Abstract
Background:
Acute Pancreatitis (AP) is an inflammatory pathology with large regional variations in incidence and etiology
Aim:
The aim of the study was to provide a description of the epidemiologic situation of AP in Albanian population, regarding incidence, etiology and severity of the disease
Methods:
We have studied all the files of all patients with acute pancreatitis admitted at the UHC ‘Mother Theresa” during an eight year period (2005-2012).
The results:
We had 964 admissions with the diagnosis acute pancreatitis, making an incidence of 5.64 per 100 000 inhabitants per year. Mean age of patients was 54.5 ± 16.93 years old. Among risk factors, alcohol consumption was found in 382 patients (39.6%), gallstone in 362 patients (37.6%), and others in 220 patients (22.8%).
Conclusion:
The incidence of acute pancreatitis in Albania ranges from 3.6 – 5.64 new cases per 100 000 inhabitants per year, with an increasing trend during the last years. The incidence of AP among females almost doubles during 2005-2012. Alcohol consumption is the predominating etiologic factor among young males.
Keywords: Acute pancreatitis, incidence, etiology, risk factors
1. INTRODUCTION
Acute pancreatitis (AP) is a pathology with acute inflammatory manifestations, and constitutes around 3% of all inpatient cases of abdominal pain (1). Prevalence and incidence of AP cannot be established easily (2). All prevalence and incidence values of AP are based on hospital records and their accuracy is influenced by data collection methods, etiological and diagnostic approaches and social-demographic factors. Descriptive incidence ranges from 5, 4/100,000 per year in England (3, 4), to 15/100,000 per year in Germany (5), to 79.8 – 80 /100 000 per year in the U.S (6, 7, 8). In countries where AP data are available, there is an increase in AP incidence. In Scotland AP incidence was 9.4/100,000 from 1968 to 1980, but it has been found a steep growth in 31, 8/100,000 between 1985-1995 (5, 9). A similar increase is observed in other countries as well, such as England, Denmark, Sweden, Finland, Germany and the Netherlands. Recent studies show that the incidence of AP has increased during the last years (10). The increase of incidence in Northern European countries like Finland and the Netherlands is related to the increase of excessive alcohol consumption (11), but on the other hand it might be linked with better discernibility due to better and faster diagnostic methods. Etiology of AP is diverse, even though in 80% of all cases common risk factors are excessive alcohol consumption and gallstones (12). Distribution of AP etiology consists of 48% gallstones-related, 21% alcohol-related, 18% idiopathic and 5–7% other etiologies. The importance of AP is evident, despite the variation of incidence among countries. Males are more vulnerable compared to females, especially the 40–60 years old. This disparity is due to higher excessive alcohol consumption prevalence among males and because the distribution of gallstones-related AP is similar in males and females (5, 6). Females are more vulnerable to gallstones-related and hypertriglyceridemia-related AP.
Mortality rates of AP are, on average 1.3/100,000 (3, 9, 10); 55%–65% of patients develop mild AP, 20%–30% necrosis of the pancreas and 25% severe AP with complications (13, 14). Inpatient mortality is between 2%–10% (15).
In Albania there is a lack of data and publications regarding AP and incidence rates. In our daily practice, it has been found that the hospital admissions with the diagnosis of AP have increased. We carried out a study of acute pancreatitis data in Albania country. Our study is intended to describe the epidemiological situation of AP among Albanians patients, as well as to obtain data on incidence rates, etiology and severity of the disease.
2. PATIENTS AND METHODS
This is a retrospective study based on clinical records and patient admissions during an 8 year period 2005-2012 in the UHC “Mother Theresa” in Tirana.
UHC “Mother Theresa” is a tertiary health care institution and serves as a reference for acute pancreatitis, as all cases are referred to this center from all corners of Albania. Patients with AP are admitted at the gastroenterology service, surgery service and intensive care unit depending on their situation at the moment of hospital admission.
We studied all the records of patients with the diagnosis of AP upon admission. Data collected included age, gender, symptoms, alcohol and drug consumption, coexisting diseases and pathologies. Nearly all the patient had an ultrasonography on admission and during hospitalization, CT scans and/or magnetic resonance image (MRI) when possible. Only those patients fulfilling the diagnostic criteria were seen as AP.
AP was diagnosed if there were persistent clinical data for AP (abdominal pain, nausea and vomiting), and elevated serum amylases levels 3 times over the normal values (reference values of amylase lower than 110 IU/I). In those cases where amylasemia levels were not elevated, the diagnosis was based on clinical examination and imaging findings on ultrasonography and CT scan. AP diagnosis was determined only if at least two of the below listed criteria were present:
Acute pain or sensitivity in the epigastric region
Elevation of amylase level 3 times over the normal values in blood or in the ascitic fluid (if ascites present)
Imaging data of the pancreatic gland that suggest AP
Patients with recurrent AP and chronic AP were not included in the study.
Classification of acute pancreatitis was determined by: (1) Etiology (gallstones, alcohol, hyper-triglyceridemia, hypercalcemia, traumatic, autoimmune, viral etc.) And by (2) severity (mild to moderate and severe AP) based on Atlanta classification and Ranson criteria. Statistical Package for Social Sciences, version 15.0, Chicago, Illinois (SPSS) was used for analysis of the collected data.
3. RESULTS
Out of 1428 patients included in the beginning of the study based on their files, only 964 patients fulfilled the inclusion criteria of the AP during the years 2005-2012.
The total average age was 54.50 years 16.93, range 2-104 years old. Among 964 patients, 377 or 39.1% were females and 587 or 60.9% males. Male/female ratio was 1.6/1.
Figure 1 shows the incidence of AP during the years 2005-2012 as well as the incidence of AP in females and males during these years. There is a steady increase in incidence rates of AP from 2005 to 2007. After 2007 there is a slight decrease till 2008. Incidence rates increase after 2008 and lower in 2010 with slight fluctuations. From 2010 to 2012 there is an almost doubling of incidence. According to gender, incidence among males is generally stable, but among females there is a tendency of growth from 2010 to 2012, almost doubled.
Figure 1.
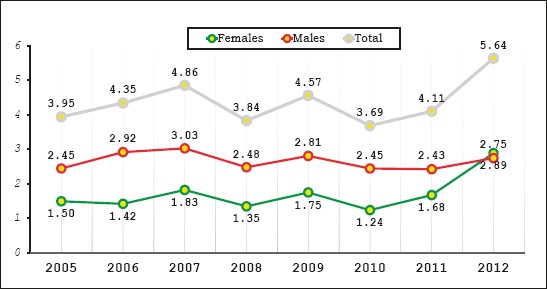
Incidence of acute pancreatitis between 2005 and 2012, by sex (per 100,000)
Incidence rates of AP in Albania range from 3, 6 – 5, 64 new cases per 100,000 inhabitants, per year. Incidence of AP is calculated based on data from our study and population data from the National Institute of Statistics of Albania (INSTAT, CENSUS 2011) (16).
Cases from urban areas were significantly more than those of rural areas. The distribution of AP cases by residence was 782 cases or 81.1% were residents of urban areas and 182 cases or 18.9% of rural areas. According to INSTAT, the resident population in urban areas was 53.5 per cent, while 46.5 per cent of the population was living in rural areas.
Table 1 represents the distribution of AP cases by risk factors and gender. Based on the risk factors, we have classified patients with AP as biliary, alcoholic, hypertriglyceridemic and other etiology, including viral, trauma, autoimmune and idiopathic causes.
Table 1.
Distribution of AP by risk factors and gender.
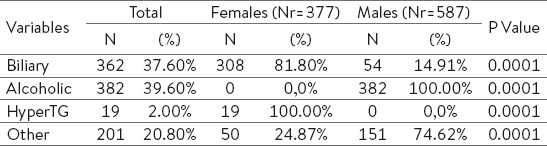
Out of 964 cases with AP, 382 (39.6%) of them reported alcohol consumption, which was greater compared with other etiological risk factors. In 362 patients (37.6%) we identified gallstones as a risk factor, hypertriglyceridemia in 19 patients (2.0%) and other etiologic factors in 201 patients (20.8%).
In males, alcoholic etiology is the most common cause of AP (382 out of 587 male patients or 65.07% had alcoholic AP), and the gallstones are more frequent in females (308 or 81.69% out of 377 female patients). Our results show that females develop more biliary pancreatitis, while men develop alcoholic pancreatitis and this is statistically significant (Kendall’s tau-b R = 0.473, P < 0,001). In Albania, males are distinctively more heavy drinkers compared to females, and this difference is expressed in the Table 1; actually we have no females identified to be alcohol consumer.
Mean age of patients with biliary etiology was 62 ± 14, while the mean age of patients with alcoholic etiology was 51 ± 14 (p < 0, 05). Mean age of female patients with biliary etiology was 59 ± 17, while the mean age of male patients with biliary etiology was 62 ± 13.
Males with alcoholic pancreatitis have younger age than females with biliary etiology who develop pancreatitis at an older age.
During the study period, 690 cases (71.57%) have been classified with mild or moderate AP. 274 patients (28.42%) have been determined to have a severe manifestation of the disease, Table 2. Markedly more man with alcoholic etiology developed severe pancreatitis P < 0.005). Overall mortality was 2.385% (23 deaths out of 964 cases). Mortality among severe cases was 8.39%.
4. DISCUSSION
The incidence of AP ranges from 10 to 80 new cases/100,000 inhabitants worldwide, but in most European studies, it has a much narrower range from 20 to 30 new cases/100,000/year (18), higher than the results of our study. Although several European studies have shown a significant increase in the incidence over the last 20 years, our study showed a slight increasing trend over an eight year period (19, 20, and 21).
Our study has also shown a pattern in the incidence of AP with regards to the etiology. The incidence of alcohol AP seems to be almost steady, with slight fluctuations over the years. On the other hand, the incidence of biliary AP is almost doubled among females.
In our clinical practice, the general impression is that we see more alcoholic than biliary pancreatitis, but our data do not support our impression. Our data clearly show increased biliary pancreatitis.
Most of the studies have demonstrated that specific etiologic factors dominate within a certain region of the country. For example, a higher proportion of cases with alcoholic AP were found in the Grampian and Highland region of Scotland (22). The different distribution of afore mentioned etiologies is not entirely clear but can be explained by the difference in alcohol consumption and the incidence of gallstone AP between North and South Europe.
Continental Europe has a particularly high incidence of alcoholic pancreatitis, not only because of high alcohol consumption per capita, but also due to the fact that alcoholic beverages in this climate contain a high percentage of alcohol, and as such may have a stronger toxic effect on the pancreas (25).
A reasonable explanation of this epidemiologic situation might be that the incidence of alcoholic AP is steady because but most of our alcoholic AP cases have developed severe AP, and mild to moderate cases are not recovering at the hospital, as the symptoms of the disease are mild. According to the Albanian Demographic and Health Survey of 2008-09, 16. 3% of men aged 15-49 are heavy drinkers and a sharp increase is observed among the group age of 35-49 from 19.5 in 24. 8 % (17).
On the other hand, the biliary AP is almost doubled. This might be because of the better diagnosis and discernibility of the gallstones and improved diagnosis of the biliary tree during the last years. During the last years, we more widely use CT scans and MRI. Imaging results of CT and MRI have enhanced the diagnostic tools of pancreatitis.
It has been demonstrated that increased prevalence of gallstones and gallbladder disease in the obese patients is accompanied with an increasing incidence of acute pancreatitis in those patients, and this might be another reason (23) and data from ADHS show an increasing prevalence of overweight and obesity during the last two decades (17).
The frequency of alcohol and biliary etiology varies between sexes, but without significant (P>0.05) and as we would expect, significantly more women than man had biliary AP, and more man than women suffered considerably from alcohol-induced AP.
Men develop alcoholic AP at a younger age 51 ± 14 years, while females develop biliary pancreatitis at an older age 59 ± 17 years, and this is statistically significant (P<0.05)
In our analysis, there were 19 patients (2%) with hypertriglyceridemia as the etiologic cause of AP. According to the literature, it is assumed that hypertriglyceridemia is the cause of AP in 4% of cases, and we are relatively close to this figure (19, 24).
Additional causes compound 20.8% of all of our cases. We had 4 patients with traumatic pancreatitis. In 4 patients we have identified a virus as the etiological factor for AP, one of them was a two year old baby boy. We had 2 patients with autoimmune pancreatitis. In 192 patients (19.81%) we were not able to identify an etiological factor for AP. These data are in agreement with other epidemiological studies, especially with the results published in our neighboring countries, Italy, Greece and Croatia, even that the incidence rates are lower (25).
5. CONCLUSION
The incidence of acute pancreatitis in Albania varies from 3.6 to 5.64 new cases per 100,000 inhabitants, which is lower than the incidence of pancreatitis in the region, but with an increasing trend. The majority of cases have an alcoholic etiology (39.6%) and the young age of male patients. The incidence of AP among females almost doubles during 2005-2012.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Banerjee A, Kaul A, Bache E, Parberry A, Doran J, Nicholson M. Multicentre audit of death from acute pancreatitis. Br J Surg. 1994;81:1541. doi: 10.1002/bjs.1800811048. [DOI] [PubMed] [Google Scholar]
- 2.Toouli J, Brooke-Smith M, Bassi C, Carr-Locke D, Telford J, Freeny P, et al. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17(Suppl):S15–39. doi: 10.1046/j.1440-1746.17.s1.2.x. [DOI] [PubMed] [Google Scholar]
- 3.Corfield A, Cooper M, Williamson R. Acute pancreatitis: a lethal disease of increasing incidence. Gut. 1985;26:724–729. doi: 10.1136/gut.26.7.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giggs J, Bourke J, Katschinski B. The epidemiology of primary acute pancreatitis in Greater Nottingham: 1969-1983. Soc Sci Med. 1988;26:79–89. doi: 10.1016/0277-9536(88)90047-0. [DOI] [PubMed] [Google Scholar]
- 5.Assmus C, Petersen M, Gottesleben F, Drüke M, Lankisch P. Epidemiology of acute pancreatitis in a defined German population. Digestion. 1996;57:A217. [Google Scholar]
- 6.Lankisch PG, Pflichthofer D, Lehnick D. Acute pancreatitis: which patient is most at risk? Pancreas. 1999;19:321–324. [PubMed] [Google Scholar]
- 7.Trapnell J. Management of the complications of acute pancreatitis. Ann R Coll Surg Engl. 1975;49:361–372. [PMC free article] [PubMed] [Google Scholar]
- 8.Go VLW, Di Magno ER, Gardner JD, Lebenthal FP, Reber HA, Scheele G. The Pancreas: Biology, Pathobiology, and Disease. 2nd ed. New York, NY, USA: Raven Press; 1993. pp. 1041–1081. [Google Scholar]
- 9.McKay C, Evans S, Sinclair M, Carter C, Imrie C. High early mortality rate from acute pancreatitis in Scotland 1984-1995. Br J Surg. 1999;86:1302–1306. doi: 10.1046/j.1365-2168.1999.01246.x. [DOI] [PubMed] [Google Scholar]
- 10.Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther. 2013;38:539–548. doi: 10.1111/apt.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaakkola M, Nordback I. Pancreatitis in Finland between 1970 and 1989. Gut. 1993;34:1255–1260. doi: 10.1136/gut.34.9.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karne S, Gorelick F. Etiopathogenesis of acute pancreatitis. Surg Clin North Am. 1999;79:699–710. doi: 10.1016/s0039-6109(05)70036-0. [DOI] [PubMed] [Google Scholar]
- 13.Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340:1412–1417. doi: 10.1056/NEJM199905063401807. [DOI] [PubMed] [Google Scholar]
- 14.Imrie CW. Classification of acute pancreatitis and the role of prognostic factors in assessing severity of disease. Schweiz Med Wochenschr. 1997;127:798–804. [PubMed] [Google Scholar]
- 15.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 16.INSTAT, National Institute of Statistics Albania. www.instat.gov.al/en/census/census-2011.aspx .
- 17.Albania Demographic and Health Survey. 2008-09. http://www.instat.gov.al/media/171075/revised_-_albania_dhs_2008-09_-_mar_05.pdf .
- 18.Gislason H, Horn A, Hoem D, et al. Acute pancreatitis in Bergen, Norway: a study on incidence, etiology and severity. Scandinavian Journal of Surgery. 2004;93(1):29–33. doi: 10.1177/145749690409300106. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell RMS, Byrne MF, Baillie J. Pancreatitis. The Lancet. 2003;361(9367):1447–1455. doi: 10.1016/s0140-6736(03)13139-x. [DOI] [PubMed] [Google Scholar]
- 20.Thomson SR, Hendry WS, McFarlane GA, Davidson AI. Epidemiology and outcome of acute pancreatitis. British Journal of Surgery. 1987;74(5):398–401. doi: 10.1002/bjs.1800740526. [DOI] [PubMed] [Google Scholar]
- 21.Eland IA, Strukenboom MJCM, Wilson JHP, Stricker BHC. Incidence and mortality of acute pancreatitis between 1985 and 1995. Scandinavian Journal of Gastroenterology. 2000;35:1110–1116. doi: 10.1080/003655200451261. [DOI] [PubMed] [Google Scholar]
- 22.Thomson HJ. Acute pancreatitis in north and northeast Scotland. Journal of the Royal College of Surgeons of Edinburgh. 1985;30(2):104–111. [PubMed] [Google Scholar]
- 23.Torgerson JS, Lindroos AK, Näslund I, Peltonen M. Gallstones, gallbladder disease, and pancreatitis: cross-sectional and 2-year data from the Swedish Obese Subjects (SOS) and SOS reference studies. Am J Gastroenterol. 2003 May;98(5):1032–1041. doi: 10.1111/j.1572-0241.2003.07429.x. [DOI] [PubMed] [Google Scholar]
- 24.Fortson MR, Freedman SN, Webster PD. Clinical assessment of hyperlipidemic pancreatitis. American Journal of Gastroenterology. 1995;90(12):2134–2139. [PubMed] [Google Scholar]
- 25.Stimac D, Mikolasevic I, Krznaric-Zrnic I, Radic M, Milic S. Epidemiology of Acute Pancreatitis in the North Adriatic Region of Croatia during the Last Ten Years, Gastroenterol Res Pract. 2013;9 doi: 10.1155/2013/956149. doi:10.1155/2013/9. [DOI] [PMC free article] [PubMed] [Google Scholar]


