Abstract
AIM: To describe the pathophysiology, clinical presentation, natural history, and therapy of portal hypertensive gastropathy (PHG) based on a systematic literature review.
METHODS: Computerized search of the literature was performed via PubMed using the following medical subject headings or keywords: “portal” and “gastropathy”; or “portal” and “hypertensive”; or “congestive” and “gastropathy”; or “congestive” and “gastroenteropathy”. The following criteria were applied for study inclusion: Publication in peer-reviewed journals, and publication since 1980. Articles were independently evaluated by each author and selected for inclusion by consensus after discussion based on the following criteria: Well-designed, prospective trials; recent studies; large study populations; and study emphasis on PHG.
RESULTS: PHG is diagnosed by characteristic endoscopic findings of small polygonal areas of variable erythema surrounded by a pale, reticular border in a mosaic pattern in the gastric fundus/body in a patient with cirrhotic or non-cirrhotic portal hypertension. Histologic findings include capillary and venule dilatation, congestion, and tortuosity, without vascular fibrin thrombi or inflammatory cells in gastric submucosa. PHG is differentiated from gastric antral vascular ectasia by a different endoscopic appearance. The etiology of PHG is inadequately understood. Portal hypertension is necessary but insufficient to develop PHG because many patients have portal hypertension without PHG. PHG increases in frequency with more severe portal hypertension, advanced liver disease, longer liver disease duration, presence of esophageal varices, and endoscopic variceal obliteration. PHG pathogenesis is related to a hyperdynamic circulation, induced by portal hypertension, characterized by increased intrahepatic resistance to flow, increased splanchnic flow, increased total gastric flow, and most likely decreased gastric mucosal flow. Gastric mucosa in PHG shows increased susceptibility to gastrotoxic chemicals and poor wound healing. Nitrous oxide, free radicals, tumor necrosis factor-alpha, and glucagon may contribute to PHG development. Acute and chronic gastrointestinal bleeding are the only clinical complications. Bleeding is typically mild-to-moderate. Endoscopic therapy is rarely useful because the bleeding is typically diffuse. Acute bleeding is primarily treated with octreotide, often with concomitant proton pump inhibitor therapy, or secondarily treated with vasopressin or terlipressin. Nonselective β-adrenergic receptor antagonists, particularly propranolol, are used to prevent bleeding after an acute episode or for chronic bleeding. Iron deficiency anemia from chronic bleeding may require iron replacement therapy. Transjugular-intrahepatic-portosystemic-shunt and liver transplantation are highly successful ultimate therapies because they reduce the underlying portal hypertension.
CONCLUSION: PHG is important to recognize in patients with cirrhotic or non-cirrhotic portal hypertension because it can cause acute or chronic GI bleeding that often requires pharmacologic therapy.
Keywords: Portal hypertensive gastropathy, Congestive gastropathy, Portal hypertension, Cirrhosis, Cirrhotic, Chronic liver disease, Nonvariceal upper gastrointestinal bleeding, Esophageal varices, Hepatic fibrosis
Core tip: Portal hypertensive gastropathy (PHG) is diagnosed by characteristic endoscopic findings of variably erythematous, small, polygonal areas surrounded by a whitish, reticular border in a mosaic pattern in the gastric fundus/body in a patient with portal hypertension of any etiology. The pathophysiology of PHG is inadequately understood. Portal hypertension is a prerequisite to develop PHG. PHG increases in frequency with increasing portal hypertension, liver disease progression, duration of liver disease, presence of esophageal varices, and endoscopic variceal obliteration. Pathogenesis is related to a hyperdynamic circulation induced by portal hypertension. Gastric mucosa in PHG exhibits greater susceptibility to gastrotoxic chemicals and poor wound healing. Acute or chronic gastrointestinal bleeding are the only clinical complications. Bleeding is typically mild-to-moderate and rarely fatal. Endoscopic therapy is rarely useful. Pharmacotherapy for acute bleeding includes octreotide with concomitant proton-pump-inhibitor therapy, or alternatively vasopressin. Nonselective β-adrenergic receptor antagonists, particularly propranolol, are used to prevent re-bleeding after acute bleeding or for chronic bleeding. Transjugular-intrahepatic-portosystemic-shunt and liver transplantation is ultimate therapies because they treat the underlying portal hypertension.
INTRODUCTION
Portal hypertensive gastropathy (PHG) is an important, but underappreciated, cause of morbidity in patients with cirrhotic or non-cirrhotic portal hypertension. Researchers have recently intensely focused on this inadequately understood disease. However, the research studies have been published in a wide spectrum of journals including basic or biomedical journals not readily accessible to clinicians and a review incorporating the recent basic and clinical advances in this rapidly evolving subject is needed. This work systematically reviews this entity including pathophysiology, clinical presentation, natural history, and established, evolving, or experimental therapy, with a focus on data relevant to clinicians and an emphasis on recent data. This work aims to describe what is known about the disease and to expose gaps, requiring further research, in our current understanding of this disease.
MATERIALS AND METHODS
Computerized search of the literature was performed via PubMed using the following medical subject headings or keywords: “portal” and “gastropathy”; or “portal” and “hypertensive”; or “congestive” and “gastropathy”; or “congestive” and “gastroenteropathy”. The following criteria were applied for study inclusion: Publication in peer-reviewed journals, and publication since 1980, except for publications from 1957-1980 of historical significance reviewed in the history section. Articles were independently evaluated by each author and selected for inclusion by consensus after a thorough discussion based on the following criteria: Well-designed, prospective trials; recent studies; large study populations; and study emphasis on PHG. However, data from retrospective series, reviews from internationally recognized authorities, and even case reports were included when prospective trials were unavailable.
RESULTS
History
Palmer[1], in 1957, proposed that the pathogenesis of erosive gastritis in cirrhotic patients was different than that in non-cirrhotic patients and that erosive gastritis in cirrhotic patients resulted from mechanical venous back-pressure from portal hypertension, rather than a circulating, mucosal, or intraluminal toxic factor. This proposal was supported by successful reversal of erosive gastritis in cirrhotic patients with portal decompression by surgical shunts[1]. In 1984, Sarfeh et al[2] recognized a distinct form of gastric mucosal hemorrhage in patients who had portal hypertension, demonstrated by cirrhosis and gastroesophageal varices, which they called “portal hypertensive gastritis”. They proposed that gastric mucosa in portal hypertension reacts differently from gastric mucosa without portal hypertension and these patients with portal hypertension may benefit from portal decompressive surgery. One year later, McCormack et al[3] reported that the gastritis in patients with portal hypertension differed from that in patients without portal hypertension in mucosal histology, nonresponse to standard therapy for conventional gastritis, and in occasionally having very similar histological changes in other gastrointestinal (GI) organs such as the colon. They called these gastritis-like changes in patients with portal hypertension “congestive gastropathy”[3], and classified it as “mild” or “severe”, using criteria described by Taor et al[4].
Epidemiology
PHG can present at any age, including pediatric or adult patients. The reported prevalence of PHG varies greatly from 20% to 75% in patients with portal hypertension (Table 1)[3,5-23], and varies greatly from about 35% to 80% in patients with cirrhosis (Table 2)[21,23-68]. For example, in a study of 373 cirrhotic patients, 299 (80.2%) had PHG[34]. In the HALT-C trial, 374 (37%) of 1011 patients with biopsy-proven cirrhosis or bridging fibrosis from hepatitis C had PHG[69]. This wide variability likely reflects variability in classification criteria, interpretation of endoscopic lesions, study populations, and natural history of PHG[10,70,71].
Table 1.
Rates of portal hypertensive gastropathy in patients with portal hypertension
| Ref. | Analyzed patients | Total number | No. (%) with PHG | No. (%) with mild PHG | No. (%) with severe PHG |
| McCormack et al[3] | Portal hypertension | 127 | 65 (51%) | 37 (29%) | 28 (22%) |
| Sarin et al[5] | Portal hypertension | 136 | 10 (7%) | ||
| DeWeert et al[6] | Non-alcoholic liver disease | 81 | 23 (28%) | Not reported | Not reported |
| McCormick et al[7] | Portal hypertension | 93 endoscopies in 74 patients | 85 endoscopies (91%) | 6 (6%), moderate 61 (66%) | 18 (19%) |
| Sarin et al[8] | Portal hypertension | 107 | 4 (3.7%) (only cirrhotic) | Not reported | Not reported |
| Parikh et al[9] | Portal hypertension | 118 | 71 (60%) | 41 (58%) | 30 (42%) |
| Sarin et al[10] | Portal hypertension with prior variceal bleeding | 967 | 86 (9%) | 56 (5.8%) | 30 (3.1%) |
| Itha et al[11] | EHPVO in children | 163 | (12%) | Not reported | Not reported |
| Rana et al[12] | Portal hypertension | 41 | 27 (66%) | 19 (46%) | 8 (20%) |
| El-Rifai et al[13] | Portal hypertension | 24 | 14 (58%) | 10 (42%) - moderate | 4 (16%) |
| Sogaard et al[14] | Portal vein thrombosis | 67 | 28 (42%) | Not reported | Not reported |
| Figueiredo et al[15] | Portal hypertension; cirrhosis | 36 | 27 (75%) | 5 (46%) | |
| Erden et al[16] | Portal hypertension | 57 | 15 (26.3%) | Not reported | Not reported |
| Duché et al[17] | Children, portal hypertension with biliary atresia | 125 | 27 (21%) | Not reported | Not reported |
| Aydoğan et al[18] | Portal hypertension | 51 | 30 (58%) | Not reported | Not reported |
| dos Santos et al[19] | Portal hypertension | 43 | 22 (51%) | Not reported | Not reported |
| Pantham et al[20] | Esophageal varices undergoing TEE | 24 | 12 (50%) | Not reported | Not reported |
| Abdollahi et al[21] | Autoimmune hepatitis | 60 | 27 (45%) | Not reported | Not reported |
| de Alcantara et al[22] | Chronic liver disease vs EHPVO | 35 vs 18 | 7 (20%) vs 8 (44.4%) | Not reported | Not reported |
| Aoyama et al[23] | Portal hypertension | 119 | 35 (29%) | Not reported | Not reported |
PHG: Portal hypertensive gastropathy; EHPVO: Extrahepatic portal vein obstruction; TEE: Transesophageal echocardiogram.
Table 2.
Rates of portal hypertensive gastropathy in patients with cirrhosis
| Ref. | Patients | Total number | PHG | Mild | Severe |
| Sacchetti et al[24] | Cirrhosis | 142 | 38 (27%) | 28 (20%) | 10 (7%) |
| D'Amico et al[25] | Cirrhosis | 212 | 130 (61%) | 110 (52%) | 20 (9%) |
| Calès et al[26] | Cirrhosis | 100 | 98 (98%) | 57 (57%) | 41 (41%) |
| Rabinovitz et al[27] | Cirrhosis | 510 | (43%) | Not reported | Not reported |
| Iwao et al[28] | Cirrhosis | 47 | 32 (68%) | 15 (32%) | 17 (36%) |
| Taranto et al[29] | Cirrhosis | 394 | 317 (80.5%) | Not reported | Not reported |
| Gupta et al[30] | Cirrhosis | 230 | (61%) | (52%) | (9%) |
| Iwao et al[31] | Cirrhosis | 476 | 254 (53%) | 208 (43%) | 46 (9%) |
| Carpinelli et al[32] | Cirrhosis | 566 | 362 (64%) | 192 (34%) | 170 (30%) |
| Zaman et al[33] | Cirrhosis | 120 | 74 (62%) | 47 (39%) | 27 (23%) |
| Primignani et al[34] | Cirrhosis | 373 | 299 (80%) | 127 (34%) | 172 (46%) |
| Chaves et al[35] | Cirrhosis vs schistosomiasis | 43 | 18 (81%) vs 7 (33%) | Not reported | Not reported |
| Merkel et al[36] | Cirrhosis | 62 | 49 (79%) | 29 (46%) | 20 (32%) |
| Merli et al[37] | Cirrhosis, with mild portal hypertension | 222 | 48 (21%) | 43 (19%) | 5 (2%) |
| Ito et al[38] | Cirrhosis | 47 | 13 (27%) | 10 (21%) | 3 (6%) |
| De Palma et al[39] | Cirrhosis | 37 | 23 (62%) | Not reported | Not reported |
| Menchén et al[40] | Cirrhosis | 549 | 353 (64%) | 275 (50%) | 77 (14%) |
| Yüksel et al[41] | Cirrhosis | 114 total | 76 (66%) | 38 (33%) | 38 (33%) |
| Fontana et al[42] | Cirrhosis or bridging fibrosis from hepatitis C | 1016 | 374 (37%) | 345 (34%) | 29 (3%) |
| Bresci et al[43] | Cirrhosis | 85 | 36 (42%) | Not reported | Not reported |
| Akatsu et al[44] | End stage liver disease | 29 | 19 (65.5%) | 18 (62.1%) | 1 (3.4%) |
| Zardi et al[45] | Cirrhosis | 266 | 84 (31%) | Not reported | Not reported |
| Barakat et al[46] | Cirrhosis with portal hypertensive duodenopathy | 105 | 105 (100%) | 17 (16.2%) | 88 (83.8%) |
| Bellis et al[47] | Cirrhosis | 59 | 44 (76%) | 16 (27%) | 28 (47%) |
| Gravante et al[48] | Liver transplant candidates with cirrhosis | 80 | 41 (51.2%) | Not reported | Not reported |
| Canlas et al[49] | Cirrhosis | 19 | 13 (68.4%) | Not reported | Not reported |
| Kim et al[50] | Cirrhosis | 83 | 48 (57.8%) | Not reported | Not reported |
| Higaki et al[51] | Cirrhosis | 21 | 8 (38%) | Not reported | Not reported |
| Frenette et al[52] | Cirrhosis | 50 | 45 (90%) | 28 (56%) | 17 (34%) moderate |
| Tarantino et al[53] | Cirrhosis | 153 | 88 (57.5%) | Not reported | Not reported |
| Curvêlo et al[54] | Cirrhosis | 46 | 43 (93.4%) | 21 (45%) | 22 (47%) |
| Anegawa et al[55] | Cirrhosis | 70 | 49 (70%) | 32 (46%) | 17 (24%) |
| Kumar et al[56] | Cirrhosis | 254 | 140 (55%) | Not reported | Not reported |
| Kim et al[57] | Cirrhosis | 331 | 298 (90%) | Mild 84 (25.4%) | 214 (64.7%) |
| De Lisi et al[58] | Cirrhosis | 611 | 448 (73.3%) | 37.3% | 36% |
| Abbasi et al[59] | Cirrhosis | 102 | 87 (85%) | Not reported | Not reported |
| Ahmed et al[60] | Cirrhosis from hepatitis B or hepatitis C | 360 | 300 (83%) | 229 (64%) | 71 (20%) |
| Garcia-Saenz-de-Sicilia et al[61] | Cirrhosis | 105 | 72 (68.6%) | Not reported | Not reported |
| Abbasi et al[62] | Cirrhosis | 217 | 172 (79.3%) | 56 (25.8%) | 116 (53.5%) |
| Aoyama et al[63] | Cirrhosis | 60 | 13 (22%) | Not reported | Not reported |
| Laleman et al[64] | Cirrhosis with refractory chronic hepatic encephalopathy | 36 | 13 (36%) | 9 (25%) | 4 (11%) |
| Giannini et al[65] | Cirrhosis and undergoing surgery for hepatocellular carcinoma | 152 | 23 (15.1%) | Not reported | Not reported |
| Abdollahi et al[21] | Autoimmune hepatitis | 60 | 27 (45%) | Not reported | Not reported |
| Aoyama et al[23] | Portal hypertension | 119 | 35 (29%) | Not reported | Not reported |
| Aoyama et al[66] | Cirrhosis | 134 | 42 (31%) | Not reported | Not reported |
| Zardi et al[67] | Cirrhosis without gastroesophageal varices | 145 | 75 (51%) | 45 (31%) | 30 (20%) |
| Wu et al[68] | Cirrhosis | 700 | 449 (64%) | Mild 208 (29.7), moderate 160 (22.9%) | Severe 81 (11.6%) |
PHG: Portal hypertensive gastropathy.
PHG is usually mild as reported by McCormack et al[3] or in the NIEC study[32,70]. The prevalence of mild PHG in patients with portal hypertension ranges from 29%-57%, and of severe PHG ranges from 9%-46%[71].
Risk factors for PHG
The main predictors of PHG are portal hypertension and severe liver disease[72].
Portal hypertension: Most studies show that the frequency and severity of PHG is strongly correlated with the severity of portal hypertension, as indicated by multiple parameters, including hepatic venous pressure gradient (HVPG)[36,57], esophageal intravariceal pressure[29], and presence or size of esophageal varices[34,42,57,62,73]. Merkel et al[36] reported that the severity of PHG was correlated with the severity of portal hypertension as determined by HVPG, but this correlation was significant only for severe PHG (HVPG = 20.5 ± 4.0 mmHg) vs no PHG (HVPG = 17.4 ± 5.2 mmHg, P = 0.0004), and not for mild PHG (HVPG = 16.1 ± 3.2 mmHg) vs no PHG [17.4 ± 5.2 mmHg, not significant (NS)]. In a prospective study of 331 cirrhotic patients, Kim et al[57] found that patients with severe PHG had significantly higher HVPG (15.6 ± 4.6 mmHg) than patients with mild PHG (10.7 ± 4.1 mmHg) or no PHG (4.9 ± 1.7 mmHg) (P < 0.001). Merkel et al[36] similarly reported in a small study that HVPG was significantly higher in patients with severe PHG as compared to mild or no PHG.
Primignani et al[34] confirmed the correlation of PHG with severity of portal hypertension, by correlating PHG with presence and size of esophageal varices. The rate of PHG was significantly higher in patients with esophageal varices [80 of 104 patients (76.9%)] than in patients without esophageal varices [51 of 84 (60.7%), P < 0.007]. The rate of PHG also significantly increased with increasing variceal size (χ2 = 13.2; df = 1, P = 0.0003). Abbasi et al[62] reported a significantly positive correlation between esophageal variceal size and rate of PHG (r = 0.46; P < 0.001). Taranto et al[29] reported more severe PHG in cirrhotic patients with more severe portal hypertension, as measured by esophageal intravariceal pressure. Iwao et al[28] reported that patients with severe PHG had elevated HVPG, high hepatic sinusoidal resistance, and low hepatic blood flow, all markers of severe portal hypertension. For example, patients without PHG had hepatic sinusoidal resistance of 1218 ± 528 dyne × s-1 × cm-5, patients with mild PHG had resistance of 1968 ± 944 dyne × s-1 × cm-5 (P < 0.05), and patients with severe PHG had resistance of 2082 ± 672 dyne × s-1 × cm-5 (P < 0.01). Presence of PHG was independent of patient age, sex, or cirrhosis etiology[28].
As discussed below, other data supporting an association between PHG and portal hypertension include resolution of PHG after intervention to decrease portal hypertension, including pharmacotherapy[74-78], transjugular intrahepatic portosystemic shunt (TIPS), or liver transplantation[74].
Contrariwise, a decided minority of studies showed no significant association between severity of portal hypertension and rate of PHG[8,9,16,26,28,30,31,47,54,67]. Curvêlo et al[54] found no significant difference in HVPG in cirrhotic patients with vs without PHG. Bellis et al[47] demonstrated similar findings. Among patients with portal hypertension from cirrhosis without esophageal varices, Zardi et al[67] reported that patients with PHG vs patients without PHG had similar mean portal vein diameter, splenic vein diameter, and portal flow volume, all markers of severity of portal hypertension. Erden et al[16] showed that the mean diameters of the left gastric, paraesophageal, and azygos veins, which are markers of portal hypertension, were not significantly different between patients with vs without PHG. The preponderance of data strongly suggest that the severity of portal hypertension is associated with the severity or frequency of PHG.
Cirrhotic vs non-cirrhotic portal hypertension: Primary liver disease usually occurs in PHG, but is not a prerequisite for PHG provided another cause of portal hypertension exists. PHG can occur among patients with non-cirrhotic portal fibrosis (NCPF), extrahepatic portal vein obstruction (EHPVO), hepatic veno-occlusive disease, and schistosomiasis[8,14,35,75,79].
The frequency of PHG appears to be higher in portal hypertension with cirrhosis than in portal hypertension without cirrhosis. Sarin et al[8] reported that patients with cirrhosis had a significantly higher frequency of PHG (37.1%) than that in patients with NCPF (16.7%; P < 0.05), or non-cirrhotic EHPVO (8.7%; P < 0.01) and had a more aggressive course of PHG with progression to more severe PHG with time. These phenomena are attributed to the worse liver function in patients with cirrhosis as compared to patients with NCPF or EHPVO[8]. Chaves et al[35] similarly reported a higher incidence of PHG in patients with cirrhosis vs patients with portal hypertension from etiologies including schistosomiasis or postsinusoidal hypertension. Chaves et al[35] reported that PHG occurred in 18 (81.8%) of 22 patients with cirrhosis vs only 7 (33.3%) of 21 patients with portal hypertension from schistosomiasis (P < 0.05). Parikh et al[9] reported a non-significant trend of more frequent PHG in patients with cirrhosis [64 of 102 patients (63%)] vs NCPF [7 of 16 patients (44%)], but the lack of statistical significance may have resulted from the small number of patients with NCPF.
Chaves et al[35] reported that the mosaic pattern was significantly more prevalent in patients with cirrhosis [12 of 22 patients (54.5%)] than in patients with schistosomiasis [2 of 21 patients (9.5%); P < 0.05]. Sarin et al[6] in a study of 50 patients with portal hypertension from various etiologies undergoing endoscopy, reported 6 (16.6%) of 36 patients with underlying cirrhosis had a mosaic pattern of PHG, whereas only 1 (8.5%) of 12 patients with EHPVO had a mosaic pattern of HPG (NS).
Cirrhosis etiology: Several research groups reported that the underlying etiology of cirrhosis did not affect PHG frequency or severity[13,71,80]. For example, Abbasi et al[62] reported among 217 patients with cirrhosis that PHG was unassociated with cirrhosis etiology (r = 0.056; P = 0.414), among 144 patients with hepatitis C, 36 patients with hepatitis B, 21 patients with cryptogenic cirrhosis, 15 patients with hepatitis C and hepatitis B coinfection, and 1 patient with hepatitis B and hepatitis D coinfection. Kim et al[57] similarly did not find a correlation between cirrhosis etiology and severity of PHG in a prospective study of 331 patients with cirrhosis, including cirrhosis etiologies of alcohol in 250, hepatitis B in 68, hepatitis C in 15, and cryptogenic cirrhosis in 8. Gupta et al[30] in a study of 230 patients with cirrhosis and esophageal varices found no significant difference in the rate of PHG between patients with cirrhosis from alcohol [32 of 52 patients (62%)] vs cirrhosis from other causes [110 of 178 patients (62%), P = NS]. Iwao et al[31] in an endoscopic study of 47 patients with histologically-proven cirrhosis reported no significant differences in etiology of cirrhosis between patients without PHG vs patients with mild or severe PHG.
Iwao et al[31] reported no association between etiology of cirrhosis and PHG severity. The etiologies of cirrhosis in this study included 7 from alcoholism vs 8 from chronic hepatitis in patients without PHG, 5 from alcoholism vs 10 from chronic hepatitis in patients with mild PHG, and 8 from alcoholism vs 9 from chronic hepatitis in patients with severe PHG (NS).
Liver disease duration: Generally, duration of liver disease positively correlates with development of PHG[5]. Merli et al[37] reported a cumulative incidence of 3% at 1 year, 10% at 2 years, and 24% at 3 years. Most cases were mild, with only 10% of cases reported as severe PHG in cirrhotic patients undergoing esophagogastroduodenoscopy (EGD) to screen for esophageal varices. Primignani et al[34] reported that the prevalence of PHG was only 56% in patients with newly diagnosed cirrhosis, rose to 75% in patients with previously diagnosed cirrhosis and no prior variceal bleeding, and rose further to 91% in patients with previously diagnosed cirrhosis and prior variceal bleeding treated with sclerotherapy (χ2 = 34.25; df = 1; P < 0.0001). The frequency of PHG increased by 46% after 5 years of follow-up in patients with cirrhosis[34]. In 30%-60% of cases, preexistent PHG remained stable with time[72], but it can fluctuate in severity with time, with progression in 30%, and regression in 20% of cases[25,34,37]. Child-Pugh stage C cirrhosis was associated with faster progression of PHG[34].
Liver disease severity: Numerous studies reported PHG is correlated with liver disease severity, as measured by Child-Pugh stage[8,9,29,35,37,55,57,67]. The reported strength of this correlation is variable. Some studies showed correlation between all stages of cirrhosis and PHG, whereas other studies showed correlation only for specific stages of cirrhosis. Sarin et al[8] reported an 87% prevalence of PHG in patients with Child-Pugh stage C, vs only 13% prevalence in patients with Child-Pugh stage A. Another study reported that only Child-Pugh stage C was independently associated with PHG (OR = 2.68; 95%CI: 1.16-6.20, P = 0.021)[56]. Merli et al[37] reported, in a study of 48 patients with PHG among 222 patients with cirrhosis, that Child-Pugh stage B or C, and presence of esophageal varices were independent risk factors for developing PHG. De Lisi et al[58] reported a significantly higher prevalence of PHG in Child-Pugh stages B or C, as compared to stage A. Zardi et al[67] reported that cirrhotic patients without esophageal varices with severe PHG had significantly more frequently Child-Pugh stage C than patients with mild PHG. In another study, the MELD (model for end-stage liver disease) score was significantly correlated with PHG severity (mean MELD score in patients without PHG = 7.6 ± 1.7, in patients with mild PHG = 10.2 ± 4.0, and in patients with severe PHG = 11.3 ± 3.5; P < 0.001)[57]. In the HALT-C trial, hypoalbuminemia and hyperbilirubinemia, biochemical markers of advanced liver disease, were independent predictors of PHG in a logistic regression model (OR = 0.53, 95%CI: 0.37-0.76 for hypoalbuminemia; OR = 1.77, 95%CI: 1.25-2.51, for hyperbilirubinemia). Markers of portal hypertension (thrombocytopenia) and of insulin resistance (hyperglycemia) were also significant independent predictors of PHG.
Contrariwise, a minority of studies found no correlation between liver disease severity, as determined by Child-Pugh stage, and presence or severity of PHG[34,36,40,47,54,62,70,79]. For example, Primignani et al[34] reported the prevalence of severe PHG was lowest in Child-Pugh stage C. In the NIEC study, patients with Child-Pugh stage B had a higher prevalence of PHG than patients with stages A or C. Zardi et al[67] reported no significant differences in Child-Pugh stage or in MELD score among cirrhotic patients with vs without PHG. The preponderance of the data, however, suggest that severity of cirrhosis, as measured by Child-Pugh score, is correlated with frequency of PHG.
Correlation with varices: Many studies report a correlation between the presence and size of esophageal varices and severity of PHG. For example, among the 188 of 373 patients with cirrhosis not undergoing variceal sclerotherapy in the NIEC study, the prevalence of PHG was significantly higher in patients with esophageal varices [80 of 104 patients (77%)] than in patients without esophageal varices [51 of 84 patients (61%); P = 0.007]; and the prevalence of PHG significantly increased with increasing variceal size (χ2 = 13.2; P < 0.0003)[34]. Numerous other studies also demonstrated significant correlation between presence of esophageal varices and PHG, and several studies also demonstrated significant correlations between variceal size and PHG[9,13,29,42,56,57,62]. For example, Abbasi et al[62] reported that esophageal variceal size was significantly correlated with PHG frequency among 217 cirrhotic patients (r = 0.46; P < 0.001).
However, a few studies showed no correlation between presence or size of varices and PHG[26,28,30,47]. All these negative studies but one were relatively small. Gupta et al[30] reported no significant association between frequency of PHG and size of esophageal varices among 230 cirrhotic patients. Similarly, in a study of 59 patients with cirrhosis, Bellis et al[47] showed a non-significant trend towards more severe PHG in patients with large vs small varices. For example, three (50%) of 6 patients without esophageal varices had PHG, 6 of 10 patients (60%) with small varices had PHG, 19 of 25 patients (76%) with medium-sized varices had PHG, and 16 of 18 patients (89%) with large varices had PHG (NS). Iwao et al[28] further reported that the frequency of PHG was not correlated with esophageal variceal size. The mean grade of gastroesophageal varices was 1.4 ± 0.9 for no PHG, 2.0 ± 0.9 for mild PHG, and 1.9 ± 1.0 for severe PHG (all NS), and the mean grade of gastric varices was 0.5 ± 0.8 for no PHG, 1.3 ± 1.3 for mild PHG, and 0.9 ± 1.2 for severe PHG (all NS).
Location of varices: Regarding variceal location, Sarin et al[8] reported in a study of 107 patients with cirrhosis, NCPF or EHPVO, that PHG was significantly more common in patients with coexistent gastric and esophageal varices as compared to solely esophageal varices. PHG occurred in 15 (42%) of 36 patients with concomitant esophageal and gastric varices, but occurred in only 8 (11%) of 71 patients with solely esophageal varices (P < 0.01). Likewise, Gupta et al[30] reported a significantly higher prevalence of PHG in patients with esophageal and gastric varices [74 of 107 patients (69%)] compared to solely esophageal varices [68 of 123 patients (55%), P < 0.05].
Iwao et al[31] reported a significantly higher incidence of PHG in cirrhotic patients with esophageal varices as compared to fundal gastric varices. Merkel et al[36] reported that patients with severe PHG localized to the gastric body or fundus had significantly higher HVPG than patients with severe PHG localized to the gastric antrum.
Portal hypertension is usually associated with portosystemic collateral circulation, commonly including esophageal varices, gastric varices, and abdominal or umbilical or hemorrhoidal vein dilatation; and uncommonly including splenorenal, gastric, renal, retroperitoneal, or cardiac angle venous shunts. Wu et al[68] reported that the rate of moderate or severe PHG was higher in patients with common collaterals [296 of 439 patients (67.4%)] vs uncommon collaterals [70 of 118 patients (59.3%)], but this difference was not statistically significant.
In 2007, Zardi et al[45] proposed that PHG is promoted by minimal collateral circulation because a significant collateral circulation would otherwise reduce portal pressure and gastric mucosal congestion. They found that the portal vein diameter in cirrhotic patients was larger in patients with PHG and no esophageal varices (13.0 ± 2.6 mm) than in patients with F1 esophageal varices (12.6 ± 2.3 mm) or F2 esophageal varices (12.9 ± 2.0 mm) (NS). They further supported this concept by finding that patients with portal vein diameter < 12 mm have a significantly higher prevalence of F1 and F2 esophageal varices than patients with a portal diameter between 12-13 mm, and argued that the absence of hepatofugal collateral circulation created by flow inversion, in patients without esophageal varices, left the entire pressure gradient over the portal vein[45].
Esophageal variceal eradication: Numerous studies demonstrated that PHG increased in incidence and that preexistent PHG increased in severity after eradication of esophageal varices by either endoscopic variceal ligation (Table 3)[41,73,80-82] or endoscopic variceal sclerotherapy (Table 4)[8,10,11,25,30,41,73,81-83] in cirrhotic patients with portal hypertension. Both phenomena also occurred after endoscopic variceal eradication in patients with non-cirrhotic portal hypertension, as shown in two studies in pediatric patients with EHPVO. For example, Poddar et al[83,84] reported in a prospective study of 274 children undergoing surveillance EGD after endoscopic sclerotherapy for EHPVO that the number of patients with PHG increased from 46 (24.7%) at baseline to 95 (51.6%) after sclerotherapy among 186 patients completing the study (P < 0.001). Likewise, Itha et al[11] reported that the rate of PHG increased from 12% to 41% after endoscopic sclerotherapy (P < 0.001) in a prospective study of 163 children undergoing surveillance EGD at 3 and 6 mo after endoscopic sclerotherapy. In the study by Sarin et al[10], 86 (9%) of 967 patients with prior variceal bleeding treated with endoscopic sclerotherapy, had PHG at EGD, of whom 22 (26%) had PHG before variceal eradication and 64 (74%) developed PHG after variceal eradication.
Table 3.
Effects of variceal ligation on frequency of portal hypertensive gastropathy
| Ref. | No. of patients and etiology | Study type | PHG rate before variceal ligation | PHG aggravation after variceal ligation | P value of pre vs post EVL |
| Hou et al[73] | 90 patients with cirrhosis and recent variceal bleeding, 46 patients underwent EVL | Randomized, controlled trial | No PHG-4, mild PHG-33, severe-PHG-9 | At eradication: 17/37; 17/37 (45.9%) in EVL; at 3 mo: 17/30 (56.7%); at 6 mo 18/29 (62.1%) | P > 0.05 |
| Elnaser et al[80] | 125 patients with upper GI bleeding undergoing variceal ligation, followed for mean of 31 mo | Retrospective study | 22/125 (17.6%) | 50/125 (50%) | P < 0.05 |
| Yüksel et al[41] | 114 patients with cirrhosis and portal hypertension undergoing EVL in 85 patients | Retrospective study | 27/85 (31.8%) none; 28/85 (32.9%) mild; 30/85 (35.3%) severe | 14/85 (16.5%) none; 30/85 (35.3%) mild; 41/85 (48.2%) severe | P < 0.05 |
| Lo et al[81] | 77 patients with bleeding from EV underwent variceal ligation and were randomized to receive propranolol (37/77) or control (40/77); patients with severe PHG prior to treatment excluded from the study | Prospective, randomized, controlled trial | Control group: 7/40 (17%); propranolol group: 8/37 (22%) | At variceal ligation: Control group: 67% (does not state number); Propranolol group: 31% (number not stated); 6 mo after treatment: Control group: 85% (number not stated) propranolol group: 48% (number not stated) | Pre vs post ligation, both groups; P < 0.05; frequency of PHG significantly higher in control group post ligation when compared to propranolol group; P = 0.002 |
| de la Peña et al[82] | 93 patients with history of variceal hemorrhage and cirrhosis, randomized to receive either EVS (46/88) or EVL (42/88); 5 patients excluded due to diagnosis of hepatoma, non-cirrhotic portal hypertension or portal vein thrombosis | Randomized, prospective study | Not reported | PHG significantly worsened in 23 patients, including 17 patients undergoing EVL | P < 0.01 |
PHG: Portal hypertensive gastropathy; EVL: Endoscopic variceal ligation; EVS: Endoscopic variceal sclerotherapy.
Table 4.
Effects of variceal sclerotherapy on frequency of portal hypertensive gastropathy
| Ref. | No. of patients and etiology | Study type | PHG before procedure | PHG aggravation after procedure | P value |
| Hou et al[73] | 90 cirrhotic patients with recent variceal bleeding; EVS 44, EVL 46 | Randomized, controlled trial | Pre EVS group: 6 none/24 mild/14 severe; pre EVL group: 4 none/33 mild/9 severe; total: 10 none/57 mild/23 severe | At eradication: 14/29 (48.3%) in EVS; 17/37 (45.9%) in EVL; at 3 mo: 15/26 (57.7%) in EVS; 17/30 (56.7%) in EVL; at 6 mo 15/25 (60%) in EVS; 18/29 (62.1%) in EVL | Non-significant difference in PHG aggravation between EVS and EVL; P > 0.05 |
| Itha et al[11] | 163 children with extrahepatic portal vein obstruction presenting with variceal bleeding underwent endoscopic injection sclerotherapy | Not reported | 12% overall PHG (actual number not stated), 1 patient with severe PHG | 41% overall PHG (actual number not stated), 12 patients with severe PHG | P < 0.001 for overall PHG; P < 0.001 for severe PHG |
| Poddar et al[83] | 186 children with extrahepatic portal vein obstruction presenting with variceal bleeding undergoing endoscopic sclerotherapy, and mean follow up of 38 ± 30 mo | Retrospective study | PHG: 46/186 (24.7%), severe PHG: 6/186 (3.2%) | PHG: 96/186 (51.6%), severe PHG: 29/186 (15.6%) | P < 0.001 for overall PHG; P < 0.05 for severe PHG |
| Yüksel et al[41] | 114 patients with cirrhosis and portal hypertension undergoing EVS (29/114) or EVL (85/114) | Retrospective study | Pre EVS group: 11/29 (37.9%) none; 10/29 (24.5%) mild; 8/29 (27.6%) severe; pre EVL group: 27/85 (31.8%) none; 28/85 (32.9%) mild; 30/85 (35.3%) severe | Post EVS group: 4/29 (13.8%) none; 8/29 (27.6%) mild; 17/29 (58.6%) severe; post EVL group: 14/85 (16.5%) none; 30/85 (35.3%) mild; 41/85 (48.2%) severe | Pre EVS vs post EVS; P < 0.05; pre EVL vs post EVL; P < 0.05; pre EVS vs pre EVL; P > 0.05; post EVS vs post EVL; P > 0.05 |
| Sarin et al[10] | 967 patients with variceal bleeding underwent endoscopic sclerotherapy; out of whom 88 patients fulfilled the inclusion criteria (including presence of endoscopic lesions consistent with PHG or GAVE, before or within 4 wk after obliteration) were prospectively followed (out of whom 2 had only GAVE) | Prospective study | 22 patients had PHG prior to EVS; 2/22 transient (9%); 17/22 persistent (77%); 3/22 progressive (14%) | Additional development in 64 patients post procedure, 28/64 transient (44%), 31/64 persistent (48%), 5/64 progressive (8%) | Only statistically significant difference was the transient PHG that disappeared in 28 (44%) of patients in the group that developed PHG post procedure; P < 0.05 |
| Gupta et al[30] | 230 patients with liver cirrhosis; of which 44 underwent variceal eradication with sclerotherapy | Prospective study | 24/44 (54%) | 33/44 (75%) | P < 0.05 |
| Sarin et al[8] | 107 patients with portal hypertension presenting with variceal bleeding that underwent sclerotherapy with mean follow-up of 23.2 ± 3.4 mo | Prospective study | 4/107 (3.7%) | 21 additional patients, 25/107 (23%) | Does not state if this was statistically significant |
| de la Peña et al[82] | 93 patients with history of variceal hemorrhage and cirrhosis, randomized to receive either EVS (46/88) or EVL (42/88); 5 patients were excluded due to diagnosis of hematoma, non-cirrhotic portal hypertension or portal vein thrombosis | Prospective study | Not reported | PHG worsened in 23 patients total; statistically significantly more in EVL group than EVS group (17 vs 6 patients respectively) | P < 0.01 |
| D'Amico et al[25] | 212 cirrhotic patients of which 75 had an episode of variceal bleeding and were treated with sclerotherapy; 137 without bleeding were not treated with sclerotherapy | Prospective study | No EVS group at admission: 104/137 (75%) none; 28/137 (20%) mild; 5/137 (4%) severe; EVS group at admission: 50/75 (66%) none; 17/75 (22%) mild; 8/75 (11%) severe | No EVS group at end of study 69/137 (50%) none; 61/137 (45%) mild; 7/137 (5%) severe; EVS group at end of study: 13/75 (17%) none; 49/75 (65%) mild; 13/75 (17%) severe | The conclusion was that sclerotherapy is a significant indicator of the risk of PHG in a multivariate analysis (P = 0.00032) |
PHG: Portal hypertensive gastropathy; EVL: Endoscopic variceal ligation; EVS: Endoscopic variceal sclerotherapy.
PHG also increases in frequency after angiographic variceal obliteration. Duan et al[85] reported de novo PHG developed in 13 of 34 patients (38%) after percutaneous transhepatic variceal embolization for massive esophagogastric variceal hemorrhage.
These phenomena are attributed to increased portal pressure and flow after eradication of esophageal varices because of redistribution of residual blood flow that had passed through the previously patent varices[5,41,45,81,86-90]. Itha et al[11] concluded that the significant increase in frequency and severity of PHG after variceal eradication resulted from decreasing collateral blood flow through esophageal varices causing increasing PHG from gastric mucosal congestion. This mechanism is supported by finding that gastric mucosal blood flow increases after variceal ligation[91]. Another theory is that delayed gastric emptying after sclerotherapy from extravasation of sclerosant, may cause development of PHG[69]. No direct evidence exists for delayed gastric emptying in PHG[71].
Data on which technique of endoscopic variceal eradication leads to quantitatively more de novo PHG is contradictory. Most studies showed no differences in frequency or severity of PHG after variceal ligation vs sclerotherapy[19,34,41,73,92,93], but some studies showed worse outcomes after variceal ligation[82,93,94], while some other studies showed worse outcomes after sclerotherapy[95].
De novo PHG after variceal obliteration is often transitory and less severe than PHG that predated the variceal obliteration[10,73]. For example, Sarin et al[10] reported in a study of 84 patients followed for a mean of 25 ± 14 mo that PHG resolved in 28 (44%) of 64 patients who developed PHG after sclerotherapy, but resolved in only 2 (9%) of 22 patients who had PHG present before sclerotherapy (P < 0.05). Hou et al[73] similarly reported that the increased severity of PHG after variceal obliteration was generally transitory and returned to baseline status. The return to baseline severity of PHG was significantly faster after variceal ligation than after sclerotherapy (P = 0.03), attributed to ligation achieving subtotal variceal obliteration and permitting faster redistribution of blood flow[10].
Some investigators believe the higher rate of PHG in patients undergoing endoscopic variceal sclerotherapy merely reflects a longer duration of portal hypertension, more advanced liver disease, or more severe portal hypertension in patients selected to undergo variceal sclerotherapy compared to controls rather than the performance of sclerotherapy per se[34,78,96]. Primignani et al[34,97] demonstrated an almost identical increase in frequency of PHG with time in patients undergoing vs not undergoing sclerotherapy, and suggested that PHG evolved identically with time regardless of performance vs nonperformance of sclerotherapy.
Additional risk factors: PHG severity is significantly associated with thrombocytopenia or splenomegaly[42,57,58]. In a prospective study of 331 cirrhotic patients performed in South Korea, PHG severity was correlated with splenic diameter: Splenic diameter with severe PHG = 13.1 ± 2.4 cm, diameter with mild PHG = 12.2 ± 2.5 cm, and diameter with no PHG = 10.7 ± 2.9 cm, P < 0.001)[57]. In this study, PHG severity was inversely correlated with platelet count: count with no PHG = 174600 ± 109400 platelets/mm3, count with mild PHG = 132000 ± 100700 platelets/mm3, count with severe PHG = 102800 ± 68800 platelets/mm3 (P < 0.001). Among 1016 patients with bridging fibrosis or compensated cirrhosis undergoing EGD in the HALT-C trial, including 374 (37%) with PHG, PHG was negatively correlated with platelet count in a logistic regression model (negative estimate: -0.00407, OR = 0.99, 95%CI: 0.99-0.998; P = 0.0007)[42].
In one study, PHG in patients with chronic liver disease was correlated with increasing thickness of the lesser omentum, and presence of a splenorenal shunt[22]. This study found that PHG frequency was not associated with severity of hypersplenism[62]. The HALT-C trial showed no association between prevalence or severity of PHG and lifetime alcohol consumption, nonsteroidal anti-inflammatory drugs (NSAIDs) use, COX- (cyclooxygenase-) 2 inhibitor use, or smoking[42]. The lack of association with alcoholism may reflect the need for near abstinence from alcohol to have enrolled in the clinical trial; at study enrollment 86% of patients reported abstinence and 14% reported minimal drinking of alcohol. Table 5[8,9,11,14,34,41,55,62,67,71,83,85] lists well-established risk factors for PHG; Table 6[11,41,44,55,75-77,83,85,98-107] lists therapies that affect the severity of PHG or the risk of bleeding from PHG, and Table 7[8,14,28,30,35,42,84,108-110] lists the factors that do not affect the risk of PGH.
Table 5.
Well-established, important risk factors for portal hypertensive gastropathy
| Parameters | Ref. |
| Portal hypertension | |
| Non-cirrhotic portal hypertension | [8,14] |
| Cirrhotic portal hypertension | [8,9,34] |
| Cirrhosis | |
| Longer duration of cirrhosis | [34,71] |
| Greater severity of cirrhosis | [55,67] |
| Greater size of esophageal varices | [34,62] |
| Eradication of esophageal varices | |
| Endoscopic therapies | |
| Endoscopic variceal ligation | [11,41] |
| Endoscopic sclerotherapy | [11,83] |
| Angiographic | |
| Percutaneous transhepatic variceal embolization | [85] |
Table 6.
Therapies affecting the severity or the risk of bleeding from portal hypertensive gastropathy
| Therapies reducing severity of PHG | Ref. |
| TIPS | [76,77,98] |
| Transcatheter splenic arterial embolization | [99] |
| Surgical shunt | |
| Portocaval shunt | [100] |
| Central splenorenal shunt | [101] |
| Laparoscopic splenectomy (in patients with hypersplenism) | [55] |
| Liver transplantation | [44] |
| Therapies reducing risk of bleeding from PHG | |
| TIPS | [75,98,102] |
| Surgical shunt (portocaval or splenorenal) | [100,101] |
| Nonselective b β-adrenergic receptor antagonists (e.g., propranolol) | [103 (in rats),104] |
| Somatostatin family of drugs | |
| Somatostatin | [105] |
| Octreotide | [106] |
| Vasopressin family of drugs | |
| Vasopressin | [106] |
| Terlipressin | [107] |
| Therapies that increase incidence or risk of bleeding from PHG | |
| Endoscopic therapies for varices | |
| Variceal ligation | [11,41] |
| Variceal sclerotherapy | [11,83] |
| Interventional angiography | |
| Percutaneous transhepatic variceal embolization | [85] |
TIPS: Transjugular intrahepatic portosystemic shunt; PHG: Portal hypertensive gastropathy.
Table 7.
Factors not affecting risk of portal hypertensive gastropathy
| Factors not affecting risk of portal hypertensive gastropathy | Ref. |
| Etiology of cirrhosis | [8,28,30] |
| Etiology of non-cirrhotic portal hypertension | [8,14,35,83,108] |
| Alcoholism | [30,42] |
| NSAID use | [42] |
| Use of COX-2 inhibitors | [42] |
| Smoking tobacco | [42] |
| Gastric infection with Helicobacter pylori | [109,110] |
NSAID: Nonsteroidal anti-inflammatory drug; COX-2: Cyclooxygenase-2.
Pathogenesis
Hemodynamic changes: The pathogenesis of PHG is inadequately understood[96]. Hemodynamic changes, especially increased portal pressure, are the suspected underlying cause because PHG develops only with established portal hypertension[72]. However, portal hypertension cannot be the sole factor because many patients with portal hypertension do not develop PHG[36,111]. Hemodynamic changes in patients with portal hypertension lead to hyperdynamic congestion with a change in gastric mucosal blood flow[112], that leads to activation of cytokines, growth factors, and hormones that perpetuate this hyperdynamic gastric circulation[113]. Vascular congestion in PHG alters the gastric microcirculation, but the nature and extent of this alteration is somewhat controversial. The hyperdynamic circulation of portal hypertension is characterized by increased intrahepatic vascular resistance, generalized splanchnic vasodilatation, decreased mean arterial pressure, decreased systemic vascular resistance, increased gastric blood flow, and most likely decreased gastric mucosal flow[110,114,115]. Hashizume et al[116] reported that cirrhotic patients have dilated small gastric blood vessels, including arterioles, precapillaries, capillaries, submucosal veins, and subserosal veins, with decreased arteriovenous resistance and straightening of arterioles.
This hyperdynamic circulation impairs gastric mucosal defense mechanisms, causes release of proinflammatory mediators, and inhibits growth factors which render gastric mucosa more susceptible to injury[67,117] and impair mucosal healing[113,114,118,119]. This vulnerable mucosa becomes predisposed to bleeding[117,120]. Decreased gastric mucosal perfusion may explain the increased rate of erosions, ulcers, and bleeding in PHG[118]. Abnormal regulation of the gastric microcirculation in PHG may render gastric mucosa more vulnerable to hypoxia[112,122], and more susceptible to noxious gastric factors, such as aspirin and ethanol[123-125].
Misra et al[126] showed that gastric mucosal capillaries, obtained by endoscopic mucosal biopsies, have a much thicker wall in patients with cirrhosis than in healthy volunteers. Ichikawa et al[127] reported a narrower diameter in gastric mucosal capillaries and less capillary angiogenesis, measured as percentage of buds in microvessels, after exposure to ethanol in individuals with PHG as compared to healthy controls. Tarnawski et al[121] reported prominent cytoplasm in endothelial cells of mucosal microvessels, that narrowed the capillary lumina, in rats with PHG. This finding was confirmed by electron microscopy which showed significantly larger cytoplasmic and pinocytic vesicular areas and increased capillary basement membrane thickness. Additionally, there was arterialization of submucosal veins and thickening of arterioles in the muscularis mucosae and submucosa[121].
The level of gastric mucosal blood flow in PHG is controversial. Most studies reported decreased mucosal blood flow in patients with PHG[76,128-131], whereas several studies reported increased gastric mucosal blood flow in experimental animals and in humans with PHG[132-136]. Makhija et al[118] described in PHG a decrease in gastric mucosal blood flow, an increase in the submucosal and muscular layer blood flow, and a net increase in total gastric blood flow. Mezawa et al[76] using a laser Doppler flowmeter to measure gastric mucosal blood flow and near-infrared endoscopy to measure total gastric blood flow, reported decreased mucosal blood flow and increased total blood flow in patients with PHG. These results reversed after undergoing TIPS, with an increase in mucosal blood flow and a decrease in total blood flow. These finding support the hypothesis of decreased mucosal blood flow in patients with PHG. Ohta et al[113] reported a decrease in superficial mucosal blood flow rendering mucosa more susceptible to injury, but noted a net increase in total gastric blood flow. Variability in study results arise from study biases including chronic anemia in some patients, variable measurement techniques, different techniques of applying endoscopic probes in laser-Doppler flowmetry, and differences in gastric mucosal angioarchitecture[113]. Laser-Doppler flowmetry, moreover, has limited utility in clinical practice[137,138].
Portal hypertension increases the splenic circulation[139]. Pan et al[79] reported that PHG severity was strongly correlated with hypersplenism (P = 0.003). However, Abbasi et al[62] did not show this correlation. The difference between these studies may reflect use of different classifications for PHG. Figure 1 describes the hypothesized pathophysiology of PHG. This current mechanism is currently sketchy and likely incomplete.
Figure 1.
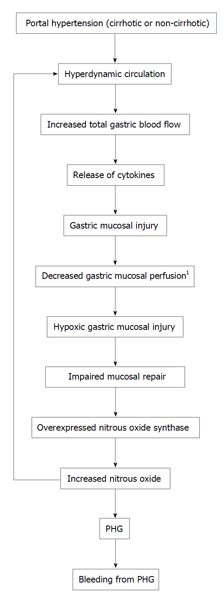
Hypothesized mechanism of portal hypertensive gastropathy. 1The finding of decreased gastric mucosal perfusion in PHG is somewhat controversial (see text). PHG: Portal hypertensive gastropathy.
Patients with secondary polycythemia A have decreased blood flow and oxygen carrying capacity because of sluggish movement of viscous blood; this phenomenon produced endoscopic and histopathologic findings of congestive gastropathy similar to those in PHG that reversed after the patient underwent serial phlebotomies to reverse the polycythemia[140].
Molecular mechanisms: Numerous molecular and cellular mechanisms have been investigated regarding the pathogenesis of PHG.
Apoptosis: Wu et al[141] showed that rats with PHG had increased gastric mucosal apoptosis and decreased mucosal proliferation. Recently, a p53-upregulated modulator of apoptosis (PUMA) was reported markedly induced in gastric mucosa in patients or mouse models of PHG. PUMA is modulated by endoplasmic reticulum - stress-induced mucosal epithelial apoptosis in PHG[142]. This effect could promote mucosal injury in PHG.
Free radicals and antioxidants: Kaur et al[143] showed elevated levels of injurious free radicals and lysosomal enzymes and decreased levels of protective antioxidant enzymes in gastric mucosal homogenates from rats with portal hypertension. Kawanaka et al[144] showed impaired endoplasmic reticulum serine/threonine kinase-2 (ERK2) activation after oxidative stress in rat gastric mucosa; ERK2 normally protects against cellular stress by inducing cell proliferation in gastric mucosa. Kinjo et al[145] showed that enhanced nitration of ERK by peroxynitrite is involved in impaired MAPK (ERK) signaling in PHG, which impairs mucosal healing and promotes mucosal injury. The levels of lipid peroxide and nitrotyrosine that tend to promote gastric injury increased significantly in rats with PHG as compared to controls.
Mucin: Wang et al[146] reported significantly reduced expression of mucin mRNA in rat models of portal hypertension induced by partial portal vein ligation. Decreased mucin production may impair gastric mucosal protection. Rats with portal hypertension had significantly greater injury to gastric mucosa than healthy controls after exposure to gastrotoxic compounds. Tomikawa et al[135] reported decreased mucosal gel layer thickness, surface epithelial cell intracellular pH, and oxygenation of gastric mucosal surface in rats with PHG.
Angiogenesis: As aforementioned, the number of angiogenic buds decreased after injury to PHG mucosa. This phenomenon may decrease the reparative capacity of PHG mucosa[127]. However, Tsugawa et al[136] reported humans with PHG had increased vascular endothelial growth factor (VEGF), a potent angiogenic factor. Additionally, rats with PHG had a significant decrease in the SaO2 and PaO2 of the arterial blood gas, and increased levels of VEGF, proliferating cell nuclear antigen (PCNA) expression, and gastric mucosal blood flow in gastric mucosa. They proposed that gastric mucosal hypoxia in portal hypertension and elevation of VEGF and PCNA levels might accelerate mucosal angiogenesis and increase blood flow[147].
Tumor necrosis factor alpha: Tumor necrosis factor alpha (TNF-α) may directly contribute to the hyperdynamic circulation in PHG. Patients and animal models with portal hypertension had an elevated TNF-α level which stimulated release of nitric oxide (NO) and prostacyclin, important mediators of a hyperdynamic circulation[148]. For example, in one study, 96 healthy rats were injected with either anti-TNF-α polyclonal antibodies or placebo before surgically creating portal vein stenosis (PVS) to induce portal hypertension and 4 d after in the short-term inhibition group and 1, 4, 7 and 10 d after PVS in the long term-inhibition group. Anti-TNF-α treated PVS rats exhibited lower serum levels of TNF-α, which normally stimulates the synthesis of NO and prostacyclin, and exhibited lower serum levels of nitrates and nitrites and of 6-keto-PGF-1-α (6-keto-PGF1α), used to monitor NO and prostacyclin release, respectively. The combined nitrate and nitrite level was significantly reduced from 68 ± 9 nmol/mL in controls to 42 ± 8 nmol/mL in the short-term inhibition group (P < 0.05), and from 66 ± 6 nmol/mL in controls to 44 ± 4 nmol/mL in the long-term inhibition group (P < 0.05). Similarly the 6-keto-PGF1α was significantly reduced from 484 ± 92 pg/mL in the controls to 174 ± 12 pg/mL in the short-term inhibition group (P < 0.05), and from 522 ± 98 pg/mL in the controls to 169 ± 18 (SD) pg/mL in the long-term inhibition group (P < 0.05). Kaviani et al[149] reported that TNF-α increased by 50% and inducible nitric oxide synthase (iNOS) mRNA levels increased by 300% in gastric strips after ligating the portal vein in rats (P < 0.01 for both). These data are consistent with TNF-α playing a role in the hyperdynamic circulation in PHG via NO and prostacyclin.
Baseline constitutional NOS (cNOS) mRNA expression increased by 75% in the PHG group as compared to placebo (P < 0.01)[149]. NOS was significantly reduced after injecting a TNF-α neutralizing antibody during incubation of mucosal strips from portal hypertensive rats; the expression of inducible NOS mRNA levels was incrementally decreased by 40%, 70% and 80% after 1, 2, and 6 h of incubation, respectively (P < 0.05)[149]. Ohta et al[150] similarly successfully used TNF-α antibody to normalize gastric mucosal blood flow in rats with PHG and to significantly reverse overexpression of gastric NOS isoform 3. In PHG rats, treatment with TNF-α antibody significantly reduced the elevated NOS isoform 3 mRNA expression by 48% (P < 0.01). Moreover, administration of thalidomide, which enhances TNF-α mRNA degradation, decreased levels of TNF-α and NOS in animals with portal hypertension produced by partial portal vein ligation[114].
Nitric oxide: Patients with portal hypertension and PHG have increased serum levels of NO, a potent vasodilator released by endothelial cells. Ohta et al[151] demonstrated gastric cNOS significantly increased, by 67%, in portal hypertensive rats, experimentally produced by portal vein and splenic vein occlusion, as compared to sham-operated rats at 14 d after surgery (P < 0.05). In portal hypertensive rats, cNOS fluorescence intensity was significantly higher in endothelia of submucosal veins [96.2 ± 5.9 (SD) U] as compared to endothelia of mucosal collecting veins (69.5 ± 1.7 (SD) U, P < 0.01), or endothelia of veins of muscularis mucosae (55.7 ± 10.0 U, P < 0.01). The average fluorescence area in submucosal vein endothelia was significantly higher in portal hypertensive rats than in normal controls (1038.5 ± 459.5 (SD) μm2 vs 372.4 ± 180.3 (SD) μm2, P < 0.01). This finding may provide a molecular mechanism for submucosal vascular dilation in the hyperdynamic circulation in PHG. In another study, gastric mucosal cNOS levels were significantly higher in patients with cirrhosis and severe PHG compared to healthy controls [125.4 ± 4.3 (SD) pmol/mg protein/minute vs 88 ± 8.6 (SD) pmol/mg protein/minute, P < 0.002]. Likewise, gastric mucosal iNOS levels were significantly higher in patients with cirrhosis and severe PHG than in healthy controls [259.7 ± 5.5 (SD) pmol/mg protein/min vs 130.8 ± 6.6 (SD) pmol/mg protein/min, P < 0.0001][152]. Serum nitrate/nitrite levels were 30.1 ± 3.2 nmol/mL in the first group vs 15.5 ± 0.09 (SD) nmol/mL in the second group (P < 0.001)[152]. In another study, iNOS and cNOS levels were also higher in gastric mucosa of patients with PHG than in controls[153], and were significantly higher in patients with severe PHG as compared to patients with mild or no PHG[154]. Nitrous oxide may underlie the gastric vascular dilation[152], and hyperdynamic circulation in PHG[148].
However, Lee et al[155] reported administration of aminoguanidine, an iNOS inhibitor, successfully corrected the hyperdynamic circulation without affecting PHG, suggesting that iNOS and NO are important in the hyperdynamic circulation in portal hypertension, but play a limited role in PHG development. They argued that PHG should be treated by reducing portal pressure rather than reversing the hyperdynamic circulation[155,156].
Glucagon: Glucagon levels are elevated in patients with portal hypertension[118]. Curvêlo et al[54] found in 43 patients with PHG from portal hypertension with cirrhosis, that the mean serum glucagon level after an overnight fast was significantly higher than the level in healthy controls. Serum glucagon levels were significantly correlated with high systemic vascular resistance index (r = -0.523; P = 0) and HVPG (r = 0.34; P = 0.019). Glucagon significantly increases portal pressure[157-159], and causes splanchnic vasodilation[148]. Geraghty et al[158] found a strong correlation between portal pressure and glucagon levels (r = 0.85). Tsui et al[160] reported that glucagon significantly increased portal pressure in rats with portal vein ligation, but did not alter portal pressure in sham-operated rats. The effect of glucagon occurred only in rats with preexisting portal hypertension. Exogenous glucagon rendered gastric mucosa more susceptible to injury from toxins, such as ethanol, which was attenuated by somatostatin[160]. For example, the lesion area was significantly higher at > 60% of gastric mucosa after glucagon administration, compared to somatostatin or glucagon and somatostatin administration (P < 0.05, ANOVA)[160].
Prostaglandins: Studies in patients or animal models with portal hypertension failed to show significant differences in prostaglandin E2 (PGE2) levels as compared to healthy controls[125,141,161-163]. Low prostaglandin levels significantly decreased gastric perfusion velocity in cirrhotic rats, whereas misoprostol, a PGE2 analogue, significantly increased gastric perfusion in cirrhotic rats as compared to controls[125]. For example, Beck et al[125] found that administration of indomethacin did not affect gastric perfusion velocity in healthy control rats, despite reducing gastric PGE2 synthesis by > 95%, but reduced gastric perfusion velocity by 30% within 10 min in cirrhotic rats achieved by ligating the common bile duct (P < 0.05). The hyperemic response to application of ethanol was significantly reduced in cirrhotic rats compared to healthy rats (56.3% ± 21.7% (SD) vs 66.1% ± 17.1% (SD) increase, P < 0.05). Misoprostol applied to gastric mucosa caused concentration-dependent increase in perfusion velocity, with a significantly greater increase in perfusion velocity in cirrhotic rats with concentrations of misoprostol > 0.8 mcg/mL (P < 0.05).
Beck et al[164] further reported that administration of misoprostol to cirrhotic rats for 1 mo restored the hyperemia in response to ethanol that sham-operated, non-cirrhotic rats showed, whereas placebo-treated cirrhotic rats failed to increase gastric blood flow in response to ethanol. PGE2-treated cirrhotic rats exhibited significantly less spontaneous gastric mucosal damage [0.2% ± 0.07% (SD)] than placebo-treated cirrhotic rats [3.0% ± 0.8% (SD); P < 0.05]. The mean microscopic gastric injury score was significantly less in PGE2-treated cirrhotic rats [0.7 ± 0.3 (SD)] than in placebo-treated cirrhotic rats [2.1 ± 0.4 (SD); P < 0.05].
Rats with PHG exhibited suppression of gastric mucosal COX-1 levels, but exhibited normal COX-2 levels compared to healthy controls[141]. Nonselective COX inhibitors, such as aspirin, decrease PGE2 levels resulting in more apoptosis of cells. Payen et al[123] reported that gastric mucosal potential difference, an index of mucosal integrity, decreased with increasing severity of PHG, suggesting greater vulnerability of gastric mucosa in patients with PHG. Payen et al[123] further reported a significantly greater decline of potential difference after aspirin administration of 11.1 ± 3.6 (SD) mV in 9 patients with severe PHG, vs 9.2 ± 3.6 mV in 21 patients with moderate PHG (P < 0.05), and vs 6.4 ± 1.9 (SD) mV in 10 healthy controls (P < 0.05). Also, PGE2 administration suppressed the increased apoptosis which occurred in rats with PHG.
Prostacyclin: Prostacyclin, a vasodilator that inhibits gastric acid secretion, has been proposed as a mediator of the hyperdynamic circulation in PHG from portal hypertension[148,163]. Ohta et al[161] found significantly elevated serum levels of 6-keto-PGF1α, a metabolite of prostacyclin, in cirrhotic patients with PHG. They also reported that these patients had significantly elevated levels of 6-keto-PGF1α in the mucosa of the gastric fundus.
Other cytokines and growth factors: Several studies have analyzed the roles of endothelin-1, VEGF, and other cytokines in PHG, but further research is required[165]. The gastric concentrations of epidermal growth factor were comparable between patients with and without PHG, and its significance in PHG remains unclear[166]. A high serum level of autotaxin, involved in liver fibrosis, was associated with advanced stage of cirrhosis, presence of esophageal varices, and PHG[167].
Helicobacter pylori: Numerous studies demonstrated that Helicobacter pylori (H. pylori) infection is not associated with PHG[7,9,25,79,109,110,154,168-172]. Indeed, several studies reported that patients with PHG less frequently have H. pylori infection than controls. H. pylori does not appear to play a pathogenic role in ulcers associated with PHG[173]. Contrariwise, Sathar et al[174] reported an association between H. pylori infection and PHG in cirrhotic patients, but this study apparently had limitations, including low specificity and low sensitivity of H. pylori serology in cirrhotic patients, potential selection bias, and underreporting of H. pylori seroprevalence[174-178].
Diagnosis
Endoscopy: PHG is diagnosed by EGD[72]. The characteristic endoscopic appearance is a mosaic-like pattern or a diffuse, erythematous and reticular cobblestone pattern of gastric mucosa consisting of small polygonal areas, with superimposed red punctate lesions, > 2 mm in diameter and a depressed white border[78,96,177]. The red lesions vary in size and in color depending on PHG severity. The lesions range from pink speckled lesions within a mosaic or snakeskin pattern in mild cases, to localized small areas of intense erythema, resembling a scarlatina rash, in severe cases[70,71,112,139]. These findings occur predominantly in the gastric body and fundus, and rarely in the antrum[71,72,139]. Figure 2 illustrates a patient with classic endoscopic findings of portal hypertensive gastropathy. Toyonaga et al[178] reported in a meta-analysis of 6 studies that the mosaic-like pattern had high specificity at 98% (range: 93%-100%), but low sensitivity at 38% (range: 7%-94%) for PHG, with an accuracy of 78% (range: 63%-98%). In severe PHG numerous petechiae and bleeding spots present as a diffuse hemorrhagic gastropathy[112,139].
Figure 2.
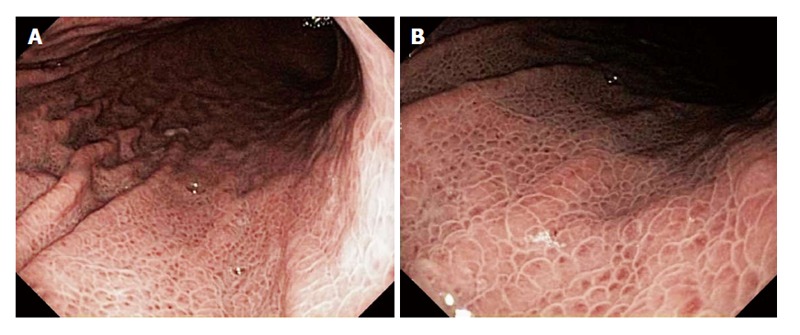
A 60-year-old man presented for routine endoscopic screening for esophageal varices due to a history of Child-Pugh class B cirrhosis, with a model for end-stage liver disease score = 18, from hepatitis C secondary to former intravenous drug use. The patient denied a history of gastrointestinal bleeding. The hematocrit was 40.1%. Esophagogastroduodenoscopy revealed the classic findings of portal hypertensive gastropathy, including a pale white reticular (mosaic) pattern surrounding small polygonal areas of mucosa, with variable erythema, in the entire stomach, but most prominently in the gastric fundus and body. B is a relatively close-up view of the lesions seen in A.
Endoscopic classification: Endoscopic classification of PHG severity is clinically important because severity is correlated with bleeding risk[31,71,72,179]. PHG can be simply categorized as mild with a mosaic-like pattern without red spots, or as severe, with superimposed red lesions present[78,96]. Multiple formal endoscopic classifications exist (Table 8[3,7,8,70,180-183]), with no consensus as to which classification is the best[5,182-184]. The following classifications are commonly used: Classifications by McCormack et al[3], Tanoue et al[180], the New Italian Endoscopic Classification (NIEC)[70], and the Baveno scoring system[181].
Table 8.
Different classification systems for portal hypertensive gastropathy
| Ref. | Mild | Moderate | Severe |
| McCormack et al[3] | Fine pink speckling (scarlatina type rash) | Discrete red spots (analogous to cherry | |
| Superficial reddening, especially on rugal surface (striped appearance) | red spots in esophagus) | ||
| Fine white reticular pattern separating areas of raised edematous mucosa (snake skin) | Diffuse hemorrhagic gastritis | ||
| McCormick et al[7] | Mosaic or snake skin appearance | Presence of erythema | Presence of erosions or hemorrhagic gastritis |
| Tanoue et al[180] | Mild reddening, congestive mucosa, no mosaic - like pattern | Severe redness and a fine reticular pattern separating the areas of raised edematous mucosa (mosaic-like pattern) or a fine speckling | Grade III (severe) |
| Point bleeding + grade II (moderate) | |||
| Spina et al[70] (NIEC) | Mosaic-pattern: Presence of small, | Red point lesions (1 mm in diameter, flat) | |
| polygonal areas surrounded by a | Cherry-red spots (2 mm, slight protrusion) | ||
| whitish-yellow depressed border | Black-brown spots (irregularly shaped, persistently present after washing) | ||
| Sarin et al[8] | Discrete cherry red spots, with or without mosaic pattern | Presence of confluent red spots, diffusely distributed in a large portion of the stomach | |
| Sarin et al[181] (Baveno II Consensus Workshop)[181] | Mild ≤ 3 points1 | Severe ≥ 4 points1 | |
| Gastric antral ectasia | |||
| Absent (0) | |||
| Present (2) | |||
| Yoo et al[182] | Fine pink speckling (scarlatina type rash) | Discrete red spots | |
| 2-category classification | Superficial reddening | Diffuse hemorrhagic lesion | |
| Mosaic pattern | |||
| Yoo et al[182] | Mild reddening | Flat red spot in center of a pink areola | Diffusely red areola |
| 3-category classification | Congestive mucosa | Severe redness and a fine reticular pattern | Pinpoint bleeding |
| Diffuse pink areola | Discrete or confluent red mark lesion |
Points assigned for Baveno II consensus according to the following: Mild mucosal mosaic pattern = 1 point, severe mucosal mosaic pattern = 2 points; isolated red markings = 1 point, confluent red markings = 2 points; gastric antral ectasia present = 2 points.
In 1985, McCormack et al[3] classified PHG according to presence of red spots into mild and severe disease. In 1991, McCormick et al[7] divided the prior single category of mild PHG into mild and moderate PHG based on absence vs presence of erythema, respectively. This moderate category is infrequently used[7]. In 1992 Tanoue et al[180] and Parker et al[185] also expanded the scoring system by providing a grade between mild and severe. This classification is rendered cumbersome by a lack of sharply defined differences between the added intermediate category and the original categories[71]. This system is, however, simple and can help predict bleeding risk[71,182]. Iwao et al[186] found McCormack’s classification was accurate for fine pink speckling in 54%, for snakeskin pattern in 76%, and for cherry-red spots in 64%. The NIEC produced a better definition in 1992 of mild and severe HPG[32,34,70]. Elementary lesions of PHG according to the NIEC classification include: (1) mosaic-like pattern defined as small, polygonal areas surrounded by a whitish-yellow, depressed border. This mosaic pattern is mild when the areola is uniformly pink, moderate if the center is red, and severe if the areola is uniformly red; (2) red-point lesions defined as small, flat, 1-mm-wide, punctate, red lesions; (3) cherry-red spots defined as red, 2-mm-wide, round lesions which protrude slightly into the gastric lumen; and (4) black-brown spots defined as irregularly shaped flat black or brown spots from intramucosal hemorrhage that remain after endoscopic irrigation. PHG is defined as mild when only a mosaic-like pattern of any degree was present, and severe when red-point lesions, cherry red spots, or black-brown spots were present. Due to variable data on the classification systems, Hashizume et al[184] proposed a simplified classification that divides PHG into three stages by presence of: Non-specific redness, a mosaic pattern, and red spots.
Yoo et al[182] demonstrated substantial limitations in intra-observer and inter-observer reproducibility in the most common 2-scoring and 3-scoring systems. Nevertheless, the 2-scoring system by McCormack et al[3,7] produced better and more reproducible results than the 3-scoring system by Tanoue et al[180]. The mean inter-observer kappa value was 32% higher and mean intra-observer kappa value was 15% higher for the 2-scoring system compared with the 3-scoring system. However, both inter-observer and intra-observer kappa values in both classification systems were below the desirable value of > 0.75[187]. Kappa values represent the degree of agreement as compared with that expected by chance alone, with one being perfect agreement, and zero being no greater agreement than expected by chance alone[182].
The Baveno scoring system uses point calculations to define PHG as mild (≤ 3 points) vs severe (≥ 4 points)[181]. This system adds gastric antral vascular ectasia (GAVE) into the classification[179]. Stewart et al[179] showed that this scoring system was reproducible and accurately reflected the risk of PHG-related bleeding in cirrhotic patients. Kappa values for mucosal mosaic pattern, red marks, and GAVE were > 0.75, indicating good reproducibility. Kappa values for lesion severity were lower, attributed to loss of details in endoscopic photographs.
de Macedo et al[187] proposed analyzing binary criteria such as presence vs absence of mosaic-like pattern, red punctate lesions, and cherry-red spots, without subdivisions or classification systems. These binary criteria were associated with high inter-observer reliability and accuracy (94%, 81% and 83% respectively). Mosaic-like pattern was associated with high sensitivity (100%). The previously used classifications and subdivisions showed unsatisfactory reliability and low inter-observer agreement.
Current classification systems are suboptimal. The ideal classification system should be simple, clinically useful, accurate, and reproducible with high levels of intra-observer and inter-observer agreement[183]. The 2-scoring system is most commonly used due to relative simplicity and reasonable reproducibility[71].
Capsule endoscopy: Several studies evaluated the diagnostic accuracy of capsule endoscopy. In a study using the PillCam ESO capsule, capsule endoscopy had an overall concordance with EGD of 90.6% for PHG[188]. This study included only 32 patients of whom 19 had PHG, and a large trial is underway to confirm these findings. In another study, PHG was identified by capsule endoscopy in 13 (68.4%) of 19 patients with cirrhosis, portal hypertension, and chronic anemia, but the 19 patients did not undergo EGD to determine capsule endoscopy test sensitivity and specificity[49]. In a study of 50 patients with cirrhosis undergoing both EGD and capsule endoscopy for screening or surveillance of esophageal varices, capsule endoscopy had an accuracy of 57%, sensitivity of 96%, and specificity of 17% compared to EGD. Inter-observer reliability was 0.61. The researchers concluded that more data are required to assess accuracy of capsule endoscopy for diagnosis and staging of PHG[52]. In another study of 50 patients with portal hypertension undergoing EGD and capsule endoscopy, only 24 of 35 patients with PHG diagnosed by EGD had PHG detected by capsule endoscopy (sensitivity = 69%)[23]. Capsule endoscopy was somewhat more sensitive at detecting severe than mild PHG (82% vs 63%), but this difference was not significant (P = 0.44). The accuracy was significantly higher in diagnosing PHG in the gastric body (100%) than the fundus (48%) (P = 0.0009).
Dynamic CT: Kim et al[50] proposed using dynamic CT to diagnose PHG by demonstrating the transient perfusion defect sign, defined as the presence of transient segmental or subsegmental hypo-attenuating mucosa in the gastric fundus or body during hepatic arterial imaging that returns to normal attenuation on portal venous or equilibrium-phase imaging. This sign had a sensitivity of 75%, specificity of 88.6%, positive predictive value of 90%, and negative predictive value of 72.1% for diagnosing PHG in patients with cirrhosis. Further prospective trials are required to validate this diagnostic modality.
Screening for PHG is currently not recommended in patients with liver disease[189]. To identify predictors of PHG and varices noninvasively in patients with chronic liver disease to increase the cost-benefits of EGD, Min et al[190] combined three independent parameters in a multivariate analysis into a “Varices and PHG” (VAP) score. The score = platelets/mm3 × albumin in g/dL/multidimensional index for spleen volume (M-Index) in cm3. The M-Index is calculated from spleen length, width, and thickness, as determined by helical computerized tomography, which is designed to reflect splenomegaly as a predictor of esophageal varices and PHG. A VAP cut-off value of 861 had a sensitivity of 85%, positive likelihood ratio of 3.17, and negative predictive value of 86%. This scoring system requires prospective validation[190].
Differentiation from GAVE: Differentiation of PHG from GAVE is important because they have distinct pathologic, clinical, and endoscopic characteristics, and different therapies (Table 9)[34,37,71,72,75,77,103,106,191-213]. Treatments that reduce portal pressure are effective for PHG but ineffective for GAVE[193]. PHG and GAVE also affect different gastric locations. PHG generally affects the proximal stomach, whereas GAVE generally affects the distal stomach[139]. A mosaic-like pattern surrounding polygonal areas of erythema is typical for PHG, but GAVE has erythema most commonly arranged linearly along folds in the antrum, less commonly arranged as diffuse erythema in the antrum, and least commonly arranged as diffuse gastric erythema[75,78,96].
Table 9.
Differences between portal hypertensive gastropathy and gastric antral vascular ectasia
| Parameter | Portal hypertensive gastropathy | Gastric antral vascular ectasia | Ref. |
| Associated conditions | Conditions associated with portal hypertension: cirrhotic or non-cirrhotic portal hypertension | Cirrhosis, autoimmune disorders, and connective tissue diseases (scleroderma, pernicious anemia, hypothyroidism) | [72] |
| Association with portal hypertension | Strong association | Only 30% of cases | [191,192] |
| Sex | Mildly more common in males (alcoholic cirrhosis more common in males than females) | Much more common in females (80%) | [193,194] |
| Age | Can occur at any age in patients with portal hypertension or cirrhosis | Typically elderly (average age > 70 years old) | |
| Location | Proximal stomach: Fundus, body | Distal stomach: Antrum | [72,192] |
| Diagnosis | Endoscopy (endoscopic biopsy sometimes useful). Radiologic imaging usually not helpful | Endoscopy (endoscopic biopsy sometimes useful) | [72,195] |
| Appearance at endoscopy | Mosaic/snakeskin mucosa with red or brown spots | Tortuous columns of ectatic vessels in "watermelon" or diffuse pattern; erythematous or hemorrhagic | [191] |
| Histology | Ectatic capillaries, mildly dilated mucosal and submucosal veins; no vascular inflammation, no vascular thrombi | Marked dilation of capillaries and venules in gastric mucosa and submucosa with areas of intimal thickening, fibrin thrombi, fibromuscular hyperplasia and spindle cell proliferation | [72,191,196,197] |
| Clinical presentation/ complications | Gastrointestinal bleeding: Usually chronic, but sometimes acute | Almost exclusively chronic gastrointestinal bleeding with guaiac positive stools | [37,193] |
| Primary prophylaxis | Not indicated | Not indicated (unless associated with large varices) | [198] |
| Medical therapy | Non-selective β-adrenergic receptor antagonists (propranolol), octreotide (for acute bleeding) | No benefit of β-adrenergic receptor antagonists | [103,106,198-201] |
| Oral contraceptive pills to temporarily control bleeding | |||
| Questionable benefit of octreotide | |||
| Endoscopic therapy | Occasionally helpful (for focal bleeding) | Very helpful at reducing risk of bleeding: Argon plasma coagulation; EBL; Radiofrequency ablation; YAG laser therapy | [202-207] |
| Argon plasma coagulation | |||
| Local hemostasis with hemospray | |||
| TIPS | Significantly reduces severity and risk of bleeding by reducing portal hypertension. Option for very severe bleeding from PHG or for moderate PHG in patients with variceal bleeding | Not recommended. Does not affect severity of GAVE or risk of bleeding | [75,77] |
| Liver transplantation | Resolves. Ultimate therapy mostly reserved for patients with end-stage liver disease | Improves or resolves with liver transplantation | [75,200,208-210] |
| Other surgery | Usually resolves with shunt surgery that lowers portal pressure. Partial gastrectomy not recommended | Limited surgical resection (partial gastrectomy) recommended for refractory cases. Shunt surgery not recommended | [75,200,211-213] |
| Prognosis from bleeding | Bleeding rarely severe and very rarely fatal | Bleeding occasionally severe | [34,71,72] |
YAG: Yttrium aluminum garnet; TIPS: Transjugular intrahepatic portosystemic shunt; PHG: Portal hypertensive gastropathy; GAVE: Gastric antral vascular ectasia; EBL: Endoscopic band ligation.
PHG is usually diagnosed by endoscopic criteria. When endoscopic features are uncertain, histologic analysis of gastric biopsies is useful to differentiate PHG from GAVE[86,183,197,212]. Superficial mucosal biopsies are frequently falsely negative because the lesions of PHG are generally submucosal[214,215]. Endoscopists are reluctant to perform deep biopsies in patients with known portal hypertension or suspected PHG because of increased risks of bleeding because of a coagulopathy from underlying cirrhosis or a bleeding diathesis from underlying portal hypertension[137,183]. However, deep biopsies may be necessary for the histologic diagnosis of PHG.
Characteristic histologic findings of PHG include capillary and venule dilatation, and markedly congested and tortuous submucosal venules[137]. Stromal fibrosis and edema of lamina propria can occurs[137]. Inflammatory cells and fibrin thrombi are generally absent[3,139]. Characteristic histologic features of GAVE include presence of fibrin thrombi in dilated capillaries and fibromuscular proliferation within the lamina propria[96,216].
Differentiating GI bleeding from varices vs PHG: PHG may occasionally resemble gastric varices at EGD. PHG can be prominent on gastric rugae in the gastric body and fundus. The intraluminal linear projections of gastric rugae might superficially resemble that of gastric varices. However, gastric varices tend to be more serpiginous than linear and tend to be grayish due to the presence of deoxygenated venous blood within varices, whereas the lesions of PHG on gastric rugae tend to be erythematous and surrounded by a prominent mosaic pattern. It is also important to distinguish between GI bleeding from esophageal varices vs PHG in patients having both lesions. Table 10 outlines differences in GI bleeding from PGH vs esophageal varices.
Table 10.
Differences in gastrointestinal bleeding from portal hypertensive gastropathy vs esophageal varices
| Parameter | Portal hypertensive gastropathy | Esophageal varices |
| Etiology | Portal hypertension: Cirrhotic or non-cirrhotic | Portal hypertension: Cirrhotic or non-cirrhotic |
| Concurrence | Frequently occur simultaneously with esophageal varices because the two diseases share common risk factors | Frequently occurs simultaneously with PHG because the two diseases have common risk factors |
| Location | Stomach: Predominantly fundus and body | Distal esophagus: Also can have gastric varices or ectopic varices in other gastrointestinal regions, particularly duodenum |
| Diagnosis | Esophagogastroduodenoscopy | Esophagogastroduodenoscopy |
| Endoscopic appearance | Erythematous small polygonal areas of mucosa surrounded by a fine, whitish, reticular or mosaic/snakeskin mucosa with red or brown spots | Serpiginous mucosal greyish luminal projections in distal esophagus |
| Clinical presentation | Mild acute or chronic bleeding | Acute gastrointestinal bleeding-typically massive |
| Severity of bleeding | Typically mild and not life-threatening | Typically severe and life-threatening |
| Histology | Not biopsied at endoscopy | |
| Endoscopic therapy | Limited role | Variceal ligation recommended as initial therapy. Sclerotherapy an alternative therapy |
| Medical therapy | Octreotide | Octreotide |
| Propranolol | Propranolol | |
| Vasopressin or vasopressin analogues-infrequently recommended any more | Vasopressin or vasopressin analogues-infrequently recommended any more | |
| Blakemore tube | Not recommended | Sometimes used for refractory bleeding especially as a temporizing measure before performing more definitive therapy |
| Angiographic therapy | TIPS used as a last resort | TIPS recommended if endoscopic therapy fails |
| Transfusion of packed erythrocytes | Transfuse only to hematocrit of about 28. Over-transfusion may increase portal pressure and induce greater bleeding | Transfuse only to hematocrit of about 28. Over-transfusion may increase portal pressure and induce greater bleeding |
| Liver transplantation | Improves or resolves with liver transplantation | Improves or resolves with liver transplantation |
| Prognosis | Rarely fatal | Frequently fatal |
TIPS: Transjugular intrahepatic portosystemic shunt; PHG: Portal hypertensive gastropathy.
Clinical presentation
Acute GI bleeding: GI bleeding is the only known clinically relevant complication of PHG. PHG is responsible for < 1% of upper GI bleeding in the general population, and for about 8% of non-variceal upper GI bleeding in patients with liver disease[217]. The reported frequency of acute upper GI bleeding in patients with PHG ranges in incidence from 2%-20% (Table 11[3,8,25,34,37,103,217,218]). Primignani et al[34] reported acute GI bleeding in 2.7% of patients with PHG, whereas Stewart et al[179] reported a 20% incidence of acute bleeding in patients with PHG. McCormack et al[3] reported that 29 (44.6%) of 65 patients with PHG bled from this lesion. In this study, PHG was the second most common cause of GI bleeding, after esophageal varices[3]. The reported variability is partly due to inaccuracies in the endoscopic diagnosis of PHG and in the endoscopic diagnosis of PHG as the cause of bleeding[71]. Diagnosis of PHG as the cause of bleeding can be challenging if a bleeding point is not visualized at EGD[71].
Table 11.
Rates of gastrointestinal bleeding from portal hypertensive gastropathy
| Ref. | Year published | Population | Bleeding from PHG | Transfusions required |
| McCormack et al[3] | 1985 | 127 patients with portal hypertension of various etiologies | 29 patients out of 65 with PHG, representing 25% of the total number of bleeds from all sources; 9 episodes presenting with bleeding; 71 episodes of subsequent bleeding | 2-15 units required for 60 bleeds |
| D'Amico et al[25] | 1990 | 212 patients with cirrhosis; 75 being treated with sclerotherapy | ||
| Sarin et al[8] | 1992 | 107 patients with portal hypertension presenting with variceal bleeding, undergoing sclerotherapy | No bleeding before sclerotherapy from PHG (4/107 had PHG); 2/13 post-sclerotherapy patients who developed PHG | Average of 4 units per patient with range of 2-8 units |
| Pérez-Ayuso et al[103] | 1991 | 54 cirrhotic patients with PHG, in a RCT to look for rebleeding; propranolol 26 vs control 28 | First hemorrhage: Acute/chronic bleeding in propranolol group 12/14; in control 12/16, rebleeding: Acute/chronic; in propranolol 6/6; in control 10/12 | |
| Gostout et al[217] | 1993 | Patients admitted for GI bleeding (1496) | 12 patients (0.8%), representing 8% of nonvariceal bleeding in patients with liver disease | |
| Primignani et al[34] | 2000 | 373 patients with cirrhosis; PHG in 299 patients (80.1%) | 8 PHG patients with acute bleeding; chronic bleeding in 34 patients | |
| Merli et al[37] | 2004 | 222 cirrhotic patients with portal hypertension; 48 patients with PHG on enrollment | During follow up for 47 ± 28 (SD) mo, acute bleeding 9, chronic bleeding 7 from PHG | |
| Kimura et al[218] | 2014 | 297 patients with living donor liver transplantation; retrospective analysis | 2 patients bled from PHG within 3 mo after transplantation |
PHG: Portal hypertensive gastropathy; RCT: Randomized controlled trial; GI: Gastrointestinal.
Major risk factors for bleeding from PHG are increasing PHG duration, extent, and severity[72,179]. For example, > 90% of acute bleeding occurs with severe PHG[10,35,71,72], and < 10% of acute bleeding occurs with mild PHG[112]. Other risk factors for bleeding from PHG include advanced cirrhosis, and prior endoscopic eradication of esophageal varies[3,5,10,81,179,180,219]. Unlike bleeding from GAVE, acute bleeding from PHG is rarely severe, very rarely fatal, and typically requires transfusion of only one-to-two units of packed erythrocytes or less[34,71,72,139].
Chronic bleeding: The frequency of chronic bleeding ranges from 3%-26%[25,34,37]. Stewart et al[179] reported a 6% incidence of chronic bleeding from PHG, whereas Primignani et al[34] reported chronic bleeding in 11% of patients with PHG (Table 11[3,8,25,34,37,103,217,218]).
The incidence of chronic gastrointestinal bleeding from PHG is difficult to determine precisely because of variable definitions of chronic GI bleeding[67,68]. Common definitions include: (1) > 2 g/dL decrease in hemoglobin level during > 6 mo in patients without acute GI bleeding and not receiving NSAID therapy; (2) presence of anemia in patients with cirrhosis; and (3) positive fecal occult blood (Baveno II)[181]. Moreover, chronic GI bleeding can be overestimated if hemoglobin decline is solely used for the diagnosis. Patients with chronic liver disease frequently have anemia without GI bleeding, from causes including alcoholism, chronic kidney disease, hypersplenism, or bone marrow suppression. No studies have objectively quantified chronic blood loss from PHG.
Chronic bleeding from PHG is usually mild to moderate but occasionally severe[37,139]. Patients after endoscopic variceal obliteration have a higher incidence of chronic GI bleeding from PHG[10,25,72]. Chronic GI bleeding from PHG can cause iron deficiency anemia[220,221].
Pharmacotherapy for PHG
Current pharmacologic therapies aim to reduce portal pressure to decrease bleeding from PHG[191,214].
β-adrenergic receptor antagonists: Nonselective β-adrenergic receptor antagonists reduce portal pressure and gastric mucosal blood flow, and thereby reduce bleeding from PHG[103,104,132,191,222-224]. Several studies evaluated the efficacy of propranolol, a nonselective β-adrenergic receptor antagonist in primary and secondary prevention of bleeding from PHG[104,225]. Pérez-Ayuso et al[103] reported in a multi-center, randomized, controlled trial of 57 patients with acute or chronic bleeding from severe PHG with cirrhosis that the 26 patients administered propranolol at 20-160 mg twice daily rebled significantly less frequently than the 31 controls receiving only iron therapy as needed at 12 mo (38% vs 65%; P < 0.05), and at 30 mo follow-up (7% vs 52%, P < 0.05). Patients receiving propranolol were transfused less units of packed erythrocytes than the controls, but this difference was not statistically significant [0.10 ± 0.06 (SD) units/mo vs 0.60 ± 0.20 (SD) units/mo, P = 0.08].
Propranolol also reduced the risk of developing PHG after esophageal variceal eradication[81]. Lo et al[81] reported in a randomized, controlled trial that 40 patients receiving placebo had a significant increase in PHG severity after variceal ligation (P < 0.01, ANOVA), but the 37 patients receiving propranolol [mean dose = 96 ± 20 (SD) mg/d] had no significant increase in PHG severity. The frequency of PHG 6 mo after variceal ligation was significantly less in patients receiving propranolol than in the controls (48% vs 85%, P = 0.002). This difference gradually decreased over time and became not significant at 12 mo. Also, the mean PHG severity score was lower in patients receiving propranolol than in the controls at 6 mo after variceal ligation (P < 0.05).
Propranolol at 240-480 mg/d has been used to arrest acute bleeding from PHG. Bleeding stopped within 3 d in 13 (93%) of 14 patients with portal hypertension administered propranolol in one study[101]. None of these patients rebled while receiving propranolol during a median of 23 mo of follow-up, but 4 out of 7 patients rebled after electively discontinuing propranolol therapy[104]. Nonresponse to β-adrenergic receptor antagonists, defined as continued bleeding despite this therapy and transfusion-dependency despite iron replacement therapy, should prompt consideration of interventional therapies[214].
Nonspecific β-adrenergic receptor antagonists are a first line therapy for secondary prophylaxis of PHG bleeding[191,208]. Nadolol alone was as effective as nadolol with isosorbide mononitrate in preventing the first episode of PHG bleeding[226]. No studies have analyzed the efficacy of carvedilol, another nonspecific β-adrenergic receptor antagonist, in controlling bleeding from PHG[227].
Somatostatin and octreotide: Somatostatin and octreotide, a synthetic somatostatin analogue, cause splanchnic vasoconstriction, reduce portal pressure, reduce portal blood flow, and decrease gastric perfusion in animal models and in patients with PHG[105,106,160,228-231]. For example, Chan et al[228] found octreotide infusion, compared to placebo, significantly increased systemic vascular resistance [3.4 ± 0.2 (SD) mmHg/mL per minute per 100 g vs 2.7 ± 0.2 (SD) mmHg/mL per minute per 100 g, P < 0.05], and significantly decreased portal pressure [9.9 ± 0.5 (SD) mmHg vs 12.5 ± 1.2 (SD) mmHg, P < 0.05] in cirrhotic rats. Another study found that somatostatin only modestly reduced portal pressure in PHG[232]. Octreotide treatment also significantly reduced mean cross-sectional area of gastric mucosal vessels compared to placebo [1810 ± 101 (SD) microns vs 2290 ± 145 (SD) microns, P < 0.05], and significantly inhibited release of several vasoactive gastrointestinal polypeptides, gastric acid, and pepsinogen[106,233]. In the stomach, somatostatin activates K+-ATP channels (adenosine triphosphate-dependent potassium channels) which normally protect against gastric mucosal injury in the presence of portal hypertension, and antagonizes the portal hypertensive and injury-promoting effects of glucagon[160].
Octreotide is a first-line treatment for acute bleeding from PHG. In a randomized controlled trial, octreotide, at 100 mcg bolus followed by infusion of 25 mcg/min for the first 24 h and then 20 mcg/min for the second 24 h, controlled bleeding from PHG in 20 (83%) of 24 patients at 24 h and in 24 (100%) of 24 patients at 48 h[106]. Octreotide significantly more frequently controlled the bleeding than vasopressin (64%, P < 0.005), or omeprazole (59%, P < 0.005), and tended to be more effective than both vasopressin and omeprazole (88%, NS)[106]. Octreotide also controlled the bleeding significantly faster and required significantly less transfusions of packed erythrocytes than vasopressin or omeprazole[106]. Octreotide even successfully stopped bleeding within 48 h in the patients with bleeding refractory to vasopressin and omeprazole therapy.
Kouroumalis et al[105] reported that somatostatin or octreotide infusion for 3 d stopped severe bleeding from PHG in all 26 study patients. Only 3 patients experienced recurrent bleeding which stopped after retreatment with somatostatin, and only one patient required gastrectomy for refractory bleeding.
Somatostatin and octreotide only temporarily reduce portal pressure. Escorsell et al[232] reported rapid desensitization to the effects of octreotide with prolonged administration in cirrhotic patients with portal hypertension. Therefore somatostatin and octreotide are useful for acute bleeding but not for preventing chronic bleeding from PHG. Octreotide did not significantly change the severity of PHG at endoscopy.
Vasopressin and terlipressin: Vasopressin, a systemic vasoconstrictor, reduces splanchnic blood flow, lowers portal pressure, and decreases gastric mucosal blood flow[106]. In a randomized, controlled trial, vasopressin, administered intravenously (IV) at a rate of 1 unit/min for the first 10 min, followed by continuous infusion at 0.1 unit/min for 48 h, arrested bleeding from PHG in 14 (64%) of 22 patients[106]. However, as aforementioned, this result was inferior to that achieved by octreotide, possibly because vasopressin does not inhibit release of peptides, including glucagon and vasoactive intestinal polypeptide, and does not inhibit gastric acid secretion[106]. Concomitant omeprazole therapy may modestly increase vasopressin efficacy[106]. Vasopressin is considered an alternative therapy to octreotide.
Vasopressin causes significantly more frequent side effects (41%) than octreotide, especially abdominal pain[106]. Vasopressin reduces oxygen saturation of gastric mucosa, but this reduction can be partly reversed by administering supplemental oxygen[234,235]. Two analogues of vasopressin have been studied. Glypressin showed similar reduction in gastric mucosal perfusion as vasopressin by laser-Doppler flowmetry, but less impairment in gastric mucosal oxygenation[235]. Terlipressin may be useful in controlling acute bleeding from PHG, especially when used at a dose of 1 mg IV every 4 h for 5 d[103,107,192].
Antioxidants: Antioxidants help in free radical scavenging and in reversing impairment of oxidative stress-induced ERK2 activation. They may decrease susceptibility of PHG gastric mucosa to alcoholic injury, as demonstrated by administration of vitamin E, an antioxidant[144]. Vitamin E reduced oxidative state, normalized MKP-1 expression, and reversed impairment of oxidative stress-induced ERK2 activation[144]. Vitamin E helped reduce gastric injury from alcohol exposure in rats with PHG[71].
Vitamin E administration decreased mucosal lipid peroxidation, decreased lysosomal enzymes, and increased levels of antioxidants. Kaur et al[143] administered vitamin E 240 mg subcutaneously to rats with common bile duct ligation vs sham surgery. Seven days after ligation or sham surgery, the level of thiobarbituric acid reactive substances in gastric mucosa was significantly higher in ligated rats not receiving vitamin E [0.78 ± 0.22 (SD) nmol of malondialdehyde formed/mg protein] as compared to sham surgery rats administered vitamin E [0.56 ± 0.07 (SD) nmol of malondialdehyde formed/mg protein, P < 0.01]. These data suggest that vitamin E decreased mucosal lipid peroxidation. In the ligation group vitamin E administered preoperatively significantly reduced the levels of β-glucuronidase as compared to untreated or post-operatively-treated groups (P < 0.01). Preoperative vitamin E therapy significantly lowered levels of acid phosphatase as compared to untreated or postoperatively treated groups (P < 0.01). Preoperative administration of vitamin E in the ligation group led to significantly increased levels of the three major antioxidant enzymes, including superoxide dismutase (P < 0.005), glutathione peroxidase (P < 0.01), and catalase (P < 0.05) when compared to the levels of these antioxidant enzymes in untreated groups or in groups treated postoperatively with vitamin E. These data provide a theoretical basis that vitamin E administration may potentially improve PHG.
Kawanaka et al[144] administered vitamin E or saline for 13 d in rats with PHG vs sham-operated rats used as a control. Rats with PHG had a 2.3-fold increased area of gastric mucosal necrosis after gastric exposure to ethanol than sham-operated rats (P < 0.05), but vitamin E treatment in rats with PHG almost completely reversed this increased necrosis compared to controls (P < 0.05). Vitamin E treatment did not significantly change portal pressure or gastric mucosal blood flow in PHG gastric mucosa.
Rebamipide: Rebamipide, an antiulcer medication, protects against oxygen-derived production of free radicals by scavenging free radicals. Intragastric administration ameliorates oxidative stress, reduces nitration of tyrosine residues of ERK, and reverses delayed mucosal healing occurring in PHG. It reversed the increased susceptibility to ethanol-induced injury and reversed the delayed healing after gastric injury in rats with PHG[145]. Kinjo et al[145] reported that administration of rebamipide in rats with PHG, significantly decreased lipid peroxide and nitrotyrosine and nitration of ERK by peroxynitrite in PHG mucosa, therefore normalizing ERK activation and restoring normal gastric mucosal healing after ethanol injury. Rebamipide requires further study as a therapy for PHG[119].
Estrogen and progesterone: Estrogen and progesterone therapy reduced gastric mucosal blood flow, portal pressure, and porto-collateral resistance in rats with surgically-induced portal hypertension[236]. Panés et al[236] reported that treatment with estradiol, dihydroxyprogesterone, or low dose combination estradiol-dihydroxyprogesterone significantly decreased gastric mucosal blood flow in rats that underwent portal vein ligation as compared to placebo [56 ± 3.5 (SD) mL/min per 100 g for placebo; 43 ± 3.4 (SD) mL/min per 100 g for estrogen; 32 ± 2.6 mL/min per 100 g for dihydroxyprogesterone, and 42 ± 6.1 (SD) mL/min per 100 g for low dose estrogen/dihydroxyprogesterone, P < 0.05]. Estrogen and progesterone have not been studied in patients with PHG.
Thalidomide: Thalidomide blunts development of a hyperdynamic circulation and decreases portal pressure by reducing NO production[114]. Thalidomide, at a low dose of 100 mg daily, was successful as a last resort therapy in one case report of bleeding from PHG caused by neoplastic invasion of the portal vein[237]. Before thalidomide therapy, the patient had required transfusion of 30 units of packed erythrocytes during 35 d while treated with propranolol and terlipressin.
Corticosteroids: There is one case report of cessation of PHG bleeding with corticosteroid therapy, using prednisolone 20 mg/d, after being admitted for five times during 5 mo with severe iron deficiency anemia from chronic GI bleeding from PHG that was refractory to propranolol therapy. At 2 mo follow-up the hemoglobin was rising and at 4 mo follow-up repeat EGD showed an improved endoscopic appearance of PHG[238]. The patient was stable during 3-years of follow-up using 15 mg prednisolone every other day, with no recurrence of the anemia.
Losartan: Hepatic stellate cells help modulate sinusoidal resistance and the sinusoidal microcirculation. These cells are influenced by vasoconstrictors such as endothelin and angiotensin II. The angiotensin II receptor antagonist, losartan, lowers portal pressure by inhibiting stellate cell contraction and by reducing sinusoidal resistance[239]. Administration of losartan at 25 or 50 mg/d resulted in improvement of PHG in 9 (56.3%) of 16 patients during 4 wk of follow-up. The higher dose had greater efficacy. There was also evidence of decreased portal pressure. Further studies are needed to evaluate the effect of losartan on PHG[239].
Sucralfate and acid-suppressing medications: Proton pump inhibitors, sucralfate[74], and histamine-2 receptor antagonists are not very effective at reducing bleeding from PHG because most patients with PHG already have hypochlorhydria[79,106,240-242]. However, proton pump inhibitors may indirectly stop bleeding from the stomach by raising intraluminal gastric pH and thereby stabilizing blood clots[243,244]. Zhou et al[106] reported that omeprazole at 40 mg IV bolus every 12 h for 48 h successfully stopped bleeding in 59% of patients with PHG bleeding. Additionally, patients whose bleeding was refractory to vasopressin, benefited from omeprazole co-administration and vice versa.
Teprenone: In a controlled clinical trial, teprenone (geranylgeranyl acetone) administered to 15 patients with PHG decreased VEGF and hexosamine content in the gastric antrum, and thereby significantly decreased the severity of PHG[136]. Among these 15 treated patients, one patient decreased from severe to moderate PHG and another patient decreased from moderate to mild PHG[180]. Contrariwise, all 15 patients receiving placebo experienced no change in PHG severity.
Endoscopic therapies: Endoscopic therapies play a minor role in PHG bleeding because the bleeding is typically diffuse and obscure. Little data exist on efficacy of endoscopic therapy for PHG bleeding[71,245]. No single predominant site of bleeding is identified that can be locally treated at endoscopy. The role of endoscopic therapy is limited to the rare circumstances in which a single active bleeding site identified that is amenable to point therapy such as cauterization or sclerotherapy.
Argon plasma coagulation (APC) or hemospray, a rapid hemostatic agent, are experimental endoscopic therapies for PHG. These therapies can treat a larger bleeding surface area than cauterization or sclerotherapy. Nine (81%) of 11 patients undergoing APC for PHG bleeding achieved hemostasis, with a significant rise in hematocrit from baseline values after a mean of 2.2 ± 2.0 (SD) endoscopic therapy sessions. The other two treated patients required fewer blood transfusions after APC therapy[202].
Hemospray has recently shown promise in halting active PHG bleeding while long-term therapy is being initiated[203,246]. Ibrahim et al[246] reported complete cessation of diffuse bleeding from severe PHG after spraying hemospray TC-325 (a nanopowder hemostatic agent[247]) in a 41-year-old woman who presented with a hemoglobin of 8.8 g/dL. No active bleeding was seen on follow-up endoscopy performed 24 h later. In another study, however, one of four patients treated with hemospray for bleeding from PHG expired from GI perforation[203].
Endoscopic cryotherapy has been used for PHG bleeding after all other modalities failed. In one patient salvage cryotherapy was successful after failed TIPS and APC, with normal hemoglobin levels maintained during 4 wk of follow-up[248].
TIPS: TIPS increases gastric mucosal blood flow while decreasing total gastric blood flow due to decreasing portal hypertension[76]. TIPS reduces the frequency and severity of PHG because it lowers portal pressure[76]. PHG can sometimes resolve completely after TIPS[75-77,249-253]. Urata et al[77] reported in a prospective study of 12 Japanese patients undergoing TIPS for portal hypertension that portal pressure declined from 25.1 ± 8.8 (SD) mmHg before TIPS to 17.1 ± 6.2 (SD) mmHg after TIPS (P < 0.005). This decline in portal pressure was correlated with a significant decrease in PHG severity after TIPS (P < 0.01), and a significant decrease in number of patients with PHG (10 before vs 4 after TIPS, P < 0.01). In particular, PHG disappeared in 2 of 5 patients who had severe PHG before TIPS. Mezawa et al[76], likewise, reported that mean portal pressure declined from 23.4 to 14.0 mmHg in 16 cirrhotic patients after TIPS (P < 0.01). All four patients with severe PHG before TIPS, had significant improvement in PHG severity after lowering portal pressure with TIPS, and five of 12 patients with mild PHG had resolution of PHG after TIPS. In contrast, GAVE does not resolve after lowering portal pressure with TIPS, and GAVE is, therefore, likely related to hepatic dysfunction rather than portal hypertension[78].
TIPS also decreased the risk of PHG bleeding[75-77,100,249-253]. For example, Kamath et al[75] reported TIPS led to improvement or resolution of PHG findings and decreased transfusion requirements from 2.9 ± 2.0 (SD) units/mo of packed erythrocytes before TIPS to 0.6 ± 0.8 units/mo after TIPS performed for severe PHG (P = 0.04). Ashraf et al[98] reported one patient with chronic liver disease from hepatitis C who presented with chronic bleeding from PHG that required transfusions of 28 units of packed erythrocytes during the prior 6 mo. The bleeding ceased after TIPS with marked improvement in the severity of PHG. Contrariwise, GAVE does not respond to TIPS or other measures that lower portal pressure.
Shunt surgery: Shunt surgery decreases portal hypertension, decreases PHG severity, decreases risk of PHG bleeding, and may sometimes completely resolve the endoscopic features of PHG[100]. Shunt surgery is rarely used today to control bleeding because TIPS is preferred because of less invasiveness[100,101,238,254].
TIPS and shunt surgery are therapies of last resort for patients who fail other therapies for PHG because they entail more morbidity and mortality than pharmacologic therapy[71]. TIPS, however, is very useful for refractory bleeding from esophageal varices from portal hypertension[255], and is a reasonable option in patients having recurrent severe bleeding from PHG despite administration of β-adrenergic receptor antagonists[102].
Other invasive therapies: Liver transplantation is the ultimate therapy for PHG[118]. In a study of 29 patients undergoing living donor liver transplantation for end stage liver disease, PHG resolved in all 19 patients who had PHG before transplantation[44].
Kimura et al[218] retrospectively examined the 19 patients experiencing gross GI bleeding, defined as gross melena or hematemesis, within 3 mo after liver transplantation among 297 patients undergoing living donor liver transplantation. The etiologies included PHG in 2 patients, varices in 1, anastomotic ulcer in 13, and other in 3.
Splenic embolization, by transcatheter splenic arterial embolization, significantly improves PHG in patients with hypersplenism as compared to controls[99,255]. Ohmagari et al[99] evaluated 30 patients with hypersplenism who underwent transcatheter splenic arterial embolization in 17 vs no interventional therapy in 13 patients. Splenic embolization significantly reduced the frequency of PHG (reduction of 71% vs 8%, P < 0.05). Partial splenic embolization also successfully controlled PHG bleeding in one case report[256].
Laparoscopic splenectomy, for various indications, was reported to decrease PHG severity[55]. Anegawa et al[55] prospectively analyzed the effect of laparoscopic splenectomy on preexistent PHG in 70 patients with liver cirrhosis from various etiologies. All patients underwent EGD before and 1 mo after splenectomy. Splenectomy was performed for indications of bleeding diathesis, interferon induction, hepatocellular cancer treatment, and sclerotherapy-resistant varices. Before splenectomy, 49 of 70 patients had PHG, including mild PHG in 32 and severe PHG in 17 patients. After splenectomy, PHG resolved completely in 7 patients with prior severe PHG, resolved completely in 12 patients with mild PHG, and was reduced from severe to mild PHG in 9 patients (P < 0.0001).
Summary of clinical treatment
Acute bleeding: Variceal bleeding must be excluded by performing EGD before initiating treatment for PHG[257,258]. General measures for patients presenting with acute bleeding from PHG include volume resuscitation and cautious transfusion of packed erythrocytes, as necessary, to maintain the hemoglobin level at 8 g/dL[192,259,260]. Over-transfusion to a higher hemoglobin level could promote bleeding from PHG by raising portal pressure, as reported for bleeding from esophageal varices[261,262]. However, patients with cardiopulmonary disease or severe other comorbidities may require a hemoglobin level of 9-10 g/dL[263]. Antibiotic prophylaxis is generally recommended for bleeding from PHG[214], just as it is recommended for esophageal variceal bleeding in cirrhotic patients[189,264-266] because of an increased risk of systemic infections, particularly spontaneous bacterial peritonitis, in cirrhotic patients with GI bleeding[267].
Bleeding from PHG may be exacerbated by a coagulopathy with an elevated international normalized ratio from advanced liver disease. This coagulopathy may require transfusion of fresh frozen plasma. Severe thrombocytopenia may occur from bone marrow suppression in alcoholic cirrhosis and from hypersplenism with portal hypertension in any cirrhotic patient. Platelet transfusion may be necessary for severe thrombocytopenia in the setting of active bleeding from PGH.
Somatostatin or octreotide are first line therapies. Vasopressin or terlipressin are second-line therapies for acute bleeding[106]. Once the acute bleeding is controlled and the patient is hemodynamically stable, a nonselective β-adrenergic receptor antagonist is instituted for secondary prevention of PHG bleeding[103,258,268]. Propranolol is the recommended drug in this class because it has been the most studied. Propranolol should be started at a dose of 20 mg twice daily and gradually escalated to 160 mg twice daily or the maximum tolerated dose, maintaining it as long as the portal hypertension is present[214]. The dose is titrated to slow the pulse to 60 beats/min. This class of drugs is not used in the acute setting because it can blunt the physiologic tachycardia to restore end-organ perfusion in response to hypovolemia and requires gradual dose titration to achieve an adequate response[214]. This class of drugs also prevents bleeding from concomitant esophageal varices.
Treatment of chronic bleeding: Scant data exist regarding management of chronic bleeding from PHG[72]. In patients with suspected chronic bleeding from PHG, after excluding other etiologies, iron replacement therapy should be initiated to avoid depleting iron reserves[260]. Propranolol is the primary therapy to reduce portal pressure and prevent chronic bleeding[72,214,260]. However, propranolol was not superior to placebo in one study, as determined by percentage of patients free from chronic GI bleeding during long term follow-up[103].
Liver transplantation is an option in patients with decompensated cirrhosis and PHG bleeding. Table 12 summarizes the recommendations for the treatment of acute and chronic bleeding from PHG.
Table 12.
Treatment of acute or chronic gastrointestinal bleeding from portal hypertensive gastropathy
| Acute bleeding |
| Patient stabilization |
| Treat severe coagulopathy with highly elevated INR associated with cirrhosis with fresh frozen plasma |
| Treat severe thrombocytopenia associated with hypersplenism and bone marrow suppression from alcoholism with platelet transfusions |
| Transfuse packed erythrocytes to main hemoglobin level at about 8 g/dL |
| Consider antibiotic prophylaxis in patient with cirrhosis |
| Endoscopic therapy from bleeding-rarely used |
| Consider argon plasma coagulation |
| Hemospray - an experimental therapy |
| Pharmacotherapy |
| Octreotide - first line therapy |
| Vasopressin or terlipressin - second line therapy |
| Proton pump inhibitor therapy - adjunct therapy |
| Propranolol - can be instituted after bleeding controlled and patient stabilized |
| Interventional therapy |
| TIPS - for uncontrolled hemorrhage or for bleeding from PHG associated with variceal bleeding |
| Liver transplantation - for advanced end stage liver disease |
| Chronic bleeding |
| Treatment of anemia |
| Transfusions of packed erythrocytes as necessary |
| Iron replacement therapy |
| Pharmacotherapy |
| Consider propranolol |
TIPS: Transjugular intrahepatic portosystemic shunt; PHG: Portal hypertensive gastropathy; INR: International normalized ratio.
Prevention
The risk of bleeding from mild PHG is low. Primary prophylaxis is, therefore, not recommended for patients with mild PHG[72,191,269]. Propranolol can be used for primary prophylaxis for severe PHG, and can significantly reduce the risk of bleeding. However, scant evidence exists that this reduction will affect the risk of a primary bleeding episode in PHG patients[72,103,104,269,270]. Propranolol is recommended regardless of the severity or presence of PHG in patients with esophageal varices because it treats both entities by reducing portal pressure[105-107,198,271,272].
Prophylaxis of bleeding from PHG is not recommended[260]. Current guidelines do not recommend endoscopic surveillance in patients with cirrhosis who have asymptomatic PHG, without evident esophageal varices, other than the standard surveillance for development of esophageal varices in these patients[189].
Mortality: Limited data exist on mortality from bleeding from PHG, but this bleeding is rarely fatal[72]. It contributes little to overall morbidity and mortality from portal hypertension, especially in comparison to variceal bleeding[118]. It represents a minor cause (< 1%) of mortality in cirrhotic patients because the bleeding is typically mild[25,34,37,71]. Only one patient expired from PHG in one series of 38 deaths among 373 study patients with cirrhosis[34]. Bleeding-related mortality was much lower for PHG [1 of 8 patients (12.5%)] than that for esophageal varices [9 of 20 patients (45%)][34].
Lesions resembling PHG in other gastrointestinal regions: PHG-like lesions can occur in other parts of the GI tract and are named according to the involved segment as portal hypertensive duodenopathy[40], portal hypertensive biliopathy, small intestinal vasculopathy[139], and portal hypertensive colopathy[96,139]. These uncommon extragastric lesions occur particularly in patients with extrahepatic portal hypertension[71]. Portal hypertensive duodenopathy has been defined as the endoscopic appearance of patchy or diffuse congestion of duodenal mucosa associated with portal hypertension[40], but a consensus definition is not established[273]. Histologically, vascular changes predominate, including capillary angiogenesis, dilatation and congestion, as well as fibrous proliferation and apoptosis[46]. This duodenopathy is significantly more severe in patients having severe than mild PHG (56.8% vs 23.5%, P < 0.05)[46]. Portal congestive jejunopathy is defined histologically by the presence of ectatic capillaries and venules in the villi, with an increase in the number of vessels to > 6/villus[274]. Portal hypertensive ileopathy[275], and portal hypertensive colopathy[38,276] are also associated with portal hypertension. The colopathy histologically appears as dilatation of mucosal blood vessels and is classified into four different types by Ito et al[38], including solitary vascular ectasias, diffuse vascular ectasias, erythema, and blue vein.
DISCUSSION
The pathophysiology of PHG is not well understood. Portal hypertension plays a central role in the pathogenesis, and liver disease a subsidiary role in the disease, but a hyperdynamic circulation likely also plays an important role. However, the precise nature and pathophysiology of the hyperdynamic circulation must be further elucidated. The pathophysiology of more severe gastric mucosal injury and blunted reparative response after exposure to toxic substances in PHG must be clarified in terms of molecular mediators and histopathology. In particular, the pathophysiologic basis of decreased superficial mucosal perfusion in PHG must be better characterized in terms of its molecular mechanisms.
The current pharmacotherapy for bleeding from PHG focuses on decreasing portal pressure because portal hypertension is a prerequisite for developing PHG. Understanding the molecular mechanisms of this disease may permit development of better targeted and more effective pharmacotherapies.
COMMENTS
Background
Portal hypertensive gastropathy (PHG) is characterized at endoscopy by characteristic lesions present in the proximal stomach; characterized pathophysiologically by a hyperdynamic circulation induced by portal hypertension by inadequately understood mechanisms; and characterized clinically by mild-to-moderate acute or chronic gastrointestinal bleeding from the endoscopically identified lesions. However, much about the pathophysiology and clinical therapy of PHG is inadequately understood. This work systematically reviews the literature on the pathophysiology, natural history and therapy of PHG to report what is known and what is not known or controversial about PHG.
Research frontiers
This work systematically reviews gaps or controversies in the current understanding of PHG. First, this work exposes gaps in the current understanding of the pathophysiology. Portal hypertension is necessary but insufficient to develop PHG because many patients have portal hypertension without PHG. The pathogenesis is related to a hyperdynamic circulation, induced by portal hypertension, characterized by increased intrahepatic resistance to flow, increased splanchnic flow, increased total gastric flow, and most likely decreased gastric mucosal flow. However, this review shows that the cellular and molecular mechanisms for this hyperdynamic circulation are inadequately characterized. Nitrous oxide, free radicals, tumor necrosis factor-alpha, and glucagon may be important mediators of PHG. Second, this work reports the inadequacies of the current recommended therapies for PHG and for bleeding from PHG based on the currently inadequate understanding of the pathophysiology. This work should be useful to clinicians, clinical researchers, and basic researchers by describing what is known, controversial, or unknown about the pathophysiology, natural history, and therapy of PHG. It is hoped that this work stimulates further research in this field by exposing gaps in the current understanding of PHG.
Innovations and breakthroughs
This systematic review extensively reviews what is known and what is not known or controversial about PHG. This work is particularly helpful to clinicians in reporting the current recommended therapy for PHG, the clinical trials supporting the current recommendations, and the limitations of the current therapies. This is also helpful to clinical and basic researchers in systematically reviewing the current state of knowledge about its pathophysiology, including gaps, uncertainties, and controversies in the current understanding of the pathophysiology.
Applications
This systematic review extensively reviews what is known and what is not known or controversial about PHG. First, this work exposes gaps in the understanding of the pathophysiology. Portal hypertension is necessary but insufficient to develop PHG because many patients have portal hypertension without PHG. The pathogenesis is related to a hyperdynamic circulation, induced by portal hypertension, characterized by increased intrahepatic resistance to flow, increased splanchnic flow, increased total gastric flow, and most likely decreased gastric mucosal flow. However, this review shows that the cellular and molecular mechanisms for this hyperdynamic circulation are inadequately characterized. Nitrous oxide, free radicals, tumor necrosis factor-alpha, and glucagon may be important mediators of PHG development. Second, this work describes the natural history of PHG. PHG increases in frequency with more severe portal hypertension, advanced liver disease, longer liver disease duration, presence of esophageal varices, and endoscopic variceal obliteration. Acute and chronic gastrointestinal bleeding are the only clinical complications. Bleeding is typically mild-to-moderate. Third, this work reports the current therapies for PHG and for bleeding from PHG and characterizes their inadequacies based on the currently inadequate understanding of the pathophysiology. In particular, this work reviews clinical trials of the therapeutic efficacy of octreotide; proton pump inhibitors; nonselective β-adrenergic receptor antagonists, particularly propranolol; and vasopressin or terlipressin. This work should be useful to clinicians, clinical researchers, and basic researchers by describing what is known, controversial, and uncertain about the pathophysiology, natural history, and therapy of PHG.
Terminology
This work systematically reviews portal hypertensive gastropathy, characterized at endoscopy by characteristic lesions present in the proximal stomach; characterized pathophysiologically by a hyperdynamic circulation induced by portal hypertension by inadequately understood mechanisms, and characterized clinically by mild-to-moderate acute or chronic gastrointestinal bleeding from the endoscopically identified lesions.
Peer-review
This review by Mihajlo Gjeorgjievski on portal hypertensive gastropathy is well written and helpful to understand its pathophysiology, clinical presentation, natural history and therapy. This review is informative and helpful to readers who are interested in the topic or subtopics.
Footnotes
Conflict-of-interest statement: None for all authors. This paper does not discuss any confidential pharmaceutical industry data reviewed by Cappell MS as a consultant for the United States Food and Drug Administration (FDA) Advisory Committee on Gastrointestinal Drugs. Cappell MS is a member of the speaker’s bureau for AstraZeneca. This paper does not discuss any drug manufactured or marketed by AstraZeneca.
Data sharing statement: The paper has no original data or original statistics presented. No additional data are, therefore, available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 4, 2015
First decision: November 4, 2015
Article in press: January 19, 2016
P- Reviewer: Dang SS, He JY, Mihaila RG, Pan JJ, Umit A S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
References
- 1.Palmer ED. Erosive gastritis in cirrhosis; influence of portal hypertension on the gastric mucosa. Am J Dig Dis. 1957;2:31–36. doi: 10.1007/BF02232594. [DOI] [PubMed] [Google Scholar]
- 2.Sarfeh IJ, Tarnawski A. Portal hypertensive gastritis. Gastroenterology. 1984;86:592. [PubMed] [Google Scholar]
- 3.McCormack TT, Sims J, Eyre-Brook I, Kennedy H, Goepel J, Johnson AG, Triger DR. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut. 1985;26:1226–1232. doi: 10.1136/gut.26.11.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taor RE, Fox B, Ware J, Johnson AG. Gastritis: gastroscopic and microscopic. Endoscopy. 1975;7:209–215. [Google Scholar]
- 5.Sarin SK, Misra SP, Singal A, Thorat V, Broor SL. Evaluation of the incidence and significance of the “mosaic pattern” in patients with cirrhosis, noncirrhotic portal fibrosis, and extrahepatic obstruction. Am J Gastroenterol. 1988;83:1235–1239. [PubMed] [Google Scholar]
- 6.DeWeert TM, Gostout CJ, Wiesner RH. Congestive gastropathy and other upper endoscopic findings in 81 consecutive patients undergoing orthotopic liver transplantation. Am J Gastroenterol. 1990;85:573–576. [PubMed] [Google Scholar]
- 7.McCormick PA, Sankey EA, Cardin F, Dhillon AP, McIntyre N, Burroughs AK. Congestive gastropathy and Helicobacter pylori: an endoscopic and morphometric study. Gut. 1991;32:351–354. doi: 10.1136/gut.32.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarin SK, Sreenivas DV, Lahoti D, Saraya A. Factors influencing development of portal hypertensive gastropathy in patients with portal hypertension. Gastroenterology. 1992;102:994–999. doi: 10.1016/0016-5085(92)90188-5. [DOI] [PubMed] [Google Scholar]
- 9.Parikh SS, Desai SB, Prabhu SR, Trivedi MH, Shankaran K, Bhukhanwala FA, Kalro RH, Desai HG. Congestive gastropathy: factors influencing development, endoscopic features, Helicobacter pylori infection, and microvessel changes. Am J Gastroenterol. 1994;89:1036–1042. [PubMed] [Google Scholar]
- 10.Sarin SK, Shahi HM, Jain M, Jain AK, Issar SK, Murthy NS. The natural history of portal hypertensive gastropathy: influence of variceal eradication. Am J Gastroenterol. 2000;95:2888–2893. doi: 10.1111/j.1572-0241.2000.03200.x. [DOI] [PubMed] [Google Scholar]
- 11.Itha S, Yachha SK. Endoscopic outcome beyond esophageal variceal eradication in children with extrahepatic portal venous obstruction. J Pediatr Gastroenterol Nutr. 2006;42:196–200. doi: 10.1097/01.mpg.0000189351.55666.45. [DOI] [PubMed] [Google Scholar]
- 12.Rana SS, Bhasin DK, Jahagirdar S, Raja K, Nada R, Kochhar R, Joshi K. Is there ileopathy in portal hypertension? J Gastroenterol Hepatol. 2006;21:392–397. doi: 10.1111/j.1440-1746.2005.04037.x. [DOI] [PubMed] [Google Scholar]
- 13.El-Rifai N, Mention K, Guimber D, Michaud L, Boman F, Turck D, Gottrand F. Gastropathy and gastritis in children with portal hypertension. J Pediatr Gastroenterol Nutr. 2007;45:137–140. doi: 10.1097/MPG.0b013e318049cbe2. [DOI] [PubMed] [Google Scholar]
- 14.Sogaard KK, Astrup LB, Vilstrup H, Gronbaek H. Portal vein thrombosis; risk factors, clinical presentation and treatment. BMC Gastroenterol. 2007;7:34. doi: 10.1186/1471-230X-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueiredo P, Almeida N, Lérias C, Lopes S, Gouveia H, Leitão MC, Freitas D. Effect of portal hypertension in the small bowel: an endoscopic approach. Dig Dis Sci. 2008;53:2144–2150. doi: 10.1007/s10620-007-0111-z. [DOI] [PubMed] [Google Scholar]
- 16.Erden A, Idilman R, Erden I, Ozden A. Veins around the esophagus and the stomach: do their calibrations provide a diagnostic clue for portal hypertensive gastropathy? Clin Imaging. 2009;33:22–24. doi: 10.1016/j.clinimag.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Duché M, Ducot B, Tournay E, Fabre M, Cohen J, Jacquemin E, Bernard O. Prognostic value of endoscopy in children with biliary atresia at risk for early development of varices and bleeding. Gastroenterology. 2010;139:1952–1960. doi: 10.1053/j.gastro.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Aydoğan A, Güllüoğlu M, Onder SY, Gökçe S, Celtik C, Durmaz O. Portal gastropathy and duodenopathy in children with extrahepatic and intrahepatic portal hypertension: endoscopic diagnosis and histologic scoring. J Pediatr Gastroenterol Nutr. 2011;52:612–616. doi: 10.1097/MPG.0b013e3182125e7c. [DOI] [PubMed] [Google Scholar]
- 19.dos Santos JM, Ferreira AR, Fagundes ED, Ferreira AP, Ferreira LS, Magalhães MC, Bittencourt PF, Carvalho SD, Figueiredo Filho PP, Penna FJ. Endoscopic and pharmacological secondary prophylaxis in children and adolescents with esophageal varices. J Pediatr Gastroenterol Nutr. 2013;56:93–98. doi: 10.1097/MPG.0b013e318267c334. [DOI] [PubMed] [Google Scholar]
- 20.Pantham G, Waghray N, Einstadter D, Finkelhor RS, Mullen KD. Bleeding risk in patients with esophageal varices undergoing transesophageal echocardiography. Echocardiography. 2013;30:1152–1155. doi: 10.1111/echo.12274. [DOI] [PubMed] [Google Scholar]
- 21.Abdollahi MR, Somi MH, Faraji E. Role of international criteria in the diagnosis of autoimmune hepatitis. World J Gastroenterol. 2013;19:3629–3633. doi: 10.3748/wjg.v19.i23.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Alcantara RV, Yamada RM, Cardoso SR, de Fátima M, Servidoni CP, Hessel G. Ultrasonographic predictors of esophageal varices. J Pediatr Gastroenterol Nutr. 2013;57:700–703. doi: 10.1097/MPG.0b013e3182a7bc2e. [DOI] [PubMed] [Google Scholar]
- 23.Aoyama T, Oka S, Aikata H, Nakano M, Watari I, Naeshiro N, Yoshida S, Tanaka S, Chayama K. Is small-bowel capsule endoscopy effective for diagnosis of esophagogastric lesions related to portal hypertension? J Gastroenterol Hepatol. 2014;29:511–516. doi: 10.1111/jgh.12372. [DOI] [PubMed] [Google Scholar]
- 24.Sacchetti C, Capello M, Rebecchi P, Roncucci L, Zanghieri G, Tripodi A, Ponz de Leon M. Frequency of upper gastrointestinal lesions in patients with liver cirrhosis. Dig Dis Sci. 1988;33:1218–1222. doi: 10.1007/BF01536669. [DOI] [PubMed] [Google Scholar]
- 25.D’Amico G, Montalbano L, Traina M, Pisa R, Menozzi M, Spanò C, Pagliaro L. Natural history of congestive gastropathy in cirrhosis. The Liver Study Group of V. Cervello Hospital. Gastroenterology. 1990;99:1558–1564. doi: 10.1016/0016-5085(90)90458-d. [DOI] [PubMed] [Google Scholar]
- 26.Calès P, Zabotto B, Meskens C, Caucanas JP, Vinel JP, Desmorat H, Fermanian J, Pascal JP. Gastroesophageal endoscopic features in cirrhosis. Observer variability, interassociations, and relationship to hepatic dysfunction. Gastroenterology. 1990;98:156–162. [PubMed] [Google Scholar]
- 27.Rabinovitz M, Yoo YK, Schade RR, Dindzans VJ, Van Thiel DH, Gavaler JS. Prevalence of endoscopic findings in 510 consecutive individuals with cirrhosis evaluated prospectively. Dig Dis Sci. 1990;35:705–710. doi: 10.1007/BF01540171. [DOI] [PubMed] [Google Scholar]
- 28.Iwao T, Toyonaga A, Sumino M, Takagi K, Oho K, Nishizono M, Ohkubo K, Inoue R, Sasaki E, Tanikawa K. Portal hypertensive gastropathy in patients with cirrhosis. Gastroenterology. 1992;102:2060–2065. doi: 10.1016/0016-5085(92)90332-s. [DOI] [PubMed] [Google Scholar]
- 29.Taranto D, Suozzo R, Romano M, di Sapio M, Caporaso N, Del Vecchio Blanco C, Coltorti M. Gastric endoscopic features in patients with liver cirrhosis: correlation with esophageal varices, intra-variceal pressure, and liver dysfunction. Digestion. 1994;55:115–120. doi: 10.1159/000201135. [DOI] [PubMed] [Google Scholar]
- 30.Gupta R, Saraswat VA, Kumar M, Naik SR, Pandey R. Frequency and factors influencing portal hypertensive gastropathy and duodenopathy in cirrhotic portal hypertension. J Gastroenterol Hepatol. 1996;11:728–733. doi: 10.1111/j.1440-1746.1996.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 31.Iwao T, Toyonaga A, Oho K, Sakai T, Tayama C, Masumoto H, Sato M, Nakahara K, Tanikawa K. Portal-hypertensive gastropathy develops less in patients with cirrhosis and fundal varices. J Hepatol. 1997;26:1235–1241. doi: 10.1016/s0168-8278(97)80457-6. [DOI] [PubMed] [Google Scholar]
- 32.Carpinelli L, Primignani M, Preatoni P, Angeli P, Battaglia G, Beretta L, Bortoli A, Capria A, Cestari R, Cosentino F, et al. Portal hypertensive gastropathy: reproducibility of a classification, prevalence of elementary lesions, sensitivity and specificity in the diagnosis of cirrhosis of the liver. A NIEC multicentre study. New Italian Endoscopic Club. Ital J Gastroenterol Hepatol. 1997;29:533–540. [PubMed] [Google Scholar]
- 33.Zaman A, Hapke R, Flora K, Rosen H, Benner K. Prevalence of upper and lower gastrointestinal tract findings in liver transplant candidates undergoing screening endoscopic evaluation. Am J Gastroenterol. 1999;94:895–899. doi: 10.1111/j.1572-0241.1999.984_g.x. [DOI] [PubMed] [Google Scholar]
- 34.Primignani M, Carpinelli L, Preatoni P, Battaglia G, Carta A, Prada A, Cestari R, Angeli P, Gatta A, Rossi A, et al. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis. The New Italian Endoscopic Club for the study and treatment of esophageal varices (NIEC) Gastroenterology. 2000;119:181–187. doi: 10.1053/gast.2000.8555. [DOI] [PubMed] [Google Scholar]
- 35.Chaves DM, Sakai P, Mucenic M, Iriya K, Iriya Y, Ishioka S. Comparative study of portal hypertensive gastropathy in schistosomiasis and hepatic cirrhosis. Endoscopy. 2002;34:199–202. doi: 10.1055/s-2002-20291. [DOI] [PubMed] [Google Scholar]
- 36.Merkel C, Schipilliti M, Bighin R, Bellini B, Angeli P, Bolognesi M, Vescovi F, Gatta A. Portal hypertension and portal hypertensive gastropathy in patients with liver cirrhosis: a haemodynamic study. Dig Liver Dis. 2003;35:269–274. doi: 10.1016/s1590-8658(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 37.Merli M, Nicolini G, Angeloni S, Gentili F, Attili AF, Riggio O. The natural history of portal hypertensive gastropathy in patients with liver cirrhosis and mild portal hypertension. Am J Gastroenterol. 2004;99:1959–1965. doi: 10.1111/j.1572-0241.2004.40246.x. [DOI] [PubMed] [Google Scholar]
- 38.Ito K, Shiraki K, Sakai T, Yoshimura H, Nakano T. Portal hypertensive colopathy in patients with liver cirrhosis. World J Gastroenterol. 2005;11:3127–3130. doi: 10.3748/wjg.v11.i20.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Palma GD, Rega M, Masone S, Persico F, Siciliano S, Patrone F, Matantuono L, Persico G. Mucosal abnormalities of the small bowel in patients with cirrhosis and portal hypertension: a capsule endoscopy study. Gastrointest Endosc. 2005;62:529–534. doi: 10.1016/s0016-5107(05)01588-9. [DOI] [PubMed] [Google Scholar]
- 40.Menchén L, Ripoll C, Marín-Jiménez I, Colón A, Gómez-Camarero J, González-Asanza C, Menchén P, Cos E, Bañares R. Prevalence of portal hypertensive duodenopathy in cirrhosis: clinical and haemodynamic features. Eur J Gastroenterol Hepatol. 2006;18:649–653. doi: 10.1097/00042737-200606000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Yüksel O, Köklü S, Arhan M, Yolcu OF, Ertuğrul I, Odemiş B, Altiparmak E, Sahin B. Effects of esophageal varice eradication on portal hypertensive gastropathy and fundal varices: a retrospective and comparative study. Dig Dis Sci. 2006;51:27–30. doi: 10.1007/s10620-006-3078-2. [DOI] [PubMed] [Google Scholar]
- 42.Fontana RJ, Sanyal AJ, Mehta S, Doherty MC, Neuschwander-Tetri BA, Everson GT, Kahn JA, Malet PF, Sheikh MY, Chung RT, et al. Portal hypertensive gastropathy in chronic hepatitis C patients with bridging fibrosis and compensated cirrhosis: results from the HALT-C trial. Am J Gastroenterol. 2006;101:983–992. doi: 10.1111/j.1572-0241.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 43.Bresci G, Parisi G, Capria A. Clinical relevance of colonic lesions in cirrhotic patients with portal hypertension. Endoscopy. 2006;38:830–835. doi: 10.1055/s-2006-944629. [DOI] [PubMed] [Google Scholar]
- 44.Akatsu T, Yoshida M, Kawachi S, Tanabe M, Shimazu M, Kumai K, Kitajima M. Consequences of living-donor liver transplantation for upper gastrointestinal lesions: high incidence of reflux esophagitis. Dig Dis Sci. 2006;51:2018–2022. doi: 10.1007/s10620-006-9362-3. [DOI] [PubMed] [Google Scholar]
- 45.Zardi EM, Uwechie V, Gentilucci UV, Dobrina A, Petitti T, Laghi V, Picardi A, Afeltra A. Portal diameter in the diagnosis of esophageal varices in 266 cirrhotic patients: which role? Ultrasound Med Biol. 2007;33:506–511. doi: 10.1016/j.ultrasmedbio.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Barakat M, Mostafa M, Mahran Z, Soliman AG. Portal hypertensive duodenopathy: clinical, endoscopic, and histopathologic profiles. Am J Gastroenterol. 2007;102:2793–2802. doi: 10.1111/j.1572-0241.2007.01536.x. [DOI] [PubMed] [Google Scholar]
- 47.Bellis L, Nicodemo S, Galossi A, Guarisco R, Spilabotti L, Durola L, Dell’Unto O, Puoti C. Hepatic venous pressure gradient does not correlate with the presence and the severity of portal hypertensive gastropathy in patients with liver cirrhosis. J Gastrointestin Liver Dis. 2007;16:273–277. [PubMed] [Google Scholar]
- 48.Gravante G, Delogu D, Venditti D. Upper and lower gastrointestinal diseases in liver transplant candidates. Int J Colorectal Dis. 2008;23:201–206. doi: 10.1007/s00384-007-0386-8. [DOI] [PubMed] [Google Scholar]
- 49.Canlas KR, Dobozi BM, Lin S, Smith AD, Rockey DC, Muir AJ, Agrawal NM, Poleski MH, Patel K, McHutchison JG. Using capsule endoscopy to identify GI tract lesions in cirrhotic patients with portal hypertension and chronic anemia. J Clin Gastroenterol. 2008;42:844–848. doi: 10.1097/MCG.0b013e318038d312. [DOI] [PubMed] [Google Scholar]
- 50.Kim TU, Kim S, Woo SK, Lee JW, Lee TH, Jeong YJ, Heo J. Dynamic CT of portal hypertensive gastropathy: significance of transient gastric perfusion defect sign. Clin Radiol. 2008;63:783–790. doi: 10.1016/j.crad.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Higaki N, Matsui H, Imaoka H, Ikeda Y, Murakami H, Hiasa Y, Matsuura B, Onji M. Characteristic endoscopic features of portal hypertensive enteropathy. J Gastroenterol. 2008;43:327–331. doi: 10.1007/s00535-008-2166-9. [DOI] [PubMed] [Google Scholar]
- 52.Frenette CT, Kuldau JG, Hillebrand DJ, Lane J, Pockros PJ. Comparison of esophageal capsule endoscopy and esophagogastroduodenoscopy for diagnosis of esophageal varices. World J Gastroenterol. 2008;14:4480–4485. doi: 10.3748/wjg.14.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tarantino G, Citro V, Esposito P, Giaquinto S, de Leone A, Milan G, Tripodi FS, Cirillo M, Lobello R. Blood ammonia levels in liver cirrhosis: a clue for the presence of portosystemic collateral veins. BMC Gastroenterol. 2009;9:21. doi: 10.1186/1471-230X-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curvêlo LA, Brabosa W, Rhor R, Lanzoni V, Parise ER, Ferrari AP, Kondo M. Underlying mechanism of portal hypertensive gastropathy in cirrhosis: a hemodynamic and morphological approach. J Gastroenterol Hepatol. 2009;24:1541–1546. doi: 10.1111/j.1440-1746.2009.05871.x. [DOI] [PubMed] [Google Scholar]
- 55.Anegawa G, Kawanaka H, Uehara H, Akahoshi T, Konishi K, Yoshida D, Kinjo N, Hashimoto N, Tomikawa M, Hashizume M, et al. Effect of laparoscopic splenectomy on portal hypertensive gastropathy in cirrhotic patients with portal hypertension. J Gastroenterol Hepatol. 2009;24:1554–1558. doi: 10.1111/j.1440-1746.2009.05906.x. [DOI] [PubMed] [Google Scholar]
- 56.Kumar A, Mishra SR, Sharma P, Sharma BC, Sarin SK. Clinical, laboratory, and hemodynamic parameters in portal hypertensive gastropathy: a study of 254 cirrhotics. J Clin Gastroenterol. 2010;44:294–300. doi: 10.1097/MCG.0b013e3181b37ea1. [DOI] [PubMed] [Google Scholar]
- 57.Kim MY, Choi H, Baik SK, Yea CJ, Won CS, Byun JW, Park SY, Kwon YH, Kim JW, Kim HS, et al. Portal hypertensive gastropathy: correlation with portal hypertension and prognosis in cirrhosis. Dig Dis Sci. 2010;55:3561–3567. doi: 10.1007/s10620-010-1221-6. [DOI] [PubMed] [Google Scholar]
- 58.De Lisi S, Peralta S, Arini A, Simone F, Craxì A. Oesophagogastroduodenoscopy in patients with cirrhosis: Extending the range of detection beyond portal hypertension. Dig Liver Dis. 2011;43:48–53. doi: 10.1016/j.dld.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Abbasi A, Butt N, Bhutto AR, Munir SM. Correlation of thrombocytopenia with grading of esophageal varices in chronic liver disease patients. J Coll Physicians Surg Pak. 2010;20:369–372. [PubMed] [Google Scholar]
- 60.Ahmed S, Mumtaz K, Ahmed US, Shah HA, Abid S, Hamid S, Jafri W. Frequency and characteristic features of portal hypertensive gastropathy in patients with viral cirrhosis. J Coll Physicians Surg Pak. 2010;20:714–718. [PubMed] [Google Scholar]
- 61.Garcia-Saenz-de-Sicilia M, Sanchez-Avila F, Chavez-Tapia NC, Lopez-Arce G, Garcia-Osogobio S, Ruiz-Cordero R, Tellez-Avila FI. PPIs are not associated with a lower incidence of portal-hypertension-related bleeding in cirrhosis. World J Gastroenterol. 2010;16:5869–5873. doi: 10.3748/wjg.v16.i46.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbasi A, Bhutto AR, Butt N, Munir SM, Dhillo AK. Frequency of portal hypertensive gastropathy and its relationship with biochemical, haematological and endoscopic features in cirrhosis. J Coll Physicians Surg Pak. 2011;21:723–726. [PubMed] [Google Scholar]
- 63.Aoyama T, Oka S, Aikata H, Nakano M, Watari I, Naeshiro N, Yoshida S, Tanaka S, Chayama K. Small bowel abnormalities in patients with compensated liver cirrhosis. Dig Dis Sci. 2013;58:1390–1396. doi: 10.1007/s10620-012-2502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laleman W, Simon-Talero M, Maleux G, Perez M, Ameloot K, Soriano G, Villalba J, Garcia-Pagan JC, Barrufet M, Jalan R, et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57:2448–2457. doi: 10.1002/hep.26314. [DOI] [PubMed] [Google Scholar]
- 65.Giannini EG, Savarino V, Farinati F, Ciccarese F, Rapaccini G, Marco MD, Benvegnù L, Zoli M, Borzio F, Caturelli E, et al. Influence of clinically significant portal hypertension on survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Liver Int. 2013;33:1594–1600. doi: 10.1111/liv.12199. [DOI] [PubMed] [Google Scholar]
- 66.Aoyama T, Oka S, Aikata H, Igawa A, Nakano M, Naeshiro N, Yoshida S, Tanaka S, Chayama K. Major predictors of portal hypertensive enteropathy in patients with liver cirrhosis. J Gastroenterol Hepatol. 2015;30:124–130. doi: 10.1111/jgh.12658. [DOI] [PubMed] [Google Scholar]
- 67.Zardi EM, Ghittoni G, Margiotta D, Viera FT, Di Matteo F, Rossi S. Portal hypertensive gastropathy in cirrhotics without varices: a case-control study. Eur J Gastroenterol Hepatol. 2015;27:91–96. doi: 10.1097/MEG.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 68.Wu Q, Shen L, Chu J, Ma X, Jin B, Meng F, Chen J, Wang Y, Wu L, Han J, et al. Characterization of uncommon portosystemic collateral circulations in patients with hepatic cirrhosis. Oncol Lett. 2015;9:347–350. doi: 10.3892/ol.2014.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fontana RJ, Sanyal AJ, Ghany MG, Bonkovsky HL, Morgan TR, Litman HJ, Reid AE, Lee WM, Naishadham D. Development and progression of portal hypertensive gastropathy in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:884–893. doi: 10.1038/ajg.2010.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spina GP, Arcidiacono R, Bosch J, Pagliaro L, Burroughs AK, Santambrogio R, Rossi A. Gastric endoscopic features in portal hypertension: final report of a consensus conference, Milan, Italy, September 19, 1992. J Hepatol. 1994;21:461–467. doi: 10.1016/s0168-8278(05)80329-0. [DOI] [PubMed] [Google Scholar]
- 71.Thuluvath PJ, Yoo HY. Portal Hypertensive gastropathy. Am J Gastroenterol. 2002;97:2973–2978. doi: 10.1111/j.1572-0241.2002.07094.x. [DOI] [PubMed] [Google Scholar]
- 72.Cubillas R, Rockey DC. Portal hypertensive gastropathy: a review. Liver Int. 2010;30:1094–1102. doi: 10.1111/j.1478-3231.2010.02286.x. [DOI] [PubMed] [Google Scholar]
- 73.Hou MC, Lin HC, Chen CH, Kuo BI, Perng CL, Lee FY, Lee SD. Changes in portal hypertensive gastropathy after endoscopic variceal sclerotherapy or ligation: an endoscopic observation. Gastrointest Endosc. 1995;42:139–144. doi: 10.1016/s0016-5107(95)70070-6. [DOI] [PubMed] [Google Scholar]
- 74.Trevino HH, Brady CE, Schenker S. Portal hypertensive gastropathy. Dig Dis. 1996;14:258–270. doi: 10.1159/000171557. [DOI] [PubMed] [Google Scholar]
- 75.Kamath PS, Lacerda M, Ahlquist DA, McKusick MA, Andrews JC, Nagorney DA. Gastric mucosal responses to intrahepatic portosystemic shunting in patients with cirrhosis. Gastroenterology. 2000;118:905–911. doi: 10.1016/s0016-5085(00)70176-4. [DOI] [PubMed] [Google Scholar]
- 76.Mezawa S, Homma H, Ohta H, Masuko E, Doi T, Miyanishi K, Takada K, Kukitsu T, Sato T, Niitsu Y. Effect of transjugular intrahepatic portosystemic shunt formation on portal hypertensive gastropathy and gastric circulation. Am J Gastroenterol. 2001;96:1155–1159. doi: 10.1111/j.1572-0241.2001.03694.x. [DOI] [PubMed] [Google Scholar]
- 77.Urata J, Yamashita Y, Tsuchigame T, Hatanaka Y, Matsukawa T, Sumi S, Matsuno Y, Takahashi M. The effects of transjugular intrahepatic portosystemic shunt on portal hypertensive gastropathy. J Gastroenterol Hepatol. 1998;13:1061–1067. doi: 10.1111/j.1440-1746.1998.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 78.Kamath PS, Shah VH. Portal hypertension and bleeding esophageal varices. In: Boyer TD, Manns MP, Sanyal AJ, editors. In: Zakim and Boyer’s Hepatology: A textbook of liver disease. 6th ed. Philadelphia: Elsevier Saunders; 2012. pp. 296–326. [Google Scholar]
- 79.Pan WD, Xun RY, Chen YM. Correlations of portal hypertensive gastropathy of hepatitis B cirrhosis with other factors. Hepatobiliary Pancreat Dis Int. 2002;1:527–531. [PubMed] [Google Scholar]
- 80.Elnaser MS, Elebiary S, Bastawi MB, El Shafei A, Elmagd IM, Hamza MM. The prevalence of portal hypertensive gastropathy and duodenopathy in some Egyptian cirrhotic patients. J Egypt Soc Parasitol. 2004;34:915–923. [PubMed] [Google Scholar]
- 81.Lo GH, Lai KH, Cheng JS, Hsu PI, Chen TA, Wang EM, Lin CK, Chiang HT. The effects of endoscopic variceal ligation and propranolol on portal hypertensive gastropathy: a prospective, controlled trial. Gastrointest Endosc. 2001;53:579–584. doi: 10.1067/mge.2001.114062. [DOI] [PubMed] [Google Scholar]
- 82.de la Peña J, Rivero M, Sanchez E, Fábrega E, Crespo J, Pons-Romero F. Variceal ligation compared with endoscopic sclerotherapy for variceal hemorrhage: prospective randomized trial. Gastrointest Endosc. 1999;49:417–423. doi: 10.1016/s0016-5107(99)70036-2. [DOI] [PubMed] [Google Scholar]
- 83.Poddar U, Bhatnagar S, Yachha SK. Endoscopic band ligation followed by sclerotherapy: Is it superior to sclerotherapy in children with extrahepatic portal venous obstruction? J Gastroenterol Hepatol. 2011;26:255–259. doi: 10.1111/j.1440-1746.2010.06397.x. [DOI] [PubMed] [Google Scholar]
- 84.Poddar U, Thapa BR, Singh K. Frequency of gastropathy and gastric varices in children with extrahepatic portal venous obstruction treated with sclerotherapy. J Gastroenterol Hepatol. 2004;19:1253–1256. doi: 10.1111/j.1440-1746.2004.03470.x. [DOI] [PubMed] [Google Scholar]
- 85.Duan X, Zhang K, Han X, Ren J, Xu M, Huang G, Zhang M. Comparison of percutaneous transhepatic variceal embolization (PTVE) followed by partial splenic embolization versus PTVE alone for the treatment of acute esophagogastric variceal massive hemorrhage. J Vasc Interv Radiol. 2014;25:1858–1865. doi: 10.1016/j.jvir.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 86.Lo GH, Liang HL, Lai KH, Chang CF, Hwu JH, Chen SM, Lin CK, Chiang HT. The impact of endoscopic variceal ligation on the pressure of the portal venous system. J Hepatol. 1996;24:74–80. doi: 10.1016/s0168-8278(96)80189-9. [DOI] [PubMed] [Google Scholar]
- 87.Korula J, Ralls P. The effects of chronic endoscopic variceal sclerotherapy on portal pressure in cirrhotics. Gastroenterology. 1991;101:800–805. doi: 10.1016/0016-5085(91)90542-s. [DOI] [PubMed] [Google Scholar]
- 88.Kimura K, Ohto M, Matsutani S, Furuse J, Hoshino K, Okuda K. Relative frequencies of portosystemic pathways and renal shunt formation through the “posterior” gastric vein: portographic study in 460 patients. Hepatology. 1990;12:725–728. doi: 10.1002/hep.1840120417. [DOI] [PubMed] [Google Scholar]
- 89.Smith-Laing G, Camilo ME, Dick R, Sherlock S. Percutaneous transhepatic portography in the assessment of portal hypertension. Clinical correlations and comparison of radiographic techniques. Gastroenterology. 1980;78:197–205. [PubMed] [Google Scholar]
- 90.Watanabe K, Kimura K, Matsutani S, Ohto M, Okuda K. Portal hemodynamics in patients with gastric varices. A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology. 1988;95:434–440. doi: 10.1016/0016-5085(88)90501-x. [DOI] [PubMed] [Google Scholar]
- 91.Yoshikawa I, Murata I, Nakano S, Otsuki M. Effects of endoscopic variceal ligation on portal hypertensive gastropathy and gastric mucosal blood flow. Am J Gastroenterol. 1998;93:71–74. doi: 10.1111/j.1572-0241.1998.071_c.x. [DOI] [PubMed] [Google Scholar]
- 92.Balan KK, Grime JS, Sutton R, Critchley M, Jenkins SA. Do alterations in the rate of gastric emptying after injection sclerotherapy for oesophageal varices play any role in the development of portal hypertensive gastropathy? HPB Surg. 1999;11:141–148; discussion 148-150. doi: 10.1155/1999/27037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sarwar S, Khan AA, Alam A, Butt AK, Shafqat F, Malik K, Ahmad I, Niazi AK. Effect of band ligation on portal hypertensive gastropathy and development of fundal varices. J Ayub Med Coll Abbottabad. 2006;18:32–35. [PubMed] [Google Scholar]
- 94.Lo GH, Lai KH, Cheng JS, Hwu JH, Chang CF, Chen SM, Chiang HT. A prospective, randomized trial of sclerotherapy versus ligation in the management of bleeding esophageal varices. Hepatology. 1995;22:466–471. [PubMed] [Google Scholar]
- 95.Sarin SK, Govil A, Jain AK, Guptan RC, Issar SK, Jain M, Murthy NS. Prospective randomized trial of endoscopic sclerotherapy versus variceal band ligation for esophageal varices: influence on gastropathy, gastric varices and variceal recurrence. J Hepatol. 1997;26:826–832. doi: 10.1016/s0168-8278(97)80248-6. [DOI] [PubMed] [Google Scholar]
- 96.Feldman M, Lee EL. Gastritis. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 10th ed. Philadelphia: Elsevier Saunders; 2010. pp. 868–883. [Google Scholar]
- 97.Primignani M, Materia M, Preatoni P, Carta A, Morbin T. Does endoscopic treatment of esophageal varices with sclerotherapy (ES) or ligation (EL) influence the evolution of portal hypertensive gastropathy (PHG) in cirrhotic patients? Gastroenterology. 1998:114; A1324. [Google Scholar]
- 98.Ashraf P, Shah GM, Shaikh H, Manan A, Kumar M. Transjugular intrahepatic portosystemic stenting in portal hypertensive gastropathy. J Coll Physicians Surg Pak. 2009;19:584–585. [PubMed] [Google Scholar]
- 99.Ohmagari K, Toyonaga A, Tanikawa K. Effects of transcatheter splenic arterial embolization on portal hypertensive gastric mucosa. Am J Gastroenterol. 1993;88:1837–1841. [PubMed] [Google Scholar]
- 100.Orloff MJ, Orloff MS, Orloff SL, Haynes KS. Treatment of bleeding from portal hypertensive gastropathy by portacaval shunt. Hepatology. 1995;21:1011–1017. [PubMed] [Google Scholar]
- 101.Soin AS, Acharya SK, Mathur M, Sahni P, Nundy S. Portal hypertensive gastropathy in noncirrhotic patients. The effect of lienorenal shunts. J Clin Gastroenterol. 1998;26:64–67; discussion 68. doi: 10.1097/00004836-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 102.Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41:386–400. doi: 10.1002/hep.20559. [DOI] [PubMed] [Google Scholar]
- 103.Pérez-Ayuso RM, Piqué JM, Bosch J, Panés J, González A, Pérez R, Rigau J, Quintero E, Valderrama R, Viver J. Propranolol in prevention of recurrent bleeding from severe portal hypertensive gastropathy in cirrhosis. Lancet. 1991;337:1431–1434. doi: 10.1016/0140-6736(91)93125-s. [DOI] [PubMed] [Google Scholar]
- 104.Hosking SW, Kennedy HJ, Seddon I, Triger DR. The role of propranolol in congestive gastropathy of portal hypertension. Hepatology. 1997;7:437–441. doi: 10.1002/hep.1840070304. [DOI] [PubMed] [Google Scholar]
- 105.Kouroumalis EA, Koutroubakis IE, Manousos ON. Somatostatin for acute severe bleeding from portal hypertensive gastropathy. Eur J Gastroenterol Hepatol. 1998;10:509–512. doi: 10.1097/00042737-199806000-00013. [DOI] [PubMed] [Google Scholar]
- 106.Zhou Y, Qiao L, Wu J, Hu H, Xu C. Comparison of the efficacy of octreotide, vasopressin, and omeprazole in the control of acute bleeding in patients with portal hypertensive gastropathy: a controlled study. J Gastroenterol Hepatol. 2002;17:973–979. doi: 10.1046/j.1440-1746.2002.02775.x. [DOI] [PubMed] [Google Scholar]
- 107.Bruha R, Marecek Z, Spicak J, Hulek P, Lata J, Petrtyl J, Urbanek P, Taimr P, Volfova M, Dite P. Double-blind randomized, comparative multicenter study of the effect of terlipressin in the treatment of acute esophageal variceal and/or hypertensive gastropathy bleeding. Hepatogastroenterology. 2002;49:1161–1166. [PubMed] [Google Scholar]
- 108.Mori T, Aisa Y, Yajima T, Shimizu T, Kato J, Nakazato T, Hibi T, Ikeda Y, Okamoto S. Esophageal varices and portal hypertensive gastropathy associated with hepatic veno-occlusive disease after allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2008;87:231–232. doi: 10.1007/s12185-008-0023-5. [DOI] [PubMed] [Google Scholar]
- 109.Urso G, Interlandi D, Puglisi M, Abate G, Bertino G, Raciti C, Sciacca C, Bruno M, Panarello A, Di Prima P, et al. Role of Helicobacter pylori in patients with portal hypertensive gastropathy by liver cirrhosis hepatitis C virus-related. Minerva Gastroenterol Dietol. 2006;52:303–308. [PubMed] [Google Scholar]
- 110.Al Mofleh IA. Does Helicobacter pylori affect portal hypertensive gastropathy? Saudi J Gastroenterol. 2007;13:95–97. doi: 10.4103/1319-3767.32186. [DOI] [PubMed] [Google Scholar]
- 111.Bayraktar Y, Balkanci F, Uzunalimoglu B, Gokoz A, Koseoglu T, Batman F, Gurakar A, Van Thiel DH, Kayhan B. Is portal hypertension due to liver cirrhosis a major factor in the development of portal hypertensive gastropathy? Am J Gastroenterol. 1996;91:554–558. [PubMed] [Google Scholar]
- 112.Thuluvath PJ. Management of upper gastrointestinal hemorrhage related to portal hypertension. In: Yamada T, Alpers DH, Kalloo AN, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 5th ed. Wiley-Blackwell; 2009. pp. 2897–3017. [Google Scholar]
- 113.Ohta M, Yamaguchi S, Gotoh N, Tomikawa M. Pathogenesis of portal hypertensive gastropathy: a clinical and experimental review. Surgery. 2002;131:S165–S170. doi: 10.1067/msy.2002.119499. [DOI] [PubMed] [Google Scholar]
- 114.Lopez-Talavera JC, Cadelina G, Olchowski J, Merrill W, Groszmann RJ. Thalidomide inhibits tumor necrosis factor alpha, decreases nitric oxide synthesis, and ameliorates the hyperdynamic circulatory syndrome in portal-hypertensive rats. Hepatology. 1996;23:1616–1621. doi: 10.1002/hep.510230644. [DOI] [PubMed] [Google Scholar]
- 115.Blendis L, Wong F. The hyperdynamic circulation in cirrhosis: an overview. Pharmacol Ther. 2001;89:221–231. doi: 10.1016/s0163-7258(01)00124-3. [DOI] [PubMed] [Google Scholar]
- 116.Hashizume M, Tanaka K, Inokuchi K. Morphology of gastric microcirculation in cirrhosis. Hepatology. 1993;3:1008–1012. doi: 10.1002/hep.1840030619. [DOI] [PubMed] [Google Scholar]
- 117.Perini RF, Camara PR, Ferraz JG. Pathogenesis of portal hypertensive gastropathy: translating basic research into clinical practice. Nat Clin Pract Gastroenterol Hepatol. 2009;6:150–158. doi: 10.1038/ncpgasthep1356. [DOI] [PubMed] [Google Scholar]
- 118.Makhija S, Burak K, Beck PL. Portal hypertensive gastropathy and gastric antral vascular ectasia. In: Helmy A, editor. Portal hypertension: pathogenesis and management. New York: Nova Science Publishers Inc; 2006. pp. 137–166. [Google Scholar]
- 119.Nayeb-Hashemi H, Kaunitz JD. Gastroduodenal mucosal defense. Curr Opin Gastroenterol. 2009;25:537–543. doi: 10.1097/MOG.0b013e328330da7b. [DOI] [PubMed] [Google Scholar]
- 120.Ferraz JG, Wallace JL. Underlying mechanisms of portal hypertensive gastropathy. J Clin Gastroenterol. 1997;25 Suppl 1:S73–S78. doi: 10.1097/00004836-199700001-00012. [DOI] [PubMed] [Google Scholar]
- 121.Tarnawski AS, Sarfeh IJ, Stachura J, Hajduczek A, Bui HX, Dabros W, Gergely H. Microvascular abnormalities of the portal hypertensive gastric mucosa. Hepatology. 1988;8:1488–1494. doi: 10.1002/hep.1840080604. [DOI] [PubMed] [Google Scholar]
- 122.Albillos A, Colombato LA, Enriquez R, Ng OC, Sikuler E, Groszmann RJ. Sequence of morphological and hemodynamic changes of gastric microvessels in portal hypertension. Gastroenterology. 1992;102:2066–2070. doi: 10.1016/0016-5085(92)90333-t. [DOI] [PubMed] [Google Scholar]
- 123.Payen JL, Cales P, Pienkowski P, Sozzani P, Kervran A, Frexinos J, Pascal JP. Weakness of mucosal barrier in portal hypertensive gastropathy of alcoholic cirrhosis. Effects of propranolol and enprostil. J Hepatol. 1995;23:689–696. doi: 10.1016/0168-8278(95)80035-2. [DOI] [PubMed] [Google Scholar]
- 124.Beck PL, Lee SS, McKnight GW, Wallace JL. Characterization of spontaneous and ethanol-induced gastric damage in cirrhotic rats. Gastroenterology. 1992;103:1048–1055. doi: 10.1016/0016-5085(92)90042-w. [DOI] [PubMed] [Google Scholar]
- 125.Beck PL, McKnight W, Lee SS, Wallace JL. Prostaglandin modulation of the gastric vasculature and mucosal integrity in cirrhotic rats. Am J Physiol. 1993;265:G453–G458. doi: 10.1152/ajpgi.1993.265.3.G453. [DOI] [PubMed] [Google Scholar]
- 126.Misra V, Misra SP, Dwivedi M. Thickened gastric mucosal capillary wall: a histological marker for portal hypertension. Pathology. 1998;30:10–13. doi: 10.1080/00313029800169595. [DOI] [PubMed] [Google Scholar]
- 127.Ichikawa Y, Tarnawski A, Sarfeh IJ, Ishikawa T, Shimada H. Distorted microangioarchitecture and impaired angiogenesis in gastric mucosa of portal hypertensive rats. Gastroenterology. 1994;106:702–708. doi: 10.1016/0016-5085(94)90705-6. [DOI] [PubMed] [Google Scholar]
- 128.Gupta R, Sawant P, Parameshwar RV, Lele VR, Kulhalli PM, Mahajani SS. Gastric mucosal blood flow and hepatic perfusion index in patients with portal hypertensive gastropathy. J Gastroenterol Hepatol. 1998;13:921–926. doi: 10.1111/j.1440-1746.1998.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 129.Iwao T, Toyonaga A, Ikegami M, Oho K, Sumino M, Harada H, Sakaki M, Shigemori H, Aoki T, Tanikawa K. Reduced gastric mucosal blood flow in patients with portal-hypertensive gastropathy. Hepatology. 1993;18:36–40. [PubMed] [Google Scholar]
- 130.Kotzampassi K, Eleftheriadis E, Aletras H. Gastric mucosal blood flow in portal hypertension patients--a laser Doppler flowmetry study. Hepatogastroenterology. 1992;39:39–42. [PubMed] [Google Scholar]
- 131.Vyas K, Gala B, Sawant P, Das HS, Kulhalli PM, Mahajan SS. Assessment of portal hemodynamics by ultrasound color Doppler and laser Doppler velocimetry in liver cirrhosis. Indian J Gastroenterol. 2002;21:176–178. [PubMed] [Google Scholar]
- 132.Shigemori H, Iwao T, Ikegami M, Toyonaga A, Tanikawa K. Effects of propranolol on gastric mucosal perfusion and serum gastrin level in cirrhotic patients with portal hypertensive gastropathy. Dig Dis Sci. 1994;39:2433–2438. doi: 10.1007/BF02087662. [DOI] [PubMed] [Google Scholar]
- 133.Ohta M, Hashizume M, Higashi H, Ueno K, Tomikawa M, Kishihara F, Kawanaka H, Tanoue K, Sugimachi K. Portal and gastric mucosal hemodynamics in cirrhotic patients with portal-hypertensive gastropathy. Hepatology. 1994;20:1432–1436. doi: 10.1002/hep.1840200609. [DOI] [PubMed] [Google Scholar]
- 134.Chung RS, Bruch D, Dearlove J. Endoscopic measurement of gastric mucosal blood flow by laser Doppler velocimetry: effect of chronic esophageal variceal sclerosis. Am Surg. 1988;54:116–120. [PubMed] [Google Scholar]
- 135.Tomikawa M, Akiba Y, Kaunitz JD, Kawanaka H, Sugimachi K, Sarfeh IJ, Tarnawski AS. New insights into impairment of mucosal defense in portal hypertensive gastric mucosa. J Gastrointest Surg. 2000;4:458–463. doi: 10.1016/s1091-255x(00)80086-4. [DOI] [PubMed] [Google Scholar]
- 136.Tsugawa K, Hashizume M, Migou S, Kishihara F, Kawanaka H, Tomikawa M, Sugimachi K. Role of vascular endothelial growth factor in portal hypertensive gastropathy. Digestion. 2000;61:98–106. doi: 10.1159/000007741. [DOI] [PubMed] [Google Scholar]
- 137.De BK, Das TK. Color Doppler and laser velocimetry studies in the assessment of portal hemodynamics and severity of chronic liver disease. Indian J Gastroenterol. 2002;21:173–175. [PubMed] [Google Scholar]
- 138.Casadevall M, Panés J, Piqué JM, Bosch J, Terés J, Rodés J. Limitations of laser-Doppler velocimetry and reflectance spectrophotometry in estimating gastric mucosal blood flow. Am J Physiol. 1992;263:G810–G815. doi: 10.1152/ajpgi.1992.263.5.G810. [DOI] [PubMed] [Google Scholar]
- 139.Bhattacharya B. Non-neoplastic disorders of the stomach. In: Iacobuzio-Donahue CA, editor. Montgomery E, Goldblum JR, editors. Gastrointestinal and liver pathology. 2nd ed. Philadelphia: Elsevier Saunders; 2012. pp. 65–141. [Google Scholar]
- 140.Misra SP, Dwivedi M, Misra V, Barthwal R. Portal-hypertensive-gastropathy-like changes in a patient with secondary polycythemia: reversal of endoscopic and histopathologic changes with phlebotomy. Gastrointest Endosc. 2004;59:916–919. doi: 10.1016/s0016-5107(04)00338-4. [DOI] [PubMed] [Google Scholar]
- 141.Wu B, Zeng L, Lin Y, Wen Z, Chen G, Iwakiri R, Fujimoto K. Downregulation of cyclooxygenase-1 is involved in gastric mucosal apoptosis via death signaling in portal hypertensive rats. Cell Res. 2009;19:1269–1278. doi: 10.1038/cr.2009.97. [DOI] [PubMed] [Google Scholar]
- 142.Tan S, Wei X, Song M, Tao J, Yang Y, Khatoon S, Liu H, Jiang J, Wu B. PUMA mediates ER stress-induced apoptosis in portal hypertensive gastropathy. Cell Death Dis. 2014;5:e1128. doi: 10.1038/cddis.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kaur S, Kaur U, Tandon C, Dhawan V, Ganguly NK, Majumdar S. Gastropathy and defense mechanisms in common bile duct ligated portal hypertensive rats. Mol Cell Biochem. 2000;203:79–85. doi: 10.1023/a:1007090205886. [DOI] [PubMed] [Google Scholar]
- 144.Kawanaka H, Tomikawa M, Jones MK, Szabo IL, Pai R, Baatar D, Tsugawa K, Sugimachi K, Sarfeh IJ, Tarnawski AS. Defective mitogen-activated protein kinase (ERK2) signaling in gastric mucosa of portal hypertensive rats: potential therapeutic implications. Hepatology. 2001;34:990–999. doi: 10.1053/jhep.2001.28507. [DOI] [PubMed] [Google Scholar]
- 145.Kinjo N, Kawanaka H, Akahoshi T, Yamaguchi S, Yoshida D, Anegawa G, Konishi K, Tomikawa M, Tanoue K, Tarnawski A, et al. Significance of ERK nitration in portal hypertensive gastropathy and its therapeutic implications. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1016–G1024. doi: 10.1152/ajpgi.90329.2008. [DOI] [PubMed] [Google Scholar]
- 146.Wang JY, Hsieh JS, Lin SR, Huang TJ. The effect of portal hypertension on expression of gastric mucin mRNA in rats. Hepatogastroenterology. 2001;48:667–671. [PubMed] [Google Scholar]
- 147.Tsugawa K, Hashizume M, Tomikawa M, Migou S, Kawanaka H, Shiraishi S, Sueishi K, Sugimachi K. Immunohistochemical localization of vascular endothelial growth factor in the rat portal hypertensive gastropathy. J Gastroenterol Hepatol. 2001;16:429–437. doi: 10.1046/j.1440-1746.2001.02452.x. [DOI] [PubMed] [Google Scholar]
- 148.Muñoz J, Albillos A, Pérez-Páramo M, Rossi I, Alvarez-Mon M. Factors mediating the hemodynamic effects of tumor necrosis factor-alpha in portal hypertensive rats. Am J Physiol. 1999;276:G687–G693. doi: 10.1152/ajpgi.1999.276.3.G687. [DOI] [PubMed] [Google Scholar]
- 149.Kaviani A, Ohta M, Itani R, Sander F, Tarnawski AS, Sarfeh IJ. Tumor necrosis factor-alpha regulates inducible nitric oxide synthase gene expression in the portal hypertensive gastric mucosa of the rat. J Gastrointest Surg. 1997;1:371–376. doi: 10.1016/s1091-255x(97)80059-5. [DOI] [PubMed] [Google Scholar]
- 150.Ohta M, Tarnawski AS, Itani R, Pai R, Tomikawa M, Sugimachi K, Sarfeh IJ. Tumor necrosis factor alpha regulates nitric oxide synthase expression in portal hypertensive gastric mucosa of rats. Hepatology. 1998;27:906–913. doi: 10.1002/hep.510270403. [DOI] [PubMed] [Google Scholar]
- 151.Ohta M, Tanoue K, Tarnawski AS, Pai R, Itani RM, Sander FC, Sugimachi K, Sarfeh IJ. Overexpressed nitric oxide synthase in portal-hypertensive stomach of rat: a key to increased susceptibility to damage? Gastroenterology. 1997;112:1920–1930. doi: 10.1053/gast.1997.v112.pm9178684. [DOI] [PubMed] [Google Scholar]
- 152.El-Newihi HM, Kanji VK, Mihas AA. Activity of gastric mucosal nitric oxide synthase in portal hypertensive gastropathy. Am J Gastroenterol. 1996;91:535–538. [PubMed] [Google Scholar]
- 153.Hartleb M, Michielsen PP, Dziurkowska-Marek A. The role of nitric oxide in portal hypertensive systemic and portal vascular pathology. Acta Gastroenterol Belg. 1997;60:222–232. [PubMed] [Google Scholar]
- 154.Arafa UA, Fujiwara Y, Higuchi K, Shiba M, Uchida T, Watanabe T, Tominaga K, Oshitani N, Matsumoto T, Arakawa T. No additive effect between Helicobacter pylori infection and portal hypertensive gastropathy on inducible nitric oxide synthase expression in gastric mucosa of cirrhotic patients. Dig Dis Sci. 2003;48:162–168. doi: 10.1023/a:1021707103590. [DOI] [PubMed] [Google Scholar]
- 155.Lee FY, Wang SS, Tsai YT, Lin HJ, Lin HC, Chu CJ, Wu SL, Tai CC, Lee SD. Aminoguanidine corrects hyperdynamic circulation without ameliorating portal hypertension and portal hypertensive gastropathy in anesthetized portal hypertensive rats. J Hepatol. 1997;26:687–693. doi: 10.1016/s0168-8278(97)80436-9. [DOI] [PubMed] [Google Scholar]
- 156.Chu CJ, Lee FY, Wang SS, Chang FY, Lin HC, Hou MC, Wu SL, Tai CC, Chan CC, Lee SD. Aminoguanidine ameliorates splanchnic hyposensitivity to glypressin in a haemorrhage-transfused rat model of portal hypertension. Clin Sci (Lond) 1998;95:629–636. doi: 10.1042/cs0950629. [DOI] [PubMed] [Google Scholar]
- 157.Silva G, Navasa M, Bosch J, Chesta J, Pilar Pizcueta M, Casamitjana R, Rivera F, Rodés J. Hemodynamic effects of glucagon in portal hypertension. Hepatology. 1990;11:668–673. doi: 10.1002/hep.1840110421. [DOI] [PubMed] [Google Scholar]
- 158.Geraghty JG, Angerson WJ, Carter DC. Splanchnic haemodynamics and vasoactive agents in experimental cirrhosis. HPB Surg. 1994;8:83–87; discussion 87-88. doi: 10.1155/1994/52975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Benoit JN, Zimmerman B, Premen AJ, Go VL, Granger DN. Role of glucagon in splanchnic hyperemia of chronic portal hypertension. Am J Physiol. 1986;251:G674–G677. doi: 10.1152/ajpgi.1986.251.5.G674. [DOI] [PubMed] [Google Scholar]
- 160.Tsui CP, Sung JJ, Leung FW. Role of acute elevation of portal venous pressure by exogenous glucagon on gastric mucosal injury in rats with portal hypertension. Life Sci. 2003;73:1115–1129. doi: 10.1016/s0024-3205(03)00413-2. [DOI] [PubMed] [Google Scholar]
- 161.Ohta M, Kishihara F, Hashizume M, Kawanaka H, Tomikawa M, Higashi H, Tanoue K, Sugimachi K. Increased prostacyclin content in gastric mucosa of cirrhotic patients with portal hypertensive gastropathy. Prostaglandins Leukot Essent Fatty Acids. 1995;53:41–45. doi: 10.1016/0952-3278(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 162.Weiler H, Weiler C, Gerok W. Gastric mucosal prostaglandin E2 levels in cirrhosis and portal hypertension. J Hepatol. 1990;11:58–64. doi: 10.1016/0168-8278(90)90272-s. [DOI] [PubMed] [Google Scholar]
- 163.Arakawa T, Satoh H, Fukuda T, Nakamura H, Kobayashi K. Endogenous prostaglandin E2 in gastric mucosa of patients with alcoholic cirrhosis and portal hypertension. Gastroenterology. 1987;93:135–140. doi: 10.1016/0016-5085(87)90325-8. [DOI] [PubMed] [Google Scholar]
- 164.Beck PL, McKnight GW, Kelly JK, Wallace JL, Lee SS. Hepatic and gastric cytoprotective effects of long-term prostaglandin E1 administration in cirrhotic rats. Gastroenterology. 1993;105:1483–1489. doi: 10.1016/0016-5085(93)90155-6. [DOI] [PubMed] [Google Scholar]
- 165.Migoh S, Hashizume M, Tsugawa K, Tanoue K, Sugimachi K. Role of endothelin-1 in congestive gastropathy in portal hypertensive rats. J Gastroenterol Hepatol. 2000;15:142–147. doi: 10.1046/j.1440-1746.2000.02061.x. [DOI] [PubMed] [Google Scholar]
- 166.Romano M, Meise KS, Suozzo R, Sessa G, Persico M, Coffey RJ. Regional distribution of transforming growth factor-alpha and epidermal growth factor in normal and portal hypertensive gastric mucosa in humans. Dig Dis Sci. 1995;40:263–267. doi: 10.1007/BF02065407. [DOI] [PubMed] [Google Scholar]
- 167.Pleli T, Martin D, Kronenberger B, Brunner F, Köberle V, Grammatikos G, Farnik H, Martinez Y, Finkelmeier F, Labocha S, et al. Serum autotaxin is a parameter for the severity of liver cirrhosis and overall survival in patients with liver cirrhosis--a prospective cohort study. PLoS One. 2014;9:e103532. doi: 10.1371/journal.pone.0103532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Misra SP, Dwivedi M, Misra V, Agarwal SK, Gupta R, Gupta SC, Mital VP. Endoscopic and histologic appearance of the gastric mucosa in patients with portal hypertension. Gastrointest Endosc. 1990;36:575–579. doi: 10.1016/s0016-5107(90)71167-4. [DOI] [PubMed] [Google Scholar]
- 169.Dong L, Zhang ZN, Fang P, Ma SY. Portal hypertensive gastropathy and its interrelated factors. Hepatobiliary Pancreat Dis Int. 2003;2:226–229. [PubMed] [Google Scholar]
- 170.Giofré MR, Meduri G, Pallio S, Calandra S, Magnano A, Niceforo D, Cinquegrani M, di Leo V, Mazzon E, Sturniolo GC, et al. Gastric permeability to sucrose is increased in portal hypertensive gastropathy. Eur J Gastroenterol Hepatol. 2000;12:529–533. doi: 10.1097/00042737-200012050-00009. [DOI] [PubMed] [Google Scholar]
- 171.Bahnacy A, Kupcsulik P, Elés ZS, Jàray B, Flautner L. Helicobacter pylori and congestive gastropathy. Z Gastroenterol. 1997;35:109–112. [PubMed] [Google Scholar]
- 172.Balan KK, Jones AT, Roberts NB, Pearson JP, Critchley M, Jenkins SA. The effects of Helicobacter pylori colonization on gastric function and the incidence of portal hypertensive gastropathy in patients with cirrhosis of the liver. Am J Gastroenterol. 1996;91:1400–1406. [PubMed] [Google Scholar]
- 173.Auroux J, Lamarque D, Roudot-Thoraval F, Deforges L, Chaumette MT, Richardet JP, Delchier JC. Gastroduodenal ulcer and erosions are related to portal hypertensive gastropathy and recent alcohol intake in cirrhotic patients. Dig Dis Sci. 2003;48:1118–1123. doi: 10.1023/a:1023772930681. [DOI] [PubMed] [Google Scholar]
- 174.Sathar SA, Kunnathuparambil SG, Sreesh S, Narayanan P, Vinayakumar KR. Helicobacter pylori infection in patients with liver cirrhosis: prevalence and association with portal hypertensive gastropathy. Ann Gastroenterol. 2014;27:48–52. [PMC free article] [PubMed] [Google Scholar]
- 175.Zullo A, Hassan C, Ridola L, Francesco VD. Helicobacter pylori and portal hypertensive gastropathy: any new information? Ann Gastroenterol. 2014;27:91. [PMC free article] [PubMed] [Google Scholar]
- 176.Abbas Z, Yakoob J, Usman MW, Shakir T, Hamid S, Jafri W. Effect of Helicobacter pylori and its virulence factors on portal hypertensive gastropathy and interleukin (IL)-8, IL-10, and tumor necrosis factor-alpha levels. Saudi J Gastroenterol. 2014;20:120–127. doi: 10.4103/1319-3767.129477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vigneri S, Termini R, Piraino A, Scialabba A, Pisciotta G, Fontana N. The stomach in liver cirrhosis. Endoscopic, morphological, and clinical correlations. Gastroenterology. 1991;101:472–478. doi: 10.1016/0016-5085(91)90027-i. [DOI] [PubMed] [Google Scholar]
- 178.Toyonaga A, Iwao T. Portal-hypertensive gastropathy. J Gastroenterol Hepatol. 1998;13:865–877. doi: 10.1111/j.1440-1746.1998.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 179.Stewart CA, Sanyal AJ. Grading portal gastropathy: validation of a gastropathy scoring system. Am J Gastroenterol. 2003;98:1758–1765. doi: 10.1111/j.1572-0241.2003.07595.x. [DOI] [PubMed] [Google Scholar]
- 180.Tanoue K, Hashizume M, Wada H, Ohta M, Kitano S, Sugimachi K. Effects of endoscopic injection sclerotherapy on portal hypertensive gastropathy: a prospective study. Gastrointest Endosc. 1992;38:582–585. doi: 10.1016/s0016-5107(92)70522-7. [DOI] [PubMed] [Google Scholar]
- 181.Sarin , SK . Diagnostic issues: Portal hypertensive gastropathy and gastric varices. In: DeFranchis R, editor. Portal hypertension II. Proceedings of the second Baveno international consensus workshop on definitions, methodology and therapeutic strategies. Oxford: Blackwell Science; 1996. pp. 30–55. [Google Scholar]
- 182.Yoo HY, Eustace JA, Verma S, Zhang L, Harris M, Kantsevoy S, Lee LA, Kalloo AN, Ravich WJ, Thuluvath PJ. Accuracy and reliability of the endoscopic classification of portal hypertensive gastropathy. Gastrointest Endosc. 2002;56:675–680. doi: 10.1067/mge.2002.128539. [DOI] [PubMed] [Google Scholar]
- 183.Burak KW, Beck PL. Diagnosis of portal hypertensive gastropathy. Curr Opin Gastroenterol. 2003;19:477–482. doi: 10.1097/00001574-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 184.Hashizume M, Sugimachi K. Classification of gastric lesions associated with portal hypertension. J Gastroenterol Hepatol. 1995;10:339–343. doi: 10.1111/j.1440-1746.1995.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 185.Parker DM, Wilsoncroft WE, Olshansky T. The relationship between life change and relative autonomic balance. J Clin Psychol. 1976;32:149–153. doi: 10.1002/1097-4679(197601)32:1<149::aid-jclp2270320138>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 186.Iwao T, Toyonaga A, Ikegami M, Shigemori H, Oho K, Sumino M, Sakaki M, Nakayama M, Nishiyama T, Minetoma T. McCormack’s endoscopic signs for diagnosing portal hypertension: comparison with gastroesophageal varices. Gastrointest Endosc. 1994;40:470–473. doi: 10.1016/s0016-5107(94)70212-8. [DOI] [PubMed] [Google Scholar]
- 187.de Macedo GF, Ferreira FG, Ribeiro MA, Szutan LA, Assef MS, Rossini LG. Reliability in endoscopic diagnosis of portal hypertensive gastropathy. World J Gastrointest Endosc. 2013;5:323–331. doi: 10.4253/wjge.v5.i7.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Eisen GM, Eliakim R, Zaman A, Schwartz J, Faigel D, Rondonotti E, Villa F, Weizman E, Yassin K, deFranchis R. The accuracy of PillCam ESO capsule endoscopy versus conventional upper endoscopy for the diagnosis of esophageal varices: a prospective three-center pilot study. Endoscopy. 2006;38:31–35. doi: 10.1055/s-2005-921189. [DOI] [PubMed] [Google Scholar]
- 189.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 190.Min YW, Bae SY, Gwak GY, Paik YH, Choi MS, Lee JH, Paik SW, Yoo BC, Koh KC. A clinical predictor of varices and portal hypertensive gastropathy in patients with chronic liver disease. Clin Mol Hepatol. 2012;18:178–184. doi: 10.3350/cmh.2012.18.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Patwardhan VR, Cardenas A. Review article: the management of portal hypertensive gastropathy and gastric antral vascular ectasia in cirrhosis. Aliment Pharmacol Ther. 2014;40:354–362. doi: 10.1111/apt.12824. [DOI] [PubMed] [Google Scholar]
- 192.Ripoll C, Garcia-Tsao G. Treatment of gastropathy and gastric antral vascular ectasia in patients with portal hypertension. Curr Treat Options Gastroenterol. 2007;10:483–494. doi: 10.1007/s11938-007-0048-5. [DOI] [PubMed] [Google Scholar]
- 193.Burak KW, Lee SS, Beck PL. Portal hypertensive gastropathy and gastric antral vascular ectasia (GAVE) syndrome. Gut. 2001;49:866–872. doi: 10.1136/gut.49.6.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dulai GS, Jensen DM, Kovacs TO, Gralnek IM, Jutabha R. Endoscopic treatment outcomes in watermelon stomach patients with and without portal hypertension. Endoscopy. 2004;36:68–72. doi: 10.1055/s-2004-814112. [DOI] [PubMed] [Google Scholar]
- 195.Urrunaga NH, Rockey DC. Portal hypertensive gastropathy and colopathy. Clin Liver Dis. 2014;18:389–406. doi: 10.1016/j.cld.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Naidu H, Huang Q, Mashimo H. Gastric antral vascular ectasia: the evolution of therapeutic modalities. Endosc Int Open. 2014;2:E67–E73. doi: 10.1055/s-0034-1365525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Payen JL, Calès P, Voigt JJ, Barbe S, Pilette C, Dubuisson L, Desmorat H, Vinel JP, Kervran A, Chayvialle JA. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:138–144. doi: 10.1016/0016-5085(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 198.Siramolpiwat S. Transjugular intrahepatic portosystemic shunts and portal hypertension-related complications. World J Gastroenterol. 2014;20:16996–17010. doi: 10.3748/wjg.v20.i45.16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tran A, Villeneuve JP, Bilodeau M, Willems B, Marleau D, Fenyves D, Parent R, Pomier-Layrargues G. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with estrogen-progesterone in cirrhotic patients: an open pilot study. Am J Gastroenterol. 1999;94:2909–2911. doi: 10.1111/j.1572-0241.1999.01436.x. [DOI] [PubMed] [Google Scholar]
- 200.Spahr L, Villeneuve JP, Dufresne MP, Tassé D, Bui B, Willems B, Fenyves D, Pomier-Layrargues G. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut. 1999;44:739–742. doi: 10.1136/gut.44.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Barbara G, De Giorgio R, Salvioli B, Stanghellini V, Corinaldesi R. Unsuccessful octreotide treatment of the watermelon stomach. J Clin Gastroenterol. 1998;26:345–346. doi: 10.1097/00004836-199806000-00029. [DOI] [PubMed] [Google Scholar]
- 202.Herrera S, Bordas JM, Llach J, Ginès A, Pellisé M, Fernández-Esparrach G, Mondelo F, Mata A, Cárdenas A, Castells A. The beneficial effects of argon plasma coagulation in the management of different types of gastric vascular ectasia lesions in patients admitted for GI hemorrhage. Gastrointest Endosc. 2008;68:440–446. doi: 10.1016/j.gie.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 203.Smith LA, Morris AJ, Stanley AJ. The use of hemospray in portal hypertensive bleeding; a case series. J Hepatol. 2014;60:457–460. doi: 10.1016/j.jhep.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 204.Probst A, Scheubel R, Wienbeck M. Treatment of watermelon stomach (GAVE syndrome) by means of endoscopic argon plasma coagulation (APC): long-term outcome. Z Gastroenterol. 2001;39:447–452. doi: 10.1055/s-2001-15722. [DOI] [PubMed] [Google Scholar]
- 205.Ng I, Lai KC, Ng M. Clinical and histological features of gastric antral vascular ectasia: successful treatment with endoscopic laser therapy. J Gastroenterol Hepatol. 1996;11:270–274. doi: 10.1111/j.1440-1746.1996.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 206.Wells CD, Harrison ME, Gurudu SR, Crowell MD, Byrne TJ, Depetris G, Sharma VK. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008;68:231–236. doi: 10.1016/j.gie.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 207.McGorisk T, Krishnan K, Keefer L, Komanduri S. Radiofrequency ablation for refractory gastric antral vascular ectasia (with video) Gastrointest Endosc. 2013;78:584–588. doi: 10.1016/j.gie.2013.04.173. [DOI] [PubMed] [Google Scholar]
- 208.Turon F, Casu S, Hernández-Gea V, Garcia-Pagán JC. Variceal and other portal hypertension related bleeding. Best Pract Res Clin Gastroenterol. 2013;27:649–664. doi: 10.1016/j.bpg.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 209.Vincent C, Pomier-Layrargues G, Dagenais M, Lapointe R, Létourneau R, Roy A, Paré P, Huet PM. Cure of gastric antral vascular ectasia by liver transplantation despite persistent portal hypertension: a clue for pathogenesis. Liver Transpl. 2002;8:717–720. doi: 10.1053/jlts.2002.34382. [DOI] [PubMed] [Google Scholar]
- 210.Novitsky YW, Kercher KW, Czerniach DR, Litwin DE. Watermelon stomach: pathophysiology, diagnosis, and management. J Gastrointest Surg. 2003;7:652–661. doi: 10.1016/s1091-255x(02)00435-3. [DOI] [PubMed] [Google Scholar]
- 211.Jin T, Fei BY, Zheng WH, Wang YX. Successful treatment of refractory gastric antral vascular ectasia by distal gastrectomy: a case report. World J Gastroenterol. 2014;20:14073–14075. doi: 10.3748/wjg.v20.i38.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fuccio L, Mussetto A, Laterza L, Eusebi LH, Bazzoli F. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc. 2013;5:6–13. doi: 10.4253/wjge.v5.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Calès P, Voigt JJ, Payen JL, Bloom E, Berg P, Vinel JP, Pradère B, Broussy P, Pascal JP. Diffuse vascular ectasia of the antrum, duodenum, and jejunum in a patient with nodular regenerative hyperplasia. Lack of response to portosystemic shunt or gastrectomy. Gut. 1993;34:558–561. doi: 10.1136/gut.34.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ripoll C, Garcia-Tsao G. Management of gastropathy and gastric vascular ectasia in portal hypertension. Clin Liver Dis. 2010;14:281–295. doi: 10.1016/j.cld.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Suit PF, Petras RE, Bauer TW, Petrini JL. Gastric antral vascular ectasia. A histologic and morphometric study of «the watermelon stomach». Am J Surg Pathol. 1987;11:750–757. [PubMed] [Google Scholar]
- 216.Kar P, Mitra S, Resnick JM, Torbey CF. Gastric antral vascular ectasia: case report and review of the literature. Clin Med Res. 2013;11:80–85. doi: 10.3121/cmr.2012.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gostout CJ, Viggiano TR, Balm RK. Acute gastrointestinal bleeding from portal hypertensive gastropathy: prevalence and clinical features. Am J Gastroenterol. 1993;88:2030–2033. [PubMed] [Google Scholar]
- 218.Kimura K, Ikegami T, Bekki Y, Ninomiya M, Yamashita Y, Yoshizumi T, Yoshiya S, Soejima Y, Harada N, Shirabe K, et al. Clinical significance of gastrointestinal bleeding after living donor liver transplantation. Transpl Int. 2014;27:705–711. doi: 10.1111/tri.12325. [DOI] [PubMed] [Google Scholar]
- 219.Kotzampassi K, Eleftheriadis E, Aletras H. The ‘mosaic-like’ pattern of portal hypertensive gastric mucosa after variceal eradication by sclerotherapy. J Gastroenterol Hepatol. 1990;5:659–663. doi: 10.1111/j.1440-1746.1990.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 220.Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, Ripoll C, Maurer R, Planas R, Escorsell A, et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7:689–695. doi: 10.1016/j.cgh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mathurin SA, Agüero AP, Dascani NA, Prestera JA, Gianserra C, Londero E, Chiorra C. [Anemia in hospitalized patients with cirrhosis: prevalence, clinical relevance and predictive factors] Acta Gastroenterol Latinoam. 2009;39:103–111. [PubMed] [Google Scholar]
- 222.Pagliaro L. Lebrec D, Poynard T, Hillon P, Benhamou J-P. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis. A controlled study [N Engl J Med 1981; 305: 1371-1374] J Hepatol. 2002;36:148–150. doi: 10.1056/NEJM198112033052302. [DOI] [PubMed] [Google Scholar]
- 223.Panés J, Bordas JM, Piqué JM, García-Pagán JC, Feu F, Terés J, Bosch J, Rodés J. Effects of propranolol on gastric mucosal perfusion in cirrhotic patients with portal hypertensive gastropathy. Hepatology. 1993;17:213–218. doi: 10.1002/hep.1840170209. [DOI] [PubMed] [Google Scholar]
- 224.Triantos C, Kalafateli M. Primary prevention of bleeding from esophageal varices in patients with liver cirrhosis. World J Hepatol. 2014;6:363–369. doi: 10.4254/wjh.v6.i6.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lebrec D, Poynard T, Hillon P, Benhamou JP. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a controlled study. N Engl J Med. 1981;305:1371–1374. doi: 10.1056/NEJM198112033052302. [DOI] [PubMed] [Google Scholar]
- 226.Merkel C, Marin R, Sacerdoti D, Donada C, Cavallarin G, Torboli P, Amodio P, Sebastianelli G, Bolognesi M, Felder M, et al. Long-term results of a clinical trial of nadolol with or without isosorbide mononitrate for primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2000;31:324–329. doi: 10.1002/hep.510310210. [DOI] [PubMed] [Google Scholar]
- 227.Abid S, Ali S, Baig MA, Waheed AA. Is it time to replace propranolol with carvedilol for portal hypertension? World J Gastrointest Endosc. 2015;7:532–539. doi: 10.4253/wjge.v7.i5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chan CC, Lee FY, Wang SS, Chang FY, Lin HC, Lin HJ, Chu CJ, Wu SL, Tai CC, Lee SD. Chronic administration of octreotide ameliorates portal hypertension and portal hypertensive gastropathy in rats with cirrhosis. Clin Sci (Lond) 1998;94:367–371. doi: 10.1042/cs0940367. [DOI] [PubMed] [Google Scholar]
- 229.Li MK, Sung JJ, Woo KS, Sanderson J, Leung NW, Yu LM, Tsui CP, Chung SC, Leung FW. Somatostatin reduces gastric mucosal blood flow in patients with portal hypertensive gastropathy: a randomized, double-blind crossover study. Dig Dis Sci. 1996;41:2440–2446. doi: 10.1007/BF02100140. [DOI] [PubMed] [Google Scholar]
- 230.Sung JJ, Tsui CP, Li MK, Leung FW. Somatostatin attenuates the hyperdynamic circulatory state in the gastric mucosa of rats with portal hypertension. Scand J Gastroenterol. 1995;30:921–926. doi: 10.3109/00365529509101602. [DOI] [PubMed] [Google Scholar]
- 231.Panés J, Piqué JM, Bordas JM, Casadevall M, Terés J, Bosch J, Rodés J. Effect of bolus injection and continuous infusion of somatostatin on gastric perfusion in cirrhotic patients with portal-hypertensive gastropathy. Hepatology. 1994;20:336–341. [PubMed] [Google Scholar]
- 232.Escorsell A, Bandi JC, Andreu V, Moitinho E, García-Pagán JC, Bosch J, Rodés J. Desensitization to the effects of intravenous octreotide in cirrhotic patients with portal hypertension. Gastroenterology. 2001;120:161–169. doi: 10.1053/gast.2001.20892. [DOI] [PubMed] [Google Scholar]
- 233.Law AW, Gales MA. Octreotide or vasopressin for bleeding esophageal varices. Ann Pharmacother. 1997;31:237–238. doi: 10.1177/106002809703100216. [DOI] [PubMed] [Google Scholar]
- 234.Iwao T, Toyonaga A, Shigemori H, Oho K, Sumino M, Sato M, Tanikawa K. Vasopressin plus oxygen vs vasopressin alone in cirrhotic patients with portal-hypertensive gastropathy: effects on gastric mucosal haemodynamics and oxygenation. J Gastroenterol Hepatol. 1996;11:216–222. doi: 10.1111/j.1440-1746.1996.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 235.Panés J, Piqué JM, Bordas JM, Llach J, Bosch J, Terés J, Rodés J. Reduction of gastric hyperemia by glypressin and vasopressin administration in cirrhotic patients with portal hypertensive gastropathy. Hepatology. 1994;19:55–60. doi: 10.1002/hep.1840190110. [DOI] [PubMed] [Google Scholar]
- 236.Panés J, Casadevall M, Fernández M, Piqué JM, Bosch J, Casamitjana R, Cirera I, Bombí JA, Terés J, Rodés J. Gastric microcirculatory changes of portal-hypertensive rats can be attenuated by long-term estrogen-progestagen treatment. Hepatology. 1994;20:1261–1270. doi: 10.1002/hep.1840200525. [DOI] [PubMed] [Google Scholar]
- 237.Karajeh MA, Hurlstone DP, Stephenson TJ, Ray-Chaudhuri D, Gleeson DC. Refractory bleeding from portal hypertensive gastropathy: a further novel role for thalidomide therapy? Eur J Gastroenterol Hepatol. 2006;18:545–548. doi: 10.1097/00042737-200605000-00016. [DOI] [PubMed] [Google Scholar]
- 238.Cremers MI, Oliveira AP, Alves AL, Freitas J. Portal hypertensive gastropathy: treatment with corticosteroids. Endoscopy. 2002;34:177. doi: 10.1055/s-2002-19848. [DOI] [PubMed] [Google Scholar]
- 239.Wagatsuma Y, Naritaka Y, Shimakawa T, Kanako H, Keiichiro I, Shunichi S, Konno S, Katsube T, Ogawa K. Clinical usefulness of the angiotensin II receptor antagonist losartan in patients with portal hypertensive gastropathy. Hepatogastroenterology. 2006;53:171–174. [PubMed] [Google Scholar]
- 240.Smart HL, Triger DR. Clinical features, pathophysiology and relevance of portal hypertensive gastropathy. Endoscopy. 1991;23:224–228. doi: 10.1055/s-2007-1010663. [DOI] [PubMed] [Google Scholar]
- 241.Pique JM, Leung FW, Kitahora T, Sarfeh IJ, Tarnawski A, Guth PH. Gastric mucosal blood flow and acid secretion in portal hypertensive rats. Gastroenterology. 1988;95:727–733. doi: 10.1016/s0016-5085(88)80021-0. [DOI] [PubMed] [Google Scholar]
- 242.Benoit JN, Barrowman JA, Harper SL, Kvietys PR, Granger DN. Role of humoral factors in the intestinal hyperemia associated with chronic portal hypertension. Am J Physiol. 1984;247:G486–G493. doi: 10.1152/ajpgi.1984.247.5.G486. [DOI] [PubMed] [Google Scholar]
- 243.Yen HH, Yang CW, Su WW, Soon MS, Wu SS, Lin HJ. Oral versus intravenous proton pump inhibitors in preventing re-bleeding for patients with peptic ulcer bleeding after successful endoscopic therapy. BMC Gastroenterol. 2012;12:66. doi: 10.1186/1471-230X-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pang SH, Graham DY. A clinical guide to using intravenous proton-pump inhibitors in reflux and peptic ulcers. Therap Adv Gastroenterol. 2010;3:11–22. doi: 10.1177/1756283X09352095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gostout CJ, Ahlquist DA, Radford CM, Viggiano TR, Bowyer BA, Balm RK. Endoscopic laser therapy for watermelon stomach. Gastroenterology. 1989;96:1462–1465. doi: 10.1016/0016-5085(89)90513-1. [DOI] [PubMed] [Google Scholar]
- 246.Ibrahim M, Degré D, Devière J. Active bleeding caused by portal hypertensive gastropathy. Gastrointest Endosc. 2014;80:724. doi: 10.1016/j.gie.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 247.Giday SA. Preliminary data on the nanopowder hemostatic agent TC-325 to control gastrointestinal bleeding. Gastroenterol Hepatol (N Y) 2011;7:620–622. [PMC free article] [PubMed] [Google Scholar]
- 248.Patel J, Parra V, Kedia P, Sharaiha RZ, Kahaleh M. Salvage cryotherapy in portal hypertensive gastropathy. Gastrointest Endosc. 2015;81:1003. doi: 10.1016/j.gie.2014.05.326. [DOI] [PubMed] [Google Scholar]
- 249.Sezai S, Ito M, Sakurai Y, Kamisaka K, Abe T, Ikegami F, Yamamoto Y, Hirano M. Effects on gastric circulation of treatment for portal hypertension in cirrhosis. Dig Dis Sci. 1998;43:1302–1306. doi: 10.1023/a:1018872227652. [DOI] [PubMed] [Google Scholar]
- 250.Hassoun Z, Pomier-Layrargues G. The transjugular intrahepatic portosystemic shunt in the treatment of portal hypertension. Eur J Gastroenterol Hepatol. 2004;16:1–4. doi: 10.1097/00042737-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 251.Bosch J, Abraldes JG. Management of gastrointestinal bleeding in patients with cirrhosis of the liver. Semin Hematol. 2004;41:8–12. doi: 10.1053/j.seminhematol.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 252.Rosado B, Kamath PS. Transjugular intrahepatic portosystemic shunts: an update. Liver Transpl. 2003;9:207–217. doi: 10.1053/jlts.2003.50045. [DOI] [PubMed] [Google Scholar]
- 253.McCashland TM. Current use of transjugular intrahepatic portosystemic shunts. Curr Gastroenterol Rep. 2003;5:31–38. doi: 10.1007/s11894-003-0007-9. [DOI] [PubMed] [Google Scholar]
- 254.Babb RR, Mitchell RL. Persistent hemorrhagic gastritis in a patient with portal hypertension and esophagogastric varices: the role of portal decompressive surgery. Am J Gastroenterol. 1988;83:777–779. [PubMed] [Google Scholar]
- 255.Biecker E. Portal hypertension and gastrointestinal bleeding: diagnosis, prevention and management. World J Gastroenterol. 2013;19:5035–5050. doi: 10.3748/wjg.v19.i31.5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yoshida H, Mamada Y, Taniai N, Tajiri T. Partial splenic embolization. Hepatol Res. 2008;38:225–233. doi: 10.1111/j.1872-034X.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 257.Shimizu T, Onda M, Tajiri T, Yoshida H, Mamada Y, Taniai N, Aramaki T, Kumazaki T. Bleeding portal-hypertensive gastropathy managed successfully by partial splenic embolization. Hepatogastroenterology. 2002;49:947–949. [PubMed] [Google Scholar]
- 258.Hosking SW. Congestive gastropathy in portal hypertension: variations in prevalence. Hepatology. 1989;10:257–258. doi: 10.1002/hep.1840100223. [DOI] [PubMed] [Google Scholar]
- 259.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823–832. doi: 10.1056/NEJMra0901512. [DOI] [PubMed] [Google Scholar]
- 260.Ripoll C, Garcia-Tsao G. The management of portal hypertensive gastropathy and gastric antral vascular ectasia. Dig Liver Dis. 2011;43:345–351. doi: 10.1016/j.dld.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 261.Kravetz D, Sikuler E, Groszmann RJ. Splanchnic and systemic hemodynamics in portal hypertensive rats during hemorrhage and blood volume restitution. Gastroenterology. 1986;90:1232–1240. doi: 10.1016/0016-5085(86)90390-2. [DOI] [PubMed] [Google Scholar]
- 262.Toubia N, Sanyal AJ. Portal hypertension and variceal hemorrhage. Med Clin North Am. 2008;92:551–574, viii. doi: 10.1016/j.mcna.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 263.Hogue CW Jr, Goodnough LT, Monk TG. Perioperative myocardial ischemic episodes are related to hematocrit level in patients undergoing radical prostatectomy. Transfusion. 1998;38:924–931. doi: 10.1046/j.1537-2995.1998.381098440856.x. [DOI] [PubMed] [Google Scholar]
- 264.Hwang JH, Shergill AK, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, et al. The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc. 2014;80:221–227. doi: 10.1016/j.gie.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 265.Soares-Weiser K, Brezis M, Tur-Kaspa R, Leibovici L. Antibiotic prophylaxis for cirrhotic patients with gastrointestinal bleeding. Cochrane Database Syst Rev. 2002;(2):CD002907. doi: 10.1002/14651858.CD002907. [DOI] [PubMed] [Google Scholar]
- 266.Soares-Weiser K, Brezis M, Tur-Kaspa R, Paul M, Yahav J, Leibovici L. Antibiotic prophylaxis of bacterial infections in cirrhotic inpatients: a meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2003;38:193–200. doi: 10.1080/00365520310000690. [DOI] [PubMed] [Google Scholar]
- 267.Chavez-Tapia NC, Soares-Weiser K, Brezis M, Leibovici L. Antibiotics for spontaneous bacterial peritonitis in cirrhotic patients. Cochrane Database Syst Rev. 2009;(1):CD002232. doi: 10.1002/14651858.CD002232.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–176. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 269.Cremers I, Ribeiro S. Management of variceal and nonvariceal upper gastrointestinal bleeding in patients with cirrhosis. Therap Adv Gastroenterol. 2014;7:206–216. doi: 10.1177/1756283X14538688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Snyder P, Ali R, Poles M, Gross SA. Portal hypertensive gastropathy with a focus on management. Expert Rev Gastroenterol Hepatol. 2015;9:1207–1216. doi: 10.1586/17474124.2015.1059275. [DOI] [PubMed] [Google Scholar]
- 271.Cirera I, Feu F, Luca A, García-Pagán JC, Fernández M, Escorsell A, Bosch J, Rodés J. Effects of bolus injections and continuous infusions of somatostatin and placebo in patients with cirrhosis: a double-blind hemodynamic investigation. Hepatology. 1995;22:106–111. [PubMed] [Google Scholar]
- 272.Villanueva C, Planella M, Aracil C, López-Balaguer JM, González B, Miñana J, Balanzó J. Hemodynamic effects of terlipressin and high somatostatin dose during acute variceal bleeding in nonresponders to the usual somatostatin dose. Am J Gastroenterol. 2005;100:624–630. doi: 10.1111/j.1572-0241.2004.40665.x. [DOI] [PubMed] [Google Scholar]
- 273.Oluyemi A, Amole A. Portal hypertensive duodenopathy manifesting as “kissing” duodenal ulcers in a nigerian with alcoholic cirrhosis: a case report and brief review of the literature. Case Rep Med. 2012;2012:618729. doi: 10.1155/2012/618729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nagral AS, Joshi AS, Bhatia SJ, Abraham P, Mistry FP, Vora IM. Congestive jejunopathy in portal hypertension. Gut. 1993;34:694–697. doi: 10.1136/gut.34.5.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Misra SP, Dwivedi M, Misra V, Gupta M. Ileal varices and portal hypertensive ileopathy in patients with cirrhosis and portal hypertension. Gastrointest Endosc. 2004;60:778–783. doi: 10.1016/s0016-5107(04)02049-8. [DOI] [PubMed] [Google Scholar]
- 276.Kozarek RA, Botoman VA, Bredfeldt JE, Roach JM, Patterson DJ, Ball TJ. Portal colopathy: prospective study of colonoscopy in patients with portal hypertension. Gastroenterology. 1991;101:1192–1197. doi: 10.1016/0016-5085(91)90067-u. [DOI] [PubMed] [Google Scholar]


