Abstract
The naturally occurring isotope of hydrogen (1H), deuterium (2H), could have an important biological role. Deuterium depleted water delays tumor progression in mice, dogs, cats and humans. Hydratase enzymes of the tricarboxylic acid (TCA) cycle control cell growth and deplete deuterium from redox cofactors, fatty acids and DNA, which undergo hydride ion and hydrogen atom transfer reactions. A model is proposed that emphasizes the terminal complex of mitochondrial electron transport chain reducing molecular oxygen to deuterium depleted water (DDW); this affects gluconeogenesis as well as fatty acid oxidation. In the former, the DDW is thought to diminish the deuteration of sugar-phosphates in the DNA backbone, helping to preserve stability of hydrogen bond networks, possibly protecting against aneuploidy and resisting strand breaks, occurring upon exposure to radiation and certain anticancer chemotherapeutics. DDW is proposed here to link cancer prevention and treatment using natural ketogenic diets, low deuterium drinking water, as well as DDW production as the mitochondrial downstream mechanism of targeted anti-cancer drugs such as Avastin and Glivec. The role of 2H in biology is a potential missing link to the elusive cancer puzzle seemingly correlated with cancer epidemiology in western populations as a result of excessive 2H loading from processed carbohydrate intake in place of natural fat consumption.
Introduction
Cancer is the leading cause of death already in Australia, Canada, Denmark, New Zealand and the UK reaching epidemic levels. In the US cancer is predicted to surpass heart disease as the lead killer by 2030 [1]. Devastating cancer statistics, ineffective treatment options and escalating costs amplify the drug compliance cliff, which necessitates new working hypotheses in order to secure affordable effective cancer treatments for the future and to control cancer epidemics. The genetic, signaling hypotheses of cancer with genomic targets as primary drug design efforts are more elusive and costly than ever, resulting in dismal, if any, progress in the field. Historically, Warburg proposed first that irreversible damage to respiration was the prime cause of cancer [2–4] with increased glucose to lactic acid product yield even in the presence of sufficient oxygen supplies. Yet, with Warburg's passing away on August 1, 1970, and the discovery of oncogenes in 1971, cancer research shifted to view cancer as a genetic disease. The “re-discovery” of cancer as a metabolic disease linked oncogenesis with pentose cycle metabolism and gene clustering in 1998 using modern targeted 13C-glucose fate association studies [5] for drug development [6]. Nevertheless, progress is still slow and overshadowed by molecular biological approaches unsuited to address metabolic networks and their inherently complex control properties [7] (Table 1). Massive medical and economical failures in genetically targeted kinase inhibitor drug development efforts have first been reviewed in 2005 [8], which since became sad realities. Thus, worsening cancer statistics JUSTIFY a continued significant interest in targeting cancer as a cellular metabolic disease [9,10].
Table 1.
Disease driving hypotheses in cancer.
|
The submolecular non-genomic theory of cancer dates back by half a century and was proposed by the 1937 Nobel laureate Albert Szent-Györgyi in Medicine. His theory links abnormal charge transfer and permittivity, as well as limited electron carrying by methyl glyoxal, proteins and ascorbic acid with cancer [11]. Submolecular mechanisms offer very precise and relatively simple reaction architectures to regulate cell growth, where hydrogen and deuterium (2H) showed prominent growth regulatory effects in a study performed by Somlyai [12]. The work of Somlyai readily offered explanations for the increase in tumorigenicity of human fibroblasts expressing an ATPase dependent yeast proton (1H+) pump with strong deuterium discrimination properties [13], which accumulates in and transforms mammalian cells [14]. Hydrogen pumping from cells in the expense of deuterium depleted metabolic water production during ATP synthesis shed lights on the critical involvement of balanced mitochondrial matrix functions in normal DNA and cell functions, as explained below as the core hypothesis of this report. Hydrogen and deuterium ratios in cells are since considered primary regulators of growth signaling, where exceptional kinetic isotope effects and severely altered collective proton tunneling are evident by deuterium [15,16] in hydrogen bonding and bridging physical as well as biological networks.
Exceptional deuterium substitution effects also offer explanations for the curious observation that 2H depletion in water, although being a rare isotope species, possesses such strong anti-cancer properties. Specifically, there are 155 2H2O heavy water molecules out of 1 million 1H2O (one 2H2O out of 6420 1H2O molecules) or 155 2H atoms out of 1 million 1H in oceanic water. On the other hand, one part mono-deuterated water 2H1HO exists in approximately 3210 parts fully protiated surface water, which means there is one deuterium in approximately 3210 water molecules. In biomolecules, e.g. in NADPH and DNA, the two stable isotopes of hydrogen, protium (1H) and deuterium (2H) induce very different physicochemical behaviors [17,18]. Though 12C containing molecules undergo ambient temperature bond-breaking reactions a few percent faster than the 13C counterparts [19], covalent bonds to protium are typically cleaved >7 times faster than bonds to deuterium because of the large differences in reduced masses and quantum mechanical properties that influence primary kinetic isotope effects [20]. Deuterium in human plasma is abundant with concentrations reaching 12–14 mmol/l, in comparison with calcium’s 2.24–2.74 mmol/l, magnesium’s 0.75–1.2 mmol/l, potassium’s 5.0–5.1 mmol/l and glucose’s 3.3–6.1 mmol/l circulating concentrations. Higher magnetic and electronic dipole moments of deuterium may also play a role in DNA hydrogen bond stability as well as abnormal cell proliferation. The deuterium/hydrogen (2H/1H) mass ratio, being also the largest among stable isotopes of the same element, causes major differences in the chemical bonding and collective proton tunneling behaviors ranging from cubic ice [15] to the structural integrity and function of growth signaling proteins [21,22], anabolic products of reductive synthesis, such as DNA [23], RNA [24] and nuclear membrane lipids in newly formed cells.
Experimental and clinical evidences of deuterium depletion and cell growth control
Consistent with the above, the effect of low 2H in water has been shown to control cell proliferation in numerous biological systems in vitro and in vivo [12, 25–27]. In vitro studies, in which the only difference of the growth media was 2H concentration in water, confirmed that 2H depletion inhibits cell growth in a dose dependent manner. To the contrary, increasing the 2H concentration over the natural abundance in water stimulates cell growth. The effects observed can be modeled when the growth rate of tumor cells is significantly inhibited in culture prepared with deuterium-depleted water (DDW) and, importantly, that physically restoring 2H levels by adding heavy water restores cell growth rates [28,29].
The clinical effectiveness of DDW is perhaps the most significant. Complete or partial tumor regression has been established in mice xenografts with MDA-MB-231, MCF-7 human breast adenocarcinoma cells, and PC-3 human prostate tumor cells [12,22]. When laboratory animals are exposed to the chemical carcinogen(s): 7,12-Dimethylbenz(a)anthracene (DMBA) and cytoplasmic myelocytomatosis oncogene (c-Myc), Ha-ras, and p53 are up-regulated. Yet the consumption of DDW suppresses the expression of these genes. In addition, DDW significantly inhibited proliferation of A549 human lung carcinoma cells in vitro, while H460 lung tumor xenografts in laboratory mice showed a 30% growth regression [31]. The anti-cancer effect of 2H-depletion has already been confirmed in a double-blind, randomized, 4-month-long, phase II clinical trial on prostate cancer [30], and the extended follow up suggests that 2H-depletion delays disease progression.
Based on these preclinical study observations, DDW is a promising new modality in cancer treatment and prevention by lowering extra-mitochondrial deuterium loading into cellular DNA. 2H-depletion, in addition to conventional treatments, improves mean survival in lung cancer even in an advanced disease, complicated by distant brain metastases [32]. In breast cancer patients DDW treatment, in combination with, or as an extension of, conventional therapies, significantly improved survival in advanced disease and was also effective in the prevention of recurrences in early stage breast cancer [33].
Deuterium carrying oncometabolites - Consistency and specificity of associations regarding mitochondrial matrix water recycling and cancer
The shift from genetic thinking towards cancer being a metabolic disease has been due to the application of metabolomics platforms identifying “oncometabolites”. Oncometabolites are intracellular products which accumulate, initiate and maintain uncontrolled cell growth with metastasis. One of the first oncometabolites identified is 2-hydroxyglutarate, a relatively rare metabolite that is found in high concentrations in gliomas [34,35]. Multiple mechanisms have been suggested for oncometabolites as means of inducing cancer. Altered gene functions and signaling pathways by indirect histonemethylation contribute to oncogenesis. Since the identification of 2-hydroxyglutarate formed in the cytoplasm, formed from citrate and isocitrate, other mitochondrial products such as fumarate and succinate in renal cell carcinomas [36] and paragangliomas, respectively have been identified. These products accumulate due to the lack of metabolic hydration to form their TCA cycle product, ketoglutarate and malate, respectively, whereby molecular crowding and the lack of metabolic water deriving hydrogen transfer to NADPH by isocitrate dehydrogenase initiate and maintain aggressive tumor growth. Therefore, “oncometabolite” formation is the results of enzymatic defects that recycle low deuterium metabolic water back into carbon cycling, gluconeogenesis and nucleic acid sugar backbone synthesis. The excessive appearance, i.e. accumulation of “metabolically dry” oncometabolites is consistent with our hypothesis that cancer is formed on the basis of mitochondrial defects that lack hydration of TCA cycle intermediates with low deuterium matrix water as the result of such defects. Such claim is supported by the fact that restoring hydratase function of mitochondria reverses tumor cells back to their genetically stable non-proliferating normal phenotype [37] with normal matrix water content, composition and morphology. All of the non-TCA cycle deriving oncometabolites arise from aerobic glycolysis, glutaminolysis or one carbon metabolic cycles [38] while carrying deuterium from the environment partially through nutrition. These include glycine in breast cancer, asparagine in leukemia, choline in prostate, brain, breast cancers, glutamine in myc-dependent cancers, as well as glucose, serine, lactate and polyamines in most cancers. Common nutrients such as glucose, lactic, amino, glutaminic and glyceric acids become oncometabolites only when mitochondrial ketogenic deuterium depleting and/or metabolite hydrating processes are insufficient to exchange and replace deuterium on specific carbons before these carbohydrate carbon skeletons become functional and structural sugar backbones for DNA, RNA and membrane building fatty acids.
Biological coherence of the deuterium loading hypothesis of cancer
The core reducing equivalent in living cells to produce new DNA and fatty acids via reductive carboxylation and biomolecule synthesis is NADPH and its deuterium loading properties depend on carbon-specific positional glucose phosphate deuterium enrichments, as well as deuterium enrichment of the cytoplasmic and mitochondrial water pools [39,40]. The above mechanisms are all important because intramolecular deuterium distributions reveal disequilibrium and a chemical shift axis for example in fructose-6-phosphate and glucose-6-phosphate [41]. More specifically, natural glucose source isolated from leaf starch of common bean (Phaseolus vulgaris) or spinach (Spinacia oleracea) is depleted in deuterium in the C(2) position. Carbon specific deuterium depletion in fatty acids from plants [42] and other sources [43,44] is also evident, which generate deuterium depleted matrix water in mitochondria during complete oxidation in complex-IV. Variations in carbon specific deuterium content of oxidizable substrates in the pentose and TCA cycles point to the biological role of intramolecular deuterium distributions that may ultimately be essential to understand all details of product deuterium abundances in compartmentalized deuterium transferring intracellular systems as oncogenic initiators.
The pentose cycle (oxidative branch) uses water of cytoplasmic origin with higher natural surface water-like deuterium content, which is about 155 parts per million (please see above). The full reaction architecture of the irreversible pentose cycle (oxidative branch) is important as the ring-opening hydrolysis in the pentose cycle results in the production of 6-phospho-D-gluconate, which provides another mole of NADPH to the pentose cycle-deriving cytosolic NADPH pool by phosphogluconate dehydrogenase [EC 1.1.1.43] during the completion of the direct C(1) oxidation process. This reaction is followed by pentose cycling, cytoplasmic water hydrogen exchanges by Lobry de Bruyn-Alberda-van Ekenstein aldose-ketose transformations via glucose-phosphate isomerase (GPI), triose phosphate isomerase (TPI) [41] and channeling into various hexose, pentose and triose phosphate pools that readily mix with carbon specific hydration products of the matrix such as malate, produced by fumarase, using deuterium depleted matrix water in mitochondria. Extensive substrate hydration steps in mitochondria using nutrient-derived hydrogens, as well as the ring opening hydrolysis of the pentose cycle using cytoplasmic water certainly alter NADH-dependent deuterium enrichments that affect all reversible cytosolic NAD+-dependent shuttle systems, including malate dehydrogenase.
The deuterium loading hypothesis of cancer emphasizes that deuterium content of cytoplasmic and mitochondrial water pools are different when they contribute to NADPH synthesis and that even small perturbations in the above mentioned cellular deuterium depleting pathways that use either cytoplasmic or metabolic water in the pentose cycle readily induce aneuploidy, undifferentiated blast cell formation and alteration of nuclear DNA size and function [45–47]. For example, increased hexose isomerization and pentose cycling with substrate switching from ketogenic palmitate to glucose and glutamine within the pentose and TCA cycles [48], or simply using DDW in place of natural abundance water in culture [5,12,28,29] profoundly alter cellular phenotype and proliferation.
In conclusion, 2H depletion in water offers new adjuvant and protective cancer therapy. The effectiveness of DDW can also be related to Warburg's theory, as it is the product of and preserves healthy mitochondrial function. Matrix DDW production can thus prevent irreversible defects in OXPHOS, which is a trigger for cancer. The role of 2H in biology is a potential missing piece in the elusive cancer puzzle, explaining cancer epidemics in western populations as it seemingly correlates with excessive 2H loading from processed carbohydrate intake in place of natural fat consumption. The resulting oncometabolites may act as 2H loading substrates (glucose, glutamine serine) or block deuterium depleting gluconeogenesis in the mitochondria as a deuterium depleting carbon processing metabolic hub, which yields DNA with a sugar backbone protected from aneuploidy and instability (Figure 1) with healthy hydrogen bonding, tunneling and bridging network.
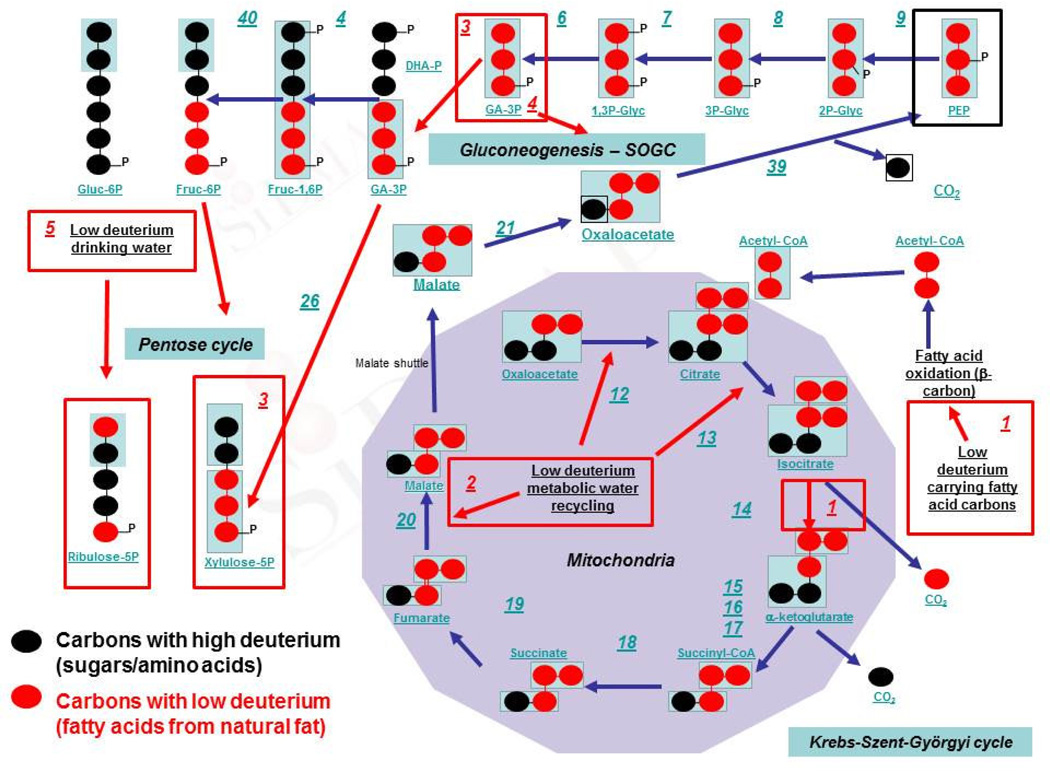
Figure 1.
Comparison of metabolic profile changes associated with 1) natural deuterium depletion by low deuterium fatty acid oxidation. Avastin® and Glivec® exert similar effect and require intact mitochondria for efficacy [49–51] (Red boxes No1), and 2) low deuterium metabolic water recycling from the mitochondrial matrix during citrate, isocitrate and malate formation; the target of fumarate hydratase activation [36,37] and hyperbaric oxygen treatment combined with a ketogenic diet [52] (Red box No2). Mitochondrial shuttles, such as the malate shuttle, pass low deuterium carrying fatty acid carbons to gluconeogenesis, where glyceraldehyde-3-phosphate becomes the source of extensive carbon exchange reactions [53] for the non-oxidative pentose cycle to maintain low deuterium saturation in C3’-C5’ pentose sugar carbon positions in RNA and DNA (Red box No3). These are the carbon sites where DNA stability, radiation- and chemotherapy derived hydroxyl radical sensitivities are regulated by hydrogen/deuterium [23,24] due to primary and secondary intrinsic isotope effects; as well as partially by collective proton tunneling [15,16]. Besides the C3’–C5’ nucleic acid sugar backbone fragment, de novo nucleic acid base syntheses, hydrogen bonding and deuterium channeling into hydrogen bonds are controlled by the serine oxidation glycine cleavage single carbon cycle pathways [38; SOGC] (Red box No4). When tumor cells revert to the Warburg phenotype and reductive carboxylation-driven mitochondria, deuterium depletion in free (drinking) water becomes the only deuterium depleting mechanism for specific carbon sites in nucleic acid backbone sugars and the bases (Red box No5). (Blue arabic numbers are enzyme identifiers also found in [54])
What is known about this topic
Deuterium depletion seems to protect cells by maintaining strong hydrogen bond networks.
Deuterium depleted water inhibits cancer cell growth as well as tumor progression.
Hydratases and isomerases of the TCA and pentose cycles transfer deuterium depleted mitochondrial matrix water to intermediates and subsequently stabilize DNA.
Deuterium depletion protects cells from exceptional deuterium substitution effects in hydrogen bridging biological networks.
Ketogenic substrates, water and drugs promote deuterium depletion of mitochondrial metabolic matrix water, offering a means to prevent tumor cell growth.
What this study adds
Defective mitochondrial functions, molecular oxygen deprivation and increased glycolysis induce cellular transformations which can cause a shift in 2H/1H ratio in mammalian cells.
Switching from a ketogenic to high sugar diet interferes with the deuterium depleting action of mitochondria serving as a potential oncogenic initiator.
The results of this analysis contain new data relevant to cancer prevention and treatment.
Questions for the experts
Can cancer risk be identified by metabolic profiling with 2H as a probe?
Can 13C metabolic tracers assist early cancer diagnosis and response to therapy by detecting 2H loading in DNA of circulating blood cells and tumorous tissue?
Is the chemical behavior of hydrogen isotopes consistent with the involvement of oncoisotopes in nutrition?
Acknowledgments
We thank Eszter Boros and Szandra Szentjóbi-Szabó for their technical help in preparing the manuscript including visual fitting of Table 1 and Figure 1.
Funding
The work was supported by the Hirshberg Foundation for Pancreatic Cancer Research, the UCLA Clinical & Translational Science Institute (UL1TR000124) and UCLA Center for Excellence in Pancreatic Diseases - Metabolomics Core (1-P01 AT003960-01A1) to LGB. Targeted 13C tracer drug efficacy marker data diagnostics for cancer were partially supported by the European Regional Development Fund, Central Hungary Operative Program, New Széchenyi Plan (KMOP-1.1.4-11/A-2011-01-05) to GS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
GS is the CEO and Director of Research and Development at HYD, LLC. LGB is an academic advisor for SiDMAP, LLC and a time-reimbursed consultant for HYD, LLC and Comix system LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) Research Data. released April 2015. [Google Scholar]
- 2.Warburg O. The Metabolism of Tumours. New York Richard R Smith. 1931;24:2. [Google Scholar]
- 3.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 4.Warburg O. The prime cause of cancer and prevention - Part 2. Annual meeting of Nobelists at Lindau; Germany. 1969. Doi: http://goo.gl/KgdcqQ. [Google Scholar]
- 5.Boros LG, Lee PW, Brandes JL, et al. Nonoxidative pentose phosphate pathways and their direct role in ribose synthesis in tumors: is cancer a disease of cellular glucose metabolism? Med Hypotheses. 1998;50:55–59. doi: 10.1016/s0306-9877(98)90178-5. [DOI] [PubMed] [Google Scholar]
- 6.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 7.Cascante M, Boros LG, Comin-Anduix B, de Atauri P, Centelles JJ, Lee PW. Metabolic control analysis in drug discovery and disease. Nat Biotechnol. 2002;20:243–249. doi: 10.1038/nbt0302-243. [DOI] [PubMed] [Google Scholar]
- 8.Boros LG. Metabolic targeted therapy of cancer: Current tracer technologies and future drug design strategies in the old metabolic network. Metabolomics. 2005;1:11–15. [Google Scholar]
- 9.Wishart DS. Is Cancer a Genetic Disease or a Metabolic Disease? E Bio Medicine. 2015;2:478–479. doi: 10.1016/j.ebiom.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seyfried T. Cancer as metabolic disease: on the origin, management, and prevention of cancer. Vol. 438. Hoboken NJ: Wiley; 2012. [Google Scholar]
- 11.Szent-Györgyi A. The Living State and Cancer. In: Wolstenholme GEW, Fitzsimons DW, Whelan J, editors. Submolecular Biology and Cancer, Ciba Foundation Symposium 67. 1979. pp. 3–18. [DOI] [PubMed] [Google Scholar]
- 12.Somlyai G, et al. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett. 1993;317:1–4. doi: 10.1016/0014-5793(93)81479-j. [DOI] [PubMed] [Google Scholar]
- 13.Kotyk A, Dvoráková M, Koryta J. Deuterons cannot replace protons in active transport processes in yeast. FEBS Lett. 1990;264:203–205. doi: 10.1016/0014-5793(90)80248-h. [DOI] [PubMed] [Google Scholar]
- 14.Perona R, Serrano R. Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature. 1988;334:438–440. doi: 10.1038/334438a0. [DOI] [PubMed] [Google Scholar]
- 15.Drechsel-Grau C, Marx D. Exceptional isotopic-substitution effect: breakdown of collective proton tunneling in hexagonal ice due to partial deuteration. Angew Chem Int Ed Engl. 2014;53:10937–10940. doi: 10.1002/anie.201405989. [DOI] [PubMed] [Google Scholar]
- 16.Sobczyk L, Obrzud M, Filarowski A. H/D isotope effects in hydrogen bonded systems. Molecules. 2013;18:4467–4476. doi: 10.3390/molecules18044467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz JJ, Crespi HL. Isotope Effects in Biological Systems. In: Collins CJ, Bowman N, editors. Isotope Effects in Chemical Reactions. New York: Van Nostrand Reinhold; 1971. pp. 286–363. [Google Scholar]
- 18.Rundel PW, Ehleringer JR, Nagy KA. Stable Isotopes in Ecological Research. New York: Springer; 1988. [Google Scholar]
- 19.Jancsó G. Isotope Effects. In: Vértes A, Nagy S, Klencsár Z, editors. Handbook of Nuclear Chemistry. Vol. 2. Dordrecht, Netherland: Kluwer Academic Publishers; 2013. pp. 85–116. [Google Scholar]
- 20.Zuev PS. Carbon tunneling from a single quantum state. Science. 2003;299:867–870. doi: 10.1126/science.1079294. [DOI] [PubMed] [Google Scholar]
- 21.Swain C, et al. Use of hydrogen isotope effects to identify the attacking nucleophile in the enolization of ketones catalyzed by acetic acid. J Am Chem Soc. 1958;80:5885–5893. [Google Scholar]
- 22.Somlyai G, Laskay G, Berkényi T, Jákli G, Jancsó G. Naturally occurring deuterium may have a central role in cell signalling. In: Heys JR, Melillo DG, editors. Synthesis and Applications of Isotopically Labelled Compounds. New York: John Wiley and Sons Ltd; 1998. pp. 137–141. [Google Scholar]
- 23.Balasubramanian B, Pogozelski WK, Tullius TD. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc Natl Acad Sci U S A. 1998;95:9738–9743. doi: 10.1073/pnas.95.17.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingle S, Azad RN, Jain SS, Tullius TD. Chemical probing of RNA with the hydroxyl radical at single-atom resolution. Nucleic Acids Res. 2014;42:12758–12767. doi: 10.1093/nar/gku934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somlyai G, et al. The biological effects of deuterium-depleted water, a possible new tool in cancer therapy. Zeitschrift für Onkologie (ger) Journal of Oncology. 1998;30:91–94. [Google Scholar]
- 26.Gyöngyi Z, Somlyai G. Deuterium depletion can decrease the expression of c-myc, Ha-ras and p53 gene in carcinogen-treated mice. In Vivo. 2000;14:437–439. [PubMed] [Google Scholar]
- 27.Cong FS, et al. Deuterium-Depleted Water Inhibits Human Lung Carcinoma Cell Growth by Apoptosis. Exp Ther Med. 2010;1:277–283. doi: 10.3892/etm_00000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boros LG, et al. Fumarate hydratase and deuterium depletion control oncogenesis via NADPH-dependent reductive synthesis: mitochondrial matrix water, DNA deuteration and epigenetic events. Cancer Res. 2014;74(19 Suppl) Abstract nr 1426. [Google Scholar]
- 29.Somlyai G, et al. Deuterium has a key role in tumor development: A new submolecular regulatory system. 75th Anniversary Publication for Albert-Szent-Gyorgyi's Nobel Award, Szeged, Hungary, EU; 2013; Jubilee Publication on the 75th Anniversary of Albert Szent-Györgyi’s Nobel Prize Award. doi: https://goo.gl/J4kFXC. [Google Scholar]
- 30.Kovács A, et al. Deuterium depletion may delay the progression of prostate cancer. J Cancer Ther. 2011;2:548–556. [Google Scholar]
- 31.Gyöngyi Z, et al. Deuterium-depleted Water Effects on Survival of Lung Cancer Patients and Expression of Kras and Bcl2 Genes in Mouse Lung. Nutr Cancer. 2013;65:240–246. doi: 10.1080/01635581.2013.756533. (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krempels K, Somlyai I, Somlyai G. A retrospective evaluation of the effects of deuterium-depleted water consumption on four patients with brain metastases from lung cancer. Integr Cancer Ther. 2008;7:172–181. doi: 10.1177/1534735408322851. [DOI] [PubMed] [Google Scholar]
- 33.Krempels K, et al. A retrospective study of survival in breast cancer patients undergoing deuterium depletion in addition to conventional therapies. J Cancer Res Ther. 2013;1:194–200. [Google Scholar]
- 34.Ward PS, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alphaketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reitman ZJ, et al. Cancer-associated isocitrate dehydrogenase 1 (IDH1) R132H mutation and d-2-hydroxyglutarate stimulate glutamine metabolism under hypoxia. J Biol Chem. 2014;289:23318–23328. doi: 10.1074/jbc.M114.575183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, et al. Metabolic reprogramming for producing energy and reducing power in fumarate hydratase null cells from hereditary leiomyomatosis renal cell carcinoma. PLoS One. 2013;8:e72179. doi: 10.1371/journal.pone.0072179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong WH, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20:315–327. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tedeschi PM, et al. Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell Death Dis. 2013;4:e877. doi: 10.1038/cddis.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis CA, et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell. 2014;55:253–263. doi: 10.1016/j.molcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boros LG, Somlyai G. Compartmentalized NADPH Synthesis, Intramolecular Deuterium Disequilibrium and Water Pools of Mammalian Cells. Molecular Cell. 2014;55:253–263. doi: 10.1016/j.molcel.2014.05.008. (Comment) doi: http://goo.gl/4CW4Dx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schleucher J, Vanderveer P, Markley Intramolecular deuterium distributions reveal disequilibrium of chloroplast phosphoglucose isomerase. Plant, Cell & Environment. 1999;22:525–533. [Google Scholar]
- 42.Billault I, Guiet S, Robins RJ. Quantitative Deuterium Isotopic Profiling at Natural Abundance Indicates Mechanistic Differences for Δ12-Epoxidase and Δ12-Desaturase in Vernonia galamensis. The Journal of Biological Chemistry. 2005;280:17645–17651. doi: 10.1074/jbc.M500909200. [DOI] [PubMed] [Google Scholar]
- 43.Duan JR, Billault I, Mabon F, Robins RJ. Natural deuterium distribution in fatty acids isolated from peanut seed oil: a site-specific study by quantitative 2H NMR spectroscopy. Chem biochem. 2002;3:752–759. doi: 10.1002/1439-7633(20020802)3:8<752::AID-CBIC752>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 44.Billault I, Guiet S, Mabon F, Robins RJ. Natural deuterium distribution in long-chain fatty acids is nonstatistical: a site-specific study by quantitative (2)H NMR spectroscopy. Chem biochem. 2001;2:425–431. doi: 10.1002/1439-7633(20010601)2:6<425::AID-CBIC425>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 45.Fleming JC. Characterization of a murine high-affinity thiamine transporter, Slc19a2. Mol Genet Metab. 2001;74:273–280. doi: 10.1006/mgme.2001.3241. [DOI] [PubMed] [Google Scholar]
- 46.Green R. Mystery of thiamine-responsive megaloblastic anemia unlocked. Blood. 2003;102:3464–3465. doi: http://goo.gl/C9kXYo. [Google Scholar]
- 47.Boros LG, Steinkamp MP, Fleming JC, Lee WN, Cascante M, Neufeld EJ. Defective RNA ribose synthesis in fibroblasts from patients with thiamine-responsive megaloblastic anemia (TRMA) Blood. 2003;102:3556–3561. doi: 10.1182/blood-2003-05-1537. [DOI] [PubMed] [Google Scholar]
- 48.Lamonte G, et al. Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer Metab. 2013;1:23. doi: 10.1186/2049-3002-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu J, et al. Colorectal cancer cells refractory to anti-VEGF treatment are vulnerable to glycolytic blockade due to persistent impairment of mitochondria. Mol Cancer Ther. 2013;12:717–724. doi: 10.1158/1535-7163.MCT-12-1016-T. [DOI] [PubMed] [Google Scholar]
- 50.Kominsky DJ, et al. Abnormalities in glucose uptake and metabolism in imatinib-resistant human BCR-ABL-positive cells. Clin Cancer Res. 2009;5:3442–3450. doi: 10.1158/1078-0432.CCR-08-3291. [DOI] [PubMed] [Google Scholar]
- 51.Boren J, et al. Gleevec (STI571) influences metabolic enzyme activities and glucose carbon flow toward nucleic acid and fatty acid synthesis in myeloid tumor cells. J Biol Chem. 2001;276:37747–37753. doi: 10.1074/jbc.M105796200. [DOI] [PubMed] [Google Scholar]
- 52.Poff A, et al. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS One. 2013;8:e65522. doi: 10.1371/journal.pone.0065522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centelles JJ, et al. Metabolic profile and quantification of deoxyribose synthesis pathways in HepG2 cells. Metabolomics. 2007;3:105–111. [Google Scholar]
- 54.Boros LG, et al. Targeted 13C-labeled tracer fate associations for drug efficacy testing in cancer. Tumor Cell Metabolism – Pathways, Regulation & Biology, Eds. Shoshan, M. (Karolinska Institute, Stockholm, Sweden) & Mazurek, S. L. (Justus-Liebig-University, Giessen, Germany), Chapter 15. 2014:349–370. [Google Scholar]