Abstract
The hypothalamus is composed of many heterogeneous nuclei that control distinct physiological functions. Investigating molecular mechanisms that regulate the specification of these nuclei and specific neuronal subtypes, and their contribution to diverse hypothalamic functions, is an exciting research focus. Here, we begin by summarizing the hypothalamic functions of feeding regulation, sleep-wake cycles, stress responses, and circadian rhythm, and describing their anatomical bases. Next, we review the molecular regulation of formation of hypothalamic territories, specification of nuclei and subnuclei, and generation of specific neurons. Finally, we highlight physiological and behavioral consequences of altered hypothalamic development. Identifying molecules that regulate hypothalamic development and function will increase our understanding of hypothalamus-related disorders, such as obesity and diabetes, and aid in the development of therapies aimed specifically at their etiologies.
Keywords: Hypothalamus, Hypothalamic functions, Neurogenesis, Hypothalamic development, Hypothalamus-related disorders
Introduction
The hypothalamus can be rostrocaudally divided into four regions: preoptic, supraoptic, tuberal, and mammillary regions (Fig. 1). Each region consists of several nuclei, whose functions were defined mainly using lesions, stimulations, and genetic approaches. It has been found that besides the conventional neurotransmitters such as 5-hydroxytryptamine (5-HT), dopamine (DA), glutamate (Glu), and gamma-aminobutyric acid (GABA), the hypothalamus predominantly and selectively produces various restricted neuropeptides and hormones, which contribute to diverse basic physiological functions, such as feeding, sleep-wake cycles, body temperature, sex behaviors and reproduction, stress responses, and circadian rhythm [1]. In addition, these nuclei in the hypothalamus are frequently connected by neuronal projections, which indicates neurobiological interactions between different physiological functions. In this review, we first summarize hypothalamic functions that are regulated by specific nuclei. We then highlight molecules such as mitogens, transcription factors, and microRNAs (miRNAs), which play crucial roles in the development of the hypothalamus. Finally, we elaborate pathophysiologic functional consequences of abnormal development of the hypothalamus.
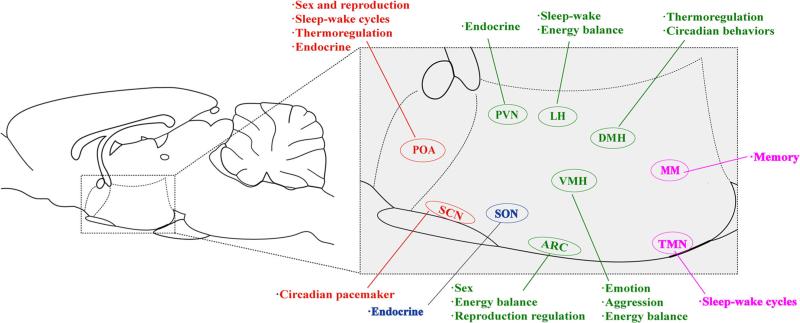
Fig. 1.
An overview of hypothalamic nuclei and functions. Nuclei and their functions in the preoptic region are denoted in red, including the preoptic area (POA) and the suprachiasmatic nucleus (SCN). Relative locations and functions of the supraoptic region are denoted in blue, including the supraoptic nucleus (SON). The tuberal region is denoted in green, including the arcuate nucleus (ARC), the paraventricular nucleus (PVN), the lateral hypothalamus (LH), the ventromedial hypothalamus (VMH), and the dorsomedial hypothalamus (DMH). The mammillary region, including the mammillary area (MM) and the tuberomammillary nucleus (TMN), is denoted in purple
Functions of the hypothalamus
Feeding and energy expenditure
Roles of the hypothalamus in feeding and expenditure were recognized as early as 1940s, as some studies showed that ablation of the arcuate nucleus (ARC) and ventromedial nucleus (VMN) led to excessive eating and obesity, while ablation of the lateral hypothalamus (LH) caused aphagia and weight loss [2–4]. Later, the dorsomedial nucleus (DMN) and paraventricular nucleus (PVN) were also proven to regulate food intake and body weight, with aphagia and weight loss caused by lesions in the DMN, and overeating and obesity due to lesions in the PVN [5, 6]. Neurocircuits composed of these hypothalamic nuclei and other brain areas seem to play important roles in determining whether to eat, when to eat, what to eat, and how much to eat [7].
The ARC is a major site of feeding regulation, whose neurons mainly project to other nuclei of the hypothalamus, including the LH, PVN, and VMN [8, 9] (Fig. 2), and release the orexigenic neuropeptides (neuropeptide Y, NPY, and agouti-related protein, AgRP) to increase food intake, and the anorexigenic neuropeptides (proopiomelanocortin, POMC; and cocaine- and amphetamine-regulated transcript, CART) to decrease food intake [8, 10, 11]. Nutrient-related signals and hormones such as leptin [12], insulin [13], glucose [14], peptide YY3-36 (PYY3-36) [15], ghrelin [16], and cholecystokinin (CCK) [17] have been reported to repress these orexigenic neuropeptides and promote anorexigenic neuropeptides to decrease food intake. VMN neurons have been found to project to the ARC and activate anorexigenic pathways [18], as well as express abundant oxytocin receptor to mediate oxytocin action on food intake [19, 20]. In addition, LH and PVN neurons have been found to relay information from other food-related neurons, such as the parabrachial nucleus (PBN) in the midbrain and the nucleus of the solitary tract (NTS) in the hindbrain, which receive visceral inputs, to the ARC to influence satiety [7]. LH and PVN neurons also receive reward-related inputs from the nucleus accumbens (NAc) and amygdala (AMY), and project to the ARC to regulate appetite [7] (Fig. 2). Moreover, studies have shown that LH neurons produce orexigenic neuropeptides orexin, melanin concentrating hormone (MCH), and CART to promote food intake [21], while PVN neurons produce hormones such as the corticotropin-releasing hormone (CRH), the thyrotropin-releasing hormone (TRH), and oxytocin (OXT) to participate in the metabolic process to regulate food intake [20, 22]. The DMN has been shown to receive information from the suprachiasmatic nucleus (SCN) and export to the PVN and LH, and participate in regulating circadian rhythm of feeding [23–25] (Fig. 2).
Fig. 2.
Hypothalamic neurocircuits involved in feeding and energy expenditure. The brain outline (left) shows relative locations of the hypothalamus and other related nuclei including the nucleus accumbens (NAc) and amygdala (AMY) in the forebrain, the parabrachial nucleus (PBN) in the midbrain, and the nucleus of the solitary tract (NTS) in the hindbrain. The highlighted image (right) shows nuclei and projections among nuclei and other brain sites in the hypothalamus. Projections between the arcuate nucleus (ARC) and other nuclei, which play important roles in mediating intrahypothalamic interactions, are denoted in purple.
Pathways between hypothalamic subnuclei and other feeding-related (PBN and NTS) and reward-related (NAc and AMY) information are denoted in red. The paraventricular nucleus (PVN) and lateral hypothalamus (LH) seem to relay nuclei from feeding-related and reward-related sites to the ARC. Projections from the supraoptic nucleus, which are denoted in blue, indicate that circadian rhythm influences when to feed during the day. The interrupted arrows stand for indirect projections from suprachiasmatic nucleus (SCN) to the dorsomedial nucleus (DMN)
Roles of specific neuronal circuits and substances involved in food intake and expenditure in the adult hypothalamus were well studied in the past. Not surprisingly, because maternal obesity and diabetes increase risks for obesity and diabetes in the offspring, studies on correlations between hypothalamic development and feeding-related disorders are now emerging [26–28].
Sleep and wakefulness
Sleep and wakefulness are complex behaviors whose regulation is involved in coordination of neural networks, homeo-static drive, and circadian system, which are all associated with the hypothalamus [29, 30]. The ventrolateral preoptic nucleus (VLPO) and the median preoptic nucleus (MnPO) of the hypothalamus are two main sleep centers that promote sleep and repress wakefulness (Fig. 3). GABA and galanin neurons in the VLPO and MnPO are active during sleep and, in turn, repress the ascending arousal system that consists of the brain stem, hypothalamus, thalamus, basal forebrain, and cortex [31, 32]. On the other hand, as a part of the ascending arousal system, the LH and tuberomammillary nucleus (TMN) consist of arousal neurons that produce orexin and histamine/gamma-aminobutyric acid (His/GABA), respectively, which are all active during wakefulness [32–35] (Fig. 3). It has been found that orexin mutation in human or deletion in mice causes narcolepsy [36–38]. Moreover, the SCN, the subparaventricular zone (SPZ), and the dorsomedial nucleus (DMN) form a SCN-SPZ-DMN network, which is responsible for the regulation of the sleep-wake circadian rhythm [29, 39] (Fig. 3). Notably, diverse neuropeptides and hormones such as NPY, CRH, and Growth Hormone Releasing Hormone (GHRH) in the hypothalamus take part in the homeostatic regulation of sleep and wakefulness likely by the interaction with neuronal networks [31].
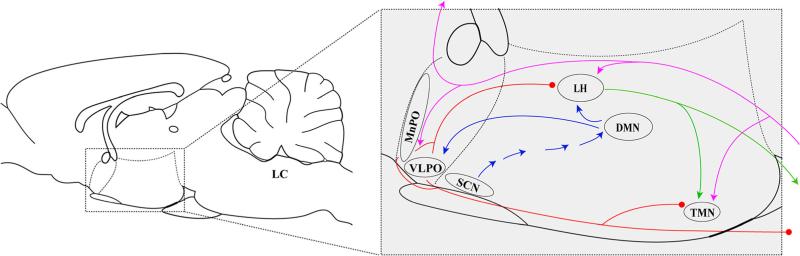
Fig. 3.
Hypothalamic neurocircuits involved in sleep and wakefulness. The ascending arousal pathways, which originate from the locus coeruleus (LC) in the hindbrain and are all active during wakefulness, are denoted in purple. Sleep-promoting pathways, in which neurons in the lateral hypothalamus (LH) and tuberomammillary nucleus (TMN) are repressed by neurons producing GABA and galanin in the ventrolateral preoptic nucleus (VLPO) and median preoptic nucleus (MnPO) during sleep, are denoted in red. Outputs of orexin, which are important for stabilizing sleep, are shown in green, and pathways of circadian rhythm involved in sleep-wake cycles are shown in blue
Dysregulation of hypothalamic development has been found to be associated with sleep disturbance, such as in Prader-Willi syndrome [40]. As a result, the hypothalamus is considered an important target for potential therapies for sleep disorders, for example, dexmedetomidine (a sleep-promoting drug) promotes sleep by activating the VLPO [41].
Responses to stress
When an organism confronts stress such as an adverse environment or threats, a series of physiological responses, also called stress responses, are triggered to adapt to these changes. Multiple nuclei and molecules of the hypothalamus participate in stress responses. The PVN, which interacts with the medial preoptic area (mPOA), DMN, LH, SCN, and some other non-hypothalamus nuclei, has been found to regulate stress by activating the hypothalamic–pituitary–adrenal (HPA) axis [42, 43]. Under stress, the PVN secrets CRH and arginine vasopressin (AVP) to stimulate release of the adrenocortico-tropic hormone (ACTH) in the pituitary, in turn to regulate the level and activity of the cortisol in the adrenal [44]. In addition, peptides such as orexin, NPY, and OXT participate in stress responses by regulating the HPA activity in a specific stress context, for example sleep loss and fear [45, 46].
Other functions of the hypothalamus
It has been shown that the SCN is a major circadian pacemaker in vertebrates. Lesions of the SCN lead to arrhythmic motor activity in rodents [47, 48]. The vasoactive intestinal peptide (Vip) or its receptor Vipr2, gastrin-releasing peptide (Grp), AVP, and prokineticin-2 have been shown to be crucial for regulating rhythm of clock gene expression and neuronal firing in the SCN [49–53]. Moreover, lesion studies have shown that the mammillary body in the hypothalamus is related to memory, but its molecular mechanism is still unknown [54–57]. Thus, the hypothalamus regulates complex physiological behaviors related to feeding, sleep, stress responses, and rhythms. Investigation of molecular mechanisms that regulate formation of distinct nuclei and productions of specific neurons will shed light on understanding neurological bases of hypothalamus functions.
Molecular regulation of hypothalamic development and functions
The development of the hypothalamus, derived from prechordal mesoderm, is a complex series of events that includes establishment of the hypothalamic regional territory, specification of subdivision of regional territories, neuronal differentiation, neuronal migration, and formation of subnuclei. A gene expression profiling study has identified altered expression of over 1000 transcripts in the mouse hypothalamus during different developmental stages, suggesting that a large number of genes contribute to hypothalamic development and may play multiple roles in the process [58]. We here summarize major signaling pathways and molecules that regulate the process of hypothalamic development, and highlight their effects on behaviors.
Signaling pathways that determine hypothalamic regional territories
The Wnt and sonic hedgehog (Shh) signaling pathways play essential roles in neural development. They normally function as mitogens that control proliferation of progenitor cells and affect expression of downstream genes. Studies have shown that the Wnt and Shh pathways are also crucial for formation of hypothalamic regional territories (Fig. 4a).
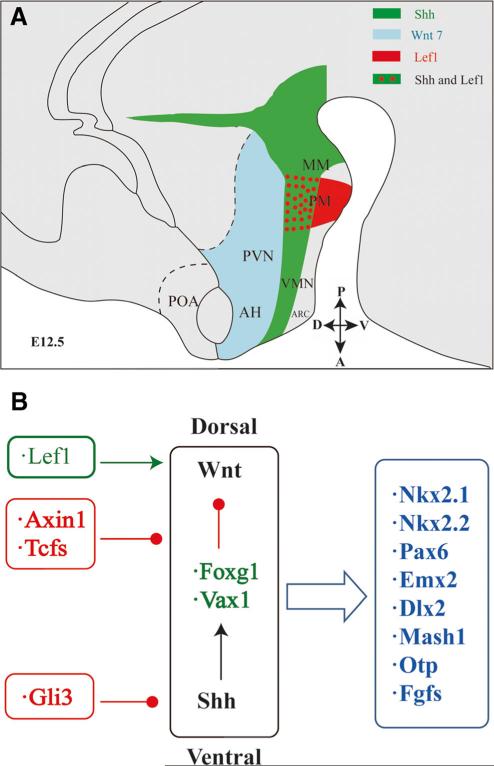
Fig. 4.
The Wnt-Shh signaling network and hypothalamic development. a Schematic drawing of expression regions of Shh and Wnt components in the hypothalamus of the E12.5 mouse brain. The expression region of Shh is denoted in green; Lef1 is denoted in red; Wnt7 is denoted in blue. Both Shh and lef1 are expressed in regions with red dots and green background. Other brain areas are shown in gray. AH anterior hypothalamus, ARC arcuate nucleus, MM mammillary area, PM premammillary region, POA preoptic area, PVN paraventricular nucleus, VMN ventrome-dial nucleus. The anterior-posterior (A-P) and dorsal-ventral (D-V) directions of the hypothalamus in the E12.5 mouse brain are shown. b Genes of the Wnt-Shh signaling network in the developing hypothalamus. Shh signaling is mainly located in the ventral hypothalamus while Wnt signaling is in the dorsal area. The essential mediator of the Wnt pathway Lef1 and inhibitors of Wnt signaling Axin1 and Tcfs regulate Wnt activity. Shh components Gli3 represses Shh activity in the developing hypothalamus. Early developmental genes Vax1 and Foxg1 regulate interactions between Wnt and Shh pathways. The Wnt-Shh signaling network regulates expression of downstream genes related to neurogenesis such as Nkx2.1, Nkx2.2, Pax6, Emx2, and Dlx2, and molecules that specify subnuclei and neuronal lineage such as Mash1, Otp, and Fgfs
Wnt signaling pathway
It has been shown that a gradient expression of the Wnt signaling contributes to the formation of the anterior-posterior (A-P) axis in the neural plate [59–61]. Wnt components are expressed in a distinct pattern to form a temporal local gradient Wnt activity and to participate in self-renewal and differentiation of prechordal mesoderm cells [62, 63]. For example, Wnt7b and Wnt8b are highly expressed in the central nervous system in mice between embryonic day 11 (E11) and E16 (http://www.brain-map.org/). Specifically, Wnt7 is expressed in the anterior hypothalamus and Lef1 is expressed in the premammillary area [58] (Fig. 4a). In zebrafish embryos, Wnt8 is expressed in the posterior area, Lef1 in the mammillary area, and Axin1 and Tcf3 in the posterior area [64–67]. Specific expression of molecules in the Wnt signaling pathway implies their roles in regulating hypothalamic regional territories.
In the zebrafish, functional deletion of Wnt8b leads to loss of expression of the proneuron marker Zash1a and the neurogenesis marker Sox3 [64]. Moreover, abrogation of Lef1, the essential mediator of the Wnt pathway, results in absence of expression of Sox3, Zash1a, and the postmitotic neuron marker HuC/D [64]. This suggests that the Wnt-Lef1 signaling is crucial for hypothalamic neurogenesis. Deletion of Axin1, a Wnt signaling inhibitor, leads to a reduced expression of the early posterior hypothalamic marker Nkx2.1a, and adding Axin1 to the mutant can restore its expression in the zebrafish [65]. Similarly, it has been found that Tcf3, another Wnt repressor, is expressed in the posterior hypothalamus of the zebrafish [67], and overexpression of Tcf3 results in an ectopic expression of Nkx2.1a [68]. This suggests that Axin1 and Tcf3 play an important role in specifying identity of the posterior hypothalamus (Fig. 4b).
Shh signaling pathway
Dynamic and transient expression of Shh in the prechordal plate has been shown to control the dorsal and ventral patterning of the developing brain [69, 70]. Sonic hedgehog (Shh) has a peak expression at E9.5 when it mainly plays a role in patterning the mouse hypothalamus. Later on, its expression becomes progressively reduced and does not reach another peak until E12.5 when it participates in hypothalamic glial differentiation [58, 71].
At the early stage of the hypothalamic development, Shh is specifically expressed in the ventral area [58] (Fig. 4a). Conditional knockout of Shh using the Foxb1-Cre line in the neural plate in mice disrupts the expression of Dbx1 and Dlx2, and causes a reduction of the ventral domain and a reversion to dorsal identity in the hypothalamus [72]. Similarly, Shh deletion in the hypothalamic basal plate using the Nkx2.1-Cre line results in loss of the anterior hypothalamus including the ARC, PVN, and VMN, and a thinner posterior hypothalamus [58]. Moreover, the Shh signaling repressor Gli3 is expressed in presumptive hypothalamus region by E7.5 in mice and plays a role of specifying the hypothalamic subregions by balancing the Shh-Gli pathway [73] (Fig. 4). These studies suggest that Shh is required for formation of the ventral and anterior hypothalamus.
Genes interacting with the Wnt and Shh pathways
Previous work has shown that BMP7, expressed in the posteroventral hypothalamus, is crucial for repressing Shh expression and in turn contributes to the Shh dynamic expression [71, 74, 75]. Deletion of Foxg1, which marks the boundary between the telencephalon and basal diencephalon [58], results in expansion of the hypothalamus [76]. It has been found that Foxg1 is induced by Shh to directly repress the expression of Wnt8b ligand [76], suggesting that Foxg1 may regulate formation of the hypothalamic regional territory by integrating the Wnt and Shh signalings (Fig. 4b). Furthermore, Vax1, which marks the ventral anterior area, is induced by Shh to repress Wnt activity by activating the inhibitor Tcf4 (also called Tcf7l2) [77, 78] (Fig. 4b). Six3 is expressed in the ventral area and may promote Shh expression by functioning as a remote enhancer [79].
Studies have shown that the combinatory expression of transcription factors such as Nkx2.1, Emx2, Dlx2, and growth factors such as fibroblast growth factors (Fgfs) also regulates hypothalamic borders [80]. For instance, Nkx2.1 specifies the posteroventral hypothalamus [81], while Rx3 is important for the anterior hypothalamus [82]. Irx5 is expressed in the supramammillary nucleus, and SF-1 and Pomc are expressed in the VMN and ARC, respectively [83, 84]. The combination of Lhx6, Arx, and Nkx2.1 expression demarcates the tuberomammillary area, and positive expression of Foxg1 and negative expression of Sim1 and Nkx6.2 define the preoptic area [58]. These transcription factors appear to be regulated by the combinatory effect of the Wnt and Shh signalings directly or indirectly [80], suggesting downstream genes of the Wnt and Shh pathways (Fig. 4b).
Genes determining formation of hypothalamic nuclei and their functions
Formation of hypothalamic nuclei is organized by generation and migration of specific neurons. Neurogenesis in the developing hypothalamus occurs between E10 and E16 in the mouse. Genetic studies have shown that deletion of specific genes leads to neurogenesis defects and failure in organization of diverse nuclei, and in turn affects distinct hypothalamic functions, and results in physiological or behavioral changes (Table 1).
Table 1.
Roles of developmental genes in hypothalamic formation and physiological functions
| Gene mutations | Developmental phenotypes | Functional consequences | References |
|---|---|---|---|
| Mash1 deletion | Failure of neurogenesis in the ARC and VMN | Unknown | [85–87] |
| Otp deletion | Failure of formation of the aPV/PVN/SON | Disrupted stress responses | [107–109, 111] |
| Sim1 heterozygous deletion | Reduction of OXT | Obesity | [121–123] |
| Ngn3 deletion | Decrease in neurons expressing POMC and SF-1, and increase in NPY cells | Obesity | [89, 90] |
| NHLH2 deletion | Disrupted GnRH migration | Infertility | [92, 93] |
| SF-1 deletion | Failure of formation of VMN | Obesity and anxiety | [94–99] |
| Lhx1 deletion | Disrupted production of neuropeptides AVP, Vip, and Grp | Disrupted SCN rhythm | [103] |
Genes regulating development and functions of the ARC and VMN
Studies have shown that Mash1 is expressed in the ARC and VMN of the hypothalamus and contributes to neurogenesis. Mash1 deletion results in loss of distinct neurons, including neurons secreting neuropeptides, GHRH, and dopamine neurons [85–87]. It has been shown that Mash1 is induced by the Shh signaling in vivo, suggesting that the Shh signaling controls the development of the ARC and VMN by regulating Mash1 expression [88] (Fig. 4b). Moreover, Ngn3 plays a role in mediating differentiation of the ARC and VMN. It has been found that Ngn3 promotes the development of neurons expressing POMC and SF-1 (a VMN marker), and inhibits the expansion of the NPY neuronal population [89]. Conditional deletion of Ngn3 in the ventral hypothalamus causes hyper-phagia and reduces energy expenditure, and eventually results in obesity [90]. This indicates that Ngn3 may control energy balance by promoting differentiation of appetite-related neurons. Moreover, a recent work has found that Ngn3 is regulated by the sex-determining gene Sry on the Y chromosome and mediates sex-specific development in the hypothalamus [91].
In addition, NHLH2 is expressed in the hypothalamus, and NHLH2 knockout mice present adult-onset obesity and infertility [92]. It appears that NHLH2 mediates infertility by regulating migration of neurons expressing GnRH, while it affects energy balance by mediating cleavage of the proneuropeptide POMC [93]. Furthermore, SF-1 is specifically expressed in the VMN, and SF-1 deletion in the VMN makes cells more scattered, and in turn results in failure in VMN formation [94, 95]. The VMN-specific SF-1 knockout mice are less sensitive to leptin and subsequently become obese in high fat diet (HFD), which suggests that the VMN regulates energy balance possibly by the leptin signal [96–98].
Moreover, VMN specific SF-1 knockout mice display anxiety-like behaviors, which indicates that the VMN also participates in stress regulation [99].
SCN formation and rhythm
Studies have shown that Six3, Six6, and Lhx2 are required for SCN neuronal development, and deletion of any of them fails to specify the SCN [100–102]. Moreover, deletion of Lhx1 specifically in the SCN selectively affects development of SCN neurons that produce neuropeptides AVP, Vip, and Grp, but has no effects on generation of neurons that secrete GABA, Gal, and SST [103]. Lhx1-deficient mice show disrupted SCN cellular rhythm and circadian activity rhythm [103]. These studies indicate that normal development of SCN is also important for acquirement of its circadian properties.
Genes determining formation of nuclei related to the neuroendocrine system
The aPV, PVN, and SON consist of parvicellular neurons that secrete CRH, TRH, GHRH, and somatostatin (SST), and magnocellular neurons that produce AVP and OXT. These nuclei are the major developmental centers of the neuroendocrine system, where many endocrine progenitors are produced and then migrate to other brain sites [104–106].
The homeobox gene Orthopedia (Otp) is strongly expressed in the putative anterior paraventricular (aPV), PVN, and the supraoptic nucleus (SON) at the early stage, and deletion of Otp leads to failure in formation of the aPV/PVN/SON and causes loss of distinct neurons including neuroendocrine neurons secreting SST, AVP, OXT, CRH, TRH, and dopamine neurons [107–109]. Otp is found to be promoted by Shh and repressed by Fgf8 in the zebrafish [110], which indicates that Shh may participate in specification of endocrine cells (Fig. 4b). While deletion of Otp leads to embryonic lethality in mice, deletion of Otp only affects stress adaptation in the zebrafish [111].
Sim1 is an important gene for organization and neuronal neurogenesis of the aPV/PVN/SON, and its expression is controlled by Otp [112, 113]. It has been shown that Sim1 mutation causes decrease of almost all endocrine cells in mice. Together with Arnt2, Sim1 regulates Brn2 expression to promote differentiation of cells secreting AVP, CRH, and OXT in the PVN and SON, and also controls Sim2 expression to determine differentiation of cells secreting SST and TRH in the aPV [114–116]. In humans, patients without SIM1 show Prader-Willi-like symptoms [117–119]. Decreased SIM1 expression in humans has been reported to cause obesity [120]. Similarly, Sim1 haploinsufficient mice show hyperphagic obesity due to profound reduction of OXT [121–123]. In contrast, Sim1 overexpressing mice show reduced diet-induced obesity [124]. This suggests that OXT is important for weight control and its function is regulated by Sim1 expression.
Formation of neurocircuits and hypothalamic functions
Besides main outputs to pituitary and autonomic neurons in the midbrain and hindbrain, one of the most significant characterizations of the hypothalamus is that its efferent fibers reach the internal nuclei, especially the ARC. Neurons that produce NPY and POMC in the ARC send a bundle of projections to the VMN, LH, and PVN to regulate feeding and expenditure (Fig. 2). Interestingly, the number of these efferent fibers in the ARC influences energy metabolism. For example, decrease of outputs of ARC neurons due to the maternal HFD causes obesity and disorders of glucose metabolism in the offspring [125]. It has been found that Fgf8 is expressed in the hypothalamus and contributes to growth of GnRH axons, and netrin-1 and its receptor DCC participate in orientation of retinal ganglion cell (RGC) axons in the ventral hypothalamus [126]. However, it is unclear what developmental genes play a role in formation of hypothalamic functional circuits.
Roles of miRNAs in the development and functions of hypothalamus
miRNAs are a large family of endogenous noncoding small RNAs. They are ~22 nucleotides long and function through degradation or posttranscriptional repression of target genes by imperfect complementary pairing. Studies have shown that miRNAs participate in neurogenesis, neuronal differentiation and migration, and regulate brain functions by silencing specific target molecules [127–129]. It has been found that some miRNAs are highly expressed in the hypothalamus, for example, miR-7, let-7, and miR-9 [130, 131]. Of these, let-7 has been shown to inhibit Mash1 to reduce neurogenesis in vitro [132]. Additionally, specific deletion of Dicer, an essential enzyme that processes miRNA precursors, in the POMC-expressing cells leads to failure in development of neurons secreting POMC, and in turn causes obesity and disrupted glucose metabolism [133]. However, what and how specific miRNAs affect development and functions of hypothalamic nuclei and its neuronal lineages are still unknown.
Perspectives
The hypothalamus is a brain region consisting of many nuclei that control a variety of endocrine, autonomic, and behavioral functions. Besides functions discussed above, the hypothalamus also participates in many other basic life functions including fluid and electrolyte balance, aggressive behaviors, and so on [1]. Molecular mechanisms that regulate hypothalamic development, especially specification of subnuclei and neuronal lineages, and formation of neurocircuits, and their consequences to hypothalamic physiological functions, need to be further studied. Genetic rescue will be helpful to further reveal the role of specific genes in regulating hypothalamic development and physiological functions. Roles of noncoding RNAs such as miRNAs in development of distinct hypothalamic subnuclei and functions are of great interests to be further explored. Revealing molecular mechanisms of genes that regulate hypothalamic development and physiological functions will provide significant insights into understanding the etiology of hypothalamus-associated diseases such as adolescent obesity, diabetes, and depression, and developing a means for treatment.
Acknowledgments
This work was supported by a grant from the National Science Foundation of China (81471152), the Hirschl/Weill-Caulier Trust (T.S.), and an R01-MH083680-06 grant from the NIH/ NIMH (T.S.).
References
- 1.Clifford BS, Bradford BL. The hypothalamus. Curr Biol. 2015;24(23):1111–1116. [Google Scholar]
- 2.Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat Rec. 1940;78:24. [Google Scholar]
- 3.Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol Rev. 1946;26(4):541–559. doi: 10.1152/physrev.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- 4.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24(2):123–140. [PMC free article] [PubMed] [Google Scholar]
- 5.Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76(3):431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- 6.Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav. 1981;27(6):1031–1040. doi: 10.1016/0031-9384(81)90366-8. [DOI] [PubMed] [Google Scholar]
- 7.Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15(6):367–378. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercer RE, Chee MJ, Colmers WF. The role of NPY in hypothalamic mediated food intake. Front Neuroendocrinol. 2011;32(4):398–415. doi: 10.1016/j.yfrne.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, Ding T, Xu F, et al. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat. 2015;9:40. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 2013;36(2):65–73. doi: 10.1016/j.tins.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23(4):775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 13.Woods SC, Lotter EC, Mckay LD, Porte D. Chronic intracerebroventricular infusion of insulin reduces food-intake and body-weight of baboons. Nature. 1979;282(5738):503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 14.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449(7159):228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 15.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, et al. Gut hormone PYY (3–36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 16.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973;84(3):488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 18.Sternson SM, Shepherd GMG, Friedman JM. Topographic mapping of VMH–>arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8(10):1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 19.Sabatier N, Rowe I, Leng G. Central release of oxytocin and the ventromedial hypothalamus. Biochem Soc Trans. 2007;35(Pt 5):1247–1251. doi: 10.1042/BST0351247. [DOI] [PubMed] [Google Scholar]
- 20.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18(2):158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36(2):199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 22.Foster MT, Song CK, Bartness TJ. Hypothalamic paraventricular nucleus lesion involvement in the sympathetic control of lipid mobilization. Obesity (Silver Spring) 2010;18(4):682–689. doi: 10.1038/oby.2009.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9(3):398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 24.Acosta-Galvan G, Yi CX, van der Vliet J, Jhamandas JH, Panula P, Angeles-Castellanos M, Basualdo MD, Escobar C, et al. Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc Natl Acad Sci U S A. 2011;108(14):5813–5818. doi: 10.1073/pnas.1015551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23(33):10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. 2008;31(12):2288–2293. doi: 10.2337/dc08-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Damm P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes the role of intrauterine hyper-glycemia. Diabetes Care. 2008;31(2):340–346. doi: 10.2337/dc07-1596. [DOI] [PubMed] [Google Scholar]
- 28.Deierlein AL, Siega-Riz AM, Chantala K, Herring AH. The association between maternal glucose concentration and child BMI at age 3 years. Diabetes Care. 2011;34(2):480–484. doi: 10.2337/dc10-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 30.Alóe F. Sleep-wake cycle mechanisms. Rev Bras Psiquiatr. 2005;27(I):9. [Google Scholar]
- 31.Richter C, Woods IG, Schier AF. Neuropeptidergic control of sleep and wakefulness. Annu Rev Neurosci. 2014;37:503–531. doi: 10.1146/annurev-neuro-062111-150447. [DOI] [PubMed] [Google Scholar]
- 32.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68(6):1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nature Reviews | Neuroscience. 2002;3:15. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- 34.Mieda M, Tsujino N, Sakurai T. Differential roles of orexin receptors in the regulation of sleep/wakefulness. Front Endocrinol (Lausanne) 2013;4:57. doi: 10.3389/fendo.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 36.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 37.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 38.Singh AK, Mahlios J, Mignot E. Genetic association, seasonal infections and autoimmune basis of narcolepsy. J Autoimmun. 2013;43:26–31. doi: 10.1016/j.jaut.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21(6):482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- 40.Camfferman D, Lushington K, O’Donoghue F, Doug McEvoy R. Obstructive sleep apnea syndrome in Prader-Willi syndrome: an unrecognized and untreated cause of cognitive and behavioral deficits? Neuropsychol Rev. 2006;16(3):123–129. doi: 10.1007/s11065-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 41.Nelson LE, Lu J, Guo TZ, Saper CB, Franks NP, Maze M. The alpha (2)-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98(2):428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 42.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 44.De Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 45.Galvao MDL, Sinigaglia-Coimbra R, Kawakami SE, Tufik S, Suchecki D. Paradoxical sleep deprivation activates hypothalamic nuclei that regulate food intake and stress response. Psychoneuroendocrinology. 2009;34(8):1176–1183. doi: 10.1016/j.psyneuen.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 47.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69(6):1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 49.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8(4):476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci. 2006;26(45):11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maywood ES, Chesham JE, O'Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci U S A. 2011;108(34):14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown TM, Hughes AT, Piggins HD. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling. J Neurosci. 2005;25(48):11155–11164. doi: 10.1523/JNEUROSCI.3821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maywood ES, Reddy AB, Wong GK, O'Neill JS, O'Brien JA, McMahon DG, Harmar AJ, Okamura H, et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16(6):599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 54.Aggleton JP, Dumont JR, Warburton EC. Unraveling the contributions of the diencephalon to recognition memory: a review. Learn Mem. 2011;18(6):384–400. doi: 10.1101/lm.1884611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.CM D. How do mammillary body inputs contribute to anterior thalamic function? Neurosci Biobehav Rev. 2014;54:108–119. doi: 10.1016/j.neubiorev.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci. 2004;5(1):35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- 57.Vann SD. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia. 2010;48(8):2316–2327. doi: 10.1016/j.neuropsychologia.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 58.Shimogori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, et al. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13(6):767–776. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128(21):4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- 60.Cavodeassi F. Integration of anterior neural plate patterning and morphogenesis by the Wnt signaling pathway. Dev Neurobiol. 2014;74(8):759–771. doi: 10.1002/dneu.22135. [DOI] [PubMed] [Google Scholar]
- 61.Altmann CR, Brivanlou AH. Neural patterning in the vertebrate embryo-wnt. International Review of Cytology-a Survey of Cell Biology. 2001;203:447–482. doi: 10.1016/s0074-7696(01)03013-3. [DOI] [PubMed] [Google Scholar]
- 62.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Kopinke D, Lin J, McPherson AD, Duncan RN, Otsuna H, Moro E, Hoshijima K, et al. Wnt signaling regulates postembryonic hypothalamic progenitor differentiation. Dev Cell. 2012;23(3):624–636. doi: 10.1016/j.devcel.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee JE, Wu SF, Goering LM, Dorsky RI. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development. 2006;133(22):4451–4461. doi: 10.1242/dev.02613. [DOI] [PubMed] [Google Scholar]
- 65.Kapsimali M, Caneparo L, Houart C, Wilson SW. Inhibition of Wnt/Axin/beta-catenin pathway activity promotes ventral CNS midline tissue to adopt hypothalamic rather than floorplate identity. Development. 2004;131(23):5923–5933. doi: 10.1242/dev.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benzler J, Andrews ZB, Pracht C, Stohr S, Shepherd PR, Grattan DR, Tups A. Hypothalamic WNT signalling is impaired during obesity and reinstated by leptin treatment in male mice. Endocrinology. 2013;154(12):4737–4745. doi: 10.1210/en.2013-1746. [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Lee JE, Dorsky RI. Identification of Wnt-responsive cells in the zebrafish hypothalamus. Zebrafish. 2009;6(1):49–58. doi: 10.1089/zeb.2008.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407(6806):913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dessaud E, Ribes V, Balaskas N, Yang LL, Pierani A, Kicheva A, Novitch BG, Briscoe J, et al. Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen sonic hedgehog. Plos Biology. 2010;8(6) doi: 10.1371/journal.pbio.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, et al. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450(7170):717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- 71.Alvarez-Bolado G, Paul FA, Blaess S. Sonic hedgehog lineage in the mouse hypothalamus: from progenitor domains to hypothalamic regions. Neural Dev. 2012;7:4. doi: 10.1186/1749-8104-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Szabo NE, Zhao T, Cankaya M, Theil T, Zhou X, Alvarez-Bolado G. Role of neuroepithelial Sonic hedgehog in hypothalamic patterning. J Neurosci. 2009;29(21):6989–7002. doi: 10.1523/JNEUROSCI.1089-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haddad-Tovolli R, Paul FA, Zhang Y, Zhou X, Theil T, Puelles L, Blaess S, Alvarez-Bolado G. Differential requirements for Gli2 and Gli3 in the regional specification of the mouse hypothalamus. Front Neuroanat. 2015;9:34. doi: 10.3389/fnana.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manning L, Ohyama K, Saeger B, Hatano O, Wilson SA, Logan M, Placzek M. Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev Cell. 2006;11(6):873–885. doi: 10.1016/j.devcel.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 75.Ohyama K, Das R, Placzek M. Temporal progression of hypothalamic patterning by a dual action of BMP. Development. 2008;135(20):3325–3331. doi: 10.1242/dev.027078. [DOI] [PubMed] [Google Scholar]
- 76.Danesin C, Peres JN, Johansson M, Snowden V, Cording A, Papalopulu N, Houart C. Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1. Dev Cell. 2009;16(4):576–587. doi: 10.1016/j.devcel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 77.Hallonet M, Hollemann T, Wehr R, Jenkins NA, Copeland NG, Pieler T, Gruss P. Vax1 is a novel homeobox-containing gene expressed in the developing anterior ventral forebrain. Development. 1998;125(14):2599–2610. doi: 10.1242/dev.125.14.2599. [DOI] [PubMed] [Google Scholar]
- 78.Vacik T, Stubbs JL, Lemke G. A novel mechanism for the transcriptional regulation of Wnt signaling in development. Genes Dev. 2011;25(17):1783–1795. doi: 10.1101/gad.17227011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeong Y, Leskow FC, El-Jaick K, Roessler E, Muenke M, Yocum A, Dubourg C, Li X, et al. Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein. Nat Genet. 2008;40(11):1348–1353. doi: 10.1038/ng.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pearson CA, Placzek M. Development of the medial hypothalamus: forming a functional hypothalamic-neurohypophyseal interface. Curr Top Dev Biol. 2013;106:49–88. doi: 10.1016/B978-0-12-416021-7.00002-X. [DOI] [PubMed] [Google Scholar]
- 81.Pera EM, Kessel M. Demarcation of ventral territories by the homeobox gene NKX2.1 during early chick development. Dev Genes Evol. 1998;208(3):168–171. doi: 10.1007/s004270050170. [DOI] [PubMed] [Google Scholar]
- 82.Chuang JC, Mathers PH, Raymond PA. Expression of three Rx homeobox genes in embryonic and adult zebrafish. Mech Dev. 1999;84(1–2):195–198. doi: 10.1016/s0925-4773(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 83.Davis AM, Seney ML, Stallings NR, Zhao LP, Parker KL, Tobet SA. Loss of steroidogenic factor. 1 alters cellular topography in the mouse ventromedial nucleus of the hypothalamus. J Neurobiol. 2004;60(4):424–436. doi: 10.1002/neu.20030. [DOI] [PubMed] [Google Scholar]
- 84.Watson SJ, Barchas JD, Li CH. beta-Lipotropin: localization of cells and axons in rat brain by immunocytochemistry. Proc Natl Acad Sci U S A. 1977;74(11):5155–5158. doi: 10.1073/pnas.74.11.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mcnay DEG, Pelling M, Claxton S, Guillemot F, Ang SL. Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol Endocrinol. 2006;20(7):1623–1632. doi: 10.1210/me.2005-0518. [DOI] [PubMed] [Google Scholar]
- 86.Sommer L, Shah N, Rao M, Anderson DJ. The cellular function of MASH1 in autonomic neurogenesis. Neuron. 1995;15(6):1245–1258. doi: 10.1016/0896-6273(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 87.Yi SH, Jo AY, Park CH, Koh HC, Park RH, Suh-Kim H, Shin I, Lee YS, et al. Mash1 and neurogenin. 2 enhance survival and differentiation of neural precursor cells after transplantation to rat brains via distinct modes of action. Mol Ther. 2008;16(11):1873–1882. doi: 10.1038/mt.2008.189. [DOI] [PubMed] [Google Scholar]
- 88.Voronova A, Fischer A, Ryan T, Al Madhoun A, Skerjanc IS. Ascl1/Mash1 is a novel target of Gli2 during Gli2-induced neurogenesis in P19 EC cells. Plos One. 2011;6(4) doi: 10.1371/journal.pone.0019174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pelling M, Anthwal N, McNay D, Gradwohl G, Leiter AB, Guillemot F, Ang SL. Differential requirements for neurogenin. 3 in the development of POMC and NPY neurons in the hypothalamus. Dev Biol. 2011;349(2):406–416. doi: 10.1016/j.ydbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 90.Anthwal N, Pelling M, Claxton S, Mellitzer G, Collin C, Kessaris N, Richardson WD, Gradwohl G, et al. Conditional deletion of neurogenin-3 using Nkx2.1iCre results in a mouse model for the central control of feeding, activity and obesity. Disease Models Mechanisms. 2013;6(5):1133–1145. doi: 10.1242/dmm.011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scerbo MJ, Freire-Regatillo A, Cisternas CD, Brunotto M, Arevalo MA, Garcia-Segura LM, Cambiasso MJ. Neurogenin. 3 mediates sex chromosome effects on the generation of sex differences in hypothalamic neuronal development. Front Cell Neurosci. 2014;8:188. doi: 10.3389/fncel.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Good DJ, Porter FD, Mahon KA, Parlow AF, Westphal H, Kirsch IR. Hypogonadism and obesity in mice with a targeted deletion of the Nhlh2 gene. Nat Genet. 1997;15(4):397–401. doi: 10.1038/ng0497-397. [DOI] [PubMed] [Google Scholar]
- 93.Good DJ, Braun T. NHLH2: at the intersection of obesity and fertility. Trends Endocrinol Metab. 2013;24(8):385–390. doi: 10.1016/j.tem.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor. 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9(4):478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- 95.Kim KW, Zhao L, Parker KL. Central nervous system-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol. 2009;300(1–2):132–136. doi: 10.1016/j.mce.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 96.Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor. 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143(2):607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 97.Zhao L, Bakke M, Hanley NA, Majdic G, Stallings NR, Jeyasuria P, Parker KL. Tissue-specific knockouts of steroidogenic factor 1. Mol Cell Endocrinol. 2004;215(1–2):89–94. doi: 10.1016/j.mce.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 98.Kim KW, Zhao L, Donato J, Jr, Kohno D, Xu Y, Elias CF, Lee C, Parker KL, et al. Steroidogenic factor. 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci U S A. 2011;108(26):10673–10678. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao LP, Kim KW, Ikeda Y, Anderson KK, Beck L, Chase S, Tobet SA, Parker KL. Central nervous system-specific knockout of steroidogenic factor. 1 results in increased anxiety-like behavior. Mol Endocrinol. 2008;22(6):1403–1415. doi: 10.1210/me.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clark DD, Gorman MR, Hatori M, Meadows JD, Panda S, Mellon PL. Aberrant development of the suprachiasmatic nucleus and circadian rhythms in mice lacking the homeodomain protein Six6. J Biol Rhythms. 2013;28(1):15–25. doi: 10.1177/0748730412468084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roy A, De Melo J, Chaturvedi D, Thein T, Cabrera-Socorro A, Houart C, Meyer G, Blackshaw S, et al. LHX2 is necessary for the maintenance of optic identity and for the progression of optic morphogenesis. J Neurosci. 2013;33(16):6877–6884. doi: 10.1523/JNEUROSCI.4216-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.VanDunk C, Hunter LA, Gray PA. Development, maturation, and necessity of transcription factors in the mouse suprachiasmatic nucleus. J Neurosci. 2011;31(17):6457–6467. doi: 10.1523/JNEUROSCI.5385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bedont JL, et al. Lhx1 controls terminal differentiation and circadian function of the suprachiasmatic nucleus. Cell Reports. 2014;5(8):609–622. doi: 10.1016/j.celrep.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 105.Morales-Delgado N, Merchan P, Bardet SM, Ferran JL, Puelles L, Diaz C. Topography of somatostatin gene expression relative to molecular progenitor domains during ontogeny of the mouse hypothalamus. Front Neuroanat. 2011;5:10. doi: 10.3389/fnana.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morales-Delgado N, Castro-Robles B, Ferran JL, Martinez-de-la-Torre M, Puelles L, Diaz C. Regionalized differentiation of CRH, TRH, and GHRH peptidergic neurons in the mouse hypothalamus. Brain Struct Funct. 2014;219(3):1083–1111. doi: 10.1007/s00429-013-0554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang W, Lufkin T. The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev Biol. 2000;227(2):432–449. doi: 10.1006/dbio.2000.9902. [DOI] [PubMed] [Google Scholar]
- 108.Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Vaccarino FM, Michaud J, Simeone A. Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 1999;13(21):2787–2800. doi: 10.1101/gad.13.21.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blechman J, Borodovsky N, Eisenberg M, Nabel-Rosen H, Grimm J, Levkowitz G. Specification of hypothalamic neurons by dual regulation of the homeodomain protein Orthopedia. Development. 2007;134(24):4417–4426. doi: 10.1242/dev.011262. [DOI] [PubMed] [Google Scholar]
- 110.Del Giacco L, Sordino P, Pistocchi A, Andreakis N, Tarallo R, Di Benedetto B, Cotelli F. Differential regulation of the zebrafish orthopedia1 gene during fate determination of diencephalic neurons. BMC Dev Biol. 2006;6 doi: 10.1186/1471-213X-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amir-Zilberstein L, Blechman J, Sztainberg Y, Norton WNJ, Reuveny A, Borodovsky N, Tahor M, Bonkowsky JL, et al. Homeodomain protein Otp and activity-dependent splicing modulate neuronal adaptation to stress. Neuron. 2012;73(2):279–291. doi: 10.1016/j.neuron.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Michaud JL, DeRossi C, May NR, Holdener BC, Fan CM. ARNT2 acts as the dimerization partner of SIM1 for the development of the hypothalamus. Mech Dev. 2000;90(2):253–261. doi: 10.1016/s0925-4773(99)00328-7. [DOI] [PubMed] [Google Scholar]
- 113.Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLHPAS transcription factor SIM1. Genes Dev. 1998;12(20):3264–3275. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jo YH, Chua S., Jr Transcription factors in the development of medial hypothalamic structures. Am J Physiol Endocrinol Metab. 2009;297(3):E563–567. doi: 10.1152/ajpendo.00064.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goshu E, Jin H, Fasnacht R, Sepenski M, Michaud JL, Fan CM. Sim2 mutants have developmental defects not overlapping with those of Sim1 mutants. Mol Cell Biol. 2002;22(12):4147–4157. doi: 10.1128/MCB.22.12.4147-4157.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakai S, Kawano H, Yudate T, Nishi M, Kuno J, Nagata A, Jishage K, Hamada H, et al. The POU domain transcription factor Brn-2 is required for the determination of specific neuronal lineages in the hypothalamus of the mouse. Genes Dev. 1995;9(24):3109–3121. doi: 10.1101/gad.9.24.3109. [DOI] [PubMed] [Google Scholar]
- 117.Faivre L, Cormier-Daire V, Lapierre JM, Colleaux L, Jacquemont S, Genevieve D, Saunier P, Munnich A, et al. Deletion of the SIM1 gene (6q16.2) in a patient with a Prader-Willi-like pheno-type. J Med Genet. 2002;39(8):594–596. doi: 10.1136/jmg.39.8.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bonnefond A, Raimondo A, Stutzmann F, Ghoussaini M, Ramachandrappa S, Bersten DC, Durand E, Vatin V, et al. Loss-of-function mutations in SIM1 contribute to obesity and Prader-Willi-like features. J Clin Invest. 2013;123(7):3037–3041. doi: 10.1172/JCI68035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Varela MC, Simoes-Sato AY, Kim CA, Bertola DR, De Castro CI, Koiffmann CP. A new case of interstitial 6q16.2 deletion in a patient with Prader-Willi-like phenotype and investigation of SIM1 gene deletion in. 87 patients with syndromic obesity. Eur J Med Genet. 2006;49(4):298–305. doi: 10.1016/j.ejmg.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 120.Ramachandrappa S, Raimondo A, Cali AM, Keogh JM, Henning E, Saeed S, Thompson A, Garg S, et al. Rare variants in single-minded 1 (SIM1) are associated with severe obesity. J Clin Invest. 2013;123(7):3042–3050. doi: 10.1172/JCI68016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22(7):1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal Sim1 deficiency causes hyper-phagic obesity and reduced Mc4r and oxytocin expression. J Neurosci. 2010;30(10):3803–3812. doi: 10.1523/JNEUROSCI.5444-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, Levy E, Mitchell GA, Himms-Hagen J, et al. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet. 2001;10(14):1465–1473. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- 124.Kublaoui BM, Holder JL, Jr, Tolson KP, Gemelli T, Zinn AR. SIM1 overexpression partially rescues agouti yellow and diet-induced obesity by normalizing food intake. Endocrinology. 2006;147(10):4542–4549. doi: 10.1210/en.2006-0453. [DOI] [PubMed] [Google Scholar]
- 125.Vogt MC, Paeger L, Hess S, Steculorum SM, Awazawa M, Hampel B, Neupert S, Nicholls HT, et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell. 2014;156(3):495–509. doi: 10.1016/j.cell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deiner MS, Sretavan DW. Altered midline axon pathways and ectopic neurons in the developing hypothalamus of netrin-1-and DCC-deficient mice. J Neurosci. 1999;19(22):9900–9912. doi: 10.1523/JNEUROSCI.19-22-09900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bian S, Xu TL, Sun T. Tuning the cell fate of neurons and glia by microRNAs. Curr Opin Neurobiol. 2013;23(6):928–934. doi: 10.1016/j.conb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bian S, Sun T. Functions of noncoding RNAs in neural development and neurological diseases. Mol Neurobiol. 2011;44(3):359–373. doi: 10.1007/s12035-011-8211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Iyengar BR, Choudhary A, Sarangdhar MA, Venkatesh KV, Gadgil CJ, Pillai B. Non-coding RNA interact to regulate neuronal development and function. Front Cell Neurosci. 2014;8:47. doi: 10.3389/fncel.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meister B, Herzer S, Silahtaroglu AI. MicroRNAs in the hypothalamus. Neuroendocrinology. 2013;98(4):243–253. doi: 10.1159/000355619. [DOI] [PubMed] [Google Scholar]
- 131.Amar L, Benoit C, Beaumont G, Vacher CM, Crepin D, Taouis M, Baroin-Tourancheau A. MicroRNA expression profiling of hypothalamic arcuate and paraventricular nuclei from single rats using Illumina sequencing technology. J Neurosci Methods. 2012;209(1):134–143. doi: 10.1016/j.jneumeth.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 132.Cimadamore F, Amador-Arjona A, Chen C, Huang CT, Terskikh AV. SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc Natl Acad Sci U S A. 2013;110(32):E3017–3026. doi: 10.1073/pnas.1220176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schneeberger M, Altirriba J, Garcia A, Esteban Y, Castano C, Garcia-Lavandeira M, Alvarez CV, Gomis R, et al. Deletion of miRNA processing enzyme Dicer in POMC-expressing cells leads to pituitary dysfunction, neurodegeneration and development of obesity. Mol Metab. 2012;2(2):74–85. doi: 10.1016/j.molmet.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]