Abstract
PURPOSE
Rotator cuff injuries are associated with atrophy and fat infiltration into the muscle, commonly referred to as "fatty degeneration." As the poor function of chronically torn muscles may limit recovery after surgical repair, there is considerable interest in finding therapies to enhance muscle regeneration. Stromal vascular stem cells (SVFCs) can improve muscle regeneration in other chronic injury states, and our objective was to evaluate the ability of SVFCs to reduce fibrosis and fat accumulation, and enhance muscle fiber specific force production after chronic rotator cuff tear.
METHODS
Chronic supraspinatus tears were induced in adult immunodeficient rats, and repaired one month following tear. Rats received vehicle control, or injections of 3×105 or 3×106 human SVFCs into supraspinatus muscles.
RESULTS
Two weeks following repair, we detected donor human DNA and protein in SVFC treated muscles. There was a 40% reduction in fibrosis in the treated groups compared to controls (p=0.03 for 3×105, p=0.04 for 3×106), and no differences between groups for lipid content or force production were observed.
CONCLUSIONS
As there has been much interest in the use of stem cell-based therapies in musculoskeletal regenerative medicine, the reduction in fibrosis and trend towards an improvement in single fiber contractility suggest that SVFCs may be beneficial to enhance the treatment and recovery of patients with chronic rotator cuff tears.
Keywords: rotator cuff, fatty degeneration, fibrosis, myosteatosis, stromal vascular stem cell
Introduction
Large rotator cuff tears are often associated with fibrosis, muscle atrophy, and inter- and intramuscular fat infiltration, commonly referred to as "fatty degeneration." Surgical repair of torn rotator cuff muscles does not reverse fatty degeneration, and due to the shortening of the muscle that occurs in chronic tears, as much as 90% of muscle fibers can be injured at the time of surgical repair [1]. This massive second injury induced by repair can lead to further lipid infiltration and fibrosis which can further limit tissue regeneration and prevent full recovery [1, 2]. The fibrosis that commonly occurs in injured muscles increases the passive stiffness of the muscle extracellular matrix (ECM), and can also disrupt the lateral transmission of forces between muscle fibers, causing increased shearing and damage of the muscle fiber membrane [3]. The combination of increased tissue stiffness and disrupted lateral force transmission can greatly increase the susceptibility of the muscle to further injury and re-tear, complicate rehabilitation, and prevent the full restoration of function [4].
Mesenchymal stem cells (MSCs) are a pluripotent population of adult stem cells found in various compartments across the body, and can give rise to a diverse lineage of differentiated cell types [5]. In addition to serving as progenitor cells, MSCs can secrete growth factors that function in a paracrine fashion to enhance tissue regeneration [5]. The stromal vascular fraction (SVF) of adipose tissue is highly enriched with a population of endothelial progenitor cells and adipose derived stem cells, which are a subclass of MSCs [6]. SVF cells (SVFCs) can be easily obtained from lipoaspirates of subcutaneous adipose tissue, and several studies have demonstrated these cells can promote tissue regeneration and prevent fibrosis after injury or disease [6, 7]. Given the therapeutic potential of SVFCs and the relative ease at which they can be obtained, our objective was to evaluate the ability of SVFCs to prevent fibrosis and enhance muscle regeneration following chronic rotator cuff tear. We hypothesized that administration of human SVFCs into the chronically torn supraspinatus muscles of immunodeficient rats would reduce fibrosis and fat accumulation, and enhance muscle fiber force production. To test this hypothesis, we obtained SVFCs using a point-of-care isolation system, administered cells to the chronically torn muscles of immunodeficient rats, and measured changes in supraspinatus muscle collagen content, contractility, and lipid accumulation two weeks after administering SVFCs.
Methods
Animals
This study was approved by the University of Michigan Committee for the Use and Care of Animals and the US Army Animal Care and Use Review Office. Four-month-old male athymic nude rats (NIH-Foxn1rnu) that lack a specific immune system were obtained from Charles River Laboratories (Wilmington, MA, USA). This animal model is commonly used for the study of xenografts, and we have previously demonstrated that nude rats develop fatty degeneration in a similar fashion to immune competent rats [8]. Rats were assigned to either serve as vehicle-treated controls or receive injections of SVFCs at a dose of either 3×105 cells or 3×106 cells in a randomized fashion.
Surgeries
Bilateral, chronic full-thickness supraspinatus tears and subsequent repairs after a period of twenty eight days were performed using sterile technique in a laminar flow hood [1, 9]. Postoperative care, antibiotics and analgesic medications were administered as described [1]. After the torn tendon was repaired, intramuscular injections of SVFCs that were isolated as described below and suspended in a vehicle of Lactated Ringers solution or vehicle alone were performed using a 25-gauge needle. Injections occurred in the proximal, middle and distal thirds of the supraspinatus muscle. Fourteen days after repair surgery, rats were anaesthetized with sodium pentobarbital (50 mg/kg), and supraspinatus muscles were harvested and weighed. Muscles were prepared for histology, single muscle fiber contractility, or finely minced to isolate DNA, lipid, and protein. Rats were then humanely euthanized by anesthetic overdose and induction of a bilateral pneumothorax.
SVFC Isolation
This procedure was approved by the University of Michigan Medical School IRB, and conducted based on previously described techniques [10, 11]. Briefly, an Icellator system (Tissue Genesis, Honolulu, HI, USA) and Adipase (Tissue Genesis) were used to gently digest and isolate the SVFCs according to the manufacturers protocols from approximately 60cc of subcutaneous lipoaspirate obtained from a patient undergoing a cosmetic procedure. The concentration of viable cells was measured using a NucleoCounter (ChemoMetec, Davis, CA, USA), and greater than 90% of the isolated cells were determined to be viable. Previous studies have demonstrated that the stromal vascular fraction of cells obtained with the Icellator system is highly enriched with CD31−/CD34+/CD45− adipose derived MSCs [10].
Permeabilized fiber contractility
Single fiber contractility was performed as previously described [8, 9]. The cross sectional area (CSA) of fibers was measured, and maximum isometric force (Fo) was elicited by immersing the fiber in a high calcium solution. Specific force (sFo) was calculated by dividing Fo by CSA. Ten to 20 fast fibers were tested from each muscle.
Genomic DNA content
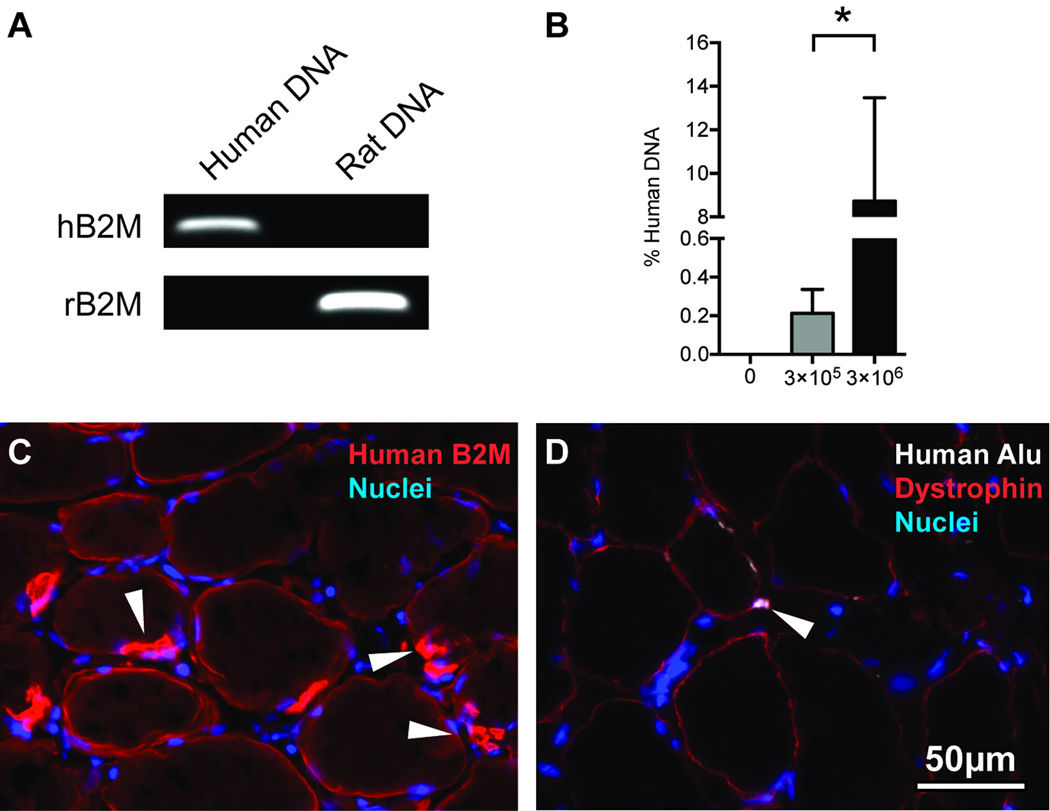
To determine the amount of human genomic DNA (gDNA) that was present in treated muscles, we designed human- and rat-specific primers against the β2-microglobulin gene and then measured the abundance of human gDNA relative to rat gDNA in treated supraspinatus muscles using a quantitative PCR (qPCR) based detection strategy modified from Becker [12]. DNA was isolated from samples using a DNeasy kit (Qiagen, Valencia, CA, USA) per the manufacturer's instructions, and treated with RNase A (Qiagen). DNA concentration was obtained using a NanoDrop ND2000 (ThermoFisher, Rockford, IL, USA), and all samples had A260/A280 ratios > 1.8. qPCR was conducted using 300ng of total DNA along with primers we designed to recognize either human specific β2-microglobulin or rat specific β2-microglobulin (Table 1 and Figure 1A) and Ssofast qPCR reagents (BioRad, Hercules, CA, USA) in a CFX96 optical thermal cycler system (BioRad). The presence of single amplicons was verified using melt-curve analysis, and electrophoretic separation of PCR reactions in a 2% agarose gel that was stained with ethidium bromide (Sigma Aldrich, St. Louis, MO, USA) and visualized in a ChemiDoc system (BioRad).
Table 1.
Primer sequences for detection of human or rat specific β2-microglobulin from genomic DNA.
| Species | Primer Sequence | Amplicon Size |
|---|---|---|
| Human | Forward: 5'-GGCCAGGTCATGAGGAGTAT-3' Reverse: 5'-AGGGAGACCAAGGGATGATT-3' |
104bp |
| Rat | Forward: 5'-CCCAAAGAGACAGTGGGTGT-3' Reverse: 5'-TGTTCTGCCTTGGAGTCCTT-3' |
101bp |
Figure 1.
Human DNA content, immunohistochemistry, and in situ hybridization. (A) Agarose gels demonstrating species specificity for primers designed for human specific β2-microglobulin (hB2M) and rat specific β2-microglobulin (rB2M) using DNA isolated from a pure population of human SVFCs or from rat EDL muscle. (B) Percent human DNA in torn rotator cuff muscles from control (N=10), 3×105 SVFC (N=8), and 3×106 SVFC (N=8) groups. Values are mean±SE. Differences between 3×105 SVFC and 3×106 SVFC were evaluated using a t-test (α=0.05). *, significantly different (P<0.05). Representative immunohistochemistry of (C) human β2-microglobulin staining (blue, nuclei; red, human β2M) and (D) human Alu probe staining (blue, nuclei; red, dystrophin; white, human Alu probe)
Histology
Supraspinatus muscles were cryosectioned at a thickness of 10μm, and slides were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. To detect the presence of human protein or gDNA, slides were incubated with antibodies against the human cell membrane protein β2-microglobulin (AbCam, Cambridge, MA, USA) or biotinylated probes against Alu sequences found only in the human genome. Muscle fiber membranes were identified using antibodies against dystrophin (DSHB, Iowa City, IA, USA). Cell nuclei were labeled with DAPI (Sigma Aldrich). Primary antibodies and probes were detected with highly cross-adsorbed secondary antibodies, avidin conjugated to AlexaFluor probes or a tyramide amplification kit (Life Technologies, Carlsbad, CA, USA). Images were obtained using an Axioplan 2 (Zeiss, Jena, Germany) microscope equipped with AxioCam (Zeiss) cameras.
Hydroxyproline content
Measurement of hydroxyproline, which is commonly used as a marker of collagen content, was performed using a colorimetric assay as previously described [9] and normalized to the dry mass of the muscle tissue.
Lipid analysis
Lipid extraction from muscle tissue and analysis was performed as described [9]. Samples and known lipid standards were spotted on HPTLC plates (EMD Millipore, Billerica, MA, USA), developed to separate lipid species and visualized with Rhodamine 6G in a ChemiDoc (BioRad).
Statistical Analysis
Data are presented as mean±SE. Differences between groups were tested using a one-way ANOVA (α=0.05) followed by Fisher's LSD post-hoc sorting, with the exception of percent human DNA, which was measured using t-tests (α=0.05). All analysis was performed in Prism 6.0 (GraphPad, La Jolla, CA).
Results
Two weeks following the repair of torn rotator cuff muscles, there were no changes in supraspinatus mass between groups (Table 2). We evaluated changes in functional muscle cell contractility, and no significant differences in muscle fiber CSA or Fo were observed (Table 2). While no statistically significant changes were observed, compared to controls there was a statistical trend in the 3×106 group for a 12% decrease in fiber CSA (p=0.07) and a 25% increase sFo production (p=0.07, Table 2).
Table 2.
Muscle mass and permeabilized muscle fiber size and contractility of control (N=10), 3×105 SVFCs (N=8), and 3×106 SVFCs (N=8) groups. Values are mean±SE. Differences between groups were tested using a one-way ANOVA (α=0.05) followed by Fisher's LSD post-hoc sorting.
| 0 SVFCs | 3×105 SVFCs | 3×106 SVFCs | |
|---|---|---|---|
| Supraspinatus Mass (mg) | 294 ± 7 | 311 ± 6 | 304 ± 15 |
| Fibre CSA (µm2) | 4312 ± 179 | 3980 ± 225 | 3796 ± 187 |
| Fibre Fo (mN) | 0.26 ± 0.03 | 0.26 ± 0.03 | 0.28 ± 0.03 |
| Fibre sFo (kPa) | 58 ± 6 | 65 ± 5 | 73 ± 5 |
We next measured the abundance of human DNA in samples. There was a dose-dependent increase from 0.2% human DNA in the 3×105 SVFC group to 8.7% human DNA in the 3×106 SVFC group (Figure 1B). As the 3×106 SVFC group demonstrated a high level of human DNA content, we then evaluated the localization of human protein and DNA in histological sections of muscle. Using an antibody that specifically detects the human β2-microglobulin membrane protein, we observed the presence of this protein on the surface of treated muscle fibers, and in cells located in a position under the muscle fiber basal lamina (Figure 1C). To further verify the presence of human cells, we used probes against human Alu DNA sequences, and human nuclei were observed in and around muscle fibers (Figure 1D).
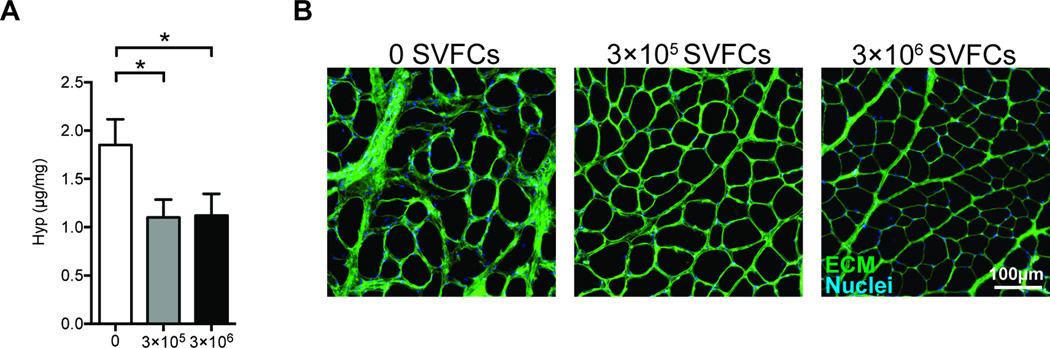
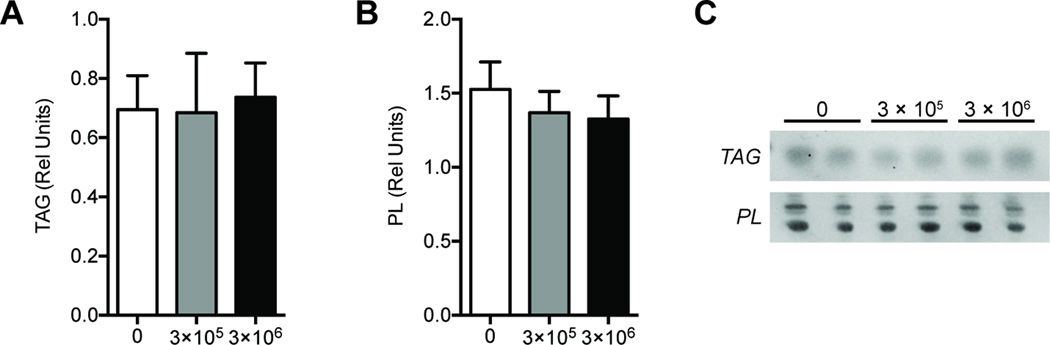
We next evaluated quantitative changes in collagen and lipid content between groups. There was an approximately 40% reduction in hydroxyproline content in the treated samples compared to controls, but there was no dose dependent change in hydroxyproline content between the 3×105 cell group and the 3×106 cell group (Figure 2A). A grossly apparent reduction in ECM abundance was also apparent in histological sections (Figure 2B). Despite the reduction in fibrosis, there were no differences between groups for triglyceride levels, nor were any differences observed for phospholipid levels, which serve as a loading control for total lipid measurements (Figure 3).
Figure 2.
Hydroxyproline content and ECM accumulation. (A) Hydroxyproline content from control (N=10), 3×105 SVFC (N=8), and 3×106 SVFC (N=8) groups. Values are mean±SE. Differences between groups were tested using a one-way ANOVA (α=0.05) followed by Fisher's LSD post-hoc sorting. *, significantly different from control group (P<0.05). (B) Representative images stained with DAPI (blue, nuclei) and WGA (green, ECM) from control, 3×105 SVFC, and 3×106 SVFC groups.
Figure 3.
Lipid content. Relative abundance of (A) triglyceride (TAG), and (B) phospholipid (PL) species in control (N=10), 3×105 SVFC (N=8), and 3×106 SVFC (N=8) groups. A representative plate is displayed in (C) showing the TAG and PL bands in each group. Values are mean±SE. Differences between groups were tested using a one-way ANOVA (α=0.05) followed by Fisher's LSD post-hoc sorting.
Discussion
Chronic rotator cuff tears are one of the most frequent and debilitating injuries of the upper extremity. Because SVFC treatment has been beneficial in other models of muscle injury [7], we sought to assess the effect of SVFC treatment on rotator cuff regeneration following chronic tear. To our knowledge, this was the first study to evaluate SVFC transplantation on muscle function and fibrosis following chronic rotator cuff tear and repair. Using a fully automated point of care cell isolation system, we demonstrated that human SVFCs can be stably introduced into torn rotator cuff muscles of immunodeficient rats, resulting in the production of functional human protein and a significant reduction in fibrosis.
Chronic fibrosis after rotator cuff tear leads to increased muscle stiffness and tension at the repair site [4], and along with the poor quality of the muscle due to atrophy and fatty infiltration [8], the potential for healing of torn rotator cuff muscles even after repair remains low. SVFC transplantation has been associated with a reduction in fibrosis and improved regeneration in other tissue injury and disease models, such as liver injury [13], Peyronie's disease [14], myocardial infarctions [15], and Dupuytren's disease [16], and supports our finding of reduced fibrosis after rotator cuff tear. The mechanism behind the reduction in fibrosis following SVFC treatment in various tissues is unclear. SVFCs may act by inhibiting collagen synthesis and reducing the proliferation of fibroblasts either directly [16] or indirectly through the regulation of macrophages [17]. Combined with data from Oh and colleagues [18] that found SVFCs augment tendon to bone healing following rotator cuff repair, SVFC treatment in chronically torn rotator cuff muscles of patients may improve muscle quality, reduce fibrosis, and lead to a more stable repair.
Previous work in dystrophin-deficient mdx mice has shown that human adipose derived MSCs are able to differentiate into the myogenic lineage, and fuse with existing mouse muscle fibers in vivo to restore dystrophin expression and ameliorate the disease phenotype [19, 20]. The current work demonstrates that human SVFCs, when injected into injured rat muscle tissue, are also able to differentiate into the myogenic lineage and enhance the regeneration of torn rotator cuff muscles. Despite the location of SVFCs within adipose tissue, we did not find any human β2M or Alu+ adipocytes within torn rotator cuff muscles, and we did not detect any changes in total lipid content between groups. The abundance of total triglyceride content of muscles in the current study is consistent with levels observed in previous rat studies [8, 9]. Although there were no differences between groups with regards to lipid content, there was a trend towards an improvement in muscle fiber sFo production in the 3×106 cell group, indicating that the treatment with SVFCs did not exacerbate fatty degeneration within these muscles. Overall, while SVFC therapy did not have an impact on force generating capacity or lipid content, given the profound impact on fibrosis, these results suggest that SVFC therapy has the potential to be both safe and successful when used to treat patients with chronic, full-thickness rotator cuff tears.
This study has many limitations. Although T-cell deficient rats develop fatty degeneration in a similar fashion to immunocompetent rats [8], no rat model develops the extent of fatty degeneration as seen in humans [21–23]. We chose a single time point of two weeks following repair and injection for our measurements and did not assess muscle function or SVFC viability past this time. We also used a single subject and did not assess the activity of SVFCs from multiple subjects. Despite these limitations, we believe this study provided important insight into the practicality and efficacy of SVFC therapy on the regeneration of chronic rotator cuff tears.
Using bone marrow aspirate as a stem cell source, the potential for MSCs to augment rotator cuff repair has shown tremendous promise in improving outcomes and reducing re-ruptures in patients [24, 25]. In the current study, we used a fully automated cell isolation system and were able to quickly obtain an enriched population of SVFCs with no post-isolation treatment or expansion in culture. These cells were directly injected into chronically torn rotator cuff muscles, and two weeks following injection we demonstrated that rotator cuff muscles contained human DNA, functional human protein and had a striking reduction in fibrosis. This cell isolation approach is likely to be practical as a point-of-care system in an operating suite as well. For most patients, 60cc of abdominal fat can be harvested with little donor site morbidity. After harvesting the fat tissue via liposuction and repositioning of the patient, SVFCs can be isolated in about an hour which would allow the surgeon to begin the repair procedure while the cells are being sorted and prepared for injection. While there is encouraging long-term results from patients who received bone marrow MSC augmentation at the time of rotator cuff repair [24], the results from the current study suggest that SVFCs might also have the potential to enhance the recovery of chronic rotator cuff tears.
Acknowledgements
This study was funded by the US Army Medical Research and Materiel Command (DOD contract number W81XWH-10-2-0108) and NIH fellowships (F31-AR065931 and F32-AR067086).
Footnotes
Conflicts of Interest
Paul E Kosnik is an employee of Tissue Genesis, Inc., which received funding support from the Department of Defense for this study. The authors otherwise report no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Michigan Institutional Review Board and with the 1964 Helsinki declaration and its later amendments. All procedures performed with animals were in accordance with the ethical standards of the University of Michigan Committee on the Use and Care of Animals.
References
- 1.Davis ME, Stafford PL, Jergenson MJ, et al. Muscle fibers are injured at the time of acute and chronic rotator cuff repair. Clin Orthop Relat Res. 2015;473:226–232. doi: 10.1007/s11999-014-3860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladstone JN, Bishop JY, Lo IKY, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. American Journal of Sports Medicine. 2007;35:719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 3.Ramaswamy KS, Palmer ML, van der Meulen JH, et al. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol (Lond) 2011;589:1195–1208. doi: 10.1113/jphysiol.2010.201921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieber RL, Ward SR. Cellular Mechanisms of Tissue Fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am J Physiol, Cell Physiol. 2013;305:C241–C252. doi: 10.1152/ajpcell.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray IR, Corselli M, Petrigliano FA, et al. Recent insights into the identity of mesenchymal stem cells: Implications for orthopaedic applications. Bone Joint J. 2014;96-B:291–298. doi: 10.1302/0301-620X.96B3.32789. [DOI] [PubMed] [Google Scholar]
- 6.Williams SK, Kosnik PE, Kleinert LB, et al. Adipose Stromal Vascular Fraction Cells Isolated Using an Automated Point of Care System Improve the Patency of Expanded Polytetrafluoroethylene Vascular Grafts. Tissue Engineering Part A. 2013;19:1295–1302. doi: 10.1089/ten.TEA.2012.0318. [DOI] [PubMed] [Google Scholar]
- 7.Hwang JH, Kim IG, Piao S, et al. Combination therapy of human adipose-derived stem cells and basic fibroblast growth factor hydrogel in muscle regeneration. Biomaterials. 2013;34:6037–6045. doi: 10.1016/j.biomaterials.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 8.Gumucio J, Flood M, Harning J, et al. T lymphocytes are not required for the development of fatty degeneration after rotator cuff tear. Bone Joint Res. 2014;3:262–272. doi: 10.1302/2046-3758.39.2000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oak NR, Gumucio JP, Flood MD, et al. Inhibition of 5-LOX, COX-1, and COX-2 Increases Tendon Healing and Reduces Muscle Fibrosis and Lipid Accumulation After Rotator Cuff Repair. The American Journal of Sports Medicine. 2014 doi: 10.1177/0363546514549943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi K, Tanaka S, Iida H, et al. Stromal vascular fraction isolated from lipo-aspirates using an automated processing system: bench and bed analysis. J Tissue Eng Regen Med. 2013;7:864–870. doi: 10.1002/term.1478. [DOI] [PubMed] [Google Scholar]
- 11.Williams SK, Kosnik PE, Kleinert LB, et al. Adipose Stromal Vascular Fraction Cells Isolated Using an Automated Point of Care System Improve the Patency of Expanded Polytetrafluoroethylene Vascular Grafts. Tissue Engineering Part A. 2013;19:1295–1302. doi: 10.1089/ten.TEA.2012.0318. [DOI] [PubMed] [Google Scholar]
- 12.Becker M, Nitsche A, Neumann C, et al. Sensitive PCR method for the detection and real-time quantification of human cells in xenotransplantation systems. Br J Cancer. 2002;87:1328–1335. doi: 10.1038/sj.bjc.6600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harn H-J, Lin S-Z, Hung S-H, et al. Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function. cell transplant. 2012;21:2753–2764. doi: 10.3727/096368912X652959. [DOI] [PubMed] [Google Scholar]
- 14.Castiglione F, Hedlund P, Van der Aa F, et al. Intratunical Injection of Human Adipose Tissue-derived Stem Cells Prevents Fibrosis and Is Associated with Improved Erectile Function in a Rat Model of Peyronie's Disease. European Urology. 2013;63:551–560. doi: 10.1016/j.eururo.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeBlanc AJ, Nguyen QT, Touroo JS, et al. Adipose-derived cell construct stabilizes heart function and increases microvascular perfusion in an established infarct. Stem Cells Transl Med. 2013;2:896–905. doi: 10.5966/sctm.2013-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhoekx JSN, Mudera V, Walbeehm ET, Hovius SER. Adipose-derived stem cells inhibit the contractile myofibroblast in Dupuytren's disease. Plastic and Reconstructive Surgery. 2013;132:1139–1148. doi: 10.1097/PRS.0b013e3182a3bf2b. [DOI] [PubMed] [Google Scholar]
- 17.Adutler-Lieber S, Ben-Mordechai T, Naftali-Shani N, et al. Human macrophage regulation via interaction with cardiac adipose tissue-derived mesenchymal stromal cells. J Cardiovasc Pharmacol Ther. 2013;18:78–86. doi: 10.1177/1074248412453875. [DOI] [PubMed] [Google Scholar]
- 18.Oh JH, Chung SW, Kim SH, et al. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. Journal of Shoulder and Elbow Surgery. 2014;23:445–455. doi: 10.1016/j.jse.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez A-M, Pisani D, Dechesne CA, et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Yan X, Sun Z, et al. Flk-1+ adipose-derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in mdx mice. Stem Cells and Development. 2007;16:695–706. doi: 10.1089/scd.2006.0118. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Manzano G, Kim HT, Feeley BT. A rat model of massive rotator cuff tears. J Orthop Res. 2011;29:588–595. doi: 10.1002/jor.21266. [DOI] [PubMed] [Google Scholar]
- 22.Soslowsky LJ, Carpenter JE, DeBano CM, et al. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 23.Kim HM, Galatz LM, Lim C, et al. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg. 2012;21:847–858. doi: 10.1016/j.jse.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. International Orthopaedics (SICOT) 2014;38:1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 25.Hernigou P. Bone transplantation and tissue engineering, part IV. Mesenchymal stem cells: history in orthopedic surgery from Cohnheim and Goujon to the Nobel Prize of Yamanaka. International Orthopaedics (SICOT) 2015;39:807–817. doi: 10.1007/s00264-015-2716-8. [DOI] [PubMed] [Google Scholar]