Abstract
Among recently investigated stroke therapies, stem cell treatment holds great promise by virtue of their putative ability to replace lost cells, promote endogenous neurogenesis and produce behavioral and functional improvement through their “bystander effects.” Translating stem cell in the clinic, however, presents a number of technical difficulties. A strategy suggested to enhance therapeutic utility of stem cells is combination therapy, i.e., cotransplantation of stem cells or adjunct treatment with pharmacological agents and substrates, which is assumed to produce more profound therapeutic benefits by circumventing limitations of individual treatments, and facilitating complementary brain repair processes. We previously demonstrated enhanced functional effects of co-treatment with granulocyte-colony stimulating factor (G-CSF) and human umbilical cord blood cell (hUCB) transplantation in animal models of traumatic brain injury (TBI). Here, we suggest that the aforementioned combination therapy may also produce synergistic effects in stroke. Accordingly, G-CSF treatment may reduce expression of pro-inflammatory cytokines and enhance neurogenesis rendering a receptive microenvironment for hUCB engraftment. Adjunct treatment of G-CSF with hUCB may facilitate stemness maintenance and guide neural lineage commitment of hUCB cells. Moreover, regenerative mechanisms afforded by G-CSF-mobilized endogenous stem cells, secretion of growth factors by hUCB grafts and G-CSF-recruited endothelial progenitor cells (EPCs) , as well as the potential graft–host integration that may promote synaptic circuitry re-establishment could altogether produce more pronounced functional improvement in stroked rats subjected to a combination G-CSF treatment and hUCB transplantation. Nevertheless, differences in pathology and repair processes underlying TBI and stroke deserve consideration when testing effects of combinatorial G-CSF and hUCB cell transplantation for stroke treatment. Further studies are also required to determine safety and efficacy of this intervention in both preclinical and clinical stroke studies.
Keywords: G-CSF, hUCB cells, stroke, combination therapy
Introduction
Stroke is a worldwide public health concern causing 5.5 million deaths and the annual loss of 49 million disability-adjusted life-years [1,2]. Despite years of research, therapeutic options for acute ischemic stroke remain very limited [3,4]. To date, there is no specific treatment available for either focal cerebral ischemia or global ischemic event other than the recombinant protein therapy called tissue plasminogen activator or tPA, which dissolves thrombi in affected blood vessels following stroke [5,6]. However, a major limitation with tPA treatment is its very narrow therapeutic window of 4.5 hours after stroke onset [7]. Administering tPA beyond the therapeutic time window in stroke patients presents with detrimental side effects, most notably, hemorrhagic transformation, which can exacerbate stroke injury and counteract the benefits provided by reperfusion of the occluded artery, and even lead to high mortality in stroke patients [7,8]. Thus, a mere 3 percent of ischemic stroke patients actually benefit from tPA therapy [9,10]. Moreover, most of the currently used stroke therapies (e.g. endovascular procedures using stents, surgical treatments) show limited efficacy in restoring lost neurological functions [11]. The conventional medical and rehabilitation therapies are only designed to enhance endogenous recovery, prevent the recurrence of stroke, adapt to loss of function, and avoid dysfunctional behavior, but fail to address the permanent loss of brain tissue following stroke [12], which must be considered for optimal recovery. The lack of effective therapies and their significant adverse effects for stroke prompted both preclinical and clinical research for novel stroke interventions. The potential for small molecules and other pharmacological treatments to enhance the recovery process is currently being investigated, but the greatest expectations are pinned on the potential of stem cells, which are painted by the media as “magic bullets” for various diseases.
Stem cells for stroke treatment
In contrast with pharmacologic agents, stem cell-based interventions show efficacy when initiated in acute and sub-acute phases, as well as at later time-points following stroke onset and address the complex pathophysiology of stroke, providing neurological improvement [13,14]. Stem cells exert therapeutic benefits against ischemic stroke via transplantation of exogenous stem cells or stimulation of endogenous stem cells within the neurogenic niches of subventricular zone (SVZ) and subgranular zone (SGZ), or recruited from the bone marrow through peripheral circulation [15]. The safety and efficacy of several sources of stem cells have been demonstrated in animal models of stroke [e.g. 15,16]. We have recently reviewed these various kinds of stem cell sources in a previous report [16]. The major types of cells transplanted in stroke include fetal-derived cells, neuroteratocarcinoma cells (NT2N), xenogenic pig-derived cells, embryonic stem (ES) cells, adult stem cells (bone marrow, human umbilical cord, placenta, amnion fluid, menstrual blood), and induced pluripotent stem cells (iPS) [16]. A number of preclinical studies on the effects of stem cell treatment in stroke reported the ability of transplanted stem cells to improve stroke-induced brain and behavioral pathology robustly early on and stably over long-term post-insult than any available stroke treatments [e.g. 17-19, for reviews see 15,16].
As opposed to ES cells, the use of adult stem cells has flourished over the last decade, due to ethical and logistical concerns associated with the use of ES cells, and the moratorium for using federal funds on ES research [15,16]. Adult stem cells as potential transplantation sources are also more feasible than ES cells, as they circumvent ethical and moral problems associated with stem cell therapy, and also minimize teratogenic and oncogenic risks usually presented with transplantation of embryonal or fetal-derived stem cells [20]. Currently, the ongoing FDA-approved stem cell clinical trials in stroke use adult stem cells. For this paper, we highlight the potential of human umbilical cord blood (hUCB)-derived cells of which our group and several others have assessed their potential clinical utility for various intractable disorders, including stroke [17-19, 21; for review see 22].
Transplantation of umbilical cord blood-derived stem cells resulted in functional recovery, reduction in infarct size, and higher expression of neuroprotective factors, such as brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) [19,23-26] in stroked animals. Limited clinical trials of hUCB cells for stroke are ongoing, as well as in the treatment of other neurological disorders [for review see 27]. There are several advantages to using hUCB in cell transplantation therapy such as providing an unlimited supply of cells in culture thereby circumventing ethical and logistical issues, availability in significant quantities and producing higher yields of hematopoietic progenitor cells, retained capacity of stem or progenitor cell from cord blood to proliferate and differentiate despite years of cryopreservation, low incidence of graft-versus-host disease when compared to that of the adult bone marrow, and long-standing and successful clinical history in the hematopoietic field [for review see 28]. hUCB stem cells have also been used experimentally in animal models of traumatic brain injury (TBI) [e.g. 28-30]. Albeit there are distinct differences in the pathology and mechanisms that govern repair in TBI and stroke (see below), some overlapping pathophysiological mechanisms in both neurological conditions indicate that hUCB treatment may be beneficial for both TBI and stroke [31]. Moreover, some studies showed that transplantation of cells obtained from the mononuclear fraction of hUCB conferred neuroprotection by decreasing inflammation and brain tissue loss, promoting neurogenesis, and rescue of neurological dysfunctions [e.g., 32-34].
The hurdles of stem cell therapy for stroke
Despite the rising trend of stem cell research quickly translating into clinical trials, it is imperative to gain insights into the mechanisms of action of stem cells. These information will guide optimization of both safety and efficacy of these interventions. In respect to safety, the extent to which a stem cell treatment needs to be tested is risk-based, thus, whether stem cells are generally safe or not remain controversial [12]. With regard to efficacy, there is a need for rigorous translational research which should primarily determine optimal transplantation regimen and provide important details on the regenerative mechanisms of transplanted stem cells, which, by far, remain to be established [35]. This necessitates demonstration of a well-defined stem cell source for quality assurance and quality control of graft origin and also for validity and reproducibility of experimental outcomes [35]. In the case of hUCB cells, apart from identifying the optimal transplantable hUCB cell phenotype, low graft survival may be expected in the injured (e.g. stroked) brain, potentially due to the host tissue microenvironment that does not promote cell integration, maturation and survival, likely created by the secondary neuroinflammatory response post-injury [35-37]. Despite the fact that robust graft survival may not be necessary to promote behavioral recovery, the alternative mechanism of graft-induced “bystander effects” still implores modulation of the hostile ischemic tissue microenvironment as well as providing an appropriate array of local and developmental cues for the maturation, integration and/or survival of implanted or existing cells in order to facilitate important clinical outcomes [35-38]. Thus, enhancing the therapeutic effects of transplanted stem cells by rendering a receptive (i.e., less neuroinflammatory) microenvironment and that which promotes maturation, integration and/or survival of stem cells appeal to advancing regenerative medicine for stroke treatment.
Enhancing therapeutic utility of stem cells through combination therapy: the potential of co-treatment of GCSF and stem cells in stroke
Synergistic effects on stem cell survival were documented with co-transplantation of stem cells. Co-transplantation therapy also decreased adverse events as in the case of co-transplanting bone marrow-derived stromal cells with embryonic stem cells which reduced the incidence of tumor production, and combined treatment of neural stem cells and epithelial cells which reportedly enhanced cell survival and stem cell differentiation [39,40].
The ability to enhance therapeutic effects of a stem cell type is not just limited to adjunct treatment with another stem cell source. The use of pharmacological agents and substrates to enhance the efficacy of a stem cell line being transplanted was also reported, for instance, combination treatment of bone marrow-derived stromal cells and trophic factors which enhanced survival and potentiation or provided a scaffold for stem cell adherence [41,42]. Here we discuss the potential therapeutic benefits of adjunct treatment of granulocyte-colony stimulating factor (GCSF) with hUCB in stroke. G-CSF, per se, is considered as an attractive stroke therapy in view of its diverse pharmacologic actions such as activation of several neuroprotective pathways, anti-inflammatory and anti-apoptotic effects, and also its ability to induce mobilization of hematopoietic stem cells which stimulate differentiation of adult neuronal stem cells, promote angiogenesis, vasculogenesis and repair of the blood-brain barrier (BBB) (see below). Thus, by combining G-CSF and hUCB cells, the therapeutic limitations of hUCB transplantation in stroke may be overcome, or the limitations of G-CSF as a stroke treatment may also be circumvented.
G-CSF as an attractive stroke therapeutics
First discovered in the mid-1960s as a hematopoietic glycoprotein that regulates the survival, proliferation, differentiation, and function of neurotrophil granulocyte progenitor cells and mature neutrophils, G-CSF is a myeloid growth factor produced by activated macrophages, endothelial cells, and fibroblasts [43,44]. G-CSF exerts a wide range of actions such as enhancement of the growth of mainly neutrophilic granulocyte colonies in a CFUGM assay, and improving the functions of mature neutrophils, for example, enhancement of chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine binding on mature neutrophils [45-47; for review see 35].
Produced in the bone marrow in response to cellular stimuli, G-CSF also binds to specific receptors (i.e., G-CSF receptor) in hematopoietic progenitor cells, monocytes, platelets, neurons, endothelial cells, and small-cell lung cancer cells [35]. When G-CSF receptors are activated, induction of signaling cascades follows including the Janus kinase/signal transducer and transcription activator, Ras/mitogen-activated protein kinase and phosphotidyl inositol 3-kinase/Protein kinase B/Akt pathways, which play important roles in cellular proliferation, anti-inflammatory, and antiapoptotic processes and also in mobilizing stem cells to target sites (e.g., sites of injury) [for review see 35]. The key roles of G-CSF in the CNS have also been identified in recent studies [46]. Notably, G-CSF passes the BBB allowing its delivery from the periphery to reach the site of brain injury, and acts on neurons to afford its therapeutic effects [47,48]. In view of these findings, G-CSF is a novel neurotrophic factor and a highly attractive candidate for the treatment of neurologic and neurodegenerative conditions and various types of brain injuries [49].
Efficacy of G-CSF in animal models of stroke
The efficacy of G-CSF in animal models of stroke in different species has been shown in previous studies [e.g., 50-52). Treatment with optimal doses of G-CSF increased CD34+ (endothelial progenitor cell [EPC] marker) cells in peripheral blood, which decreased infarct volumes in stroked animal [53]. In other studies, treatment with G-CSF reduced glutamate-induced neurotoxicity [54], influenced apoptotic pathways [55,56], attenuated edema formation and interleukin-1β expression [57], and activated the cerebral G-CSF receptor resulting in reduction of infarct volume in stroked animals [54,56]. G-CSF also stimulated endogenous neurogenesis and vascularization [56].
As mentioned above, the only FDA-approved treatment for acute ischemic stroke, tPA, presents deleterious side effects, notably, hemorrhage, when administered beyond the 4.5 therapeutic window. Recent studies indicate that cerebral hemorrhage after delayed tPA treatment is due to tPA’s effect on the neurovascular unit which is comprised of microvascular endothelium, astrocytes, pericytes, neurons, and the extracellular matrix, and subsequent breakdown of the BBB [56]. We have recently investigated whether treatment with G-CSF in conjunction with tPA at a delayed time point (6 h post stroke) reduces hemorrhagic transformation associated with delayed tPA treatment [58]. Quantitative analysis of cerebral hemorrhage volume showed reduction of delayed tPA-induced hemorrhage in stroked rats treated with G-CSF. Rats treated with G-CSF also displayed significant improvement of neurological and motor deficits compared to rats given vehicle or tPA only [58]. Concomitant with the reduction in HT, increased levels of angiogenesis marker Ang-2, vasculogenesis marker vWF, phosphorylated eNOS, as well as EPC markers CD34+ and vascular endothelial growth factor (VEGFR)-2 were observed in the ischemic hemispheres of G-CSF+tPA-treated stroke rats compared to those given tPA alone. Together, these findings indicate that G-CSF reduces delayed tPA-induced HT and consequently improves neurological improvement post-stroke via angiogenic and vasculogenic activities of G-CSF and/or proliferative or regenerative actions of G-CSF-recruited EPCs [58]. Of note, apart from G-CSF, we have earlier proposed the therapeutic efficacy of EPC transplantation in stroke in view of their ability to reconstitute the BBB [59,60]. Together, the above studies indicate the potential clinical worth of G-CSF in stroke treatment, not only through its capacity to reduce functional post-stroke histological and functional deficits, but also to attenuate lethal hemorrhage due to delayed treatment of the only FDA-approved stroke intervention, tPA.
G-CSF in clinical trials for stroke
G-CSF is indeed a promising therapy for stroke due to its diverse therapeutic actions. The clinical studies of G-CSF in acute ischemic stroke reported that G-CSF (Filgrastim) was effective at mobilizing bone marrow CD34+ stem cells in stroke patients [61], was feasible, and safe and improved neurological outcomes in stroke patients [62], and was also well-tolerated even at high dosages in stroke patients [63]. The results of recent clinical trial [63], however, reported contradicting findings, in that G-CSF (Filgrastim; AX200) treatment failed to improve clinical outcomes in adult ischemic stroke patients. Among the reasons cited for the clinical failure of G-CSF were excessively long therapeutic time window for G-CSF (i.e., the timepoint of treatment with G-CSF was 6.8 h post-stroke), the limited number of centers involved in the clinical trial [64], and co-treatment with tPA. Since irreversible damage could have already occurred at 6.8 h post-stroke, administering G-CSF at this timepoint may be expected to produce limited efficacy [65-67]. Co-treatment of G-CSF with tPA may have also attenuated the beneficial effects of G-CSF due to the prior effects of the tPA to weaken the endothelium [64]. This is, however, controversial in view of the findings of an association study which reported that G-CSF did not enhance hemorrhage in G-CSF-treated stroke patients administered with tPA [68]. Moreover, the latter study reported that serum levels of G-CSF was associated with good functional outcomes even at 90 days post treatment [68].
Currently, there are other ongoing clinical trials evaluating G-CSF treatment for stroke of which findings are not yet reported: “Study to determine the effect of a drug called Neupogen on stroke recovery (GIST),” “Establishment of clinical basis for hematopoietic growth factors therapy in brain injury,” and “The variation of movement related cortical potential, cortio-cortical inhibition, and motor evoked potential in intracerebral implantation of autologous peripheral blood stem cells (CD34) in old ischemic stroke”[69]. In summary, albeit the promise of G-CSF as a stroke intervention, there is still much to be proven with regard to both safety and efficacy before it can be used in the clinic.
Combining G-CSF and stem cells for stroke treatment: translational perspectives and efficacy
Adjunct treatment of G-CSF with stem cells (e.g., bone marrow mononuclear cell) produced synergistic beneficial effects in an experimental mouse model of cerebral ischemia [70]. These effects were assumed to involve, at least in part, enhancement of proliferation and differentiation of bone marrow stem cells resulting in improved host brain regeneration and functional recovery [70]. The effects of G-CSF treatment alone or in combination with bone marrow mesenchymal stem cells (BM-MSCs) after stroke were recently evaluated in aged rats [71, 72]. While the combination therapy produced remarkable angiogenesis in the formerly infarct core and beyond in the “islet of regeneration,” it did not afford better neuroprotection and recovery post-stroke compared with G-CSF treatment alone [71]. That effects of G-CSF were more robust than those exerted by combination treatment with BM-MSCs is consistent with the previous observation on survival-enhancing capacity and beneficial effects on functional outcomes of G-CSF treatment in aged rats [72].
Recently, we tested the putative therapeutic benefits of combination therapy of hUCB and G-CSF in a controlled cortical impact model of moderate TBI in adult rats [73]. Our studies showed that the combination therapy produced more pronounced functional improvement in TBI rats than that exerted by monotherapy with hUCB or G-CSF [73]. Notably, beneficial effects of combination therapy were longer lasting than those exerted by hUCB transplantation or G-CSF administration alone. Hence, complementary brain repair processes distinctly or mutually afforded by these two therapies may have mediated improved functional recovery exerted by combination treatment of hUCB and G-CSF.
The efficacy of hUCB transplantation to reduce inflammation and facilitate neurogenesis and angiogenesis has been shown in preclinical studies involving animal models of TBI, stroke, and aging studies [74-76]. Furthermore, treatment with G-CSF has been shown to modulate neurogenesis in TBI and in other neurodegenerative disorders (e.g., hypoxic injury, Alzheimer’s disease, etc.) [75,77]. Our histological studies indicated that concomitant with functional improvement, adjunct treatment of G-CSF and hUCB resulted in more pronounced reduction of TBI-induced upregulation of MHCII+ cells (putatively activated microglia) in the cortex, striatum, thalamus, SVZ, and the dentate gyrus (DG) of the hippocampus, compared with hUCB and/or G-CSF monotherapy [73]. We also noted a decrease in the number of MHCII+ cells not only in the corpus callosum and fornix, but also in the cerebral peduncle of TBI rats subjected to combined therapy of hUCB and G-CSF. Moreover, combination hUCB and GCSF therapy exerted synergistic effects in attenuating TBI-induced impairment in endogenous neurogenesis and hippocampal cell loss, and reduced neuroinflammation, which coincided with enhanced neurogenesis in DG and SVZ while increasing the survival of CA3 neurons in TBI rats [73]. Together, the above results show that combined therapy of hUCB and G-CSF synergistically dampened TBI-induced neuroinflammation and also enhanced endogenous neurogenesis and reduced hippocampal cell loss.
In view of these results, it would be worthwhile to examine the efficacy of combination hUCB and G-CSF therapy in stroke. The interactions between hUCB and G-CSF may produce more profound functional recovery post-stroke by producing widespread effects in diverse brain regions. G-CSF via its ability to pass the BBB to act upon neurons and glial cells through the G-CSF receptor [76], may further reduce expression of pro-inflammatory cytokines and enhance neurogenesis [51,78]. G-CSF-mobilized stem cells, which can infiltrate injured tissues may enhance neural repair [56,77,79]. Adjunct treatment of G-CSF with hUCB may facilitate stemness maintenance and, under appropriate circumstances (such as in combination with SCF), may guide neural lineage commitment of hUCB [80,81]. Moreover, G-CSF-mobilized bone marrow cells (i.e. EPCs) and hUCB cells may also altogether exert therapeutic benefits via a paracrine mechanism, whereby transplanted cells secrete growth factors, trophic factors, chemokines, and immune-modulating cytokines to the injured brain tissue microenvironment, in keeping with the concept of bystander effects of transplanted stem cells [32,82-84]. hUCB contains many cell types e.g., hematopoietic stem/progenitor cells, endothelial progenitors, lymphocytes, and mesenchymal stem/progenitor cells that have been shown to exert neuroprotective and immunomodulatory effects in animal models of neurological diseases, including stroke [for review see 85]. Freshly isolated hUCB mononuclear cells are known to express BDNF transcripts, glial cell line–derived neurotrophic factor (GDNF), nerve growth factor (NGF), neurotrophin (NT)-3, and NT-5 in higher amounts than peripheral blood mononuclear cells [86]. Furthermore, culture supernatants of hUCB cells also contain VEGF, BDNF, NT-4, NT-5, and several cytokines and chemokines [28,88]. G-CSF, per se, has been suggested as a neurotrophic factor [46,87], which enhanced neuronal differentiation of adult neural stem cells in the brain, and improved long-term recovery in more chronic stroke models [46]. It has also been reported that Flk1/CD34-double-positive EPCs can express pro-recovery mediators such as the BDNF and basic fibroblast growth factor [88]. In summary, regenerative mechanisms such as those accomplished by G-CSF-mobilized endogenous stem cells as well as secretion of growth and neurotrophic factors by hUCB grafts and G CSF-recruited EPCs may altogether cause more profound functional improvement in stroke rats subjected to a combination hUCB and G-CSF therapy. An important goal for future research is to determine whether this approach will also facilitate graft–host integration and promote reconstruction of synaptic circuitry [89], and to prove that these events correlate with functional recovery. So far, circuit integration of newly formed cells remains to be proven as an important mechanism of repair of stem cell transplantation in stroke and in other neurological disorders [for review see 90].
Differences in pathology and repair processes underlying TBI and stroke deserve consideration when evaluating effects of combining G-CSF and hUCB for stroke treatment. It is quite known that TBI and stroke arise from different types of primary insults (traumatic vs. ischemic) producing different cellular vulnerability patterns, injury and recovery processes [for review see 31]. In TBI, head trauma results in shear forces that produce primary membrane damage to neuronal cell bodies, white matter structures and vascular beds and a spectrum of secondary injury mechanisms. Cerebral ischemia produces metabolic stress, disturbances in intracellular ionic homeostasis, and a cascade of biochemical and molecular events ultimately leading to neuronal death [31]. While stroke involves greater damage in gray matter structures, damages in white matter are more pronounced in TBI [31]. Nevertheless, as mentioned above, certain similarities in the pathogenesis of these neurological injuries imply that therapeutic approaches which are beneficial in TBI may also be helpful in stroke [31]. Thus, it is still very interesting to test efficacy of combination G-CSF and hUCB treatment in both preclinical and clinical stroke studies. Importantly, the fact that both G-CSF and hUCB cell transplantation showed promise as stand-alone treatments in both preclinical and clinical stroke studies indicate rationality and potentiality of combinatorial G-CSF and hUCB cells treatment for stroke. Moreover, while the pathology of TBI (axonal injury) differs from stroke (infarction), the acute cortical cell death followed by progressive secondary cell death may allow testing of treatments effective in TBI to stroke, and vice versa.
Conclusion
Therapeutic interventions for stroke remain very limited, and there is a substantial need for restorative or regenerative stroke therapies in view of the permanent disability that ensue in at least 50% of stroke survivors [for review see 12]. Stem cell treatment holds promise to address the latter clinical need in view of the putative ability of stem cells to replace lost cells and promote endogenous neurogenesis, and their bystander effects. However, the diverse functional outcomes of stem cell treatment suggest the necessity for adjunctive treatments (e.g. pharmacologic) to enhance their therapeutic benefits. Our recent studies on the effects of combination therapy of hUCB and G-CSF in TBI models support the potential of this approach in stroke treatment and demonstrate how the limitations of stand-alone therapies (i.e., hUCB and G-CSF) could be overcome when combination therapy is pursued instead of monotherapy [73]. Further studies are necessary to address safety and efficacy of this approach in both preclinical and clinical settings and to determine the mechanism of action. With regard to the latter, it might be challenging to identify the exact mechanism of action of this combination therapy in stroke, considering that molecular events underlying functional recovery following stem cell transplantation are not yet well-defined, and could interface with the molecular effects G-CSF. Furthermore, it is also important to consider that despite similarities in the pathogenesis of stroke and TBI, these neurological injuries also show differences in certain aspects in their pathology (e.g. tissue responses to differing injury severities and types) which may complicate treatment strategies not tailored to individual cases [31].
As different types of combination therapies continue to surface and demonstrate their efficacy, many lab-to-clinic translational variables persist necessitating laboratory investigations prior to human application. In respect to the use of stem cells, factors including optimal dose, route of administration, and sex of donor/recipient, all of which are likely to be dependent upon the cell type being tested need to be determined [91]. We erstwhile investigated many of these parameters with umbilical cord blood for conditions such as Alzheimer’s disease, Amyotrophic Lateral Sclerosis, and Sanfilippo syndrome [91,92], but there is still much to be understood in regard to stroke therapy. With regard to the use of G-CSF, although the safety of this intervention has been proven in the clinic, it remains important to determine appropriate dosing of the drug when combined with other interventions (e.g. stem cells), as well as the timepoint of G-CSF treatment with respect to stem cell treatment, to facilitate enhanced neuroprotection and induction of neurogenesis, and also vascular and BBB repair afforded by G-CSF-recruited EPCs.
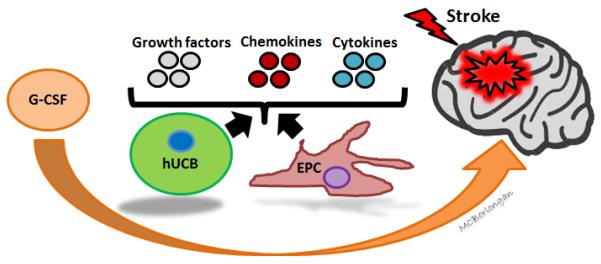
Figure 1. Perspectives on efficacy, mechanism of action and therapeutic utility of combinatorial G-CSF and hUBC treatment in stroke.
Combination therapy of G-CSF and hUCB treatment is likely to produce more meaningful therapeutic effects and circumvent therapeutic limitations of G-CSF or hUCB treatment alone. Accordingly, interactions between G-CSF and hUCB may produce numerous effects in diverse brain regions. GCSF treatment may reduce expression of pro-inflammatory cytokines and enhance neurogenesis by acting upon GCSF receptors on neurons and glial cells. It may also enhance neural repair through G-CSF mobilized cells, e.g. EPCs. Adjunct treatment of G-CSF with hUCB may promote stemness maintenance and guide neural lineage commitment of hUCB. Furthermore, G-CSF-mobilized EPCs and hUCB cells may altogether exert therapeutic benefits by secreting growth and neurotrophic factors, such as BDNF, GDNF, VEGF, NGF, NT-3, NT-4, NT-5, chemokines, and immune-modulating cytokines to the injured brain tissue microenvironment. Together, regenerative mechanisms accomplished by G-CSF-mobilized EPCs and EPC- and hUCB graft-secreted growth and neurotrophic factors and chemokines may cause more profound functional improvement after stroke. Abbreviations: hUCB: human umbilical cord blood cell, G-CSF: granulocyte-colony stimulating factor, EPC: endothelial progenitor cells, BDNF: brain-derived neurotrophic factor, GDNF: glial cell line–derived neurotrophic factor, NGF: nerve growth factor, NT: neurotrophin, VEGF: vascular endothelial growth factor.
Acknowledgments
This research was funded by the National Institutes of Health National Institute of Neurological Disorders and Stroke 1R01NS071956-01A1, 1R21NS089851-0, James and Esther King Biomedical Research Foundation 1KG01-33966, and Loma Linda University School of Pharmacy. The authors thank Mia Borlongan for help with drawing of the figure.
Footnotes
Compliance with Ethical Standards
Ethical Approval
This article does not contain studies with human participants or animals performed by any of the authors.
Conflict of Interest
C. V. Borlongan has patents and patent applications on stem cell therapy. I. dela Peña declares that he has no conflict of interest.
References
- 1.Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312(3):259–68. doi: 10.1001/jama.2014.7692. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . World health report 2004-changing history. World Health Organization; Geneva: 2004. [Google Scholar]
- 3.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–23. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 4.Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl. Stroke Res. 2014;5(4):442–53. doi: 10.1007/s12975-014-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nature Rev. Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 6.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N. Eng. J. Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 7.The NINDS rt-PA Stroke Study Group Intracerebral hemorrhage after intravenous tPA therapy for ischemic stroke. Stroke. 2007;28:2109–18. doi: 10.1161/01.str.28.11.2109. (1997) [DOI] [PubMed] [Google Scholar]
- 8.Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116(16):e391–413. doi: 10.1161/CIRCULATIONAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 9.Graham GD. Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta-analysis of safety data. Stroke. 2003;34:2847–50. doi: 10.1161/01.STR.0000101752.23813.C3. [DOI] [PubMed] [Google Scholar]
- 10.Yip TR, Demaerschalk BM. Estimated cost savings of increased use of intravenous tissue plasminogen activator for acute ischemic stroke in Canada. Stroke. 2007;38:1952–55. doi: 10.1161/STROKEAHA.106.479477. [DOI] [PubMed] [Google Scholar]
- 11.Stone LL, Grande A, Low WC. Neural repair and neuroprotection with stem cells in ischemic stroke. Brain Sci. 2013;3(2):599–614. doi: 10.3390/brainsci3020599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinden JD, Muir KW. Stem cells in stroke treatment: the promise and the challenges. Int. J. Stroke. 2012;7(5):426–34. doi: 10.1111/j.1747-4949.2012.00840.x. [DOI] [PubMed] [Google Scholar]
- 13.Borlongan CV, Koutouzis TK, Jorden JR, Martinez R, Rodriguez AI, Poulos SG, et al. Neural transplantation as an experimental treatment modality for cerebral ischemia. Neurosci. Biobehav. Rev. 1997;21:79–90. doi: 10.1016/0149-7634(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 14.Nishino H, Borlongan CV. Restoration of function by neural transplantation in the ischemic brain. Prog. Brain Res. 2000;127:461–76. doi: 10.1016/s0079-6123(00)27022-2. [DOI] [PubMed] [Google Scholar]
- 15.Shinozuka K, Dailey T, Tajiri N, Ishikawa H, Kim DW, Pabon M, et al. Stem cells for neurovascular repair in stroke. J. Stem Cell Res. Ther. 2013;4(4):12912. doi: 10.4172/2157-7633.S4-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinozuka K, Dailey T, Tajiri N, Ishikawa H, Kaneko Y, et al. Stem cell transplantation for neuroprotection in stroke. Brain Sci. 2013;3:239–61. doi: 10.3390/brainsci3010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui X, Chopp M, Shehadah A, Zacharek A, Kuzmin-Nichols N, Sanberg D, et al. Therapeutic benefit of treatment of stroke with simvastin and human umbilical cord blood cells: neurogenesis, synaptic plasticity, and axon growth. Cell Transplant. 2012;21:845–56. doi: 10.3727/096368911X627417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe D, Leonardo C, Recio J, Collier L, Willing A, Pennypacker K. Human umbilical cord blood cells protect oligodendrocytes from brain ischemia through Akt signal transduction. J. Biol. Chem. 2012;287:4177–87. doi: 10.1074/jbc.M111.296434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao J, Nan Z, Motooka Y, Low WC. Transplantation of a novel cell line population of umbilical cord blood stem cells ameliorates neurological deficits associated with ischemic brain injury. Stem Cells Dev. 2005;14:722–33. doi: 10.1089/scd.2005.14.722. [DOI] [PubMed] [Google Scholar]
- 20.Sanberg PR, Eve DJ, Metcalf C, Borlongan CV. Advantages and challenges of alternative sources of adult-derived stem cells for brain repair in stroke. Prog. Brain Res. 2012;201:99–117. doi: 10.1016/B978-0-444-59544-7.00006-8. [DOI] [PubMed] [Google Scholar]
- 21.Acosta SA, Franzese N, Staples M, Weinbren NL, Babilonia M, Patel J, et al. Human umbilical cord for transplantation therapy in myocardial infarction. J. Stem Cell Res. Ther. 2013;1(Suppl 4):S4–005. [PMC free article] [PubMed] [Google Scholar]
- 22.Sanberg PR, Willing AE, Garbuzova-Davis S, Saporta S, Liu G, Sanberg CD, et al. Umbilical cord blood-derived stem cells and brain repair. Ann. N. Y. Acad. Sci. 2005;1049:67–83. doi: 10.1196/annals.1334.008. [DOI] [PubMed] [Google Scholar]
- 23.Chung DJ, Choi CB, Lee SH, Kang EH, Lee JH, Hwang SH, et al. Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. J. Neurosci. Res. 2009;87:3554–67. doi: 10.1002/jnr.22162. [DOI] [PubMed] [Google Scholar]
- 24.Copeland N, Harris D, Gaballa MA. Human umbilical cord blood stem cells, myocardial infarction and stroke. Clin. Med. 2009;9(4):342–5. doi: 10.7861/clinmedicine.9-4-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, et al. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–95. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- 26.Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J. Neurosci. Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- 27.Ilic D, Miere C, Lazic E. Umbilical cord blood cells: clinical trials in non-hematological disorders. Brit. Med. Bull. 2012;102:43–57. doi: 10.1093/bmb/lds008. [DOI] [PubMed] [Google Scholar]
- 28.Newman MB, Willing AE, Manresa JJ, Sanberg CD, Sanberg PR. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: implications for brain repair. Exp Neurol. 2006;199(1):201–8. doi: 10.1016/j.expneurol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Liu N, Yi B, Zhang X, Gao BB, Zhang Y, et al. Transplanted hUCB-MSCs migrated to the damaged area by SDF-1/CXCR4 signaling to promote functional recovery after traumatic brain injury in rats. Neurol Res. 2015;37(1):50–6. doi: 10.1179/1743132814Y.0000000399. [DOI] [PubMed] [Google Scholar]
- 30.Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, Sanchez-Ramos J, et al. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002;11(3):275–81. [PubMed] [Google Scholar]
- 31.Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J. Cereb. Blood Flow Metab. 2004;24(2):133–50. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- 32.Boltze J, Reich DM, Hau S, Reymann KG, Strassburger M, Lobsien D, et al. Assessment of neuroprotective effects of human umbilical cord blood mononuclear cell subpopulations in vitro and in vivo. Cell Transplant. 2012;21(4):723–37. doi: 10.3727/096368911X586783. [DOI] [PubMed] [Google Scholar]
- 33.Henning RJ, Shariff M, Eadula U, Alvarado F, Vasko M, Sanberg PR, et al. Human cord blood mononuclear cells decrease cytokines and inflammatory cells in acute myocardial infarction. Stem Cells Dev. 2008;17(6):1207–19. doi: 10.1089/scd.2008.0023. [DOI] [PubMed] [Google Scholar]
- 34.Pimentel-Coelho PM, Rosado-de Castro PH, Barbosa da Fonseca LM, Otero RM. Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic-ischemic encephalophaty. Pediatr. Res. 2012;71:464–73. doi: 10.1038/pr.2011.59. [DOI] [PubMed] [Google Scholar]
- 35.dela Peña I, Sanberg PR, Acosta S, Lin SZ, Borlongan CV. G-CSF as an adjunctive therapy with umbilical cord blood cell transplantation for traumatic brain injury. Cell Transplant. 2015;24(3):447–57. doi: 10.3727/096368915X686913. [DOI] [PubMed] [Google Scholar]
- 36.dela Peña I, Sanberg PR, Acosta S, Lin SZ, Borlongan CV. Umbilical cord blood cell and granulocyte-colony stimulating factor: combination therapy for traumatic brain injury. Regen Med. 2014;9(4):409–12. doi: 10.2217/rme.14.32. [DOI] [PubMed] [Google Scholar]
- 37.dela Peña I, Sanberg PR, Acosta S, Tajiri N, Lin SZ, Borlongan CV. Stem cells and G-CSF for treating neuroinflammation in traumatic brain injury: aging as a comorbidity factor. J. Neurosurg. Sci. 2014;58(3):145–49. [PMC free article] [PubMed] [Google Scholar]
- 38.Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16(11):1033–9. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda R, Yoshikawa M, Kimura H, Ouji Y, Nakase H, Nishimura F, et al. Cotransplantation of mouse embryonic stem cells and bone marrow stromal cells following spinal cord injury suppresses tumor development. Cell Transpl. 2009;18:39–54. [PubMed] [Google Scholar]
- 40.Nakagomi N, Nakagomi T, Kubo S, Nakano-Doi A, Saino O, Takata M, et al. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells. 2009;27:2185–95. doi: 10.1002/stem.161. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Yan Q, Zeng YS, Zhang XB, Xiong Y, Wang JM, et al. Implantation of adult bone marrow-derived mesenchymal stem cells transfected with the neurotrophin-3 gene and pretreated with retinoic acid in completely transected spinal cord. Brain Res. 2010;1359:256–71. doi: 10.1016/j.brainres.2010.08.072. [DOI] [PubMed] [Google Scholar]
- 42.Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y, et al. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J. Cereb. Blood Flow Metab. 2010;30:534–44. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metcalf D. The colony-stimulating factors and cancer. Nat. Rev. Cancer. 2010;10:425–34. doi: 10.1038/nrc2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welte K. Discovery of G-CSF and early clinical studies. In: Molineux G, et al., editors. Twenty years of G-CSF, milestones in drug therapy. Springer Basel AG; Basel, Switzerland: 2012. pp. 15–24. [Google Scholar]
- 45.Platzer E, Welte K, Gabrilove JL, Lu L, Harris P, Mertelsmann R, et al. Biological activities of a human pluripotent hematopoietic colony stimulating factor on normal and leukemic cells. J. Exp. Med. 1985;162:1788–1801. doi: 10.1084/jem.162.6.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider A, Kuhn HG, Schabitz WR. A role for G-CSF (granulocyte colony stimulating factor) in the central nervous system. Cell Cycle. 2005;4:1753–57. doi: 10.4161/cc.4.12.2213. [DOI] [PubMed] [Google Scholar]
- 47.Diederich K, Sevimli S, Dörr H, Kösters E, Hoppen M, Lewejohann L, et al. The role of granulocyte-colony stimulating factor (G-CSF) in the healthy brain. A characterization of G-CSF-deficient mice. J. Neurosci. 2009;29(37):11572–581. doi: 10.1523/JNEUROSCI.0453-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minnerup J, Sevimli S, Schabitz WR. Granulocyte-colony stimulating factor for stroke treatment: mechanisms of action and efficacy in preclinical studies. Exp. Transl. Stroke Med. 2009;1:2. doi: 10.1186/2040-7378-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bao-Guo X. Cell biology and clinical promise of G-CSF: immunomodulation and neuroprotection. J. Cell. Mol. Med. 2007;11(6):1272–90. doi: 10.1111/j.1582-4934.2007.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schabitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Scholzke MN, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34:745–51. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- 51.Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Invest. 2005;115:2083–98. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komine-Kobayashi M, Zhang N, Liu M, Tanaka R, Hara H, Osaka A, et al. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J. Cereb. Blood Flow. Metab. 2006;26:402–13. doi: 10.1038/sj.jcbfm.9600195. [DOI] [PubMed] [Google Scholar]
- 53.Han JL, Blank T, Schwab S, Kollmar R. Inhibited glutamate release by granulocyte-colony stimulating factor after experimental stroke. Neurosci. Lett. 2008;432:167–69. doi: 10.1016/j.neulet.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 54.Solaroglu I, Tsubokawa T, Cahill J, Zhang JH. Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience. 2006;143:965–74. doi: 10.1016/j.neuroscience.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solaroglu I, Cahill J, Tsubokawa T, Beskonakli E, Zhang JH. Granulocyte colony-stimulating factor protects the brain against experimental stroke via inhibition of apoptosis and inflammation. Neurol. Res. 2009;31(2):167–72. doi: 10.1179/174313209X393582. [DOI] [PubMed] [Google Scholar]
- 56.Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–54. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–30. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 58.dela Peña IC, Yoo A, Tajiri N, Acosta SA, Ji X, Kaneko Y, et al. Granulocyte colony-stimulating factor attenuates delayed tPA-induced hemorrhagic transformation in ischemic stroke rats by enhancing angiogenesis and vasculogenesis. J. Cereb. Blood Flow Metab. 2015;35(2):338–46. doi: 10.1038/jcbfm.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishikawa H, Tajiri N, Shinozuka K, Vasconcellos J, Kaneko Y, Lee HJ, et al. Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke. 2013;4(12):3473–81. doi: 10.1161/STROKEAHA.113.001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaneko Y, Tajiri N, Shinozuka K, Glover LE, Weinbren NL, Cortes L, et al. Cell therapy for stroke: emphasis on optimizing safety and efficacy profile of endothelial progenitor cells. Curr. Pharm. Des. 2012;18(25):3731–4. doi: 10.2174/138161212802002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprigg N, Bath PM, Zhao L, Willmot MR, Gray LJ, Walker MF, et al. Granulocyte-colony-stimulating factor mobilizes bone marrow stem cells in patients with subacute ischemic stroke: the Stem cell Trial of recovery enhanceMent after etroke (STEMS) pilot randomized, controlled trial (ISRCTN 16784092) Stroke. 2006;37:2979–83. doi: 10.1161/01.STR.0000248763.49831.c3. [DOI] [PubMed] [Google Scholar]
- 62.Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomizedcontrolled trial. CMAJ. 2006;174:927–33. doi: 10.1503/cmaj.051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schabitz WR, Laage R, Vogt G, Koch W, Kollmar R, Schwab S, et al. AXIS: a trial of intravenous granulocyte colony-stimulating factor in acute ischemic stroke. Stroke. 2010;41:2545–51. doi: 10.1161/STROKEAHA.110.579508. [DOI] [PubMed] [Google Scholar]
- 64.Ringelstein EB, Thijs V, Norrving B, Chamorro A, Aichner F, Grond M, et al. Granulocyte colony-stimulating factor in patients with acute ischemic stroke: results of the AX200 for Ischemic Stroke trial. Stroke. 2013;44(10):2681–7. doi: 10.1161/STROKEAHA.113.001531. [DOI] [PubMed] [Google Scholar]
- 65.An C, Shi Y, Li P, Hu X, Gan Y, Stetler RA, et al. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog. Neurobiol. 2014;115:6–24. doi: 10.1016/j.pneurobio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 2013;4(3):279–85. doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li L, McBride DW, Doycheva D, Dixon BJ, Krafft PR, Zhang JH, et al. G-CSF attenuates neuroinflammation and stabilizes the blood-brain barrier via the PI3K/Akt/GSK-3β signaling pathway following neonatal hypoxiaischemia in rats. Exp Neurol. 2015 doi: 10.1016/j.expneurol.2014.12.020. S0014-4886(15)00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sobrino T, Millán M, Castellanos M, Blanco M, Brea D, Dorado L, et al. Association of growth factors with arterial recanalization and clinical outcome in patients with ischemic stroke treated with tPA. J. Thromb. Haemost. 2010;8(7):1567–74. doi: 10.1111/j.1538-7836.2010.03897.x. [DOI] [PubMed] [Google Scholar]
- 69.Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, et al. Cell based therapies for ischemic stroke: from basic science to bedside. Prog. Neurobiol. 2014;115:92–115. doi: 10.1016/j.pneurobio.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang XM, Du F, Yang D, Wang R, Yu C, Huang XN, et al. Granulocyte colony-stimulating factor increases the therapeutic efficacy of bone marrow mononuclear cell transplantation in cerebral ischemia in mice. BMC Neurosci. 2011;12:61. doi: 10.1186/1471-2202-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balseanu AT, Buga AM, Catalin B, Wagner DC, Boltze J, Zagrean AM, et al. Multimodal approaches for regenerative stroke therapies: combination of granulocyte colony-stimulating factor with bone marrow mesenchymal stem cells is not superior to G-CSF alone. Front. Aging Neurosci. 2014;6:130. doi: 10.3389/fnagi.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Popa-Wagner A, Stöcker K, Balseanu AT, Rogalewski A, Diederich K, Minnerup J, et al. Effects of granulocyte-colony stimulating factor after stroke in aged rats. Stroke. 2010;41(5):1027–31. doi: 10.1161/STROKEAHA.109.575621. [DOI] [PubMed] [Google Scholar]
- 73.Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Sanberg PR, Sanchez-Ramos J, et al. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS One. 2014;12(9(3)):e90953. doi: 10.1371/journal.pone.0090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iskander A, Knight RA, Zhang ZG, Ewing JR, Shankar A, Varma NR, et al. Intravenous administration of human umbilical cord blood-derived AC133+ endothelial progenitor cells in rat stroke model reduces infarct volume: magnetic resonance imaging and histological findings. Stem Cells Transl. Med. 2013;2:703–14. doi: 10.5966/sctm.2013-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shahaduzzaman M, Golden JE, Green S, Gronda AE, Adrien E, Ahmed A, et al. A single administration of human umbilical cord blood T cells produces long-lasting effects in the aging hippocampus. Age (Dordr) 2013;35:2071–87. doi: 10.1007/s11357-012-9496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao LR, Piao CS, Murikinati SR, Gonzalez-Toledo ME. The role of stem cell factor and granulocyte-colony stimulating factor in treatment of stroke. Recent Pat. CNS Drug Discov. 2013;8(1):2–12. doi: 10.2174/1574889811308010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez-Ramos J, Song S, Sava V, Catlow B, Lin X, Mori T, et al. Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer's mice. Neuroscience. 2009;163:55–72. doi: 10.1016/j.neuroscience.2009.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toth ZE, Leker RR, Shahar T, Pastorino S, Szalayova I, Asemenew B, et al. The combination of granulocyte colony-stimulating factor and stem cell factor significantly increases the number of bone marrow-derived endothelial cells in brains of mice following cerebral ischemia. Blood. 2008;111:5544–52. doi: 10.1182/blood-2007-10-119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.England TJ, Abaei M, Auer DP, Lowe J, Jones DR, Sare G, et al. Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: the stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke. 2012;43:405–11. doi: 10.1161/STROKEAHA.111.636449. [DOI] [PubMed] [Google Scholar]
- 80.Stachura DL, Svoboda O, Campbell CA, Espín-Palazón R, Lau RP, Zon LI, et al. The zebrafish granulocyte colony stimulating factors (Gcsfs): two paralogous cytokines and their roles in hematopoietic development and maintenance. Blood. 2013;122(24):3918–28. doi: 10.1182/blood-2012-12-475392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsuji T, Nishimura-Morita Y, Watanabe Y, Hirano D, Nakanishi S, Mori KJ, et al. A murine stromal cell line promotes the expansion of CD34high+-primitive progenitor cells isolated from human umbilical cord blood in combination with human cytokines. Growth Factors. 1999;16(3):225–40. doi: 10.3109/08977199909002132. [DOI] [PubMed] [Google Scholar]
- 82.Borlongan CV. Bone marrow stem cell mobilization in stroke: a ‘bonehead’ may be good after all! Leukemia. 2011;25:1674–86. doi: 10.1038/leu.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang DY, Chen YJ, Wang MF, Pan HC, Chen SY, Cheng FC. Granulocyte colony-stimulating factor enhances cellular proliferation and motor function recovery on rats subjected to traumatic brain injury. Neurol. Res. 2010;32(10):1041–49. doi: 10.1179/016164110X12807570510013. [DOI] [PubMed] [Google Scholar]
- 84.Yang M, Wei X, Li J, Heine LA, Rosenwasser R, Iacovitti L. Changes in host blood factors and brain glia accompanying the functional recovery after systemic administration of bone marrow stem cells in ischemic stroke rats. Cell Transplant. 2010;19:1073–84. doi: 10.3727/096368910X503415. [DOI] [PubMed] [Google Scholar]
- 85.Pimentel-Coelho PM, Rosado-de-Castro PH, da Fonseca LM, Mendez-Otero R. Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2012;71(4 Pt 2):464–73. doi: 10.1038/pr.2011.59. [DOI] [PubMed] [Google Scholar]
- 86.Fan CG, Zhang QJ, Tang FW, Han ZB, Wang GS, Han ZC. Human umbilical cord blood cells express neurotrophic factors. Neurosci Lett. 2005;380:322–5. doi: 10.1016/j.neulet.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 87.Diederich K, Sevimli S, Dörr H, Kösters E, Hoppen M, Lewejohann L, et al. The role of granulocyte-colony stimulating factor (G-CSF) in the healthy brain: a characterization of G-CSF-deficient mice. J Neurosci. 2009;29(37):11572–81. doi: 10.1523/JNEUROSCI.0453-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hayakawa K, Miyamoto N, Seo JH, Pham LD, Kim KW, Lo EH, et al. High-mobility group box 1 from reactive astrocytes enhances the accumulation of endothelial progenitor cells in damaged white matter. J. Neurochem. 2013;125(2):273–80. doi: 10.1111/jnc.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Willing AE, Vendrame M, Mallery J, Cassady CJ, Davis CD, Sanchez-Ramos J, et al. Mobilized peripheral blood cells administered intravenously produce functional recovery in stroke. Cell Transplant. 2003;12:449–54. doi: 10.3727/000000003108746885. [DOI] [PubMed] [Google Scholar]
- 90.Sullivan R, Duncan K, Dailey T, Kaneko Y, Tajiri N, Borlongan CV. A possible new focus for stroke treatment - migrating stem cells. Expert Opin Biol Ther. 2015;15(7):949–58. doi: 10.1517/14712598.2015.1043264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dailey T, Metcalf C, Mosley YI, Sullivan R, Shinozuka K, Tajiri N, et al. An update on translating stem cell therapy for stroke from bench to bedside. J. Clin. Med. 2013;2(4):220–41. doi: 10.3390/jcm2040220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanberg PR, Eve DJ, Cruz LE, Borlongan CV. Neurological disorders and the potential role for stem cells as a therapy. Br. Med. Bull. 2012;101:163–81. doi: 10.1093/bmb/lds001. [DOI] [PMC free article] [PubMed] [Google Scholar]