Abstract
Up to date, in literature, it is still debated the role of anti-tumor necrosis factors (TNF)-α treatments in hepatitis C virus (HCV) patients. TNF-α performs a lot of functions, it is an important pro-inflammatory cytokine and it is involved in the host’s immunity. Since TNF-α is implicated in the apoptotic signaling pathway of hepatocytes infected by HCV, anti TNF-α therapy may increase the risk of viral replication or their reactivation. However the treatment of anti TNF-α could have a healthful role because TNF-α appears to be engaged in the pathogenesis of liver fibrosis, inducing apoptotic pathways. We describe the case of a patient with plaque-type psoriasis and concomitant chronic HCV, who was treated successfully with anti-TNF agents simultaneously to cyclosporine without sign of reactivation of HCV and increase of liver enzymes. Our personal experience shows that anti-TNF-α agents are not only effective but also safe. Furthermore the combination therapy of cyclosporine and anti-TNF-α appears to be well-tolerated and able to reduce the amount of liver enzymes as well as HCV-viral-load. However systematic, large-scale studies with long follow-ups will be needed to confirm our results, in association with close liver function monitoring.
Keywords: Hepatitis C virus infection, Cyclosporine, Psoriasis, Safety, Anti-tumor necrosis factors-α agents
Core tip: Our paper reports a patient with plaque-type psoriasis and concomitant chronic hepatitis C virus (HCV), focusing on the safety and efficacy of cyclosporine therapy and anti tumor necrosis factors-α agents. This therapeutic association is also able to decrease liver enzymes as well as HCV load with general clinical improvement. Our topic may be useful in the clinical setting of patients with simultaneous severe psoriasis and chronic HCV infection.
INTRODUCTION
Psoriasis is a chronic inflammatory disorder, showing an incidence in the worldwide population of 2%-3%, where the genetic predisposition plays a pivotal role[1,2]. Typically it shows well-bordered erythemato-squamous patches and plaques, often covered by a characteristic silvery white scale formed by the hyperproliferation of epidermal keratinocytes. In addition itching and burning occur together with the clinical manifestations of the disease, compromising the patient’s quality of life[3-5].
According to the literature, in psoriatic patients, above all in the ones with a moderate and severity psoriasis, there is an increased risk of cardiovascular and metabolic disorders including diabetes mellitus, hypertension, obesity, dyslipidaemia, non-alcoholic fatty liver disease and arthritis[6,7].
In the last decade, the addition of tumor necrosis factors (TNF)-α antagonists has introduced new therapeutic perspectives for effective treatment of psoriasis and psoriatic arthritis. These treatments decrease the disease activity, also limiting and improving the pathological articular involvement. However, at the same time, TNF-α is also a critical cytokine implicated in the host’s defence against infective pathogens, performing a key function in the management of viral infections[8].
Consequently, the inhibition of the TNF-α pathway is supposed to increase the susceptibility to various infections, including viral diseases as well as their reactivation and immune response by recruiting and activating macrophages, natural killer cells, T cells and antigen-presenting cells[9].
According to these observations, patients qualified for anti-TNF-α therapy require to be carefully assessed about possible infections, in particular, positive medical history for hepatitis B virus (HBV) and/or chronic hepatitis C virus (HCV).
However, to date, the safety of anti-TNF-α agents in HCV patients with psoriasis is quite restricted and controversial[10-13].
The current case describes our personal experience with TNF-α blockers in the treatment of a patient with plaque-type psoriasis and concomitant chronic HCV infection, evaluating their efficacy and safety.
CASE REPORT
A 47-year-old man was diagnosed with an moderate-severe plaque-type psoriasis ever since 7 years. His medical history included familiarity for psoriasis and chronic hepatitis C infection (genotype 4) first diagnosed in 1993. The patient received combined treatment with Peg-interferon (INF)-α-2a and ribavirin which he continued for 24 wk. At the time of the diagnosis, HCV infection was under control, with a quantitative RNA PCR value of 3.02 × 105 IU/mL. Laboratory evaluation revealed a moderate increase both in erythrocyte sedimentation rate (44 mm/H) and in liver enzymes [aspartate aminotransferase (AST): 78 U/L; alanine aminotransferase (ALT): 80 U/L; gamma glutamyl transferase (γ-GT): 60 U/L]. The ultrasonic imaging of the liver showed moderate hepatomegaly with sharp and diffuse dishomogenity and minimal steatosis.
Assessment of cryoglobulins, complete blood count, total protein, albumin, total cholesterol, prothrombin time, blood urea nitrogen (BUN), creatinine, and urine examination were also performed.
His baseline Psoriasis Area and Severity Index (PASI) score was 11.5 and Dermatology Life Quality Index (DLQI) was 16. Treatments with several different topical therapies for psoriasis, including corticosteroids, vitamin D derivatives (calcipotriol, calcitriol or tacalcitol) were ineffective. Joint ultrasound showed no signs of psoriatic arthropathy; chest X-ray and quantiferon tb gold were negative and the patient started 4 mg/kg per day cyclosporine (Figure 1). At the first follow up after 12 wk of treatment the patient showed a PASI score improvement (2.7) with a reduction of DLQI. At the same time his HCV RNA status remained unaltered.
Figure 1.
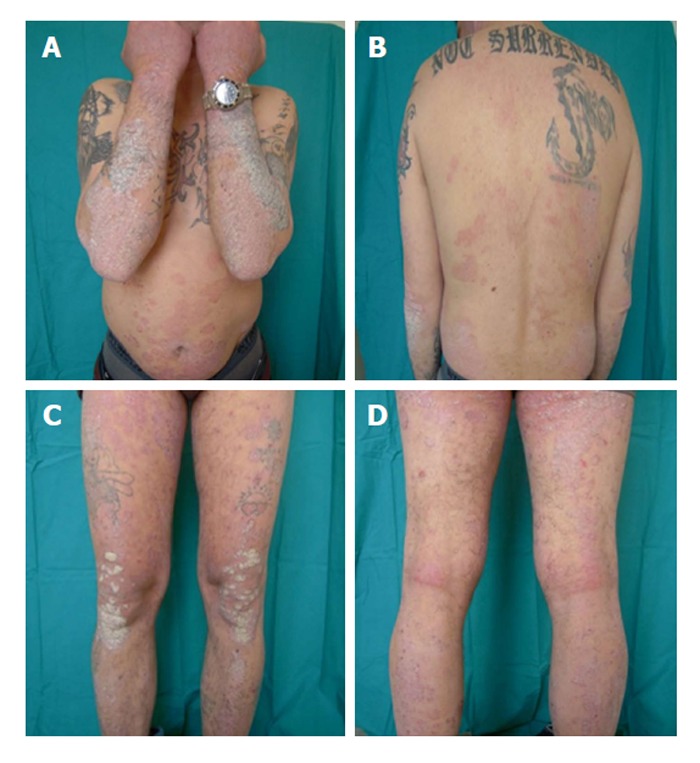
Basaline features: Psoriasis Area and Severity Index score 11.5, starting dose of cyclosporine 4 mg/kg per day.
At week 48 we observed an increase of BUN, creatinine and electrolytes, so we decided to reduce cyclosporine to a dose of 200 mg/d. However after further 4 wk the patient showed a worsened PASI score of 22.8 and DLQI 16 (Figure 2). At week 6, the patient began the first biological treatment with Etanercept at the dose of 50 mg weekly and at week 12 the PASI and DLQI were improved. Furthermore even if quantitative HCV RNA remained stable, we detected an increase of liver enzymes with the following values: AST: 99 U/L, ALT: 88 U/L and γ-GT: 99 U/L (Figure 3).
Figure 2.

Week 48: Clinical worsening (Psoriasis Area and Severity Index score 22.8) after reduction cyclosporine therapy.
Figure 3.
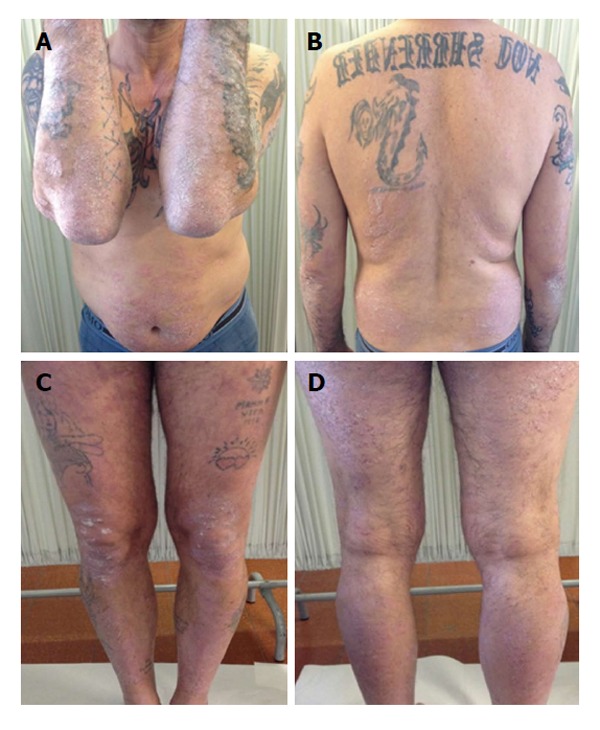
Treatment with etanercept: At week 12 Psoriasis Area and Severity Index score 16.2.
Etanercept was discontinued and the patient was started on intravenous anti TNF-α, infliximab, administration. At week 16 the liver enzymes decreased (AST: 60 U/L, ALT: 78 U/L, γ-GT: 96 U/L) while PASI score and HCV RNA maintained stable (Figure 4).
Figure 4.
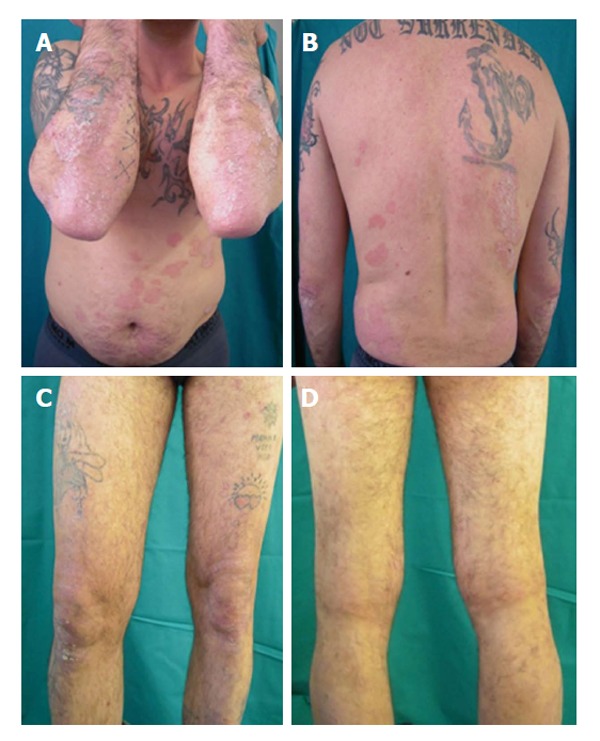
Treatment with infliximab: Week 16 Psoriasis Area and Severity Index score 15.
Since cyclosporine was discontinued because of kidney toxicity, Etanercept because of hepatic toxicity and clinical inefficacy and infliximab was stopped due to clinical failure, the patient was administrated the third anti TNF-α, that is adalimumab. At week 12 of treatment PASI score increased again whereas liver indexes were further restricted and HCV RNA serum levels decreased significantly (102 × 106 IU/mL) after 3 mo of therapy with adalimumab (Figure 5).
Figure 5.
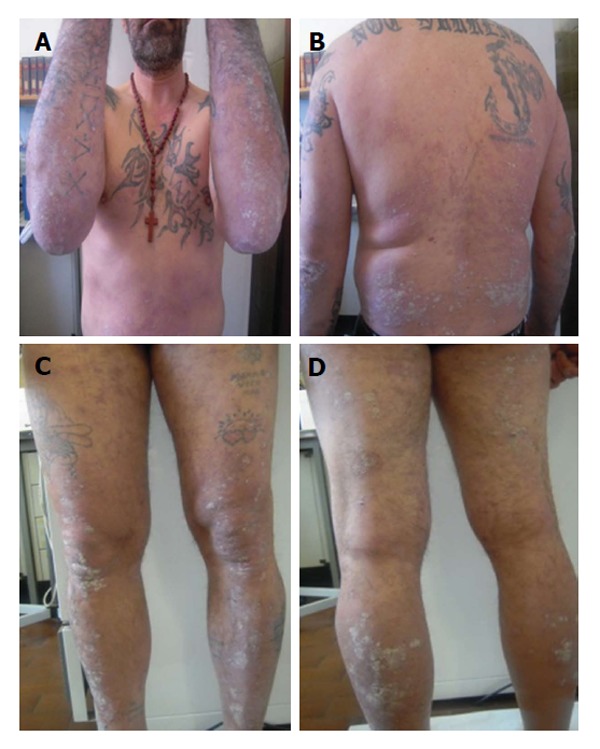
Treatment with adalimumab: Week 12 Psoriasis Area and Severity Index score 22.4.
Nevertheless cyclosporine therapy was associated to adalimumab to achieve a reduction of the PASI score.
After 12 wk the patient showed a PASI score and DLQI improvement with further reduction of liver enzymes (AST: 40 U/L, ALT: 48 U/L, γ-GT: 60 U/L); liver ultrasonic imaging documented a mild steatosis and the HCV RNA status remained unaffected (Figure 6).
Figure 6.
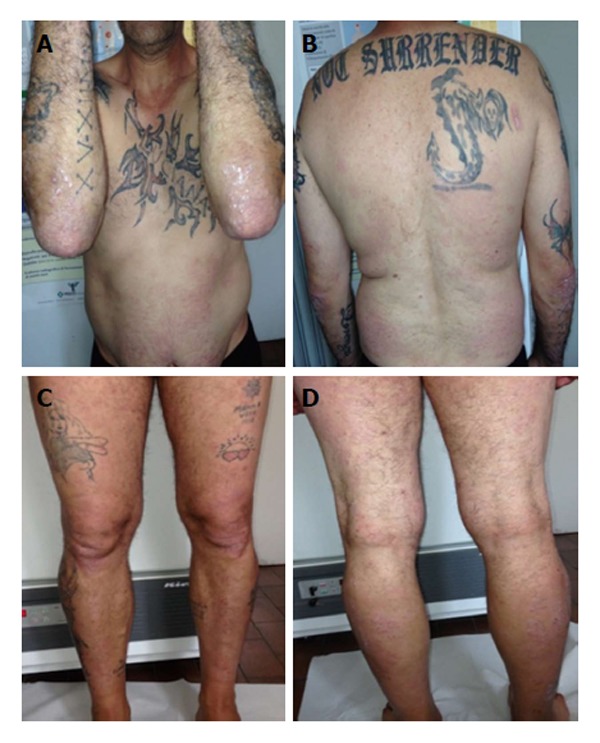
Combination therapy with cyclosporine and adalimumab: Week 12 Psoriasis Area and Severity Index score 2.3.
Currently, after a follow-up of 24 mo the patient performs the combined treatment with adalimumab and cyclosporine in association with clinical and instrumental investigations, showing a good clinical control of the skin lesions, as well as of the chronic HCV infection.
DISCUSSION
The present article reports our personal experience with TNF-α blockers in the handling of a patient with plaque-type psoriasis and concomitant chronic HCV infection, evaluating their efficacy and safety. Furthermore it shows that these treatments did not amplify the viral load and liver enzyme values.
Direct liver toxicity in psoriatic patients under treatment with TNF-α blockade is rare[14], although they may exacerbate the concurrent infections as well as reactivation of infections (e.g., tuberculosis); however their effects on the course of HBV or HCV infections are not well estimate[15].
Thus, in patients qualified for anti TNF-α therapy, a screening for tuberculosis (chest radiography, quantiferon tb gold assay or mantoux test), HBV, human immunodeficiency virus, cytomegalovirus, herpes viruses and HCV are mandatory[16,17].
TNF-α is a cytokine involved in several inflammatory reactions and autoimmune disorders, at the same time, playing a crucial role in the pathogenesis of HCV through the mediation of apoptosis and the maintenance of inflammatory processes. TNF-α is produced by hepatocytes in patients who are chronically infected with HCV, regulating viral replication and the relative hepatocyte damage[18,19].
According to the literature, in HCV patients, serum and hepatic levels of TNF-α and TNF-α receptor (p75) are increased, showing a direct correlation with serum transaminase levels, histological activity and fibrosis, but not with serum HCV RNA levels and/or viral genotype[20-22]. Besides, it is known that over-expression of TNF-α is associated with a negative prognosis and a relative resistance to INF therapy[23].
Therefore in the context of HCV the effect of TNF-α blockade could have potential healthful role because TNF-α appears to be engaged in the pathogenesis of liver fibrosis, inducing apoptotic pathways[24].
On the contrary, it is known that TNF-α is able to recruit and activate macrophages, natural kille (NK) cells, T cells and antigen-presenting cells; so, this cytokine induces the host’s immune response against infective pathogens and plays an important role in the control of viral infection. Thus, the reduction of TNF-α levels by biological drugs may increase the risk of an excessive viral replication as well as of a reactivation of chronic HCV infection[9]. However, there are not sufficient data about the assessment of the potential specific differences among the anti TNF-α drugs regarding their effect on viral replication[25].
The occurrence of autoimmune phenomena, encompassing non-organ specific autoantibody formation [including anti-double-stranded-DNA (dsDNA), rheumatoid factors and anti-cardiolipin] is a frequent event in patients treated with biologic agents[26,27]. In this regard, Vauloup et al[28] showed the formation of anti-nuclear and anti-dsDNA antibodies in HCV patients treated with anti-TNF-α inhibitors. Finally, in literature, are reported also seventeen cases of TNF-α-induced hepatitis (in patients without a positive medical history of liver disease), mainly caused by infliximab and resembling to hepatitis type 1[29-33].
Data on the efficacy and safety of anti-TNF-α in psoriatic patients with concomitant chronic HCV are very limited[9,11,12,34,35].
In literature there are reports about patients showing an increasing liver function, during treatment with biologic therapies (infliximab, adalimumab and etanercept), although the simultaneous administration of other therapies render difficult the evaluation of this effect[36,37]. At the same time, studies regarding psoriatic arthritis patients affected by HCV, after a follow-up of 12 mo did not reveal any significant increase in viral load, without showing alterations of liver enzymes and, neither clinical evidence of flaring of their liver disease[10].
In addition, a retrospective analysis showed that the risk of TNF-α inhibitors (etanercept and adalimumab) related to HBV or HCV reactivation is very low in these patients[14].
In this case report, our patients, before begin the treatment showed high liver enzymes and HCV RNA serum levels. During adalimumab and cyclosporine treatment the viral load and transaminases decreased together with a clinical improvement.
Cyclosporine is a powerful immunosuppressive agent that has clinical application in the treatment of autoimmune disorders, but several studies in the literature, both in vitro and in vivo, suggests that this drug also exerts an inhibitory effect on HCV replication at standard therapeutic dose[38]. Besides, literature reports also studies about the treatment of rheumatoid arthritis (RA) or autoimmune disorders with the combination therapy of anti-TNF-α and cyclosporine in HCV patients, highlighting the efficacy and safety in controlling HCV viremia and liver toxicity[39,40]. In this regard, Giannitti et al[39] described that 7 RA patients with chronic HCV have been treated so far, 4 with Etanercept and 3 with adalimumab combined with cyclosporine. After 6 mo of therapy, viral load decreased of 67% of the initial value and both aminotransferases also remained within normal limits in all patients over time.
This study focuses on the safety and efficacy in the short time of anti-TNF-α therapy in a HCV patient with psoriasis. However, to date, the literature lacks of results on the use of biologic treatments in long term, in this class of patients.
Serum aminotransferase, gammaglutamyl-transferase, total bilirubin, cryoglobulins, complete blood count, creatinine, urine exam, serum anti-HCV antibodies, assessment of HCV-RNA, liver ultrasonography in addition to an evaluation of the hepatologist are recommended in patients candidate for anti TNF-α agents, in order to evaluate the liver disease stage and a possible need for antiviral therapy. In addition, liver function tests should be performed every three months during treatment with TNF-α inhibitors.
In patients with plaque-type psoriasis and concomitant chronic HCV infection the treatment with cyclosporine and anti TNF-α agents should be considered safe, efficacy and well-tolerated as well as able to decrease liver enzymes and viral load.
Large-scale studies and long follow-ups are needed to successfully evaluate the risks and benefits of TNF-α blockades in psoriatic patients with a HCV infection.
COMMENTS
Case characteristics
A 47-year-old man was diagnosed with a moderate-severe plaque-type psoriasis ever since 7 years. His medical history included familiarity for psoriasis and chronic hepatitis C infection.
Clinical diagnosis
Baseline Psoriasis Area and Severity Index score was 11.5 and Dermatology Life Quality Index was 16. Treatments with several different topical therapies for psoriasis, including corticosteroids, vitamin D derivatives (calcipotriol, calcitriol or tacalcitol) were ineffective, while joint ultrasound showed no signs of psoriatic arthropathy.
Differential diagnosis
Hepatotoxicity induced by etanercept, viral reactivation.
Laboratory diagnosis
At week 6 after the first biological treatment with etanercept, the authors detected an increase of liver enzymes with the following values: Aspartate aminotransferase: 99 U/L, alanine aminotransferase: 88 U/L and gamma glutamyl transferase: 99 U/L; while hepatitis C virus (HCV) RNA remained always stable. Once Etanercept was discontinued and adalimumab was started, liver enzymes decreased concurrently.
Imaging diagnosis
The ultrasonic imaging of the liver showed moderate hepatomegaly with sharp and diffuse dishomogenity and minimal steatosis, while joint ultrasound showed no signs of psoriatic arthropathy.
Pathological diagnosis
A cutaneous punch biopsy revealed a plaque-type psoriasis.
Treatment
Cyclosporine (4 mg/kg per day), etanercept (50 mg/wk) and adalimumab (80 mg for the induction and 40 mg for the maintenance).
Related reports
This study shows that anti-tumor necrosis factors (TNF)-α inhibitors in patients with psoriasis and HCV appear to be effective and safe in the short term, but there are still insufficient data to estimate their long term safety.
Experiences and lessons
Combination therapy with cyclosporine and anti TNF-α agents in patients with plaque-type psoriasis and concomitant chronic HCV infection should be considered safe, efficacy and well-tolerated as well as able to decrease liver enzymes and viral load.
Peer-review
This is an interesting case that deserves to be published in the journal.
Footnotes
Institutional review board statement: This case report was exempt from the Institutional Review Board Standards of the Department of Dermatology at La Sapienza University of Rome.
Informed consent statement: The patient involved in this study gave his written informed consent authorizing use and disclosure of his protected health information.
Conflict-of-interest statement: All the authors have not conflict of interest to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 24, 2015
First decision: September 8, 2015
Article in press: November 11, 2015
P- Reviewer: Nahum MS S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
References
- 1.Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26:314–320. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 2.Sticherling M, Augustin M, Boehncke WH, Christophers E, Domm S, Gollnick H, Reich K, Mrowietz U. Therapy of psoriasis in childhood and adolescence - a German expert consensus. J Dtsch Dermatol Ges. 2011;9:815–823. doi: 10.1111/j.1610-0387.2011.07668.x. [DOI] [PubMed] [Google Scholar]
- 3.Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine. 2015;73:342–350. doi: 10.1016/j.cyto.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yosipovitch G, Goon A, Wee J, Chan YH, Goh CL. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol. 2000;143:969–973. doi: 10.1046/j.1365-2133.2000.03829.x. [DOI] [PubMed] [Google Scholar]
- 5.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280–284. [PubMed] [Google Scholar]
- 6.Piérard-Franchimont C, Piérard GE, Delvenne P, Hermanns-Lê T. [Psoriasis syndrome with its comorbidities] Rev Med Liege. 2014;69:555–558. [PubMed] [Google Scholar]
- 7.Okan G, Baki AM, Yorulmaz E, Doğru-Abbasoğlu S, Vural P. Serum Visfatin, Fetuin-A, and Pentraxin 3 Levels in Patients With Psoriasis and Their Relation to Disease Severity. J Clin Lab Anal. 2015:Epub ahead of print. doi: 10.1002/jcla.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Punzi L, Podswiadek M, Sfriso P, Oliviero F, Fiocco U, Todesco S. Pathogenetic and clinical rationale for TNF-blocking therapy in psoriatic arthritis. Autoimmun Rev. 2007;6:524–528. doi: 10.1016/j.autrev.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Modesti V, Ramonda R, Ortolan A, Lorenzin M, Lo Nigro A, Frallonardo P, Oliviero F, Campana C, Punzi L. Infection relapse in spondyloarthritis treated with biological drugs: a single-centre study. Scand J Rheumatol. 2012;41:490–491. doi: 10.3109/03009742.2012.698393. [DOI] [PubMed] [Google Scholar]
- 10.Costa L, Caso F, Atteno M, Giannitti C, Spadaro A, Ramonda R, Vezzù M, Del Puente A, Morisco F, Fiocco U, et al. Long-term safety of anti-TNF-α in PsA patients with concomitant HCV infection: a retrospective observational multicenter study on 15 patients. Clin Rheumatol. 2014;33:273–276. doi: 10.1007/s10067-013-2378-0. [DOI] [PubMed] [Google Scholar]
- 11.Aslanidis S, Vassiliadis T, Pyrpasopoulou A, Douloumpakas I, Zamboulis C. Inhibition of TNFalpha does not induce viral reactivation in patients with chronic hepatitis C infection: two cases. Clin Rheumatol. 2007;26:261–264. doi: 10.1007/s10067-006-0394-z. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese LH, Zein N. Biologic agents and liver toxicity: an added concern or therapeutic opportunity? Nat Clin Pract Rheumatol. 2007;3:422–423. doi: 10.1038/ncprheum0537. [DOI] [PubMed] [Google Scholar]
- 13.Rokhsar C, Rabhan N, Cohen SR. Etanercept monotherapy for a patient with psoriasis, psoriatic arthritis, and concomitant hepatitis C infection. J Am Acad Dermatol. 2006;54:361–362. doi: 10.1016/j.jaad.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 14.Prignano F, Ricceri F, Pescitelli L, Zanieri F, Lotti T. Tumour necrosis factor-α antagonists in patients with concurrent psoriasis and hepatitis B or hepatitis C: a retrospective analysis of 17 patients. Br J Dermatol. 2011;164:645–647. doi: 10.1111/j.1365-2133.2010.10140.x. [DOI] [PubMed] [Google Scholar]
- 15.Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor alpha therapy. Arthritis Rheum. 2003;48:3013–3022. doi: 10.1002/art.11301. [DOI] [PubMed] [Google Scholar]
- 16.Nathan DM, Angus PW, Gibson PR. Hepatitis B and C virus infections and anti-tumor necrosis factor-alpha therapy: guidelines for clinical approach. J Gastroenterol Hepatol. 2006;21:1366–1371. doi: 10.1111/j.1440-1746.2006.04559.x. [DOI] [PubMed] [Google Scholar]
- 17.Fabris P, Baldo V, Baldovin T, Bellotto E, Rassu M, Trivello R, Tramarin A, Tositti G, Floreani A. Changing epidemiology of HCV and HBV infections in Northern Italy: a survey in the general population. J Clin Gastroenterol. 2008;42:527–532. doi: 10.1097/MCG.0b013e318030e3ab. [DOI] [PubMed] [Google Scholar]
- 18.Park J, Kang W, Ryu SW, Kim WI, Chang DY, Lee DH, Park do Y, Choi YH, Choi K, Shin EC, et al. Hepatitis C virus infection enhances TNFα-induced cell death via suppression of NF-κB. Hepatology. 2012;56:831–840. doi: 10.1002/hep.25726. [DOI] [PubMed] [Google Scholar]
- 19.Suryaprasad AG, Prindiville T. The biology of TNF blockade. Autoimmun Rev. 2003;2:346–357. doi: 10.1016/s1568-9972(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 20.Tilg H, Wilmer A, Vogel W, Herold M, Nölchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–274. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- 21.Nelson DR, Lim HL, Marousis CG, Fang JW, Davis GL, Shen L, Urdea MS, Kolberg JA, Lau JY. Activation of tumor necrosis factor-alpha system in chronic hepatitis C virus infection. Dig Dis Sci. 1997;42:2487–2494. doi: 10.1023/a:1018804426724. [DOI] [PubMed] [Google Scholar]
- 22.Zylberberg H, Rimaniol AC, Pol S, Masson A, De Groote D, Berthelot P, Bach JF, Bréchot C, Zavala F. Soluble tumor necrosis factor receptors in chronic hepatitis C: a correlation with histological fibrosis and activity. J Hepatol. 1999;30:185–191. doi: 10.1016/s0168-8278(99)80060-9. [DOI] [PubMed] [Google Scholar]
- 23.Larrea E, Garcia N, Qian C, Civeira MP, Prieto J. Tumor necrosis factor alpha gene expression and the response to interferon in chronic hepatitis C. Hepatology. 1996;23:210–217. doi: 10.1002/hep.510230203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghavami S, Hashemi M, Kadkhoda K, Alavian SM, Bay GH, Los M. Apoptosis in liver diseases--detection and therapeutic applications. Med Sci Monit. 2005;11:RA337–RA345. [PubMed] [Google Scholar]
- 25.Pompili M, Biolato M, Miele L, Grieco A. Tumor necrosis factor-α inhibitors and chronic hepatitis C: a comprehensive literature review. World J Gastroenterol. 2013;19:7867–7873. doi: 10.3748/wjg.v19.i44.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeire S, Noman M, Van Assche G, Baert F, Van Steen K, Esters N, Joossens S, Bossuyt X, Rutgeerts P. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn’s disease: a prospective cohort study. Gastroenterology. 2003;125:32–39. doi: 10.1016/s0016-5085(03)00701-7. [DOI] [PubMed] [Google Scholar]
- 27.Elkayam O, Burke M, Vardinon N, Zakut V, Yitzhak RB, Paran D, Levartovsky D, Litinsky I, Caspi D. Autoantibodies profile of rheumatoid arthritis patients during treatment with infliximab. Autoimmunity. 2005;38:155–160. doi: 10.1080/08916930400021378. [DOI] [PubMed] [Google Scholar]
- 28.Vauloup C, Krzysiek R, Greangeot-Keros L, Wendling D, Goupille P, Brault R, Brousse C, Mariette X, Emilie D. Effects of tumor necrosis factor antagonist treatment on hepatitis C-related immunological abnormalities. Eur Cytokine Netw. 2006;17:290–293. [PubMed] [Google Scholar]
- 29.García Aparicio AM, Rey JR, Sanz AH, Alvarez JS. Successful treatment with etanercept in a patient with hepatotoxicity closely related to infliximab. Clin Rheumatol. 2007;26:811–813. doi: 10.1007/s10067-006-0253-y. [DOI] [PubMed] [Google Scholar]
- 30.Wahie S, Alexandroff A, Reynolds NJ. Hepatitis: a rare, but important, complication of infliximab therapy for psoriasis. Clin Exp Dermatol. 2006;31:460–461. doi: 10.1111/j.1365-2230.2006.02086.x. [DOI] [PubMed] [Google Scholar]
- 31.Kluger N, Girard C, Guillot B, Bessis D. Efficiency and safety of etanercept after acute hepatitis induced by infliximab for psoriasis. Acta Derm Venereol. 2009;89:332–334. doi: 10.2340/00015555-0619. [DOI] [PubMed] [Google Scholar]
- 32.Mancini S, Amorotti E, Vecchio S, Ponz de Leon M, Roncucci L. Infliximab-related hepatitis: discussion of a case and review of the literature. Intern Emerg Med. 2010;5:193–200. doi: 10.1007/s11739-009-0342-4. [DOI] [PubMed] [Google Scholar]
- 33.Coffin CS, Fraser HF, Panaccione R, Ghosh S. Liver diseases associated with anti-tumor necrosis factor-alpha (TNF-α) use for inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:479–484. doi: 10.1002/ibd.21336. [DOI] [PubMed] [Google Scholar]
- 34.Cavazzana I, Ceribelli A, Cattaneo R, Franceschini F. Treatment with etanercept in six patients with chronic hepatitis C infection and systemic autoimmune diseases. Autoimmun Rev. 2008;8:104–106. doi: 10.1016/j.autrev.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Magliocco MA, Gottlieb AB. Etanercept therapy for patients with psoriatic arthritis and concurrent hepatitis C virus infection: report of 3 cases. J Am Acad Dermatol. 2004;51:580–584. doi: 10.1016/j.jaad.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Hyrich KL, Silman AJ, Watson KD, Symmons DP. Anti-tumour necrosis factor alpha therapy in rheumatoid arthritis: an update on safety. Ann Rheum Dis. 2004;63:1538–1543. doi: 10.1136/ard.2004.024737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khanna D, McMahon M, Furst DE. Safety of tumour necrosis factor-alpha antagonists. Drug Saf. 2004;27:307–324. doi: 10.2165/00002018-200427050-00003. [DOI] [PubMed] [Google Scholar]
- 38.Galeazzi M, Bellisai F, Giannitti C, Manganelli S, Morozzi G, Sebastiani GD. Safety of cyclosporin A in HCV-infected patients: experience with cyclosporin A in patients affected by rheumatological disorders and concomitant HCV infection. Ann N Y Acad Sci. 2007;1110:544–549. doi: 10.1196/annals.1423.058. [DOI] [PubMed] [Google Scholar]
- 39.Giannitti C, Benucci M, Caporali R, Manganelli S, Bellisai F, Sebastiani GD, Galeazzi M. Efficacy and safety of anti-TNF-alpha therapy combined with cyclosporine A in patients with rheumatoid arthritis and concomitant hepatitis C virus infection. Int J Immunopathol Pharmacol. 2009;22:543–546. doi: 10.1177/039463200902200233. [DOI] [PubMed] [Google Scholar]
- 40.Bellisai F, Giannitti C, Donvito A, Galeazzi M. Combination therapy with cyclosporine A and anti-TNF-alpha agents in the treatment of rheumatoid arthritis and concomitant hepatitis C virus infection. Clin Rheumatol. 2007;26:1127–1129. doi: 10.1007/s10067-006-0412-1. [DOI] [PubMed] [Google Scholar]


