Abstract
Arrhythmia-induced cardiomyopathy (AIC) is a potentially reversible condition in which left ventricular dysfunction is induced or mediated by atrial or ventricular arrhythmias. Cellular and extracellular changes in response to the culprit arrhythmia have been identified, but specific pathophysiological mechanisms remain unclear. Early recognition of AIC and prompt treatment of the culprit arrhythmia using pharmacological or ablative techniques results in symptom resolution and recovery of ventricular function. Although cardiomyopathy in response to an arrhythmia may take months to years to develop, recurrent arrhythmia can result in rapid decline in ventricular function with development of heart failure, suggesting residual ultrastructural abnormalities. Reports of sudden death in patients whose left ventricular ejection fraction have normalized cast doubt on the complete reversibility of this condition. Several aspects of AIC, including specific pathophysiological mechanisms, predisposing factors, optimal therapeutic strategies to prevent ultrastructural changes, and long-term risk of sudden death remain unresolved and need further research.
Keywords: atrial fibrillation, atrial flutter, catheter ablation, heart failure, heart rate, tachycardia
Introduction
Identifying, stabilizing, and correcting the causes of cardiomyopathy improves outcomes and quality of life in patients with systolic heart failure (HF). Arrhythmias can initiate or exacerbate acute HF in patients with pre-existing heart disease. Less well-recognized is the effect of persistent tachycardia or frequent ectopy on cardiomyopathy in patients with or without concomitant heart disease (1–3). Early recognition of the relationship of a culprit arrhythmia to cardiomyopathy is paramount in providing treatment that improves symptoms, left ventricular ejection fraction (LVEF), and functional status.
This state-of-the-art review addresses the significance, mechanisms, diagnosis, current management strategies, and prognosis of arrhythmia-induced cardiomyopathy (AIC).
What is AIC?
AIC is a condition in which atrial or ventricular tachyarrhythmias or frequent ventricular ectopy result in left ventricular (LV) dysfunction, leading to systolic HF (1,2). The hallmark of this condition is partial or complete reversibility once arrhythmia control is achieved. AIC can be classified into 2 categories: one where the arrhythmia is the sole reason for ventricular dysfunction (arrhythmia-induced), and another where the arrhythmia exacerbates ventricular dysfunction and/or worsens HF in a patient with concomitant heart disease (arrhythmia-mediated) (3).
Epidemiology
The incidence and prevalence of AIC are uncertain, but AIC appears under-recognized. Atrial fibrillation (AF) is present in 10% to 50% of patients with HF; many patients with cardiomyopathy and AF have worsening symptoms and ventricular function due solely to poorly-controlled ventricular rates (4). In 2 studies of adult patients with focal atrial tachycardia (AT), the incidence of AIC was 8.3% to 10% (5,6), whereas in a pediatric multicenter study of atrial ectopic tachycardia (AET), 28% had AIC (7). The incidence of AIC was 9% to 34% in patients with frequent premature ventricular complexes (PVCs) and/or nonsustained ventricular tachycardia referred for electrophysiological evaluation (8–10).
Pathophysiology, Mechanisms, and Predisposing Factors
Pathophysiology: Insights from Animal Models
Chronic rapid pacing has long been recognized to cause HF in animal models (11). LV remodeling and HF occur in a time-dependent and highly predictable manner, similar to the phenotype of AIC (12–18). Due to the rapidly progressive and predictable nature of pacing to induce cardiomyopathy, the natural history of LV remodeling and failure, neurohormonal activation and signaling, as well as cell viability and molecular pathways have been examined in detail. The Central Illustration shows the natural history and contributing cellular and molecular events in rapid pacing-induced cardiomyopathy and HF.
Central Illustration.
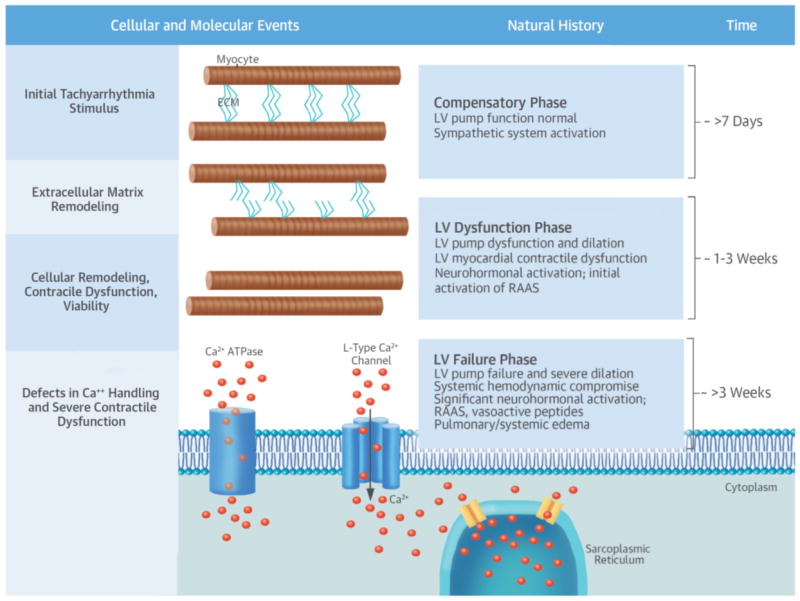
In response to rapid pacing in animals, left ventricular (LV) remodeling and heart failure occur in a time-dependent and highly predictable manner, similar to the phenotype of arrhythmia-induced cardiomyopathy. Several cellular and molecular events in response to the initial tachyarrhythmia stimulus take place over a period of approximately 3 to 4 weeks and involve both extracellular matrix (ECM) and myocyte remodeling. Specifically, there is loss of normal extracellular matrix and architecture. This, coupled with alterations in cellular growth and viability, defects in Ca2+ handling, and neurohormonal activation, results in LV dilation and severe contractile dysfunction. The natural history of rapid pacing–induced cardiomyopathy progresses from a compensatory phase (approximately >7 days) to an LV dysfunction phase (approximately 1 to 3 weeks) to an LV failure phase (approximately >3 weeks), characterized by progressive LV dilation and dysfunction and increasing neurohormonal activation. ATPase - adenosine triphosphatase; RAAS - renin-angiotensin-aldosterone system.
LV Remodeling and HF
During the early phase (the first 3 to 7 days) of rapid pacing, LV dilation occurs with a decline in LVEF (12–15). This early remodeling phase is not accompanied by compromises in cardiac output or systemic perfusion pressures. By the second week, LV dilation, a fall in LVEF, and elevations in central venous and pulmonary capillary wedge pressures and systemic vascular resistance ensue (12–17). Eventually, HF develops.
Whereas initial manifestations are adaptive, sustained rapid pacing affects LV load-ejection relationships, specifically, the pre-load recruitable LV stroke-work relationship (17), the LV systolic wall stress-ejection relationship, and the slope of the LV end-systolic pressure-volume relationship (18). LV dilation and dysfunction are accompanied by a decline in intrinsic myocardial contractile function.
Neurohormonal Activation
Rapid pacing in animals results in a predictable, time–dependent change in neurohormonal pathways and synthesis and release of bioactive peptides (19–25). Plasma atrial natriuretic peptide and B-type natriuretic peptide (BNP) increase early, concomitant with LV dilation (19,21); eventually, natriuretic peptides plateau or decrease, likely due to suppression of synthesis and increased degradation by endopeptidases. Another hallmark is activation of sympathetic pathways, resulting in norepinephrine spillover (20,21). Consistent with the phenotype of progressive LV failure, rapid pacing invariably causes activation of the renin-angiotensin-aldosterone system (20,25). Other bioactive molecules activated and released include endothelin and inflammatory cytokines, such as tumor necrosis factor alpha (24).
Whereas mediators of vasoconstriction are induced, mediators of vasodilation, such as the response to nitric oxide, become impaired (22,23). Several key aspects regarding these temporal changes in the neurohormonal/bioactive signaling pathways hold relevance. First, shifts in key neurohormonal pathways, such as the natriuretic peptides and the renin-angiotensin-aldosterone system, likely reflect shifts in underlying LV functional status from a “compensated” to a “decompensated” state. Thus, neurohormonal measurements may serve as useful biomarkers for the progressive nature/status of AIC. Secondly, it is likely that stimulation of key pathways, such as the adrenergic and cytokine systems, contribute directly to myocardial dysfunction.
Cell Signaling and Viability
Under normal conditions, increased contraction frequency causes a progressive rise in myocardial contractile performance due to appropriate changes in calcium (Ca2+) handling and in the myofilament response to Ca2+. However, this positive force-frequency relationship is blunted with pacing-induced cardiomyopathy (26), with accompanied prolongation of myocardial Ca2+ transients and defects in Ca2+ cycling (27). These changes in the force-frequency relation and Ca2+ handling are a manifestation of prolonged rapid pacing and when definable changes in LV systolic function have already occurred (27–30). The summation of changes in Ca2+ handling results in fundamental defects in the excitation-contraction coupling process, leading to defects in absolute myocyte contractile function and inotropic responsiveness (27–30).
In isolated myocyte preparations, intrinsic defects in the rate and extent of shortening, as well as defects in the rate of relaxation, have been identified as cardiomyopathy develops and progresses (20,28,31). Changes in cellular growth and viability ensue (32,33). Chronic rapid pacing induces several proapoptotic cascades (34). LV dilation is not associated with an absolute increase in myocyte cross-sectional area, but rather with lengthening of individual myocytes, with alterations in cytoskeletal architecture (20,29,31,35). If these changes in myocyte function and viability were placed in a temporal context, then LV myocyte remodeling (cell shape and architecture) is likely to occur early, followed by abnormalities in excitation-contraction and changes in cell viability.
Extracellular Remodeling
Although remodeling occurs within the myocyte, robust changes also occur within the extracellular matrix (35,36). Specifically, there is a loss of normal fibrillar collagen content and distribution (35,36), which, in turn, initiates alterations in myocyte support and alignment within the LV wall. Concomitantly, the capacity of myocytes to bind to the extracellular matrix is diminished (35), suggesting that abnormalities in the extracellular matrix-integrin interface have occurred. Increased matrix metalloproteinase activity and expression contribute to loss of normal extracellular matrix support and architecture (37). Activation of matrix metalloproteinases precedes the defects in myocyte contractile function (37). These findings suggest that the likely mechanism for LV remodeling is loss of normal myocardial extracellular matrix structure, composition, and function.
Implications of Animal Studies
Chronic rapid pacing in animals causes dilated cardiomyopathy resembling clinical AIC. With rapid atrial pacing in pigs, an atrioventricular conduction pattern is maintained and ventricular dyssynchrony as the stimulus for adverse remodeling and HF is unlikely (12,13,20). However, rapid ventricular pacing, where myocardial dyssynchrony is inevitable, results in a more rapid and precipitous fall in LV systolic performance (14–17). Thus, the tachycardia stimulus in and of itself is sufficient to drive the cardiomyopathy process, whereby electrical dyssynchrony would constitute an additive factor accelerating the process.
Phases and structural milestones that occur with chronic arrhythmias in animal models translate to clinical forms of AIC. Specifically, there is an adaptive/compensatory phase whereby LV dilation, extracellular matrix remodeling, and neurohormonal system activation occurs, but severe LV dysfunction does not. This phase could be identified in patients by LV imaging and biomarker profiling, and is followed by a phase whereby LV dysfunction becomes manifest and associated with defects in excitation-contraction coupling, LV myocyte remodeling, and dysfunction. This phase likely represents a critical transition whereby this process may not completely reverse, leading to neurohormonal activation accompanied by the full pathophysiological spectrum of HF.
Several challenges and limitations exist in translating animal data to humans. The time course for development of AIC and HF in patients with otherwise structurally normal hearts is usually months to years, which is quite different from the acute changes seen in pacing models. Some arrhythmias (e.g., AF and PVCs) may have mechanisms leading to AIC that are not explained fully by rapid-pacing animal models (10,38–40). Although ultrastructural changes may explain the initial development of AIC, the relationship between identifiable ultrastructural changes and the mechanisms by which cardiomyopathy develops are less clear. An overview of the current understanding of the relationship between arrhythmias and cardiomyopathy, from mechanisms to management and prognosis, is shown in Figure 1.
Figure 1. Overview of the Current Understanding of AIC, from Mechanisms to Management and Prognosis.
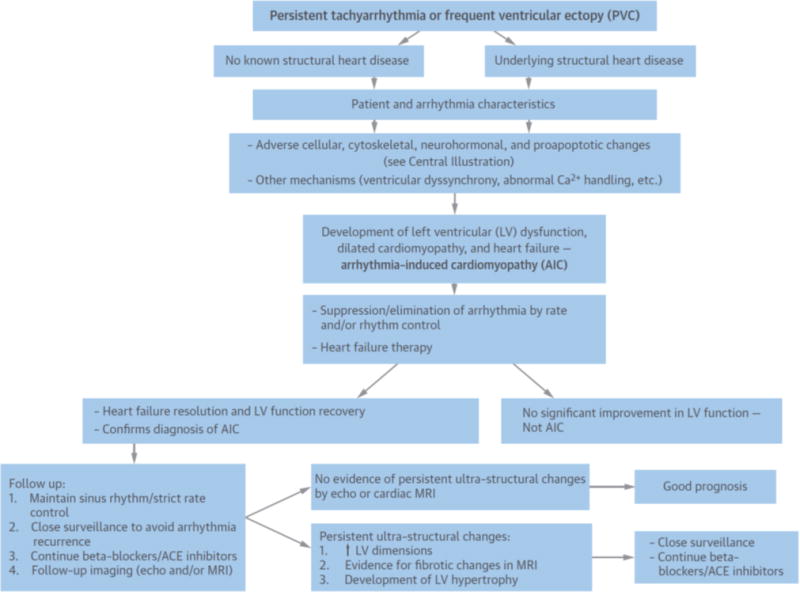
Persistent tachyarrhythmias or frequent ventricular ectopy can lead to dilated cardiomyopathy and decompensated heart failure in patients with or without concomitant structural heart disease. Cellular and extracellular changes in response to the culprit arrhythmia have been identified (see Central Illustration), but specific pathophysiological mechanisms have not been well elucidated. Early recognition of the relationship of a culprit arrhythmia to cardiomyopathy and aggressive arrhythmia control results in improved symptoms, left ventricular (LV) ejection fraction, and functional status and helps establish the diagnosis of arrhythmia-induced cardiomyopathy (AIC). Cellular and extracellular ultrastructural changes can, however, persist and can contribute to a rapid decline in cardiac function with arrhythmia recurrence. Close surveillance is thus crucial to ensure good long-term prognosis. ACE - angiotensin-converting enzyme; MRI - magnetic resonance imaging; PVC - premature ventricular complex.
Arrhythmia and Patient Characteristics
Arrhythmia characteristics contributing to AIC include arrhythmia type (Table 1), rate, duration, rhythm irregularity, persistence (5,41–43), and concomitant heart disease (44). An arrhythmia that is insidious, persistent, and well-tolerated is more likely to result in AIC (5). Lack of persistent tachycardia from autonomic influences and resultant slower rates during sleep, are likely to be the reason AIC is rare or nonexistent with inappropriate sinus tachycardia and postural tachycardia syndrome (POTS), although the average heart rate can be >100 beats/min. There is no specific heart rate cutoff at which AIC develops. The rate is not well-defined, may be age-dependent, and is likely lower than initially suspected. Little is known about patient factors that increase vulnerability to AIC, but 1 report indicates that patients with a homozygous deletion polymorphism in the angiotensin-converting enzyme gene (DD) had a higher propensity to develop AIC when faced with persistent tachycardia, suggesting a potential genetic link (45). In a longitudinal study of patients with high PVC burden, those who developed cardiomyopathy had wider PVC QRS width versus those who did not, suggesting baseline myocardial fiber disruption that portends an increased risk (46).
Table 1.
Causes of Arrhythmia-Induced Cardiomyopathy
| Supraventricular |
| Atrial fibrillation |
| Atrial flutter |
| Atrial tachycardia |
| AV nodal reentrant tachycardia |
| AV re-entrant tachycardia |
| Permanent junctional reciprocating tachycardia (PJRT) |
| Junctional Ectopic Tachycardia (JET) |
| Ventricular |
| Idiopathic ventricular tachycardia |
| Fascicular tachycardia |
| Ectopy |
| Frequent premature ventricular contractions |
| Frequent premature atrial contractions |
| Pacing |
| Persistent rapid atrial and/or ventricular pacing |
Adapted with permission from (2). AV = atrioventricular.
Clinical Features and Diagnosis
The key diagnostic feature of AIC is the presence of a pathologic tachycardia or persistent arrhythmia (PVCs) in the presence of an otherwise unexplained cardiomyopathy. The relationship of arrhythmia to cardiomyopathy can be difficult to determine because an arrhythmia could exist for years before its recognition and before cardiomyopathy develops. Although a high index of clinical suspicion may point to subtle diagnostic clues, the condition may go unrecognized for years. The presentation can be late only after manifest systolic HF develops. Similarly, if the arrhythmia is detected early but a nonaggressive approach is taken, progressive worsening of symptoms and insidious development of cardiomyopathy ensue. It can be difficult to determine whether an arrhythmia is the initiator or consequence of cardiomyopathy in a patient with tachycardia and HF. Thus, AIC raises a “chicken or egg” question (47). Frequently, the arrhythmia is considered secondary and not treated effectively when, in fact, arrhythmia control is necessary for full recovery.
History, physical examination, and clinical investigations should focus on determining the etiology of cardiomyopathy. Specific patient characteristics may suggest a higher likelihood of AIC. Patients with AIC have a smaller LV end-diastolic diameter and mass index versus those with pre-existing dilated cardiomyopathy and concomitant tachyarrhythmia (48,49). Cardiac magnetic resonance imaging (MRI) may help differentiate AIC from dilated cardiomyopathy. In a study of 27 patients with frequent PVCs and cardiomyopathy, 22 had improvement in LVEF following PVC suppression; 5 did not. Four of the 5 patients whose LVEF did not improve had evidence for late gadolinium enhancement, suggesting underlying scar (8). Campos et al. compared patients with irreversible nonischemic dilated cardiomyopathy, reversible PVC-mediated cardiomyopathy, and patients with PVCs and normal LVEF who underwent electroanatomic mapping. These investigators noted that those with irreversible cardiomyopathy had a significantly larger percentage of the LV endocardium with abnormal unipolar voltage (64% vs. 5.2% for the reversible group and 0.1% for the normal LVEF group). An abnormal unipolar voltage area ≥32% of LV endocardium predicted the irreversibility of cardiomyopathy with >95% sensitivity and specificity, but prospective validation is lacking (49).
Serial assessment of the N-terminal pro-B-type natriuretic peptide (NT-pro BNP) ratio (NT-BNP at baseline/NT-BNP during follow-up) can differentiate AIC from irreversible dilated cardiomyopathy. Forty patients with AF or atrial flutter with ventricular rates >100 beats/min and LVEF <40% were cardioverted and NT-proBNP was measured on day 1, and weekly for 4 weeks. An NT-proBNP ratio cutoff >2.3 at 1 week, suggesting rapid decline in NT-proBNP levels, was associated with reversible cardiomyopathy, with an accuracy of 90% (50).
When AIC cannot be easily differentiated from dilated cardiomyopathy with consequent tachycardia, treatment for both problems becomes necessary (2). The diagnosis may be evident only after restoration and maintenance of sinus rhythm, or after aggressive rate control.
Principles of Management
AIC management should focus on concerted attempts to eliminate or control the arrhythmia, with the goal of improving symptoms, reversing LV dysfunction, and preventing arrhythmia recurrence (1,5,43,51,52). A favorable response to arrhythmia elimination/control establishes the diagnosis of AIC. Along with arrhythmia control, use of neurohormonal antagonists can result in favorable ventricular remodeling. Controversy remains about the need to continue these medications if there is complete recovery of LVEF with arrhythmia control.
AIC Associated with Specific Arrhythmias in Adults
Atrial Fibrillation
AF is the most common cause of AIC in adults (1,43). Of patients presenting with HF, 10% to 50% have AF (4,53), and many such patients likely have a component of AIC. The mechanisms underlying development or progression of cardiomyopathy in patients with persistent AF are not clearly elucidated (39). Rapid heart rates, loss of atrial contraction, and rhythm irregularity may contribute (1,39,40,54). Persistent tachycardia can impair myocardial contractility, either directly or through alterations in cellular and neurohormonal mechanisms. Resting tachycardia, as well as a rapid increase in heart rate with exercise, can impair diastolic filling. Lack of an atrial contribution to ventricular filling can further worsen diastolic function. A vicious cycle can thus develop, with HF and the associated increases in left-sided filling pressures and functional mitral regurgitation resulting in mechanoelectrical changes in the left atrium, perpetuating AF and cardiomyopathy (54).
Management of AF consists of rate and/or rhythm control. In those with AF and cardiomyopathy, the ideal target heart rate remains uncertain. For those with permanent AF, lenient rate control (resting heart rate <110 beats/min) has been advocated over strict rate control (resting heart rate <80 beats/min and heart rate with moderate exercise <110 beats/min) (55). However, in the RACE II trial (55), most patients were rate controlled before enrollment (baseline heart rate 96 beats/min), and were asymptomatic without cardiomyopathy. Follow-up was short and development of AIC was not specifically considered.
Several methods are available for AF rate control (56). Achieving adequate rate control may require drug combinations and frequent regimen changes (57). However, these data may not directly apply to AF-mediated AIC, as rate irregularity may contribute to symptoms and facilitate development of AIC (40). Although AV nodal ablation with pacemaker implantation can provide effective rate control (58) and regularize the rate, this strategy changes ventricular activation, even if cardiac resynchronization therapy is used, and therefore should only be considered if rhythm and/or rate control cannot be established. In contrast, electrical or pharmacologic cardioversion, antiarrhythmic drugs, and catheter ablation can achieve rhythm control. In AF-mediated cardiomyopathy, restoration and maintenance of sinus rhythm can hasten clinical recovery and reverse cardiomyopathy over several months (1,40,59).
The AF-CHF trial randomized 1,376 patients with AF (70% persistent) and HF to either a rhythm control (amiodarone with cardioversion) or a rate control strategy. The mean LVEF was 27% and patients were followed for 37 months. A rhythm control strategy using antiarrhythmics did not improve all-cause mortality or prevent worsening HF. However, the AF-CHF patient population may not fully represent the AIC population, as 40% of patients in the rate control arm were in sinus rhythm during follow-up (60). The CAFÉ-II study randomized 61 patients with persistent AF (median duration of 14 months) and moderate LV dysfunction to a rate control and rhythm control strategy (amiodarone with cardioversion). During a follow-up of 1.2 years, 66% of patients maintained sinus rhythm, whereas 90% achieved target rate control (<80 beats/min at rest and <110 beats/min with 6-minute walk). Rhythm control was superior to rate control in improving LV function, pro-BNP levels, and quality of life (61). Attempts to control rate might not be as aggressive or carefully monitored in the real world as in clinical trials. It is also possible that side effects and proarrhythmic risks from antiarrhythmic drugs may offset any salutary effects from restoring and maintaining sinus rhythm (62).
Restoring and maintaining sinus rhythm by catheter ablation of AF can improve and reverse AIC (63,64). Pulmonary vein isolation appears superior to AV node ablation and biventricular pacing in patients with drug-refractory AF and HF (64). A systematic review of 19 studies (914 patients) evaluating AF ablation in patients with concomitant LV dysfunction showed that sinus rhythm was maintained in 57% after a single procedure, with an increase to 82% with >1 procedure and/or use of antiarrhythmic drugs (65). LVEF increased by 13.3% (95% CI: 11% to 16%), suggesting the effectiveness of catheter ablation for maintaining sinus rhythm and reversing AIC.
The recent AATAC-AF trial randomized 203 persistent AF patients with HF and cardiomyopathy (LVEF <40%) to either amiodarone or catheter ablation. During a 24-month follow-up, 70% of patients in the ablation arm were free of AT/AF (vs. 34% in the amiodarone arm [p < 0.001]) and had significant improvements in mortality, hospitalization rates, and quality of life. LVEF improved 9.6 ± 7.4% in the ablation arm versus 4.2 ± 6.2% in the amiodarone arm (p < 0.01) (66). Catheter ablation thus offers an effective rhythm control strategy and avoids potentially toxic long-term antiarrhythmic therapy. The available data argues for catheter ablation as the best choice for rhythm control in the patient with AF-mediated AIC.
Atrial Flutter
Atrial flutter is more difficult to rate control than AF, given less concealed conduction into the AV node. Therefore, despite intense efforts at pharmacological rate control minimal exertion can lead to rapid ventricular rates. Given the inherent difficulty with rate control and the high success rate and low risk of complications with catheter ablation (67,68), ablation to eliminate atrial flutter is recommended when AIC is suspected. For those in whom catheter ablation is not feasible or desired, cardioversion with antiarrhythmic therapy or aggressive rate control should be employed.
Supraventricular Tachycardias
Persistent supraventricular tachycardias can result in AIC by several mechanisms (5). Near simultaneous AV relationships can have a negative hemodynamic effect. A curative strategy by catheter ablation should be pursued whenever possible as first-line therapy for supraventricular tachycardia-mediated AIC. Successful catheter ablation can normalize LVEF and is usually associated with excellent long-term outcomes (5,6).
PVCs and Ventricular Tachycardia
Idiopathic ventricular tachycardia and, more commonly, frequent PVCs, can lead to AIC in patients without structural heart disease and can exacerbate cardiomyopathy in patients with structural disease (10,52,69,70). PVCs associated with cardiomyopathy usually arise in the right or left ventricular outflow tract, but PVCs from non-outflow tract sites can also result in AIC (10,46,52).
The mechanism of PVC-mediated AIC is not fully understood. A large animal model using a pacing protocol to simulate paroxysmal PVCs produced an AIC phenotype that resolved completely within 2 to 4 weeks after pacing cessation. Tissue analysis did not show evidence of inflammation, fibrosis, or apoptosis, suggesting that PVC-induced cardiomyopathy in structurally normal hearts could be a functional abnormality (38). Potential mechanisms postulated include ventricular dyssynchrony, especially related to a left bundle branch block PVC morphology, abnormal calcium handling from the short coupling intervals, and abnormal ventricular filling from the post-PVC pause (10,37,38).
The development of a myopathy with atrial premature depolarizations (71) and the absence of convincing site-specific association in PVC-mediated AIC suggest that it may not be a simple matter of LV dyssynchrony. The causal relationship between PVCs and AIC has been firmly established on the basis of reversal of the cardiomyopathy with suppression and/or elimination of PVCs (72,73).
The most prominent predictor of cardiomyopathy in patients with frequent PVCs appears to be the daily burden of PVCs. A high PVC burden has been variably defined as ranging from >10,000 to 25,000 PVCs/day and as >10% to 24% of total heartbeats/day (74–76). There appears to be a threshold burden of ~10,000 PVCs/day for developing AIC. Ventricular function can improve if the PVC burden is reduced to <5,000/day (72). This is an important target when elimination of all PVCs may not be possible, especially in the setting of multiform PVCs.
Retrospective studies suggest multiple potential patient and PVC characteristics associated with the development of AIC (9,10,74,77,78). These characteristics include male sex, increased body mass index, asymptomatic PVCs, higher PVC coupling interval dispersion, interpolated PVCs, and presence of retrograde P waves (10,77–80). In a study of patients with high PVC burden followed for 14 months, 17 (38%) developed AIC. A PVC QRS duration >153 ms and non-outflow tract origin predicted development of AIC (46). Importantly, most patients with frequent PVCs will not develop cardiomyopathy (76). Currently, although an important goal of future investigation, no risk profile defines a group of patients requiring prophylactic PVC elimination to prevent AIC. However, it is advisable for patients with a high PVC burden to have periodic echocardiographic assessment to confirm stable LV chamber size and function.
Therapy for PVC-mediated AIC should be targeted at suppressing or eliminating the PVCs, and include antiarrhythmic therapy and catheter ablation. Beta-blockade and non-dihydropyridine calcium channel blockade are low-risk therapies, but with limited effectiveness. Beta-blockers are frequently considered first-line treatment because of the benign nature of the treatment. Dofetilide, mexiletine, sotalol, or amiodarone may be more effective, although with greater risk of side effects and proarrhythmia. Antiarrhythmic drug use is frequently reserved for patients who fail or are reluctant to undergo catheter ablation.
Catheter ablation has emerged as the definitive therapy for PVC-mediated AIC, with success rates ranging from 70% to 90% (52). Elimination of PVCs with ablation has been shown to improve LVEF, ventricular dimensions, mitral regurgitation, and functional status. In an observational series, ablation was superior to antiarrhythmic therapy in reducing PVCs and improving LVEF (51). Successful ablation of PVCs can improve the efficacy of cardiac resynchronization therapy in nonresponders (81). The elimination of high PVC burden (>10%) in patients with impaired LVEF can be associated with improvement of function, even when structural cardiac abnormalities are present (72,73,82).
Management in Adults–Summary
Our approach to manage patients with suspected AIC is to attempt careful and aggressive control of rate and rhythm, with the focus on arrhythmia elimination by catheter ablation whenever possible. The only tachyarrhythmias that do not appear to require aggressive treatment to prevent AIC are sinus tachycardia and POTS. Continued therapy with neurohormonal antagonists is advisable for favorable remodeling, although the duration of such therapy is not well-defined (83).
AIC in Children
In children, dilated cardiomyopathy is the most common reason for heart transplantation (84). Nearly 40% of children with cardiomyopathy undergo heart transplantation or die within 2 years of presentation (85). AIC must be considered in this setting. In the largest pediatric series of AIC, AET (59%) and permanent junctional reciprocating tachycardia (PJRT; 23%) were the most common arrhythmias represented. Ventricular arrhythmias were uncommon (86).
Tachyarrhythmias are a reversible cause of cardiomyopathy from fetal life onward (87–90). Children often present late because they fail to recognize palpitations or are unable to verbalize symptoms and come to medical attention only after the development of HF. Compared with adults, different arrhythmia mechanisms are represented in pediatric AIC.
Supraventricular arrhythmias are more common than ventricular arrhythmias in children and therefore are more frequently associated with AIC. In the newborn, a single, sustained episode of typical supraventricular tachycardia may be unrecognized until HF symptoms emerge; thus, neonates may present with decreased LV function, or even shock. In this group, prognosis and recovery of cardiac function are excellent after control of supraventricular tachycardia. This is in contrast to the more incessant tachycardias, where control is more challenging and recovery less rapid.
Etiologically, sustained rapid rates, QRS duration, AV dyssynchrony and heart rate irregularity all could contribute to AIC, yet not all children with incessant tachycardia develop AIC. In children, the tachycardias most often associated with AIC have a narrow QRS complex and 1:1 AV conduction. Heart rate irregularity occurs in pediatric AIC, but as salvos of tachycardia interspersed with periods of sinus rhythm, rather than as the persistent heart rate irregularity seen in AF.
As evident in many cardiac conditions, genetic factors may underlie the development of AIC. Serum- and glucocorticoid-regulated kinase-1 (SGK1), a component of the cardiac phosphatidylinositol 3-kinase signaling pathway has proarrhythmic effects and has been linked to biochemical and functional changes in the cardiac sodium (Na+) channel. These effects are reversed by treatment with ranolazine, which blocks the late Na+ current (91). Conversely, inhibition of SGK1 in the heart protects against fibrosis, HF, and Na+ channel alterations after hemodynamic stress. One might speculate that an underlying cardiomyopathic process is unmasked by a tachyarrhythmia, or that genetic factors are responsible for both arrhythmic and myopathic outcomes. Recent advancement in our understanding of channelopathic conditions and the overlapping of arrhythmic and myopathic phenotypes seen in some patients lends credibility to this theory (92–95). Mutations in the cardiac Na+ channel and in the ryanodine receptor, long known to be involved in arrhythmogenesis, can now be linked to abnormalities of contractile function (92).
Pediatric Arrhythmias Associated with AIC
Atrial Ectopic Tachycardia
AET is the most common arrhythmia associated with AIC in children (96). Although P-wave morphology and axis usually differ from sinus rhythm, AET foci near the sinus node are hard to differentiate from sinus tachycardia, especially in HF patients where tachycardia is expected. Increased automaticity is the most likely mechanism; others include triggered activity and micro-reentry (97–100). AET usually occurs without structural heart disease, but has been described after congenital heart disease surgery (100) and in the setting of channelopathies (101).
In a multicenter study of 249 children with AET, 28% had AIC (7). The cardiomyopathy varied from asymptomatic mild LV dysfunction to the need for HF medications in 52%; 3 required extracorporeal membrane oxygenation (7). Multiple antiarrhythmic medications or combinations of medications were used, with beta-blockers being the most common first-line therapy. No trends emerged to define the most successful medication. Catheter ablation was effective in 81% of patients in whom it was used. The use of electroanatomical mapping for ablation improved success (102) and decreased recurrence (103).
Spontaneous resolution of AET can occur, especially in those presenting within the first year of life, where 74% had resolution (7). Ablation proved safe and effective, but the authors suggest a trial of medical therapy in the youngest patients, in whom ablation may have more risk (104,105) and where spontaneous resolution is more likely.
Permanent Junctional Reciprocating Tachycardia
PJRT is an accessory pathway-mediated tachycardia with a long RP interval and occurs predominantly in infants and children. The pathway can be located anywhere in the AV junction, but is usually posteroseptal (106). Pathways are tortuous and slow-conducting; thus, tachycardia is often incessant.
In a recent review, 27% of PJRT patients presented in fetal life, 7% with hydrops, a fetal manifestation of AIC (107). Isolation of the AV junction is a continuing process that may not be complete at birth, and the presence of accessory connections crossing the annulus fibrosis could result in persistent perinatal supraventricular tachycardia. Although most children with PJRT present with palpitations or are noted to have rapid heart rates, in a series of 194 children, 18% presented with AIC (107). Cardiomyopathy is more likely to occur in children with longer RP interval/cycle length ratios, consistent with an accessory pathway with slow retrograde conduction and a wide, excitable gap (108). PJRT is often incessant and those with incessant PJRT had longer RP intervals, were younger at diagnosis, and more often had AIC (107). The clinical course of PJRT is not benign and spontaneous resolution is unlikely (107).
A number of antiarrhythmic medications are used, but a single most effective agent has not emerged (107). Beta-blockers are the common first choice, likely reflecting physician comfort, rather than proven efficacy. Complete tachycardia suppression with medications varies from 25% in the recent series (107) to >80% in a study using regimens that included amiodarone (109). Medical therapy is commonly employed in neonates and infants, whereas older children undergo ablation. Catheter ablation is the primary treatment for PJRT, with reported success rates of 90% (107). Thus, the role for medical therapy is limited to the neonate and small infant as a temporary measure to allow for the rare patient with spontaneous resolution, to suppress the tachycardia, and to prevent AIC and allow time and growth before undertaking catheter ablation.
Junctional Ectopic Tachycardia
Junctional ectopic tachycardia (JET), most commonly seen in small children following congenital heart surgery (110), is due to abnormal automaticity in the region of the AV junction. JET unassociated with cardiac surgery can present at any age, and congenital JET, presenting in infancy, is associated with high morbidity and mortality (110–112). In an early study, mortality was 34%, with sudden death occurring in infants (113). In a multicenter study of nonoperative JET, overall mortality was low, with all deaths occurring in children ≤6 months of age (114). Although only 16% presented with HF, the tachycardia was incessant in >40%. JET can be incessant or paroxysmal, although infants <6 months of age are more likely to have incessant tachycardia.
Medical management is commonly undertaken (89%) as the first step, but was variably effective, with a variety of drugs used. Beta-blockers were the most common first-line agent, but the majority required ≥2 drugs for control, with complete suppression seen in only 11%. Amiodarone alone or in combination was cited as the most effective for rate or rhythm control. Catheter ablation for JET can be accomplished with the preservation of AV nodal conduction (115,116), and cryoablation has been associated with success rates similar to radiofrequency ablation, but without AV block (114,117).
Ventricular Tachycardia and PVCs
Although rare in children, ventricular tachycardia can result in AIC. Incessant ventricular tachycardia of infancy occurs in association with ventricular Purkinje cell tumors or histiocytoid or lymphocytoid tumors (118–121). Both left and right ventricular tachycardias can result in pediatric AIC (86), and there are reports of PVC-induced AIC in children (122,123). The burden of ectopy needed, or other features associated with AIC risk, have not been defined.
AIC in Children–Summary
Tachyarrhythmias resulting in AIC in children differ from those in the adult. There is a predictable pattern of resolution with treatment and although previous small reports describe weeks to months for functional recovery and years for reverse remodeling, the median time to recovery in a larger study was <2 months (86). Recovery seems independent of treatment strategy (ablation vs. medical therapy). Recovery is predictable following arrhythmia control, and failure to recover should instigate a search for factors, such as subclinical arrhythmia recurrence or an underlying cardiomyopathy.
Recovery, Prognosis, and Impact of Recurrent Arrhythmia on AIC
Clinical and animal studies document the resolution of signs and symptoms of HF and recovery of LV dysfunction with termination of culprit arrhythmia. In several large animal models of pacing-induced cardiomyopathy, cessation of pacing resulted in significant improvements in LV function and a relative normalization of neurohormonal systems (13,32,36,124,125). For example, LV volumes and systolic performance will progress toward normalcy and biomarker measurements of renin-angiotensin activation fall sharply by 7 to 14 days following cessation of rapid pacing. Clinically, studies have shown that recovery from AIC usually happens over weeks to several months following arrhythmia suppression (1,9,52,83).
In 75 patients with PVC-mediated cardiomyopathy with successful catheter ablation, LVEF normalized in 5 ± 6 months, with 68% recovering by 4 months (9). A similar time course to recovery of LVEF was seen in another study of 24 patients with AIC (predominantly from AT) (1). Elimination of PVCs in patients with AIC resulted in progressive improvement in LVEF and clinical profile, irrespective of underlying structural heart disease (82).
Although originally considered a completely reversible form of cardiomyopathy, several observations have cast doubt on whether the improvement in LVEF in AIC really means “cure.” Whether myocardial structure and function fully normalizes remains unresolved. Resolution of HF and recovery of LVEF may not imply normalization of LV structure and function. Identifying predictors of recovery has therefore been of great interest. Early improvement (>25% improvement in 1 week) of LVEF following catheter ablation was predictive of complete normalization in LVEF during long-term follow-up (126). In a study of 69 patients with AIC from outflow tract PVCs, significant PVC suppression (>80% reduction in PVC burden and always <5,000 PVCs/day) showed LVEF improvement comparable to complete elimination (72). Longstanding and persistent AIC prior to recognition and curative therapy of the culprit arrhythmia could result in irreversible adverse LV remodeling and can limit complete recovery (83). Another major factor affecting recovery and outcomes is the effect of recurrent arrhythmia. In a study of 24 patients with AIC and NYHA Class III/IV HF, the median time from onset of arrhythmia to cardiomyopathy and development of HF was 4.2 years. As one would expect, aggressive rate and/or rhythm control of the culprit arrhythmia resulted in significant improvement in LVEF and resolution of HF in all patients within 6 months. Five of 24 patients developed recurrent arrhythmia, and all had a rapid decline in LVEF (within 6 months of arrhythmia recurrence) with recurrence of HF, which was again reversed with aggressive arrhythmia control.
In 69 patients with AF and LVEF <40% who underwent catheter ablation, those who maintained sinus rhythm (65%) had complete recovery of LVEF and maintained normal LVEF after 28 ± 11 months of follow-up, compared with those who had recurrent AF/AT. Interestingly, recovery of LVEF at 6 months was similar in both groups, but further improvement was seen only in the sinus rhythm group (40). An example of a patient with recurrent AF and AIC is shown in Table 2, where the time course of LVEF changes in relation to recurrence of AF is apparent. These data demonstrate that AIC is reversible if the culprit arrhythmia is controlled, but recurrence of the arrhythmia can result in HF and an abrupt decline in LVEF (1).
Table 2.
An Example of the Clinical Course of AIC When the Culprit Arrhythmia was Not Eliminated: A 54-Year-Old Male With AF and LVEF of 30%
| Time (months) | LVEF % | Intervention |
|---|---|---|
| 0 | 30 | Diuretics, ACE inhibitors, TEE, cardioversion |
| 1 | 47 | |
| 6 | 52 | AIC diagnosed |
| 14 | - | Recurrent AF per patient. Did not see a doctor |
| 18 | 25 | Cardioverted. Started on sotalol, but discontinued due to ↑ QTc interval. Amiodarone started |
| 23 | 50 | |
| 35 | 20 | Admitted with heart failure. Recurrent AF with rapid rates. Underwent AF ablation. Amiodarone continued |
| 40 | 55 | |
| 43 | 40 | Left atrial flutter with ventricular rate of 110 beats/min. Underwent perimitral LA flutter ablation |
| 53 | 52 | |
| 63 | 53 | Sinus rhythm. Taking amiodarone (200 mg daily), beta-blockers, ACE inhibitor, warfarin |
ACE = angiotensin-converting enzyme; AF = atrial fibrillation; AIC = arrhythmia-induced cardiomyopathy; LA = left atrial; LVEF = left ventricular ejection fraction; TEE = transesophageal echocardiography.
In AIC patients who have had resolution of arrhythmia, echocardiographic evaluations noted persistently elevated stroke volumes and LV end-systolic and end-diastolic volume indexes, suggesting persistent negative remodeling (127). In patients with AIC from focal AT who had normalization of LVEF with successful ablation, MRI scanning 5 years after ablation showed higher LV end-diastolic volumes and evidence for diffuse fibrosis when compared with focal AT patients without AIC and with healthy controls (128).
Current evidence thus suggests that myocardial structural and functional impairment can persist in AIC patients, even after successful elimination of the causal arrhythmia and normalization of LVEF and symptoms, suggesting that recovery is incomplete at an ultrastructural level. The “recovery” from AIC perhaps represents a transition to another form of LV remodeling and dysfunction. The mechanisms underlying these abnormalities, and whether patterns of recovery from AIC differ among different arrhythmias, require further study.
Risk of Sudden Death
Long-term survival of AIC patients following arrhythmia resolution is likely, but concerns remain. Sudden cardiac death has been reported in AIC patients following symptom recovery and LVEF normalization (1,6,129). Nerheim et al. reported 3 patients with AF-mediated AIC who died suddenly during follow-up while asymptomatic and having preserved LVEF and good functional status. Two patients were on adequate rate control and the third was on rhythm control. All 3 had index LVEFs that were markedly lower than those in the rest of the study group (n = 24), suggesting a greater risk in patients with severe baseline LV dysfunction (1). Available reports thus suggest that certain AIC patients could be at risk for sudden cardiac death, despite normalization of LVEF. However, the true risk of sudden death in AIC patients, especially following arrhythmia suppression and LVEF recovery, is likely low and there is no present justification for ICD implantation in patients with reversible cardiomyopathy (on the basis of improvement in LVEF).
Close surveillance, as well as continuation of neurohormonal antagonists to aid positive remodeling, is warranted in AIC, even after arrhythmia suppression and normalization of LVEF. A follow-up cardiac MRI for assessment of residual fibrosis/scar could provide valuable prognostic information (128). Arrhythmia recurrence can be devastating (1,6,129).
Future Directions
Since the original observations by Philips and Levine in 1949 of the association between rapid AF and a reversible form of HF (130), AIC has become a recognized disease entity. Diligent basic and clinical investigations have shed light on the etiology, pathophysiology, clinical features, treatment, and prognosis of AIC. However, several gaps in our understanding still exist. The true incidence and prevalence of AIC need to be defined. Future research should focus on specific pathophysiological mechanisms for cardiomyopathy in relation to a particular arrhythmia. Identifying clinical, genetic, and demographic characteristics that predispose a particular individual to AIC in the setting of a culprit arrhythmia will be crucial for early recognition of this disease process, before cardiomyopathy develops. Questions still exist concerning optimal therapeutic strategies: Is rhythm control really better than rate control for improving long-term outcomes in AF-mediated AIC? How can we best prevent residual ultrastructural abnormalities? What are the inter-relationships between structural heart disease, arrhythmia, and risk of sudden death?
The “Big Picture”: Clinical and Global Health Impact of AIC
Heart failure is increasing globally. Tachyarrhythmias and frequent PVCs remain an important cause of nonischemic cardiomyopathy and manifest HF that is partially or completely reversible with control of the arrhythmia. However, this condition remains under-recognized, often leading to worsening HF and deterioration of LV function. Given that limited therapeutic options exist for advanced HF patients, the ability to detect a potentially reversible cause is critical. Raising awareness about this condition globally is paramount.
Conclusions
AIC can affect patients of any age and has a wide range of clinical manifestations, from asymptomatic tachycardia or ectopy to cardiomyopathy to end-stage HF. Early recognition is critical, and aggressive treatment aimed at controlling or eliminating the inciting arrhythmia results in symptom resolution and recovery of ventricular function. However, cellular and extracellular ultrastructural changes can persist and can contribute to a rapid decline in cardiac function with arrhythmia recurrence, as well as confer a risk of sudden cardiac death. Thus, this supposedly “benign” and “reversible” condition poses unique challenges to the practicing cardiologist. Novel approaches for early detection, as well as targeted intervention, are warranted and may ultimately improve long-term outcomes for these patients.
Acknowledgments
Funding: Dr. Spinale’s research is supported by NIH grants HL057952, HL059165, and HL095608 and a Merit Award from the Veterans’ Affairs Health Administration.
Abbreviations
- AET
atrial ectopic tachycardia
- AF
atrial fibrillation
- AIC
arrhythmia-induced cardiomyopathy
- AT
atrial tachycardia
- BNP
B-type natriuretic peptide
- HF
heart failure
- LV
left ventricle/ventricular
- LVEF
left ventricular ejection fraction
- PVC
premature ventricular complexes
- PJRT
permanent junctional reciprocating tachycardia
Footnotes
DISCLAIMER: The views expressed in this paper by the American College of Cardiology’s (ACC’s) Electrophysiology Section Leadership Council do not necessarily reflect the views of the Journal of the American College of Cardiology or the ACC.
Disclosures: Dr. Gopinathannair serves as a consultant to St. Jude Medical and Abiomed, and is on the Speaker’s Bureau for Pfizer/Bristol Myers Squibb. Dr. Etheridge has reported that she has no relationships relevant to the contents of this paper to disclose. Dr. Etheridge serves as the vice-president of Sudden Arrhythmic Death Syndrome Society and is a member of the ACC’s EP council, which are both voluntary positions. Dr. Marchlinski’s research is sponsored by Biosense Webster, Boston Scientific, St. Jude Medical, and Medtronic. Dr. Marchlinski also serves on the Advisory Panel/Board for Biosense Webster, Boston Scientific, and Medtronic; and has received lecture honoraria from Biosense Webster, Biotronik, Boston Scientific, St. Jude Medical, and Medtronic. Dr. Spinale’s research is supported by NIH grants HL057952, HL059165, and HL095608 and a Merit Award from the Veterans’ Affairs Health Administration. Dr. Lakkireddy serves as a speaker and consultant to Janssen, Pfizer, St. Jude Medical, and Biosense Webster. Dr. Olshansky serves as a consultant to Boston Scientific, Medtronic, Daichi Sankyo, Biotronik, Cardionomics, BioControl, Amarin, Boehringer Ingelheim, and On-X.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nerheim P, Birger-Botkin S, Piracha L, et al. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation. 2004;110:247–52. doi: 10.1161/01.CIR.0000135472.28234.CC. [DOI] [PubMed] [Google Scholar]
- 2.Gopinathannair R, Sullivan R, Olshansky B. Tachycardia-mediated cardiomyopathy: recognition and management. Curr Heart Fail Rep. 2009;6:257–64. doi: 10.1007/s11897-009-0035-3. [DOI] [PubMed] [Google Scholar]
- 3.Fenelon G, Wijns W, Andries E, et al. Tachycardiomyopathy: mechanisms and clinical implications. Pacing Clin Electrophysiol. 1996;19:95–106. doi: 10.1111/j.1540-8159.1996.tb04796.x. [DOI] [PubMed] [Google Scholar]
- 4.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 5.Medi C, Kalman JM, Haqqani H, et al. Tachycardia-mediated cardiomyopathy secondary to focal atrial tachycardia: long-term outcome after catheter ablation. J Am Coll Cardiol. 2009;53:1791–7. doi: 10.1016/j.jacc.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Ju W, Yang B, Li M, et al. Tachycardiomyopathy complicated by focal atrial tachycardia: incidence, risk factors, and long-term outcome. J Cardiovasc Electrophysiol. 2014;25:953–7. doi: 10.1111/jce.12428. [DOI] [PubMed] [Google Scholar]
- 7.Kang KT, Etheridge SP, Kantoch MJ, et al. Current management of focal atrial tachycardia in children: a multicenter experience. Circ Arrhythm Electrophysiol. 2014;7:664–70. doi: 10.1161/CIRCEP.113.001423. [DOI] [PubMed] [Google Scholar]
- 8.Hasdemir C, Yuksel A, Camli D, et al. Late gadolinium enhancement CMR in patients with tachycardia-induced cardiomyopathy caused by idiopathic ventricular arrhythmias. Pacing Clin Electrophysiol. 2012;35:465–70. doi: 10.1111/j.1540-8159.2011.03324.x. [DOI] [PubMed] [Google Scholar]
- 9.Yokokawa M, Good E, Crawford T, et al. Recovery from left ventricular dysfunction after ablation of frequent premature ventricular complexes. Heart Rhythm. 2013;10:172–5. doi: 10.1016/j.hrthm.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura M, Badhwar N, Vedantham V, et al. Coupling interval dispersion and body mass index are independent predictors of idiopathic premature ventricular complex-induced cardiomyopathy. J Cardiovasc Electrophysiol. 2014;25:756–62. doi: 10.1111/jce.12391. [DOI] [PubMed] [Google Scholar]
- 11.Whipple GHSL, Woodman EG, Thoephilis C, et al. Reversible congestive heart failure due to rapid stimulation of the normal heart. Proc N Engl Cardiovasc Soc. 1961;20:39–40. [Google Scholar]
- 12.Spinale FG, Hendrick DA, Crawford FA, et al. Chronic supraventricular tachycardia causes ventricular dysfunction and subendocardial injury in swine. Am J Physiol. 1990;259:H218–29. doi: 10.1152/ajpheart.1990.259.1.H218. [DOI] [PubMed] [Google Scholar]
- 13.Tomita M, Spinale FG, Crawford FA, et al. Changes in left ventricular volume, mass, and function during the development and regression of supraventricular tachycardia-induced cardiomyopathy. Disparity between recovery of systolic versus diastolic function. Circulation. 1991;83:635–44. doi: 10.1161/01.cir.83.2.635. [DOI] [PubMed] [Google Scholar]
- 14.Moe GW, Angus C, Howard RJ, et al. Evaluation of indices of left ventricular contractility and relaxation in evolving canine experimental heart failure. Cardiovasc Res. 1992;26:362–6. doi: 10.1093/cvr/26.4.362. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong PW, Stopps TP, Ford SE, et al. Rapid ventricular pacing in the dog: pathophysiologic studies of heart failure. Circulation. 1986;74:1075–84. doi: 10.1161/01.cir.74.5.1075. [DOI] [PubMed] [Google Scholar]
- 16.Shannon RP, Komamura K, Stambler BS, et al. Alterations in myocardial contractility in conscious dogs with dilated cardiomyopathy. Am J Physiol. 1991;260:H1903–11. doi: 10.1152/ajpheart.1991.260.6.H1903. [DOI] [PubMed] [Google Scholar]
- 17.Komamura K, Shannon RP, Ihara T, et al. Exhaustion of Frank-Starling mechanism in conscious dogs with heart failure. Am J Physiol. 1993;265:H1119–31. doi: 10.1152/ajpheart.1993.265.4.H1119. [DOI] [PubMed] [Google Scholar]
- 18.Cheng CP, Noda T, Nozawa T, et al. Effect of heart failure on the mechanism of exercise-induced augmentation of mitral valve flow. Circ Res. 1993;72:795–806. doi: 10.1161/01.res.72.4.795. [DOI] [PubMed] [Google Scholar]
- 19.Moe GW, Grima EA, Wong NL, et al. Dual natriuretic peptide system in experimental heart failure. J Am Coll Cardiol. 1993;22:891–8. doi: 10.1016/0735-1097(93)90208-i. [DOI] [PubMed] [Google Scholar]
- 20.Spinale FG, Holzgrefe HH, Mukherjee R, et al. Angiotensin-converting enzyme inhibition and the progression of congestive cardiomyopathy. Effects on left ventricular and myocyte structure and function. Circulation. 1995;92:562–78. doi: 10.1161/01.cir.92.3.562. [DOI] [PubMed] [Google Scholar]
- 21.Riegger GA, Elsner D, Kromer EP, et al. Atrial natriuretic peptide in congestive heart failure in the dog: plasma levels, cyclic guanosine monophosphate, ultrastructure of atrial myoendocrine cells, and hemodynamic, hormonal, and renal effects. Circulation. 1988;77:398–406. doi: 10.1161/01.cir.77.2.398. [DOI] [PubMed] [Google Scholar]
- 22.Sun D, Huang A, Zhao G, et al. Reduced NO-dependent arteriolar dilation during the development of cardiomyopathy. Am J Physiol Heart Circ Physiol. 2000;278:H461–8. doi: 10.1152/ajpheart.2000.278.2.H461. [DOI] [PubMed] [Google Scholar]
- 23.Recchia FA, McConnell PI, Bernstein RD, et al. Reduced nitric oxide production and altered myocardial metabolism during the decompensation of pacing-induced heart failure in the conscious dog. Circ Res. 1998;83:969–79. doi: 10.1161/01.res.83.10.969. [DOI] [PubMed] [Google Scholar]
- 24.Bradham WS, Bozkurt B, Gunasinghe H, et al. Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res. 2002;53:822–30. doi: 10.1016/s0008-6363(01)00503-x. [DOI] [PubMed] [Google Scholar]
- 25.Spinale FG, de Gasparo M, Whitebread S, et al. Modulation of the renin-angiotensin pathway through enzyme inhibition and specific receptor blockade in pacing-induced heart failure: I. Effects on left ventricular performance and neurohormonal systems. Circulation. 1997;96:2385–96. doi: 10.1161/01.cir.96.7.2385. [DOI] [PubMed] [Google Scholar]
- 26.Eising GP, Hammond HK, Helmer GA, et al. Force-frequency relations during heart failure in pigs. Am J Physiol. 1994;267:H2516–22. doi: 10.1152/ajpheart.1994.267.6.H2516. [DOI] [PubMed] [Google Scholar]
- 27.Cory CR, McCutcheon LJ, O’Grady M, et al. Compensatory downregulation of myocardial Ca channel in SR from dogs with heart failure. Am J Physiol. 1993;264:H926–37. doi: 10.1152/ajpheart.1993.264.3.H926. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee R, Hewett KW, Walker JD, et al. Changes in L-type calcium channel abundance and function during the transition to pacing-induced congestive heart failure. Cardiovasc Res. 1998;37:432–44. doi: 10.1016/s0008-6363(97)00128-4. [DOI] [PubMed] [Google Scholar]
- 29.Perreault CL, Shannon RP, Komamura K, et al. Abnormalities in intracellular calcium regulation and contractile function in myocardium from dogs with pacing-induced heart failure. J Clin Invest. 1992;89:932–8. doi: 10.1172/JCI115674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vatner DE, Sato N, Kiuchi K, et al. Decrease in myocardial ryanodine receptors and altered excitation-contraction coupling early in the development of heart failure. Circulation. 1994;90:1423–30. doi: 10.1161/01.cir.90.3.1423. [DOI] [PubMed] [Google Scholar]
- 31.Spinale FG, Fulbright BM, Mukherjee R, et al. Relation between ventricular and myocyte function with tachycardia-induced cardiomyopathy. Circ Res. 1992;71:174–87. doi: 10.1161/01.res.71.1.174. [DOI] [PubMed] [Google Scholar]
- 32.Spinale FG, Zellner JL, Johnson WS, et al. Cellular and extracellular remodeling with the development and recovery from tachycardia-induced cardiomyopathy: changes in fibrillar collagen, myocyte adhesion capacity and proteoglycans. J Mol Cell Cardiol. 1996;28:1591–608. doi: 10.1006/jmcc.1996.0150. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Cigola E, Cheng W, et al. Myocyte nuclear mitotic division and programmed myocyte cell death characterize the cardiac myopathy induced by rapid ventricular pacing in dogs. Lab Invest. 1995;73:771–87. [PubMed] [Google Scholar]
- 34.Kajstura J, Zhang X, Liu Y, et al. The cellular basis of pacing-induced dilated cardiomyopathy. Myocyte cell loss and myocyte cellular reactive hypertrophy. Circulation. 1995;92:2306–17. doi: 10.1161/01.cir.92.8.2306. [DOI] [PubMed] [Google Scholar]
- 35.Zellner JL, Spinale FG, Eble DM, et al. Alterations in myocyte shape and basement membrane attachment with tachycardia-induced heart failure. Circ Res. 1991;69:590–600. doi: 10.1161/01.res.69.3.590. [DOI] [PubMed] [Google Scholar]
- 36.Spinale FG, Tomita M, Zellner JL, et al. Collagen remodeling and changes in LV function during development and recovery from supraventricular tachycardia. Am J Physiol. 1991;261:H308–18. doi: 10.1152/ajpheart.1991.261.2.H308. [DOI] [PubMed] [Google Scholar]
- 37.Spinale FG, Coker ML, Thomas CV, et al. Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res. 1998;82:482–95. doi: 10.1161/01.res.82.4.482. [DOI] [PubMed] [Google Scholar]
- 38.Huizar JF, Kaszala K, Potfay J, et al. Left ventricular systolic dysfunction induced by ventricular ectopy: a novel model for premature ventricular contraction-induced cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:543–9. doi: 10.1161/CIRCEP.111.962381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira JP, Santos M. Heart failure and atrial fibrillation: from basic science to clinical practice. Int J Mol Sci. 2015;16:3133–47. doi: 10.3390/ijms16023133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nedios S, Sommer P, Dagres N, et al. Long-term follow-up after atrial fibrillation ablation in patients with impaired left ventricular systolic function: the importance of rhythm and rate control. Heart Rhythm. 2014;11:344–51. doi: 10.1016/j.hrthm.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 41.Zupan I, Rakovec P, Budihna N, et al. Tachycardia induced cardiomyopathy in dogs; relation between chronic supraventricular and chronic ventricular tachycardia. Int J Cardiol. 1996;56:75–81. doi: 10.1016/0167-5273(96)02728-3. [DOI] [PubMed] [Google Scholar]
- 42.Shinbane JS, Wood MA, Jensen DN, et al. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–15. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 43.Calò L, De Ruvo E, Sette A, et al. Tachycardia-induced cardiomyopathy: mechanisms of heart failure and clinical implications. J Cardiovasc Med (Hagerstown) 2007;8:138–43. doi: 10.2459/01.JCM.0000260841.30415.62. [DOI] [PubMed] [Google Scholar]
- 44.Tibayan FA, Lai DT, Timek TA, et al. Alterations in left ventricular torsion in tachycardia-induced dilated cardiomyopathy. J Thorac Cardiovasc Surg. 2002;124:43–9. doi: 10.1067/mtc.2002.121299. [DOI] [PubMed] [Google Scholar]
- 45.Deshmukh PM, Krishnamani R, Romanyshyn M, et al. Association of angiotensin converting enzyme gene polymorphism with tachycardia cardiomyopathy. Int J Mol Med. 2004;13:455–8. [PubMed] [Google Scholar]
- 46.Carballeira Pol L, Deyell MW, Frankel DS, et al. Ventricular premature depolarization QRS duration as a new marker of risk for the development of ventricular premature depolarization-induced cardiomyopathy. Heart Rhythm. 2014;11:299–306. doi: 10.1016/j.hrthm.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 47.Gallagher JJ. Tachycardia and cardiomyopathy: the chicken-egg dilemma revisited. J Am Coll Cardiol. 1985;6:1172–3. doi: 10.1016/s0735-1097(85)80328-4. [DOI] [PubMed] [Google Scholar]
- 48.Jeong YH, Choi KJ, Song JM, et al. Diagnostic approach and treatment strategy in tachycardia-induced cardiomyopathy. Clin Cardiol. 2008;31:172–8. doi: 10.1002/clc.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campos B, Jauregui ME, Park KM, et al. New unipolar electrogram criteria to identify irreversibility of nonischemic left ventricular cardiomyopathy. J Am Coll Cardiol. 2012;60:2194–204. doi: 10.1016/j.jacc.2012.08.977. [DOI] [PubMed] [Google Scholar]
- 50.Nia AM, Gassanov N, Dahlem KM, et al. Diagnostic accuracy of NT-proBNP ratio (BNP-R) for early diagnosis of tachycardia-mediated cardiomyopathy: a pilot study. Clin Res Cardiol. 2011;100:887–96. doi: 10.1007/s00392-011-0319-y. [DOI] [PubMed] [Google Scholar]
- 51.Zhong L, Lee YH, Huang XM, et al. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm. 2014;11:187–93. doi: 10.1016/j.hrthm.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 52.Bogun F, Crawford T, Reich S, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007;4:863–7. doi: 10.1016/j.hrthm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Ellison KE, Stevenson WG, Sweeney MO, et al. Management of arrhythmias in heart failure. Congest Heart Fail. 2003;9:91–9. doi: 10.1111/j.1527-5299.2003.00271.x. [DOI] [PubMed] [Google Scholar]
- 54.Cha YM, Redfield MM, Shen WK, et al. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation. 2004;109:2839–43. doi: 10.1161/01.CIR.0000132470.78896.A8. [DOI] [PubMed] [Google Scholar]
- 55.Van Gelder IC, Groenveld HF, Crijns HJ, et al. RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–73. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 56.Farshi R, Kistner D, Sarma JS, et al. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: a crossover open-label study of five drug regimens. J Am Coll Cardiol. 1999;33:304–10. doi: 10.1016/s0735-1097(98)00561-0. [DOI] [PubMed] [Google Scholar]
- 57.Olshansky B, Rosenfeld LE, Warner AL, et al. AFFIRM Investigators. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: approaches to control rate in atrial fibrillation. J Am Coll Cardiol. 2004;43:1201–8. doi: 10.1016/j.jacc.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 58.Narasimhan C, Blanck Z, Akhtar M. Atrioventricular nodal modification and atrioventricular junctional ablation for control of ventricular rate in atrial fibrillation. J Cardiovasc Electrophysiol. 1998;9:S146–50. [PubMed] [Google Scholar]
- 59.Gentlesk PJ, Sauer WH, Gerstenfeld EP, et al. Reversal of left ventricular dysfunction following ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:9–14. doi: 10.1111/j.1540-8167.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 60.Roy D, Talajic M, Nattel S, et al. Atrial Fibrillation and Congestive Heart Failure Investigators. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 61.Shelton RJ, Clark AL, Goode K, et al. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II Study) Heart. 2009;95:924–30. doi: 10.1136/hrt.2008.158931. [DOI] [PubMed] [Google Scholar]
- 62.The AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–13. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 63.Hsu LF, Jaïs P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–83. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 64.Khan MN, Jaïs P, Cummings J, et al. PABA-CHF Investigators. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778–85. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 65.Ganesan AN, Nandal S, Luker J, et al. Catheter ablation of atrial fibrillation in patients with concomitant left ventricular impairment: a systematic review of efficacy and effect on ejection fraction. Heart Lung Circ. 2015;24:270–80. doi: 10.1016/j.hlc.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 66.Di Biase L, Mohanty S, Mohanty P, et al. Ablation vs. amiodarone for treatment of atrial fibrillation in patients with congestive heart failure and an implanted ICD/CRTD. Paper presented at: ACC Scientific Sessions; March 16, 2015; San Diego, CA. [Google Scholar]
- 67.Perez FJ, Schubert CM, Parvez B, et al. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis. Circ Arrhythm Electrophysiol. 2009;2:393–401. doi: 10.1161/CIRCEP.109.871665. [DOI] [PubMed] [Google Scholar]
- 68.Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary. a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Supraventricular Arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol. 2003;42:1493–531. doi: 10.1016/j.jacc.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Del Carpio Munoz F, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol. 2011;22:791–8. doi: 10.1111/j.1540-8167.2011.02021.x. [DOI] [PubMed] [Google Scholar]
- 70.Taieb JM, Maury P, Shah D, et al. Reversal of dilated cardiomyopathy by the elimination of frequent left or right premature ventricular contractions. J Interv Card Electrophysiol. 2007;20:9–13. doi: 10.1007/s10840-007-9157-2. [DOI] [PubMed] [Google Scholar]
- 71.Hasdemir C, Simsek E, Yuksel A. Premature atrial contraction-induced cardiomyopathy. Europace. 2013;15:1790. doi: 10.1093/europace/eut141. [DOI] [PubMed] [Google Scholar]
- 72.Mountantonakis SE, Frankel DS, Gerstenfeld EP, et al. Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation: effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011;8:1608–14. doi: 10.1016/j.hrthm.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 73.Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm. 2009;6:1543–9. doi: 10.1016/j.hrthm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–9. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 75.Kanei Y, Friedman M, Ogawa N, et al. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol. 2008;13:81–5. doi: 10.1111/j.1542-474X.2007.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart. 2009;95:1230–7. doi: 10.1136/hrt.2008.159558. [DOI] [PubMed] [Google Scholar]
- 77.Yokokawa M, Kim HM, Good E, et al. Relation of symptoms and symptom duration to premature ventricular complex-induced cardiomyopathy. Heart Rhythm. 2012;9:92–5. doi: 10.1016/j.hrthm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 78.Yokokawa M, Kim HM, Good E, et al. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012;9:1460–4. doi: 10.1016/j.hrthm.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 79.Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm. 2011;8:1046–9. doi: 10.1016/j.hrthm.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 80.Ban JE, Park HC, Park JS, et al. Electrocardiographic and electrophysiological characteristics of premature ventricular complexes associated with left ventricular dysfunction in patients without structural heart disease. Europace. 2013;15:735–41. doi: 10.1093/europace/eus371. [DOI] [PubMed] [Google Scholar]
- 81.Lakkireddy D, Di Biase L, Ryschon K, et al. Radiofrequency ablation of premature ventricular ectopy improves the efficacy of cardiac resynchronization therapy in nonresponders. J Am Coll Cardiol. 2012;60:1531–9. doi: 10.1016/j.jacc.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 82.Penela D, Van Huls Van Taxis C, Aguinaga L, et al. Neurohormonal, structural, and functional recovery pattern after premature ventricular complex ablation is independent of structural heart disease status in patients with depressed left ventricular ejection fraction: a prospective multicenter study. J Am Coll Cardiol. 2013;62:1195–202. doi: 10.1016/j.jacc.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 83.Ilkhanoff L, Gerstenfeld EP, Zado ES, et al. Changes in ventricular dimensions and function during recovery of atrial tachycardia-induced cardiomyopathy treated with catheter ablation. J Cardiovasc Electrophysiol. 2007;18:1104–6. doi: 10.1111/j.1540-8167.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 84.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–55. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz ML, Cox GF, Lin AE, et al. Clinical approach to genetic cardiomyopathy in children. Circulation. 1996;94:2021–38. doi: 10.1161/01.cir.94.8.2021. [DOI] [PubMed] [Google Scholar]
- 86.Moore JP, Patel PA, Shannon KM, et al. Predictors of myocardial recovery in pediatric tachycardia-induced cardiomyopathy. Heart Rhythm. 2014;11:1163–9. doi: 10.1016/j.hrthm.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 87.Kleinman CS, Nehgme RA. Cardiac arrhythmias in the human fetus. Pediatr Cardiol. 2004;25:234–51. doi: 10.1007/s00246-003-0589-x. [DOI] [PubMed] [Google Scholar]
- 88.Strasburger JF, Cuneo BF, Michon MM, et al. Amiodarone therapy for drug-refractory fetal tachycardia. Circulation. 2004;109:375–9. doi: 10.1161/01.CIR.0000109494.05317.58. [DOI] [PubMed] [Google Scholar]
- 89.Salerno JC, Kertesz NJ, Friedman RA, et al. Clinical course of atrial ectopic tachycardia is age-dependent: results and treatment in children < 3 or ≥3 years of age. J Am Coll Cardiol. 2004;43:438–44. doi: 10.1016/j.jacc.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 90.Horenstein MS, Saarel E, Dick M, et al. Reversible symptomatic dilated cardiomyopathy in older children and young adolescents due to primary non-sinus supraventricular tachyarrhythmias. Pediatr Cardiol. 2003;24:274–9. doi: 10.1007/s00246-002-0274-5. [DOI] [PubMed] [Google Scholar]
- 91.Das S, Aiba T, Rosenberg M, et al. Pathological role of serum- and glucocorticoid-regulated kinase 1 in adverse ventricular remodeling. Circulation. 2012;126:2208–19. doi: 10.1161/CIRCULATIONAHA.112.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ai X, Curran JW, Shannon TR, et al. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–22. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 93.Beckermann TM, McLeod K, Murday V, et al. Novel SCN5A mutation in amiodarone-responsive multifocal ventricular ectopy-associated cardiomyopathy. Heart Rhythm. 2014;11:1446–53. doi: 10.1016/j.hrthm.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McNair WP, Ku L, Taylor MR, et al. Familial Cardiomyopathy Registry Research Group. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation. 2004;110:2163–7. doi: 10.1161/01.CIR.0000144458.58660.BB. [DOI] [PubMed] [Google Scholar]
- 95.Olson TM, Michels VV, Ballew JD, et al. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA. 2005;293:447–54. doi: 10.1001/jama.293.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koike K, Hesslein PS, Finlay CD, et al. Atrial automatic tachycardia in children. Am J Cardiol. 1988;61:1127–30. doi: 10.1016/0002-9149(88)90144-0. [DOI] [PubMed] [Google Scholar]
- 97.Kammeraad JA, Balaji S, Oliver RP, et al. Nonautomatic focal atrial tachycardia: characterization and ablation of a poorly understood arrhythmia in 38 patients. Pacing Clin Electrophysiol. 2003;26:736–42. doi: 10.1046/j.1460-9592.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 98.Moltedo JM, Cannon BC, Fenrich AL, et al. Radiofrequency ablation of nonautomatic focal atrial tachycardia in children with structurally normal hearts. J Interv Card Electrophysiol. 2009;26:225–9. doi: 10.1007/s10840-009-9430-7. [DOI] [PubMed] [Google Scholar]
- 99.Seslar SP, Alexander ME, Berul CI, et al. Ablation of nonautomatic focal atrial tachycardia in children and adults with congenital heart disease. J Cardiovasc Electrophysiol. 2006;17:359–65. doi: 10.1111/j.1540-8167.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- 100.Kato Y, Horigome H, Takahashi-Igari M, et al. Focal atrial tachycardia originating from inside the inferior vena cava late after surgical repair of congenital heart defects. Pediatr Cardiol. 2011;32:846–8. doi: 10.1007/s00246-011-9978-8. [DOI] [PubMed] [Google Scholar]
- 101.Di Pino A, Caruso E, Costanzo L, et al. A novel RyR2 mutation in a 2-year-old baby presenting with atrial fibrillation, atrial flutter, and atrial ectopic tachycardia. Heart Rhythm. 2014;11:1480–3. doi: 10.1016/j.hrthm.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 102.Toyohara K, Fukuhara H, Yoshimoto J, et al. Electrophysiologic studies and radiofrequency catheter ablation of ectopic atrial tachycardia in children. Pediatr Cardiol. 2011;32:40–6. doi: 10.1007/s00246-010-9809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cummings RM, Mahle WT, Strieper MJ, et al. Outcomes following electroanatomic mapping and ablation for the treatment of ectopic atrial tachycardia in the pediatric population. Pediatr Cardiol. 2008;29:393–7. doi: 10.1007/s00246-007-9137-4. [DOI] [PubMed] [Google Scholar]
- 104.Kugler JD, Danford DA, Deal BJ, et al. Pediatric Electrophysiology Society. Radiofrequency catheter ablation for tachyarrhythmias in children and adolescents. N Engl J Med. 1994;330:1481–7. doi: 10.1056/NEJM199405263302103. [DOI] [PubMed] [Google Scholar]
- 105.Saul JP, Hulse JE, Papagiannis J, et al. Late enlargement of radiofrequency lesions in infant lambs. Implications for ablation procedures in small children. Circulation. 1994;90:492–9. doi: 10.1161/01.cir.90.1.492. [DOI] [PubMed] [Google Scholar]
- 106.Ticho BS, Saul JP, Hulse JE, De W, et al. Variable location of accessory pathways associated with the permanent form of junctional reciprocating tachycardia and confirmation with radiofrequency ablation. Am J Cardiol. 1992;70:1559–64. doi: 10.1016/0002-9149(92)90457-a. [DOI] [PubMed] [Google Scholar]
- 107.Kang KT, Potts JE, Radbill AE, et al. Permanent junctional reciprocating tachycardia in children: a multicenter experience. Heart Rhythm. 2014;11:1426–32. doi: 10.1016/j.hrthm.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 108.Gaztañaga L, Marchlinski FE, Betensky BP. Mechanisms of cardiac arrhythmias. Rev Esp Cardiol (Engl Ed) 2012;65:174–85. doi: 10.1016/j.recesp.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 109.Vaksmann G, D’Hoinne C, Lucet V, et al. Permanent junctional reciprocating tachycardia in children: a multicentre study on clinical profile and outcome. Heart. 2006;92:101–4. doi: 10.1136/hrt.2004.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garson A, Jr, Gillette PC. Junctional ectopic tachycardia in children: electrocardiography, electrophysiology and pharmacologic response. Am J Cardiol. 1979;44:298–302. doi: 10.1016/0002-9149(79)90320-5. [DOI] [PubMed] [Google Scholar]
- 111.Cilliers AM, du Plessis JP, Clur SA, et al. Junctional ectopic tachycardia in six paediatric patients. Heart. 1997;78:413–5. doi: 10.1136/hrt.78.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sarubbi B, Musto B, Ducceschi V, et al. Congenital junctional ectopic tachycardia in children and adolescents: a 20 year experience based study. Heart. 2002;88:188–90. doi: 10.1136/heart.88.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Villain E, Vetter VL, Garcia JM, et al. Evolving concepts in the management of congenital junctional ectopic tachycardia. A multicenter study. Circulation. 1990;81:1544–9. doi: 10.1161/01.cir.81.5.1544. [DOI] [PubMed] [Google Scholar]
- 114.Collins KK, Van Hare GF, Kertesz NJ, et al. Pediatric nonpost-operative junctional ectopic tachycardia medical management and interventional therapies. J Am Coll Cardiol. 2009;53:690–7. doi: 10.1016/j.jacc.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 115.Hamdan M, Van Hare GF, Fisher W, et al. Selective catheter ablation of the tachycardia focus in patients with nonreentrant junctional tachycardia. Am J Cardiol. 1996;78:1292–7. doi: 10.1016/s0002-9149(96)00616-9. [DOI] [PubMed] [Google Scholar]
- 116.Fishberger SB, Rossi AF, Messina JJ, et al. Successful radiofrequency catheter ablation of congenital junctional ectopic tachycardia with preservation of atrioventricular conduction in a 9-month-old infant. Pacing Clin Electrophysiol. 1998;21:2132–5. doi: 10.1111/j.1540-8159.1998.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 117.Law IH, Von Bergen NH, Gingerich JC, et al. Transcatheter cryothermal ablation of junctional ectopic tachycardia in the normal heart. Heart Rhythm. 2006;3:903–7. doi: 10.1016/j.hrthm.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 118.Ruszkiewicz AR, Vernon-Roberts E. Sudden death in an infant due to histiocytoid cardiomyopathy. A light-microscopic, ultrastructural, and immunohistochemical study. Am J Forensic Med Pathol. 1995;16:74–80. doi: 10.1097/00000433-199503000-00017. [DOI] [PubMed] [Google Scholar]
- 119.Gelb AB, Van Meter SH, Billingham ME, et al. Infantile histiocytoid cardiomyopathy--myocardial or conduction system hamartoma: what is the cell type involved? Hum Pathol. 1993;24:1226–31. doi: 10.1016/0046-8177(93)90219-7. [DOI] [PubMed] [Google Scholar]
- 120.Garson A, Jr, Smith RT, Jr, Moak JP, et al. Incessant ventricular tachycardia in infants: myocardial hamartomas and surgical cure. J Am Coll Cardiol. 1987;10:619–26. doi: 10.1016/s0735-1097(87)80205-x. [DOI] [PubMed] [Google Scholar]
- 121.Davis AM, Gow RM, McCrindle BW, et al. Clinical spectrum, therapeutic management, and follow-up of ventricular tachycardia in infants and young children. Am Heart J. 1996;131:186–91. doi: 10.1016/s0002-8703(96)90068-x. [DOI] [PubMed] [Google Scholar]
- 122.Pfammatter JP, Paul T. Idiopathic ventricular tachycardia in infancy and childhood: a multicenter study on clinical profile and outcome. J Am Coll Cardiol. 1999;33:2067–72. doi: 10.1016/s0735-1097(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 123.Kakavand B, Ballard HO, Disessa TG. Frequent ventricular premature beats in children with a structurally normal heart: a cause for reversible left ventricular dysfunction? Pediatr Cardiol. 2010;31:986–90. doi: 10.1007/s00246-010-9740-7. [DOI] [PubMed] [Google Scholar]
- 124.Spinale FG, Holzgrefe HH, Mukherjee R, et al. LV and myocyte structure and function after early recovery from tachycardia-induced cardiomyopathy. Am J Physiol. 1995;268:H836–47. doi: 10.1152/ajpheart.1995.268.2.H836. [DOI] [PubMed] [Google Scholar]
- 125.Moe GW, Stopps TP, Howard RJ, et al. Early recovery from heart failure: insights into the pathogenesis of experimental chronic pacing-induced heart failure. J Lab Clin Med. 1988;112:426–32. [PubMed] [Google Scholar]
- 126.Hasdemir C, Kartal Y, Simsek E, et al. Time course of recovery of left ventricular systolic dysfunction in patients with premature ventricular contraction-induced cardiomyopathy. Pacing Clin Electrophysiol. 2013;36:612–7. doi: 10.1111/pace.12087. [DOI] [PubMed] [Google Scholar]
- 127.Dandamudi G, Rampurwala AY, Mahenthiran J, et al. Persistent left ventricular dilatation in tachycardia-induced cardiomyopathy patients after appropriate treatment and normalization of ejection fraction. Heart Rhythm. 2008;5:1111–4. doi: 10.1016/j.hrthm.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 128.Ling LH, Kalman JM, Ellims AH, et al. Diffuse ventricular fibrosis is a late outcome of tachycardia-mediated cardiomyopathy after successful ablation. Circ Arrhythm Electrophysiol. 2013;6:697–704. doi: 10.1161/CIRCEP.113.000681. [DOI] [PubMed] [Google Scholar]
- 129.Watanabe H, Okamura K, Chinushi M, et al. Clinical characteristics, treatment, and outcome of tachycardia induced cardiomyopathy. Int Heart J. 2008;49:39–47. doi: 10.1536/ihj.49.39. [DOI] [PubMed] [Google Scholar]
- 130.Philips E, Levine SA. Auricular fibrillation without other evidence of heart disease: a reversible cause of heart failure. Am J Med. 1949;7:478–89. doi: 10.1016/0002-9343(49)90397-6. [DOI] [PubMed] [Google Scholar]


