Abstract
Background
Genetic and environmental factors are implicated in the onset and evolution of pediatric bipolar disorder, and may be associated to structural brain abnormalities. The aim of our study was to assess the impact of the interaction between the Brain-Derived Neurotrophic Factor (BDNF) rs6265 polymorphism and family functioning on hippocampal volumes of children and adolescents with bipolar disorder, and typically-developing controls.
Methods
We evaluated the family functioning cohesion subscale using the Family Environment Scale-Revised, genotyped the BDNF rs6265 polymorphism, and performed structural brain imaging in 29 children and adolescents with bipolar disorder, and 22 healthy controls.
Results
We did not find significant differences between patients with BD or controls in left or right hippocampus volume (p=0.44, and p=0.71, respectively). However, we detected a significant interaction between low scores on the cohesion subscale and the presence of the Met allele at BNDF on left hippocampal volume of patients with bipolar disorder (F=3.4, p=0.043). None of the factors independently (BDNF Val66Met, cohesion scores) was significantly associated with hippocampal volume differences.
Limitations
small sample size, cross-sectional study.
Conclusions
These results may lead to a better understanding of the impact of the interaction between genes and environment factors on brain structures associated to bipolar disorder and its manifestations.
Keywords: bipolar disorder, BDNF, family functioning, gene-environment, hippocampus, neuroimaging, pediatric
Introduction
The most consistently reported contributing factor to bipolar disorder (BD) occurrence in children and adolescents (Pediatric Bipolar Disorder – PBD) is the presence of BD in first-degree relatives (Pavuluri et al., 2006). Specifically, 60% of phenotypic variance in BD has been attributed to genes. The BDNF val66met polymorphism (rs6265) has been consistently implicated in the pathophysiology of BD (Geller et al., 2004). This genetic variation is located on the chromosome 11p13 and results into a valine (G) to methionine (A) substitution at codon 66. BDNF is a molecule related to neuronal survival, growth and differentiation - neural and synaptic plasticity (Grande et al., 2010). Previous studies have extensively discussed the impact of BDNF in many aspects of BD, and BDNF polymorphisms have been associated with alterations in brain structure and function (Kapczinski et al., 2008). Furthermore, neuroimaging studies have demonstrated that BDNF allelic variations are associated with different anterior cingulate and hippocampal volumes, and abnormal hippocampal activation (Matsuo et al., 2009; Szeszko et al., 2005). However, there has been a failure to replicate many candidate gene associations findings, including those for BDNF, probably due to the small effect size of individual genes in such a heterogeneous disorder as PBD (Gottesman & Gould, 2003). Assessment of the impact that interactions between candidate genes and environmental factors have on structural brain changes implicated in BD episode onset and disease progression, may be an important intermediate step towards understanding BD pathophysiology.
Families of children with BD present high levels of dysfunction, possibly due to the rollercoaster of emotions caused by BD mood swings. (Miklowitz et al., 2004). Low cohesion has been the most consistent finding across studies assessing the relationships between families with BD. (Nader et al., 2013; Belardinelli et al., 2008; Romero et al., 2005). Cohesion can be defined as the emotional bonding that family members have toward one another, and strong emotional bonds are expected to promote family support. Psychosocial treatment models for PBD focusing on family functioning such as the Family Focused Treatment have promoted lower relapse rates and reductions in mood symptoms. Following such interventions, the presence of low cohesion was associated with lower response in terms of overall psychiatric severity (Weinstein et al., 2015).
Hippocampus plays a key role in mood and behavior regulation, and the association between abnormal hippocampal volumes and BD has been extensively reported in the literature of adult BD (Frey et al., 2007). Available data suggests children and adolescents with BD present a decrease in hippocampal volumes when compared to healthy controls (Frazier et al., 2005; Blumberg et al., 2003), but other studies fail to detect such differences (Chang et al., 2005; Dickstein et al., 2005; Chen et al., 2004). These conflicting results may reflect methodological differences across studies, the effect of illness evolution (i.e., the recently reported negative correlation between the right hippocampal volume and the duration of bipolar disorder in adolescents – Inal-Emiroglu et al., 2015), or the participation of environmental factors.
We report in this article an interaction between genetic and environmental factors impacting brain structures in pediatric bipolar disorder. Specifically, we assessed the interaction between the BDNF val66met polymorphism and family functioning - cohesion on hippocampal volume. We hypothesized that both gene and family functioning would predict differences in hippocampal volumes, and a synergistic effect would be observed when the Met allele and worse family functioning are combined.
Material and methods
a) Sample
This study was approved by the local institutional review board at The University of Texas Health Science Center at Houston. Written informed assent and consent were obtained from all subjects and their guardians.
The inclusion criteria for the patient group was DSM-IV diagnosis of bipolar disorder [bipolar I disorder, bipolar II disorder, or bipolar disorder not otherwise specified (NOS)] through a structured clinical interview for the Diagnostic and Statistical Manual of Mental Disorders-IV (APA, 1994). Patients were included if they were 8–18 years old, male or female, and free of any psychotropic drugs for at least two weeks prior to MRI scans. The exclusion criteria were: history of substance abuse, pregnancy, neurologic disorders, head injury with loss of consciousness, family history of hereditary neurologic disorders, and presence of metallic objects in the body impeding MRI. The inclusion criteria for healthy controls were no history of any personal psychiatric or neurologic disorders, or in first-degree relatives.
b) Diagnostic and Functioning Assessment
Participants were evaluated for DSM-IV-TR Axis I disorders using the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). (Sheehan et al., 2008). A senior psychiatrist (JCS) reviewed all clinical information, including history of medical and neurological conditions, and confirmed that all subjects met DSM-IV-TR diagnostic criteria for bipolar disorder, and that healthy controls would not have any Axis I DSM-IV disorder.
Family functioning ratings were assessed using the parent-rated scale Family Environment Scale (FES – Moos & Moos, 2002). This is a likert scale which provides information about family strength and problem areas. The FES is composed of 90 items distributed in 10 subscales (Cohesion, Expressiveness, Conflict, Independence, Achievement Orientation, Intellectual-Cultural Orientation, Active-Recreational Orientation, Moral-Religious Emphasis, Organization, and Control). These can be grouped into three dimensions (Family Relationship, Personal Growth, and System Maintenance). Due to our small sample size, we only assessed the effect of cohesion, since this subscale has been the most consistently altered factor in family functioning of BD (Nader et al., 2013; Belardinelli et al., 2008; Romero et al., 2005).
c) Magnetic resonance imaging (MRI) acquisition and processing protocol
All structural MRI images were visually inspected for gross artifacts, hippocampal volumetric measurements were extracted through a standard procedure using Freesurfer software (Greve and Fischl 2009; Postelnicu et al. 2009; Fischl, 2012) version 5.3 (http://surfer.nmr.mgh.harvard.edu/). Freesurfer volumetric measurements have successfully been used in previous pediatric neuroimaging studies (Ecker et al., 2010). All the analyses were performed considering volumes for the hippocampus corrected according to each participant total intracranial volume, due to the high variation in our age range, and also to account brains size variability between males and females.
d) BDNF gene polymorphism determination
Blood was drawn by venipuncture and DNA extracted from white blood cells using the PUREGENE kit (Gentra Systems). The BDNF genotype (rs6265) was determined using a TaqMan® primer-probe assay ID C_11592758_10. PCR amplification was performed at 50°C for 2 min, 95°C for 10 min, and then 50 cycles of 95°C for 15 s, and 60°C for 1 min. The amplification products were analyzed using an Applied Biosystems Prism 7900 sequence detection system and SDS 2.2 software (Applied Biosystems). TaqMan® assays were performed in duplicate by an individual unaware of the clinical status of the subjects. In subsequent analyses, individuals with Val/Met or Met/Met genotypes were combined (Met carriers) and compared with individuals with the Val/Val genotype.
Statistical Analysis
Analysis-of-variance (ANOVA) was performed with corrected left and right hippocampal volumes as outcome variables. Independent variables were genotypes: Val/Val, or Met carriers, and high/low cohesion scores. Categorization of scores was based on the normative data of the scale (Moos & Moos, 2002). Thus, there were three groups in our analysis: healthy controls, patients with BD and low cohesion scores, and patients with BD and high cohesion scores. Age and gender were included in the analysis as covariates. Post-hoc analysis was performed between the three diagnosis-cohesion groups with Bonferroni correction.
Results
The study participants included 29 children and adolescents with a DSM-IV diagnosis of bipolar disorder, and 22 healthy controls. Characteristics of the sample are shown in Table 1. Specifically in BD, 18 patients (62%) presented comorbid attention-deficit/hyperactivity disorder, 12 (41%) presented disruptive behavior disorders, and 9 (31%) presented anxiety disorders.
Table 1.
Characteristics of the sample
| Bipolar Disorder (n=29) |
Controls (n=22) | |||
|---|---|---|---|---|
| Val/Val (19) |
Val/Met-Met/Met (10) |
Val/Val (12) |
Val/Met-Met/Met (10) |
|
| Gender | Male 8 (42%) | Male 7 (70%) | Male 9 (75%) | Male 5 (50%) |
| Ethnicity | ||||
| Caucasian | 16 (84.2%) | 9 (90%) | 10 (83.3%) | 10 (100%) |
| African-American | 3 (15.8%) | 1 (10%) | 1 (8.3%) | 0 |
| Other | 0 | 0 | 1 (8.3%) | 0 |
| Age (years) | 14.84±2.267 | 11.80±2.57 | 12.75±2.63 | 13.4± 3.44 |
| Min-Max | 11-17 | 8-15 | 9-17 | 9-17 |
| Age at onset* | 10.05±3.12 | 7.40±3.20 | ||
| Presence of psychosis | 7 (36.8%) | 3 (30%) | ||
Values are presented as absolute count (relative frequencies), or as means ± standard deviation.
We did not find significant differences between patients with BD or controls in left or right hippocampus volume (p=0.44, and p=0.71, respectively). Also, no significant differences were observed between carriers and non-carriers of the Met allele at the BDNF gene and left or right hippocampus volume (p=0.97, and p=0.31, respectively). Family cohesion scores were significantly different between BD and control groups (p=0.002). There was no significant correlation between family cohesion scores and left or right hippocampus volume (r=0.18; p=0.2, and r=−0.03, p=0.84, respectively).
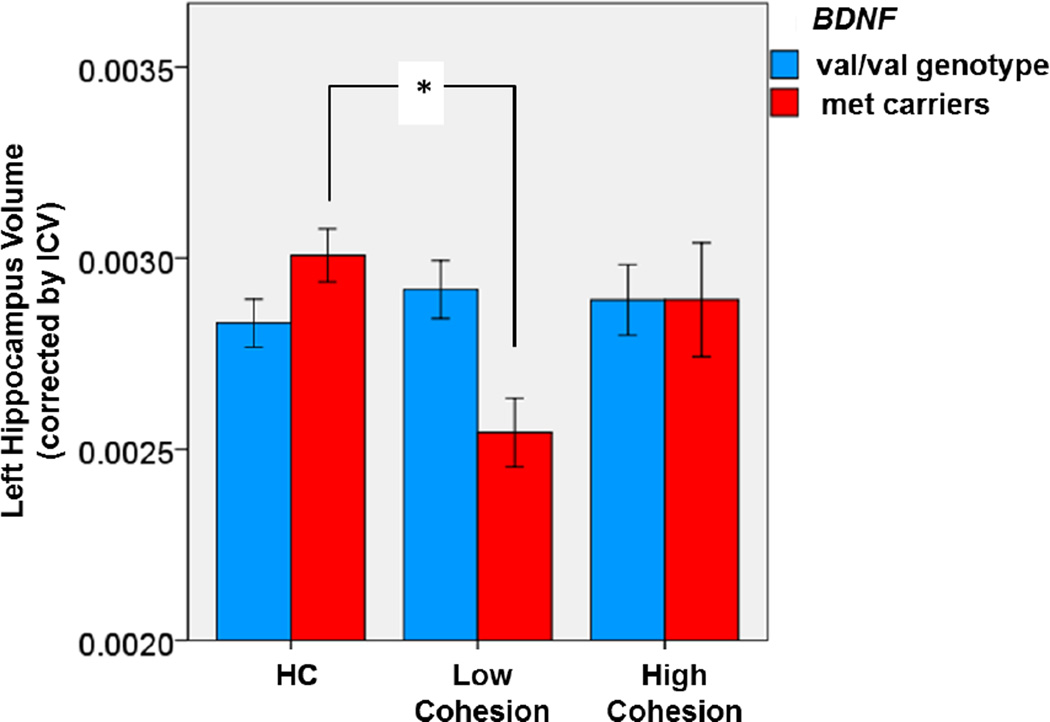
We detected a significant interaction between the cohesion subscale of the FES and presence of the Met allele at BNDF on left hippocampal volume in patients with BD (F2,43=3.399, p=0.043; Figure 1). In the post-hoc test, we found that patients who were Met carriers and had low cohesion scores had smaller left hippocampus than healthy controls (p=0.027). No significant differences were observed on right hippocampus (F2,43=0.69, p=0.51).
Figure 1.
Association between Left Hippocampal Volume and the interaction between BDNF Met allele and cohesion levels in children and adolescents with BD and controls
Marginally main effect of cohesion (F(2,43) = 2.467, p=0.097).
Significant interaction between BDNF and cohesion (F(2,43) = 3.399, p=0.043).
Post-hoc test: *p = 0.027
Discussion
In this pilot study, the combination of the BDNF rs6265 Met allele and low family cohesion levels in children and adolescents with bipolar disorder was associated with smaller left hippocampus volumes. To the best of our knowledge, this is the first attempt in the literature to evaluate the combined effect of both genetic and environmental factors on brain structures in pediatric bipolar disorder.
Our findings do not replicate previous genetic association studies that reported different hippocampal volumes according to BDNF Val66Met in healthy children, adolescents, and adults (n=36; Bueller et al., 2006), and also patients with bipolar disorder (n=38; Chepenik et al., 2009). However, the data we encountered is corroborated by previous studies where a combination of genetic and environmental factors provides a better explanation for brain structure. In a recent study, Frodl et al. (2014) showed that the BDNF Val66Met polymorphism influenced formation of hippocampal subfields, interacting with childhood adversity.
Based on the results of our study, we postulate two hypothesis, to be tested in future longitudinal studies. First, we hypothesize that children and adolescents with BD may have a dysfunctional neural system for the identification of emotionally salient environmental information, leading to inappropriate affective states and behaviors. The absence of bonding (low cohesion) would then decrease the possibility of help, leading these subjects to mood episodes. Second, we hypothesize that the continuous distress caused by a dysfunctional family system may play an epigenetic role in decreasing synaptic plasticity and impairing connectivity according to the presence of the BDNF Met allele. This has been demonstrated in studies of environmental stressors such as trauma (Angelucci et al., 2014), and also physical stress such as infections (Kapczinski et al., 2010). To date, no studies of psychotherapeutic interventions on family functioning have evaluated genetic effects.
Our data are preliminary and should be interpreted in the light of some limitations. The sample size is small and therefore our findings should be replicated using data from larger cohorts. Also, the cross-sectional design of this investigation does not allow conclusions regarding causality of any of the assessed factors. The vast majority of healthy controls (18 out of 21) had high cohesion scores on the FES-R, so we were not able to further separate controls into low and normal cohesion score groups.
Given the consequences of such an impairing condition as bipolar disorder, clues for a better understanding of its pathophysiology are crucial. Further investigations considering other environmental stressors and biological factors promoting brain alterations and their behavioral consequences are warranted.
Highlights.
-
-
Genetic and environmental factors are implicated in etiopathology of pediatric bipolar disorder (BD) and its associated structural brain characteristics.
-
-
We assessed the effect of the BDNF rs6265 polymorphism, family cohesion scores, and their interaction in hippocampal volumes of children and adolescents with BD and healthy controls.
-
-
A significant interaction was observed between low scores on the cohesion subscale and the presence of the Met allele at BNDF on left hippocampal volume of patients with bipolar disorder.
-
-
Gene-Environment Studies in Pediatric Bipolar Disorders may provide new insights on this severe mental disorder.
Acknowledgments
This study was supported in part by NIMH grant R01 085667, the Pat Rutherford, Jr. Endowed Chair in Psychiatry to Jair C. Soares and the Dunn Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 2.Angelucci F, Ricci V, Gelfo F, Martinotti G, Brunetti M, Sepede G, Signorelli M, Aguglia E, Pettorruso M, Vellante F, Di Giannantonio M, Caltagirone C. BDNF serum levels in subjects developing or not posttraumatic stress disorder after trauma exposure. Brain Cogn. 2014 Feb;84(1):118–122. doi: 10.1016/j.bandc.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Belardinelli C, Hatch JP, Olvera RL, Fonseca M, Caetano SC, Nicoletti M, Pliszka S, Soares JC. Family environment patterns in families with bipolar children. J Affect Disord. 2008 Apr;107(1-3):299–305. doi: 10.1016/j.jad.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003 Dec;60(12):1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 5.Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006 May 1;59(9):812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005 Jun;44(6):565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 7.Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M, Brambilla P, Keshavan MS, Ryan ND, Birmaher B, Soares JC. Crosssectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry. 2004 Sep 15;56(6):399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Chepenik LG, Fredericks C, Papademetris X, Spencer L, Lacadie C, Wang F, Pittman B, Duncan JS, Staib LH, Duman RS, Gelernter J, Blumberg HP. Effects of the brain-derived neurotrophic growth factor val66met variation on hippocampus morphology in bipolar disorder. Neuropsychopharmacology. 2009 Mar;34(4):944–951. doi: 10.1038/npp.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, Leibenluft E. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005 Jul;62(7):734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 10.Ecker C, Marquand A, Mourão-Miranda J, Johnston P, Daly EM, Brammer MJ, Maltezos S, Murphy CM, Robertson D, Williams SC, Murphy DG. Describing the brain in autism in five dimensions--magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J Neurosci. 2010 Aug 11;30(32):10612–10623. doi: 10.1523/JNEUROSCI.5413-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischl B. FreeSurfer. Neuroimage. 2012 Aug 15;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischl B, Stevens AA, Rajendran N, Yeo BT, Greve DN, Van Leemput K, Polimeni JR, Kakunoori S, Buckner RL, Pacheco J, Salat DH, Melcher J, Frosch MP, Hyman BT, Grant PE, Rosen BR, van der Kouwe AJ, Wiggins GC, Wald LL, Augustinack JC. Predicting the location of entorhinal cortex from MRI. Neuroimage. 2009 Aug 1;47(1):8–17. doi: 10.1016/j.neuroimage.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005 Jul;162(7):1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 14.Frey BN, Andreazza AC, Nery FG, Martins MR, Quevedo J, Soares JC, Kapczinski F. The role of hippocampus in the pathophysiology of bipolar disorder. Behav Pharmacol. 2007 Sep;18(5-6):419–430. doi: 10.1097/FBP.0b013e3282df3cde. [DOI] [PubMed] [Google Scholar]
- 15.Frodl T, Skokauskas N, Frey EM, Morris D, Gill M, Carballedo A. BDNF Val66Met genotype interacts with childhood adversity and influences the formation of hippocampal subfields. Hum Brain Mapp. 2014 Dec;35(12):5776–5783. doi: 10.1002/hbm.22584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geller B, Badner JA, Tillman R, Christian SL, Bolhofner K, Cook EH Jr. Linkage disequilibrium of the brain-derived neurotrophic factor Val66Met polymorphism in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2004 Sep;161(9):1698–1700. doi: 10.1176/appi.ajp.161.9.1698. [DOI] [PubMed] [Google Scholar]
- 17.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003 Apr;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 18.Grande I, Fries GR, Kunz M, Kapczinski F. The role of BDNF as a mediator of neuroplasticity in bipolar disorder. Psychiatry Investig. 2010 Dec;7(4):243–250. doi: 10.4306/pi.2010.7.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. http:// surfer.nmr.mgh.harvard.edu/ [Google Scholar]
- 20.Inal-Emiroglu FN, Resmi H, Karabay N, Guleryuz H, Baykara B, Cevher N, Akay A. Decreased right hippocampal volumes and neuroprogression markers in adolescents with bipolar disorder. Neuropsychobiology. 2015;71(3):140–148. doi: 10.1159/000375311. [DOI] [PubMed] [Google Scholar]
- 21.Kapczinski F, Frey BN, Kauer-Sant'Anna M, Grassi-Oliveira R. Brainderived neurotrophic factor and neuroplasticity in bipolar disorder. Expert Rev Neurother. 2008 Jul;8(7):1101–1113. doi: 10.1586/14737175.8.7.1101. [DOI] [PubMed] [Google Scholar]
- 22.Kapczinski F, Dal-Pizzol F, Teixeira AL, Magalhaes PV, Kauer-Sant'Anna M, Klamt F, Pasquali MA, Quevedo J, Gama CS, Post R. A systemic toxicity index developed to assess peripheral changes in mood episodes. Mol Psychiatry. 2010 Aug;15(8):784–786. doi: 10.1038/mp.2009.112. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo K, Walss-Bass C, Nery FG, Nicoletti MA, Hatch JP, Frey BN, Monkul ES, Zunta-Soares GB, Bowden CL, Escamilla MA, Soares JC. Neuronal correlates of brain-derived neurotrophic factor Val66Met polymorphism and morphometric abnormalities in bipolar disorder. Neuropsychopharmacology. 2009 Jul;34 (8):1904–1913. doi: 10.1038/npp.2009.23. [DOI] [PubMed] [Google Scholar]
- 24.Miklowitz DJ. The role of family systems in severe and recurrent psychiatric disorders: a developmental psychopathology view. Dev Psychopathol. 2004;16(3):667–688. doi: 10.1017/s0954579404004729. [DOI] [PubMed] [Google Scholar]
- 25.Miklowitz DJ, George EL, Richards JA, Simoneau TL, Suddath RL. A randomized study of family-focused psychoeducation and pharmacotherapy in the outpatient management of bipolar disorder. Arch Gen Psychiatry. 2003 Sep;60 (9):904–912. doi: 10.1001/archpsyc.60.9.904. [DOI] [PubMed] [Google Scholar]
- 26.Moos RH, Moos BS. Family Environment Scale Manual. 3rd. Palo Alto, CA: Mind Garden, Inc; 2002. [Google Scholar]
- 27.Nader EG, Kleinman A, Gomes BC, Bruscagin C, dos Santos B, Nicoletti M, Soares JC, Lafer B, Caetano SC. Negative expressed emotion best discriminates families with bipolar disorder children. J Affect Disord. 2013 Jun;148(2-3):418–423. doi: 10.1016/j.jad.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Pavuluri MN, Henry DB, Nadimpalli SS, O'Connor MM, Sweeney JA. Biological risk factors in pediatric bipolar disorder. Biol Psychiatry. 2006 Nov 1;60(9):936–941. doi: 10.1016/j.biopsych.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Postelnicu G, Zollei L, Fischl B. Combined volumetric and surface registration. IEEE Trans Med Imaging. 2009 Apr;28(4):508–522. doi: 10.1109/TMI.2008.2004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero S, Delbello MP, Soutullo CA, Stanford K, Strakowski SM. Family environment in families with versus families without parental bipolar disorder: a preliminary comparison study. Bipolar Disord. 2005;7(6):617–622. doi: 10.1111/j.1399-5618.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 31.Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, Milo KM, Stock SL, Wilkinson B. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) J Clin Psychiatry. 2010 Mar;71(3):313–326. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- 32.Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, Ashtari M, Napolitano B, Bilder RM, Kane JM, Goldman D, Malhotra AK. Brain- derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005 Jul;10(7):631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein SM, Henry DB, Katz AC, Peters AT, West AE. Treatment moderators of child- and family-focused cognitive-behavioral therapy for pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2015 Feb;54(2):116–125. doi: 10.1016/j.jaac.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]