Abstract
Functional localizer tasks allow researchers to identify brain regions in each individual's brain, using a combination of anatomical and functional constraints. In this study we compare three social cognitive localizer tasks, designed to efficiently identify regions in the “Pain Matrix”, recruited in response to a person's physical pain, and the “Theory of Mind network”, recruited in response to a person's mental states (i.e. beliefs and emotions). Participants performed three tasks: first, the verbal false-belief stories task; second, a verbal task including stories describing physical pain versus emotional suffering; and third, passively viewing a nonverbal animated movie, which included segments depicting physical pain, and beliefs and emotions. All three localizers were efficient in identifying replicable, stable networks in individual subjects. The consistency across tasks makes all three tasks viable localizers. Nevertheless, there were small reliable differences in the location of the regions and the pattern of activity within regions, hinting at more specific representations. The new localizers go beyond those currently available: first, they simultaneously identify two functional networks with no additional scan time, and second, the non-verbal task extends the populations in whom functional localizers can be applied. These localizers will be made publicly available.
Keywords: fMRI, functional localizer, empathy, pain, theory of mind
Introduction
When people read a story or watch a movie depicting another person's experiences, remarkably reliable and robust patterns of activity are elicited in the observer's brain. For example, if the protagonist is in physical pain, observers have increased activity in ‘Pain Matrix’ brain regions, including bilateral anterior insula and anterior middle cingulate cortex (AMCC; Botvinick et al., 2005; Bruneau et al., 2012; Singer et al., 2004); if the protagonist is befuddled by a false belief, observers have increased activity in ‘theory of mind’ brain regions, including bilateral temporoparietal junction (TPJ) and medial prefrontal cortex (MPFC; C. D. Frith and U. Frith, 1999; Saxe and Kanwisher, 2003). These functional profiles have been observed across thousands of participants in hundreds of neuroimaging studies utilizing dozens of different tasks (for review, Lamm et al., 2011; Schurz et al., 2014) a challenge for social cognitive neuroscience remains how to relate the results of each new study to the previous ones.
The most common approach, in social cognitive neuroscience, is to compare results via meta-analyses (Costafreda, 2009; Mar, 2011; Wager et al., 2007). For example, a researcher might run a group analysis on her own data, identify the locations of maximal differences between conditions (i.e. peaks), and then compare those locations to a “library” of previously observed peaks. If the activation in her study is close to activation previously reported for many other studies examining pain empathy, she can conclude that she has activated regions involved in processing others’ pain. The advantage of this approach is that it allows the researcher to compare her results to hundreds of prior studies simultaneously, with no extra cost or scan time. However, the disadvantage of this approach is that group analyses and meta-analyses lead to substantial spatial blurring, which translates to reduced sensitivity and under estimation of effect sizes (Nieto-Castañón and Fedorenko, 2012). Individual brains vary in both anatomy and function. Alignment of brains to a common space provides an approximate correspondence (Amunts et al., 2000; Crum et al., 2003; Tomaiuolo et al., 1999). That means that neighboring but functionally distinct brain regions may be aligned to the same place, and also that the functional loci in different individuals might be aligned to varying locations in the common space (Nieto-Castañón and Fedorenko, 2012; Saxe et al., 2006). Due to that blurring, important functional differences between neighboring regions may be impossible to detect.
An alternative way to link current and past results in support of theoretical progress is to identify functional regions in individual subjects. To use this strategy, the researcher would run her own experiment, and also a short, robust “localizer” task that identifies regions involved in e.g. physical pain perception in each individual subject. By running an individual localizer in each subject, the functional regions of interest identified are tailored to each individual's functional organization and constrained by either their anatomy or a common functional search space. In visual cognitive neuroscience, for example, almost all researchers use retinotopic mapping to identify primary visual areas (Sereno et al., 1995; Wandell et al., 2007; Warnking et al., 2002). Under some circumstances, independent localizers also allow hypotheses to be tested in a handful of “regions” instead of hundreds of thousands of voxels, thus reducing the problems of multiple comparisons and increasing the study's sensitivity.
Functional localizer tasks are already in widespread use to identify brain regions involved in a number of social cognitive processes: for example, viewing faces versus other objects, to identify regions involved in human face processing (Kanwisher et al., 1997); viewing human bodies versus other objects, to identify regions involved in human body form recognition (Downing et al., 2001); viewing biological motion versus other motion, to identify regions involved in perceiving biological motion (Grossman et al., 2000); attributing personality traits to one's self as opposed to making other judgments about the same traits, to identify regions involved in explicit self conception(Kelley et al., 2002); and reading stories about a person's mental representations versus stories about physical representations, to identify regions involved in Theory of Mind (ToM) (Dodell-Feder et al., 2011). Using these localizer tasks has allowed researchers to aggregate data across many studies (Berman et al., 2010; Dufour et al., 2013; Spunt and Adolphs, 2014) and build strong empirical and theoretical connections across different experiments (Fedorenko and Thompson-Schill, 2014; Kanwisher, 2010).
However, there are significant practical and theoretical obstacles to using localizer tasks in social cognitive neuroscience. First, the use of functional localizers is expensive, in both time and money. The cost of localizers can easily compound, too, as important scientific questions in social cognitive neuroscience often concern the relative or interacting roles of multiple regions or networks. Second, there are no established “localizer” tasks for some key cognitive functions. For example, Pain Matrix brain regions can be identified by having participants experience painful shocks in the scanner, but these experiments require special expertise and materials, and current protocols are impractically long. In addition, localizing Pain Matrix through felt pain may not target part of the Pain Matrix that are specifically sensitive to observed or perceived pain (Morrison and Downing, 2007), which might be of specific interest for social cognitive neuroscientists studying empathy, for example. Third, many existing localizer tasks require participants to follow complicated instructions, or read sophisticated verbal texts. These tasks therefore cannot be used to identify relevant networks in lower functioning participants or preverbal children. Finally, localizer tasks are a relatively blunt tool, identifying large regions involved in many aspects of a task. For example, “face localizer” tasks identify many different brain regions associated with face processing. Consistently localizing the set of brain regions allows for follow-up experiments, which could help to clarify which regions are involved in processes such as recognizing face identity versus facial expressions.
The central goal of the current study is to introduce two novel functional localizers for social cognitive neuroscience. Both of these localizer tasks are designed to circumvent some of the challenges described above. In one task, participants read short stories about characters experiencing physical pain, or emotional suffering (the E/P stories task). Participants were explicitly instructed to rate the pain or suffering the character was experiencing. In the second task, participants watched a short non-verbal animated cartoon (that was made for broad entertainment by Pixar Studios, and not designed for an experiment). During the movie, characters experience physical pain, and consider other characters’ thoughts (the Movie task). Participants passively viewed the movie, so any activity was elicited spontaneously by the events depicted.
The localizer tasks were designed to be short – each novel localizer task defined both ToM and Pain Matrix brain regions in less than 10 minutes of scanner time – and they were required to be robust and reliable; that is, activity in response to physical pain versus mental states should be observed in the same regions within individuals, and should be identifiable in the vast majority of participants. Each task allows the user to identify two distinct functional networks simultaneously: regions involved in processing of perceived pain and bodily states (e.g. insula, middle cingulate, secondary sensory regions), and regions involved in ToM (e.g. bilateral temporo-parietal junction, posterior cingulate and medial prefrontal cortex). In addition, the movie task has other advantages: it is extremely short, non-verbal, and requires no instructions, and thus could in principle be used with younger, lower-functioning, or non-native English speaking participants.
As a benchmark, we compared both tasks to the most commonly used localizer task for identifying ToM regions, the false-belief task (Dodell-Feder et al., 2011). Because the false-belief task has been used in many prior studies, it is important to validate any new localizer task against this benchmark (Spunt and Adolphs, 2014). Directly comparing the three tasks also allows us to test the similarity and stability of responses to ToM tasks across verbal versus non-verbal stimuli, across three different explicit tasks, and across a range of emotional contents.
Methods
Participants
Twenty right-handed adults (12 females, mean age 25.3, range 18-39) participated in the study for payment. All participants were fluent English speakers, with no neurological or psychiatric conditions, and had normal or corrected to normal vision. All participants gave written informed consent in accordance with the requirement of MIT's Committee on the Use of Humans as Experimental Subjects.
False-Belief Task (FB)
The publicly available false-belief (FB) localizer (Dodell-Feder et al., 2011) includes twenty stories, all of which describe an outdated representation. The false representation is either mentally held by a person (belief condition – 10 stories) or physically present on an object, such as a photo or map (photo condition – 10 stories). The stories were presented in two functional runs with 5 belief and 5 photo stories per run. Each story was presented for 10 seconds, followed by a true/false question about the either the true state of the world or the false representation (4 s). Stimuli were separated by 12 s inter-stimulus intervals, resulting in a total task runtime of 9 minutes, 4 seconds. The contrast of interest in the task is the belief condition relative to the photo condition (belief > photo).
Emotional/Physical Pain Stories Task (E/P)
In the emotional/physical pain stories task (E/P), participants read short verbal narratives describing people experiencing events that were either physically painful (P condition – 10 stories) or emotionally painful (E condition – 10 stories). The stimuli were pulled from a larger set of 24 E and 24 P stories (Bruneau et al., 2012) and represent the 10 E and 10 P stories that were rated to involve the most “emotional pain” and “physical suffering”, respectively, by an independent group of online participants. The stories were presented in two functional runs with 5 E and 5 P stories per run. Each story was presented for 12 seconds, followed by 4 seconds in which participants rated how much pain or suffering the protagonist experienced, from (1) ‘None’ to (4) ‘A lot’. Stimuli were separated by 12 s inter-stimulus intervals, resulting in a total task runtime of 9 minutes, 44 seconds. The contrasts of interest in the task are E > P (ToM network contrast) and P > E (Extended Pain Matrix contrast).
Passive Animated Movie Watching Task (MOV)
In the passive animated movie watching task (MOV), participants viewed “Partly Cloudy” (Pixar Animation Studios), an animated short film. Events in the movie were coded by the third author and 4 additional observers into 4 conditions: “Control”, in which there are no specific character related events (e.g. flying birds, wide shot of clouds; 3 events, 24 seconds total); “Social”, in which characters interact without engaging mental/emotional representations (e.g. cloud wrapping and handing over babies to storks, cloud and stork playing; 5 events, 28 seconds total); “Pain”, in which a character is undergoing a physically painful event (e.g. bitten by a crocodile, electrocuted by an electric eel; 7 events, 26 seconds total); and “Mental”, in which the viewer is led to think about the character's thoughts (e.g. a character who has just experienced pain watches others interacting happily, a character falsely believing he has been abandoned by his companion; 4 events, 44 seconds total). The total length of the movie is 5 minutes, 36 seconds; total coded time is 2 minutes, 2 seconds. The two contrasts of interest in the task are Mental > Pain (ToM network contrast) and Pain > Mental (Pain Matrix contrast). Due to technical problems, three subjects did not perform this task.
fMRI acquisition and analysis
Participants were scanned using a Siemens Magnetom Tim Trio 3T system (Siemens Solutions, Erlangen, Germany) in the Athinoula A. Martinos imaging center at the McGovern Institute for Brain Research at MIT using a 32-channel head coil. Functional images were acquired with near whole brain coverage, in 32 near axial 64×64 slices (voxel size: 3.125×3.125×3.13 mm; 0.313 mm interslice spacing, TR = 2s, TE = 30ms, flip angle = 90). High-resolution structural (anatomical) images were acquired using T1MPRAGE sequence (voxel size: 1×1×1mm).
MRI data were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/), SnPM (http://www2.warwick.ac.uk/snpm), and custom software. Each participant's data were motion corrected and registered to the first image of each run, which was registered to the first image of the first run. All functional runs were coregistered with the individual's anatomical scan and all images (functional and anatomical) were normalized to a common (Montreal Neurological Institute, EPI template) brain space, using a non-linear warping algorithm. Functional images were smoothed using a 5 mm FWHM Gaussian kernel filter.
First-level analyses were performed by applying a general linear model (GLM) to the functional data. All models included condition regressors, modeled as boxcar functions matching the onset and duration of the stimulus convolved with a canonical (double gamma) hemodynamic response function. Nuisance covariates were included in each model for run effects, and the time series’ were subjected to a high pass filter (1/128 Hz). For group effect analyses, all individual contrast images were submitted to a second level random-effects analysis and corrected for multiple comparisons at p < 0.05 using Monte-Carlo Simulations (SnPM voxel-cluster correction, with θ = 0.5 (Hayasaka and Nichols, 2004).
fROI detection rate
An effective localizer is one that is able to reliably identify functional ROIs (fROIs) within single subjects. To measure the detection rate of individual fROIS, we first created two sets of search spaces, one for the ToM network and one for the Extended Pain Matrix using Neurosynth probabilistic maps (Yarkoni et al., 2011, http://neurosynth.org). For the ToM network, we used the Reverse Inference map for ‘mentalizing’ feature, masked with anatomical definitions of 7 ROIs, which generated search spaces in dorsomedial prefrontal Cortex (DMPFC), ventromedial prefrontal cortex (VMPFC), precuneus (PC), left/right temporoparietal junction (L/RTPJ), and left/right anterior superior semporal sulcus (L/RASTS). For the Extended Pain Matrix, we used the Reverse Inference Map for the ‘pain’ feature masked with 5 anatomical ROIs for areas of the Pain Matrix that have been implicated in both felt and perceived pain, which generated search spaces in anterior middle cingulate cortex (AMCC), left/right insula (L/RIns), and left/right secondary sensory cortex (L/RSII).
Individual subjects’ T-maps were first masked by the pre-defined search spaces and then thresholded at p < 0.001 for the FB and E/P tasks; a more lenient threshold of p < 0.005 was used for the MOV task, since the overall task was shorter and there were fewer events per condition.
To compare the efficacy of the localizers under different ROI picking procedures, we used two common fROI picking procedures and applied them to the first-level T-maps generated by each of the contrasts of interest. In the first picking procedure, all supra-threshold voxels in each of the search spaces were picked as the fROI, without any contiguity constraint (as in Julian et al., 2012). In the second procedure, in each of the search spaces, the cluster with highest T-value and 10 or more contiguous voxels was identified and all supra-threshold voxels in that cluster within a 9mm sphere were picked as the fROI (as in Kuhl et al., 2010; Zaki et al., 2011). For brevity and because the results are very similar across both methods, all the results of this and subsequent analyses use the non-contiguous voxels method; for results on the contiguous 9mm sphere method, see Supplementary Materials.
Task generalizability
To compare generalizability of fROIs across tasks, we identified individual fROIs in one task, and used them as independent localizers to probe for activity in another task. Specifically, we picked fROIs using the verbal tasks (FB and E/P) as localizers and tested whether those voxels were also sensitive to the condition differences in the MOV task despite the differences in nature of contrasts and stimuli. We extracted the beta values for all the MOV conditions and tested if the response to Mental condition in ToM brain regions is significantly higher than to Pain condition. Conversely in the Pain Matrix, we tested if the response to Pain condition significantly higher than to Mental. The statistical testing was done with a t-test with a significance threshold of p < 0.0071 for ToM brain regions (Bonferroni corrected for 7 ROIs), and p < 0.01 for Pain Matrix ROIs (Bonferroni corrected for 5 ROIs).
Overlap analysis
To determine the extent to which the tasks elicited overlapping patterns of activation within individual subjects, we compared the number of voxels showing a significant response in each task (i.e. the conjunction across tasks) to the number of voxels showing a significant response across runs, within a task (i.e. a measure of test-retest reliability, TRR) – this allowed us to ask how much the two tasks overlap relative to the maximum observable overlap, given the noise in the measurement. This analysis (and all analyses that require two runs for cross-validation) was only performed on the FB and E/P tasks, for which we had two runs (10 trials per condition) per participant.
For this overlap analysis, we applied the two fROI picking procedures (contiguous and non-contiguous) to the individual first-level analysis maps of each run separately. This procedure allowed us to match the statistical power of the maps in each of the voxel sets. TRR voxels were defined as the conjunction between the union of voxels responsive (p < 0.001) to either task in the first and second run:
The between-task overlap (TO) was defined as the conjunction of the voxels that were responsive (p < 0.001) to both tasks:
This allowed us to quantify the across-task overlap against a measurement of test-retest reliability:
Location of fROIs
To determine whether the spatial relations between tasks were stable within participants, we calculated the average x, y and z coordinates across all active voxels in each fROIs (for both fROI picking methods separately), per subject per task. We then used a two-tailed t-test on the mean individual activation in each coordinate to identify systematic differences in activation across individuals, between tasks (e.g. how close the average z coordinate of one functional region as identified by the FB task is to the average z coordinate of that functional region as identified by the E/P task). The statistical threshold for significance was set to p < 0.0024 (Bonferroni corrected accounting for 7 ROIs and 3 directions, as family-wise errors). Trends (0.0024 < p < 0.05) that did not survive this conservative correction for multiple comparisons are also reported.
Spatial patterns
A complementary spatial distribution analysis using multi-voxel pattern analysis (MVPA) was performed to examine whether the different tasks elicit stable spatial patterns inside ROIs. If the different tasks activate the same locations, there could still be systematic differences in activity that is not driven by the concentration of task responsive voxels within an ROI (the measurement used to pick voxels in that analysis is a threshold on the p value of a voxel's fit with the model), but instead by the spatial distribution of task responsivity (as measured by the contrast of beta values) within the ROI. In order to test for such differences, we extracted the contrast responses per run to the FB and E/P tasks from all the voxels in each of the search spaces.. following Haxby et al., 2001). We then calculated the correlation between the spatial patterns (i.e. response of all voxels in an ROI) in the first run of each task to the spatial patterns in the second run of both task's contrasts. The results were then z-scored using fisher transformation. The within task correlations (correlation between first and second run of each task) were averaged across task, as were the between task correlations (correlations between first run of FB and second run of E/P and vice-versa). The average within-task and between-task z scores were calculated for each individual, and then a paired-samples one tailed t-test (Bonferroni corrected for 7 ROIs) was used to identify reliably higher within- than across-task correlations.
Localizer choice effect
Finally, we examined the effect of choosing the FB versus E/P localizer tasks for subsequent analysis of the MOV activity. To do this, we used the beta values extracted from MOV from the fROIs defined by FB and E/P in the generalizability analysis, and examined activity across all conditions. We ran a mixed model effect with subjects as a random variable, and localizer (FB or E/P) and condition (Mental, Pain, Social or Control) as fixed variables. We also conducted paired-samples t-tests to identify effects of fROI definition on specific conditions. All the tests were Bonferroni corrected for multiple comparisons.
Results
Whole brain analysis
Whole brain analyses were used to determine the general extent of activity generated by each of the localizer tasks, and to visualize gross overlap across tasks. Whole brain analysis results of the respective ToM contrasts across each of the 3 tasks showed reliable recruitment of the ToM network (bilateral middle temporal lobes extending up through the STS to the TPJ, PC, VMPFC, and DMPFC; Figure 1a-c, Table 1). These results replicate previous studies using the false-belief (Dodell-Feder et al., 2011; Saxe and Kanwisher, 2003) and the E/P stories task (Bruneau et al., 2012; 2013; 2015) and extend the findings to the novel MOV task. Figure 1d shows the extent of ToM overlap across all three tasks.
Figure 1.
Whole brain response (p<0.05 corrected) to the different ToM contrasts (a) False-Belief task: Belief>Photo; (b) Emotional/Physical Pain stories: Emotional>Physical; (c) Passive Animated Movie Watching Task: Mental>Pain; (d) overlap in additive color scheme corresponding to the colors in panes (a)-(c).
Abbreviations: D/VMPFC - Dorsal/Venrtal Medial Prefrontal Cortex; L/RSTS - Left/Right Superior Temporal Sulcus; L/RTPJ - Left/Right Temopro-parietal Junction; PC - Precuneus.
Table 1.
Brain regions active (p<0.05 corrected) for the ToM contrast in the 3 different tasks. Showing brain regions, cluster extent, local peaks in MNI, peak (pseudo) t value.
| False-Belief Task | Emotional/Physical Pain stories task | Movie Watching Task | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster | Region | n voxels | x | y | z | Peak t | n voxels | x | y | z | Peak t | n voxels | x | y | z | Peak t |
| ToM network regions | ||||||||||||||||
| 1 | Precuneus | 2775 | 0 | −52 | 34 | 10.14 | 3095 | −2 | −56 | 32 | 13.69 | 5494 | 14 | −58 | 26 | 7.50 |
| 2 | Dorsal Medial Prefrontal Cortex | 1549 | −6 | 54 | 22 | 6.25 | cluster 3 | 2 | 54 | 10 | 6.24 | cluster 11 | −10 | 56 | 32 | 5.29 |
| 3 | Ventral Medial Prefrontal Cortex | 383 | 4 | 46 | −16 | 6.07 | 4297 | 0 | 38 | −18 | 12.48 | cluster 11 | 2 | 54 | −12 | 5.02 |
| 4 | R Temporoparietal Junction | cluster 6 | 50 | −54 | 22 | 8-42 | 1396 | 50 | −56 | 26 | 10.06 | duster 16 | 52 | −56 | 30 | 5.19 |
| 5 | R Superior Temporal Sulcus | cluster 6 | 50 | −18 | −12 | 8.33 | 2459 | 60 | 0 | −16 | 8.45 | |||||
| 6 | R Temporal Pole | 4721 | 52 | 4 | −34 | 9.07 | cluster 5 | 46 | 16 | −38 | 8-45 | |||||
| 7 | L Temporoparietal Junction | 3853 | −50 | −60 | 24 | 10.13 | cluster 14 | −50 | −60 | 24 | 7.54 | cluster 14 | −52 | −58 | 34 | 4.88 |
| B | L Superior Temporal Sulcus | cluster 7 | −52 | −2 | −22 | 7.51 | 3348 | −64 | −10 | −22 | 12.36 | 645 | −56 | −24 | −6 | 6.03 |
| 9 | L Temporal Pole | 52 | 6 | −32 | 6.82 | cluster 8 | −48 | 14 | −36 | 7.75 | ||||||
| Other Regions | ||||||||||||||||
| 10 | R Middle Frontal Gyrus | 323 | 26 | 30 | 42 | 5.16 | cluster 3 | 32 | 28 | 48 | 5.26 | 648 | 24 | 28 | 40 | 6.81 |
| 11 | L Middle Frontal Gyrus | 277 | −20 | 30 | 36 | 5.42 | −42 | 6 | 51 | 5.39 | 3328 | −40 | 18 | 38 | 5.72 | |
| 12 | R Hippocampus | 157 | 26 | −18 | −18 | 6.31 | ||||||||||
| 13 | L Hippocampus | 233 | −24 | 18 | −20 | 7.16 | ||||||||||
| 14 | L Angular Gyrus | 1325 | 46 | 68 | 34 | 8.72 | 1503 | −44 | −54 | 24 | 5.26 | |||||
| 15 | Calcerine Sulcus | 560 | −10 | −94 | −2 | 6.21 | ||||||||||
| 16 | R Lateral Occipita | 1525 | 40 | −74 | 38 | 7.14 | ||||||||||
Whole brain analysis of the Pain contrasts from the E/P and MOV tasks show significant recruitment of both brain regions associated with self/perceived pain (i.e. ‘Pain Matrix’: bilateral Insula, anterior middle cingulate cortex (AMCC), secondary sensory (SII), premotor, middle frontal gyrus (MFG)) and brain regions associated with action and body perception (extrastriate body area (EBA)) in both tasks (Figure 2a-b, Table 2). The results from the E/P task replicate previous studies using a superset of the stimuli used in the current study (Bruneau et al., 2012; 2013; 2015), and extend the findings to the MOV task (Figure 2b, Table 2). Figure 2c shows the extent of overlap in activation between both tasks.
Figure 2.
Whole brain response (p<0.05 corrected) to the different Pain Matrix contrasts (a) Emotional/Physical Pain stories: Physical>Emotional; (b) Passive Animated Movie Watching Task: Pain>Mental; (c) overlap in additive color scheme corresponding to the colors in panes (a)-(b).
Abbreviations: AMCC - anterior middle cingulate cortex; L/RINS - left/right insula; L/RSII - left/right secondary sensory.
Table 2.
Brain regions active (p<0.05 corrected) for the Pain Matrix contrast in the 2 different relevant tasks. Showing brain regions, cluster extent, local peaks in MNI, peak t value and Broadman Area.
| Emotional/Physical Pain stories task | Movie Watching Task | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster # | Region | n voxels | x | y | z | Peak t | n voxels | x | y | z | Peak t |
| Pain Matrix regions | |||||||||||
| 1 | Anterior Middle Cingulate Cortex | 1377 | −2 | 4 | 32 | 8.78 | 181 | 2 | 2 | 34 | 6.72 |
| 2 | R Anterior Insula | cluster 7 | 34 | 18 | 2 | 6.00 | cluster 7 | 42 | 4 | −6 | 6.65 |
| 3 | R Postcentral Gvrus | 1358 | 60 | −28 | 38 | 9.60 | 3158 | 64 | −24 | 28 | 7.85 |
| 4 | L Anterior Insula | cluster 8 | −28 | 16 | 4 | 5.23 | cluster 8 | −38 | 10 | −6 | 5.38 |
| 5 | L Postcentral Gyrus | 2487 | −60 | −26 | 34 | 9.19 | 2887 | −58 | −26 | 40 | 8.61 |
| Other Regions | |||||||||||
| 6 | L Posterior Cingulate Cortex | 1146 | −12 | −30 | 40 | 10.56 | 53 | −8 | −26 | 42 | 5.94 |
| 7 | R Inferior Frontal Gyrus / Insula | 3503 | 44 | 40 | 2 | 10.52 | 2051 | 38 | −2 | 14 | 7.48 |
| 8 | L Insula | 4098 | −36 | −14 | −4 | 8.84 | 2409 | −40 | −2 | −4 | 7.72 |
| 9 | L Orbital Frontal Cortex | 546 | −26 | 34 | −16 | 9.68 | 111 | −28 | 32 | −14 | 7.44 |
| 10 | L Middle Temporal Gyrus | 1478 | −52 | −64 | 2 | 8.04 | cluster 18 | −48 | −60 | −10 | 7.00 |
| 11 | R Middle Temporal Gyrus | 535 | 56 | −62 | 0 | 7.85 | 1489 | 56 | −56 | −12 | 7.80 |
| 12 | R Thalamus | 257 | 10 | −12 | −4 | 5.48 | 591 | 14 | −30 | −2 | 5.20 |
| 13 | R Posterior Cingulate Cortex | 499 | 16 | −34 | 40 | 7.64 | |||||
| 14 | L Middle Frontal Gyrus | 425 | −24 | −4 | 56 | 6.75 | |||||
| 15 | L Uncus | 223 | −30 | −2 | −40 | 6.67 | |||||
| 16 | Cerebellum | 87 | 26 | −68 | −24 | 5.89 | |||||
| 17 | Superior Occipital Gyrus | 100 | −34 | −86 | 34 | 5.59 | |||||
| 18 | L Lateral/Ventral Occipital | 5078 | −14 | −84 | 36 | 7.50 | |||||
| 19 | L Precentral Gyrus | 334 | −40 | −8 | 56 | 6.27 | |||||
| 20 | R Cuneus | 698 | 32 | −66 | 24 | 6.22 | |||||
| 21 | L Amygdala | 133 | −24 | 6 | −16 | 6.05 | |||||
Together, these results indicate that verbal stimuli from the FB localizer and E/P task activate very similar ToM brain regions across subjects, and that regions identified by the novel, non-verbal MOV task were remarkably similar to those generated by the verbal tasks, at the group level.
Detection rate
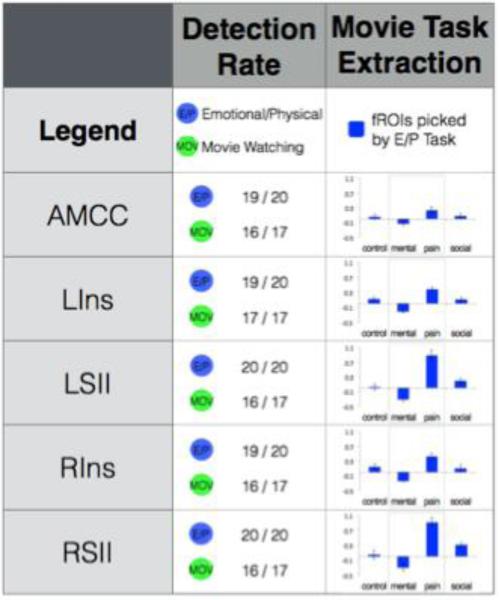
Requisite for an effective functional localizer is the ability to reliably identify fROIs within individual subjects. To determine the robustness of each localizer task, we determined the number of participants in which each of the localizer's fROIs could be identified. Both verbal tasks (FB and E/P) led to extremely high fROI detection rates (every fROI identified in >80% of participants). The fROI detection rate for the MOV task also showed a very high identification rate (at the reduced threshold of p<0.005) for most ROIs (every fROI identified in >70% of participants; Figure 3, 4 “Detection Rate”).
Figure 3.
The results of all analyses on ToM brain regions, presented by Region of Interest. Detection rate: minimum of 10 voxels with p<0.001 (p<0.005 in movie task) in individual; Movie Task Extraction: Beta estimate to all conditions in the Movie task, extracted from fROIs defined with either FB or E/P task; Overlap Analysis: Relative portions of overlapping voxels and non-overlapping voxels in relation to the number of reliably activated voxels in each ROI; Relative Location: relation between the mean activation coordinate of the different tasks on 3 axis; Spatial Correlation: mean correlations within task and correlations between task.
Figure 4.
The results of all analyses on Pain Matrix brain regions, presented by Region of Interest. Detection rate: minimum of 10 voxels with p<0.001 (p<0.005 in movie task) in individual; Movie Task Extraction: Beta estimate to all conditions in the Movie task, extracted from fROIs defined with E/P task.
Task generalizability
To determine how generalizable the fROI identification was across tasks, we cross-validated each verbal localizer by identifying fROIs with one task, and extracting activity for each of the MOV task conditions: Mental, Pain, Social and Control. In particular, we wanted to determine if the activity in the MOV-Mental condition is reliably higher than to the MOV-Pain condition in the ToM fROIs identified by the verbal tasks, and if activity during MOV-Pain is reliably higher than during MOV-Mental in the Extended Pain Matrix fROIs identified by the verbal tasks.
Activity in the MOV Mental > Pain contrast was significant (at a corrected threshold of p < 0.0071) across all ToM fROIs picked by the FB and the E/P localizers, except for trends in: E/P-picked RASTS (t(15) = 3.02, p = 0.008), FB-picked LASTS (t(15) = 2.77, p = 0.014) and FB-picked VMPFC (t(13) = 2.72, p = 0.017). Activity in the MOV Pain > Mental contrast was significant (at a corrected threshold of p < 0.01) across all Extended Pain Matrix fROIs picked by the E/P localizer (Figure 3, 4 “Movie Task Extraction”).
These results indicate that the fROIs can be identified with localizers that present others’ thoughts/feelings or pain, across modalities (verbal to visual) and task demand (active judgments vs. passive viewing).
Overlap analysis
In order to directly compare the similarity of ToM activity generated across the FB and E/P localizer tasks, we examined how many supra-threshold voxels identified by the FB and E/P tasks overlapped in comparison to the number of test-retest reliably activated voxels. In all of the ROIs we found a high rate of over 50% overlapping voxels (DMPFC – 83%; VMPFC – 94%; PC – 88%; LTPJ – 72%; RTPJ – 90%; LASTS – 59%; RASTS – 95%; Figure 3, “Overlap Analysis”). This pattern suggests two things. First, the numbers are remarkably high, especially given that the picking procedures were iterated on single runs of single subjects. Second, although the fROIs identified by the different tasks follow the same network structure, small differences in overlaps suggest that they may nevertheless have subtle spatial differences.
Location of fROIs
In order to further characterize the differences identified by the overlap analysis in ToM activity generated by FB and E/P tasks, we compared the mean location of activation between the tasks (Figure 3, “Relative Location”’). Overall, the mean coordinates identified by the two tasks were very similar, but there were some reliable differences in location of some of the ROIs. Most notably, the LTPJ is more anterior and inferior in FB activation compared to E/P activation (y axis: t(19) = 5.6, p < 0.001; z axis: t(19) = 4.99, p < 0.001). This result is consistent with the difference observed in the overlap analysis. A similar pattern is observed in the RTPJ (y axis: t(19) = 4.44, p < 0.001; z axis: t(19) = 3.7, p = 0.0015).
A few results showed trends of differences (i.e. 0.05 < p < 0.0024). In the PC, activation in the FB task showed a trend to be superior to E/P activation (t(19) = 3.02, p = 0.007). Another trend was observed in the VMPFC, where FB activation was anterior to E/P activation (t(15) = 2.76, p = 0.0145). More trends that were observed are LTPJ was more lateralized in FB than E/P (t(19) = 3.15, p = 0.005) and LASTS activation in FB was superior to E/P (t(17) = 2.68, p = 0.0158).
Spatial patterns
To examine overlap at a smaller spatial scale, we also compared voxel-level pattern differences observed across the FB and E/P tasks. We extracted the beta response of all voxels in the search spaces, from the first and second runs of the two verbal tasks and calculated the spatial correlation within and across tasks (Figure 3, ‘Spatial Correlation’). In 6 out of 7 of the search spaces used, the within-task correlation was significantly higher than the across-task (at a corrected threshold of p < 0.0071); and the last ROI, DMPFC, was below the statistical corrected threshold (t(19) = 2.02, p = 0.029). These results show that the global similarity in overlap and peak activity across tasks belies a reliable difference at a finer grained level: at the individual voxel level, multi-voxel pattern activity can be used to reliably decode task (FB and E/P) in a number of ToM brain regions. This is true for both cases where there is a noticeable difference in the distribution of the univariate signal such as bilateral TPJ (as identified by the location of fROIs analysis above), but also in ROIs where the differences in distribution are smaller or negligible.
Localizer choice effect
Given that fROIs selected from the FB and E/P tasks are not perfectly overlapping, how does the difference in the ROI that has been picked affect response of the fROI measured in an independent task? In other words, do the two localizers identify fROIs that are similar in function/functional profile even though they are not exactly similar in space? To examine this question, we compared the beta responses extracted from the FB versus E/P fROIs for the MOV task conditions (Figure 3, 4 “Movie Task Extraction”).
We tested for differences in response across ToM fROIs defined by FB versus E/P using a mixed effect model. We found a main effect of condition (p < 0.0071) in DMPFC, LTPJ, RTPJ, and PC, and a trend that did not survive correction for multiple comparisons in all other fROIs: LASTS (F(3,45) = 4.37, p = 0.0087); RASTS (F(3,45) = 4.41, p = 0.0084); VMPFC (F(3,36) = 4.51, p = 0.0087). There was no main effect of localizer in any of the ROIs (all Fs < 2.62, NS) and no significant or trend interaction between Localizer and Condition (significant nor trends) in any of the ROIs, except LTPJ (F(3,48) = 6.06, p = 0.0014).
This indicates that the functional profile of the picked fROI is similar between the two tasks, both when looking at conditions of interest, and when looking at the neural representations of other conditions in the same fROIs.
Discussion
We used two novel “localizer” tasks to identify brain regions involved in Theory of Mind and brain regions involved in perception of physical pain. We compared these tasks to the most widely used existing localizer for ToM, the false-belief task. Both of the novel tasks were robust, allowing us to identify the majority of the targeted functional regions of interest in almost every participant. Furthermore, the three different tasks converged, producing largely overlapping regions in individual participants, showing that these regions are stable across varying stimuli and tasks. We hope that these two novel tasks will be useful to many social cognitive neuroscientists, whose experiments often involve consideration of characters’ minds, bodies or both. All three localizer tasks are now publicly available at http://saxelab.mit.edu/.
There are three main advantages to the novel localizers. First, both of the novel localizers identify two distinct networks simultaneously, and thus are more efficient than the false-belief task, which only identifies one functional network. Second, the movie watching task has the lowest demands of any existing localizer task, and so could be used in children, non-native English speakers, and lower-functioning participants. Third, although hundreds of prior studies have examined activity in the Extended Pain Matrix, there is no simple robust localizer task that can be used to identify these regions in individual subjects without application of direct pain to the subjects (as in Corradi-Dell'Acqua et al., 2011). By manipulating vicarious experiences of pain, the current localizers will allow researchers to identify these regions safely, without requiring participants to undergo physical pain themselves and without the need for a special MR safe setup. Each localizer task identifies two brain networks in less than 10 minutes of scantime.
In addition to revealing robust and stable regions of activity across tasks, our results also suggest subtle differences in the response of ToM regions to the two verbal tasks. For example, the average coordinates of response to the two tasks was reliably different in left temporo-parietal junction, and in almost all regions the pattern of response within the region was reliably different for the two tasks. One possibility is that these differences reflect distinct pattern of response to affective (emotional) versus non-affective (false belief) mental states. However, a prior study that directly tested this hypothesis found different patterns of response to affective versus cognitive states in medial prefrontal cortex, but not in bilateral TPJ (Corradi-Dell'Acqua et al., 2014). Our results suggest an alternative possibility: that these differences in patterns of activity within fROIs associated with Theory of Mind are driven by the different ‘control’ conditions in the two tasks (Berman et al., 2010). The E/P task uses stories about bodily physical pain as the control condition and yields overall group activity similar to the Movie task (which also uses physical pain as the control condition). On the other hand, the FB task uses a non-human “photograph” control condition.
Note that these small but reliable differences in the regions’ responses to these three tasks reflect one of the key limits of localizer tasks. The ideal localizer task is a robust but blunt instrument, identifying functional regions that almost certainly contain many distinct functions and neural sub-populations (i.e. populations with different functional profiles within the same voxel/region). For example, both of the networks described here are spatially similar to two ‘intrinsically connected’ networks commonly found in resting state analysis (Fox et al., 2005; Thomas Yeo et al., 2011): the ToM brain regions are similar to the ‘default mode network’ (DMN; Buckner et al., 2008), implicated in rumination and internally directed thoughts, while the Pain Matrix regions are similar to the ‘salience network’, which shows increased activity during externally directed attention across a wide range of experiments (Bzdok et al., 2013; Yarkoni et al., 2011). Thus, it is important to ask whether the regions identified by these localizers are entirely overlapping with these two functional networks; and if so, whether these regions’ true functions are specific to the social domain, or more general. We hypothesize that the regions identified by our localizers do play a specific role in thinking about other's minds and bodies, partly because our studies include control conditions designed to match ‘salience’, and partly because prior studies have identified both spatial and functional dissociations, for example, between brain regions involved in Theory of Mind and the DMN (Andrews-Hanna et al., 2010; 2014; Lombardo et al., 2010). However, a more definitive answer to this question could be obtained by identifying the loci of responses for ToM and DMN, and Pain Matrix and Salience Network, using resting state and localizer tasks, within individual subjects. The localizers described here would provide an efficient means of examining questions such as these.
Another distinct localizer task for the ToM regions was recently developed and validated by Spunt and colleagues (Spunt and Adolphs, 2014). The ‘Why/How’ task requires participants to watch the same photograph of a character's action, while performing two distinct explicit tasks: either judging how (i.e. with which muscle movements) the action was performed, or why (i.e. in what context, or with what goals) the action was performed. Activity during the ‘Why’ task was largely overlapping with the false-belief task, suggesting that the ‘Why’ task activates ToM. On the other hand, within ToM regions, the ‘Why’ task elicited a distinct pattern of activation from the false-belief task. Thus, as in the current data, two different localizers identify the same region, but activate different sub-populations within that region. More generally, distinct sub-populations within the same ToM region may contain information about distinct features or aspects of mental states (Contreras et al., 2013; Skerry and Saxe, 2015). A promising strategy for future research is therefore to identify brain regions implicated in ToM using a localizer task, and then directly study the information represented in those regions using more minimal experimental manipulations and finer grained analysis techniques like multivoxel pattern analyses and representational similarity analyses (Haxby et al., 2014; Kriegeskorte, 2008; Kriegeskorte and Kievit, 2013).
An alternative approach to using a separate localizer task is to functionally identify ROIs by building an orthogonal contrast into the main experiment as suggested by (Friston et al., 2006). In some cases, this could be efficient, because it does not require collecting any additional data and uses a contrast that is directly related to the experimental design and psychological processes under consideration. On the other hand, this approach has the disadvantage that each new experiment will use a slightly different contrast to localize the “same” regions or networks. Our current results suggest that differences in the precise contrast can result in subtle spatial differences in the regions localized. Using standardized, separate localizers is the only way to ensure that the “same” region is under investigation across studies and labs. Also, the standardized localizers are highly powered, so experimenters know in advance that regions will be identified in most individual subjects, whereas novel orthogonal contrasts may turn out to have less power than expected.
Given the largely similar but still reliably distinct patterns of activation observed across the current three localiser tasks, can different localizers be used interchangeably, and can we directly compare experiments that used different localizers? The generalizability analysis and the overlap analysis suggest an answer to the first question. The overall voxel overlap as measured in the overlap analysis was very high in all the ROIs (59% overlap in the most divergent ROI). Moreover, when we extracted all experimental conditions of the movie task from the voxels picked by the two verbal localizer tasks, the only ROI where there was a condition by localizer interaction was the LTPJ. This ROI showed one of the lowest overlap rate (72%) and the most stable between-tasks difference in both location and pattern. Overall, this suggests that, for the most part, the localizers identify the same voxels. Therefore, if the goal is to identify voxels that are involved in theory of mind processing, the tasks can be used largely interchangeably (and indeed, the main ToM contrast from the movie task remained highly significant regardless of the choice of localizer). On the other hand, the significant differences observed here in average location and within-region patterns suggest that for analyses that depend on relatively subtle effects, such as multi-voxel pattern analyses, it may be important to compare results only across studies that use the same localizer task.
Another question not addressed by the current research is: how stable would the results of these localizers be, within an individual over time? Although anecdotal evidence suggests that activation patterns remain stable over many decades in adulthood, this claim has yet to be formally tested, especially for brain regions involved in social cognition (though Mahowald and Fedorenko, under review, have tested that question as it pertains to the language system, showing some promising results). Changes in patterns activation may also occur related to both social experiences (e.g. college) and maturation in early adulthood. An additional related area for future work is individual variability in mentalizing skills. How do different mentalizing skills relate to one another within different individuals behaviorally and neurally. Such research will have to use paradigms that create substantial performance variability between subjects, and would benefit from the methodological advances of utilizing functional localizers.
When choosing a localizer task, there are also practical considerations. Localizers vary in both the extent and reliability of activation (Berman et al., 2010) which should be taken into account. Another consideration is efficiency, in this case, both of the two novel localizers, the Emotion/Pain stories task and the Movie task, have two contrasts of interest and are designed to localize two theoretically important networks at the same time. Thus, they are more efficient than the traditional false-belief localizer, and offer a built in “control network” for hypotheses that are specific to one network. In addition, a key practical advantage of the Movie task is that is an ecologically valid task: participants passively view a non-verbal cartoon, with no explicit task instructions. The movie is engaging and approachable, making it more appropriate for use in children and lower-functioning populations.
Conclusions
In sum, here we introduce and validate two novel localizer tasks for use in social cognitive neuroscience. The Emotion/Pain stories task and the Movie task can both be used to identify, in individual participants, functional regions implicated in Theory of Mind and in processing pain and bodily states. Both tasks are short (<12 minutes), and robust in individual participants. The identified networks of activity converge across task modality and stimulus content with the commonly used false-belief Localizer task. There are small reliable differences between the localizers, in the location of the regions activated and in the pattern of activity within each region, hinting at more specific representations within each region. Still, the consistency across tasks makes both novel tasks viable localizers, and we hope many researchers in social cognitive neuroscience will find them useful.
Supplementary Material
Highlights.
Two novel functional localizers task for social cognitive neuroscience
Localizers simultaneously identify ToM network and pain matrix in individuals
All localizers are validated against the currently most widely used task
All localizers will be made public
Acknowledgments
This work was supported by National Institutes of Health Grant 1R01 MH096914-01A1. Data were collected at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research, MIT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann's Areas 17 and 18 Brought into Stereotaxic Space—Where and How Variable? NeuroImage. 2000;11:66–84. doi: 10.1006/nimg.1999.0516. doi:10.1006/nimg.1999.0516. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-Anatomic Fractionation of the Brain's Default Network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. doi:10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Saxe R, Yarkoni T. Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. 2014;91:324–335. doi: 10.1016/j.neuroimage.2014.01.032. doi:10.1016/j.neuroimage.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Park J, Gonzalez R, Polk TA, Gehrke A, Knaffla S, Jonides J. Evaluating functional localizers: The case of the FFA. NeuroImage. 2010;50:56–71. doi: 10.1016/j.neuroimage.2009.12.024. doi:10.1016/j.neuroimage.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. NeuroImage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. doi:10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Bruneau E, Dufour N, Saxe R. How we know it hurts: item analysis of written narratives reveals distinct neural responses to others’ physical pain and emotional suffering. PLoS ONE. 2013;8:e63085. doi: 10.1371/journal.pone.0063085. doi:10.1371/journal.pone.0063085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau EG, Jacoby N, Saxe R. Empathic control through coordinated interaction of amygdala, theory of mind and extended pain matrix brain regions. NeuroImage. 2015;114:105–119. doi: 10.1016/j.neuroimage.2015.04.034. doi:10.1016/j.neuroimage.2015.04.034. [DOI] [PubMed] [Google Scholar]
- Bruneau EG, Pluta A, Saxe R. Distinct roles of the “Shared Pain’ and ‘Theory of Mind’ networks in processing others” emotional suffering. Neuropsychologia. 2012;50:219–231. doi: 10.1016/j.neuropsychologia.2011.11.008. doi:10.1016/j.neuropsychologia.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Buckner RL, ANDREWS-HANNA JR, SCHACTER DL. The Brain's Default Network: Anatomy, Function, and Relevance to Disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. doi:10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, Laird AR, Fox PT, Zilles K, Eickhoff SB. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. NeuroImage. 2013;81:381–392. doi: 10.1016/j.neuroimage.2013.05.046. doi:10.1016/j.neuroimage.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JM, Schirmer J, Banaji MR, Mitchell JP. Common Brain Regions with Distinct Patterns of Neural Responses during Mentalizing about Groups and Individuals. J Cogn Neurosci. 2013;25:1406–1417. doi: 10.1162/jocn_a_00403. doi:10.1162/jocn_a_00403. [DOI] [PubMed] [Google Scholar]
- Corradi-Dell'Acqua C, Hofstetter C, Vuilleumier P. Cognitive and affective theory of mind share the same local patterns of activity in posterior temporal but not medial prefrontal cortex. Soc Cogn Affect Neurosci. 2014;9:1175–1184. doi: 10.1093/scan/nst097. doi:10.1093/scan/nst097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi-Dell'Acqua C, Hofstetter C, Vuilleumier P. Felt and Seen Pain Evoke the Same Local Patterns of Cortical Activity in Insular and Cingulate Cortex. J. Neurosci. 2011;31:17996–18006. doi: 10.1523/JNEUROSCI.2686-11.2011. doi:10.1523/JNEUROSCI.2686-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda S. Pooling fMRI data: meta-analysis, mega-analysis and multi-center studies. Front. Neuroinform. 2009;3:1–8. doi: 10.3389/neuro.11.033.2009. doi:10.3389/neuro.11.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum WR, Griffin LD, Hill DLG, Hawkes DJ. Zen and the art of medical image registration: correspondence, homology, and quality. NeuroImage. 2003;20:1425–1437. doi: 10.1016/j.neuroimage.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D, Koster-Hale J, Bedny M, Saxe R. fMRI item analysis in a theory of mind task. 2011;55:705–712. doi: 10.1016/j.neuroimage.2010.12.040. doi:10.1016/j.neuroimage.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. doi:10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Dufour N, Redcay E, Young L, Mavros PL, Moran JM, Triantafyllou C, Gabrieli JDE, Saxe R. Similar Brain Activation during False Belief Tasks in a Large Sample of Adults with and without Autism. PLoS ONE. 2013;8:e75468. doi: 10.1371/journal.pone.0075468. doi:10.1371/journal.pone.0075468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Thompson-Schill SL. Reworking the language network. Trends Cogn Sci (Regul Ed) 2014;18:120–126. doi: 10.1016/j.tics.2013.12.006. doi:10.1016/j.tics.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. doi:10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Rotshtein P, Geng JJ, Sterzer P, Henson RN. A critique of functional localisers. NeuroImage. 2006;30:1077–1087. doi: 10.1016/j.neuroimage.2005.08.012. doi:10.1016/j.neuroimage.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999 doi: 10.1126/science.286.5445.1692. doi:10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, Blake R. Brain areas involved in perception of biological motion. J Cogn Neurosci. 2000;12:711–720. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Connolly AC, Guntupalli JS. Decoding Neural Representational Spaces Using Multivariate Pattern Analysis. Annu. Rev. Neurosci. 2014;37:435–456. doi: 10.1146/annurev-neuro-062012-170325. doi:10.1146/annurev-neuro-062012-170325. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. doi:10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Combining voxel intensity and cluster extent with permutation test framework. NeuroImage. 2004;23:54–63. doi: 10.1016/j.neuroimage.2004.04.035. doi:10.1016/j.neuroimage.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Julian JB, Fedorenko E, Webster J, Kanwisher N. An algorithmic method for functionally defining regions of interest in the ventral visual pathway. NeuroImage. 2012;60:2357–2364. doi: 10.1016/j.neuroimage.2012.02.055. doi:10.1016/j.neuroimage.2012.02.055. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Functional specificity in the human brain: a window into the functional architecture of the mind. Presented at the Proceedings of the National Academy of ... 2010 doi: 10.1073/pnas.1005062107. doi:10.1073/pnas.1005062107/-/DCSupplemental/pnas.201005062SI.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. doi:10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N. Representational similarity analysis – connecting the branches of systems neuroscience. Front. Sys. Neurosci. 2008:1–28. doi: 10.3389/neuro.06.004.2008. doi:10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Kievit RA. Representational geometry: integrating cognition, computation, and the brain. Trends Cogn Sci (Regul Ed) 2013;17:401–412. doi: 10.1016/j.tics.2013.06.007. doi:10.1016/j.tics.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nat Neurosci. 2010;13:501–506. doi: 10.1038/nn.2498. doi:10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. doi:10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, MRC AIMS Consortium. Baron-Cohen S. Shared neural circuits for mentalizing about the self and others. J Cogn Neurosci. 2010;22:1623–1635. doi: 10.1162/jocn.2009.21287. doi:10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Mahowald K, Fedorenko E. Reliable individual-level neural markers of high-level language processing: A necessary precursor for relating neural variability to behavioral and genetic variability. doi: 10.1016/j.neuroimage.2016.05.073. Under Review. [DOI] [PubMed] [Google Scholar]
- Mar RA. The Neural Bases of Social Cognition and Story Comprehension. Annu. Rev. Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. doi:10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Morrison I, Downing PE. Organization of felt and seen pain responses in anterior cingulate cortex. NeuroImage. 2007;37:642–651. doi: 10.1016/j.neuroimage.2007.03.079. doi:10.1016/j.neuroimage.2007.03.079. [DOI] [PubMed] [Google Scholar]
- Nieto-Castañón A, Fedorenko E. Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. NeuroImage. 2012;63:1646–1669. doi: 10.1016/j.neuroimage.2012.06.065. doi:10.1016/j.neuroimage.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. Divide and conquer: A defense of functional localizers. NeuroImage. 2006;30:1088–1096. doi: 10.1016/j.neuroimage.2005.12.062. doi:10.1016/j.neuroimage.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. doi:10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Singer T, Ben Seymour, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for Pain Involves the Affective but not Sensory Components of Pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. doi:10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Skerry AE, Saxe R. Neural Representations of Emotion Are Organized around Abstract Event Features. Current Biology. 2015:1–11. doi: 10.1016/j.cub.2015.06.009. doi:10.1016/j.cub.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Adolphs R. Validating the Why/How contrast for functional MRI studies of Theory of Mind. NeuroImage. 2014;99:301–311. doi: 10.1016/j.neuroimage.2014.05.023. doi:10.1016/j.neuroimage.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. doi:10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, Petrides M. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur. J. Neurosci. 1999;11:3033–3046. doi: 10.1046/j.1460-9568.1999.00718.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2:150–158. doi: 10.1093/scan/nsm015. doi:10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Dumoulin SO, Brewer AA. Visual Field Maps in Human Cortex. Neuron. 2007;56:366–383. doi: 10.1016/j.neuron.2007.10.012. doi:10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Warnking J, Dojat M, Guérin-Dugué A, Delon-Martin C, Olympieff S, Richard N, Chéhikian A, Segebarth C. fMRI retinotopic mapping--step by step. NeuroImage. 2002;17:1665–1683. doi: 10.1006/nimg.2002.1304. doi:10.1006/nimg.2002.1100. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. doi:10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Schirmer J, Mitchell JP. Social Influence Modulates the Neural Computation of Value. Psychological Science. 2011;22:894–900. doi: 10.1177/0956797611411057. doi:10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.