Abstract
Stress-related mucosal disease is a typical complication of critically ill patients in the intensive care unit (ICU). It poses a risk of clinically relevant upper gastrointestinal (GI) bleeding. Therefore, stress ulcer prophylaxis (SUP) is recommended in high-risk patients, especially those mechanically ventilated > 48 h and those with a manifest coagulopathy. Proton pump inhibitors (PPI) and, less effectively, histamine 2 receptor antagonists (H2RA) prevent GI bleeding in critically ill patients in the ICU. However, the routine use of pharmacological SUP does not reduce overall mortality in ICU patients. Moreover, recent studies revealed that SUP in the ICU might be associated with potential harm such as an increased risk of infectious complications, especially nosocomial pneumonia and Clostridium difficile-associated diarrhea. Additionally, special populations such as patients with liver cirrhosis may even have an increased mortality rate if treated with PPI. Likewise, PPI can be toxic for both the liver and the bone marrow, and some PPI show clinically relevant interactions with important other drugs like clopidogrel. Therefore, the agent of choice, the specific balance of risks and benefits for individual patients as well as the possible dose of PPI has to be chosen carefully. Alternatives to PPI prophylaxis include H2RA and/or sucralfate. Instead of routine SUP, further trials should investigate risk-adjusted algorithms, balancing benefits and threats of SUP medication in the ICU.
Keywords: Proton pump inhibitors, Clostridium difficile, Intensive care unit, Gastrointestinal hemorrhage, Stress, Histamine H2 antagonists, Risk assessment, Pneumonia, Physiological, Sucralfate
Core tip: To prevent gastrointestinal (GI) bleeding due to stress-related mucosal disease, critically ill patients are often routinely treated with proton pump inhibitors (PPI) or histamine 2 receptor antagonists (H2RA) for stress ulcer prophylaxis (SUP) in the intensive care unit (ICU). While major GI bleeding is currently rare in the ICU, SUP has not improved the overall survival of ICU patients in large clinical trials. Moreover, PPI and H2RA pose significant risks including toxicity, drug-drug-interactions and infectious complications (e.g., nosocomial pneumonia or Clostridium difficile-associated diarrhea). Instead of routine SUP, risk-adjusted algorithms may better balance benefits and threats of SUP in the ICU.
INTRODUCTION
The gastric mucosa is sensitive to both hemodynamic changes and inflammatory signals in critical illness. The term stress-related mucosal disease (SRMD) has been introduced to describe the resulting mucosal damage ranging from single lesions to multiple gastric ulcers that may lead to major bleeding complications in critical ill patients[1].
With proton pump inhibitors (PPI) and histamine 2 receptor antagonists (H2RA) potent options for pharmacological prophylaxis of such lesions are available. Both are able to decrease the risk of a bleeding event effectively[2] and are usually well tolerated. However, pharmacological stress ulcer prophylaxis (SUP) in the intensive care unit (ICU) has not translated into a mortality benefit in prospective trials. Thus, recently, some intensivists have expressed concerns about the safety of SUP, especially with respect to infectious complications.
EPIDEMIOLOGY
SRMD, as defined by clinical, endoscopic or histological characteristics, is present in most critically ill patients[3]. However, only a few patients experience overt bleeding complications. The fraction of ICU patients with SMRD-related gastrointestinal (GI) bleeding has been reported to be as high as 17% in earlier trials and in patients without prophylaxis[4,5] but has remarkably decreased at present to rates as low as 1% or below[2,6,7].
PATHOPHYSIOLOGY
In most critically ill patients, the gastric mucosal blood flow is impaired. Reasons include systemic hemodynamic changes (hypotension and/or vasopressor therapy) and/or local alterations, e.g., reduced splanchnic blood flow because of positive end-expiratory pressure in mechanical ventilated patients[8]. In addition to the ischemic tissue damage itself, hypoperfusion leads to a reduced production of several protective mechanisms that exist in a healthy stomach (Figure 1)[4]. The latter include various components such as mucus, phospholipids, bicarbonate, trefoil factor family peptides and heat-shock proteins[9]. For example, gastric ischemia/reperfusion in an experimental rat model led to an inhibition of both cyclooxygenase and lipoxygenase pathways, resulting in lower prostaglandin levels (especially PGE2), lower bicarbonate levels and decreased gastric mucosal defense[10,11]. Moreover, two important molecular regulators of vascular tension are dysregulated in critical illness. While the production of the vasodilator nitric oxide is reduced, the level of endothelin-1, a strong vasoconstrictor, is significantly increased[12,13]. This shift can further harm the mucosa.
Figure 1.
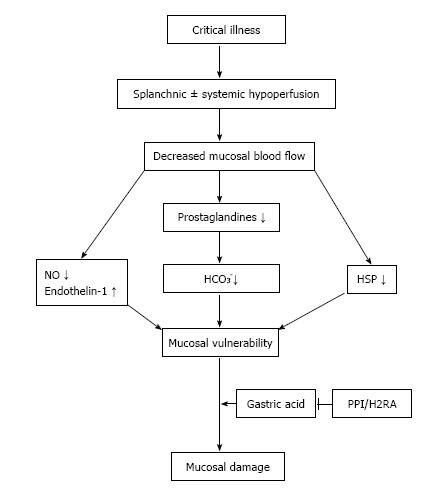
Pathophysiology of stress-related mucosal disease and rationale for the routine use of proton pump inhibitor/histamine 2 receptor antagonists at the intensive care unit. NO: Nitric oxide; PPI: Proton pump inhibitor(s); H2RA: Histamine 2 receptor antagonists; HSP: Heat-shock proteins; HCO3−: Bicarbonate.
While these mechanisms can cause mucosal damage, they are often insufficient by themselves to cause major ulcerations and gastric bleeding. A crucial component for overt damage is the presence of gastric acid. Without acid, mucosal damage is only minimal. In animal models of gastric ischemia, the addition of acid increased the damage by factor of ten[12]. This provides the rationale for the use of acid-suppressive drugs such as PPI or H2RA for pharmacological prophylaxis.
MORTALITY RISK OF STRESS ULCER-RELATED BLEEDING
An acute bleeding episode due to a stress ulcer is associated with an increased risk of death in the ICU. In a large prospective trial by Cook et al[14] the mortality of patients with stress ulcer bleeding was 49% compared to 9% in those without an episode of GI bleeding. This latter figure, however, appears unusually low for a general ICU population, raising the concern that related co-factors (e.g., co-morbidities, medication) might have affected the mortality risk of ICU patients who experienced bleeding.
Moreover, the patients in this study mainly underwent cardiovascular surgery and only 1.6% presented with sepsis, provoking the question whether the numbers can be extrapolated to other settings of critical illness[14]. Nonetheless, a more recent study by the same authors using multivariate analysis for adjustment showed an increased relative risk (RR) of 1 to 4 (dependent on the model used) as well as an extension of the ICU stay by up to eight days in ICU patients with GI hemorrhage[15].
In contrast to these findings, in a more recent study including 1034 patients in 97 ICUs, GI bleeding was not associated with an increased mortality in multivariate analysis after adjusting for severity of comorbidity, other organ failure and age[7], in line with two meta-analyses reported in 2012 and 2013[2,16]. However, these recent studies all reported a very low incidence of stress ulcer-related bleeding due to effective pharmacological and non-pharmacological prophylactic measures, which may not allow proper assessment of true mortality risk.
RISK FACTORS FOR STRESS ULCER-RELATED BLEEDING
Multiple investigations have been conducted to identify patients at risk for stress ulcer-related bleeding. A large, prospective multicenter trial of 2252 ICU patients was able to identify at multiple regression two main risk factors: mechanical ventilation (OR = 15.6; P < 0.001) and coagulopathy (OR = 4.3; P < 0.001). In the absence of both risk factors the bleeding rate was as low as 0.1%[14]. A smaller, earlier trial came to the same conclusion[17]. A more recent inception cohort study (n = 1034) identified the presence of more than three or more comorbidities (OR = 8.9; 95%CI: 2.7-28.8), liver disease (OR = 7.6; 95%CI: 3.3-17.6); use of renal replacement therapy (OR = 6.9; 95%CI: 2.7-17.5); a coexisting (OR = 5.2; 95%CI: 2.3-11.8) or acute coagulopathy (OR = 4.2; 95%CI: 1.7-10.2) and higher SOFA-score (OR = 1.4; 95%CI: 1.2-1.6) as significant risk factors after multivariate analysis. Interestingly, mechanical ventilation was not associated with an increased risk of GI bleeding in this trial[7].
Other risk factors with a lower degree of evidence include patients with severe head trauma, those who have had extended surgeries with operation times exceeding 4 h as well as patients with acute kidney or hepatic failure, sepsis, hypotension, a history of gastrointestinal bleeding, high-dose corticosteroids, burn patients, advanced age and male sex[1,3,17,18]. This wide spectrum of suggested risk factors has prompted the rather unselected use of pharmacological SUP in the ICU setting, resulting in the routine use of PPI and/or H2RAs in > 80% of critically ill patients as reported in in many observational studies[6,7].
INDICATIONS FOR PHARMACOLOGICAL PROPHYLAXIS
While SRMD-related bleeding can have severe clinical impact, acid-suppressive medication effectively decreases bleeding rates as demonstrated by multiple meta-analyses on this topic[19-22]. Although the quality of the available data has been criticized[23], both national and international guidelines recommend stress ulcer prophylaxis (SUP) in critically ill patients with sepsis and other risk factors[24,25].
In our ICU, patients with at least one of the following risk factors are recommended to receive pharmacological ulcer prophylaxis based upon current evidence: Mechanical ventilation, coagulopathy, history of an upper gastrointestinal bleeding within the past 12 mo, severe sepsis or septic shock, or cardiogenic shock. Additionally, we consider ulcer prophylaxis for the following patients based on weaker evidence: burn patients, those with cranio-cerebral injury, acute renal failure, known peptic ulcer disease, those post kidney or liver transplantation and patients taking non-steroidal anti-inflammatory drugs (NSAID) or high-dose glucocorticoids. The algorithm that we propose for SUP in the ICU is presented as Figure 2.
Figure 2.

Proposed algorithm for stress ulcer prophylaxis. For the different indications for SUP, the level of evidence is provided [A: Multiple randomized trials or meta-analysis, B: Single randomized or large non-randomized trial(s), C: Expert opinion or retrospective studies]. GI: Gastrointestinal; ICU: Intensive care unit; INR: International normalized ratio; NO: Nitric oxide; NSAID: Nonsteroidal anti-inflammatory drugs; PLT: Platelets; PTT: Partial thromboplastin time; SUP: Stress ulcer prophylaxis.
However, it is mandatory to frequently re-evaluate the individual indication both during and after ICU stay. Buckley et al[26] could show that 14.4% of patients in an ICU received acid suppression without proper indication, which resulted in unnecessary risk of side effects (see below) and unnecessary costs (> 200000 dollar annually in the study hospital).
While prophylaxis effectively decreases the risk of stress ulcer-related bleeding, it is important to stress that no single trial and/or meta-analysis has been able to convincingly demonstrate a benefit regarding survival. Outside an ICU or even in outpatients, very little evidence supports the use of stress ulcer prophylaxis; for instance, patients with cardiovascular diseases who have concomitant newly prescribed with the oral anticoagulant dabigatran may be at lower risk for severe GI bleedings if PPI are administered[27]. Without a proper indication or a clear high-risk assessment, SUP should be discontinued, because it might cause unnecessary harm (see below) as well as costs[22].
PHARMACOLOGICAL PROPHYLAXIS
If a stress ulcer prophylaxis is necessary, different options are available: Options include the acid-suppressing drugs, PPI and H2RA, or the mucosa-protective agent sucralfate. Sucralfate is a reasonable option and reduces the risk of stress ulcer-related bleeding. However, a large trial revealed its inferiority to H2RA[28], so that an acid-suppressive medication is preferred for SUP.
There are several trials and meta-analyses comparing PPI to H2RA. Most of them favor PPI with respect to reduction of bleeding rates (Table 1). Regarding mortality, no analysis has been able to show a significant difference. Currently, PPI are the agents of choice in SUP.
Table 1.
Efficacy of proton pump inhibitor compared to histamine 2 receptor antagonists at the intensive care unit
| Meta-analysis | n | Risk reduction (bleeding) | Risk reduction (mortality) |
| Alhazzani et al[2] | 1720 | RR = 0.36 | RR = 1.01 |
| (95%CI: 0.19-0.67) | (95%CI: 0.83-1.24) | ||
| Pongprasobchai et al[59] | 569 | OR = 0.42 | n/a |
| (95%CI: 0.20-0.91) | |||
| Barkun et al[60] | 1587 | OR = 0.30 | OR = 1.19 |
| (95%CI: 0.17-0.54) | (95%CI: 0.84-1.68) | ||
| Lin et al[61] | 936 | RD = 0.04 | RD = 0.00 |
| (95%CI: 0.09-0.01) | (95%CI: 0.04-0.05) |
n/a: Not assessed; n: Patients included in the meta-analysis; RR: Relative risk; OR: Odds ratio; PPI: Proton pump inhibitor(s); RD: Risk difference.
ADVERSE EVENTS
Gastric acid is a natural physiological barrier against ingested pathogens. Pharmacological acid suppression alters this barrier significantly. Subsequently, it is associated with gastric and duodenal bacterial overgrowth[29]. This effect is stronger in patients receiving PPI than in those taking H2RA[30]. The loss of this natural barrier may lead to intestinal (e.g., Clostridium difficile-associated diarrhea), but also to extra-intestinal infections (e.g., pneumonia, possibly via retrograde microaspiration). In addition, both PPI and H2RA potentially affect leucocyte function: Experimental studies have shown an effect of these drugs on both phagocytosis by neutrophils itself and the acidification of the phagolysosome in neutrophils necessary to kill its contents[31,32].
As the effects of acid-suppressing drugs may render patients susceptible for infections, two main complications have to be considered: Clostridium difficile-associated diarrhea (CDAD) and pneumonia. In outpatients and patients on standard care wards, it has been shown that PPI increase the risk of both significantly[6,33-44]. Additionally, experiments in mice suggest that acid suppression favors intestinal colonization with multi-resistant bacteria such as Vancomycin-resistant Enterococcus faecium (VRE) or multi-resistant Klebsiella pneumonia[45].
In the setting of SUP in the ICU, the data are controversial (Table 2). Two meta-analyses failed to show any effect on the rate of nosocomial and/or ventilator-associated pneumonia[2,16]. However, only seven of the original studies included reported on pneumonia. In contrast, a small (n = 137) but prospective and randomized trial showed a strong increase in ventilator-associated pneumonia within the PPI group compared to placebo (36.4% vs 14.1%, P < 0.001)[46].
Table 2.
Acid suppression as a risk factor for pneumonia at the intensive care unit
| Acid suppression as a risk factor for | Pneumonia | |
| Barkun et al[16] | Meta-analysis | OR = 1.05 (95%CI: 0.69-1.62) |
| Alhazzani et al[2] | Meta-analysis | RR = 1.06 (95%CI: 0.73-1.52) |
| Khorvash et al[6] | Randomized controlled trial | 14.1% without vs 36.4% with PPI, P < 0.001 |
| Buendgens et al[6] | Retrospective cohort study | OR = 1.28 (95%CI: 0.95-1.73) |
OR: Odds ratio; RR: Relative risk; PPI: Proton pump inhibitor.
A retrospective study from our group found a significant association of PPI with pneumonia only by univariate but not by multivariate analysis[6]. A prevalence study including over 10000 patients from 17 countries identified SUP as an independent risk factor for infections[47]. Thus, the role of acid suppression as a risk factor for pneumonia is unclear but remains likely. Larger randomized prospective trials are warranted to resolve this issue.
The main infection route of C. difficile is via ingestion of its spores and its vegetative forms. While the spores are naturally resistant to acid, the vegetative form is normally killed by acid in the stomach. If the stomach pH is raised above 5, Clostridia species show drastically improved survival. Given that the stool of infected individuals contains tenfold more vegetative forms than spores, this might explain an association of PPI and H2RA with CDAD[48].
Although no prospective data is available on this matter for critically ill patients, studies suggest an association between pharmacological SUP and CDAD in the ICU (Table 3). A small case-control study showed a positive association between the duration of PPI therapy and the risk of CDAD[49]. A retrospective study with 3286 ICU patients demonstrated PPI as an independent risk factor for CDAD by multivariate analysis (OR = 3.11; 95%CI: 1.11-8.74), comparable to the risk for CDAD associated with the use of fluoroquinolones or third-generation cephalosporins. Moreover, in this trial an ICU-onset CDAD was associated with an increased mortality (OR = 1.59; 95%CI: 1.06-2.41)[6]. Another recent study from Canada revealed a significant association with CDAD recurrence rates and continuation of PPI therapy (OR = 1.5; 95%CI: 1.1-2.0), similar to antibiotic reexposure (OR = 1.3; 95%CI: 0.9-1.7)[50].
Table 3.
Proton pump inhibitor as a risk factor for Clostridium difficile-associated diarrhea at the intensive care unit
| PPI as a risk factor for | Clostridium difficile-associated diarrhea (OR, 95%CI) | |
| Barletta et al[49] | Case control study | 1.14 (1.02-1.27) |
| Buendgens et al[6] | Retrospective cohort study | 3.11 (1.11-8.74) |
OR: Odds ratio; PPI: Proton pump inhibitor.
Patients with liver cirrhosis appear to pose a population particularly prone to adverse effects of SUP. A prospective study including 272 patients with cirrhosis found the use of PPI to be an independent risk factor for overall mortality by multivariate analysis in those patients (HR = 2.3; 95%CI: 1.3-4.3)[51]. Reasons for this might be an increased risk of spontaneous bacterial peritonitis in addition to higher rates of pneumonia and CDAD[52-54].
Drug-drug-interactions are another concern for using PPI, especially in ICU patients. An important possible interaction exists between the antiplatelet agent clopidogrel and various PPI. In 2009, a study reported increased cardiovascular events in patients taking both clopidogrel and PPI[55]. The antiplatelet agent clopidogrel is a prodrug, dependent on the enzyme CYP2C19. In vitro PPI inhibit CYP2C19 and potentially inhibit clopidogrel. It remains unclear if this experimental finding is of clinical importance, since the patients with concomitant use of PPI and clopidogrel might have had a higher intrinsic risk due to greater age and more cardiovascular risk factors. In order to overcome this potential interaction, independent ingestion times, the use of pantoprazole (a PPI with low interaction potential) and/or replacing clopidogrel with ticagrelor, which is not a prodrug, have been suggested.
Other side effects of PPI potentially relevant for critically ill patients include toxicity to liver or bone marrow and hypomagnesaemia. The latter has resulted in a recent warning from the Food and Drug Administration of the United States[56]. Osteopenia, another known association, seems less important acutely in ICU patients[57]. It is currently unknown if those adverse effects affect the prognosis of patients in an ICU.
ENTERAL NUTRITION
With regard to the potential adverse effects of SUP as described above, potential alternatives have been discussed. One should also keep in mind that both PPI and H2RA do not have a direct effect on the SRMD pathophysiology of reduced blood flow and altered balance between vasoconstrictors and dilatators (Figure 1). Enteral nutrition, in contrast, potentially has a positive impact on both[58]. Enteral nutrition could therefore be a viable alternative to pharmacological SUP. However, no prospective data is available on this subject. A meta-analysis of data available on 1836 patients disclosed that in presence of enteral nutrition a pharmacological SUP did not significantly change the risk of stress ulcer-related bleeding. Interestingly, in those patients that were enterally fed and treated with SUP the risk of pneumonia was increased (OR = 2.81; 95%CI: 1.2-6.6) compared to patients on parenteral nutrition. In this subgroup, even an increase in mortality was observed[21]. Therefore, the role of enteral nutrition in SUP should be further explored in randomized prospective trials.
CONCLUSION
Critically ill patients often develop gastrointestinal lesions due to altered perfusion of the gastric mucosa, reduced protective mucosal factors and increased gastric acid, rendering them at risk for GI bleeding due to SRMD or ulcers. Pharmacological SUP is performed in the majority of ICU patients at present, with PPI or H2RA effectively preventing GI bleeding. However, this common practice is currently debated, due to the fact that SUP does not significantly improve mortality of ICU patients, while acid suppression poses relevant risks. Specifically, nosocomial pneumonia and Clostridium difficile associated diarrhea are potential serious complications of SUP. Thus, SUP should follow a clear algorithm balancing risks and benefits (Figure 2). Alternative strategies like enteral feeding or restricting SUP to the early phase of ICU treatment or to patients with an exceptional high-risk profile deserve evaluation in prospective randomized trials.
Footnotes
Supported by The German Research Foundation, No. DFG Ta434/5-1; and the Interdisciplinary Center for Clinical Research (IZKF) Aachen.
Conflict-of-interest statement: The authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 9, 2015
First decision: November 4, 2015
Article in press: January 7, 2016
P- Reviewer: Kozarek R, Li YY, Manguso F, Rabago L S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
References
- 1.Stollman N, Metz DC. Pathophysiology and prophylaxis of stress ulcer in intensive care unit patients. J Crit Care. 2005;20:35–45. doi: 10.1016/j.jcrc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Alhazzani W, Alenezi F, Jaeschke RZ, Moayyedi P, Cook DJ. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2013;41:693–705. doi: 10.1097/CCM.0b013e3182758734. [DOI] [PubMed] [Google Scholar]
- 3.Bardou M, Quenot JP, Barkun A. Stress-related mucosal disease in the critically ill patient. Nat Rev Gastroenterol Hepatol. 2015;12:98–107. doi: 10.1038/nrgastro.2014.235. [DOI] [PubMed] [Google Scholar]
- 4.Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Shuman RB, Schuster DP, Zuckerman GR. Prophylactic therapy for stress ulcer bleeding: a reappraisal. Ann Intern Med. 1987;106:562–567. doi: 10.7326/0003-4819-106-4-562. [DOI] [PubMed] [Google Scholar]
- 6.Buendgens L, Bruensing J, Matthes M, Dückers H, Luedde T, Trautwein C, Tacke F, Koch A. Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridium difficile-associated diarrhea. J Crit Care. 2014;29:696.e11–696.e15. doi: 10.1016/j.jcrc.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Krag M, Perner A, Wetterslev J, Wise MP, Borthwick M, Bendel S, McArthur C, Cook D, Nielsen N, Pelosi P, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med. 2015;41:833–845. doi: 10.1007/s00134-015-3725-1. [DOI] [PubMed] [Google Scholar]
- 8.Fujita Y. Effects of PEEP on splanchnic hemodynamics and blood volume. Acta Anaesthesiol Scand. 1993;37:427–431. doi: 10.1111/j.1399-6576.1993.tb03742.x. [DOI] [PubMed] [Google Scholar]
- 9.de Foneska A, Kaunitz JD. Gastroduodenal mucosal defense. Curr Opin Gastroenterol. 2010;26:604–610. doi: 10.1097/MOG.0b013e32833f1222. [DOI] [PubMed] [Google Scholar]
- 10.Nakagiri A, Murakami M. Roles of NADPH oxidase in occurrence of gastric damage and expression of cyclooxygenase-2 during ischemia/reperfusion in rat stomachs. J Pharmacol Sci. 2009;111:352–360. doi: 10.1254/jphs.09169fp. [DOI] [PubMed] [Google Scholar]
- 11.Peskar BM, Ehrlich K, Schuligoi R, Peskar BA. Role of lipoxygenases and the lipoxin A(4)/annexin 1 receptor in ischemia-reperfusion-induced gastric mucosal damage in rats. Pharmacology. 2009;84:294–299. doi: 10.1159/000244017. [DOI] [PubMed] [Google Scholar]
- 12.Michida T, Kawano S, Masuda E, Kobayashi I, Nishimura Y, Tsujii M, Takei Y, Tsuji S, Nagano K, Fusamoto H, et al. Endothelin-1 in the gastric mucosa in stress ulcers of critically ill patients. Am J Gastroenterol. 1997;92:1177–1181. [PubMed] [Google Scholar]
- 13.Björne H, Govoni M, Törnberg DC, Lundberg JO, Weitzberg E. Intragastric nitric oxide is abolished in intubated patients and restored by nitrite. Crit Care Med. 2005;33:1722–1727. doi: 10.1097/01.ccm.0000171204.59502.aa. [DOI] [PubMed] [Google Scholar]
- 14.Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R, Winton TL, Rutledge F, Todd TJ, Roy P. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med. 1994;330:377–381. doi: 10.1056/NEJM199402103300601. [DOI] [PubMed] [Google Scholar]
- 15.Cook DJ, Griffith LE, Walter SD, Guyatt GH, Meade MO, Heyland DK, Kirby A, Tryba M. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care. 2001;5:368–375. doi: 10.1186/cc1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barkun AN, Bardou M, Pham CQ, Martel M. Proton pump inhibitors vs. histamine 2 receptor antagonists for stress-related mucosal bleeding prophylaxis in critically ill patients: a meta-analysis. Am J Gastroenterol. 2012;107:507–520; quiz 521. doi: 10.1038/ajg.2011.474. [DOI] [PubMed] [Google Scholar]
- 17.Schuster DP, Rowley H, Feinstein S, McGue MK, Zuckerman GR. Prospective evaluation of the risk of upper gastrointestinal bleeding after admission to a medical intensive care unit. Am J Med. 1984;76:623–630. doi: 10.1016/0002-9343(84)90286-9. [DOI] [PubMed] [Google Scholar]
- 18.Quenot JP, Thiery N, Barbar S. When should stress ulcer prophylaxis be used in the ICU? Curr Opin Crit Care. 2009;15:139–143. doi: 10.1097/MCC.0b013e32832978e0. [DOI] [PubMed] [Google Scholar]
- 19.Kahn JM, Doctor JN, Rubenfeld GD. Stress ulcer prophylaxis in mechanically ventilated patients: integrating evidence and judgment using a decision analysis. Intensive Care Med. 2006;32:1151–1158. doi: 10.1007/s00134-006-0244-0. [DOI] [PubMed] [Google Scholar]
- 20.Cook DJ, Reeve BK, Guyatt GH, Heyland DK, Griffith LE, Buckingham L, Tryba M. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA. 1996;275:308–314. [PubMed] [Google Scholar]
- 21.Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med. 2010;38:2222–2228. doi: 10.1097/CCM.0b013e3181f17adf. [DOI] [PubMed] [Google Scholar]
- 22.Messori A, Trippoli S, Vaiani M, Gorini M, Corrado A. Bleeding and pneumonia in intensive care patients given ranitidine and sucralfate for prevention of stress ulcer: meta-analysis of randomised controlled trials. BMJ. 2000;321:1103–1106. doi: 10.1136/bmj.321.7269.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krag M, Perner A, Wetterslev J, Wise MP, Hylander Møller M. Stress ulcer prophylaxis versus placebo or no prophylaxis in critically ill patients. A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med. 2014;40:11–22. doi: 10.1007/s00134-013-3125-3. [DOI] [PubMed] [Google Scholar]
- 24.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinhart K, Brunkhorst FM, Bone HG, Bardutzky J, Dempfle CE, Forst H, Gastmeier P, Gerlach H, Gründling M, John S, et al. [Prevention, diagnosis, treatment, and follow-up care of sepsis. First revision of the S2k Guidelines of the German Sepsis Society (DSG) and the German Interdisciplinary Association for Intensive and Emergency Care Medicine (DIVI)] Anaesthesist. 2010;59:347–370. doi: 10.1007/s00101-010-1719-5. [DOI] [PubMed] [Google Scholar]
- 26.Buckley MS, Park AS, Anderson CS, Barletta JF, Bikin DS, Gerkin RD, O’Malley CW, Wicks LM, Garcia-Orr R, Kane-Gill SL. Impact of a clinical pharmacist stress ulcer prophylaxis management program on inappropriate use in hospitalized patients. Am J Med. 2015;128:905–913. doi: 10.1016/j.amjmed.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Chan EW, Lau WC, Leung WK, Mok MT, He Y, Tong TS, Wong IC. Prevention of Dabigatran-Related Gastrointestinal Bleeding With Gastroprotective Agents: A Population-Based Study. Gastroenterology. 2015;149:586–595.e3. doi: 10.1053/j.gastro.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Cook D, Guyatt G, Marshall J, Leasa D, Fuller H, Hall R, Peters S, Rutledge F, Griffith L, McLellan A, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791–797. doi: 10.1056/NEJM199803193381203. [DOI] [PubMed] [Google Scholar]
- 29.Thorens J, Froehlich F, Schwizer W, Saraga E, Bille J, Gyr K, Duroux P, Nicolet M, Pignatelli B, Blum AL, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39:54–59. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K, Lin HJ, Perng CL, Tseng GY, Yu KW, Chang FY, Lee SD. The effect of H2-receptor antagonist and proton pump inhibitor on microbial proliferation in the stomach. Hepatogastroenterology. 2004;51:1540–1543. [PubMed] [Google Scholar]
- 31.Agastya G, West BC, Callahan JM. Omeprazole inhibits phagocytosis and acidification of phagolysosomes of normal human neutrophils in vitro. Immunopharmacol Immunotoxicol. 2000;22:357–372. doi: 10.3109/08923970009016425. [DOI] [PubMed] [Google Scholar]
- 32.Zedtwitz-Liebenstein K, Wenisch C, Patruta S, Parschalk B, Daxböck F, Graninger W. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit Care Med. 2002;30:1118–1122. doi: 10.1097/00003246-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107:1011–1019. doi: 10.1038/ajg.2012.108. [DOI] [PubMed] [Google Scholar]
- 34.Herzig SJ, Vaughn BP, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171:991–997. doi: 10.1001/archinternmed.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292:1955–1960. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther. 2010;31:1165–1177. doi: 10.1111/j.1365-2036.2010.04284.x. [DOI] [PubMed] [Google Scholar]
- 37.Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308–2313. doi: 10.1111/j.1572-0241.2008.01975.x. [DOI] [PubMed] [Google Scholar]
- 38.Giuliano C, Wilhelm SM, Kale-Pradhan PB. Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert Rev Clin Pharmacol. 2012;5:337–344. doi: 10.1586/ecp.12.20. [DOI] [PubMed] [Google Scholar]
- 39.Filion KB, Chateau D, Targownik LE, Gershon A, Durand M, Tamim H, Teare GF, Ravani P, Ernst P, Dormuth CR. Proton pump inhibitors and the risk of hospitalisation for community-acquired pneumonia: replicated cohort studies with meta-analysis. Gut. 2014;63:552–558. doi: 10.1136/gutjnl-2013-304738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ. 2011;183:310–319. doi: 10.1503/cmaj.092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 42.Tleyjeh IM, Bin Abdulhak AA, Riaz M, Alasmari FA, Garbati MA, AlGhamdi M, Khan AR, Al Tannir M, Erwin PJ, Ibrahim T, et al. Association between proton pump inhibitor therapy and clostridium difficile infection: a contemporary systematic review and meta-analysis. PLoS One. 2012;7:e50836. doi: 10.1371/journal.pone.0050836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 44.Pant C, Madonia P, Minocha A. Does PPI therapy predispose to Clostridium difficile infection? Nat Rev Gastroenterol Hepatol. 2009;6:555–557. doi: 10.1038/nrgastro.2009.128. [DOI] [PubMed] [Google Scholar]
- 45.Stiefel U, Rao A, Pultz MJ, Jump RL, Aron DC, Donskey CJ. Suppression of gastric acid production by proton pump inhibitor treatment facilitates colonization of the large intestine by vancomycin-resistant Enterococcus spp. and Klebsiella pneumoniae in clindamycin-treated mice. Antimicrob Agents Chemother. 2006;50:3905–3907. doi: 10.1128/AAC.00522-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khorvash F, Abbasi S, Meidani M, Dehdashti F, Ataei B. The comparison between proton pump inhibitors and sucralfate in incidence of ventilator associated pneumonia in critically ill patients. Adv Biomed Res. 2014;3:52. doi: 10.4103/2277-9175.125789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, Wolff M, Spencer RC, Hemmer M. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- 48.Jump RL, Pultz MJ, Donskey CJ. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob Agents Chemother. 2007;51:2883–2887. doi: 10.1128/AAC.01443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barletta JF, El-Ibiary SY, Davis LE, Nguyen B, Raney CR. Proton Pump Inhibitors and the Risk for Hospital-Acquired Clostridium difficile Infection. Mayo Clin Proc. 2013;88:1085–1090. doi: 10.1016/j.mayocp.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 50.McDonald EG, Milligan J, Frenette C, Lee TC. Continuous Proton Pump Inhibitor Therapy and the Associated Risk of Recurrent Clostridium difficile Infection. JAMA Intern Med. 2015;175:784–791. doi: 10.1001/jamainternmed.2015.42. [DOI] [PubMed] [Google Scholar]
- 51.Dultz G, Piiper A, Zeuzem S, Kronenberger B, Waidmann O. Proton pump inhibitor treatment is associated with the severity of liver disease and increased mortality in patients with cirrhosis. Aliment Pharmacol Ther. 2015;41:459–466. doi: 10.1111/apt.13061. [DOI] [PubMed] [Google Scholar]
- 52.Goel GA, Deshpande A, Lopez R, Hall GS, van Duin D, Carey WD. Increased rate of spontaneous bacterial peritonitis among cirrhotic patients receiving pharmacologic acid suppression. Clin Gastroenterol Hepatol. 2012;10:422–427. doi: 10.1016/j.cgh.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 53.Trikudanathan G, Israel J, Cappa J, O’Sullivan DM. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients - a systematic review and meta-analysis. Int J Clin Pract. 2011;65:674–678. doi: 10.1111/j.1742-1241.2011.02650.x. [DOI] [PubMed] [Google Scholar]
- 54.Deshpande A, Pasupuleti V, Thota P, Pant C, Mapara S, Hassan S, Rolston DD, Sferra TJ, Hernandez AV. Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. J Gastroenterol Hepatol. 2013;28:235–242. doi: 10.1111/jgh.12065. [DOI] [PubMed] [Google Scholar]
- 55.Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, Rumsfeld JS. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–944. doi: 10.1001/jama.2009.261. [DOI] [PubMed] [Google Scholar]
- 56.Tamura T, Sakaeda T, Kadoyama K, Okuno Y. Omeprazole- and esomeprazole-associated hypomagnesaemia: data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2012;9:322–326. doi: 10.7150/ijms.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep. 2010;12:448–457. doi: 10.1007/s11894-010-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacLaren R, Jarvis CL, Fish DN. Use of enteral nutrition for stress ulcer prophylaxis. Ann Pharmacother. 2001;35:1614–1623. doi: 10.1345/aph.1A083. [DOI] [PubMed] [Google Scholar]
- 59.Pongprasobchai S, Kridkratoke S, Nopmaneejumruslers C. Proton pump inhibitors for the prevention of stress-related mucosal disease in critically-ill patients: a meta-analysis. J Med Assoc Thai. 2009;92:632–637. [PubMed] [Google Scholar]
- 60.Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843–857. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]
- 61.Lin PC, Chang CH, Hsu PI, Tseng PL, Huang YB. The efficacy and safety of proton pump inhibitors vs histamine-2 receptor antagonists for stress ulcer bleeding prophylaxis among critical care patients: a meta-analysis. Crit Care Med. 2010;38:1197–1205. doi: 10.1097/CCM.0b013e3181d69ccf. [DOI] [PubMed] [Google Scholar]


