Abstract
Scaffold diversity is key in the ongoing exercise of discovery of novel bioactive compounds using high throughput screening (HTS). Based on the Ugi tetrazole synthesis we have designed novel bi- and tri-cyclic scaffolds featuring interesting pharmacophore properties. The compounds of the scaffold (B) are synthesizable in large diversity and numbers in two steps using (hetero)phenylethylamines, HN3, oxo components and iscyanoacetaldehyde(dimethylacetale). The chemistry is amenable to parallel synthesis and is used to enhance and fill the screening decks of the European Lead factory (ELF). Here, we are reporting full experimental details, scope and limitations of the reaction, cheminformatic analysis and the 3D structures of selected compounds.
Keywords: Ugi, Tetrazol, Pictet-spengler, Isocyanide, Parallel, Synthesis
1. Introduction
High throughput screening (HTS), is the current screening paradigm: most medicinal chemistry programs start with hits from HTS. However, historical compound collections, narrow property definitions and low diversity often hamper the discovery of hits especially for novel unconventional targets such as protein–protein interactions or intrinsically disordered proteins. The European Lead Factory (ELF) is an Innovative Medicines Initiative (IMI) aiming to boost academic and industrial drug discovery by creating a library of 500,000 compounds by the end of 2017.1 The availability of molecular probes (small molecule or antibody) has been recently and impressively demonstrated to be a key determinant of progress in basic biology and disease areas.2 Multicomponent reaction chemistry (MCR) is very well suited for the creation of large libraries of compounds for several reasons:3 MCR leads to target scaffolds in only one or few synthetic steps; this is of major importance to minimize the time and effort to accomplish libraries of suitable size. Next MCR and suitable post transformations provide a very large number of scaffolds thus not compromising diversity.4 This feature ensures scaffold diversity in a library. Recently, many MCRs employ common functional groups with many derivatives commercially accessible; thus a large chemical space around the scaffold can be projected. Based on our focus of chemical diversity based MCR chemistry and its leverage to discover novel bioactive compounds we describe here the design of a novel tetracyclic tetrazole scaffold with full experimental detail.5–9 The scope, limitations, exemplified 3D structures and the general pharmacophore are discussed.
2. Results and discussion
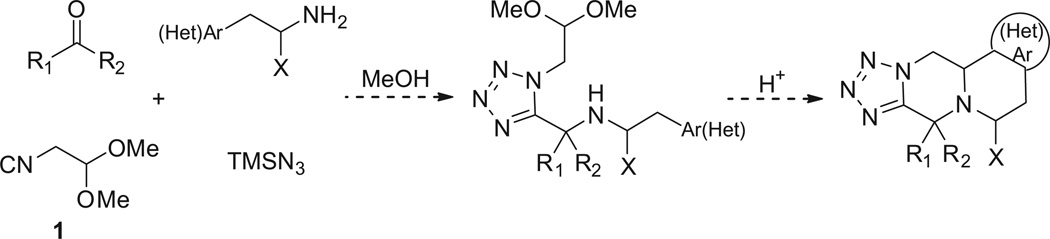
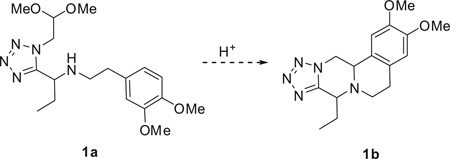
Isocyanoacetaldehyde dimethylacetale 1 is a stable, well described isocyanide with a robust multi gram scale synthesis described.10 It reacts well in isocyanide-based MCRs and provides a protected aldehyde functionality which is useful for post transformations. 11–18 We recently described its use in several isocyanide based MCRs and here we intended to use this isocyanide in the classical Ugi tetrazole MCR (submitted). This isocyanide was used in the development of a short, scalable synthesis of a largely diversified scaffold using phenylethylamines, oxo components, and TMS-N3 by an Ugi-Pictet-Spengler reaction (Scheme 1).
Scheme 1.
The general reaction sequence.
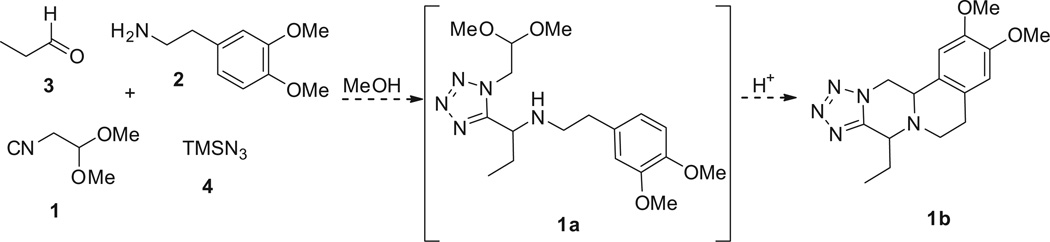
We first tested this reaction using 3,4-dimethoxyphenethyl-amine (2) with propionaldehyde (3), trimethylsilylazide (TMS-N3) (4), and isocyanoacetaldehyde (dimethylacetal) (1) in MeOH (1 M) at room temperature for 18 h. The classical Ugi product 5a afforded good yield as judged by supercritical fluid chromatography-mass spectrometry (SFC–MS) and TLC analysis. Then various acids such as formic acid, trifluroacetic acid and methanesulfonic acid were screened to the same reaction flask and heated at 50– 80°C to form the Pictet–Spengler reaction product. Surprisingly, the cyclized Pictet-Spengler product 1b as described in Scheme 2 was not observed.
Scheme 2.
Synthesis of Ugi–Pictet–Spengeler 5H-tetrazoleo[1′,5′:4,5]pyrazino[2,1-a]isoquinoline 1b.
This forced us to develop a methodology for the final cyclization step. The development of such reaction conditions was optimized as shown in Table 1.
Table 1.
Optimization of the Pictet–Spengler cyclization stepa
 | |||||
|---|---|---|---|---|---|
| Entrya | Acid | Solvent | Tempb (°C) | Time (h) | Convc (%) |
| 1 | HCOOH | MeOH | rt | 18 | — |
| 2 | HCOOH | MeOH | 80 °C | 18 | — |
| 3 | HCOOH | — | rt | 18 | — |
| 4 | HCOOH | — | 80 °C | 18 | — |
| 5 | CF3COOH | MeOH | 80 °C | 18 | Traces |
| 6 | CF3COOH | CH2Cl2 | rt | 18 | Traces |
| 7 | CF3COOH | CH3CN | 60 °C | 18 | Traces |
| 8 | CF3COOH | Toluene | 80 °C | 18 | Traces |
| 9 | CF3COOH | — | 50 °C | 18 | Traces |
| 10 | CH3SO3H | Toluene | 80 °C | 18 | Traces |
| 11 | CH3SO3H | MeOH | 80 °C | 18 | Traces |
| 12 | CH3SO3H | — | 80 °C | 18 | Traces |
| 13 | CH3SO3H | — | rt | 18 | 67d |
Reaction conditions: 1a (0.3 mmol), acid (30.0 mmol), solvent (3.0 mL).
The reaction was performed under classical heating conditions.
Determined by SFC–MS analysis of crude reaction mixture.
Isolated product.
Under classical conditions, Ugi-product 1a treated in formic acid with and without methanol did not proceed as expected even when the reaction mixture was heated at room temperature to 80 °C in an oil bath for 18 h (entries 1–4, Table 1). Using trifluoroacetic acid instead of formic acid, in various solvents and temperatures (entries 5–9, Table 1) showed only traces of product formation, determined by SFC–MS analysis. Using methanesulfonic acid similar results were obtained with various solvents at 80°C for 18 h. However, when pure Ugi-product 1a was treated with methanesulfonic acid at room temperature for 18 h, surprisingly, the Pictet-Spengler cyclized product 1b (67%) (entry 13, Table 1) was afforded as diastereomeric mixture 5:3, determined by SFC–MS and NMR analysis. Although a diastereomeric mixture was obtained, this method gives us good yield of the cyclized product. This reaction condition was used for a one-pot reaction, that is, the Ugi reaction followed immediately by the Pictet-Spengler reaction without purification. Unfortunately, this one-pot reaction sequence failed to give the cyclized product. Next, the optimal conditions (entry 13, Table 1) were chosen to explore the substituent scope of this methodology.
To explore the scope of the methodology, we tested the synthesis of Ugi 4-CR adducts using different oxo components and various aryl ethyl amines (Table 2).
Table 2.
Substrate scope with various aryl ethyl amines
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | (Het)Ar | X | Ugi product A | Yielda (%) (dr)b | Pictet–Spengler product B | Yielda (%) (dr)b | |
| 1 |  |
H | 1a | 73 | 1b | 67 (5:3) | |
| 2 |  |
H | 2a | 93 | 2b | 57 | |
| 3 | 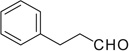 |
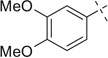 |
H | 3a | 78 | 3b | 70 |
| 4 | 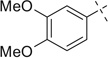 |
H | 4a | 74 | 4b | 50 | |
| 5 | 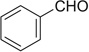 |
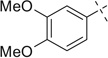 |
H | 5a | 82 | 5b | 69 |
| 6 | 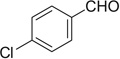 |
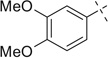 |
H | 6a | 85 | 6b | 89 |
| 7 | 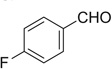 |
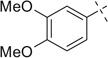 |
H | 7a | 71 | 7b | 56 |
| 8 | 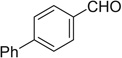 |
 |
H | 8a | 94 | 8b | 68 |
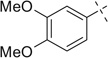
| 9 | 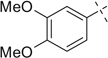 |
H | 9a | 93 | 9b | 57 (2:1) | |
| 10 | 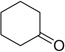 |
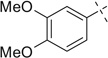 |
H | 10a | 68 | 10b | 77 |
| 11 | 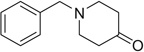 |
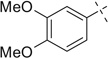 |
H | 11a | 67 | 11b | 70 |
| 12 | 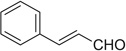 |
 |
H | 12a | tracesc | 12b | — |
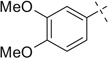
| 13 | 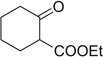 |
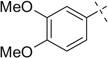 |
H | 13a | — | 13b | — |
| 14 | 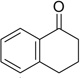 |
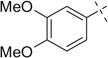 |
H | 14a | — | 14b | — |
| 15 | 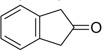 |
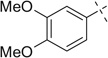 |
H | 15a | — | 15b | — |
| 16 | 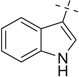 |
H | 16a | 57 | 16b | 30 (5:3) | |
| 17 | 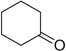 |
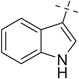 |
H | 17a | 94 | 17b | 53 |
| 18 | 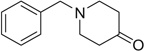 |
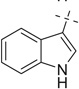 |
H | 18a | 87 | 18b | 72 |
| 19 | 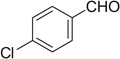 |
 |
H | 19a | 82 | 19b | 84 |
| 20 | 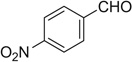 |
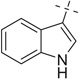 |
H | 20a | 98 | 20b | 95 |
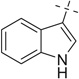
| 21 | 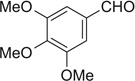 |
 |
H | 21a | 86 | 21b | 32 |
| 22 | 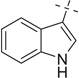 |
22a | 31 — | — | |||
| 23 | 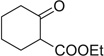 |
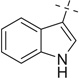 |
H | 23a | — | — | — |
| 24 | 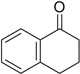 |
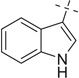 |
H | 24a | —— | — | |
| 25 | 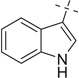 |
H | 25a | — | — | — | |
| 26 | 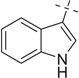 |
COOMe | 26a | 81 (5:1) |
26b | 57c | |
| 27 | 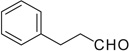 |
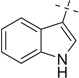 |
COOMe | 27a | 91 (5:1) |
27b | 57 (2:2:1) |
| 28 | 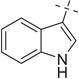 |
COOMe | 28a | 25 (3:2) |
28b | — | |
| 29 | 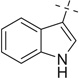 |
COOMe | 29a | 70 (5:4) |
29b | 35 (2:1) | |
| 30 | 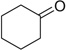 |
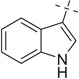 |
COOMe | 30a | 88 | 30b | 58 |
| 31 | 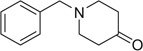 |
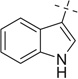 |
COOMe | 31a | 91 | 31b | 82 |
| 32 | 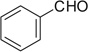 |
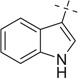 |
COOMe | 32a | 86 (3:2) |
32b | 74 (2:1) |
| 33 | 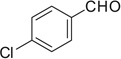 |
 |
COOMe | 33a | 85 (3:2) |
33b | 85 (9:1) |
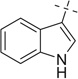
| 34 | 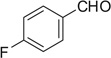 |
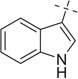 |
COOMe | 34a | 92 (5:3) |
34b | 75 (5:3) |
| 35 | 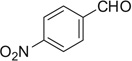 |
 |
COOMe | 35a | 89 (5:4) |
35b | 87 (5:4) |
| 36 | 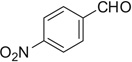 |
COOMe | 36a | 53 (5:1) |
36b | 51 (5:1) | |
| 37 |  |
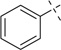 |
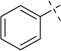
H | 37a | 93 | 37b | — |
| 38 | 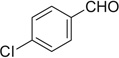 |
 |
H | 38a | 92 | 38b | — |
| 39 | 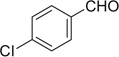 |
 |
H | 39a | 94 | 39b | — |
| 40 | 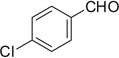 |
 |
H | 40a | 98 | 40b | |
| 41 |  |
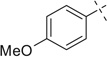 |
COOMe | 41a | 78 | 41b | — |
| 42 | 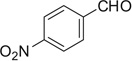 |
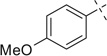 |
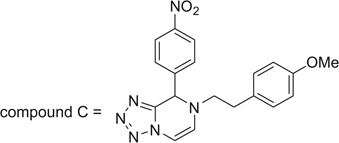
H | 42a | 92 | C | 61d |
| Compound C = | |||||||
 | |||||||
Isolated yield after silica gel column chromatography.
Diastereomeric ratio determined by 1H and 13C spectra or SFC–MS.
Determined by SFC–MS analysis of crude reaction mixture.
Isolated yield of compound C.
First we studied the reaction of 3,4-dimethoxyphenylethyl-amine with various oxo components. The reaction of 3,4-dimeth-oxyphenylethylamine with isocyanoacetaldehyde dimethylacetale 1, TMS-N3 with different aldehydes and ketones were examined. Classical Ugi-reactions were carried out with stoichiometric amounts of all four starting materials in methanol (1 M) at room temperature. Ugi-adducts 1a to 11a were synthesized in good to excellent yields (67–94%) through standard conditions. However, when cinnamicaldehyde was used only traces of product (12a) formation was observed by SFC–MS. Use of ketones like β-ketoester and cyclic ketones (entries 14 and 15) failed to give the Ugi products. These Ugi-adducts obtained, were further subjected to the Pictet-Spengler reaction with neat methansulfonic acid (100 equiv) at room temperature for 18 h. We observed that only in the cases of propyl aldehyde and ethylmethyl ketone diastereomeric mixtures were obtained in 5:3 and 2:1 ratios, respectively (1b, 9b). In all other cases, only one major isomer was formed and the other isomer was seen in less than 5%, which was confirmed before purification of crude reaction mixture by SFC–MS analysis. Major isomers could be easily separated after purification on flash column chromatography using silica gel. We observed that, in all cases, the cyclized product was obtained in moderate to good yield (50–89%). Aliphatic aldehydes gave moderate yields (1b–4b) while aromatic aldehydes and cyclic ketones gave good yields (5b–8b, 10b, 11b). p-Chlorobenzaldehyde gave excellent yield (89%) (6b). However, by replacing chlorine to more electronegative fluorine atom in the para position of phenyl ring of aldehyde, the yield of the cyclized product dropped to 56% (7b).
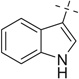
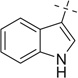
After examining the 3,4-dimethoxyphenylethylamine, we extended our scope of this reaction to hetero aryl ethyl amines like tryptamine (entries 16–25) and tryptophan (entries 26–35).
When we employed tryptamine as the amine component in the Ugi reaction, we observed good to excellent yields for the Ugi-adducts (16a–21a). 3–(Methylmercapto) propionicaldehyde gave a lower yield (3%) of the Ugi-adduct (22a); while cyclic ketones (entries 23–25) failed to give Ugi-adduct under the same conditions. The Ugi-adducts obtained (16a–21a), were further treated with methanesulfonic acid at room temperature and underwent the Pictet–Spengler reaction to give cyclized products (16b–21b) in moderate to excellent yields. It was observed that the Ugi-adducts (16a–18a) of aliphatic aldehydes and ketones gave moderate yields. The Ugi-adduct (16a) obtained from propyl aldehyde upon cyclization afforded a diastereomeric mixture (16b) in 30% yield as a 5:3 dr ratio. The Ugi-adduct of aromatic aldehydes (19a, 20a) gave excellent yields (84–95%) (19b, 20b). Surprisingly, the Pictet–Spengler reaction of the Ugi-adduct (21a) containing electron-rich aldehyde gave a lower yield, 32% (21b) and electron-deficient p-nitrobenzaldehyde (20a) gave an excellent yield (95%) (20b) with only one major stereoisomer.
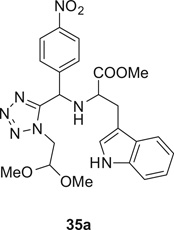
Next to further investigate scope, we introduced one more stereocenter in the hetroarylethyl amine, that is, by using d,l-tryptophan as an amine component. If a stereogenic aldehyde is used in this reaction three stereocenters are present in the product and potentially 8 stereoisomers can be formed. It was observed that yields of the Ugi-reaction were excellent (26a–35a), except with 3-(methylmercapto) propylaldehyde (28a) 25% in 3:2 dr ratio, confirmed by SFC–MS. When aromatic aldehydes were used, Ugi-adducts were obtained in diastereomeric mixtures (32a–35a). Surprisingly only two out of four possible diastereomers could be observed. Further, these Ugi-adducts treated with methanesulfonic acid gave the Pictet–Spengler cyclized product in moderate to good yields (26b–35b). The Ugi adduct from 3-(methylmercapto) propyl aldehyde (28a) failed to give the cyclized product. In the cases of isopropyl carbaldehyde the Ugi-adduct gave stereoselectively the major product 57% (26b) and the minor diastereomer was found in less than 5%. However, ethyl-methyl ketone gave diastereomeric mixtures of the Ugi-adduct (29a 70%) in 5:4 dr ratio, but after the Pictet–Spengler reaction, the dr ratio was changed to 2:1 (29b) suggesting partial epimerization during the Pictet–Spengler reaction. Similarly, in the cases of aromatic aldehydes the Ugi-adduct was obtained as diastereomeric mixtures (32a–35a) in 3:2, 3:2, 5:3 and 5:4 dr ratios, respectively. The ratio was determined by analysis of the 1H NMR, 13C NMR and SFC–MS spectrum of the crude reaction mixture. For example, in the 1H NMR signals for methyl in methyl ester of compound 35a is located at 3.79 ppm (major) and 3.62 ppm (minor) for each of these diastereomers were used to determine the diastereomeric ratio, while the signals for indole NH were located at 8.27 and 8.22 ppm for major and minor, respectively. Also, in the 13C NMR of compound 35a the carbonyl carbon of ester group is located at 174.1 ppm (minor) and 173.9 ppm (major) for each of these diastereomers were used as a confirmation of the stereoselectivity observed.
After the Pictet–Spengler reaction of these adducts, the diastereomeric ratios were changed. Furthermore, in case of hydrocinnamal-dehyde, Ugi adduct (27a, 91%) was obtained in 5:1 dr ratio but after the Pictet–Spengler reaction, three diastereomers were observed in 2:2:1 dr ratio (27b, 57%). This clearly indicates that partial epimerization takes place during the acidic Pictet–Spengler cyclization.
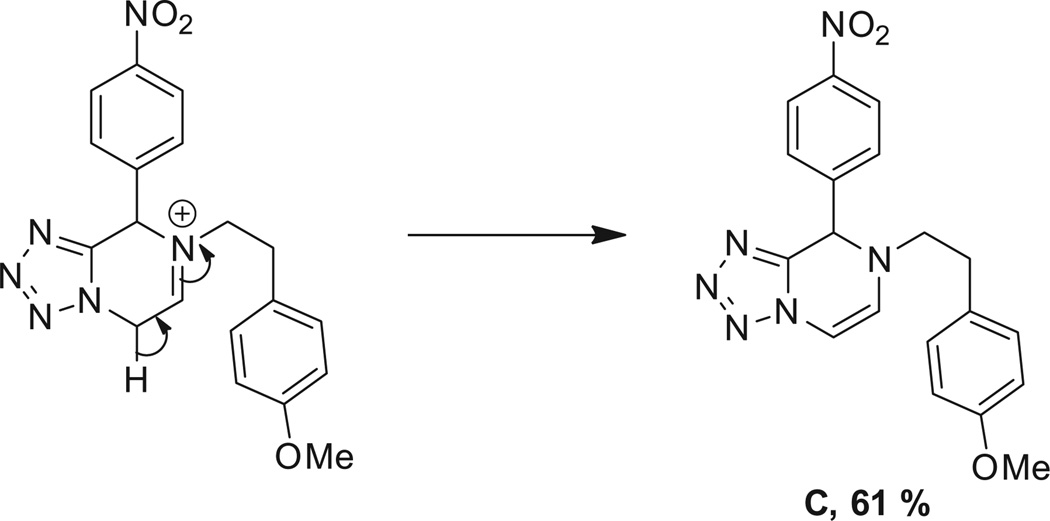
Lastly, we extended this study to various other aryl ethyl amines (entries 36–42, Table 2). All aryl ethyl amines gave excellent yields (78–98%) of the Ugi-adducts (37a–42a), except for 3-(2-thienyl)-d,l-alanine which gave moderate yield (53%) (36a). Surprisingly, it was observed that only the Ugi-adduct of 3-(2-thienyl)-d,l-alanine (36a) gave the required cyclized product (36b) in good yield (51%) in 5:1 dr ratio which was in the same as it is Ugi-adduct. When we tested the Ugi-adducts of phenylethylamine (37a, 38a) and its derivatives like tyramine (39a), o-methoxyphenyl ethylamine (40a), and o-methyl-d,l-tyrosine methyl ester (41a), we failed to obtain the required cyclized product. In these cases, multiple product formation was observed by TLC and SFC analysis. When we employed the Ugi-adduct formed by p-methoxyphenylethylamine and p-nitrobenzaldehde (42a) with methanesulfonic acid under our standard reaction conditions it gave a six member heterocycle C in good yield, 61%, instead of the expected Pictet–Spengler product 42b.
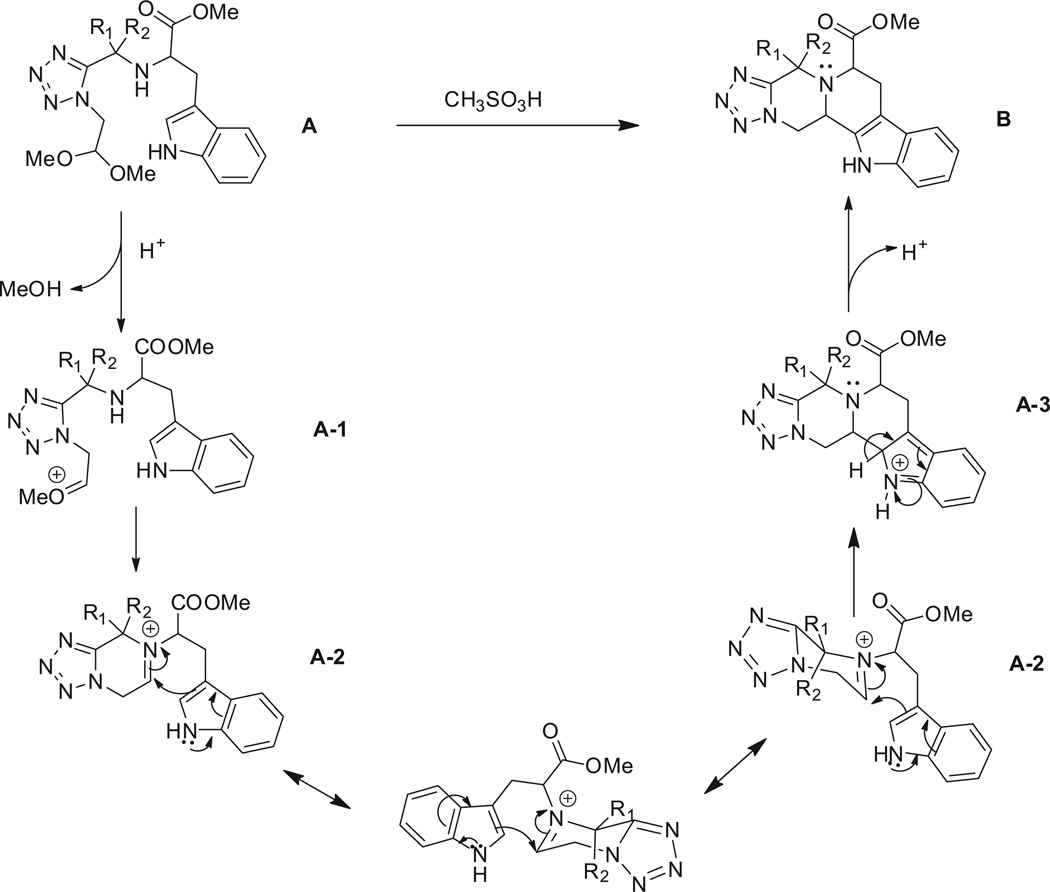
For the proposed reaction mechanism of formation of B (Scheme 3), deprotection of the acetal groups could occur under acidic conditions to form the iminium ion (A-2).19 Alternatively a hemiaminal could be postulated by direct substitution of a methoxy group. In case of tryptamine, due to the presence of the carboxylic ester group, the adjacent proton is acidic in nature and could easily undergo epimerization. In the presence of an electron-rich aryl ring, the ring could undergo electrophilic substitution (A-3). After subsequent deprotonation, the desired product (B) was obtained. In case of Ugi adduct (42a), the electrophilic substitution step did not happen due to the less electron-rich aryl ring. Instead, the cyclisation via an iminium-six-member ring followed by tautomerisation as shown in Scheme 4 can happen.
Scheme 3.
The plausible reaction mechanism for the cyclized products 1b–42b.
Scheme 4.
Side reaction to C observed.
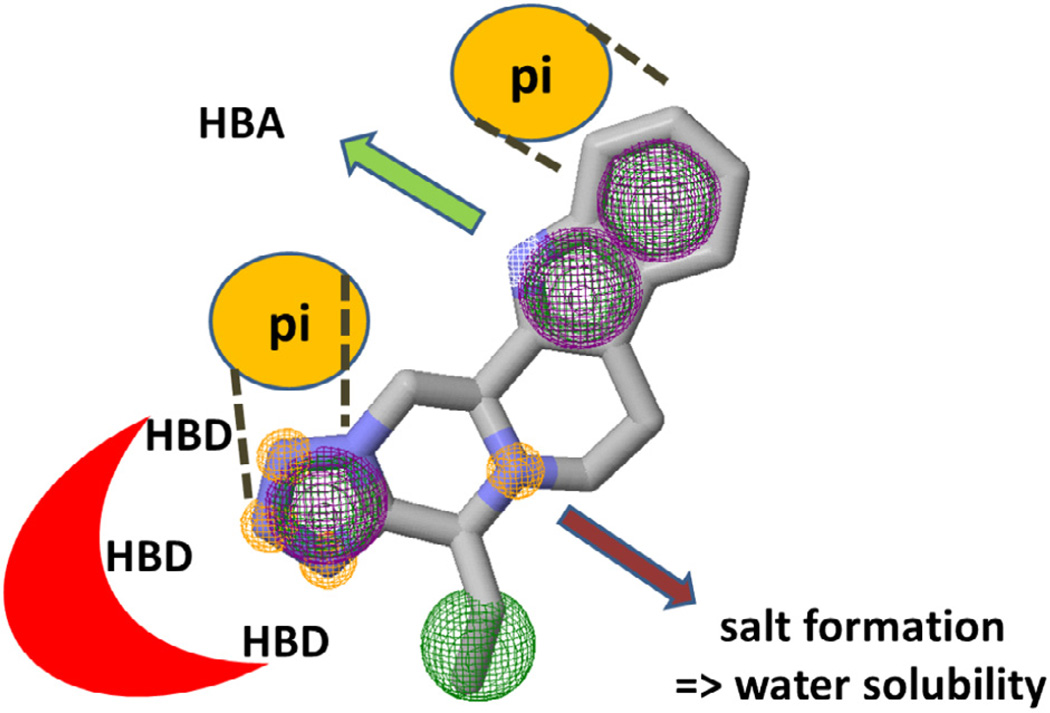
The analysis and knowledge of physicochemical properties, pharmacophore features of scaffolds is of uttermost importance to medicinal chemistry. It allows for the rational design of compounds and proper inclusion in decision processes during triaging often enhances the drug-like properties of leads.20 Key 3D and pharmacophore features of the different scaffold classes (B) are their extended polycyclic nature based on the aliphatic piperazine linking ring elements thus introducing considerable 3D character and not just indole or isoquinoline flatness (Fig. 1). The scaffolds do not show any rotational bonds except in the substituents. Scaffold stiffness is known to increase passive transport through biological membranes and thus oral bioavailability.21 All the scaffolds contain a basic tertiary amine in the piperazine moiety which can contribute to considerable water solubility, for example, by salt formation. Water solubility–a key property of bioactive compounds–not only facilitates transport but also helps to avoid false positive results during HTS. Many traditional extended Pictet–Spengler ring systems are known but the scaffolds described herein have a unique ring-fused tetrazole moiety which allows for up to three hydrogen bonds (Fig. 1).22–28 The electron rich tetrazole ring can potentially also undergo pi-stacking and electrostatic interactions and in fact all these specific possible interactions can be observed in the solid state in several crystal structures (Figs. 2–5).
Figure 1.
Physicochemical properties and pharmacophore of the new scaffolds.
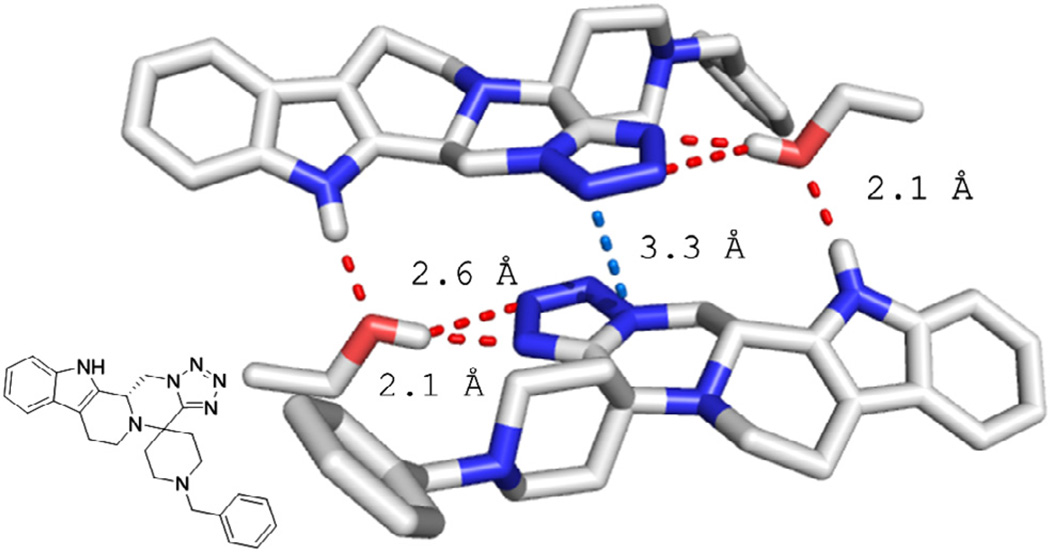
Figure 2.
Compound 18b in the solid state. Key contacts and distances are highlighted.
Figure 5.
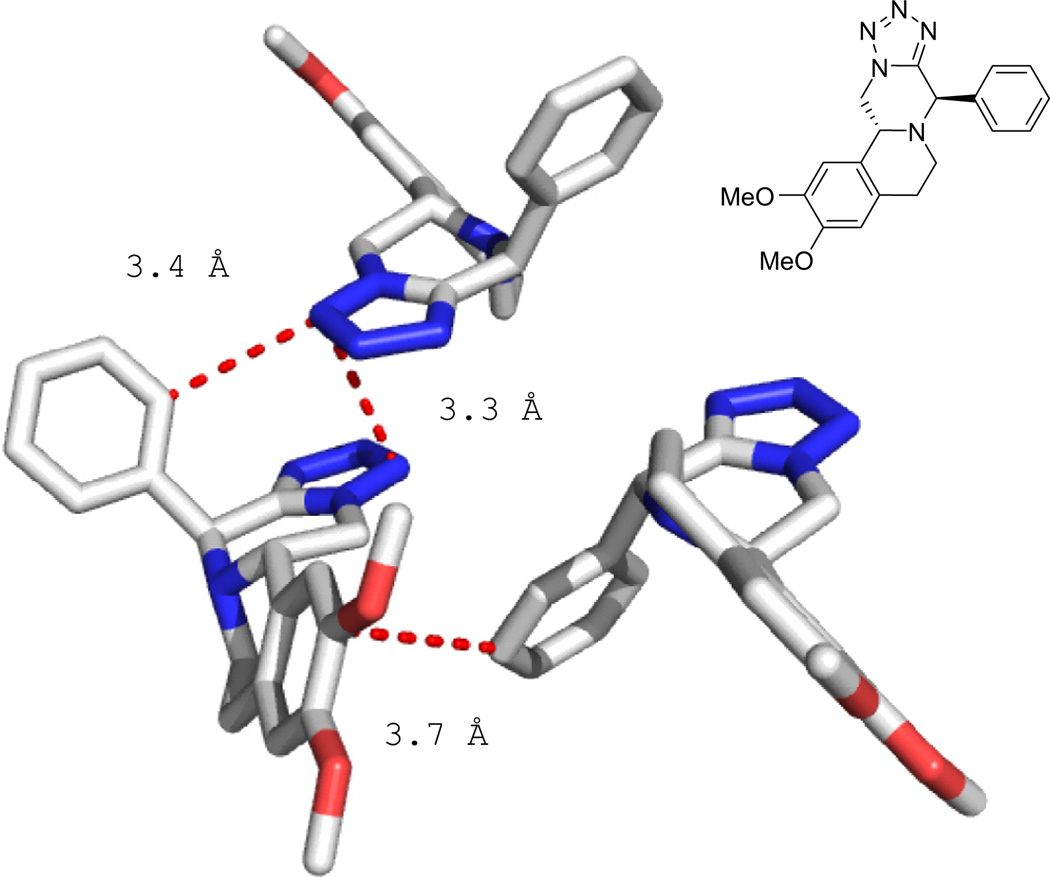
Compound 5b in the solid state. Key contacts and distances are highlighted.
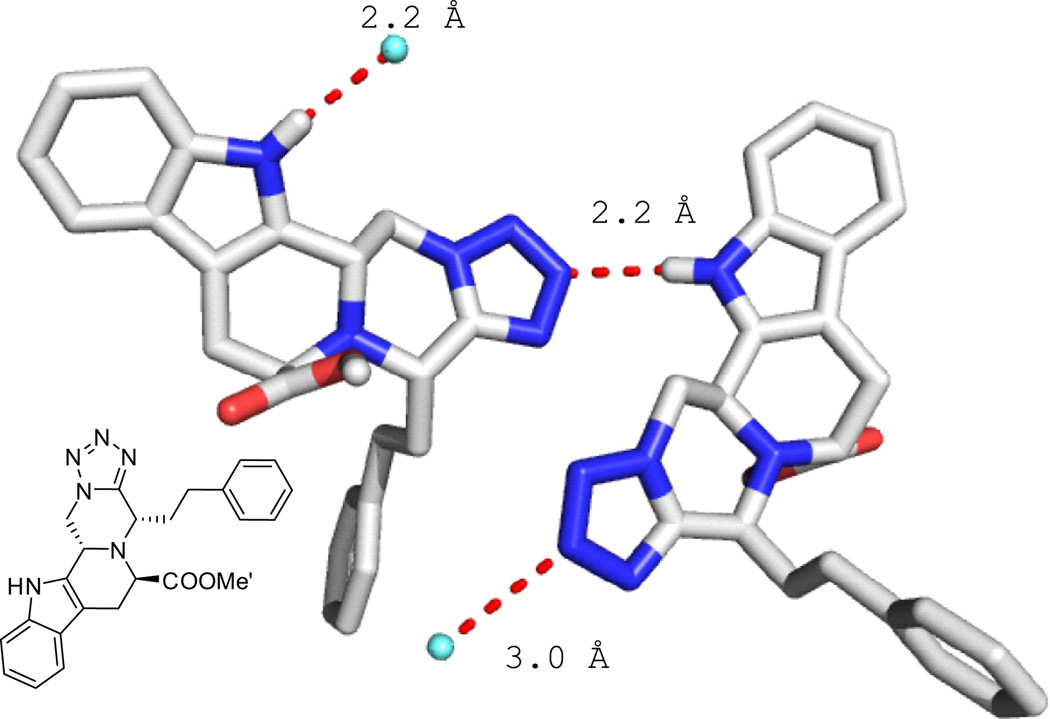
The 5-cyclic indole scaffold shown in Figs. 2–4 and an example of a 4-cyclic tetrahydroisoquinoline scaffold in Fig. 5. Compound 18b is co-crystallizing with an ethanol solvent molecule. Ethanol hydrogen forms a bifurcated hydrogen bridge to the two adjacent nitrogens of the tetrazole exhibiting short contacts of 2.1 and 2.6 Å, respectively. The ethanol oxygen forms a hydrogen bond with the indole hydrogen of 2.1 Å. The tetrazole rings of the two adjacent molecules undergo parallel stacking and short contacts of up to 3.3 Å can be observed.
Figure 4.
Compound 30b in the solid state. Key contacts and distances are highlighted.
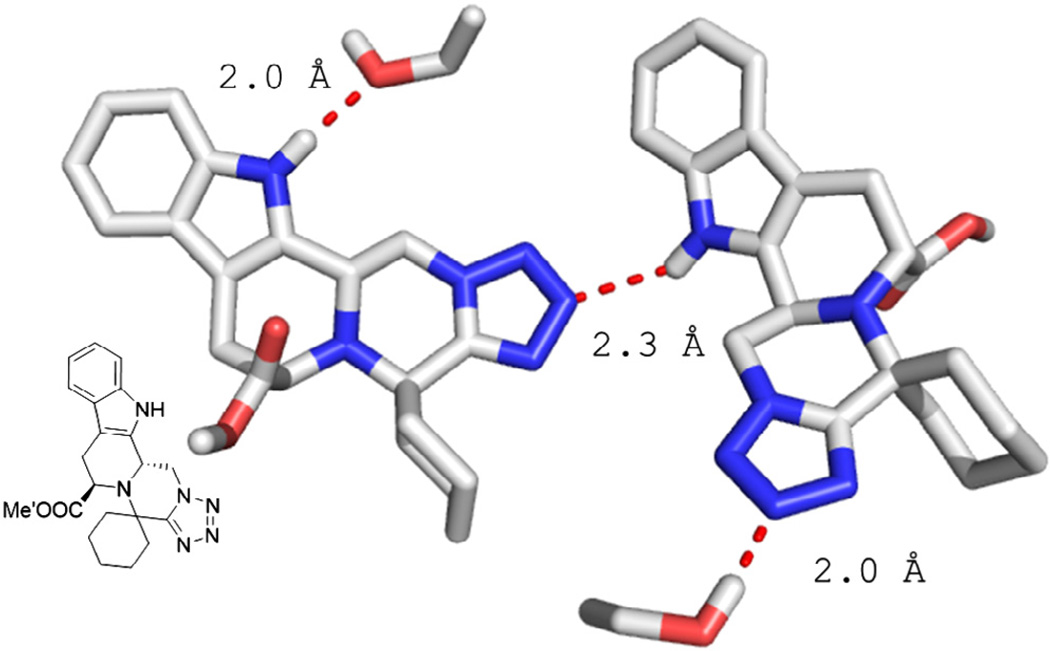
Pentacyclic 27b co-crystallize with water and forms hydrogen bonds to it. Three types of hydrogen bonds can be observed. The indole-NH of two molecules form each a 2.2 Å hydrogen bond to a water and a tetrazole N3, respectively. The tetrazole moiety of another molecule exhibits a 3 Å hydrogen bond to a water molecule.
Compound 30b is derived from tryptophan and co-crystallizes with the solvent molecule ethanol. The indole hydrogen forms two types of hydrogen bridges towards the N3 of the tetrazole moiety with 2.3 Å and a short contact of 2 Å to the ethanol oxygen. The tetrazole N3 on the other hand forms two types of hydrogen bridges to an adjacent indole nitrogen (2.3 Å) and an ethanol oxygen (2 Å).
Tetracyclic 5b exhibits no polar hydrogen atoms and thus several aromatic interactions but no hydrogen bonding. Two tetrazole moieties of adjacent molecules align parallel and form short contacts of up to 3.3 Å. The phenyl substituent introduced by the aldehyde reactant forms two pi-pi interactions to an adjacent tetrazole (3.4 Å) and to the tetrahydro isoquinoline moiety of an adjacent molecule (3.7 Å). The conformation of the two interactions is more T-shaped and not parallel.
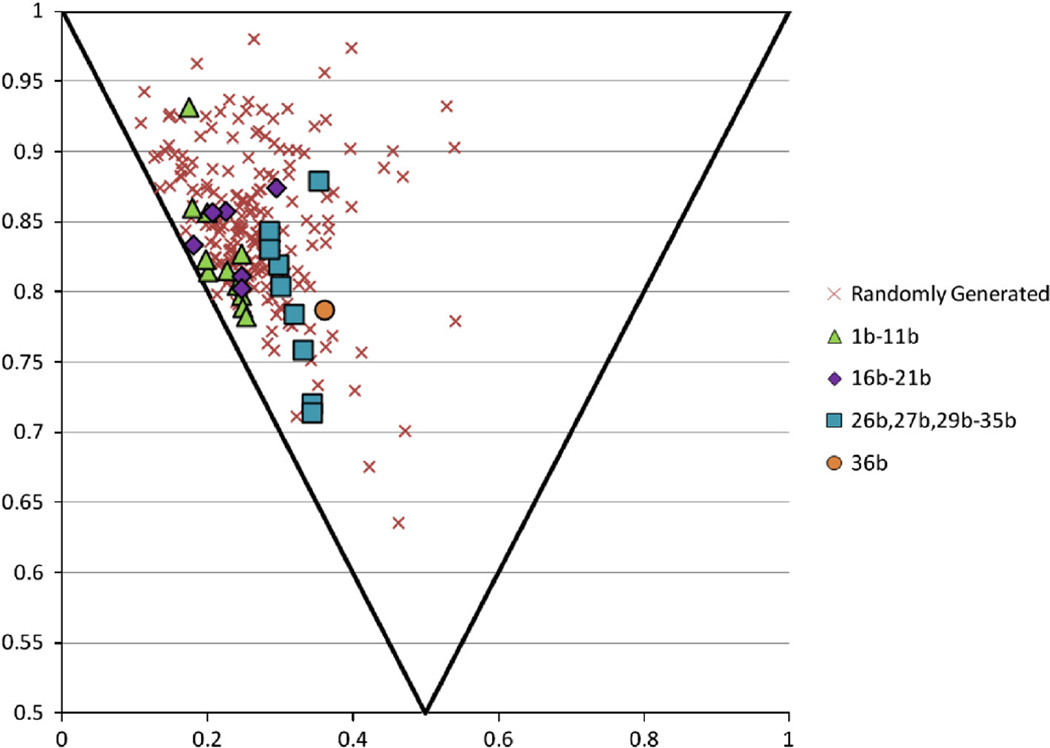
We then set out to examine some of the virtual properties of our actual compounds compared to the scaffolds potential chemical space. We randomly generated 200 examples of the scaffold described in this paper based on readily available and purchasable starting materials to compare against the specific compounds synthesized in this paper. One particular property we were interested in was the measurement of the 3D nature of our compounds which was observed by plotting the principal moment of inertia29 (Fig. 6). 3D coordinates for compounds were generated using Mol3d (Moloc).30 As can be seen the randomly generated compounds cluster in an area between flat and linear. This is to be expected as the backbone of the scaffold is comprised of 4–5 fused rings and is therefore a fairly rigid and flatter compound, however some compounds are in fact able to occupy a more 3D space. When comparing the randomly generated compounds to those specific compounds described in this paper we can see our physical compounds generally occupy the same space as those virtually generated. Again while most of the compounds tend to be more flat in nature specific compounds like 30b or 36b are able to occupy a more 3D space. When looking at other calculated properties such as molecular weight, or polar surface area (Supplemental Fig.) again we can see that our compounds synthesized compare reasonably well those that were randomly generated.
Figure 6.
Principal moment of inertia of randomly generated compounds from this class of compounds vs those compounds physically synthesized in this paper (grouped by amine starting material).
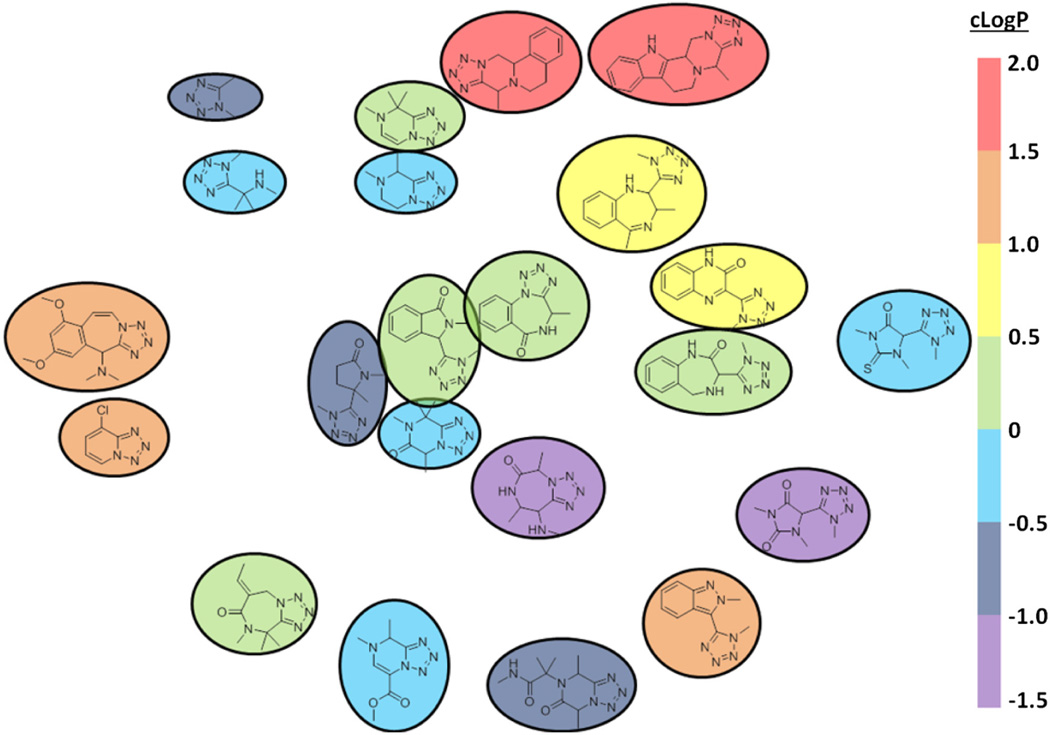
Additionally we wanted to compare the scaffold described in this paper to other tetrazole containing compounds synthesized via multi component reaction chemistry. We plotted the backbones of 20 tetrazole containing scaffolds described in literature via MCR by scaffold key31 (Fig. 7). Scaffold key distances were calculated and plotted for each pair of backbones; distances between compounds represent how similar two scaffolds are, that is: two compounds closer together are more similar then two compounds farther away. As can be seen, even though all compounds are based around the Ugi-tetrazole reaction post condensation modification allows for this to be a very diverse reaction. Given that our backbones have at a minimum 4 fused rings and no rotatable bonds we can see that this post Ugi modification allows for access to a truly new, never described scaffold. It is believed that when designing libraries for virtual drug design scaffold diversity and not size is the key.32 As novel scaffolds, such as those described in this article, are being discovered, it paves the way to address various unmet medical needs.33–35
Figure 7.
Scaffold map of tetrazole containing compounds described via MCR. plotted by scaffold key distances, and color coded by cLogP
3. Experimental
3.1. Synthesis
All reagents were purchased from commercial sources and used without further purification. The reactions were conducted under air atmosphere. Nuclear magnetic resonance spectra (NMR) were recorded on a Bruker Avance 500 spectrometer (1H NMR (500 MHz), 13C NMR (126 MHz)). Chemical shifts for 1H NMR were reported as δ values and coupling constants were in hertz (Hz). The following abbreviations were used for spin multiplicity: s = singlet, d = doublet, t = triplet, dd = double doublet, m = multiplet, brs = broad singlet. Chemical shifts for 13C NMR reported in ppm relative to the solvent peak. Thin layer chromatography was performed on Fluka precoated silica gel plates (0.20 mm thick, particle size 25 µm). Flash chromatography was performed on a Teledyne ISCO Combiflash Rf, using RediSep Rf Normal-phase Silica Flash Columns (Silica Gel 60 Å, 230–400 mesh). The isocyanides were made from the formamides by dehydration with POCl3 (Aldrich). Electrospray ionization mass spectra (ESI–MS) were recorded on a Waters Investigator semi-prep-15 SFC–MS instrument.
3.2. General procedure A: preparation of Ugi-adducts
3.2.1. Synthetic procedure A (Ugi reaction/Ugi-adduct)
To a 1 M solution of aldehyde in methanol were added successively 1.0 equiv of amine, 1.0 equiv of azidotrimethylsilane, and 1.0 equiv of isocyanoacetaldehyde dimethylacetal (1). The resulting mixture was stirred at room temperature for 18 h. The solvent was removed under reduced pressure and the residue was purified using flash chromatography to obtain the Ugi-product.
3.2.2. Synthetic procedure B (Pictet-Spengler cyclization)
To a solution of Ugi-tetrazolee (1 mmol) was stirred with methanesulfonic acid (100 mmol) at room temperature for 18 h. The reaction was quenched with saturated sodium carbonate and extracted with EtOAc (20 mL × 3). The solvent was removed under reduced pressure, and the residue was purified by crystallization or flash column chromatography to afford cyclic product.
3.3. Computational methods
A virtual library of 1000 randomly generated examples were made of the described scaffolds using previously described methods.36 200 compounds were randomly selected and physiochemical properties relating to drug likeness were calculated via ChemAxon’s Calculator Plugins (Marvin 6.1.0, 2013, ChemAxon http://www.chemaxon.com). Compound 3d generation of compounds was done using Moloc’s Mol3d software (Moloc http://www.moloc.ch).30 Principal moment of inertia was calculated using Schrodinger’s Maestro V 9.3 (Suite 2012: Maestro, version 9.3, Schrödinger, LLC, New York, NY, 2012.). Scaffold key distances were calculated using previously described methods31 and plotted using Cytoscape (Cytoscape v3.1.1, www.cytoscape.org).37
3.4. Analytical data of Ugi-adducts and cyclization products
3.4.1. Compound 1a: 1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-N-(3,4-dimethoxyphenethyl)propan-1-amine (1a)
Compound 1a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 379.22; found [M+H]+: 380.31; 1H NMR (500 MHz, CDCl3) δ 6.78 (d, J =8.0 Hz, 1H), 6.67 (m, 2H), 4.75 (t, J = 5.5 Hz, 1H), 4.48 (dd, J =14.1, 5.5 Hz, 1H), 4.41 (dd, J =14.1, 5.5 Hz, 1H), 4.09 (t, J =7.0 Hz, 1H), 3.85 (s, 6H), 3.39 (s, 3H), 3.35 (s, 3H), 2.67 (m, 4H), 1.92-1.85 (m, 2H), 1.64 (br s, 1H), 0.88 (t, J = 7.4 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 157.0, 148.8, 147.4, 132.0, 120.5, 111.7, 111.2, 102.7, 55.8, 55.7, 55.5, 55.4, 54.5, 48.9, 48.2, 35.8, 27.2, 10.2.
3.4.2. Compound 1b: 8-ethyl-2,3-dimethoxy-6,8,13,13a-tetrahydro-5H-tetrazoleo[1′,5′:4,5]pyrazino [2,1-a]isoquinoline (1b)
Compound 1b was prepared according to standard procedure B, MS (ESI) m/z calculated [M]+: 315.17; found [M+H]+: 316.21; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 6.68 (s, 1H), 6.62 (s, 1H), 4.65 (dd, J =13.0, 4.5 Hz, 1H), 4.46 (dd, J =11.0, 4.5 Hz, 1H), 4.23 (dd, J =12.9, 11.0 Hz, 1H), 4.15 (t, J = 7.3 Hz, 1H), 3.88 (s, 6H), 3.10-3.01 (m, 2H), 2.98–2.92 (m, 1H), 2.85-2.78 (m, 1H), 1.95-1.85 (m, 2H), 1.17 (t, J = 7.3 Hz, 3H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 152.9, 148.63, 147.7, 126.6, 124.7, 111.9, 109.1, 60.2, 56.1, 55.9, 50.5, 47.5, 46.5, 29.3, 25.7, 11.1.
3.4.3. Compound 2a: 1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-N-(3,4-dimethoxyphenethyl)-2-methylpropan-1-amine (2a)
Compound 2a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 393.24; found [M+H]+: 394.33; 1H NMR (500 MHz, CDCl3) δ 6.77 (d, J =8.0 Hz, 1H), 6.70–6.64 (m, 2H), 4.78 (t, J = 5.5 Hz, 1H), 4.42 (d, J = 5.5 Hz, 2H), 3.85 (s, 6H), 3.41 (s, 3H), 3.32 (s, 3H), 2.71-2.60 (m, 4H), 2.18–207 (m, 1H), 1.59 (br s, 1H), 0.99 (d, J = 6.7 Hz, 3H), 0.81 (d,J= 6.7 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 156.8, 148.8, 147.3, 132.1, 120.4, 111.7, 111.1, 102.8, 59.2, 55.8, 55.7, 55.5, 49.0, 48.6, 35.7, 32.1, 19.3, 19.0.
3.4.4. Compound 2b: 8-isopropyl-2,3-dimethoxy-6,8,13,13a-tetrahydro-5H-tetrazoleo[1′,5′:4,5] pyrazino[2,1-a]isoquinoline (2b)
Compound 2b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 329.19; found [M+H]+: 330.29; 1H NMR (500 MHz, CDCl3) δ 6.68 (s, 1H), 6.61 (s, 1H), 4.61 (dd, J = 13.1, 4.0 Hz, 1H), 4.44 (dd, J = 10.8, 4.0 Hz, 1H), 4.35–4.26 (m, 1H), 3.88 (s, 6H), 3.81 (d, J = 8.0 Hz, 1H), 3.23–3.12 (m, 1H), 3.06 (td, J = 11.3, 3.1 Hz, 1H), 2.87 (dd, J = 10.9, 6.2 Hz, 1H), 2.72 (d, J = 15.3 Hz, 1H), 2.25–2.12 (m, 1H), 1.17 (d, J = 6.6 Hz, 3H), 1.08 (d, J = 6.6 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 151.9, 148.6, 147.5, 126.3, 124.8, 111.9, 109.3, 64.8, 56.3, 56.1, 55.8, 51.4, 46.6, 46.2, 32.2, 29.1, 19.7.
3.4.5. Compound 3a: 1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-N-(3,4-dimethoxyphenethyl)-3-phenylpropan-1-amine (3a)
Compound 3a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 455.55; found [M+H]+: 456.39; 1H NMR (500 MHz, CDCl3) δ 7.28-7.24 (m, 2H), 7.19 (t, J = 7.3 Hz, 1H), 7.12 (d, J = 7.3 Hz, 2H), 6.78 (d, J = 8.0 Hz, 1H), 6.70–6.61 (m, 2H), 4.70 (t, J = 5.4 Hz, 1H), 4.37–4.30 (m, 2H), 4.11 (t, J = 7.0 Hz, 1H), 3.85 (s, 6H), 3.32 (s, 3H), 3.30 (s, 3H), 2.70-2.58 (m, 6H), 2.20-2.14 (m, 2H), 1.6 (br s, 1H); 13C NMR (126 MHz, CDCl3) δ 157.1, 148.9, 147.5, 140.7, 132.0, 128.4, 128.3, 126.1, 120.5, 111.7, 111.2, 102.5, 55.8, 55.8, 55.3, 55.2, 52.1, 48.8, 48.1, 35.8, 35.4, 31.8.
3.4.6. Compound 3b: 2,3-dimethoxy-8-phenethyl-6,8,13,13a-tetrahydro-5H-tetrazoleo[1′,5′:4,5] pyrazino[2,1-a]isoquinoline (3b)
Compound 3b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 391.20; found [M+H]+: 392.28; 1H NMR (500 MHz, CDCl3) δ 7.32–7.15 (m, 5H), 6.69 (s, 1H), 6.66 (s, 1H), 4.63 (dd, J = 13.0, 4.0 Hz, 1H), 4.48 (dd, J = 10.7, 4.0 Hz, 1H), 4.23 (t, J = 12.0 Hz, 1H), 4.16 (t, J = 7.3 Hz, 1H), 3.89 (s, 6H), 3.16– 2.75 (m, 6H), 2.18 (dd, J = 14.5, 7.2 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 152.7, 148.5, 147.5, 140.5, 128.5, 128.3, 126.3, 126.0, 124.6. 111.8, 109.2, 57.1, 56.0, 55.9, 50.2, 46.8, 46.0, 33.9, 31.8, 29.1.
3.4.7. Compound 4a: 1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-N-(3,4-dimethoxyphenethyl)-2-phenylethanamine (4a)
Compound 4a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 441.24; found [M+H]+: 442.34; 1H NMR (500 MHz, CDCl3) δ 7.26–7.18 (m, 3H), 7.02–6.95 (m, 2H), 6.77–6.72 (m, 1H), 6.66–6.59 (m, 2H), 4.54 (t, J = 5.0 Hz, 1H), 4.34 (t, J = 7.0 Hz, 1H), 4.04 (dd, J = 14.3, 5.0 Hz, 1H), 3.90–3.81 (m, 7H), 3.30 (s, 3H), 3.27–3.21 (m, 4H), 3.04 (dd, J = 13.3, 8.0 Hz, 1H), 2.73–2.60 (m, 4H), 1.76 (br s, 1H); 13C NMR (126 MHz, CDCl3) δ 157.0, 148.8, 147.3, 136.6, 131.8, 129.0, 128.6, 126.9, 120.4, 111.6, 111.1, 102.5, 55.8, 55.7, 55.5, 55.2, 55.0, 48.3, 48.2, 41.2, 35.7.
3.4.8. Compound 4b: 8-benzyl-2,3-dimethoxy-6,8,13,13a-tetrahydro-5H-tetrazoleo[1′,5′:4,5]pyrazino [2,1-a]isoquinoline (4b)
Compound 4b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 377.44; found [M+H]+: 378.30; 1H NMR (500 MHz, CDCl3) δ 7.31–7.19 (m, 5H), 6.65 (s, 1H), 6.60 (s, 1H), 4.63 (dd, J = 13.0, 4.0 Hz, 1H), 4.53 (t, J = 6.5 Hz, 1H), 4.41 (dd, J = 11.0, 4.0 Hz, 1H), 4.20 (t, J = 13.0 Hz, 1H), 3.88 (s, 6H), 3.21 (d, J = 6.5 Hz, 2H), 3.10-3.02 (m, 1H), 2.97-2.87 (m, 1H), 2.85-2.78 (m, 1H), 2.73-2.66 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 152.5, 148.4, 147.5, 137.6, 129.3, 128.2, 126.6, 124.3, 111.7, 109.1, 60.0, 56.0, 55.8, 50.6, 47.3, 46.5, 39.0, 29.2.
3.4.9. Compound 5a: N-((1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)(phenyl)methyl)-2-(3,4-dimethoxy phenyl)ethanamine (5a)
Compound 5a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 427.22; found [M+H]+: 428.33; 1H NMR (500 MHz, CDCl3) δ 7.42–7.20 (m, 5H), 6.83–6.64 (m, 3H), 5.30 (s, 1H), 4.51 (s, 1H), 4.36 (d, J = 14.0 Hz, 1H), 4.20 (dd, J = 14.0, 3.6 Hz, 1H), 3.85 (s, 6H), 3.32 (s, 3H), 3.28 (s, 3H), 2.90– 2.71 (m, 4H), 2.13 (s, 1H); 13C NMR (126 MHz, CDCl3) δ 156.5, 148.8, 147.4, 137.8, 131.9, 128.9, 128.4, 127.4, 120.5, 111.7, 111.2, 102.5, 57.2, 55.8, 55.7, 55.5, 55.3, 48.9, 48.9, 35.6.
3.4.10. Compound 5b: 2,3-dimethoxy-8-phenyl-6,8,13,13a-tetrahydro-5H-tetrazolo[1′,5′:4,5]pyrazino [2,1-a]isoquinoline (5b)
Compound 5b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 363.17; found [M+H]+: 364.29; 1H NMR (500 MHz, CDCl3) δ 7.40–7.20 (m, 5H), 6.67 (s, 1H), 6.57 (s, 1H), 5.58 (s, 1H), 4.78 (dd, J = 12.0, 4.0 Hz, 1H), 4.43 (dd, J = 12.0, 4.0 Hz, 1H), 4.27 (t, J = 12.0 Hz, 1H), 3.87 (s, 3H), 3.84 (s, 3H), 3.19–3.10 (m, 1H), 3.08–2.98 (m, 1H), 2.97–2.83 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 151.5, 148.4, 147.6, 135.1, 128.4, 128.3, 127.9, 126.3, 124.4, 111.7, 108.7, 61.0, 55.9, 55.8, 49.8, 48.7, 46.1, 29.0.
3.4.11. Compound 6a: N-((4-chlorophenyl)(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)methyl)-2-3,4-dimethoxyphenyl)ethanamine (6a)
Compound 6a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 461.18; found [M+H]+: 462.26; 1H NMR (500 MHz, CDCl3) δ 7.31 (d, J = 8.4 Hz, 2H), 7.25 (d, J = 8.4 Hz, 2H), 6.78 (d, J = 8.1 Hz, 1H), 6.70 (m, 2H), 5.26 (s, 1H), 4.56 (t, J = 5.4 Hz, 1H), 4.38 (dd, J = 14.2, 5.5 Hz, 1H), 4.23 (dd, J = 14.2, 5.5 Hz, 1H), 3.85 (s, 3H), 3.84 (s, 3H), 3.33 (s, 3H), 3.29 (s, 3H), 2.85–2.72 (m, 4H), 2.05 (br s, 1H); 13C NMR (126 MHz, CDCl3) δ 156.2, 148.9, 147.6, 136.4, 134.3, 131.8, 129.1, 128.9, 120.5, 111.8, 111.3, 102.7, 56.5, 55.9, 55.8, 55.6, 55.5, 49.1, 48.8, 35.7.
3.4.12. Compound 6b: 8-(4-chlorophenyl)-2,3-dimethoxy-6,8,13,13a-tetrahydro-5H-tetrazolo[1′,5′:4,5]pyrazino[2,1-a]isoquinoline (6b)
Compound 6b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 397.13; found [M+H]+: 398.19; 1H NMR (500 MHz, CDCl3) δ 7.31 (d, J = 8.4 Hz, 2H), 7.27 (d, J = 8.4 Hz, 2H), 6.68 (s, 1H), 6.53 (s, 1H), 5.53 (s, 1H), 4.72 (dd, J = 11.6, 3.2 Hz, 1H), 4.39–4.27 (m, 2H), 3.88 (s, 3H), 3.84 (s, 3H), 3.17–3.05 (m, 2H), 2.97–2.87 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 151.1, 148.7, 147.7, 134.4, 134.0, 129.2, 128.8, 126.2, 124.3, 111.9, 109.0, 108.7, 60.4, 56.1, 55.9, 50.1, 48.2, 46.0, 29.1.
3.4.13. Compound 7a: N-((1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)(4-fluorophenyl)methyl)-2-(3,4-dimethoxyphenyl)ethanamine (7a)
Compound 7a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 445.21; found [M+H]+: 446.35; 1H NMR (500 MHz, CDCl3) δ 7.33–7.26 (m, 2H), 7.03 (t, J = 8.5 Hz, 2H), 6.79 (d, J = 8.0 Hz, 1H), 6.74–6.66 (m, 2H), 5.27 (s, 1H), 4.56 (t, J = 5.5 Hz, 1H), 4.38 (dd, J = 14.1, 5.5 Hz, 1H), 4.22 (dd, J = 14.1, 5.5 Hz, 1H), 3.85 (s, 6H), 3.34 (s, 3H), 3.29 (s, 3H), 2.87–2.74 (m, 4H), 2.07 (s, 1H); 13C NMR (126 MHz, CDCl3) δ 162.29 (d, J = 247.6 Hz), 156.3, 148.7, 147.3, 133.6 (d, J = 2.9 Hz), 131.7, 129.2 (d, J = 8.2 Hz), 120.4, 115.7, 111.4 (d, J = 66.1 Hz), 111.1, 102.4, 56.3, 55.7, 55.6, 55.3, 55.3, 48.8, 48.6, 35.5.
3.4.14. Compound 7b: 8-(4-fluorophenyl)-2,3-dimethoxy-6,8,13,13a-tetrahydro-5H-tetrazolo[1′,5′:4,5] pyrazino[2,1-a]isoquinoline (7b)
Compound 7b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 381.16; found [M+H]+: 382.27; 1H NMR (500 MHz, CDCl3) δ 7.27 (dd, J = 8.6, 5.3 Hz, 2H), 7.00 (t, J = 8.6 Hz, 2H), 6.68 (s, 1H), 6.61 (s, 1H), 5.54 (s, 1H), 4.78 (dd, J = 12.9, 4.5 Hz, 1H), 4.40 (dd, J = 10.8, 4.5 Hz, 1H), 4.32–4.24 (m, 1H), 3.87 (s, 3H), 3.84 (s, 3H), 3.18–3.11 (m, 1H), 3.10–3.02 (m, 1H), 2.96–2.87 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 162.46 (d, J = 247.9 Hz), 151.3, 148.5, 147.6, 131.1 (d, J = 3.1 Hz),129.6 (d, J = 8.2 Hz), 126.2, 124.3, 115.4 (d, J = 21.7 Hz), 111.8, 108.8, 60.3, 56.0, 55.7, 49.9, 48.4, 46.0, 29.0.
3.4.15. Compound 8a: N-([1,1′-biphenyl]-4-yl(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)methyl)-2-(3,4-dimethoxyphenyl)ethanamine (8a)
Compound 8a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 503.25; found [M+H]+: 504.40; 1H NMR (500 MHz, CDCl3) δ 7.56 (t, J = 7.0 Hz, 4H), 7.46–7.33 (m, 5H), 6.79 (d, J = 8.0 Hz, 1H), 6.76–6.68 (m, 2H), 5.34 (s, 1H), 4.56 (t, J = 5.0 Hz, 1H), 4.41 (dd, J = 14.1, 5.0 Hz, 1H), 4.25 (dd, J = 14.1, 5.0 Hz, 1H), 3.85 (s, 3H), 3.84 (s, 3H), 3.33 (s, 3H), 3.30 (s, 3H), 2.91–2.75 (m, 4H), 2.16 (br s, 1H); 13C NMR (126 MHz, CDCl3) δ 156.5, 148.8, 147.4, 141.3, 140.1, 136.7, 131.9, 128.8, 127.9, 127.6, 127.5, 126.9, 120.5, 111.7, 111.2, 102.6, 56.9, 55.8, 55.7, 55.5, 55.3, 53.4, 49.0, 48.9, 35.7.
3.4.16. Compound 8b: 8-([1,1′-biphenyl]-4-yl)-2,3-dimethoxy-6,8,13,13a-tetrahydro-5H-tetrazolo [1′,5′:4,5]pyrazino[2,1-a]isoquinoline (8b)
Compound 8b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 439.20; found [M+H]+: 440.27; 1H NMR (500 MHz, CDCl3) δ 7.54 (t, J = 8.0 Hz, 4H), 7.42 (t, J = 7.5 Hz, 2H), 7.34 (t, J = 7.5 Hz, 3H), 6.68 (s, 1H), 6.57 (s, 1H), 5.63 (s, 1H), 4.79 (dd, J = 12.0, 4.5 Hz, 1H), 4.48 (dd, J = 10.7, 4.5 Hz, 1H), 4.30 (t, J = 12.0 Hz, 1H), 3.87 (s, 3H), 3.84 (s, 3H), 3.22–2.91 (m, 4H); 13C NMR (126 MHz, CDCl3) δ 151.6, 148.6, 147.7, 141.4, 140.2, 134.2, 128.8, 128.5, 127.6, 127.3, 127.0, 126.4, 124.5, 111.9, 108.7, 60.9, 56.0, 55.9, 50.1, 48.8, 46.2, 29.2.
3.4.17. Compound 9a: 2-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-N-(3,4-dimethoxyphenethyl)butan-2-amine (9a)
Compound 9a was prepared according to standard procedure A, 93% as pale yellow liquid; MS (ESI) m/z calculated [M]+: 393.23; found [M+H]+: 394.33; 1H NMR (500 MHz, CDCl3) δ 6.79 (d, J = 8.1 Hz, 1H), 6.69 (dd, J = 8.1 Hz, 1.7, 1H), 6.65 (d, J = 1.7 Hz, 1H), 4.94 (t, J = 5.8 Hz, 1H), 4.78 (dd, J = 13.9, 5.5 Hz, 1H), 4.60 (dd, J = 13.9, 5.5 Hz, 1H), 3.86 (s, 6H), 3.41 (s, 3H), 3.38 (s, 3H), 2.72–2.64 (m, 3H), 2.45–2.39 (m, 1H), 2.03 (dd, J = 14.0, 7.3 Hz, 1H), 1.86 (dd, J = 14.0, 7.3 Hz, 1H), 1.56 (s, 3H), 1.22 (br s, 1H), 0.71 (t, J = 7.5 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 158.5, 148.9, 147.5, 132.2, 120.6, 111.8, 111.2, 103.1, 56.7, 55.8, 55.8, 55.6, 55.3, 49.9, 43.9, 36.0, 32.3, 23.8, 7.7.
3.4.18. Compound 9b: 8-ethyl-2,3-dimethoxy-8-methyl-6,8,13,13a-tetrahydro-5H-tetrazolo[1′,5′:4,5] pyrazino[2,1-a]isoquinoline (9b)
Compound 9b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 329.19; found [M+H]+: 330.29; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 6.67 (s, 1H), 6.63 (s, 1H), 4.81 (dd, J =12.4,3.8 Hz, 1H), 4.63 (dd, J = 10.8,3.8 Hz, 1H), 4.05 (dd, J =12.4,10.8 Hz, 1H), 3.88 (s, 6H), 3.19–3.12 (m, 1H), 2.94–2.83 (m, 2H), 2.82–2.76 (m, 1H), 2.02–1.88 (m, 2H), 1.74 (s, 3H), 1.06 (t, J = 7.5 Hz, 3H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 156.7, 148.3, 147.7, 127.6, 124.9, 111.5, 108.5, 59.6, 58.8, 55.8, 51.8, 40.1, 31.7, 30.1, 28.1, 24.3, 9.3.
3.4.19. Compound 10a: 1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-N-(3,4-dimethoxyphenethyl) cyclohexanamine (10a)
Compound 10a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 419.25; found [M+H]+: 420.33; 1H NMR (500 MHz, CDC13) δ 6.81 (d, J =8.1 Hz, 1H), 6.69 (dd, J = 8.1, 1.6 Hz, 1H), 6.65 (d, J=1.6 Hz, 1H), 4.96 (t, J =5.7 Hz, 1H), 4.66 (d, J = 5.7 Hz, 2H), 3.88 (s, 3H), 3.87 (s, 3H), 3.41 (s, 6H), 2.65 (t, J = 6.5 Hz, 2H), 2.45 (t, J = 6.5 Hz, 2H), 2.14–2.04 (m, 2H), 1.99–1.90 (m, 2H), 1.68–1.56 (m, 2H), 1.51 (s, 1H), 1.40–1.31 (m, 3H); 13C NMR (126 MHz, CDC13) δ 158.9, 148.9, 147.5, 132.2, 120.7, 111.5, 111.3, 103.1, 55.8, 55.7, 55.6, 55.5, 55.5, 55.3, 49.9, 43.1, 35.9,34.6,24.9,21.3.
3.4.20. Compound 10b: 2′,3′-dimethoxy-5′,6′,13′,13a′-tetrahy-drospiro[cyclohexane-1,8′-tetrazolo [1′,5′:4,5]pyrazino[2,1-a]-isoquinoline] (10b)
Compound 10b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 355.20; found [M+H]+: 356.30; 1H NMR (500 MHz, CDC13) δ 6.69 (s, 1H), 6.61 (s, 1H), 4.66 (dd, J =11.1, 4.9 Hz, 1H), 4.56 (dd, J = 13.1, 4.9 Hz, 1H), 4.32 (dd, J = 13.1, 11.1 Hz, 1H), 3.88 (s, 6H), 3.20–3.12 (m, 1H), 3.09–3.00 (m, 1H), 2.81–2.75 (m, 1H), 2.73–2.64 (m, 1H), 2.38–2.28 (m, 1H), 2.20–2.06 (m, 2H), 2.00–1.91 (m, 1H), 1.84–1.73 (m, 2H), 1.69–1.50 (m, 4H); 13C NMR (126 MHz) δ 156.6, 148.6, 147.5, 126.6, 125.7, 111.7, 109.3, 57.1, 56.1, 56.0, 55.8, 50.3, 46.7, 37.5, 35.3, 32.3, 29.4, 25.3, 20.8.
3.4.21. Compound 11a: 1-benzyl-4-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-N-(3,4-dimethoxy phenethyl)piperidin-4-amine (11a)
Compound 11a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 510.30; found [M+H]+: 511.38; 1H NMR (500 MHz, CDC13) δ 7.34–7.22 (m, 5H), 6.80 (d,J = 8.1 Hz, 1H), 6.66 (dd,J =8.1, 1.5 Hz, 1H), 6.61 (d, J = 1.5 Hz, 1H), 4.90 (t, J = 5.8 Hz, 1H), 4.56 (d, J = 5.8 Hz, 2H), 3.88 (s, 3H), 3.85 (s, 3H), 3.41 (s, 2H), 3.36 (s, 6H), 2.61 (t, J =6.4 Hz, 2H), 2.55–2.25 (m, 8H), 1.98–1.84 (m, 2H), 1.31 (br s, 1H); 13C NMR (126 MHz, CDC13) , δ 158.0, 148.9, 147.5, 138.2, 132.1, 128.9, 128.1, 126.9, 120.6, 111.6, 111.2, 103.1, 62.9, 55.8, 55.8, 55.6, 53.8, 49.8, 49.1, 43.0, 35.8, 34.7.
3.4.22. Compound 11b: 1-benzyl-2′,3′-dimethoxy-5′,6′,13′,13a′-tetrahydrospiro[piperidine-4,8′-tetrazolo[1′,5′:4,5]pyrazino[2,1-a]isoquinoline] (11b)
Compound 11b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 446.24; found [M+H]+: 447.35; 1H NMR (500 MHz, CDC13) δ 7.43–7.19 (m, 5H), 6.69 (s, 1H), 6.63 (s, 1H), 4.64 (dd, J =11.0, 4.5 Hz, 1H), 4.58 (dd, J =12.2, 4.5 Hz, 1H), 4.31 (t, J =12.2 Hz, 1H), 3.89 (s, 3H), 3.88 (s, 3H), 3.58 (s, 2H), 3.19–2.96 (m, 3H), 2.82–2.70 (m, 2H), 2.68–2.42 (m, 4H), 2.28–2.19 (m, 1H), 2.18–2.10 (m, 1H), 2.07–1.96 (m, 1H); 13C NMR (126 MHz, CDC13) δ 156.0, 148.6, 147.6, 138.6, 128.9, 128.2, 126.9, 126.6, 125.4, 111.6, 109.1, 62.8, 56.1, 55.1, 55.9, 50.6, 48.7, 48.4, 47.0, 37.8, 34.6, 32.2, 29.3.
3.4.23. Compound 16a: N-(2-(1H-indol-3-yl)ethyl)-1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)propan-1-amine (16a)
Compound 16a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 358.21; found [M+H]+: 359.29; 1H NMR (500 MHz, CDC13) δ 8.12 (br s, 1H), 7.53 (d, J = 8.0 Hz, 1H), 7.35 (d, J =8.0 Hz, 1H), 7.18 (t, J = 7.3 Hz, 1H), 7.09 (t, J = 7.3 Hz, 1H), 6.97 (s, 1H), 4.73 (t, J = 5.5 Hz, 1H), 4.46 (dd, J =14.1, 5.5 Hz, 1H), 4.38 (dd, J =14.1, 5.5 Hz, 1H), 4.10 (t, J = 7.0 Hz, 1H), 3.35 (s, 3H), 3.32 (s, 3H), 2.90 (t, J =6.8 Hz, 2H), 2.85–2.72 (m, 2H), 1.93–1.75 (m, 3H), 0.85 (t, J =7.4 Hz, 3H); 13C NMR (126 MHz, CDC13) δ 157.1, 136.3, 127.3, 121.9, 121.8, 119.1, 118.5, 113.2, 111.2, 102.6, 55.3, 55.3, 54.6, 48.9, 47.1, 27.2, 25.7, 10.2.
3.4.24. Compound 16b: 4-ethyl-4,6,7,12,12b,13-hexahydro-tetrazolo[1″,5″:4′,5′]pyrazino[1′,2′:1,2] pyrido[3,4-b]indole (16b)
Compound 16b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 294.16; found [M−H]+: 293.17; 1H NMR (500 MHz, CDC13) (major diastereomer) δ 8.39 (br s, 1H), 7.54 (t, J = 7.6 Hz, 1H), 7.40 (t, J =7.8 Hz, 1H), 7.22 (t, J =7.6 Hz, 1H), 7.15 (dd, J =7.8 Hz, 1H), 4.81 (dd, J= 12.7 Hz, 4.2, 1H), 4.66 (dd, 7=10.4, 4.2 Hz, 1H), 4.34–4.26 (m, 2H), 3.24–3.17 (m, 1H), 3.16–3.10 (m, 1H), 3.04–2.94 (m, 1H), 2.93–2.84 (m, 1H), 2.05–1.96 (m, 1H), 1.93–1.83 (m, 1H), 1.18 (t, J = 7.4 Hz, 3H); 13C NMR (126 MHz, CDC13) (major diastereomer) δ 153.5, 136.5, 129.8, 126.7, 122.6, 120.0, 118.5, 111.3, 110.1, 59.5, 55.1, 49.4, 47.8, 24.7,22.0, 11.0.
3.4.25. Compound 17a: N-(2-(1H-indol-3-yl)ethyl)-1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl) cyclohexanamine (17a)
Compound 17a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 398.24; found [M−H]+: 397.32; 1H NMR (500 MHz, CDC13) δ 8.02 (s, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.20 (t, J =7.5 Hz, 1H), 7.10 (t, J =7.5 Hz, 1H), 6.98 (s, 1H), 4.93 (t, J =5.7 Hz, 1H), 4.66 (d, J = 5.7 Hz, 2H), 3.36 (s, 6H), 2.87 (t, J = 6.5 Hz, 2H), 2.53 (t, J = 6.5 Hz, 2H), 2.13–2.01 (m, 2H), 1.96–1.85 (m, 2H), 1.57–1.50 (m, 3H), 1.48–1.38 (m, 1H), 1.36–1.27 (m, 3H); 13C NMR (126 MHz, CDC13) δ 159.1, 136.3, 127.2, 121.9, 121.8, 119.2, 118.5, 113.4, 111.2, 102.9, 55.4, 55.3, 49.9, 42.2, 34.7, 25.8, 25.0, 21.3.
3.4.26. Compound 17b: 7′,12′,12b′,13′-tetrahydro-6′H-spiro[cyclohexane-1,4′-tetrazolo[1″,5″:4′,5′]pyrazino[1′,2′:1,2]pyrido[3,4-b]indole] (17b)
Compound 17b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 334.19; found [M−H]+: 333.25; 1H NMR (500 MHz, CDC13) δ 8.60 (br s, 1H), 7.54 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.21 (t, J =7.3 Hz, 1H), 7.15 (t, J = 7.3 Hz, 1H), 4.90–4.81 (m, 1H), 4.76–4.67 (m, 1H), 4.43–4.32 (m, 1H), 3.29–3.19 (m, 1H), 3.02–2.77 (m, 3H), 2.30– 2.18 (m, 2H), 2.14–2.02 (m, 2H), 1.83–1.52 (m, 6H); 13C NMR (126 MHz, CDC13) δ 157.1, 136.5, 131.0, 126.6, 122.3, 119.9, 118.5, 111.3, 109.9, 57.7, 47.3, 46.8, 39.3, 34.5, 32.9, 25.3, 22.6, 21.1, 21.1.
3.4.27. Compound 18a: N-(2-(1H-indol-3-yl)ethyl)-1-benzyl-4-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)piperidin-4-amine (18a)
Compound 18a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 489.29; found [M−H]+: 488.30; 1H NMR (500 MHz, CDC13) δ 8.11 (s, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.38 (d,J = 8.1 Hz, 1H), 7.31–7.17 (m, 6H), 7.10 (t, J =7.4 Hz, 1H), 6.95 (s, 1H), 4.89 (t, J = 5.7 Hz, 1H), 4.60 (d, J = 5.6 Hz, 2H), 3.34 (s, 6H), 3.31 (s, 2H), 2.85 (t, J = 6.3 Hz, 2H), 2.52 (t, J = 6.1 Hz, 2H), 2.45 (s, 2H), 2.34 (d, J = 9.4 Hz, 2H), 2.17 (s, 2H), 1.90 (s, 2H); 13C NMR (126 MHz, CDC13) δ 158.2, 138.2, 136.3, 129.0, 128.1, 127.2, 127.0, 122.1, 121.9, 119.4, 118.6, 113.4, 111.2, 103.0, 62.8, 55.4, 53.8, 49.8, 49.0, 42.0, 34.7, 25.7.
3.4.28. Compound 18b: 1-benzyl-7′,12′,12b′,13′-tetrahydro-6′H-spiro[piperidine-4,4′-tetrazolo [1″,5″:4′,5′]pyrazino[1′,2′:1,2]pyrido[3,4-b]indole] (18b)
Compound 18b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 425.53; found [M+H]+: 426.33; 1H NMR (500 MHz, CDCl3) δ 8.29 (br s, 1H), 7.55 (d, J = 8.0 Hz, 1H), 7.40 (d,J = 8.0 Hz, 1H), 7.38–7.28 (m, 4H), 7.28–7.20 (m, 2H), 7.16 (t, J = 7.3 Hz, 1H), 4.79 (dd,J = 10.4, 4.0 Hz, 1H), 4.72 (dd, J = 12.8, 4.0 Hz, 1H), 4.37 (t, J = 11.8 Hz, 1H), 3.60 (s, 2H), 3.24–3.11 (m, 2H), 3.08–2.92 (m, 2H), 2.87–2.79 (m, 1H), 2.74–2.60 (m, 3H), 2.40–2.34 (m, 2H), 2.24–2.13 (m, 1H), 2.01–1.91 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 156.6, 138.5, 136.5, 130.7, 129.0, 128.2, 127.0, 126.6, 122.3, 119.8, 118.5, 111.3, 109.8, 62.8, 56.4, 48.8, 48.5, 47.7, 47.0, 39.6, 33.7, 32.7, 22.5.
3.4.29. Compound 19a: N-((4-chlorophenyl)(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)methyl)-2-(1H-indol-3-yl)ethanamine (19a)
Compound 19a was prepared according to standard procedure A; MS (ESI) .m/z calculated [M]+: 440.17; found [M−H]+: 439.32; 1H NMR (500 MHz, CDCl3) δ 8.06 (br s, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.35 (d, J =8.0 Hz, 1H), 7.30–7.16 (m, 5H), 7.09 (t, J = 7.6 Hz, 1H), 7.03 (s, 1H), 5.26 (s, 1H), 4.53 (t, J = 5.3 Hz, 1H), 4.34 (dd, J =14.1, 5.3 Hz, 1H), 4.21 (dd, J=14.1, 5.3 Hz, 1H), 3.30 (s, 3H), 3.25 (s, 3H), 3.04–2.95 (m, 2H), 2.90 (t, J = 6.4 Hz, 2H), 2.12 (br s, 1H); 13C NMR (126 MHz, CDCl3) δ 156.4, 136.4, 136.3, 134.2, 129.90, 128.9, 127.2, 122.1, 122.0, 119.2, 118.6, 113.1, 111.2, 102.5, 56.5, 55.4, 49.0, 47.5, 25.6.
3.4.30. Compound 19b: 4-(4-chlorophenyl)-4,6,7,12,12b,13-hexahydrotetrazolo[1″,5″:4′,5′]pyrazino [1′,2′:1,2]pyrido[3,4-b]indole (19b)
Compound 19b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 376.12; found [M−H]+: 375.15; 1H NMR (500 MHz, CDCl3+MeOD) δ 7.51 (d, J = 7.7 Hz, 1H), 7.48– 7.38 (m, 5H), 7.20 (t, J = 7.5 Hz, 1H), 7.11 (t, J = 7.5 Hz, 1H), 5.32– 5.21 (m, 1H), 4.93 (s, 1H), 4.39–4.29 (m, 2H), 4.26 (s, 1H), 3.20 (dd, J =10.7, 4.0 Hz, 1H), 2.91–2.80 (m, 1H), 2.76 (d, J =15.1 Hz, 1H), 2.70–2.60 (m, 1H); 13C NMR (126 MHz, CDCl3+CD3OD)) δ 153.3, 136.6, 135.7, 134.6, 129.6, 129.0, 128.6, 126.0, 121.7, 119.0, 118.0, 111.0, 109.1, 63.0, 55.1, 49.0, 48.6, 21.2.
3.4.31. Compound 20a: N-((1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)(4-nitrophenyl)methyl)-2-(1H-indol-3-yl)ethanamine (20a)
Compound 20a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 451.20; found [M+H]+: 452.29; 1H NMR (500 MHz, CDCl3) δ 8.17 (br s, 1H), 8.09 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 8.0 Hz, 1H), 7.43 (d, J = 8.4 Hz, 2H), 7.34 (d, J = 8.0 Hz, 1H), 7.18 (t, J =7.5 Hz, 1H), 7.05 (t, J = 7.5 Hz, 1H), 7.01 (s, 1H), 5.34 (s, 1H), 4.57 (t, J= 5.1 Hz, 1H), 4.38 (dd, J =14.1, 5.0 Hz, 1H), 4.30 (dd, J = 14.1, 5.0 Hz, 1H), 3.32 (s, 3H), 3.23 (s, 3H), 3.05–2.82 (m, 4H), 2.13 (br s, 1H); 13C NMR (126 MHz, CDCl3) δ 155.8, 147.7, 145.0, 136.4, 128.7, 128.6, 127.1, 123.9, 122.2, 119.3, 118.5, 112.9, 111.3, 102.4, 56.5, 56.3, 49.2, 47.4, 25.6.
3.4.32. Compound 20b: 4-(4-nitrophenyl)-4,6,7,12,12b,13-hexahydrotetrazolo[1″,5″:4′,5′]pyrazino [1′,2′:1,2]pyrido[3,4-b]indole (20b)
Compound 20b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 387.14; found [M−H]+: 386.20; 1H NMR (500MHz, DMSO) δ 11.12 (s, 1H), 8.28 (d, J = 8.7 Hz, 2H), 7.82 (d, J =8.7 Hz, 2H), 7.42 (dd, J=13.8, 8.0 Hz, 2H), 7.12 (t, J =7.4 Hz, 1H), 7.01 (t, J =7.4 Hz, 1H), 5.39 (dd, J =12.1, 3.2 Hz, 1H), 5.36 (s, 1H), 4.57 (d, J =10.4 Hz, 1H), 4.46 (t, J = 11.4 Hz, 1H), 2.98–2.90 (m, 1H), 2.74–2.62 (m, 3H); 13C NMR (126 MHz, DMSO) δ 153.7, 148.7, 147.0, 137.3, 131.2, 131.1, 127.0, 125.0, 124.9, 122.4, 119.8, 119.0, 118.9, 112.3, 109.0, 62.8, 55.3, 49.6, 49.0, 22.1.
3.4.33. Compound 21a: N-((1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)(3,4,5-trimethoxyphenyl)methyl)-2-(1H-indol-3-yl)ethanamine (21a)
Compound 21a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 496.24; found [M−H]+: 495.29; 1H NMR (500 MHz, CDCl3) δ 8.34 (s, 1H), 7.54 (d, J =8.0 Hz, 1H), 7.34 (d, J = 8.0 Hz, 1H), 7.16 (t, J = 7.5 Hz, 1H), 7.07 (t, J = 7.5 Hz, 1H), 7.03 (s, 1H), 6.48 (s, 2H), 5.24 (s, 1H), 4.48 (t, J = 5.0 Hz, 1H), 4.37 (dd, J = 14.1, 5.0 Hz, 1H), 4.25 (dd, J = 14.1, 5.0 Hz, 1H), 3.80 (s, 3H), 3.71 (s, 6H), 3.29 (s, 3H), 3.28 (s,3H), 3.07–2.91 (m, 4H), 2.17 (s, 1H); 13C NMR (126 MHz, CDCl3) δ 156.5, 153.5, 137.7, 136.3, 133.4, 127.3, 122.2, 122.0, 119.2, 118.6, 113.2, 111.2, 104.3, 102.5, 60.7, 57.4, 56.0, 55.4, 55.2, 48.9, 47.7, 25.6.
3.4.34. Compound 21b: 4-(3,4,5-trimethoxyphenyl)-4,6,7,12,12b,13-hexahydrotetrazolo[1″,5″:4′,5′]pyrazino[1′,2′:1,2]pyrido[3,4-b]indole (21b)
Compound 21b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 432.19; found [M−H]+: 431.29; 1H NMR (500 MHz, CDCl3) δ 8.90 (s, 1H), 7.52 (d, J =7.7 Hz, 1H), 7.34 (d, J = 8.1 Hz, 1H), 7.20 (t, J = 7.5 Hz, 1H), 7.14 (t, J = 7.4 Hz, 1H), 6.69 (s, 2H), 5.02 (dd, J =12.1, 3.2 Hz, 1H), 4.70 (s, 1H), 4.29 (t, J = 11.5 Hz, 1H), 4.14 (d, J = 10.4 Hz, 1H), 3.85 (s, 3H), 3.80 (s, 6H), 3.21 (dd, J = 11.1, 4.3 Hz, 1H), 2.89–2.70 (m, 2H), 2.55–2.46 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 153.8, 153.7, 137.9, 136.8, 133.0, 129.0, 126.4, 122.4, 119.8, 118.4, 111.3, 110.3, 105.2, 64.0, 60.8, 56.1, 55.1, 49.4, 48.1, 21.7.
3.4.35. Compound 22a: N-(2-(1H-indol-3-yl)ethyl)-1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-3-(methylthio)propan-1-amine (22a)
Compound 22a was prepared according to standard procedure A, MS (ESI) m/z calculated [M]+: 404.20; found [M−H]+: 403.27; 1H NMR (500 MHz, CDCl3) δ 8.09 (s, 1H), 7.53 (d, J =8.0 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.18 (t, J = 7.5 Hz, 1H), 7.09 (t, J =7.5 Hz, 1H), 6.98 (s, 1H), 4.71 (t, J = 5.3 Hz, 1H), 4.44 (dd, J= 14.1, 5.3 Hz, 1H), 4.40–4.28 (m, 2H), 3.35 (s, 3H), 3.31 (s, 3H), 2.90 (t, J =6.5 Hz, 2H), 2.79 (t, J = 6.6 Hz, 2H), 2.58–2.40 (m, 2H), 2.11 (q, J =6.8, 2H), 2.04 (s, 3H); 13C NMR (126 MHz, CDCl3)δ 157.1, 136.2, 127.2, 121.9, 119.2, 118.5, 113.2, 111.1, 102.4, 55.3, 55.2, 51.3, 48.7, 46.9, 33.1, 30.3, 25.7, 15.3.
3.4.36. Compound 26a: methyl 2-((1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-2-methylpropyl)amino)-3-(1H-indol-3-yl)propanoate (26a)
Compound 26a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 430.23; found [M−H]+: 429.31; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.15 (s, 1H), 7.56 (d, J = 7.3 Hz, 1H), 7.40–6.90 (m, 4H), 4.77–4.70 (m, 1H), 4.36–4.21 (m, 2H), 3.89–3.71 (m, 1H), 3.57–3.42 (m, 4H), 3.36 (s, 6H), 3.19–3.08 (m, 1H), 3.07–2.91 (m, 1H), 2.17 (br s, 1H), 2.11– 1.96 (m, 1H), 0.84 (d, J = 5.6 Hz, 3H), 0.68 (d, J = 5.6 Hz, 3H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 174.4, 156.4, 136.1, 127.2, 123.0, 122.1, 119.4, 118.5, 111.3, 110.7, 102.6, 60.6, 58.4, 55.3, 51.8, 49.1, 32.0, 29.0, 19.4, 18.9, 18.7.
3.4.37. Compound 26b: methyl 4-isopropyl-4,6,7,12,12b,13-hexahydrotetrazolo[1″,5″:4′,5′]pyrazino [1′,2′:1,2]pyrido[3,4-b]indole-6-carboxylate (26b)
Compound 26b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 366.18; found [M−H]+: 365.23; 1H NMR (500 MHz, CDCl3) δ 9.14 (s, 1H), 7.54 (d, J = 7.5 Hz, 1H), 7.42 (d, J= 7.5 Hz, 1H), 7.27–7.07 (m, 2H), 5.19 (d, J=11.6 Hz, 1H), 4.89 (d, J = 9.7 Hz, 1H), 4.70 (s, 1H), 4.38 (d, J = 3.2 Hz, 1H), 4.08 (t, J= 11.2 Hz, 1H), 3.57 (s, 3H), 3.43 (d, J =15.2 Hz, 1H), 3.27–3.17 (m, 1H), 2.53–2.41 (m, 1H), 1.35 (d, J = 6.0 Hz, 3H), 0.68 (d,J = 5.8 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 172.3, 152.5, 136.8, 129.4, 126.2, 122.3, 119.5, 118.3, 118.1, 111.5, 111.4, 107.4, 61.2, 55.1, 51.8, 51.1, 30.2, 25.4, 19.1, 15.7.
3.4.38. Compound 27a: methyl 2-((1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-3-phenylpropyl) amino)-3-(1H-indol-3-yl)propanoate (27a)
Compound 27a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 492.25; found [M−H]+: 491.33; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.09 (br s, 1H), 7.55 (d, J = 7.4 Hz, 1H), 7.40–6.88 (m, 9H), 4.64–4.55 (m, 1H), 4.30–4.10 (m, 2H), 4.02 (t, J = 7.0 Hz, 1H), 3.61–3.52 (m, 4H), 3.24 (s, 6H), 3.20–3.12 (m, 1H), 3.01–2.90 (m, 1H), 2.62–2.37 (m, 2H), 2.19–1.98 (m, 3H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 174.6, 156.6, 140.8, 136.2, 128.4, 128.3, 126.0, 122.9, 122.3, 119.7, 118.5, 111.3, 111.1, 102.3, 59.4, 55.3, 53.4, 52.0, 50.7, 48.5, 34.6, 31.5, 29.3.
3.4.39. Compound 27b: methyl 4-phenethyl-4,6,7,12,12b,13-hexahydrotetrazolo[1″,5″:4′,5′]pyrazino [1′,2′:1,2]pyrido[3,4-b]indole-6-carboxylate (27b)
Compound 27b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 428.20; found [M−H]+: 427.26; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 9.00–8.87 (br s, 1H), 7.54 (d, J = 7.9 Hz, 1H), 7.39 (d, J = 7.9 Hz, 1H), 7.29–7.08 (m, 7H), 5.07–4.95 (m, 1H), 4.92–4.82 (m, 1H), 4.57–4.50 (m, 1H), 4.17 –3.97 (m, 2H), 3.54 (s, 3H), 3.46–3.34 (m, 1H), 3.25– 3.15 (m, 1H), 3.00–2.79 (m, 1H), 2.78–2.64 (m, 1H), 2.49–2.08 (m, 2H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 180.2, 172.1, 153.8, 140.8, 136.9, 129.1, 128.5, 128.5, 126.3, 126.1, 122.5, 119.8, 118.5, 111.5, 107.3, 58.5, 54.8, 54.1, 51.2, 47.0, 31.7, 29.4, 26.2.
3.4.40. Compound 28a: methyl 2-((1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-3-(methylthio)propyl) amino)-3-(1H-indol-3-yl)propanoate (28a)
Compound 28a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 462.20; found [M−H]+: 461.23; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.19 (s, 1H), 7.56 (d, J = 7.9 Hz, 1H), 7.41–7.36 (m, 1H), 7.24–7.18 (m, 1H), 7.16–7.08 (m, 1H), 6.97 (s, 1H), 4.69–4.61 (m, 1H), 4.45–4.35 (m, 1H), 4.31–4.17 (m, 2H), 3.63 (s, 3H), 3.37 (s, 3H), 3.31 (s, 3H), 3.23–3.15 (m, 1H), 3.02–2.92 (m, 1H), 2.46–2.34 (m, 2H), 2.11–2.04 (m, 2H), 1.98 (s, 3H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 174.6, 156.4, 136.1, 127.2, 122.8, 122.1, 119.5, 118.4, 111.3, 102.4, 59.2, 55.4, 54.7, 52.0, 49.9, 48.4, 32.3, 30.1, 29.3,15.1.
3.4.41. Compound 29a: methyl 2-((2-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)butan-2-yl)amino)-3-(1H-indol-3-yl)propanoate (29a)
Compound 29a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 430.23; found [M−H]+: 429.27; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.26 (s, 1H), 7.56 (d, J = 7.0 Hz, 1H), 7.39–6.99 (m, 4H), 4.92–4.62 (m, 3H), 3.71 (m, 1H), 3.51 (s, 3H), 3.43 (s, 3H), 3.35 (s, 3H), 3.14 (d, J =12.1 Hz, 1H), 3.05–2.87 (m, 1H), 2.17–1.62 (m, 3H), 1.48 (s, 3H), 0.47 (t, J = 7.8 Hz, 3H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 175.6, 158.4, 136.3, 127.1, 123.1, 122.2, 119.5, 118.3, 111.3, 110.5, 103.1, 57.4, 56.8, 55.8, 55.0, 51.9, 50.4, 32.5, 30.3, 23.9, 7.9.
3.4.42. Compound 29b: methyl 4-ethyl-4-methyl-4,6,7,12,12b,13-hexahydrotetrazolo[1″,5″:4′,5′]pyrazino[1′,2′:1,2]pyrido[3,4-b]indole-6-carboxylate (29b)
Compound 29b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 366.18; found [M−H]+: 365.23; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.57 (br s, 1H), 7.50 (t, J =7.0 Hz, 1H), 7.28–7.08 (m, 3H), 5.48 (d, J = 9.1 Hz, 1H), 5.01 (dd, J = 12.0, 2.8 Hz, 1H), 4.39 (d, J =3.3 Hz, 1H), 3.91 (td, J =12.0, 6.3 Hz, 1H), 3.58 (s, 3H), 3.27–3.13 (m, 2H), 2.05–1.95 (m, 1H), 1.87–1.76 (m, 4H), 0.98 (t, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 175.0, 156.4, 136.7,131.0, 126.5, 122.3, 119.7, 118.1, 111.3, 106.2, 60.2, 52.3, 51.9, 51.4, 47.5, 30.3, 27.3, 26.7, 10.0.
3.4.43. Compound 30a: methyl 2-((1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)cyclohexyl)amino)-3-(1H-indol-3-yl)propanoate (30a)
Compound 30a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 456.25; found [M−H]+: 455.28; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.34 (br s, 1H), 7.45 (d, J = 7.5 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 7.18 (t, J = 7.5 Hz, 1H), 7.09 (t, J = 7.5 Hz, 1H), 6.87 (s, 1H), 4.76 (t, J= 5.6 Hz, 1H), 4.46 (dd, J =14.4, 5.6 Hz, 1H), 4.09–3.98 (m, 1H), 3.49 (s, 3H), 3.42–3.35 (m, 1H), 3.32 (s, 3H), 3.29 (s, 3H), 2.99 (dd, J =14.3, 5.0 Hz, 1H), 2.87 (dd, J =14.3, 8.7 Hz, 1H), 2.31–2.15 (d, J= 13.2 Hz, 2H), 2.10 (br s, 1H), 1.78–1.53 (m, 3H), 1.50–1.11 (m, 5H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 176.0, 157.6, 136.0, 127.3, 122.6, 122.2, 119.6, 118.4, 111.2, 111.0, 102.9, 55.8, 55.7, 55.4, 55.2, 51.9, 50.1, 36.0, 35.5, 30.3, 25.1, 22.2, 21.9.
3.4.44. Compound 30b: methyl 7′,12′,12b′,13′-tetrahydro-6′H-spiro[cyclohexane-1,4′-tetrazolo[1″,5″:4′,5′]pyrazino[1′,2′:1,2]pyrido[3,4-b]indole]-6′-carboxylate (30b)
Compound 30b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 392.20; found [M−H]+: 391.24; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.51 (s, 1H), 7.50 (d, J = 8.0 Hz, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.17 (t, J = 7.5 Hz, 1H), 7.12 (t, J = 7.5 Hz, 1H), 5.30 (d, J = 9.6 Hz, 1H), 5.00 (dd, J =12.1, 3.1Hz, 1H), 4.51 (d, J = 4.5 Hz, 1H), 3.91 (t, J =11.4 Hz, 1H), 3.56 (s, 3H), 3.27 (d, J= 15.1 Hz, 1H), 3.16 (dd, J =15.1, 4.5 Hz, 1H), 2.47–2.24 (m, 2H), 2.15–2.00 (m, 2H), 1.91–1.87 (m, 1H), 1.74–1.58 (m, 3H), 1.45 (td, J =13.1, 4.1Hz, 1H), 1.28–1.15 (m, 1H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 175.2, 156.1, 136.7, 131.3, 126.5, 122.2, 119.7, 118.2, 111.4, 106.8, 60.1, 52.4, 52.0, 51.5, 48.1, 35.6, 32.3, 27.5, 25.1, 22.0, 21.8.
3.4.45. Compound 31a: methyl 2-((1-benzyl-4-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)piperidin-4-yl)amino)-3-(1H-indol-3-yl)propanoate (31a)
Compound 31a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 547.29; found [M+H]+: 546.29; 1H NMR (500 MHz, CDCl3) δ 8.22 (s, 1H), 7.48 (d, J =7.8 Hz, 1H), 7.39 (d, J = 8.1 Hz, 1H), 7.31–7.17 (m, 6H), 7.12 (t, J = 7.8 Hz, 1H), 6.90 (s, 1H), 4.74 (t, J = 5.4 Hz, 1H), 4.44 (dd, J = 14.5, 5.4 Hz, 1H), 4.19–4.01 (m, 1H), 3.51–3.39 (m, 4H), 3.32 (s, 3H), 3.26 (s, 3H), 3.19 (q, J =12.5 Hz, 2H), 3.01 (dd, J =14.2, 4.0 Hz, 1H), 2.85 (dd, J =14.2, 9.2 Hz, 1H), 2.44–2.22 (m, 4H), 2.12–1.99 (m, 2H), 1.95–1.80 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 175.5, 157.2, 138.2, 136.1, 129.0, 128.1, 127.3, 126.9, 122.8, 122.3, 119.7, 118.5, 111.3, 111.0, 103.1, 62.6, 55.7, 55.4, 55.4, 53.9, 53.4, 52.0, 50.1, 49.5, 49.0, 35.7, 30.1.
3.4.46. Compound 31b: methyl 1-benzyl-7′,12′,12b′,13′-tetrahydro-6′H-spiro[piperidine-4,4′-tetrazolo [1″,5″:4′,5′]pyrazino[1′,2′:1,2]pyrido[3,4-b]indole]-6′-carboxylate (31b)
Compound 31b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 483.24; found [M+H]+: 484.34; 1H NMR (500 MHz, CDCl3) δ 8.47 (s, 1H), 7.51 (d, J =7.5 Hz, 1H), 7.40–7.22 (m, 6H), 7.19 (t, J =7.2 Hz, 1H), 7.13 (t, J = 7.2 Hz, 1H), 5.33–5.25 (m, 1H), 5.00 (d, J = 11.5 Hz, 1H), 4.57 (d, J = 3.0 Hz, 1H), 3.93 (t, J = 11.4 Hz, 1H), 3.71 (d, J =13.0 Hz, 1H), 3.64 (d, J =13.0 Hz, 1H), 3.53 (s, 3H), 3.32 (d, J = 15.1 Hz, 1H), 3.24–3.10 (m, 3H), 2.80 (d, J= 10.5 Hz, 2H), 2.44 (t, J =10.7 Hz, 1H), 2.06–1.86 (m, 2H), 1.52 (d, J = 13.0 Hz, 1H); 13C NMR (126 MHz, CDCl3) δ 174.9, 156.1, 138.7, 136.7, 131.0, 129.2, 128.2, 126.9, 126.4, 122.2, 119.7, 118.3, 118.1, 111.4, 107.1, 99.9, 62.6, 58.4, 52.6, 49.3, 48.6, 35.4, 32.0, 27.5.
3.4.47. Compound 32a: methyl 2-(((1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)(phenyl)methyl)amino)-3-(1H-indol-3-yl)propanoate (32a)
Compound 32a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 464.22; found [M−H]+: 463.27; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.32 (s, 1H), 7.38–7.25 (m, 3H), 7.20–7.14 (m, 2H), 7.13–7.06 (m, 2H), 7.04–6.98 (m, 2H), 5.29 (s, 1H), 4.54–4.46 (m, 2H), 4.34–4.27 (m, 1H), 4.07 (dd, J = 8.5, 5.5 Hz, 1H), 3.70 (s, 3H), 3.35 (s, 3H), 3.28 (dd, J=14.2, 4.8 Hz, 1H), 3.24 (s, 3H), 3.05 (dd, J =14.4, 8.6 Hz, 1H), 2.68 (br s, 1H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 174.2, 156.5, 136.5, 136.3, 128.7, 128.4, 127.8, 127.1, 123.3, 122.1, 119.5, 118.5, 111.3, 110.4, 102.5, 59.7, 57.9, 55.4, 55.0, 52.0, 48.9, 29.1.
3.4.48. Compound 32b: methyl 4-phenyl-4,6,7,12,12b,13-hexahydrotetrazolo[1″,5″:4′,5′]pyrazino [1′,2′:1,2]pyrido[3,4-b]indole-6-carboxylate (32b)
Compound 32b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 400.17; found [M+H]+: 401.25; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.28 (s, 1H), 7.72 (d, J =7.7 Hz, 1H), 7.77–7.30 (m, 5H), 7.25–7.10 (m, 3H), 5.83 (s, 1H), 5.16 (d, J = 10.2 Hz, 1H), 5.06 (d, J =12.2 Hz, 1H), 4.34–4.22 (m, 1H), 3.89 (d, J = 4.9 Hz, 1H), 3.58 (s, 3H), 3.25 (d, J =15.4 Hz, 1H), 3.05 (3dd, J = 15.4, 5.5 Hz, 1H); 13C NMR (126 MHz, CDCl3)) (major diastereomer) δ 172.3, 154.2, 136.9, 136.5, 129.3, 129.2, 128.9, 128.6, 126.2, 122.2, 119.5, 118.1, 111.5, 107.2, 61.0, 58.1, 51.6, 51.2, 46.4, 22.6.
3.4.49. Compound 33a: methyl 2-(((4-chlorophenyl)(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)methyl) amino)-3-(1H-indol-3-yl)propanoate (33a)
Compound 33a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 498.18; found [M−H]+: 497.24; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.25 (s, 1H), 7.38–7.30 (m, 2H), 7.27–7.17 (m, 4H), 7.06–6.95 (m, 2H), 6.90 (s, 1H), 5.23 (s, 1H), 4.63–4.55 (m, 2H), 4.43–4.33 (m, 1H), 4.12–4.05 (m, 1H), 3.74 (s, 3H), 3.39 (s, 3H), 3.30–3.20 (m, 4H), 2.99 (dd, J = 14.2, 9.4 Hz, 1H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 174.1, 156.2, 136.0, 134.9, 134.3, 129.1, 128.7, 126.9, 123.3, 122.4, 119.6, 118.5, 111.3, 110.4, 102.7, 57.7, 55.7, 55.3, 54.3, 52.1, 49.0, 29.4.
3.4.50. Compound 33b: methyl 4-(4-chlorophenyl)-4,6,7,12,12b,13-hexahydrotetrazolo[1″,5″:4′,5′]pyrazino[1′,2′:1,2]pyrido[3,4-b]indole-6-carboxylate (33b)
Compound 33b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 434.13; found [M−H]+: 433.20; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.79 (s, 1H), 7.51 (d, J = 7.5 Hz, 1H), 7.40–7.28 (m, 5H), 7.21 (t, J =7.5 Hz, 1H), 7.14 (t, J = 7.5 Hz, 1H), 5.82 (s, 1H), 5.14 (d, J = 9.7 Hz, 2H), 4.21 (t, J =12.0 Hz, 1H), 3.82 (d, J = 5.0 Hz, 1H), 3.57 (s, 3H), 3.27 (d, J = 15.3 Hz, 1H), 3.04 (dd, J =15.3, 5.3 Hz, 1H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 172.2, 153.8, 136.9, 135.4, 135.3, 129.9, 129.7, 128.7, 126.2, 122.5, 119.7, 118.3, 111.5, 107.4, 60.5, 55.3, 51.9, 51.3, 50.8, 25.0.
3.4.51. Compound 34a: methyl 2-(((1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)(4-fluorophenyl) methyl)amino)-3-(1H-indol-3-yl)propanoate (34a)
Compound 11i was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 482.21; found [M−H]+: 481.28; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.20 (s, 1H), 7.39–7.32 (m, 2H), 7.30–6.91 (m, 5H), 6.73 (t,J = 8.5 Hz, 2H), 5.24 (s, 1H), 4.63–4.52 (m, 1H), 4.35 (dd, J =13.1, 3.6 Hz, 1H), 4.16– 4.04 (m, 1H), 3.73 (s, 3H), 3.55–3.50 (m, 1H), 3.32–3.23 (m, 7H), 3.00 (dd, J = 14.3, 9.2 Hz, 1H), 2.57 (br s, 1H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 174.1, 162.5 (d, J = 247.4 Hz), 156.4, 136.33, 132.1 (d, J = 3.2 Hz), δ 129.5 (d, J = 8.3 Hz), 126.9, 123.3, 122.2, 119.5, 118.5, 115.5 (d, J = 21.8 Hz), 111.3, 110.3, 102.6, 57.6, 55.6, 54.2, 52.1, 48.9, 29.0.
3.4.52. Compound 34b: methyl 4-(4-fluorophenyl)-4,6,7,12,12b,13-hexahydrotetrazolo[1″,5″:4′,5′]pyrazino[1′,2′:1,2]pyrido[3,4-b]indole-6-carboxylate (34b)
Compound 34b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 418.16; found [M−H]+: 417.24; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.93–8.72 (br s, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.37–7.28 (m, 3H), 7.20 (t, J = 7.3 Hz, 1H), 7.13 (t, J = 7.3 Hz, 1H), 7.08 (t, J =8.3 Hz, 2H), 5.82 (s, 1H), 5.12 (d, J = 9.3 Hz, 2H), 4.20 (t, J = 11.9 Hz, 1H), 3.83 (d, J = 4.7 Hz, 1H), 3.57 (s, 3H), 3.30–3.21 (m, 1H), 3.03 (dd, J =15.1, 5.1Hz, 1H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 172.3, 163.1 (d, J = 249.0 Hz), 154.0, 136.9, 132.6 (d, J = 2.9 Hz), 130.4 (d, J = 7.9 Hz), 128.8, 126.3, 122.5, 119.8, 118.3 (d, J = 18.3 Hz), 116.6, 111.5, 107.5, 60.9, 60.4, 55.3, 51.4, 51.3, 25.0.
3.4.53. Compound 35a: methyl 2-(((1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)(4-nitrophenyl)methyl) amino)-3-(1H-indol-3-yl)propanoate (35a)
Compound 35a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 509.20; found [M−H]+: 508.23; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.27 (s, 1H), 7.76 (d, J = 8.5 Hz, 2H), 7.45 (d, J = 8.6 Hz, 1H), 7.37 (t, J = 9.3 Hz, 1H), 7.24 (d, J = 8.0 Hz, 1H), 7.23–7.15 (m, 1H), 7.05 (d, J = 8.6 Hz, 2H), 6.93 (t, J =7.5 Hz, 1H), 5.32 (d, J =8.7 Hz, 1H), 4.74–4.62 (m, 2H), 4.55–4.44 (m, 1H), 4.21–4.06 (m, 1H), 3.79 (s, 3H), 3.48– 3.42 (m, 4H), 3.37–3.23 (m, 5H), 2.92 (dd, J =14.2, 10.2 Hz, 1H), 2.50 (dd, J = 8.6, 4.6 Hz, 1H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 173.9, 155.5, 147.5, 143.6, 136.4, 128.7, 126.7, 123.4, 123.0, 122.5, 119.6, 118.3, 111.5, 110.2, 102.8, 57.6, 55.7, 55.3, 54.3, 52.3, 49.0, 29.0.
3.4.54. Compound 35b: methyl 4-(4-nitrophenyl)-4,6,7,12,12b,13-hexahydrotetrazolo[1″,5″:4′,5′]pyrazino[1′,2′:1,2]pyrido[3,4-b]indole-6-carboxylate (35b)
Compound 35b was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 445.15; found [M+H]+: 446.27; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.59 (br s, 1H), 8.24 (d, J = 8.4 Hz, 2H), 7.60 (d, J = 8.4 Hz, 2H), 7.51 (d, J = 8.0 Hz, 1H), 7.34 (d, J = 8.0 Hz, 1H), 7.22 (t, J =7.6 Hz, 1H), 7.15 (t, J = 7.6 Hz, 1H), 5.99 (s, 1H), 5.22–5.15 (m, 2H), 4.29 (t, J =12.0 Hz, 1H), 3.76 (d, J = 5.3 Hz, 1H), 3.59 (s, 3H), 3.32–3.26 (m, 1H), 3.08 (dd, J = 153, 5.2 Hz, 1H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 171.9, 153.1, 148.5, 144.0, 136.9, 129.5, 128.2, 126.2, 124.7, 122.8, 120.1, 118.5, 111.4, 107.6, 60.4, 55.7, 52.1, 51.3, 50.8, 25.0.
3.4.55. Compound 36a: methyl 2-(((1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)(4-nitrophenyl)methyl) amino)-3-(thiophen-2-yl)propanoate (36a)
Compound 36a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 476.15; found [M+H]+: 477.26; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.15 (d, J = 8.5 Hz, 2H), 7.44 (d, J = 8.5 Hz, 2H), 7.21 (d, J = 4.8 Hz, 1H), 6.99–6.95 (m, 1H), 6.83 (m, 1H), 5.42 (d, J =6.3 Hz, 1H), 4.68 (t, J =5.2 Hz, 1H), 4.64–4.56 (m, 1H), 4.51 (dd, J=14.0, 4.7 Hz, 1H), 4.32 (d, J = 5.2 Hz, 1H), 3.75 (s, 3H), 3.43 (s, 3H), 3.31 (s, 3H), 3.10 (m, 1H), 2.80 (t,J = 7.0 Hz, 1H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 173.1, 155.4, 147.9, 144.2, 138.3, 129.1, 128.9, 127.0, 126.9, 126.7, 124.9, 124.8, 123.8, 102.8, 102.5, 59.9, 55.8, 55.6, 54.4, 52.3, 49.2, 33.4.
3.4.56. Compound 36b: methyl 7-(4-nitrophenyl)-5,7,12,12a-tetrahydro-4H-tetrazoleo[1,5-a]thieno [3′,2′:3,4]pyrido[1,2-d]pyrazine-5-carboxylate (36b)
Compound 15a was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 412.10; found [M+H]+: 413.12; 1H NMR (500 MHz, CDCl3) (major diastereomer) δ 8.30 (d, J = 8.5 Hz, 2H), 7.64 (d, J = 8.5 Hz, 2H), 7.29 (d, J = 5.2 Hz, 1H), 6.92 (d, J = 5.2 Hz, 1H), 6.03 (s, 1H), 5.06 (m, 2H), 4.21 (t, J = 12.3 Hz, 1H), 3.77 (d, J = 4.2 Hz, 1H), 3.67 (s, 3H), 3.34 (d, J = 16.0 Hz, 1H), 3.17 (dd, J = 16.0, 4.9 Hz, 1H); 13C NMR (126 MHz, CDCl3) (major diastereomer) δ 171.1, 152.5, 148.5, 144.0, 132.7, 130.7, 129.6, 125.3, 124.5,123.5, 60.2, 55.5, 53.3, 52.1, 51.0, 28.5.
3.4.57. Compound 37a: 1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)-N-phenethylcyclohexanamine (37a)
Compound 37a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 359.23; found [M+Na]+: 382.30; 1H NMR (500 MHz, CDCl3) δ 7.31–7.26 (m, 2H), 7.22 (d, J = 7.2 Hz, 1H), 7.12 (d, J = 7.2 Hz, 2H), 4.92 (t, J = 5.8 Hz, 1H), 4.59 (d, J = 5.8 Hz, 2H), 3.38 (s, 6H), 2.69 (t, J =6.7 Hz, 2H), 2.46 (t, J = 6.7 Hz, 2H), 2.13–2.03 (m, 2H), 1.97–1.87 (m, 2H), 1.64–1.54 (m, 2H), 1.53–1.27 (m, 4H); 13C NMR (126 MHz, CDCl3) δ 159.0, 139.8, 128.7, 128.5, 126.3, 103.1, 55.5, 55.4, 49.9, 43.3, 36.5, 34.7, 25.0, 21.4.
3.4.58. Compound 38a: N-((4-chlorophenyl)(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)methyl)-2-phenylethanamine (38a)
Compound 38a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 401.16; found [M+Na]+: 424.23; 1H NMR (500 MHz, CDCl3) δ 7.33–7.23 (m, 6H), 7.20 (t, J = 7.3 Hz, 1H), 7.16 (d, J =7.0 Hz, 2H), 5.26 (s, 1H), 4.56 (t, J = 5.4 Hz, 1H), 4.38 (dd, J =14.2, 5.4 Hz, 1H), 4.22 (dd, J =14.2, 5.4 Hz, 1H), 3.32 (s, 3H), 3.28 (s, 3H), 2.84–2.79 (m, 4H), 2.06 (br s, 1H). 13C NMR (126 MHz, CDCl3) δ 156.2, 139.3, 136.3, 134.2, 129.0, 128.9, 128.5, 128.4, 126.2, 102.5, 56.5, 56.4, 55.5, 48.7, 36.1.
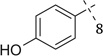
3.4.59. Compound 39a: 4-(2-(((4-chlorophenyl)(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)methyl)amino)ethyl)phenol (39a)
Compound 39a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 417.16; found [M−H]+: 416.20; 1H NMR (500 MHz, CDCl3) δ 7.30 (d, J =8.5 Hz, 2H), 7.24 (d, J = 8.5 Hz, 2H), 6.97 (d, J =8.4 Hz, 2H), 6.74 (d, J = 8.4 Hz, 2H), 5.27 (s, 1H), 4.56 (t, J = 5.4 Hz, 1H), 4.39 (dd, J = 14.2, 5.4 Hz, 1H), 4.23 (dd, J = 14.2, 5.4 Hz, 1H), 3.33 (s, 3H), 3.28 (s, 3H), 2.80–2.69 (m, 4H); 13C NMR (126 MHz, CDCl3) δ 156.3, 154.7, 136.0, 134.3, 130.5, 129.6, 129.1, 128.9, 115.6, 115.4, 102.5, 56.5, 56.4, 55.6, 55.5, 55.2, 49.0, 35.0.
3.4.60. Compound 40a: N-((4-chlorophenyl)(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)methyl)-2-(2-methoxyphenyl)ethanamine (40a)
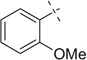
Compound 40a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 431.17; found [M+Na]+: 454.23; 1H NMR (500 MHz, CDCl3) δ 7.32–7.25 (m, 4H), 7.19 (t, J = 8.1 Hz, 1H), 7.10 (d, J =7.4 Hz, 1H), 6.86 (t, J =7.4 Hz, 1H), 6.82 (d, J = 8.6Hz,1H), 5.30 (s, 1H), 4.56 (t, J =5.5Hz,, 1H), 4.39 (dd, J =14.1, 5.6 Hz, 1H), 4.28 (dd, J=14.1, 5.4 Hz, 1H), 3.75 (s, 3H), 3.33 (s, 3H), 3.27 (s, 3H), 2.87–2.74 (m, 4H), 2.07 (br s, 1H); 13C NMR (126 MHz, CDCl3) δ 157.4, 156.2, 136.5, 134.0, 130.2, 128.9, 127.6, 127.5, 120.3, 110.2, 102.5, 102.4, 56.2, 55.3, 55.2, 55.1, 48.9, 47.2, 30.6.
3.4.61. Compound 41a: Methyl 2-((1-(1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)cyclohexyl)amino)-3-(4-methoxyphenyl)propanoate (41a)
Compound 41a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 447.25; found [M−H]+: 446.34; 1H NMR (500 MHz, CDCl3) δ 6.86 (d, J = 8.4 Hz, 2H), 6.74 (d, J = 8.4 Hz, 2H), 6.00 (br s, 1H), 4.97 (dd, J = 6.8, 4.7 Hz, 1H), 4.85 (d, J =5.6 Hz, 1H), 4.78 (dd, J =14.0, 6.8, 1H), 4.29 (dd, J =14.0, 4.7 Hz, 1H), 3.62 (s, 3H), 3.51 (s, 3H), 3.42 (s, 3H), 3.35 (s, 3H), 2.72–2.60 (m, 2H), 2.57–2.51 md, 1H), 2.38–2.29 (m, 1H), 2.19– 2.09 (m, 1H), 1.90–1.84 (m, 1H), 1.78–1.5 (m, 4H); 13C NMR (126 MHz, CDCl3) δ 178.4, 159.0, 155.0, 131.5, 127.2, 115.1, 103.6, 61.6, 56.5, 55.8, 52.1, 50.5, 49.0, 39.8, 38.0, 35.5, 25.3, 22.3, 21.0, 18.0.
3.4.62. Compound 42a: N-((1-(2,2-dimethoxyethyl)-1H-tetrazole-5-yl)(4-nitrophenyl)methyl)-2-(4-methoxyphenyl)ethanamine (42a)
Compound 42a was prepared according to standard procedure A; MS (ESI) m/z calculated [M]+: 442.20; found [M−H]+: 441.23; 1H NMR (500 MHz, CDCl3) δ 8.19 (d, J = 8.6 Hz, 2H), 7.52 (d, J = 8.6 Hz, 2H), 7.07 (d, J = 8.5 Hz, 2H), 6.82 (d, J =8.5 Hz, 2H), 5.38 (s, 1H), 4.61 (t, J = 5.4 Hz, 1H), 4.43 (dd, J = 14.1, 5.0 Hz, 1H), 4.34 (dd, J =14.1, 5.0 Hz, 1H), 3.78 (s, 3H), 3.36 (s, 3H), 3.28 (s, 3H), 2.84–2.69 (m, 4H), 2.08 (br s, 1H); 13C NMR (126 MHz, CDCl3) δ 158.1, 155.6, 147.6, 145.0, 131.0, 129.5, 128.6, 123.8, 113.8, 102.6, 56.3, 55.7, 55.5, 55.1, 49.2, 48.9, 35.1.
3.4.63. 7-(4-Methoxyphenethyl)-8-(4-nitrophenyl)-7,8-dihydrotetrazolo[1,5-a]pyrazine (C)
Compound C was prepared according to standard procedure B; MS (ESI) m/z calculated [M]+: 378.14; found [M−H]+: 377.20; 1H NMR (500 MHz, CDCl3) δ 8.21 (d, J = 8.6 Hz, 2H), 7.52 (d, J = 8.6 Hz, 2H), 6.97 (d, J = 8.4 Hz, 2H), 6.79 (d, J =8.4 Hz, 2H), 6.59 (d, J = 5.6 Hz, 1H), 6.19 (d, J =5.6 Hz, 1H), 6.03 (s, 1H), 3.78 (s, 3H), 3.43 (dt, J =14.1, 6.9 Hz, 1H), 3.35–3.23 (m, 1H), 2.87– 2.78 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 158.5, 148.1, 145.0, 144.2, 129.5, 129.1, 127.9, 127.6, 124.3, 114.2, 95.3, 58.0, 55.2, 54.7, 34.1.
Supplementary Material
Figure 3.
Compound 27b in the solid state. Key contacts and distances are highlighted.
Acknowledgments
This work was supported by Innovative Medicine Initiative European Lead Factory project (Grant agreement no. 115489) and National Institute of Health (1R01GM097082-01).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2014.12.021.
References and notes
- 1.Mullard A. Nat. Rev. Drug Discov. 2013;12:173. doi: 10.1038/nrd3956. [DOI] [PubMed] [Google Scholar]
- 2.Edwards AM, Isserlin R, Bader GD, Frye SV, Willson TM, Yu FH. Nature. 2011;470:163. doi: 10.1038/470163a. [DOI] [PubMed] [Google Scholar]
- 3.Ugi I, Heck S. Comb. Chem. High Throughput Screen. 2001;4:1. doi: 10.2174/1386207013331291. [DOI] [PubMed] [Google Scholar]
- 4.Dömling A. Chem. Rev. 2005;106:17. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 5.Dömling A, Wang W, Wang K. Chem. Rev. 2012;112:3083. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Khoury K, Chanas T, Dömling A. Org. Lett. 2012;14:5916. doi: 10.1021/ol302837h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao T, Boltjes A, Herdtweck E, Dömling A. Org. Lett. 2013;15:639. doi: 10.1021/ol303348m. [DOI] [PubMed] [Google Scholar]
- 8.Beck B, Larbig G, Mejat B, Magnin-Lachaux M, Picard A, Herdtweck E, Dömling A. Org. Lett. 2003;5:1047. doi: 10.1021/ol034077e. [DOI] [PubMed] [Google Scholar]
- 9.Boltjes A, Huang Y, van de Velde R, Rijkee L, Wolf S, Gaugler J, Lesniak K, Guzik K, Holak TA, Dömling A. ACS Comb. Sci. 2014;16:393. doi: 10.1021/co500026b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartke K. Chem. Ber. 1966;99:3163. [Google Scholar]
- 11.Liu H, Domling A. J. Org. Chem. 2009;74:6895. doi: 10.1021/jo900986z. [DOI] [PubMed] [Google Scholar]
- 12.Cao H, Liu H, Domling A. Chemistry. 2010;16:12296. doi: 10.1002/chem.201002046. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Herdtweck E, Domling A. Chem. Commun. (Camb) 2010:770. doi: 10.1039/b917660h. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Ollio S, Herdtweck E, Domling A. J. Org. Chem. 2011;76:637. doi: 10.1021/jo102058s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, William S, Herdtweck E, Botros S, Domling A. Chem. Biol. DrugDes. 2012;79:470. doi: 10.1111/j.1747-0285.2011.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury K, Sinha MK, Nagashima T, Herdtweck E, Domling A. Angew. Chem., Int. Ed. 2012;51:10280. doi: 10.1002/anie.201205366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinha MK, Khoury K, Herdtweck E, Domling A. Org. Biomol. Chem. 2013;11:4792. doi: 10.1039/c3ob40523k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha MK, Khoury K, Herdtweck E, Domling A. Chemistry. 2013;19:8048. doi: 10.1002/chem.201300962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunawan S, Hulme C. Tetrahedron Lett. 2013;54:4467. doi: 10.1016/j.tetlet.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerns EH, Di L. In: Drug-like Properties: Concepts, Structure Design and Methods. Kerns EH, Di L, editors. San Diego: Academic Press; 2008. [Google Scholar]
- 21.Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD. Med. Chem. 2002;45:2615. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 22.Kabeshov MA, Musio B, Murray PRD, Browne DL, Ley SV. Org. Lett. 2014;16:4618. doi: 10.1021/ol502201d. [DOI] [PubMed] [Google Scholar]
- 23.L’Homme C, Menard M-A, Canesi S. J. Org. Chem. 2014;79:8481. doi: 10.1021/jo501583c. [DOI] [PubMed] [Google Scholar]
- 24.Wang K-B, Di Y-T, Bao Y, Yuan C-M, Chen G, Li D-H, Bai J, He H-P, Hao X-J, Pei Y-H, Jing Y-K, Li Z-L, Hua H-M. Org. Lett. 2014;16:4028. doi: 10.1021/ol501856v. [DOI] [PubMed] [Google Scholar]
- 25.Mons E, Wanner MJ, Ingemann S, van Maarseveen JH, Hiemstra H. J. Org Chem. 2014;79:7380. doi: 10.1021/jo501099h. [DOI] [PubMed] [Google Scholar]
- 26.Painter TO, Wang L, Majumder S, Xie X-Q, Brummond KM. ACS Comb. Sci. 2011;13:166. doi: 10.1021/co100052s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kočí J, Krchnák W. J. Comb. Chem. 2009;12:168. doi: 10.1021/cc9001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Ollio S, Herdtweck E, Dömling A. J. Org. Chem. 2010;76:637. doi: 10.1021/jo102058s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauer WH, Schwarz MK. J. Chem. Inf. Comp. Sci. 2003;43:987. doi: 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
- 30.Gerber P, Müller K. J. Comp.-Aided Mol. Des. 1995;9:251. doi: 10.1007/BF00124456. [DOI] [PubMed] [Google Scholar]
- 31.Ertl P. J. Chem. Inf. Model. 2014;54:1617. doi: 10.1021/ci5001983. [DOI] [PubMed] [Google Scholar]
- 32.Galloway WRJD, Isidro-Llobet A, Spring DR. Nat. Commun. 2010;1:80. doi: 10.1038/ncomms1081. [DOI] [PubMed] [Google Scholar]
- 33.Gunawan S, Hulme C. Org. Biomol. Chem. 2013;11:6036. doi: 10.1039/c3ob40900g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunawan S, Petit J, Hulme C. ACS Comb. Sci. 2012;14:160. doi: 10.1021/co200209a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medda F, Hulme C. Tetrahedron Lett. 2012;53:5593. doi: 10.1016/j.tetlet.2011.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koes D, Khoury K, Huang Y, Wang W, Bista M, Popowicz GM, Wolf S, Holak TA, Domling A, Camacho CJ. PLoS ONE. 2012;7:e32839. doi: 10.1371/journal.pone.0032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito R, Smoot ME, Ono K, Ruscheinski J, Wang PL, Lotia S, Pico AR, Bader GD, Ideker T. Nat. Methods. 2012;9:1069. doi: 10.1038/nmeth.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.