the risk of breast cancer and of colorectal cancer and, as a secondary outcome, the risk of heart disease in postmenopausal women.21 The group-based behavioral intervention was associated with large initial changes in dietary fat intake that persisted for an average of eight years.22 It is important to evaluate the extent to which the intervention affected the overall perceived health and well-being of the participants especially in the first year of the intervention when very large dietary behavior changes were being made. In earlier published analyses, we have shown that, in spite of efforts to deliver the intervention in a calorie neutral fashion, women in the intervention group lost more weight than those in the comparison group at one year, although the weight difference between the groups decreased over the eight years of follow-up.23 It seems reasonable to assume, therefore, that while the diet tested in the WHI did not reduce the morbidity and mortality from certain chronic diseases,21,24,25 it might favorably affect health-related quality of life at one year follow-up.
Relatively few studies have examined the effect of long term dietary interventions on quality of life or functional health status.8,11 This paper proposes to study changes in quality of life measures among women enrolled in the DM trial of the Women’s Health Initiative. Because of the large dietary behavior changes attributable to the intervention at one year follow-up, our specific hypotheses include intervention-associated positive changes in global QOL, eight subscales of HRQoL, overall self-reported health, depressive symptoms, sleep quality, and cognitive functioning at Year 1.
METHODS
Study population
Recruitment of postmenopausal women aged 50 to 79 years who were interested in one or more components of the WHI Clinical Trials was conducted between 1993 and 1998 by 40 Clinical Centers throughout the U.S., as described previously.21,24,25 The Clinical Trials were: Hormone Therapy (HT), with 27,347 women; DM (48,835 women); and Calcium and Vitamin D supplementation (36,282 women who had been part of one or both HT or DM for one or two years). About 16% of the women in the DM trial also participated in the HT trial (8050 women). Eligibility criteria for the DM trial included being willing to be randomized to the intervention or comparison group and having fat intake at baseline ≥32% of total calories as evaluated by the WHI food frequency questionnaire (FFQ).26 Major exclusions at screening included prior colorectal cancer or breast cancer, other cancers in the last 10 years, type-1 diabetes, medical conditions with predicted survival less than three years, and adherence concerns, including frequent meals away from home. The Fred Hutchinson Cancer Research Center Institutional Review Board approved the study protocol and all participants provided written informed consent. Women were randomized to intervention or comparison group in the ratio of 2:3 to contain trial costs while preserving power, as has been previously described.22,27-29
Intervention
The primary nutrition goal of the WHI DM intervention was to reduce total dietary fat intake to 20% of energy. Individualized fat gram goals were set according to the person’s height, so as to reduce energy from total fat to 20% if the goals were achieved. The DM intervention was characterized as a low-fat dietary pattern, and included recommendations to increase consumption of vegetables and fruit to at least 5 servings a day and increasing grain servings to at least 6 servings a day. It was presumed that reduction of total fat to 20% energy intake would reduce the amount of energy from saturated fat to 7%. The DM intervention was delivered in a group setting by trained nutritionists delivering information and activities that reflect both nutritional and behavioral principles. During the first year, 18 group sessions were held, with quarterly sessions thereafter. Later in the intervention period, additional tailored and targeted strategies were added to enhance adherence. Details on the DM intervention are published elsewhere.25,30-32 The WHI DM intervention changed the dietary fat intake of participants at one year.22,23 In the first year of the intervention, the reduction in percent energy from fat in the intervention compared to the comparison group was 10.9% (compared to the goal of 13%).2The differential changes associated with the intervention, in percent energy from fat, percent energy from saturated fat, servings of fruits and vegetables and servings of grain, were all statistically significant (p<0.001),22. It is clear that, although short of the goal, the dietary changes made by the intervention women were substantial, demonstrating what might be possible on a population basis.33
Assessment of quality of life-related variables
All quality of life-related variables were self-reported via questionnaires completed by the women prior to their first screening visit and selected follow-up times. Completed HRQoL questionnaires were collected and reviewed for completeness during the screening clinic visit and at previously determined follow-up visits. Women who forgot to bring in their completed questionnaires could complete them in the clinic or mail them back to the clinic with a stamped, addressed envelope provided by the clinic at their visit.
Quality of Life and Functional Status
Global Quality of Life was assessed by a single item (“Overall, how would you rate your quality of life?”) with an 11-point response scale (0=”As bad or worse than being dead”, 10=”Best quality of life”). Quality of life/functional status was assessed using the RAND 36-Item Health Survey (RAND36).34 The RAND36 provides eight subscales that include; (1) general health perceptions (general health); (2) physical functioning; (3) vitality (energy and fatigue);; (4) role limitations due to physical health (role physical); (5) bodily pain; (6) social functioning; (7) role limitations due to emotional problems (role emotional); and (8) general mental health or emotional well-being (mental health). General health was assessed by asking questions about perceived health relative to another person or to the expectation of one’s health in the near future. Physical functioning included information on vigorous activities, bending, kneeling, stooping and walking one block, and were asked in terms of health limiting these functions. Vitality questions included such things as feeling full of pep, worn out, or having a lot of energy. Role physical consisted of items which measure the extent to which physical health interfered with work or activities of daily living. Bodily pain was determined by asking how much pain the participant had in the last 4 weeks and how much it interfered with her work inside and outside of the house. Social functioning consisted of how much the participant’s physical or emotional health interfered with her regular social activities (during the past 4 weeks). Role emotional consisted of questions which assessed how often emotional problems affected the respondent’s work or other activities of daily living. Mental health was assessed by asking questions about how often the participant had felt blue or “down in the dumps”, calm and happy, or nervous.
Self-Reported Health was a single item addressing a self-rating of health, that has been used commonly over the past 40 years, with five response options from excellent (coded as 5) to poor (coded as 1).35-37 It is also one of the questions that makes up the general health subscale of the RAND36, described above.
Depressive symptoms were assessed by a six-item scale described by Smoller and colleagues.38 This scale characterizes current depressive mood and is composed of six items from the Center for Epidemiological Studies Depression Scale (CES-D).
3MSE
Cognitive functioning was assessed in participants 65 years or older by the Modified Mini-Mental State Examination (3MSE) 41, a scale used in the Cardiovascular Health Study.42 The 3MSE consists of 15 parts that contain 46 separately scored items. For some items the maximum score was 1 while for others the maximum score was 8. The functions tested included orientation to time, place and person, short-term memory, reading, writing, naming, verbal fluency, praxis, and graphomotor skill. The 3MSE was not administered at close-out.
Sleep disturbance
Sleep quality was assessed by the 5-item Women’s Health Initiative Insomnia Rating Scale,39,40 which was developed and validated for use in the WHI. Items in this instrument referenced sleep during the “past 4 weeks.” Four items assessed sleep initiation and maintenance using a 5-point response scale (No, not in past four weeks; yes, less than once a week; 1- 2 times a week; 3 or 4 times a week; 5 or more times a week) and a fifth item assessed sleep quality, also using a 5-point scale (very sound or restful, sound or restful, average quality, restless, very restless).
Statistical analyses
All primary analyses focused on changes in HRQoL scores from baseline to Year 1 in relation to DM randomization assignment. For all HRQoL measures, an unadjusted linear model was used to test whether DM had a significant treatment effect on HRQoL change score (year 1 minus baseline). Statistical significance of the effect of DM on HRQoL was judged by a nominal alpha =0.05. We expected to obtain one statistically significant effect by chance. For the subgroup analyses, a nominal alpha of 0.05 was used to judge whether the DM effect was moderated by a baseline characteristic. This p-value corresponded to the interaction term, between DM and a baseline characteristic, from a linear model that also contains the main effects. Baseline characteristics, for which a differential effect of the intervention on HRQoL might be expected, included age, body mass index, physical activity, and dietary fat intake. Additional baseline characteristics other than the four mentioned were also examined and included ethnicity, income, education, geographic region, alcohol, smoking, multivitamin use, use of supplements with vitamin E, use of supplements with vitamin C, total dietary energy, total dietary fat, percent energy from fat, fruit and vegetable intake, total dietary fiber, conjugated equine estrogen (CEE) use (from HT trial), and CEE+ medroxyprogesterone acetate (MPA) use (from the HT trial). Considering all outcome measures and baseline characteristics equally, we count 247 linear models (13 HRQoL measures × 19 baseline characteristics). Twelve statistically significant subgroups would be expected by chance, of which three subgroups would be expected by chance among our endpoints and four baseline characteristics of interest. Similar analyses of the main effects were performed on data available at end of follow-up. All analyses were based on the intention-to-treat principle using SAS (version 9.1, 2002, SAS Institute Inc).43
Effect size (ES) was used to assess the clinical significance of statistically significant findings.44 ES is a distribution based approach to judge clinical significance and was defined as , where M is the average difference in quality of life measure and S the standard deviation of the difference.45 Effect sizes of 0.2 to 0.5 are generally regarded as small, 0.5 to 0.8 as medium, and 0.8 and above as large.45
RESULTS
Overall, 19,541 women were randomized into the intervention group and 29,294 into the comparison group. The mean length of follow-up was 8.1 (SD 1.7) years, with a maximum of 11.2 years. Less than one percent of women in each group did not participate in the Year 1 follow-up visit because they died or were lost to follow-up. Adherence to the study protocol was good (women in the intervention attended 78% of sessions, provided 87% of fat self-monitoring scores and reduced their percent energy from fat to 25.1%). Table 1 shows selected baseline characteristics of the DM participants in the intervention and comparison groups. The most represented group is age 60 to 69 years, nearly half the women in the DM trial. More than 80% are white, and many women are in the middle family income category of $20,000 to $50,000 per year. About 38% of participants were obese, and a further 36% were overweight. Less than 10% of participants consumed alcohol at a frequency of more than one drink per day, approximately 7% were current smokers, and 36% took multivitamin supplements. As a direct result of eligibility criteria for the DM trial, women reported consuming approximately 38% calories from fat (from the FFQ), and just under four servings daily of vegetables and fruit, and about 15 grams of dietary fiber (Table 1). Their baseline physical activity averaged 10 METs per week.
Table 1. Baseline Characteristics of the Women’s Health Initiative Dietary Modification Trial Participants (N = 48,835) by Randomization Assignment.
| Comparison (n=29294) |
Intervention (n=19541) |
||||
|---|---|---|---|---|---|
| n | (%) | n | % | P-Valuea | |
|
|
|
|
|||
| Age group at screening, years | > 0.99 | ||||
| 50-54 | 4178 | (14.3) | 2783 | (14.2) | |
| 55-59 | 6619 | (22.6) | 4423 | (22.6) | |
| 60-69 | 13626 | (46.5) | 9086 | (46.5) | |
| 70-79 | 4871 | (16.6) | 3249 | (16.6) | |
|
| |||||
| Ethnicity | 0.76 | ||||
| White | 23890 | (81.6) | 15869 | (81.2) | |
| Black | 3129 | (10.7) | 2137 | (10.9) | |
| Hispanic | 1099 | (3.8) | 755 | (3.9) | |
| American Indian | 115 | (0.4) | 88 | (0.5) | |
| Asian/Pacific Islander | 674 | (2.3) | 433 | (2.2) | |
| Unknown | 387 | (1.3) | 259 | (1.3) | |
|
| |||||
| Family income | 0.24 | ||||
| <$20K | 4303 | (15.6) | 2774 | (15.1) | |
| $20-$50K | 12682 | (46.0) | 8455 | (45.9) | |
| ≥$50K | 10610 | (38.4) | 7180 | (39.0) | |
|
| |||||
| More than high school education | 22639 | (77.8) | 15156 | (78.0) | 0.52 |
|
| |||||
| Body mass index | 0.69 | ||||
| <25 | 7585 | (26.0) | 5072 | (26.1) | |
| 25-29 | 10446 | (35.8) | 6940 | (35.7) | |
| 30-34 | 6748 | (23.1) | 4450 | (22.9) | |
| ≥35 | 4378 | (15.0) | 2992 | (15.4) | |
|
| |||||
| U.S. region | > 0.99 | ||||
| Northeast | 6855 | (23.4) | 4561 | (23.3) | |
| South | 7640 | (26.1) | 5105 | (26.1) | |
| Midwest | 5978 | (20.4) | 3984 | (20.4) | |
| West | 8821 | (30.1) | 5891 | (30.1) | |
|
| |||||
| Alcohol servings | 0.62 | ||||
| Non Drinker | 12323 | (42.2) | 8134 | (41.8) | |
| ≤ 1 drink/day | 14121 | (48.3) | 9449 | (48.5) | |
| > 1 drink/day | 2769 | (9.5) | 1881 | (9.7) | |
|
| |||||
| Smoking | 0.24 | ||||
| Never smoked | 15029 | (51.8) | 9918 | (51.4) | |
| Past smoker | 11980 | (41.3) | 8121 | (42.1) | |
| Current smoker | 1977 | (6.8) | 1273 | (6.6) | |
|
| |||||
| Multivitamin (with or without minerals) | 10398 | (35.5) | 6939 | (35.5) | 0.97 |
| Use of supplements containing vitamin E | 15032 | (51.3) | 10039 | (51.4) | 0.89 |
| Use of supplements containing vitamin C | 14852 | (50.7) | 9894 | (50.6) | 0.89 |
|
| |||||
| Menopausal hormone therapy trial arm | |||||
| CEEb-Alone | 1039 | (3.5) | 615 | (3.1) | 0.41c |
| CEE-Alone Placebo | 1068 | (3.6) | 670 | (3.4) | |
| CEE+MPAd | 1457 | (5.0) | 972 | (5.0) | 0.30e |
| CEE+MPA Placebo | 1304 | (4.5) | 925 | (4.7) | |
| Not randomized | 24426 | (83.4) | 16359 | (83.7) | |
| n | Mean | SD | n | Mean | SD | P-Value | |
|---|---|---|---|---|---|---|---|
| Total energy expended from recreational physical activity (METf-hours/week) |
26254 | 10.1 | (12.0) | 17507 | 10.0 | (11.7) | 0.45 |
| Dietary energy (kcal) | 29216 | 1789.4 | (703.0) | 19470 | 1790.2 | (710.1) | 0.90 |
| Dietary total fat (g) | 29216 | 75.7 | (33.6) | 19470 | 75.7 | (34.1) | 0.83 |
| Percent calories from fat | 29216 | 37.8 | (5.0) | 19470 | 37.8 | (5.1) | 0.84 |
| Vegetable and fruit (servings/day) | 29216 | 3.6 | (1.8) | 19470 | 3.6 | (1.8) | 0.52 |
| Dietary fiber (g) | 29216 | 15.4 | (6.4) | 19470 | 15.4 | (6.4) | 0.63 |
Test of association, based on chi-squared (categorical variables) or t-test (continuous variables), between baseline characteristic and randomization assignment.
CEE, conjugated equine estrogens
P-value corresponds to test of association between dietary modification trial group and CEE alone trial group.
MPA, medroxyprogesterone acetate
P-value corresponds to test of association between dietary modification trial group and CEE+MPA trial group.
MET, metabolic equivalent of task
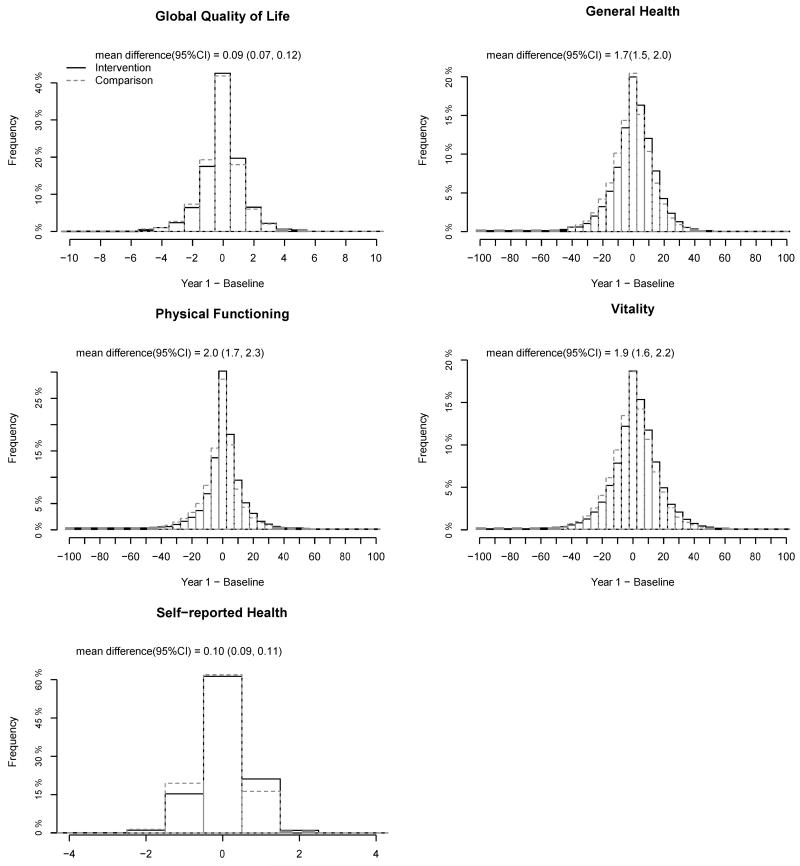
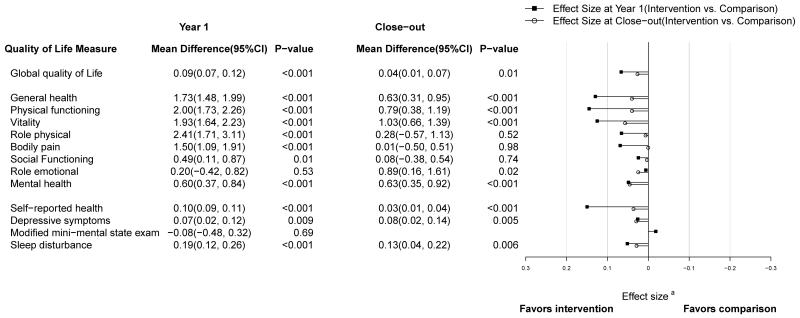
Baseline scores of women in the intervention and comparison groups were comparable. For example, mean physical functioning scores at baseline were 81.1 and 80.9 for the intervention and comparison groups, respectively (Table 2). At one year, each of these scores had improved by a small but statistically significant amount in the intervention group women relative to the comparison group. The comparison group scores declined (or, in the cases of vitality and sleep disturbance, stayed the same). Figure 1 shows the small shift in the two distributions of change in five of these measures at one year for illustrative purposes. The differential change in all the scores with its 95% confidence interval is shown in Table 2. All the intervention effects were statistically significant at the < 0.001 level except social functioning (P<0.01), depressive symptoms (p=0.009), and role emotional (p=0.53, 3MSE (p=0.69) but effect sizes were small (< 0.20) and did not meet the general criteria of clinical significance. A forest plot of effect sizes are presented in Figure 2.
Table 2. Mean Health Related Quality of Life Scores at Baseline, Year 1, and Change Scores (Year 1 minus Baseline) by Randomization Assignment in the Women’s Health Initiative Dietary Modification Trial.
| Quality of Life Measure | n | Baseline | Intervention Means Year1 |
Change scoreb | n | Baseline | Comparison Means Year1 |
Change score | Mean | Differencea (95%CI) |
P-Valuec |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Global quality of life (0 worst to 10 best) | 17780 | 8.20 | 8.19 | −0.01 | 26407 | 8.18 | 8.11 | −0.10 | 0.09 | (0.07, 0.12) | <0.001 |
| General Healthd (0 worst to 100 best) | 17518 | 74.5 | 75.8 | 1.1 | 25985 | 74.3 | 73.9 | −0.7 | 1.7 | (1.5, 2.0) | <0.001 |
| Physical Functioningd (0 worst to 100 best) | 17329 | 81.1 | 81.9 | 0.6 | 25716 | 80.9 | 79.8 | −1.4 | 2.0 | (1.7, 2.3) | <0.001 |
| Vitality d (0 worst to 100 best) | 17559 | 62.6 | 64.7 | 2.0 | 25997 | 62.8 | 63.1 | 0.0 | 1.9 | (1.6, 2.2) | <0.001 |
| Role Physical d (0 worst to 100 best) | 17592 | 75.0 | 75.7 | 0.4 | 26054 | 74.5 | 72.9 | −2.0 | 2.4 | (1.7, 3.1) | <0.001 |
| Bodily Pain d (0 worst to 100 best) | 17808 | 74.8 | 74.9 | −0.2 | 26410 | 74.5 | 73.1 | −1.7 | 1.5 | (1.1, 1.9) | <0.001 |
| Social Functioning d (0 worst to 100 best) | 17713 | 90.2 | 88.9 | −1.5 | 26253 | 90.0 | 88.3 | −2.0 | 0.5 | (0.1, 0.9) | 0.01 |
| Role Emotional d (0 worst to 100 best) | 17612 | 84.3 | 84.1 | −0.7 | 26129 | 84.2 | 83.8 | −0.9 | 0.2 | (−0.4, 0.8) | 0.53 |
| Mental Health d (0 worst to 100 best) | 17472 | 79.0 | 80.4 | 1.1 | 25905 | 79.1 | 79.8 | 0.5 | 0.6 | (0.4, 0.8) | <0.001 |
| Self-Reported Health (1 worst to 5 best) | 17815 | 3.64 | 3.72 | 0.06 | 26456 | 3.64 | 3.61 | −0.05 | 0.10 | (0.09, 0.11) | <0.001 |
| Depressive Symptoms (0 worst to 18 best) | 17335 | 15.72 | 15.71 | −0.05 | 25698 | 15.69 | 15.61 | −0.12 | 0.07 | (0.02, 0.12) | 0.009 |
| 3MSEe (0 worst to 100 best) | 762 | 95.1 | 95.6 | 0.5 | 1155 | 95.0 | 95.3 | 0.6 | −0.1 | (−0.5, 0.3) | 0.69 |
| Sleep disturbance (0 worst to 20 best) | 17242 | 13.44 | 13.65 | 0.18 | 25583 | 13.43 | 13.44 | −0.01 | 0.19 | (0.12, 0.26) | <0.001 |
Mean difference of change scores (intervention minus comparison).
Missing data, at either baseline or Year 1, account for mean change scores not equal to Year 1 mean minus baseline mean.
Based on statistical significance of randomization assignment from linear model.
RAND-36 subscales
3MSE, modified mini-mental state examination
Figure 1.
Histograms of change scores (year 1 minus baseline) for select quality of life scores by randomization assignment in the Women’s Health Initiative Dietary Modification Trial.
Figure 2. close–out (minus baseline), and the corresponding Effect Size for Health Related Quality of Life Scores in the Women’s Health Initiative Dietary Modification Trial.
a Effect size is the estimated mean difference at year 1 or close–out, divided by the respective root–mean–square error. For all measures, the sign of the effect size has been flipped (if necessary) so that adverse effects (favors comparison) extend to the right and favorable effects (favors intervention) extend to the left.
In subgroup analyses, we found significant modification of the intervention effect on physical functioning according to baseline age (p=0.02), with the greatest benefit experienced by the oldest age group at 2.3 units of change (95% CI 1.7, 3.0); baseline body mass index (p<0.001), with the greatest benefit experienced by very obese women (BMI > 35) at 2.7 (95% CI 2.0, 3.4); and baseline physical activity (p<0.001), with the greatest benefit experienced by women who were inactive 2.6 (95% CI 2.0, 3.3). We failed to find similar effect modification for the differential change in vitality, with the possible exception of baseline BMI (p=0.02), where the largest benefit in the highest BMI group: 2.7 (95% CI 1.9, 3.5). A consistent moderating effect of BMI was also found with respect to general health perceptions (p=0.004), with the greatest benefit in very obese women: 2.3 (95% CI 1.6, 2.9). The interaction with age (p=0.04) was significant even after adjusting for BMI and appeared to be in the opposite direction, with younger women experiencing a larger change in general health perceptions. There was also evidence of an interaction with dietary fat intake at baseline, with largest changes in perception of general health occurring in the group consuming the highest dietary fat (2.0 95% CI (1.5, 2.4) p<0.05). Baseline physical activity did not significantly modify the intervention effect on general health perceptions. Similar interaction effects were found with self-reported health. No additional interactions with age, BMI, physical activity and dietary fat beyond those presented in Table 3 were found. Forest plots of effect sizes for all thirteen HRQoL measures by these select subgroups are displayed in Supplemental Material 1, 2, 3 and 4. Other significant interactions between the intervention and race/ethnicity, smoking status, percent energy from fat, and CEE+MPA trial arm were seen for role-emotional (p=0.05; Hispanic women were most favorably affected), sleep disturbance (p=0.02; current smokers were most favorably affected), depressive symptoms (p=0.03; women that consumed the most fat were most favorably affected) and global QOL (p=0.005; those randomized to CEE+MPA were most favorably affected), respectively. Regardless of whether the effect of the dietary intervention was modified by baseline characteristics or due to chance, the effect sizes remained small across subgroups (ES < 0.20).
Table 3. Mean Difference (Intervention vs. Comparison) of Health Related Quality of Life Change Score (Year 1 minus Baseline) by Subgroup in the Women’s Health Initiative Dietary Modification Trial.
| Global Quality of Life | General Health | Physical Functioning | Vitality | Self-reported Health | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | Mean | (95% CI) | Pa | Mean | (95% CI) | P | Mean | (95% CI) | P | Mean | (95% CI) | P | Mean | (95% CI) | P |
| All | 0.09 | (0.07, 0.12) | <0.001 | 1.7 | (1.5, 2.0) | <0.001 | 2.0 | (1.7, 2.3) | <0.001 | 1.9 | (1.6, 2.2) | <0.001 | 0.10 | (0.09, 0.11) | <0.001 |
|
| |||||||||||||||
| Age (years) | 0.62 | 0.04 | 0.02 | 0.76 | 0.03 | ||||||||||
| 50-54 | 0.07 | (0.00, 0.14) | 1.7 | (1.0, 2.4) | 1.5 | (0.8, 2.2) | 1.8 | (1.0, 2.6) | 0.12 | (0.09, 0.16) | |||||
| 55-59 | 0.11 | (0.05, 0.17) | 2.2 | (1.6, 2.7) | 1.6 | (1.1, 2.2) | 1.8 | (1.1, 2.4) | 0.11 | (0.08, 0.14) | |||||
| 60-69 | 0.10 | (0.06, 0.13) | 1.6 | (1.2, 2.0) | 2.2 | (1.8, 2.6) | 2.0 | (1.6, 2.5) | 0.09 | (0.07, 0.11) | |||||
| 70-79 | 0.08 | (0.01, 0.14) | 1.5 | (0.8, 2.1) | 2.3 | (1.7, 3.0) | 2.0 | (1.3, 2.7) | 0.09 | (0.06, 0.12) | |||||
|
| |||||||||||||||
| Body Mass Index | 0.61 | 0.004 | <0.001 | 0.02 | 0.003 | ||||||||||
| <25 | 0.10 | (0.04, 0.15) | 1.2 | (0.7, 1.7) | 1.3 | (0.8, 1.8) | 1.6 | (1.1, 2.2) | 0.08 | (0.05, 0.10) | |||||
| 25 – 29 | 0.11 | (0.06, 0.15) | 1.7 | (1.3, 2.1) | 1.9 | (1.4, 2.3) | 1.8 | (1.3, 2.3) | 0.10 | (0.08, 0.12) | |||||
| 30 – 34 | 0.09 | (0.03, 0.14) | 2.0 | (1.5, 2.5) | 2.6 | (2.1, 3.2) | 1.9 | (1.3, 2.5) | 0.11 | (0.08, 0.14) | |||||
| >35 | 0.06 | (−0.01, 0.13) | 2.3 | (1.6, 2.9) | 2.7 | (2.0, 3.4) | 2.7 | (1.9, 3.5) | 0.13 | (0.10, 0.17) | |||||
|
| |||||||||||||||
| Physical Activity (MET-hours/week)b |
0.46 | 0.67 | <0.001 | 0.70 | 0.25 | ||||||||||
| Inactive | 0.07 | (0.01, 0.13) | 1.9 | (1.3, 2.5) | 2.6 | (2.0, 3.3) | 1.7 | (0.9, 2.4) | 0.10 | (0.07, 0.14) | |||||
| < 5 | 0.06 | (0.00, 0.11) | 1.7 | (1.2, 2.3) | 2.3 | (1.7, 2.9) | 1.9 | (1.3, 2.6) | 0.09 | (0.06, 0.11) | |||||
| 5-12 | 0.13 | (0.08, 0.19) | 1.5 | (0.9, 2.0) | 2.2 | (1.6, 2.8) | 2.2 | (1.5, 2.8) | 0.12 | (0.09, 0.14) | |||||
| > 12 | 0.09 | (0.04, 0.14) | 1.8 | (1.4, 2.3) | 1.3 | (0.8, 1.8) | 1.8 | (1.3, 2.4) | 0.09 | (0.06, 0.11) | |||||
|
| |||||||||||||||
| Dietary total Fat (g) | 0.56 | 0.05 | 0.07 | 0.34 | 0.10 | ||||||||||
| <57.6 | 0.08 | (0.03, 0.12) | 1.4 | (0.9, 1.8) | 1.7 | (1.2, 2.1) | 1.8 | (1.3, 2.3) | 0.08 | (0.06, 0.10) | |||||
| 57.6 - 82.8 | 0.08 | (0.04, 0.13) | 1.8 | (1.4, 2.3) | 2.0 | (1.6, 2.5) | 1.8 | (1.3, 2.3) | 0.11 | (0.09, 0.13) | |||||
| ≥ 82.8 | 0.11 | (0.06, 0.16) | 2.0 | (1.5, 2.4) | 2.3 | (1.9, 2.8) | 2.2 | (1.6, 2.7) | 0.11 | (0.09, 0.13) | |||||
P-value based on a test of interaction between baseline characteristic and randomization assignment.
Total energy expended from recreational physical activity; MET, metabolic equivalent of task. Participants with 0 MET-hours/week
In additional analyses, the differential gains in physical functioning and vitality remained significant after adjusting for age, ethnicity, and weight at year 1 and baseline, suggesting an independent effect of the intervention from weight loss.
The analyses at study close-out demonstrated some continuing benefit of the intervention on physical functioning, vitality and general health perceptions at an average of 8.1 years of follow-up, but the intervention effect estimates were smaller (see Figure 2), with differential change average scores of 0.8, 1.0 and 0.6 respectively (see table 4).
Table 4. Mean Health Related Quality of Life Change Scores at Close-out (Close-out minus Baseline) by Randomization Assignment in the Women’s Health Initiative Dietary Modification Trial.
| Quality of Life Measure | Intervention | Comparison | Differencea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | S.D. | n | Mean | S.D. | Mean | (95%CI) | P-Valueb | |
| Global quality of life (0 worst to 10 best) | 15788 | 0.07 | (1.41) | 24342 | 0.03 | (1.44) | 0.04 | (0.01, 0.07) | 0.01 |
| General Healthc (0 worst to 100 best) | 15504 | −5.2 | (15.9) | 23912 | −5.8 | (15.8) | 0.6 | (0.3, 1.0) | <0.001 |
| Physical Functioningc(0 worst to 100 best) | 15132 | −9.7 | (19.8) | 23365 | −10.5 | (19.7) | 0.8 | (0.4, 1.2) | <0.001 |
| Vitalityc (0 worst to 100 best) | 15501 | −2.5 | (18.0) | 23917 | −3.5 | (18.1) | 1.0 | (0.7, 1.4) | <0.001 |
| Role Physicalc (0 worst to 100 best) | 15620 | −14.3 | (42.0) | 24089 | −14.6 | (42.3) | 0.3 | (−0.6, 1.1) | 0.52 |
| Bodily Painc (0 worst to 100 best) | 15833 | −7.8 | (25.3) | 24414 | −7.8 | (25.2) | 0.0 | (−0.5, 0.5) | 0.98 |
| Social Functioningc (0 worst to 100 best) | 15709 | −5.2 | (22.9) | 24217 | −5.2 | (23.2) | 0.1 | (−0.4, 0.5) | 0.74 |
| Role Emotionalc (0 worst to 100 best) | 15664 | −4.4 | (35.3) | 24129 | −5.3 | (36.4) | 0.9 | (0.2, 1.6) | 0.02 |
| Mental Healthc (0 worst to 100 best) | 15506 | 0.7 | (13.9) | 23873 | 0.0 | (14.0) | 0.6 | (0.4, 0.9) | <0.001 |
| Self-Reported Health (1 worst to 5 best) | 15795 | −0.20 | (0.81) | 24374 | −0.23 | (0.79) | 0.03 | (0.01, 0.04) | <0.001 |
| Depressive Symptoms (0 worst to 18 best) | 15285 | −0.11 | (2.71) | 23549 | −0.19 | (2.79) | 0.08 | (0.02, 0.14) | 0.005 |
| Sleep disturbance (0 worst to 20 best) | 15190 | −0.53 | (4.33) | 23435 | −0.66 | (4.43) | 0.13 | (0.04, 0.22) | 0.006 |
Intervention minus comparison.
P-value based on statistical significance of randomization assignment from linear model.
RAND-36 subscales
DISCUSSION
The WHI DM intervention was associated with small, statistically significant, improvements in the HRQoL subscales of general health, physical functioning, and vitality, consistent with our hypothesis. Improvements in vitality and physical functioning associated with the intervention persisted after adjusting for age, ethnicity, and weight at year 1 and baseline, suggesting that not all the effects of the intervention are mediated by weight change. In other words, some effects of the intervention are likely directly attributable to the large dietary changes made by the DM intervention group.
These findings are consistent with other research, which has shown improvements in HRQoL among studies that emphasized lower intake of fat, calories or both. Most of these studies, like ours, have not used a study design to specify a dietary component responsible for the observed changes. Increases in physical and mental health were noted with a Mediterranean dietary intervention as measured by the Medical Outcomes Survey short form.10 A calorie reduction diet coupled with increased physical activity resulted in increases in physical functioning, role physical, vitality and mental health, which compared to increased vitality for diet alone, and no changes in HRQoL for physical activity alone.4 Also, a low fat intervention to induce weight loss and decrease lipid levels demonstrated improvements in HRQoL including general health, vitality, physical functioning, and mental health.9 The same scales were markedly increased at the end of a 6 month weight loss intervention that included decreased dietary fat and calories as well as increases in physical activity.14,46 Another weight loss study with modest caloric reduction comparing self help versus a commercial program demonstrated no difference between groups, but improvement in general health and vitality with weight loss in both groups.18 As in our findings, changes in HRQoL scales physical functioning, general health, mental health, vitality and role-physical were found after a 13 week intervention, but in contrast only general health and vitality persisted after a year.17
In many studies the effects of dietary change are intertwined with those of weight loss, or increased fitness, although some reports reported separate associations. The Nurses’ Health Study demonstrated that while weight loss was associated with physical functioning and vitality dimensions of HRQoL, any weight change was more associated with physical rather than mental health.16 Consistent with those conclusions, a cross sectional study of mildly hypertensive adults by Stewart found that weight and BMI were associated with different HRQoL measures (pain) than those associated with fitness (vitality).3 In contrast, intervention induced weight loss among obese participants was associated with improvements in overall psychological health, decreases in anxiety and depression, and increases in positive well-being, self-control and vitality.12 Similarly, weight loss through diet or diet and physical activity interventions among overweight or obese postmenopausal women was strongly associated with improvements in physical functioning, role-physical, vitality and mental health, as were the psychosocial measures of depression, stress and social support.4 Though the aspects of HRQoL affected by changes in diet, fitness and weight may be somewhat distinct, Blissmer, et al. determined that sustained weight loss was not necessary to maintain changes in HRQoL.14 Additionally, the changes in general health, physical functioning, vitality, and mental health identified at one year in our study, though small in magnitude, persisted to the close-out measurement. To our knowledge, longer term follow-up of HRQoL measures have not been demonstrated in other research.
Our research identified significant changes in HRQoL scales, though the average magnitude of change was small. The size of change attributable to the intervention was smaller than is typically considered clinically significant,44,45 but may nonetheless be important at the population level.47 Previous studies that found larger effects were focused on overweight and obese participants.4,14,17,18 and individuals with pre-existing coronary heart disease.9 On the other hand, the Nurses’ Health Study observed small changes, similar to our findings, among overweight or obese women who lost weight over the four years of observation.16
Intervention-associated increases in measures of HRQoL were found among women who were older, and women who at baseline were obese, or who reported minimal activity. The moderating effects in our research have not been identified by others: older women and obese women have not previously been identified as more likely to benefit in physical functioning and general health from a dietary change intervention.
There are several limitations to this study. Dietary interventions designed for weight loss may have different results from those reported in this study, since the diet intervention did not include energy reduction or weight loss goals although both occurred in the first year of the intervention.26 This was not a blinded trial, so participant’s perception of the benefits of the intervention, and perceived lack of benefit for the control group, may have biased the results in these self-reported outcomes. The social support offered to participants in the group sessions, an important part of the intervention, could also have played a role not only in their weight loss but in change in measures of HRQoL (improved vitality, general health, and physical functioning) as well. It is difficult to separate the social benefits from the dietary benefits of the intervention, though the intervention appeared to provide increased benefit for very obese women, indicating that the effect was higher for heavier women. Women who did not complete the first year follow-up had lower baseline physical functioning and global QOL scores compared to women who participated in follow-up measures. However, since the proportion of women who did not complete follow up in both groups was very low, the likelihood of these differences influencing the results observed is minimal. The Role Physical scale measures problems with work or other daily activities that are the result of physical health. It is not inconceivable that those who are successful in changing their dietary habits improve their overall health in a way that would affect their ability to perform their daily activities or work. Change in diet may result in mood changes, some weight loss, or even some increase in physical activity which can, in turn, be associated with less difficulty performing work or daily activities. On the other hand, the changes may be largely perceptual: those deciding to make healthy changes may perceive greater differences in this particular scale once they notice they have made dietary behavior changes.
There were a large number of statistical tests of significance performed and some may be due to chance. The reader should be particularly cautious in interpreting associations within subgroups (e.g., CEE+MPA randomization group) that appear to modify the effect of DM on only one HRQoL measure. In contrast, DM effects varied among levels of BMI for several HRQoL measures, so these significant interactions are more plausible, although the effect sizes remained small. In interpreting the findings, it should be borne in mind that the women in the DM trial (both experimental groups) were by design those who were consuming a high proportion of calories from fat at baseline, and likely had a high fat dietary pattern for many years prior to the study.
Though the changes in HRQoL achieved were small for the full group, it is important to place those changes in the context of an intervention study that did not aim to change HRQoL, and did not even aim to change weight. 23, 33 The intervention asked participants to make many changes in their diets. Investigators were concerned that the magnitude of these changes would be burdensome to participants. The results of this paper demonstrate that not only were the changes not excessively burdensome, but may in fact have contributed to an overall increase in HRQoL attributable to the DM intervention.
CONCLUSIONS
The WHI DM intervention was associated with small, but significant improvements in three health related quality of life subscales: general health, physical functioning, and vitality at one year follow-up, with the largest improvements seen in the women with the greatest baseline BMI. Intervention-associated increases in measures of HRQoL were found among women who were older, and women who at baseline were obese, or who reported minimal activity. Obese women might ordinarily be targeted for intervention due to the clear health benefits of even modest weight loss, but our research suggests that older women could also be targeted for weight loss interventions given the likely benefits reported by this study: benefits over and above those directly associated with diet improvement or weight loss. Additionally, dietitians might consider emphasizing improved HRQoL as an outcome expectation among all diet improvement and weight loss candidates. The study may have implications for beneficial changes in quality of life associated with dietary changes in later life more generally.
Supplementary Material
ACKNOWLEDGMENT
The Women’s Health Initiative was supported by the National Heart, Lung and Blood Institute, US Department of Health and Human Services; NHLBI N01-WH-3-2100 and NHLBI N01-WH-3-2100. Clinical trial registration NCT00000611. Moreover, we gratefully acknowledge the dedicated efforts of the Women’s Health Initiative participants and of key Women’s Health Initiative investigators and staff throughout the project. We are especially grateful to Sonia Bishop for her contributions to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Annlouise R. Assaf, Global Medical Affairs, Global Innovative Pharma Women’s Health Pfizer Inc. Eastern Point Road, MS 8260-2204 Groton, CT 06340 Tel: (860) 441-1961 Annlouise.r.assaf@pfizer.com.
Shirley A.A. Beresford, Epidemiology and Senior Associate Dean School of Public Health, University of Washington Box 357230 Seattle, WA 98195-7230 Tel: 206-543-9512 beresfrd@u.washington.edu.
Patricia Markham Risica, Department Community Health Alpert Medical School, Brown University, Box G-S121-8, 121 South Main Street, Providence, RI 02912, Tel. 401-863-6550, Fax. 401-863-6651, Patricia_Risica@brown.edu.
Aaron Aragaki, Women’s Health Initiative Clinical Coordinating Center, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue N, M3-B232, Seattle, WA 98109-1024, Tel. (206) 667-6734, Fax. (206) 667-4142, aaragaki@whi.org.
Robert L. Brunner, Department of Nutrition, University of Nevada School of Medicine, 1664 North Virginia Street, Pennington Medical Education Building, Reno, NV 89557-0342, Tel. (775) 762-2479, Fax. (775) 784-6194, bbrunner@medicine.nevada.edu.
Deborah J. Bowen, Department of Bioethics & Humanities, University of Washington, 1107 NE 45th St, Suit 305, Seattle, WA 98195-7120, Tel. (206) 616-5601, Fax. (206) 685-7515, Dbowen@u.washington.edu.
Michelle Naughton, Division of Population Sciences, Department of Internal Medicine, College of Medicine, The Ohio State University, 1590 N High St, Suite 525, Columbus, OH 43210, Tel. (614) 293-6390, Fax. (614) 293-5611, Michelle.Naughton@osumc.edu.
Milagros C. Rosal, Division of Preventive and Behavioral Medicine, Department of Medicine, University of Massachusetts Medical School, 55 Lake Ave North, Worcester, MA 01655, Tel. (508) 856-2656, Fax. (508) 856-3840, Milagros.Rosal@umassmed.edu.
Linda Snetselaar, College of Public Health, 111 Jessup Hall, University of Iowa, Iowa City, IA 52242, Tel. (319) 335-3565, Fax. (319) 335-3560, Linda-snetselaar@uiowa.edu.
Nanette Wenger, Professor Emeritus, Emory University School of Medicine, Department of Medicine, Division of Cardiology, 49 Jesse Hill Jr. Drive, SE, Atlanta, GA 30303, Tel. (404) 616-4420, Fax. (404) 616-3093, nwenger@emory.edu.
REFERENCES
- 1.Acree LS, Longfors J, Fjeldstad AS, et al. Physical activity is related to quality of life in older adults. Health Qual Life Outcomes. 2006;4:37. doi: 10.1186/1477-7525-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Women’s Health Initiative Study G Dietary adherence in the Women’s Health Initiative Dietary Modification Trial. J Am Diet Assoc. 2004;104(4):654–658. doi: 10.1016/j.jada.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Stewart KJ, Turner KL, Bacher AC, et al. Are fitness, activity, and fatness associated with health-related quality of life and mood in older persons? J Cardiopulm Rehabil. 2003;23(2):115–121. doi: 10.1097/00008483-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Imayama I, Alfano CM, Kong A, et al. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:118. doi: 10.1186/1479-5868-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henriquez Sanchez P, Ruano C, de Irala J, Ruiz-Canela M, Martinez-Gonzalez MA, Sanchez-Villegas A. Adherence to the Mediterranean diet and quality of life in the SUN Project. Eur J Clin Nutr. 2012;66(3):360–368. doi: 10.1038/ejcn.2011.146. [DOI] [PubMed] [Google Scholar]
- 6.Ruano C, Henriquez P, Bes-Rastrollo M, Ruiz-Canela M, del Burgo CL, Sanchez-Villegas A. Dietary fat intake and quality of life: the SUN project. Nutr J. 2011;10:121. doi: 10.1186/1475-2891-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye X, Scott T, Gao X, Maras JE, Bakun PJ, Tucker KL. Mediterranean diet, healthy eating index 2005, and cognitive function in middle-aged and older Puerto Rican adults. J Acad Nutr Diet. 2013;113(2):276–281. e271–273. doi: 10.1016/j.jand.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowen DJ, Kestin M, McTiernan A, Carrell D, Green P. Effects of dietary fat intervention on mental health in women. Cancer Epidemiol Biomarkers Prev. 1995;4(5):555–559. [PubMed] [Google Scholar]
- 9.Gleason JA, Bourdet KL, Koehn K, Holay SY, Schaefer EJ. Cardiovascular risk reduction and dietary compliance with a home-delivered diet and lifestyle modification program. J Am Diet Assoc. 2002;102(10):1445–1451. doi: 10.1016/s0002-8223(02)90320-2. [DOI] [PubMed] [Google Scholar]
- 10.Toobert DJ, Glasgow RE, Strycker LA, et al. Biologic and quality-of-life outcomes from the Mediterranean Lifestyle Program: a randomized clinical trial. Diabetes Care. 2003;26(8):2288–2293. doi: 10.2337/diacare.26.8.2288. [DOI] [PubMed] [Google Scholar]
- 11.Wayne SJ, Baumgartner K, Baumgartner RN, Bernstein L, Bowen DJ, Ballard-Barbash R. Diet quality is directly associated with quality of life in breast cancer survivors. Breast Cancer Res Treat. 2006;96(3):227–232. doi: 10.1007/s10549-005-9018-6. [DOI] [PubMed] [Google Scholar]
- 12.Swencionis C, Wylie-Rosett J, Lent MR, et al. Weight change, psychological well-being, and vitality in adults participating in a cognitive-behavioral weight loss program. Health Psychol. 2013;32(4):439–446. doi: 10.1037/a0029186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driscoll I, Espeland MA, Wassertheil-Smoller S, et al. Weight change and cognitive function: findings from the Women’s Health Initiative Study of Cognitive Aging. Obesity (Silver Spring) 2011;19(8):1595–1600. doi: 10.1038/oby.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blissmer B, Riebe D, Dye G, Ruggiero L, Greene G, Caldwell M. Health-related quality of life following a clinical weight loss intervention among overweight and obese adults: intervention and 24 month follow-up effects. Health Qual Life Outcomes. 2006;4:43. doi: 10.1186/1477-7525-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns CM, Tijhuis MA, Seidell JC. The relationship between quality of life and perceived body weight and dieting history in Dutch men and women. Int J Obes Relat Metab Disord. 2001;25(9):1386–1392. doi: 10.1038/sj.ijo.0801714. [DOI] [PubMed] [Google Scholar]
- 16.Fine JT, Colditz GA, Coakley EH, et al. A prospective study of weight change and health-related quality of life in women. JAMA. 1999;282(22):2136–2142. doi: 10.1001/jama.282.22.2136. [DOI] [PubMed] [Google Scholar]
- 17.Fontaine KR, Barofsky I, Bartlett SJ, Franckowiak SC, Andersen RE. Weight loss and health-related quality of life: results at 1-year follow-up. Eat Behav. 2004;5(1):85–88. doi: 10.1016/S1471-0153(03)00059-X. [DOI] [PubMed] [Google Scholar]
- 18.Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA. 2003;289(14):1792–1798. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (Lond) 2007;31(8):1248–1261. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 20.Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Health-related quality of life in obese outpatients losing weight with very-low-energy diet and behaviour modification--a 2-y follow-up study. Int J Obes Relat Metab Disord. 2003;27(10):1233–1241. doi: 10.1038/sj.ijo.0802379. [DOI] [PubMed] [Google Scholar]
- 21.Prentice RRJ, Furberg C, Johnson S, Henderson M, Cummings S, Manson J, Freedman L, Oberman A, Kuller L, Anderson G, The Women’s Health Initiative Study Group Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 22.Beresford SA, Johnson KC, Ritenbaugh C, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):643–654. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- 23.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: the Women’s Health Initiative Dietary Modification Trial. JAMA. 2006;295(1):39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 24.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 Suppl):S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 25.Ritenbaugh C, Patterson RE, Chlebowski RT, et al. The Women’s Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 Suppl):S87–97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 26.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 28.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 29.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 30.Dietary adherence in the Women’s Health Initiative Dietary Modification Trial. J Am Diet Assoc. 2004;104(4):654–658. doi: 10.1016/j.jada.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Tinker LF, Perri MG, Patterson RE, et al. The effects of physical and emotional status on adherence to a low-fat dietary pattern in the Women’s Health Initiative. J Am Diet Assoc. 2002;102(6):789–800. 888. doi: 10.1016/s0002-8223(02)90178-1. [DOI] [PubMed] [Google Scholar]
- 32.Tinker LB, Henry E, Patterson H, Rupp R, Van Horn J. The Women’s Health Initiative: Overview of the nutrition components. In: Kris-Etherton DAKM, editor. Nutrition in Women’s Health. Aspen Publishers; Gaithersburg, MD: 1996. pp. 510–549. L. [Google Scholar]
- 33.Howard BV. Dietary fat and cardiovascular disease: putting the Women’s Health Initiative in perspective. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2007;17(3):171–174. doi: 10.1016/j.numecd.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 35.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38(1):21–37. [PubMed] [Google Scholar]
- 36.Maddox GL. Some correlates of differences in self-assessment of health status among the elderly. J Gerontol. 1962;17:180–185. doi: 10.1093/geronj/17.2.180. [DOI] [PubMed] [Google Scholar]
- 37.Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. Am J Public Health. 1982;72(8):800–808. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wassertheil-Smoller S, Shumaker S, Ockene J, et al. The Women’s Health Initiative (WHI) Depression and cardiovascular sequelae in postmenopausal women. Arch Intern Med. 2004;164(3):289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- 39.Levine DW, Kaplan RM, Kripke DF, Bowen DJ, Naughton MJ, Shumaker SA. Factor structure and measurement invariance of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):123–136. doi: 10.1037/1040-3590.15.2.123. [DOI] [PubMed] [Google Scholar]
- 40.Levine DW, Kripke DF, Kaplan RM, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):137–148. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 41.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 42.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60(10):1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 43.SAS Institute, Inc. SAS Institute Inc Statistical Analysis System; Cary, NC: 2002-2003. [Google Scholar]
- 44.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 45.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Academic Press Inc; Orlando, Fla: 1988. [Google Scholar]
- 46.Riebe D, Blissmer B, Greene G, et al. Long-term maintenance of exercise and healthy eating behaviors in overweight adults. Prev Med. 2005;40(6):769–778. doi: 10.1016/j.ypmed.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 47.Young DR, Coughlin J, Jerome GJ, Myers V, Chae SE, Brantley PJ. Effects of the PREMIER interventions on health-related quality of life. Annals of behavioral medicine: a publication of the Society of Behavioral Medicine. 2010;40(3):302–312. doi: 10.1007/s12160-010-9220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.