Abstract
Alloreactive memory T cells mediate accelerated allograft rejection and transplant tolerance resistance. Recent studies have shown that B cell deficient–µMT mice fail to mount donor-specific memory T cell responses after transplantation. At the same time, other studies showed that pretransplant B cell depletion using rituximab (IgG1 anti-CD20 mAb) combined with cyclosporine A promoted the survival of islet allografts in monkeys. In this study, we investigated the effect of anti-CD20 antibody-mediated B cell depletion on the memory T cell alloresponse in mice. Wild-type and anti-OVA TCR transgenic mice were treated with an IgG2a anti-CD20 monoclonal antibody, which depleted nearly all B cells in the peripheral blood and secondary lymphoid organs but spared some B cells in the bone marrow. B cell depletion did not affect the direct alloresponse but resulted in a marked increase of indirect alloresponse after skin transplantation of naïve mice. Furthermore, in allosensitized mice, anti-CD20 mAb treatment enhanced the reactivation of allospecific memory T cells and accelerated second set rejection of skin allografts. This suggests that the effect of anti-CD20 antibodies on alloimmunity and allograft rejection might vary upon the nature of the antibodies as well as the circumstances under which they are delivered.
Introduction
Laboratory rodents raised in sterile environments display low frequencies of memory T cells (TMEMs), a feature that has been associated with their high susceptibility to allograft tolerance. This view is supported by studies showing that mice exhibiting alloreactive TMEMs (induced after microbial infection or adoptive transfer) are resistant to transplant tolerance procedures based on donor hematopoietic chimerism or donor-specific transfusion (1, 2). In contrast, nonhuman primates and patients display higher frequencies of potentially alloreactive TMEMs (3). These TMEMs are likely to derive from individuals’ exposure to allogeneic MHC molecules during blood transfusion, pregnancy, or a prior transplantation. In addition, microbial infections can induce the differentiation/expansion of TMEMs that can cross-react with allogeneic MHC antigens. This has been shown in mice after exposure to lymphocytic choriomeningitis virus (LCMV) and Leishmania parasites (1, 2). Indeed, since direct allorecognition involves up to 5% of the T cell repertoire, it is conceivable that some alloreactive T cells can recognize both self-MHC + a microbial peptide X and allo-MHC + a peptide Y (4). For instance, human T cells primed to Epstein–Barr virus peptides presented by HLA-B8 also react to the allo-MHC molecule HLA-B4402 (5). In humans, P. Heeger’s group has demonstrated that the presence of T cells, which are pre-expanded and display kinetics of cytokine production characteristic of TMEMs, increases the risk for acute rejection of kidney transplants (6). Furthermore, there is now abundant evidence that the presence of pre-existing alloreactive TMEMs in primates represents a major barrier to tolerance induction (3, 7, 8). Therefore, deletion or inactivation of alloreactive TMEMs is considered essential to the design of successful tolerance protocols in clinical transplantation.
B lymphocytes participate in the differentiation and survival of memory CD4+ T cells following infections (9). They contribute to these processes via antigen presentation, cytokine release (10), delivery of costimulation signals and the generation of antigen–antibody (Ag-Ab) complexes (11). However, the actual requirement for B cells and Ag-Ab complexes in the development and maintenance of anamnestic T cell responses varies with the TMEM subset (CD4+ vs. CD8+ T cells), the nature of infection, the cell being infected and the kinetics of infections (9). For instance, impaired memory responses by CD4+ T cells were revealed in B cell–deficient mice after lung infection with Chlamydia (12), but not after genital tract infection (13). Likewise, B cells were required for the development of CD8+ T cell anamnestic immunity ensuing chronic LCMV infection (14), but not after acute LCMV or Listeria monocytogenes infection (15). Likewise, the contribution of B cells to TMEM immunity after vaccination with nominal antigens depends on the nature of the antigen and its route of entry as well as the site of immune response and the extent of inflammation (16, 17). Altogether, this underscores the complexity of the relationships between B cells and T cell memory.
A previous report by G. Chalasani’s group showed that mice constitutionally devoid of B cells (µMT mice) reject normally allografts but fail to develop donor-specific TMEM responses (18). These results suggested that inhibition or depletion of B cells in transplant recipients could be used to prevent anamnestic alloresponses by T cells after transplantation and thereby promote graft survival. In this study, we investigated the effect of anti-CD20 antibody-mediated B cell depletion on T cell anamnestic responses after skin allotransplantation in wild-type and transgenic mice. We observed that B cell depletion enhanced both generation and reactivation of TMEMs and accelerated second set rejection of skin allografts. Possible reasons for the discrepancy between these results and previous observations in B cell–deficient mice are discussed.
Materials and Methods
Mice and transplantations
BALB/c (Kd Ad Ed Ld Dd), C3H (Kk Ak Ek Lk Dk), C57BL/6 (Kb Ab E− Lb Db), anti-OVA TCR transgenic OT1 mice (recognize MHC class I Kb + OVA peptide 254–267, SIINFEKL) and transgenic Act mOVA mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Act mOVA transgenic mice express the membrane-bound chicken ovalbumin OVA gene under the direction of the chicken β actin promoter coupled with the cytomegalovirus immediate-early enhancer. µMT mice are B cell deficient owing to the disruption in their µ heavy chain transmembrane region. All animal care and handling were performed according to institutional guidelines. Full-thickness skin grafts (2 × 3 cm) were placed on the recipients’ flank area.
T cells and T cell subsets isolation
T cells were isolated from the spleen and lymph nodes (axillary, inguinal, and brachial) of transplanted and naïve mice by negative selection using commercially available T cell purification columns according to the manufacturer’s instructions (R & D Systems, Minneapolis, MN). Purified T cells were washed in HBSS and used in ELISPOT assays. Naïve and TMEMs were separated using a fluorescence-activated Vantage cell sorter (BD Immunocytometry System, Franklin Lakes, NJ), based on their expression of CD44 surface marker (CD44low: naïve T cells, CD44high: TMEMs). The purity of sorted cells was consistently >95%.
Preparation of sonicates
Stimulator spleen cells were suspended at 3 × 107 cells/mL in AIM-V containing 0.5% fetal calf serum, and sonicated with 10 pulses of 1 s each. The resulting suspension was frozen in a dry ice/ethanol bath, thawed at room temperature, and centrifuged at 300 g for 10 min to remove remaining intact cells (19).
ELISPOT assays
Alloresponses by T cells were measured as previously described (19). Briefly, 96-well ELISPOT plates (Polyfiltronics, Rockland, MA) were coated with an anti- γ-interferon (γ-IFN) capture mAb (R4-6A2) in sterile phosphate-buffered saline (PBS) overnight. The plates were washed twice with sterile PBS, blocked for 1.5 h with PBS containing 1% bovine serum albumin, then washed three times with sterile PBS. 5 × 105 responder cells or purified T cells were cultured for 24 h with either intact irradiated allogeneic cells (5 × 105 cells/well) or syngeneic antigen-presenting cells (APCs) together with sonicates prepared from allogeneic cells. After washing, a biotinylated anti-γ-IFN detection antibody (XMG 1.2) was added overnight. The plates were developed and the resulting spots were counted and analyzed on a computer-assisted enzyme-linked immunosor-bent assay spot image analyzer (C.T.L., Cleveland, OH), as previously described.
Treatments with anti-CD20 monoclonal antibodies
Mice were injected intraperitoneally with the anti-CD20 murine IgG2a monoclonal antibody, 5D2 (250 µg given intraperitoneally) (a generous gift from Genentech Inc., South San Francisco, CA) (20).
Statistics
All statistical analyses were performed using STATView software (Abacus Concepts, Inc., Berkeley, CA). P-values were calculated using paired t-test. P-values <0.05 were considered statistically significant.
Results
Memory alloreactive T cell responses in naïve and transplanted mice
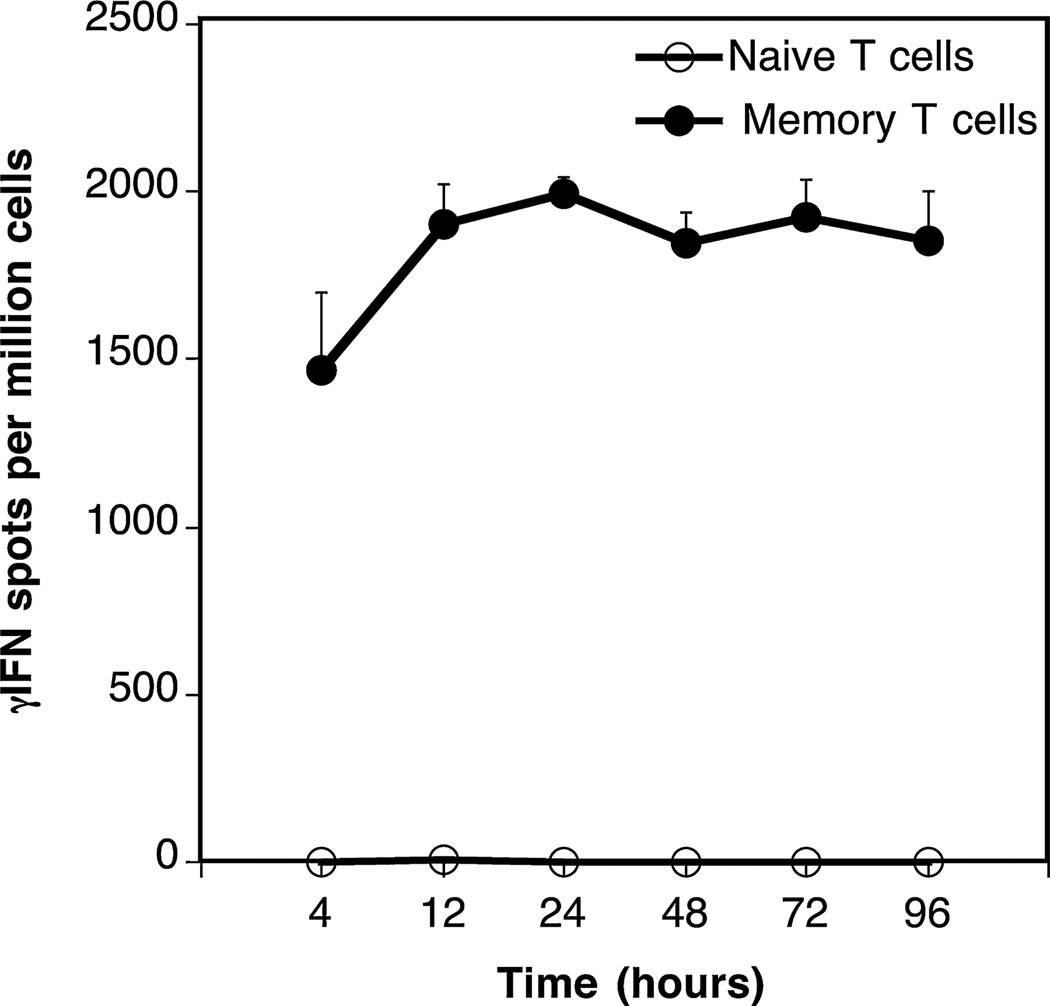
We studied the frequency of alloreactive TMEMs in transplanted mice. One characteristic feature of TMEMs is their ability to respond to short-term antigen stimulation (21). Based upon this principle, we compared the kinetics of γ-IFN production by naïve and TMEMs collected from the spleen of transplanted mice. To test this, T cells were isolated from the spleen of BALB/c mice (H-2d) 40 days after transplantation of a fully allogeneic C57/Bl6 (H-2b) skin graft (MST=8–10 days). Next, T cells were fluorescence-activated cell sorter (FACS)-sorted into naïve (CD44low) and memory (TMEMs: CD44high) cells. Each T cell subset was then cultured for various periods of time (4–96 h) with allogeneic irradiated donor spleen cells (primary mixed lymphocyte reaction). The frequencies of γ-IFN-producing T cells through direct allorecognition were measured by ELISPOT, as previously described (19). No spots were detected with naïve T cells (Figure 1, open symbols). In contrast, very high frequencies of TMEMs (2000 spots/million TMEMs) secreting γ-IFN were detected as early as 4 h after alloantigen exposure (Figure 1, solid symbols). Therefore, a 4-h allostimulation ELISPOT assay was appropriate to detect selectively “induced” allospecific TMEMs generated after transplantation in mice.
Figure 1. Kinetics of γ-IFN production by naïve and memory T cells.
Naïve (CD44−) and memory (CD44+) T cells were isolated from the spleen of BALB/c mice 40 days after placement of a B6 skin allograft. The T cells were cultured for various periods of time with irradiated B6 antigen-presenting cells and the frequencies of activated cells producing γ-interferon (γ-IFN) were assessed by ELISPOT. The results are expressed as numbers of γ-IFN spots per million T cells and represent four to six mice tested individually±standard deviation.
B cell depletion using 5D2 anti-CD20 mAb
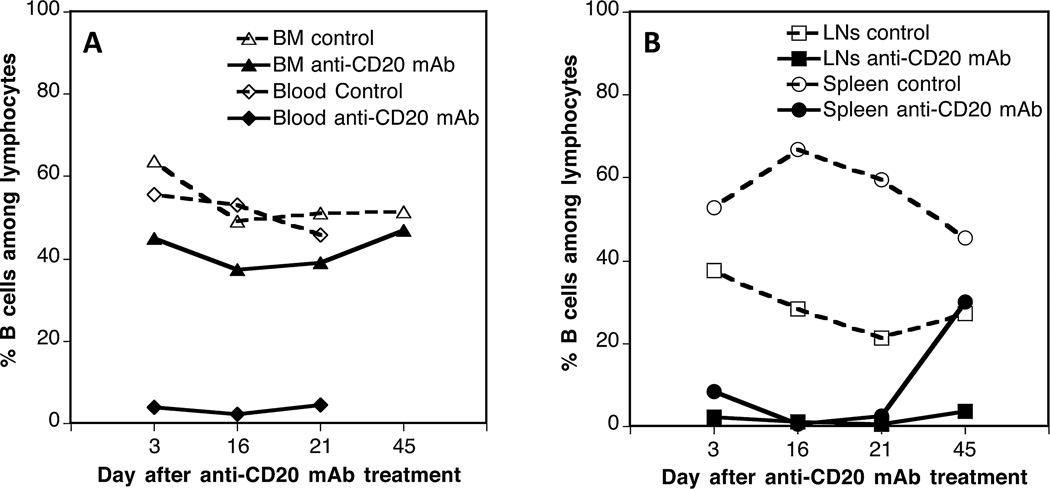
We investigated the effects of in vivo B cell depletion on the generation of allospecific TMEMs after transplantation in mice. First, we monitored the depletion of B cells after a single injection of an IgG2a anti-CD20 mAb, 5D2. OT1 mice, which express a transgenic TCR specific of MHC class I Kb bound to the peptide OVA 257–264 (SIINFEKL), were injected intraperitoneally with 250 µg of 5D2 mAb. The frequencies of B cells were measured by FACS at different time points after antibody treatment using an anti-CD19 mAb. As shown in Figure 2, virtually all B cells were depleted for 20 days in the peripheral blood, lymph nodes (axillary, inguinal, and brachial), and spleen. Normal B cells levels were recovered between 20 and 40 days after treatment. In contrast, >40% B cells were still detected in the bone marrow. We surmise that these undeleted cells correspond to CD19+ pre-B-II small B cells and/or long-lived plasma B cells, which are abundant in the bone marrow and lack expression of CD20.
Figure 2. In vivo B cell depletion using 5D2 IgG2a anti-CD20 mAbs.
OT1 mice were injected with 5D2 IgG2a anti-CD20 antibody (250 µg given intraperitoneally). The percentages of B cells among total lymphocytes were evaluated at different time points in the peripheral blood and bone marrow (BM) (A) as well as lymph nodes (LN) and spleen (B) of anti-CD20 mAb-treated (solid symbols) or control untreated (open symbols) mice via fluorescence activated cell sorter staining with an anti-CD19 antibody. The percentages of CD19+ B cells among lymphocytes of control untreated OT1 mice were as follows: bone marrow: 52%, peripheral blood: 48%, lymph nodes: 28%, and spleen: 46%. The results are representative of three to four mice tested at each time point.
Effect of B cell depletion on the generation of TMEM responses after allotransplantation
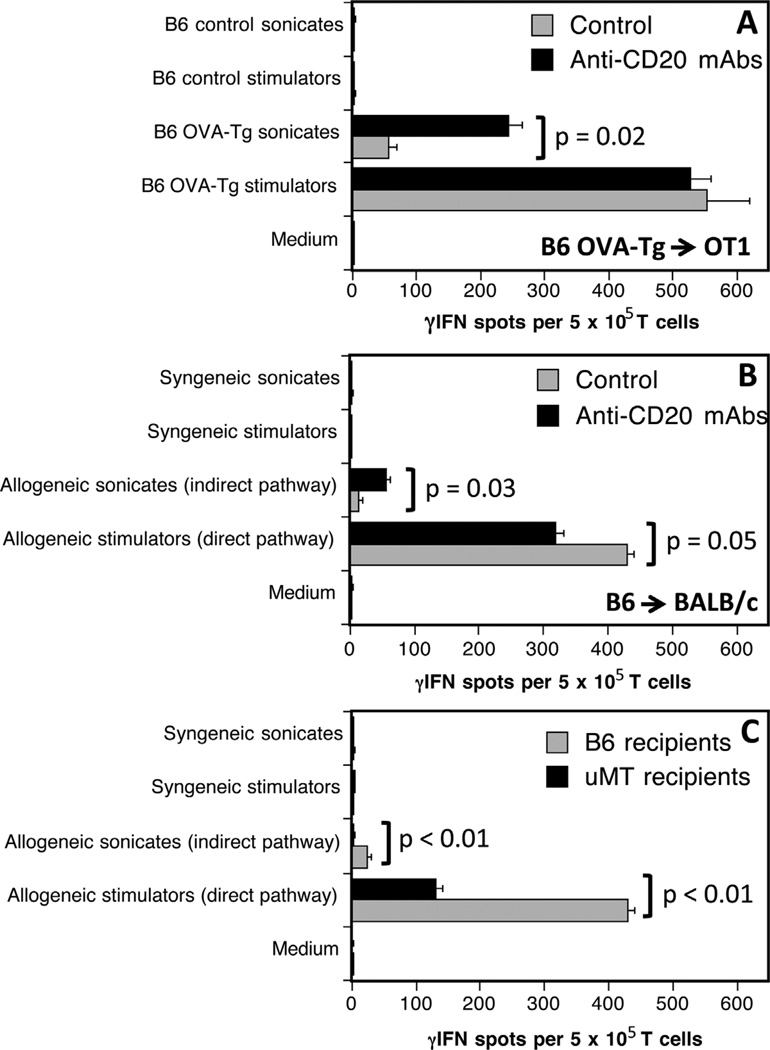
Next, we investigated the influence of B cell depletion on the development of allospecific pro-inflammatory TMEM responses after allotransplantation. OT1 mice, either treated (5 days before grafting) or not with anti-CD20 mAbs, were transplanted with a skin allograft derived from an Act mOVA transgenic mouse (OVA-Tg). All mice rejected their grafts within 13–15 days posttransplantation. The frequencies of γ-IFN producing donor-specific TMEMs were investigated by ELISPOT 40 days after transplantation, using OT1 spleen T cells stimulated in vitro with donor irradiated OVA-Tg APCs (endogenous processing of OVA) or B6 APCs + donor OVA-Tg sonicates (exogenous processing of OVA) for 4 h. Both treated and control mice mounted similar TMEMs alloresponses to intact donor APCs (Figure 3A). In contrast, mice depleted of B cells mounted much higher T cell responses to B6 APCs presenting OVA peptides than their untreated counterparts (Figure 3A). No response was observed following T cell exposure to medium, self-APCs alone or self-APCs + control B6 sonicates. Next, the same experiments were conducted using B6 mice transplanted with a fully allogeneic BALB/c skin graft. B cell depletion resulted in a marked increase of indirect but not direct memory alloreactivity (Figure 3B).
Figure 3. Effects of anti-CD20 mAbs on induction of memory T cell alloresponses.
The frequencies of γ-interferon (γ-IFN)-producing memory T cells activated through allorecognition were investigated 40 days after transplantation of OT1(A) or BALB/c (B) mice with ActmOVAorB6skin grafts, respectively. Panel (C) shows the memory responses of T cells collected from µMT B cell deficient mice transplanted with a BALB/c skin graft. Black bars correspond to anti-CD20 mAb-treated recipients while gray bars show T cell responses measured with control untreated mice.
Our results using anti-CD20 mAb-depleted mice were in apparent disagreement with previous observations in µMT mice genetically devoid of B cells having demonstrated a lack of memory T cell reactivity after allotransplantation (18). This prompted us to investigate memory direct and indirect alloreactivity in our model using µMT mice. As shown in Figure 3C, B cell–deficient mice grafted with a BALB/c skin mounted a much lower direct memory alloresponse than their wild-type B6 counterparts (Figure 3B) and no indirect response at all. This confirms the results previously reported by Ng and colleagues (18).
Effect of B cell depletion on the reactivation of TMEMs after retransplantation
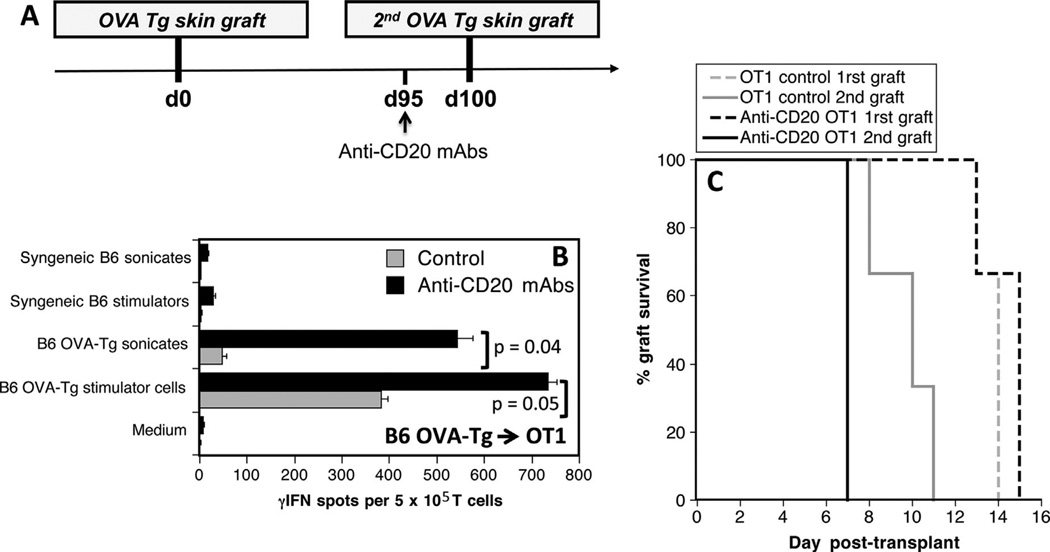
Next, OT1 mice received an Act mOVA skin graft (rejected within 13–15 days). One hundred days later, allosensitized mice were either treated or not treated with anti-CD20 mAbs 5 days prior to placement of a second Act mOVA skin graft (Figure 4A). The effect of B cell depletion on secondary TMEM alloresponses and second set rejection were investigated. Anti-CD20 mAb treatment resulted in marked increases of memory inflammatory alloresponses (Figure 4B) and accelerated second set rejection of skin allografts (p = 0.007) (Figure 4C).
Figure 4. Effects of anti-CD20 mAbs on memory T cell reactivation and allograft rejection in allosensitized mice.
(A) OT1 mice received two skin grafts from Act mOVA donors at day 0 and day 100. Five days before the second graft, some recipients were treated with anti-CD20 mAbs. (B) This panel shows the frequencies of alloreactive memory T cells producing γ-interferon (γ-IFN) in untreated (gray bars) or mice treated with anti-CD20 mAbs (black bars) measured 40 days after the second graft via in vitro stimulation with intact donor OVA transgenic antigen-presenting cells (APCs) (endogenous processing) or recipient B6 APCs + OVA-transgenic donor sonicates (exogenous processing). Controls included T cells stimulated with syngeneic APCs, syngeneic sonicates, or medium. The results are expressed as numbers of γ-IFN spots per million T cells and represent three mice tested individually±standard deviation. Panel (C) shows the percentages of first (dotted lines) and second graft (solid lines) survival overtime for untreated OT1 control mice (gray lines) and anti-CD20 mAb-treated mice (black bars).
Discussion
Our study shows that pretransplant elimination of B cells using 5D2, an IgG2a anti-CD20, did not prevent the generation of TMEMs activated directly and markedly enhanced indirect anamnestic anti-donor T cell inflammatory responses after skin transplantation in mice. Furthermore, B cell depletion increased the reactivation of TMEMs in sensitized mice and accelerated second set skin graft rejection. On the other hand, B cell depletion did not affect the activation of allospecific effector T cells (Figure S1). This challenges the conclusions drawn from previous studies showing impaired TMEM generation in µMT B cell– deficient mice (9, 18). This may be due to the fact that µMT mice display multiple immune abnormalities, including decreased T cell repertoire diversity and number and impaired T cell responses (22). Indeed, studies by Ng et al showed that B cells promoted the differentiation of wild-type effector T cells into TMEMs when adoptively cotransferred in µMT hosts (18). However, experiments showing the actual restoration of TMEM responses in µMT mice adoptively transferred with B cells were not presented. Therefore, the possibility that the lack of memory response in µMT mice is due in part to a T cell defect cannot be ruled out.
Our observation that anti-CD20 mAb treatment enhances allospecific memory T cell immunity may be explained by the expansion of CD20− long-lived plasma cells and/or the elimination of some regulatory B cells (Bregs) documented after anti-CD20 mAb administration (23, 24). This is suggested by the observation that anti-CD20 mAb treatment induced some autoimmune response in sensitized mice (Figure 4C). In further support of this view, anti- CD20 mAb treatments have been shown to enhance autoreactive responses in experimental autoimmune encephalomyelitis models and to prevent tolerance of islet allografts presumably by eliminating Bregs (24, 25). Likewise, J. Bromberg’s group has reported that B cell depletion prevented transplantation tolerance induced via donor-specific transfusion and anti-CD40L mAb-mediated costimulation blockade (26). Finally, a recent study by Clatworthy et al showed that rituximab treatment promoted acute rejection of renal allografts in patients (27). On the other hand, rituximab administration, a humanized IgG1 anti-CD20 mAb, has been shown to reduce disease severity in various chronic inflammatory diseases and promote islet allograft survival in nonhuman primates (28, 29). However, it is noteworthy that in many models anti-CD20 mAbs were delivered along with calcineurin inhibitors or T cell depletion treatments (28). Together with the present study, these conflicting observations suggest that the influence of anti-CD20mAb treatment on the auto- and alloimmune responses may be more complex than initially anticipated. Indeed, it might vary upon the nature of the clonotype and isotype of anti-CD20 antibodies as well as the timing and context of their administration. For instance, type 1 and 2 anti-CD20mAbs,which differ in their ability to translocate CD20 into lipid rafts, mediate their effects on B cells through different mechanisms (30). A better understanding of the mechanisms by which B cell depletion influences the immune response will be required for the design of more selective and effective anti-CD20 mAb-based therapies for transplantation and autoimmune disorders.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH to Gilles Benichou, NIH R21AI100278 and R03AI094235. The anti-CD20 monoclonal antibody, 5D2, was a generous gift from Genentech Inc., South San Francisco, CA.
Abbreviations
- APCs
antigen-presenting cells
- Bregs
regulatory B cells
- LCMV
lymphocytic choriomeningitis virus
- TMEMs
memory T cells
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Figure S1: B cell depletion does not affect alloresponses by effector T cells measured 10 days after transplantation. B6 mice were either treated or not with an anti-CD20 monoclonal antibody 5D2 (250 µg given intraperitoneally) and transplanted 5 days later with a BALB/c skin allograft. The frequency of recipient spleen effector T cells secreting γ-IFN through the direct or indirect allorecognition pathway was measured by ELISPOT 10 days after transplantation. Control experiments were performed with T cells cultured with syngeneic stimulators (self) or medium alone (negative controls) or ConA (positive control). The results are expressed as numbers of γ-IFN spots per million T cells±standard deviation and are representative of three mice tested individually.
References
- 1.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: An overlooked barrier to tolerance. Immunol Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 2.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 3.Nadazdin O, Boskovic S, Murakami T, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Translat Med. 2011;3:86ra51. doi: 10.1126/scitranslmed.3002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lechler R, Lombardi G. Structural aspects of allorecognition. Curr Opin Immunol. 1991;3:715–721. doi: 10.1016/0952-7915(91)90102-7. [DOI] [PubMed] [Google Scholar]
- 5.Amir AL, D’Orsogna LJ, Roelen DL, et al. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115:3146–3157. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- 6.Heeger PS, Greenspan NS, Kuhlenschmidt S, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 7.Weaver TA, Charafeddine AH, Agarwal A, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nature Med. 2009;15:746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bingaman AW, Farber DL. Memory T cells in transplantation: Generation, function, and potential role in rejection. Am J Transplant. 2004;4:846–852. doi: 10.1111/j.1600-6143.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 9.Whitmire JK, Asano MS, Kaech SM, et al. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris DP, Haynes L, Sayles PC, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature immunology. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 11.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- 13.Johansson M, Lycke N. Immunological memory in B-cell-deficient mice conveys long-lasting protection against genital tract infection with Chlamydia trachomatis by rapid recruitment of T cells. Immunology. 2001;102:199–208. doi: 10.1046/j.1365-2567.2001.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen JP, Kauffmann SO, Thomsen AR. Deficient CD4+ T cell priming and regression of CD8+ T cell functionality in virus-infected mice lacking a normal B cell compartment. J Immunol. 2003;171:4733–4741. doi: 10.4049/jimmunol.171.9.4733. [DOI] [PubMed] [Google Scholar]
- 15.Shen H, Whitmire JK, Fan X, Shedlock DJ, Kaech SM, Ahmed R. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 16.van Essen D, Dullforce P, Brocker T, Gray D. Cellular interactions involved in Th cell memory. J Immunol. 2000;165:3640–3646. doi: 10.4049/jimmunol.165.7.3640. [DOI] [PubMed] [Google Scholar]
- 17.Doherty PC, Topham DJ, Tripp RA. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 18.Ng YH, Oberbarnscheidt MH, Chandramoorthy HC, Hoffman R, Chalasani G. B cells help alloreactive T cells differentiate into memory T cells. Am J Transplant. 2010;10:1970–1980. doi: 10.1111/j.1600-6143.2010.03223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 20.Montalvao F, Garcia Z, Celli S, et al. The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. J Clin Invest. 2013;123:5098–5103. doi: 10.1172/JCI70972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 22.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: Increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192:475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahevas M, Patin P, Huetz F, et al. B cell depletion in immune thrombocytopenia reveals splenic long-lived plasma cells. J Clin Invest. 2013;123:432–442. doi: 10.1172/JCI65689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KM, Stott RT, Zhao G, et al. TGF-beta-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol. 2014;44:1728–1736. doi: 10.1002/eji.201344062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lal G, Nakayama Y, Sethi A, et al. Interleukin-10 from marginal zone precursor B-cell subset is required for costimulatory blockade-induced transplantation tolerance. Transplantation. 2015;99:1817–1828. doi: 10.1097/TP.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clatworthy MR, Watson CJ, Plotnek G, et al. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med. 2009;360:2683–2685. doi: 10.1056/NEJMc0808481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Noorchashm H, Sutter JA, et al. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nature Med. 2007;13:1295–1298. doi: 10.1038/nm1673. [DOI] [PubMed] [Google Scholar]
- 29.Barr TA, Shen P, Brown S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim SH, Levy R. Translational medicine in action: Anti-CD20 therapy in lymphoma. J Immunol. 2014;193:1519–1524. doi: 10.4049/jimmunol.1490027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.