Abstract
Sex ratio is defined as the proportion of males to females in a population. Subdivisions of the sex ratio are the primary (ratio at fertilization) and the secondary (ratio at birth). The expected secondary sex ratio is 0.5, but biological, environmental, or occupational variables can shift the secondary sex ratio from this expectation.
Keywords: real-time PCR
Sex ratio is defined as the proportion of males to females in a population. Subdivisions of the sex ratio are the primary (ratio at fertilization) and the secondary (ratio at birth). The expected secondary sex ratio is 0.5, but biological, environmental, or occupational variables can shift the secondary sex ratio from this expectation. Economic and medical benefits also exist for altering the secondary sex ratio; for example, breeding cattle for milk production and developing a method to suppress or eliminate pest populations, like the malaria mosquito, could benefit from manipulating the secondary sex ratio.
Mice are an ideal model to understand the biological mechanism of sex-ratio alterations because there are numerous wild-type and genetically altered strains. The secondary sex ratio of a mouse litter can be determined by visual inspection at birth or at weaning, but more sophisticated techniques are required to track the primary sex ratio as it evolves into the secondary sex ratio. Morphological distinction between males and females, for example, is possible after gonadal differentiation at embryonic day 12.5, while female embryos can be identified throughout embryogenesis via selective mating between a male with an X chromosome containing a fluorescent transgene and a wild-type female (Kobayashi et al. 2006).
Sexing mice using either a DNA- or RNA-based PCR method is a less technically demanding protocol that is readily available to most laboratories. The stability during embryogenesis of DNA compared to RNA, which undergoes dynamic changes during the maternal-to-zygotic genome transition and the development of sex-specific gene expression in preimplantation embryos (blastocysts), is one advantage of DNA-based sexing. Multiplex and simplex PCR methods are the two common approaches to reveal an individual’s genotypic sex. Multiplex PCR simultaneously amplifies a Y chromosome gene (e.g. sex-determining region on the Y, Sry) in combination with an endogenous control gene (e.g. interleukin 2, Il2) that functions as an internal control of PCR amplification to confirm that the inability to amplify the Y chromosome gene is a true negative for that gene. Simplex PCR, which can be easier to optimize and validate than multiplex PCR, uses a single primer pair to amplify two highly homologous genes that have an intron of different lengths (McFarlane et al. 2013). We used simplex quantitative real-time PCR (qPCR) with a primer pair that amplifies a portion of the X-chromosome gene lysine-specific demethylase 5, Kdm5c (synonyms: Jarid1c, Smcx) and the corresponding Y-chromosome gene Kdm5d (synonyms: Jarid1d, Smcy) (Clapcote and Roder 2005). The sizes of the fragments from the X (331 bp) and the Y (302 bp) chromosomes are distinct because the intron lengths are different on the X and Y chromosome, resulting in distinguishable melting curves.
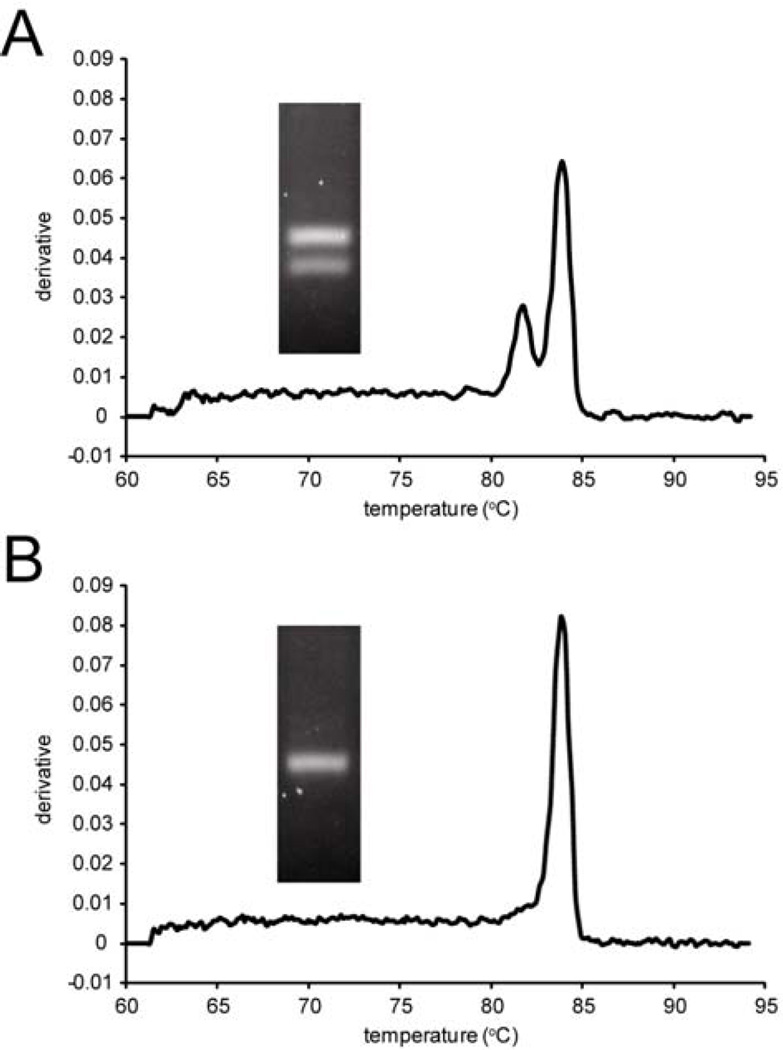
We prepared template from single blastocysts (n = 11) for sexing using conventional multiplex PCR and simplex qPCR followed by melting-curve analysis (Supplemental Materials and Methods). Two PCR bands (Sry and Il2) are observed from male blastocysts (Fig. 1A) by agarose gel electrophoresis while two peaks on the melting-curve analysis (Kdm5c/Kdm5d), whereas a single band is observed from female blastocysts (Fig. 1B) by both agarose-gel (Il2 only) and melting-curve analysis (Kdm5c). The combined data from the 11 blastocysts represent six males and five females. Our melting-curve analysis protocol thus provides a high-throughput sexing method that eliminates the time-consuming steps of running, staining, and interpreting agarose gels of PCR products. We envision that this method will contribute to high-throughput screening for factors that contribute to alterations in the primary and secondary sex ratio.
Figure 1.
Sexing of mouse blastocysts. Representative quantitative real-time PCR melting curve data (Kdm5c/Kdm5d simplex primer set) and agarose gel data (Sry and Il2 duplex primer set) for a (A) male and (B) female blastocyst.
Supplementary Material
Acknowledgments
This research was supported by grants R21-ES024527 and P30-ES013508 from the National Institute of Environmental Health Sciences. We thank Eric DeWaal (Marisa Bartolomei lab) and Lacey Luense (Shelley Berger lab) for providing blastocysts.
Grant Support:
grant sponsor: NIEHS, grant number: R21-ES024527
grant sponsor: NIEHS, grant number: P30-ES013508
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/mrd.22595]
Additional Supporting Information may be found in the online version of this article.
References
- Clapcote SJ, Roder JC. Simplex PCR assay for sex determination in mice. Biotechniques. 2005;38(5):702. doi: 10.2144/05385BM05. 704, 706. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Isotani A, Mise N, Yamamoto M, Fujihara Y, Kaseda K, Nakanishi T, Ikawa M, Hamada H, Abe K, Okabe M. Comparison of gene expression in male and female mouse blastocysts revealed imprinting of the X-linked gene, Rhox5/Pem, at preimplantation stages. Curr Biol. 2006;16(2):166–172. doi: 10.1016/j.cub.2005.11.071. [DOI] [PubMed] [Google Scholar]
- 3.McFarlane L, Truong V, Palmer JS, Wilhelm D. Novel PCR assay for determining the genetic sex of mice. Sex Dev. 2013;7(4):207–211. doi: 10.1159/000348677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.