Abstract
In the general population, obesity is associated with increased cardiovascular risk and decreased survival. In patients with end-stage renal disease (ESRD), however, an “obesity paradox” or “reverse epidemiology” (to include lipid and hypertension paradoxes) has been consistently reported, i.e. a higher body mass index (BMI) is paradoxically associated with better survival. This survival advantage of large body size is relatively consistent for hemodialysis patients across racial and regional differences, although published results are mixed for peritoneal dialysis patients.. Recent data indicate that both higher skeletal muscle mass and increased total body fat are protective, although there are mixed data on visceral (intra-abdominal) fat. The obesity paradox in ESRD is unlikely to be due to residual confounding alone and has biologic plausibility. Possible causes of the obesity paradox include protein-energy wasting and inflammation, time discrepancy among competitive risk factors (undernutrition versus overnutrition), hemodynamic stability, alteration of circulatory cytokines, sequestration of uremic toxin in adipose tissue, and endotoxin-lipoprotein interaction. The obesity paradox may have significant clinical implications in the management of ESRD patients especially if obese dialysis patients are forced to lose weight upon transplant wait-listing. Well-designed studies exploring the causes and consequences of the reverse epidemiology of cardiovascular risk factors, including the obesity paradox, among ESRD patients could provide more information on mechanisms. These could include controlled trials of nutritional and pharmacologic interventions to examine whether gain in lean body mass or even body fat can improve survival and quality of life in these patients.
Keywords: Obesity paradox, Reverse epidemiology, dialysis, visceral fat
Introduction
Patients with end-stage renal disease (ESRD) who receive maintenance dialysis therapy have a significantly higher mortality rate (about 20% per year in the United States and 10 – 15% in Europe), primarily due to cardiovascular disease (CVD) [1, 2]. Based on extrapolation of findings from the general population, treatment to reduce cardiovascular morbidity and mortality has focused on conventional risk factors, such as obesity, hypertension and hypercholesterolemia. However, survival has not improved substantially in the past 3 decades. Additional efforts have targeted other possible correlates of the high mortality associated with ESRD, such as anemia or dialysis dose. However, large clinical trials have failed to show any survival advantages of normalization of hemoglobin level [3] or increasing dialysis dose in hemodialysis (HD) [4] and peritoneal dialysis (PD) [5].
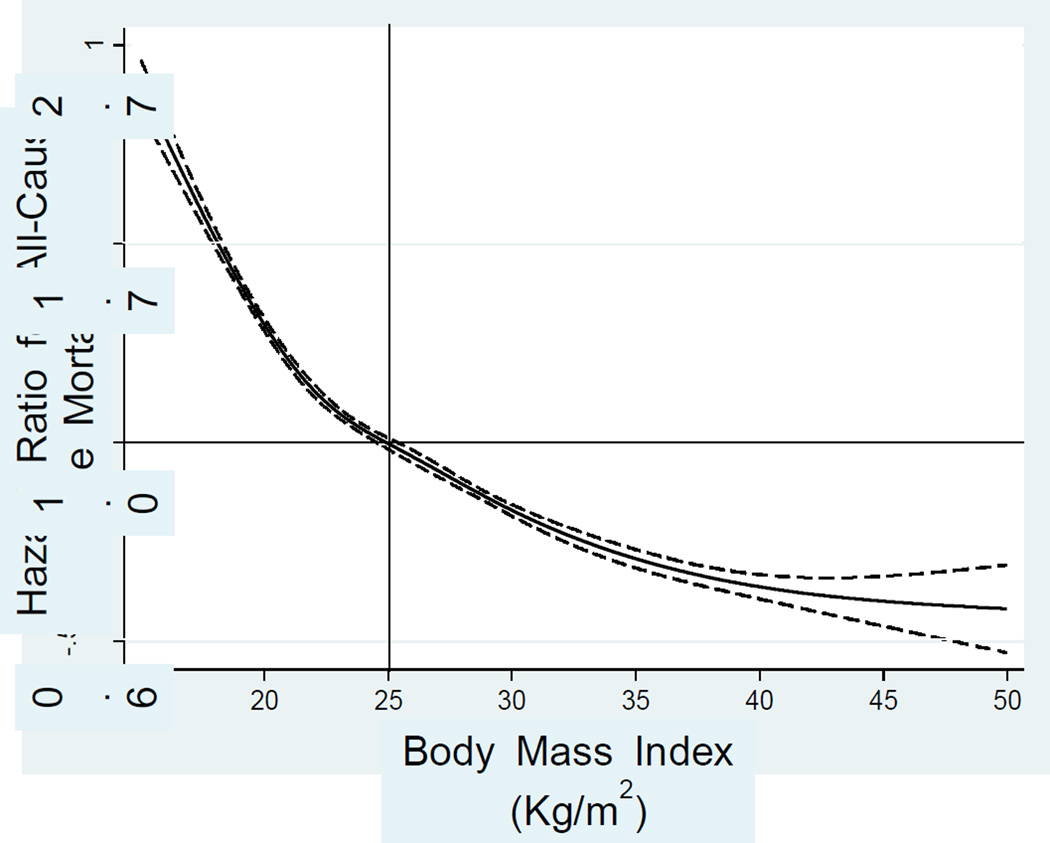
A number of epidemiologic studies with large samples of ESRD patients have indicated paradoxically inverse associations between classic risk factors for CVD and mortality [6]. In terms of obesity, worse survival has been observed with a lower body mass index (BMI), and findings have also indicated that higher values of BMI reflecting overweight or obesity seemed to be associated with better survival (Figure 1). This phenomenon has been referred to as the obesity paradox. This term may not necessarily mean that the principles of vascular pathophysiology are different in ESRD patients compared to the general population but may indicate that there are other superimposed and more dominant factors that overwhelm the traditional relation between obesity and outcomes as seen in the general population.
Figure 1.
Association of baseline BMI with mortality in 121,762 US HD patients over 5 years (July 2001–June 2006). The y-axis shows the hazard ratio for all-cause mortality over 5 years based on the spline model (log scale), adjusted for age, sex, DM, dialysis vintage, primary insurance, marital status, dialysis dose, residual renal function, hemoglobin, serum albumin, transferrin, ferritin, calcium, phosphorus, bicarbonate, peripheral white blood cell count, lymphocyte percentage, and daily protein intake. Dashed lines are 95% point-wise confidence bands.
A better understanding of the phenomenon of the obesity paradox in ESRD patients may help improve the poor outcomes in this population. In addition to earlier observations, recent studies have indicated the presence of the obesity paradox in contemporary cohorts across different races and geographic regions. In this article, the inverse association of BMI and mortality in ESRD patients and several hypotheses to it are reviewed. The distinct effects of dialysis modalities (HD versus PD) on nutritional parameters, and studies conducted exclusively in PD patients were summarized separately.
Body size and mortality in HD patients
HD patients appear to have a lower BMI than age- and sex-matched control subjects from the general population [7]. In a matched analysis comparing the lipid profiles of 285 HD patients with those of 285 non-ESRD patients matched in a one-to-one fashion on age, sex, race, and diabetes, BMI was found to be significantly lower in the HD patients than in the control subjects (26.2 ± 6.0 compared with 31.5 ± 7.8, p < 0.001) [8]. A lower BMI was consistently found to be a strong predictor of increased mortality. Unlike the general population, however, a higher BMI (overweight or obesity) was generally not associated with an increase in mortality risk [9–19]. In spite of recent advances in HD techniques and drug therapies, this phenomenon remains present in virtually all large contemporary cohorts [20–29]. Most studies have shown that the inverse association between BMI and mortality in HD patients is independent of demographics, co-morbidities and other nutritional markers, although because of methodological differences, only limited comparisons can be made . Important epidemiologic studies are summarized in Table 1. Because these are clinical studies that follow mortality prospectively in well-characterized patient populations, they are able to take into account the clinical characteristics of the patients such as severity of illness. However, the ability of this type of study to identify biological mechanisms or choose among possible explanations is inherently somewhat limited. The biological mechanisms could be investigated using other study designs, including randomized clinical trials. The first report came from the Diaphane collaborative study in France, which reported a paradoxical observation of a lack of increase in mortality with high BMI in HD patients [9]. This study included a cohort of 1,453 younger, mostly non-diabetic French HD patients followed between 1972 and 1978 in 33 French dialysis units. Leavey et al [10] confirmed the lack of association between higher BMI values and increased mortality risk in a national sample of 3,607 HD patients in the United States Renal Data System (USRDS). The mean BMI was 24.4 ± 5.3 in this study. Low BMI was independently and significantly predictive of increased mortality. With the use of time-varying effect models, it was observed that the greatest predictive value of BMI occurred early during the follow-up period but its independent predictive ability for mortality risk persisted even 5 years later. No significant interactions were identified between BMI and other demographic, co-morbid conditions or laboratory variables. Fleischmann et al.[11] reported higher survival rates for overweight and obese HD patients (BMI ≥27.5) than for patients with a normal BMI of 20.0 – <27.5. This suggested for the first time that obesity might be protective in this population. The result also showed that for every unit increase in BMI, the relative risk of mortality was reduced by 10%.
Table 1.
Summary of studies with large sample size (>1000 subjects) evaluating the association between BMI and outcomes in HD patients
| Study | Patients | F/U† (yr) |
Results |
|---|---|---|---|
| Degoulet et al, 1982 [9] | 1,453 HD |
5 | Lower BMI (<20 kg/m2) was associated with higher over-all and CV mortality |
| Leavey et al, 1998 [10] | 3,607 HD |
5 | Low BMI was independently predictive of increased mortality. |
| Fleischmann et al, 1999 [11] | 1,346 HD |
1 | Survival was significantly higher with higher BMI and lower with lower BMI. Mostly African American (89%) |
| Kopple et al, 1999 [12] | 12,965 HD |
1 | Mortality rate decreased progressively as the patients' weight-for-height increased. |
| Wolfe et al, 2000 [13] | 9,165 HD |
2 | Body weight and BMI were inversely related to mortality. |
| Leavey et al, 2001 [14] | 9,417 HD |
4 | Mortality risk decreased with increasing BMI independent of the degree of sickness |
| Port et al, 2002 [16] | 45,967 HD |
2 | The highest BMI tertile had the lowest mortality risk. |
| Lowrie et al, 2002 [17] | 43,334 HD |
1 | The log of risk decreased linearly for weight, weight- for-height, and BSA and J-shaped for weight/height and BMI. |
| Glanton et al, 2003 [18] | 151,027 HD & PD |
2 | Obesity defined as BMI ≥30 kg/m2 was associated with reduced mortality, which was stronger in African Americans. |
| Johansen et al, 2004 [19] | 418,055 HD & PD |
2 | High BMI, adiposity, and fat mass were associated with increased survival in all but Asian Americans. |
| Kalantar-Zadeh et al, 2005 [20] | 54,535 HD |
2 | Time-varying BMI and weight gain over time were associated with improved cardiovascular mortality. |
| Chazot et al, 2009 [21] | 5,592 HD |
2 | Overweight and obese patients carry a significant lower mortality risk than patients in the normal and lower BMI ranges. Low prevalence of DM (27.7%). |
| Kalantar-Zadeh et al, 2010 [22] | 121,762 HD |
2 | Higher BMI (up to 45) was incrementally associated with greater survival. Weight loss or gain over time exhibited a graded association with higher rates of mortality or survival |
| Yen et al, 2010 [23] | 959 HD |
3 | Underweight patients (BMI <18.5 kg/m2) suffer higher mortality than other groups in Asian cohort. |
| Molnar et al, 2011 [24] | 14,632 HD |
2.5 | Transplant-waitlisted patients with lower BMI and/or unintentional weight loss have higher mortality. |
| Rick et al, 2011 [25] | 109,605 HD |
2 | Survival advantage of high BMI is consistent across whites, African American and Hispanics, in which African American HD patients had the strongest association. |
| Hall et al, 2011 [26] | 21,492 HD & PD |
- | Larger body size was associated with lower mortality among Pacific Islanders, Whites and most Asians. |
| Hoogeveen et al, 2012 [27] | 1,749 HD & PD |
6 | Age-standardized mortality rate was 1.7 times higher in obese younger patients (<65 yo) than those with normal BMI. Younger patients with low or very high BMI had a substantially elevated risk for death |
| Kalantar-Zadeh et al, 2012 [28] | 121,762 HD |
2 | Lower BMI, lower muscle mass, weight and muscle loss over time were associated with higher death rates. In joint effect analysis, a decline in muscle mass estimated with serum creatinine appeared to be a stronger predictor of mortality than did weight loss. |
| Park et al, 2013 [29] | 40,818 HD |
6 | Mortality risks were lower across higher BMI, which was identical among Asian vs. white and African Americans. |
Mean or median duration of follow-up was presented according to the reports.
Thereafter, the obesity paradox was replicated in various large, nationally representative or international cohorts. Kopple et al. [12] evaluated 12,965 HD patients and found that in both men and women, the mortality rate decreased progressively as the patients' weight-for-height increased even after adjusting for clinical characteristics and laboratory measurements. These findings suggest that besides BMI other measures of body size also correlate inversely with mortality in maintenance HD patients. Port et al [16] analyzed data from 45,967 incident HD patients who started dialysis treatment between 1997 and 1998. Of the three body-size groups, the lowest BMI group had a 42% higher mortality risk compared to the highest BMI tertile. Similarly, Lowrie et al [17] analyzed survival in 43,334 HD patients treated on January, 1999 in different Kt and body-size groups defined by body weight, weight adjusted statistically for height, body surface area, weight divided by height, and BMI. The observed log-risk relationships were "reverse J-shaped" for weight divided by height and BMI. However, mortality risk was slightly increased only in patients with morbid obesity, i.e. BMI ≥34. Main effect models from that study suggested improved survival with increasing Kt and all of the size measures. The Dialysis Outcome and Practice Patterns Study (DOPPS) also helped us understand the effect of body size on mortality in ESRD patients. In 9,714 HD patients in the US and Western Europe from 1996 to 2000, an inverse BMI – mortality association was found in all subpopulations defined by continent, race, sex, severity of illness, age, smoking, and diabetic status [14]. Overall, a lower relative risk (RR) of mortality, as compared with a BMI of 23.0 – 24.9 kg/m2, was found for overweight (BMI 25.0 – 29.9 kg/m2), mild obesity (BMI 30.0 – 34.9 kg/m2) and moderate obesity (BMI 35.0 – 39.9 kg/m2) [RR 0.84, 0.73 and 0.76, respectively; all p-value <0.05]. Contrary to the investigators’ hypothesis that the obesity paradox may not exist in healthier ESRD patients, there was a survival benefit in healthy overweight patients that was even greater in obese patients. With the largest cohort of incident HD patients (n = 418,055, 4/1995 – 10/2000, USRDS), Johansen et al. [19] sought to clarify the relation between body size and outcomes, especially for patients with extremely high BMI and for alternative measures of adiposity. They found that high BMI was associated with increased survival over a 2-y average follow-up time after adjustment for confounding factors, even at extremely high BMI levels. This result was observed for whites, African Americans, and Hispanics but not for Asians. Alternative estimates of adiposity, including the Benn index and estimated fat mass, yielded similar results. Adjustments for lean body mass did not substantially alter the findings.
The obesity paradox in HD patients has been consistently observed in more contemporary cohorts. Kalantar-Zadeh et al. [20] explored the effect of both baseline BMI and change in BMI over time on cardiovascular mortality in a 2-year non-concurrent cohort of 54,535 HD patients in the US. They found that there were survival advantages of obesity for BMI cutoff values of 25, 30, and 35 across almost all strata of age, race, sex, dialysis dose, protein intake, and serum albumin level. Examining the regression slope of change in weight over time, progressively worsening weight loss was associated with poor survival, whereas weight gain showed a tendency toward decreased cardiovascular death. Chazot et al. [21] conducted a prospective observational study of 5,592 incident HD patient in Southern Europe. Patients were included between January, 2000 and September 2005 and followed for 2.0 ± 1.6 years. Notably, the prevalence of diabetes was lower (27.7%) in this cohort. The categories of baseline BMI (underweight, normal range, overweight and obese) significantly were significantly associated with survival. Relative to the normal range, estimated hazard ratios (HR) and 95% confidence intervals (CI) were 1.14 (0.96 – 1.35) for underweight, 1 (reference) for normal weight, 0.74 (0.67 – 0.9) for overweight and 0.78 (0.56 – 0.87) for obesity. Moreover, when compared to patients for whom body weight remained stable during the first year, survival was significantly lower in patients with a decrease in body weight (<-5.8% in 1 year). Molnar et al. [24] identified 14,632 HD patients waitlisted for kidney transplantation by linking the 6-year (7/2001–6/2007) national databases of a large dialysis organization and the Scientific Registry of Transplant Recipients. Dialysis patients on transplant waiting lists have indeed better survival than their nonlisted counterparts, which could be explained by the less severe comorbidities seen in waitlisted patients. Even in this selected population, each unit higher BMI was associated with a mortality HR of 0.96 (95% CI 0.95 – 0.97) in a time-dependent survival model. Compared to minimal (< ±1 kg) weight change over 6 months, those with 3 – <5 kg and ≥5 kg weight loss had mortality HRs of 1.31 (1.14 – 1.52) and 1.51 (1.30 – 1.75), respectively.
Although most of the studies showed the BMI – survival association to be independent of patients’ age, one recent study suggests that BMI may interact with age to predict long-term survival in dialysis patients. Hoogeveen et al. [27] prospectively investigated the extent to which the relation of BMI and mortality differs between younger (<65 years, n = 984)) and older (≥65 years, n = 765) dialysis patients. Baseline BMI was categorized as <20, 20 – 24 (reference), 25 – 29, and ≥30. After adjustment for age, sex, smoking, comorbidities, and treatment modality, estimated HRs (95% CI) across incremental BMI categories were 2.00 (1.30 – 3.07), 1 (reference), 0.95 (0.69 – 1.31), and 1.57 (1.08 – 2.28) for younger patients and 1.07 (0.76 – 1.52), 1 (reference), 0.88 (0.72 – 1.08), and 0.91 (0.66 – 1.27) for older patients, implying that obesity is a 1.7-fold (95% CI 1.1 to 2.9-fold) stronger risk factor in younger than older patients.
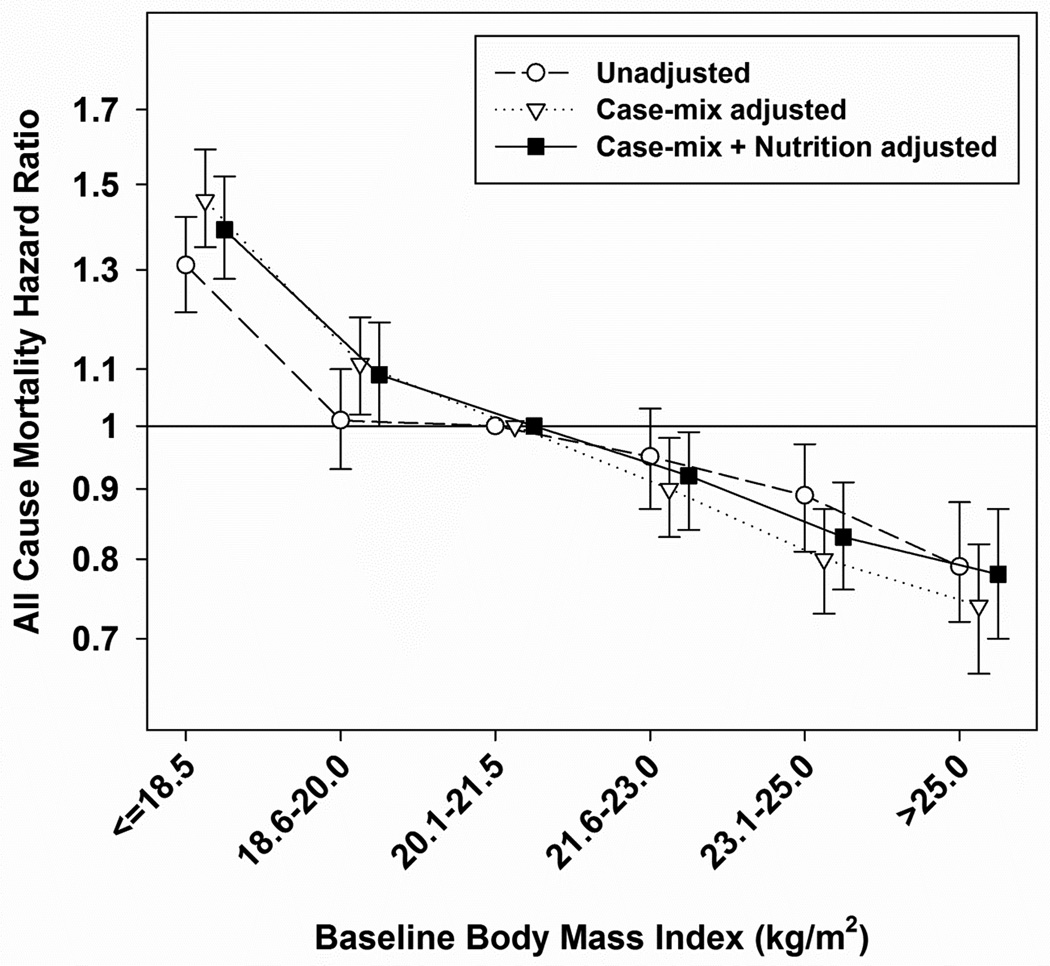
Another interesting issue is whether or not the obesity – mortality association may differ by race/ethnicity in ESRD patients. It is important to generalize the concept of the obesity paradox beyond racial and regional differences. Glanton et al. [18] performed a historical cohort study on 151,027 incident ESRD patients who had never received kidney transplantation. They found that obese patients had a higher 2-year survival after controlling for comorbidities and other potential risk factors. However, the relation was not uniform and was stronger in African Americans. Rick et al. [25] evaluated whether or not higher BMI is more strongly associated with lower mortality among blacks and Hispanics than among non-Hispanic whites. In a 6-year (2001–2007) cohort of 109,605 HD patients including 39,090 blacks, 17,417 Hispanics, and 53,098 non-Hispanic white HD outpatients, a higher BMI was associated with greater survival in all 3 racial/ethnic groups. However, Hispanic and black patients experienced higher survival gains compared with non-Hispanic whites across higher BMI categories. Hispanics and blacks in the BMI ≥40 category had the lowest estimated mortality hazard ratios (HR 0.57, 95% CI 0.49 – 0.68 and HR 0.63, 95% CI 0.58 – 0.70) compared with non-Hispanic whites in the BMI 23 – <25 group (reference). In earlier studies, Kaizu et al. [30] analyzed the association of BMI with long-term survival in 116 non-diabetic Japanese HD patients who were monitored for up to 12 years. Patients with a BMI of less than 16.9 and more than 23.0 showed lower survival relative to patients with BMI of 17.0 – 18.9. They failed to show a survival advantage of obesity in HD patients; however, patients with a BMI <16.9 were observed to have the highest risk of mortality, independent of age, gender, smoking, duration of HD, serum albumin, blood pressure and urea reduction rate. Johansen et al. [19] also reported that high BMI was not robustly associated with better survival in American Asians unlike whites, African Americans, and Hispanics. However, Yen et al. [23] examined 959 Taiwanese patients undergoing maintenance HD in a 3-year, multicenter longitudinal study. After three years, 149 (15.5%) patients had died. The mortality percentages were 21.6% in underweight (BMI <18.5), 13.0% in normal weight (BMI 18.5 – 22.9), 20.3% in overweight (BMI 23.0 – 24.9), and 15.5% in obese patients (BMI ≥25). Although they could not detect an inverse and linear relationship between BMI and mortality, BMI <18 was independently associated with increased mortality compared with BMI ≥25 in a multivariate regression model (HR 2.23, 95% CI 1.22 – 4.05). Most recently, Park et al. [29] conducted analyses to determine whether the association of body size with survival among patients undergoing long-term HD is consistent across different races, especially in East Asian versus white and African American patients. Using national data for 20,818 patients from South Korea who underwent HD from February 2001 to June 2009 and 20,000 matched patients from the US (10,000 whites and 10,000 African Americans), they found that BMI level was inversely and linearly associated with mortality even in East Asian HD patients (Figure 2); furthermore, the associations between BMI and mortality were very similar in all 3 races. They concluded that race does not modify the association of higher body size with greater survival, and the obesity paradox is a universal phenomenon irrespective of race in HD patients.
Figure 2.
Unadjusted and adjusted hazard ratios and 95% confidence intervals for all-cause mortality associated with BMI in 20,818 Korean HD patients. The model was adjusted for case-mix (age, sex, DM, dialysis history, dialysis dose, hemoglobin) and nutritional (serum albumin, and daily protein intake) covariates.
Body size and mortality in PD patients
Similar inverse associations between body size and mortality have been observed in some studies with PD patients, but a survival advantage associated with large body size seemed to be less likely in PD than HD patients [31] (Table 2), although comparisons are limited by methodological differences across studies, including the use of different BMI categories. In the Canada-USA (CANUSA) Peritoneal Dialysis Study Group, 1% lower lean body mass estimated from creatinine kinetics was associated with a 3% increase in the RR of death [32, 33]. Chung et al. [34] described a similar association between lean body mass and mortality in Korean PD patients. Snyder et al. [35] conducted a retrospective cohort study with US Medicare patients initiating dialysis between 1995 and 2000 (n = 418,021), in which 41,197 PD patients were included (11%). Among PD patients, adjusted mortality HR in the first, second, and third year were 1.45 (P < 0.05), 1.28 (P < 0.05), and 1.17 respectively for the underweight (BMI <18.5); 0.84 (P < 0.05), 0.89 (P < 0.05), and 0.98 respectively for the overweight (BMI 25.0 – 29.9); and 0.89 (P < 0.05), 0.99, and 1.00 respectively for the obese (BMI ≥30.0). These results were similar after considering switch to HD and transplantation. The investigators concluded that overweight and obese PD patients have longer survival than those with lower BMI and that this was not adequately explained by lower transplantation and technique survival rates.
Table 2.
Summary of studies with large sample size (>500 subjects) evaluating the association between BMI and outcomes in PD patients
| Study | Patients | F/U† (yr) | Results |
|---|---|---|---|
| Snyder et al, 2003 [35] | 41,197 PD |
3 | Overweight and obese PD patients have longer survival than those with lower BMI. Adjusted for transplantation rate & dialysis modality change. |
| McDonald et al, 2003 [36] | 9,679 PD |
17,973 py | Obesity was associated with increased death and technique failure, in which there was J-shaped relationship between BMI and mortality rates. Australia/New Zealand population. |
| Abbott et al, 2004 [15] | 1,662 PD |
5 | Survival for patients with BMI ≥30 kg/m2 did not differ from that for counterpart. |
| Stack et al, 2004 [37] | 17,419 PD |
1 | Risk of death was higher for patients with a BMI <20.9 kg/m2 but no survival advantage was associated with higher BMI values (reference: 23.5 – 26.1 kg/m2). |
| Ramkumar et al, 2005 [45] | 10,140 PD |
17,500 py | Both body size (BMI) and muscle mass (24-h urinary creatinine excretion) influenced survival. Patients with high BMI and normal/high muscle mass had the best survival |
| de Mutsert et al, 2009 [38] | 688 PD |
5 | Obese patients do not have a worse survival compared with patients with a normal BMI. Patients with a low BMI have a twofold increased mortality risk. |
Mean or median duration of follow-up or person-year (py) was presented according to the reports.
McDonald et al. [36] performed an analysis of all incident adult ESRD patients (n = 9,679) who underwent an episode of PD treatment in Australia or New Zealand between April, 1991 and March, 2002. In multivariate analyses, obesity (BMI ≥30 kg/m2) was independently associated with death (HR 1.36, 95% CI 1.14 – 1.54) and technique failure (HR 1.17, 95% CI 1.07 – 1.26), except among patients of New Zealand Maori/Pacific Islander origin, for whom there was no significant relationship between BMI and death. Fractional polynomial analysis modeled BMI as a continuous predictor and indicated a J-shaped relationship between BMI and patient mortality rates. Abbott et al. [15] performed a retrospective cohort study with 1,675 HD and 1,662 PD patients included in the USRDS Dialysis Morbidity and Mortality Wave II Study. They found that among PD patients, 5-year survival for patients with BMI ≥30 was 38.7% vs. 40.4% for lower BMI (P > 0.05 by log-rank test). In adjusted analysis, BMI ≥30 was associated with improved survival in HD patients (HR 0.89, 95% CI 0.81 – 0.99) but not in PD patients (HR 0.99, 95% CI 0.86 – 1.15). Results were not different when censoring at change from PD to HD. Stack et al. [37] observed similar differences in the BMI – mortality association by dialysis modality in a cohort of 134,728 new ESRD patients who were initiated on dialysis from May, 1995 to July, 1997 using data from USRDS. For HD, the adjusted RR of death was greatest for patients in the lowest BMI quintile (≤ 20.9) and lowest for patients in the highest BMI quintile (>30.0). For PD, the RR of death was higher for patients with a BMI <20.9 but no survival advantage was observed with higher BMI values compared with BMI of 23.5 – 26.1. de Mutsert et al. [38] evaluated mortality associated with obesity in the PD population of the Netherlands Co-operative Study on the Adequacy of Dialysis-2 (NECOSAD-2) cohort (n = 688). Compared with a normal BMI (18.5 – 25.0), obesity at the start of PD (BMI ≥30) was associated with a HR of 0.8 (95% CI 0.5 – 1.3). Time-dependently, this was 0.7 (0.4 – 1.2). The HR of BMI < 18.5 at the start of PD was 1.3 (95% CI 0.4 – 3.2), and time-dependently this was 2.3 (1.0 – 5.3).
Body composition and mortality in ESRD patients
BMI may not be an optimal surrogate of visceral obesity when compared to waist circumference, which better reflects intra-abdominal (truncal) fat. Indeed Postorino et al. [39] showed that surrogate measures of abdominal obesity and segmental fat distribution (waist circumference and waist/hip ratio) were stronger predictors of all-cause and cardiovascular death than BMI in 537 patients with ESRD. In this study, higher BMI was protective whereas higher waist circumference was a predictor of higher mortality.[39] Interestingly, similar “crossing curves” have also been observed in elderly patients,[40] and in kidney transplant patients.[41]
Most large epidemiologic studies used BMI to define obesity. Although BMI has been accepted as one of the most reliable anthropometric indices for obesity and has been used widely in research and for guidelines on obesity, BMI has a limited ability to differentiate adiposity from muscle mass. However, assessing fat mass or muscle mass separately is particularly difficult in large epidemiologic studies and requires elaborate tests of body composition such as dual-energy X-ray absorptiometry (DEXA) [42]. For this reason, most studies have not examined the relative contribution of fat versus muscle mass or their changes over time to the survival benefits of larger body size.
Huang et al. [43] examined the relationship between measures of fat and muscle mass and mortality in 1,709 patients from the HEMO Study (median follow-up 2.5 years). Triceps skin-fold thickness was used to assess body fat and mid-arm muscle circumference was used to assess muscle mass. In adjusted models, higher BMI and higher triceps skin-fold thickness were significantly associated with decreased hazards of mortality, while higher mid-arm muscle circumference showed a trend toward decreased mortality. Noori et al. [44] tested the hypothesis that both higher fat mass (FM) and higher lean body mass (LBM) are associated with greater survival in HD patents irrespective of sex. In 742 HD patients, they categorized men (n = 391) and women (n = 351) separately into 4 quartiles of near-infrared interactance-measured FM and LBM. After adjustment for case-mix and inflammatory markers, the highest quartiles of FM and LBM were associated with greater survival in women: estimated HRs of 0.38 (95% CI 0.20 – 0.71) and 0.34 (95% CI 0.17 – 0.67), respectively (reference: first quartile). In men, the highest quartiles of FM but not of LBM were associated with greater survival: estimated HRs of 0.51 (95% CI 0.27 – 0.96), and 1.17 (95% CI 0.60 – 2.27), respectively. Kalantar-Zadeh et al. [22] evaluated whether dry weight gain accompanied by an increase in muscle mass is associated with a survival benefit in HD patients. Serum creatinine concentration was used as a surrogate for muscle mass. Among 50,831 patients, those who gained weight and had an increase in serum creatinine concentration showed the best survival. Notably, those who lost weight but had an increased serum creatinine level had a greater observed survival rate than those who gained weight but had a decreased creatinine level. These findings may suggest that discordant muscle gain with weight loss over time confers more survival benefit than weight gain while losing muscle. The same research group also analyzed the relative role of muscle mass in the obesity paradox using a ranking analysis of joint effects in which the sums and differences of the percentiles of change for the 2 measures (weight and serum creatinine) in each patient were used as the regressors [28]. Concordant with previous observations, lower BMI, lower muscle mass, weight loss, and serum creatinine decline were associated with higher death rates. Among patients with a discordant change, persons whose weight declined but whose serum creatinine levels increased had lower death rates than those whose weight increased but whose serum creatinine level declined. These results suggest that some of the increased mortality in HD patients with lower BMIs might be explained by a lower muscle mass.
In PD patients, Ramkumar et al. [45] evaluated survival with BMI and 24-hr urinary creatinine excretion as a measure of muscle mass (n = 10,140). Patients with high BMI (≥25.0) but low muscle mass (24-hr urinary creatinine <0.64 g/day) might be considered to have high body fat mass. Compared to normal BMI – normal/high muscle mass patients, high BMI – normal/high muscle mass patients had lower hazard of cardiovascular (HR 0.88, 95% CI 0.79 – 0.97) death; high BMI patients with low muscle mass had higher hazard of cardiovascular (HR 1.21, 95% CI 1.06 – 1.39) death. The authors concluded that both body size and body composition influence survival of incident PD patients and that PD patients should be encouraged to gain muscle mass rather than fat mass. For now, the relative influence of fat mass or muscle mass on the obesity paradox is difficult to evaluate because of limited epidemiologic evidence, unclear mechanistic processes to explain the obesity paradox and lack of hard evidence from nutritional or fitness trials..
Possible Explanations for the Obesity Paradox
The obesity paradox has been also observed in other populations such as the elderly [46] and in patients with congestive heart failure [47, 48]. The obesity paradox may appear counterintuitive, because obesity is an established risk factor for cardiovascular disease and poor outcomes in the general population. Indeed, it is not only lack of an association between obesity and mortality, but the opposite direction of this relation. Hence, there must be prevailing conditions that are uniquely present in ESRD patients, as well as in similar populations with a similar risk factor reversal. These conditions may render ESRD patients more susceptible to a poor outcome when BMI is low, and in whom obesity has a favorable effect on their future well-being. Several hypothetical explanations are briefly presented here.
1) Protein-energy wasting and inflammation
Usually, malnutrition refers to abnormalities induced by an inadequate diet, whereas wasting refers to abnormalities that cannot be corrected solely by increasing dietary intake [49, 50]. To avoid confusion regarding the terms and definitions used for conditions associated with loss of muscle and fat tissue, malnutrition, and inflammation in patients with chronic kidney disease (CKD), the International Society of Renal Nutrition and Metabolism (ISRNM) expert panel recommended the term ‘protein-energy wasting (PEW)’ for loss of body protein and fuel reserves (that is, body protein and fat masses) [51]. Studies using classic measures of nutritional status indicate that wasting is frequently observed in ESRD patients [52, 53]. Recently, it has become apparent that PEW can be induced by inflammatory processes in ESRD patients [54–56]. Increased release or activation of inflammatory cytokines, such as interleukin-6 or tumor necrosis factor-α, may suppress appetite and may cause muscle proteolysis and hypoalbuminemia [57]. Moreover, loss of muscle and fat mass and inflammation are likely to increase the risk of death from cardiovascular or cerebrovascular disease, which is possibly mediated by promoting vascular endothelial damage [58–60]. On the other hand, it is possible, but not proven, that malnutrition may also predispose to inflammatory states as shown in animal models [61].
The obesity paradox in ESRD patients may be due to PEW and inflammation [6, 62]. Patients who have lower BMI or body weight may have PEW that is responsible for increased mortality. If overweight patients who have an increase in adipose tissue develop a deficiency in energy or protein intake, they would be less likely to develop frank PEW. In addition, it may be that when persons are malnourished, they are more susceptible to the ravages of inflammatory process [53, 63]. Hence, obesity may potentially attenuate the magnitude of PEW and/or inflammation, which would be favorable to ESRD patients.
The nutritional hypothesis may also explain why, in PD patients, the obesity paradox is less evident, i.e. the so-called “paradox-in-paradox” [31]. Almost all PD patients use 1.5 – 4.25% of dextrose in their peritoneal dialysate, 45% of which is estimated to be absorbed. In contrast, HD patients are exposed to 1% of dextrose in their dialysate during the 4-h, thrice-weekly dialysis. A higher caloric intake by peritoneal dialysate may attenuate a potential benefit of obesity to prevent PEW in PD patients compared with HD patients.
Another explanation similar to the PEW and inflammation hypothesis has been put forward by Lowrie et al. [64]. During inflammatory conditions or malnutrition, body protein stores are diverted to defend against inflammation and to repair injury. Thus, the increased body mass of overweight dialysis patients offers protection against or resources for responding to inflammation, infection, and subsequent CVD. This theory may explain the survival benefit of a high BMI or creatinine concentrations in ESRD patients who have low nutritional reserves.
2) Time discrepancies among competing risk factors
In the populations of most industrialized countries, overnutrition is a major risk factor for long-term cardiovascular mortality [65–67]. These are areas of the world where people have a greater life expectancy than do those in other parts of the world; hence, such populations are relatively healthy and live long enough to die of consequences of conventional cardiovascular risk factors. Studies of risk factors for cardiovascular mortality are essentially based on these long living populations. In contrast, in developing countries, which represent most of the world’s population, undernutrition is still a powerful determinant of poor clinical outcome, which leads to a shorter life expectancy [68]. Similarly, survival advantages that exist in obese ESRD patients may, in the short term, outweigh the harmful effects of obesity on CVD in the long term. Because most ESRD patients on dialysis die within 5 years of commencing dialysis treatment [69, 70], the long-term effects of obesity as a conventional risk factor on future mortality may be overwhelmed by the short-term effects of undernutrition and/or inflammation. Indeed, it may be difficult to observe mortality improvement by treating obesity in ESRD patients, who have a short life expectancy, even when such a risk reduction is beneficial in the general population that has a normal life expectancy.
3) Other potential hypotheses
Obesity may be associated with better short-term hemodynamic stability. Many ESRD patients on dialysis have some degree of heart failure and/or fluid overload. Despite having similar pulmonary capillary wedge pressure and cardiac indices, overweight and obese patients with heart failure tend to have higher systolic blood pressure values [47].Thus, obese patients might better tolerate removal of large volumes of fluid during dialysis with lower likelihood of transient hypotension. This may mitigate heightened sympathetic and rennin-angiotensin activities [71] which are associated with a poor prognosis in heart failure and fluid overload such as ESRD patients [72]. Furthermore, transient hypotension and related myocardial stunning during the HD procedure has been recently highlighted as a possible cause of the extremely high cardiovascular mortality seen in these patients [73–75]. The latter could also explain why the obesity paradox is less evident in PD patients who are more hemodynamically stable than HD patients when removing excessive fluid from the body.
Altered cytokine profiles of obese patients may play a role in conferring survival advantage to obese patients. Adipose tissue produces adiponectins, as well as soluble tumor necrosis factor alpha (TNF-α) receptors. TNF-α is elevated in heart failure and ESRD patients and may contribute to cardiac injury through its pro-apoptotic and negative inotropic effects [60, 76]. Increased soluble TNF-α receptor may play a cardioprotective role via neutralizing the adverse effect of TNF-α [77].
It is also possible that uremic toxins are more effectively sequestered when abundant adipose tissue is present. Weight loss and reduction in adipose tissue were reported to be associated with the imminent release of circulating lipophilic hexachlorobenzene and other chlorinated hydrocarbons [78]. This finding may provide one hypothesis for why body fat loss has been found to be associated with increased death risk in ESRD patients [79]. In addition, obese patients generally have higher lipid and lipoprotein concentration. Since lipopolysaccharide concentrations are increased in persons with fluid overload [80, 81] and lipoproteins bind lipopolysaccharides, it is possible that a richer pool of lipoproteins in obese patients effectively retards the deleterious effects of circulating endotoxin, i.e., inflammation and subsequent atherosclerosis [81].
4) Reverse causation
As mentioned above, it is possible that lower BMI is not a cause but a consequence of conditions that lead to poor outcomes in ESRD patients. This effect, sometimes called reverse causation, is a type of confounding that is a possible source of bias in epidemiologic studies that examine associations without considering the direction of the causal pathway. However, if clinical studies adequately took into account severity of illness and other clinical characteristics reverse causation would not explain a finding that obesity, including morbid obesity, is associated with better outcomes in ESRD patients.
5) Survival bias
ESRD patients are a very small proportion of the general population who have undergone specific processes of selection and survival; hence, they may not represent the general population. The relation between obesity and outcomes may have been modified through this patient selection. There are over 20 million patients with CKD in the US [82], but the vast majority of CKD patients will not live long enough to reach ESRD to commence maintenance dialysis [83]. Beyond severe and complex comorbid conditions, CKD itself is an independent risk factor for greater morbidity and mortality, particularly from cardiovascular and cerebrovascular diseases [83–85]. This partially explains why only a small proportion of CKD patients develop ESRD. Some CKD patients who have survived to make up the ESRD population might be “exceptional individuals” who successfully survived the conventional (traditional) risk factors such as obesity, hypertension, hypercholesterolemia, hypercreatininemia, and hyperhomocysteinemia, which are often strongly present in CKD patients. Hence, the assumption that the epidemiology of cardiovascular risk factors is the same in dialysis-dependent populations as in the general population may be flawed, because survival bias, a form of selection bias, may heavily influence the epidemiologic constellations in ESRD patients [62, 86, 87].
Remaining questions and future studies
The obesity paradox of cardiovascular mortality in ESRD patients may have indeed serious clinical and public health implications. Is the survival advantage of obesity in ESRD patients a clinically valid characteristic, or is it a statistical fallacy that needs to be ‘controlled away’? [88] Does obesity which promotes atherosclerosis and mortality in the general population, prevent cardiovascular death in ESRD patients and, if so, how? Should ESRD patients be advised to increase their nutrient intake to gain weight? In which subgroups of ESRD patients is obesity more protective than in others?
Studies presented above indicate that a higher BMI is associated with reduced mortality in these vulnerable populations. This phenomenon has been consistently observed in ESRD cohorts from the 90’s through the new millennium. Furthermore, this phenomenon does not differ by race or by region, which suggests that the concept of obesity paradox can be generalized. However, it is prudent to avoid causal inferences from such observational data until crucial clinical trial information is available. It is possible that overweight patients with ESRD suffer from more cardiovascular consequences if they survive long enough (time discrepancy hypothesis). Considering that overall mortality has not been much improved despite recent advances in dialysis techniques and drugs [70], a long-term longitudinal study is still hard to conduct.
Efforts to gain a better understanding of the existence, etiology, and components of the obesity paradox and the role of PEW and inflammation in its development in ESRD patients could provide useful information. It may be possible to identify the predominant risk factors responsible for extremely poor outcomes in ESRD patients, especially markers of PEW and inflammation. These nutritional or inflammatory states may be potentially modifiable, possibly resulting in improvement of clinical outcomes. This possibility has not yet been tested in randomized prospective clinical trials. In addition, a well-designed epidemiologic study could provide insights about the mechanisms through which PEW and/or inflammation are associated with poor outcomes in ESRD patients.[89]
Acknowledgments
Funding
KKZ and CPK are supported by the National Institute of Diabetes, Digestive and Kidney Disease grants R01-DK078106 and R01-DK096920, and KKZ is supported additionally by grant K24-DK091419 and a philanthropist grant from Mr. Harold Simmons.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and not necessarily of the Centers for Disease Control and Prevention.
Relevant Conflicts of Interests:
None declared by the authors.
References
- 1.USRDS 2011 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. [Accessed July 1, 2013]; http://www.usrds.org/atlas.asps.
- 2.Goodkin DA, Mapes DL, Held PJ. The dialysis outcomes and practice patterns study (DOPPS): how can we improve the care of hemodialysis patients? Semin Dial. 2001;14(3):157–159. doi: 10.1046/j.1525-139x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 3.Besarab A, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 4.Eknoyan G, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 5.Paniagua R, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13(5):1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, et al. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63(3):793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 7.Kopple JD. Nutritional status as a predictor of morbidity and mortality in maintenance dialysis patients. ASAIO J. 1997;43(3):246–250. [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, et al. A matched comparison of serum lipids between hemodialysis patients and nondialysis morbid controls. Hemodial Int. 2005;9(3):314–324. doi: 10.1111/j.1492-7535.2005.01147.x. [DOI] [PubMed] [Google Scholar]
- 9.Degoulet P, et al. Mortality risk factors in patients treated by chronic hemodialysis. Report of the Diaphane collaborative study. Nephron. 1982;31(2):103–110. doi: 10.1159/000182627. [DOI] [PubMed] [Google Scholar]
- 10.Leavey SF, et al. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis. 1998;31(6):997–1006. doi: 10.1053/ajkd.1998.v31.pm9631845. [DOI] [PubMed] [Google Scholar]
- 11.Fleischmann E, et al. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55(4):1560–1567. doi: 10.1046/j.1523-1755.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 12.Kopple JD, et al. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56(3):1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe RA, et al. Body size, dose of hemodialysis, and mortality. Am J Kidney Dis. 2000;35(1):80–88. doi: 10.1016/S0272-6386(00)70305-2. [DOI] [PubMed] [Google Scholar]
- 14.Leavey SF, et al. Body mass index and mortality in 'healthier' as compared with 'sicker' haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2001;16(12):2386–2394. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 15.Abbott KC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004;65(2):597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 16.Port FK, et al. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol. 2002;13(4):1061–1066. doi: 10.1681/ASN.V1341061. [DOI] [PubMed] [Google Scholar]
- 17.Lowrie EG, et al. Body size, dialysis dose and death risk relationships among hemodialysis patients. Kidney Int. 2002;62(5):1891–1897. doi: 10.1046/j.1523-1755.2002.00642.x. [DOI] [PubMed] [Google Scholar]
- 18.Glanton CW, et al. Factors associated with improved short term survival in obese end stage renal disease patients. Ann Epidemiol. 2003;13(2):136–143. doi: 10.1016/s1047-2797(02)00251-x. [DOI] [PubMed] [Google Scholar]
- 19.Johansen KL, et al. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004;80(2):324–332. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;46(3):489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Chazot C, et al. Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrol Dial Transplant. 2009;24(9):2871–2876. doi: 10.1093/ndt/gfp168. [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85(11):991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yen TH, et al. Association between body mass and mortality in maintenance hemodialysis patients. Ther Apher Dial. 2010;14(4):400–408. doi: 10.1111/j.1744-9987.2010.00818.x. [DOI] [PubMed] [Google Scholar]
- 24.Molnar MZ, et al. Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant. 2011;11(4):725–736. doi: 10.1111/j.1600-6143.2011.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricks J, et al. Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. Am J Kidney Dis. 2011;58(4):574–582. doi: 10.1053/j.ajkd.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall YN, Xu P, Chertow GM. Relationship of body size and mortality among US Asians and Pacific Islanders on dialysis. Ethn Dis. 2011;21(1):40–46. [PMC free article] [PubMed] [Google Scholar]
- 27.Hoogeveen EK, et al. Obesity and mortality risk among younger dialysis patients. Clin J Am Soc Nephrol. 2012;7(2):280–288. doi: 10.2215/CJN.05700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalantar-Zadeh K, et al. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol. 2012;175(8):793–803. doi: 10.1093/aje/kwr384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J, et al. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clin Proc. 2013;88(5):479–486. doi: 10.1016/j.mayocp.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaizu Y, et al. Overweight as another nutritional risk factor for the long-term survival of non-diabetic hemodialysis patients. Clin Nephrol. 1998;50(1):44–50. [PubMed] [Google Scholar]
- 31.Abbott KC, et al. Body mass index and peritoneal dialysis: "exceptions to the exception" in reverse epidemiology? Semin Dial. 2007;20(6):561–565. doi: 10.1111/j.1525-139X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 32.Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1996;7(2):198–207. doi: 10.1681/ASN.V72198. [DOI] [PubMed] [Google Scholar]
- 33.McCusker FX, et al. How much peritoneal dialysis is required for the maintenance of a good nutritional state? Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Kidney Int Suppl. 1996;56:S56–S61. [PubMed] [Google Scholar]
- 34.Chung SH, Lindholm B, Lee HB. Influence of initial nutritional status on continuous ambulatory peritoneal dialysis patient survival. Perit Dial Int. 2000;20(1):19–26. [PubMed] [Google Scholar]
- 35.Snyder JJ, et al. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int. 2003;64(5):1838–1844. doi: 10.1046/j.1523-1755.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 36.McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol. 2003;14(11):2894–2901. doi: 10.1097/01.asn.0000091587.55159.5f. [DOI] [PubMed] [Google Scholar]
- 37.Stack AG, Murthy BV, Molony DA. Survival differences between peritoneal dialysis and hemodialysis among "large" ESRD patients in the United States. Kidney Int. 2004;65(6):2398–2408. doi: 10.1111/j.1523-1755.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 38.de Mutsert R, et al. Is obesity associated with a survival advantage in patients starting peritoneal dialysis? Contrib Nephrol. 2009;163:124–131. doi: 10.1159/000223790. [DOI] [PubMed] [Google Scholar]
- 39.Postorino M, et al. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol. 2009;53(15):1265–1272. doi: 10.1016/j.jacc.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 40.Guallar-Castillon P, et al. BMI, waist circumference and mortality according to health status in the older adult population of Spain. Obesity (Silver Spring) 2009;17(12):2232–2238. doi: 10.1038/oby.2009.115. [DOI] [PubMed] [Google Scholar]
- 41.Kovesdy CP, et al. Body mass index, waist circumference and mortality in kidney transplant recipients. Am J Transplant. 2010;10(12):2644–2651. doi: 10.1111/j.1600-6143.2010.03330.x. [DOI] [PubMed] [Google Scholar]
- 42.Bross R, et al. Comparing body composition assessment tests in long-term hemodialysis patients. Am J Kidney Dis. 2010;55(5):885–896. doi: 10.1053/j.ajkd.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang CX, et al. Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int. 2010;77(7):624–629. doi: 10.1038/ki.2009.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noori N, et al. Survival predictability of lean and fat mass in men and women undergoing maintenance hemodialysis. Am J Clin Nutr. 2010;92(5):1060–1070. doi: 10.3945/ajcn.2010.29188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramkumar N, Pappas LM, Beddhu S. Effect of body size and body composition on survival in peritoneal dialysis patients. Perit Dial Int. 2005;25(5):461–469. [PubMed] [Google Scholar]
- 46.Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. J Am Geriatr Soc. 2001;49(7):968–979. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]
- 47.Horwich TB, et al. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38(3):789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 48.Kalantar-Zadeh K, et al. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43(8):1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 49.Mitch WE. Malnutrition: a frequent misdiagnosis for hemodialysis patients. J Clin Invest. 2002;110(4):437–439. doi: 10.1172/JCI16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stenvinkel P, Heimburger O, Lindholm B. Wasting, but not malnutrition, predicts cardiovascular mortality in end-stage renal disease. Nephrol Dial Transplant. 2004;19(9):2181–2183. doi: 10.1093/ndt/gfh296. [DOI] [PubMed] [Google Scholar]
- 51.Fouque D, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 52.Mehrotra R, Kopple JD. Nutritional management of maintenance dialysis patients: why aren't we doing better? Annu Rev Nutr. 2001;21:343–379. doi: 10.1146/annurev.nutr.21.1.343. [DOI] [PubMed] [Google Scholar]
- 53.Kalantar-Zadeh K, et al. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42(5):864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 54.Balakrishnan VS, et al. Cytokine gene polymorphisms in hemodialysis patients: association with comorbidity, functionality, and serum albumin. Kidney Int. 2004;65(4):1449–1460. doi: 10.1111/j.1523-1755.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 55.Stenvinkel P. Inflammation in end-stage renal disease--a fire that burns within. Contrib Nephrol. 2005;149:185–199. doi: 10.1159/000085525. [DOI] [PubMed] [Google Scholar]
- 56.Yao Q, Lindholm B, Stenvinkel P. Inflammation as a cause of malnutrition, atherosclerotic cardiovascular disease, and poor outcome in hemodialysis patients. Hemodial Int. 2004;8(2):118–129. doi: 10.1111/j.1492-7535.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- 57.Kalantar-Zadeh K, et al. Appetite inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004;80(2):299–307. doi: 10.1093/ajcn/80.2.299. [DOI] [PubMed] [Google Scholar]
- 58.Zimmermann J, et al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55(2):648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 59.Qureshi AR, et al. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol. 2002;(13 Suppl 1):S28–S36. [PubMed] [Google Scholar]
- 60.Kalantar-Zadeh K, et al. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transplant. 2004;19(6):1507–1519. doi: 10.1093/ndt/gfh143. [DOI] [PubMed] [Google Scholar]
- 61.Ling PR, et al. Effects of protein malnutrition on IL-6-mediated signaling in the liver and the systemic acute-phase response in rats. Am J Physiol Regul Integr Comp Physiol. 2004;287(4):R801–R808. doi: 10.1152/ajpregu.00715.2003. [DOI] [PubMed] [Google Scholar]
- 62.Kalantar-Zadeh K, et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81(3):543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 63.Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38(6):1343–1350. doi: 10.1053/ajkd.2001.29250. [DOI] [PubMed] [Google Scholar]
- 64.Lowrie EG. Acute-phase inflammatory process contributes to malnutrition, anemia, and possibly other abnormalities in dialysis patients. Am J Kidney Dis. 1998;32(6 Suppl 4):S105–S112. doi: 10.1016/s0272-6386(98)70172-6. [DOI] [PubMed] [Google Scholar]
- 65.Kushner RF. Body weight and mortality. Nutr Rev. 1993;51(5):127–136. doi: 10.1111/j.1753-4887.1993.tb03089.x. [DOI] [PubMed] [Google Scholar]
- 66.Byers T. Body weight and mortality. N Engl J Med. 1995;333(11):723–724. doi: 10.1056/NEJM199509143331109. [DOI] [PubMed] [Google Scholar]
- 67.Manson JE, et al. Body weight and mortality among women. N Engl J Med. 1995;333(11):677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 68.Collins S, Myatt M. Short-term prognosis in severe adult and adolescent malnutrition during famine: use of a simple prognostic model based on counting clinical signs. JAMA. 2000;284(5):621–626. doi: 10.1001/jama.284.5.621. [DOI] [PubMed] [Google Scholar]
- 69.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S16–S23. [PubMed] [Google Scholar]
- 70.Collins AJ, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57(1 Suppl 1)(A8):e1–e526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Weber MA, Neutel JM, Smith DH. Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. J Am Coll Cardiol. 2001;37(1):169–174. doi: 10.1016/s0735-1097(00)01103-7. [DOI] [PubMed] [Google Scholar]
- 72.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341(8):577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 73.McIntyre CW, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3(1):19–26. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McIntyre CW. Recurrent circulatory stress: the dark side of dialysis. Semin Dial. 2010;23(5):449–451. doi: 10.1111/j.1525-139X.2010.00782.x. [DOI] [PubMed] [Google Scholar]
- 75.Park J, et al. A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int. 2013 doi: 10.1038/ki.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feldman AM, et al. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000;35(3):537–544. doi: 10.1016/s0735-1097(99)00600-2. [DOI] [PubMed] [Google Scholar]
- 77.Mohamed-Ali V, et al. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277(6 Pt 1):E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 78.Jandacek RJ, et al. Effects of yo-yo diet, caloric restriction, and olestra on tissue distribution of hexachlorobenzene. Am J Physiol Gastrointest Liver Physiol. 2005;288(2):G292–G299. doi: 10.1152/ajpgi.00285.2004. [DOI] [PubMed] [Google Scholar]
- 79.Kalantar-Zadeh K, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83(2):202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 80.Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356(9233):930–933. doi: 10.1016/S0140-6736(00)02690-8. [DOI] [PubMed] [Google Scholar]
- 81.Niebauer J, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353(9167):1838–1842. doi: 10.1016/S0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 82.Jones CA, et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 1998;32(6):992–999. doi: 10.1016/s0272-6386(98)70074-5. [DOI] [PubMed] [Google Scholar]
- 83.Keith DS, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 84.Mann JF, et al. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134(8):629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 85.Ruilope LM, et al. Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. J Am Soc Nephrol. 2001;12(2):218–225. doi: 10.1681/ASN.V122218. [DOI] [PubMed] [Google Scholar]
- 86.Kalantar-Zadeh K, Kopple JD. Obesity paradox in patients on maintenance dialysis. Contrib Nephrol. 2006;151:57–69. doi: 10.1159/000095319. [DOI] [PubMed] [Google Scholar]
- 87.Kalantar-Zadeh K. Causes and consequences of the reverse epidemiology of body mass index in dialysis patients. J Ren Nutr. 2005;15(1):142–147. doi: 10.1053/j.jrn.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291(4):451–459. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 89.Kovesdy CP, Kalantar-Zadeh K. Changes in Body Weight and Subsequent Mortality: Are We Any Closer to Knowing How to Deal with Obesity in ESRD? Clin J Am Soc Nephrol. 2013 doi: 10.2215/CJN.08260813. [DOI] [PMC free article] [PubMed] [Google Scholar]