Abstract
Background and purpose
Established prognostication markers, such as clinical findings, electroencephalography (EEG), and biochemical markers, used by clinicians to predict neurologic outcome after cardiac arrest (CA) are altered under therapeutic hypothermia (TH) conditions and their validity remains uncertain.
Methods
MEDLINE and EMBASE were searched for evidence on the current standards for neurologic outcome prediction for out-of-hospital CA patients treated with TH and the validity of a wide range of prognostication markers. Relevant studies that suggested one or several established biomarkers, and multimodal approaches for prognostication were included and reviewed.
Results
While the prognostic accuracy of various tests has been questioned after TH, pupillary light reflexes and somatosensory evoked potentials (SSEP) are still strongly associated with negative outcome for early prognostication. Increasingly, EEG background activity has also been identified as a valid predictor for outcome after 72 hours after CA and a preferred prognostic method in clinical settings. Neuroimaging techniques, such as MRI and CT, can identify functional and structural brain injury, but are not readily available at the patient’s bedside because of limited availability and high costs.
Conclusions
A multimodal algorithm composed of neurological examination, EEG-based quantitative testing, and SSEP, in conjunction with newer MRI sequences, if available, holds promise for accurate prognostication in CA patients treated with TH. In order to avoid premature withdrawal of care, prognostication should be performed later than 72 hours after CA.
Keywords: Cardiac arrest, hypothermia, prognostication, neurological outcome, neuroimaging, brain injury
Introduction
Cardiac arrest (CA) is a leading cause of death and disability that has a U.S. incidence of 326,200 annually (1). The survival rate for out-of-hospital CA is 10.6 %, while 8.3% is the survival rate with good neurologic outcome, which has been gradually increasing over the past decades (1). Therapeutic hypothermia (TH) has been regarded as the most effective method for improving survival and functional outcome and has become a standard practice for treating out-of-hospital CA patients after resuscitation (1–3). TH has been associated with better functional outcome (4) and shorter duration of hospital stay (5). Moderate TH of 32–34°C for 12–24 hours is currently recommended for CA patients with ventricular fibrillation or pulseless ventricular tachycardia after return of spontaneous circulation (ROSC) (3, 6).
In patients treated with TH, the prognostic algorithm in the most recent American Academy of Neurology (AAN) practice parameter (7) may need to be modified. Several studies have shown that some predictors for poor outcome are less reliable in patients treated with TH (8–10), such as recovery of motor responses, electrophysiologic tests, and biochemical markers (11–14). The 72-hour benchmark for prognostication in the AAN practice parameter may no longer be valid in patients treated with TH (15). A study of 111 CA patients found that TH weakened the prognostic accuracy of motor responses (11), while another prospective study showed that NSE levels of >33μg/liter stated in the AAN report guidelines (7), are unreliable for poor outcome prognostication (10). These findings were also confirmed by other studies (16, 17) and have been attributed to higher sedative use, delayed metabolism of sedatives, and neuroprotective effects of TH (18). Table S1 summarizes recent findings regarding common neurophysiologic markers in CA.
The urgent need to reevaluate the accuracy of prognostication markers in TH conditions and discuss optimal prognostication methods based on one or several parameters is the main topic of this review. The vast amount of quantitative data in this dynamic field could be made further accessible by drawing meaningful conclusions based on it. This review has been chosen to give a concise and up-to-date summary of the validity of prognostication markers for neurologic outcome and discuss the benefits of using a multimodal approach in determining neurologic outcome.
Assessment of Neurologic Outcome at Discharge
There is a variability of methods for assessment of neurologic outcome. The Cerebral Performance Category (CPC) has been widely used in CA research to assess neurological status (19, 20), with 1 representing good performance, 5 signaling brain death, and the intermediate scores assessing the degree of disability (20, 21). Discharge CPC can serve as a reliable method for predicting longer term outcomes in survivors (11, 21). A recent study of CA patients treated with TH reported that good discharge CPC predicted better long-term survivor outcomes (22). Although CPC is a common assessment measure, it should be treated with caution because the definitions for good outcome vs. bad outcome vary across published literature. Standardizing these definitions should be the focus of future research, as well taking into account that CPC is taken at discharge and does not reflect the change of condition of the patient before and after the arrest, but only post-arrest functionality, which limits to a certain extent the value of this test. Finding earlier clinical prognostic markers should be the focus of future research (23).
Clinical Findings
Clinical examination is one of the most widely used tools in comatose CA patients because it can be easily recorded at the bedside. The performance of clinical prognostic markers in TH-treated patients is often confounded by sedatives, which are given during the course of hypothermia and shortly after. A study examining the effect of sedation on commonly used clinical markers (brainstem reflexes and motor responses to painful stimuli) concluded that they cannot be reliably used to determine poor outcome (17). Although most studies conclude that clinical examination is still a useful method to prognosticate poor neurological outcome 72 hours after CA (2, 10, 16), these results should be treated with caution because of reduced sensitivity. Delayed prognostication beyond the 72-hour has been proposed to avoid the sedation effects and thus increase the specificity of the individual test (15, 24–26). It is important to note that brainstem reflexes, in particular pupillary light reflexes, are not accurate at admission, but they become a very specific test to indicate bad outcome at 72 hours after CA (10) and after rewarming (27). In contrast, absence of corneal reflexes at 72 hours has been shown to not be invariably associated with poor outcome (26). Nevertheless, brainstem reflex tests should be regarded with caution because they suffer from low sensitivity, meaning that not all patients who have absent brainstem reflexes actually have a bad outcome.
Early myoclonus in comatose patients is widely associated with severe global ischemia and poor neurological outcomes before and after the advent of TH (11), although cases have been reported where patients achieve good neurologic outcome in spite of presence of early myoclonus (28).
Absent motor response after 72 hours may not be a reliable predictor of poor outcome in patients treated with TH. Absent motor response has a significantly higher false positive rate (FPR) for prediction of poor outcome when TH is applied (10, 11, 17). False positive rates are one of the important measures for the accuracy of tests because they indicate the percentage of cases when poor outcome has been wrongly predicted. A recent study found a very high FPR of 0.24 for motor response worse than flexion in their study of 111 TH-treated patients (11), possibly due to increased sedation and slowed metabolism of sedatives. This finding, in contrast to the results obtained before the advent of TH (Glasgow Coma Scale Motor Score ≤2 with FPR=0, 95% CI) (7), illustrates the need to reconsider the accuracy of motor responses for poor prognostication.
Compared to poor outcome, good neurological recovery is more difficult to predict because the absence of unfavorable markers does not guarantee good outcomes (8). For instance, the absence of somatosensory evoked potentials at day 3 is considered a good predictor for non-awakening, however, their presence does not exclude poor outcome (29). It is necessary to develop more robust independent indicators for good outcome. A promising alternative is the auditory-evoked potential wave called mismatch negativity (MMN), which when present in comatose patients, is a good indicator for awakening (30). Finding good outcome prognosticators early could prevent premature withdrawal of medical care and give hope to the families of the comatose patients.
Although often neglected in favor of more expensive tests, simple indicators such as the age of the patient, whether the arrest was witnessed and, initial rhythm, are useful to make an early prognosis. In general, younger age, witnessed arrest, and initial shockable rhythm of ventricular fibrillation or ventricular tachycaridia, are strong predictors for good neurologic outcome (31), and increase the likelihood of belated awakening of comatose patients (32). The presence of the above-mentioned characteristics are well correlated with long-term survival as measured by CPC=1 or CPC=2 (21).
Prognostication based on clinical findings is a well-known and easily performed method, which provides early information regarding discrimination between good and bad outcome, but recent findings have found that sedation accompanying hypothermia treatment could influence results of brainstem reflex tests.
Biochemical Markers
Neuron-Specific Enolase (NSE) and S-100B
Biochemical markers offer an early, non-invasive method to measure brain injury and neurologic outcome after CA (33–35). Serum S-100B protein has some predictive value for outcome after CA (34, 36) but it is not routinely measured in clinical practice (7). The 2006 AAN practice parameter included serum NSE levels >33 μg/L on days 1–3 as a predictor of poor outcome (7), but a high FPR in TH-treated patients suggests that prognostication based solely on NSE may be inaccurate (2, 8). A recent meta-analysis found an FPR of 0.12 (0.06–0.23) for NSE >33 μg/L, which was inferior to other predictors of poor outcome, such as absent corneal and pupillary light reflexes, myoclonus status epilepticus (MSE), and unfavorable EEG patterns (15). The predictive value of serum NSE is further confounded because it may vary with hemolysis, presence of NSE-secreting tumors, and changes in red blood cell concentration (37, 38). Additionally, there is a wide range of suggested threshold values with some studies reporting that a cut-off >80 μg/L increases the specificity for poor outcome at the expense of the sensitivity (10, 35). A marked reduction of NSE levels was observed in TH-treated patients which correlated with good outcome (38), however a recent study showed only slightly decreasing NSE levels are indicative of good outcome (14). This study suggested that the time course of dynamic change of NSE levels during the first 72 hours is more useful than choosing one specific time point to measure absolute NSE levels, thus implying that serial biochemical monitoring over the course of at least 3 days would be more useful for making an early prognostication than taking a single measurement. Evolution of thresholds was predicted in a recent meta-analysis as NSE > 81.8 mcg/L at 48 h, and an NSE >78.9 mcg/L at 72 h for a CPC 3–5 (27). These findings have recently been supported by another retrospective study (39) with 73 patients, which found that the NSE levels change between days 1 and 2. The patients who achieved an excellent neurologic outcome (CPC score 1) showed decreasing NSE levels at day 2 compared to day 1. The study found that a change ≥4.3 ng/ml had the greatest predictive value with a specificity of 100% (39), while absolute values should be regarded with caution.
Lactate levels
The advent of TH has required re-evaluation of the prognostic value of serum lactate levels on patient survival. Previous studies have shown that initial lactate levels post-CA could be potentially associated with survival or mortality (40), but thresholds for good and bad neurological outcome remain uncertain (41). A recent study compared initial lactate levels versus the rate of lactate clearance, and concluded that early lactate clearance (within the first 6–12 hours) is predictive of good outcome in TH-treated patients (42). These results have been supported by results showing that lower lactate levels at 12 and 24 hours after resuscitation are associated with reduced mortality (43).
Biochemical markers prognostication has the advantage of being non-invasive and providing information about the general organ dysfunction of the patients, however, the variability of results in literature regarding useful thresholds makes it risky to use this type of marker, especially in cases when sedation was used.
Electrophysiological Testing
Electroencephalography (EEG)
Continuous EEG (cEEG) recordings have been divided into benign and malignant patterns based on the predominant frequency, contour, periodicity, amplitude, and presence or absence of variability (2). Further classification of malignant patterns has been suggested to hold even greater prognostic value (44) and facilitate prediction of a wider range of outcomes. The presence of EEG reactivity – alteration of EEG amplitude and frequency in response to external stimuli – may be an indicator of recovery of consciousness, while the persistent lack of reactivity is associated with widespread brain injury and poor outcomes (2, 45). EEG, however, can be affected by temperature and use of sedative medications. A recent study confirmed that EEG reactivity to painful stimuli during TH could provide early prognostic information (45). Because cEEG monitoring was undertaken in CA patients during NT and TH, these results suggested that presence or absence of EEG reactivity is a strong predictor of outcome independent of temperature and sedatives. In order to simplify EEG monitoring, amplitude-integrated EEG (aEEG) has been suggested as an alternative (46, 47). aEEG has proven useful in detecting abnormal EEG patterns that are common in neonates treated with TH for hypoxic-ischemic injury (48), and it also provides useful information about the extent of brain dysfunction.
Poor prognostic signs for CA patients based on EEG recorded within the first 72 hours include: generalized suppression <20 μV (7), burst suppression (BS) patterns (Figure S1), electrographic seizures on a flat background (7, 49), and absence of EEG reactivity (11, 45, 50). The predictive value of isoelectric and BS EEG is dependent on the timing of recording and is not invariably associated with poor outcome when TH is applied, especially when EEG is initiated within the first 24 hours, however if present later, specificity of these markers for poor outcome is increased (51). The definition for BS varies across studies, making it difficult to determine the value of its results. Electrographic status epilepticus (ESE), defined as repetitive discharges >50 μV with frequency >1 Hz for >30 minutes, has been associated with poor neurologic outcome in both TH and NT patients (2, 49). Recovery may be possible if ESE evolves from a continuous EEG background during the application of TH, while ESE evolving from BS or isoelectric EEG is associated with poor outcomes (46). MSE and BS EEG are both considered specific signs of poor outcome in comatose CA patients (7), although some examples of subsequent awakening have been reported (9, 52). Studies have shown that the presence of status myoclonus within the first 72 hours after CA predicts poor outcome with 100% specificity in both hypothermia and normothermia groups (37). Most studies have indicated that the presence of MSE has a low FPR and it remains a valid predictor for poor outcome (2, 37). Due to sedative and paralytic agents, however, MSE may be difficult to detect during TH.
As a diagnostic tool, however, waveform-based EEG analysis is subjective and laborious, with results depending on the interpreter’s expertise. Quantitative EEG (qEEG) has been developed as a measure of neurologic recovery after CA. A new index called Cerebral Recovery Index (CRI) has recently been developed and was shown to assist prognostication of neurological outcomes in a single-center clinical study. CRI uses five different qEEG characteristics (53), but the final result is a single quantitative value that could predict outcomes within the first 24 hours after CA. CRI’s biggest advantages are that it does not seem to be affected by TH or sedative medications and the EEG characteristics that comprise the algorithm are familiar to clinicians. A simpler and more robust qEEG method, using an entropy-based measure Information Quantity (IQ), from 0 as iso-electricity to 1 as normal, was able to accurately predict the functional outcomes and mortality from 2 hours after resuscitation with temperature management in comatose animals, with a specificity of 100% and sensitivity of 81.8% to predict good neurologic outcome at 72 hours post-resuscitation (54, 55). Verification of this method still requires clinical studies to be performed in humans, however.
Somatosensory Evoked Potential (SSEP)
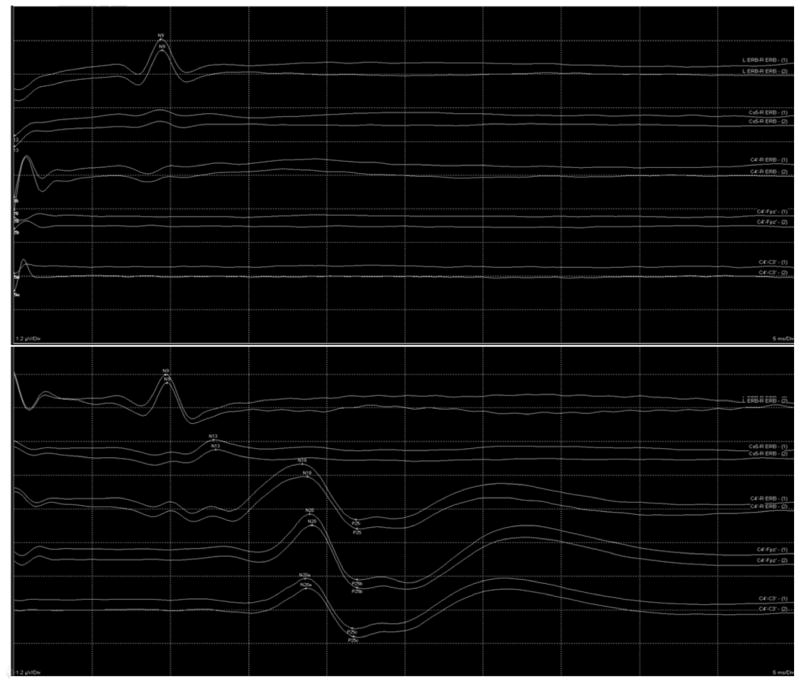
Bilateral absence of cortical N20 potentials on median nerve SSEP (Figure 1) was one of the most specific predictors of poor outcome in the 2006 AAN practice parameter (7). SSEP has retained its predictive value in TH-treated CA patients and it remains among the most robust indicators of poor neurologic outcome (10, 27). The specificity of SSEP increases when testing is delayed until after 72 hours from CA in TH-treated patients (15). In contrast, Sandroni et al. have shown that absent N20 potentials are predictive for poor outcome as early as during administration of TH (27). Bilaterally absent cortical potentials on SSEP has a very low FPR of 0.007 according to a recent meta-analysis (26). One study found that bilateral absence of N20 potentials does not guarantee poor outcome among TH-treated CA patients (13). This study reported two patients with initially absent N20 potentials who recovered after 24 hours, indicating that TH may extend the recovery period. Timing is critical for prognostic accuracy of SSEP and inconsistent findings may occur during the first 24 hours. For this reason, clinicians should be wary of early prognostication based solely on SSEP performed within 24 hours of CA. This view is consistent with the findings of a recent retrospective study that found that bilateral absence of cortical responses does not predict poor outcome independently and that recovery may be possible (56).
Figure 1.
Absent cortical N20 potentials (top) on median nerve SSEP in a comatose patient resuscitated from cardiac arrest. Normal cortical N20 potentials are also shown (bottom) for reference.
As an isolated finding, the presence of N20 potentials is not a guarantee of good outcomes. The presence of cortical potentials without other positive predictive markers has poor sensitivity (46%) for predicting good outcomes (7). Animal studies have indicated that the utility of quantitative SSEP (qSSEP), using novel algorithms to track the evolution of potentials recovery beyond the N20 or N35 waveform-based analysis and simplify the subjective interpretation, is not only restricted to poor outcome prediction, but can also be used to predict both good and bad outcomes (57). The temporal evolution of SSEP may be a good predictive tool as an indicator of recovery of arousal because it requires integrity of thalamocortical networks (57, 58). The quantitative information derived from qSSEP, similar to qEEG, may ultimately prove valuable in distinguishing between good and bad outcomes early after CA.
Neuroimaging
In the 2006 AAN practice parameter, routine structural imaging studies were found to have poor prognostic accuracy in comatose CA survivors. More recently, several neuroimaging-based markers – based on advanced MRI, CT, and PET imaging – have shown promise in predicting long-term neurological recovery of post-CA patients. Table S2 summarizes some of the recent studies performed in this area.
Computer Tomography (CT)
Computer tomography (CT) is useful to detect cytotoxic edema by measuring loss of distinction between gray and white matter. However, this finding may not be visible until post-CA day 3 in patients with poor outcome and, therefore, its further use as a predictive tool has been limited. CT has primarily been studied in conjunction with other prognostic tools such as SSEP and NSE levels (59). Due to the variable timing of CT acquisition, the lack of comparison with other prognostic methods, and the limited sample size of most studies, the European Resuscitation Council Guidelines has warned against routine use of CT as an isolated prognostic tool (6).
Reduced distinction between gray and white matter, measured by decreased gray-to-white matter ratio (GWR) of Hounsfield units (Figure 2), has been associated with mortality in comatose CA patients (60). There is no consensus on the appropriate cut-off value for GWR, however, and few studies have investigated whether TH affects the GWR. Global cerebral edema and the progressive loss of differentiation between white and gray matter have been observed with CT in both TH and NT patients (2). A recent study attempted to determine an optimal cutoff value for GWR in 62 CA patients treated with TH who also underwent prognostic testing with NSE and SSEP (59). The results showed GWR <1.16 predicted poor outcome with 100% specificity but with low sensitivity of 38%. The sensitivity increased from 43% to 53% if at least 2 out of the 3 prognostication parameters were used (59). Other studies have also used multimodal approaches with a combination of imaging and other predictive tests (2, 59, 61).
Figure 2.
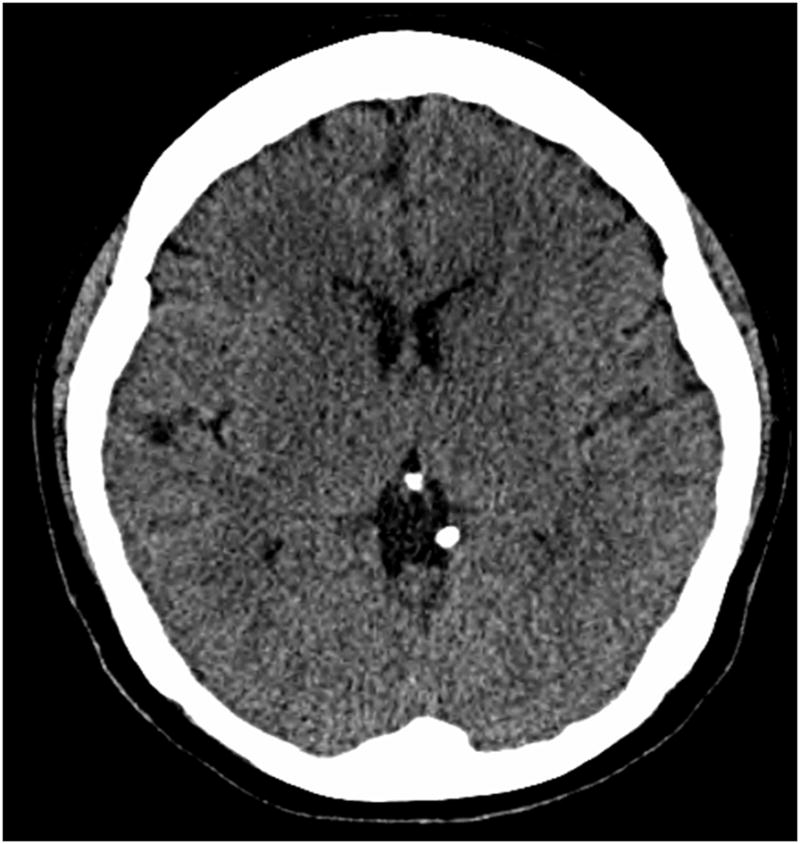
Non-contrast head CT of a comatose patient resuscitated from cardiac arrest showing a diminished gray-to-white matter ratio involving the basal ganglia and internal capsule consistent with cytotoxic edema.
Using imaging in combination with clinical findings has been shown to improve sensitivity of prognostication and allow accurate prediction of mortality within the first 48 hours after CA (62). A recent study used a similar approach, focusing on non-contrast CT in combination with GCS and Apparent Diffusion Coefficient (ADC) (63). This study compared NT and TH groups and concluded that imaging increased the specificity of neurological exams and that it maintained high specificity in patients even within the TH group (63). The CT scans were performed within 3 days after CA. In order to avoid self-fulfilling poor outcome predictions, designing future studies such that clinical decisions are not dependent upon the results of the test of interest and ensuring that all patients receive the test might be helpful to determine if CT results remain predictive when CT scans are used alone or in combination with other tests.
Magnetic Resonance Imaging (MRI) and Functional MRI (fMRI)
Diffusion-weighted imaging (DWI) is in common clinical use for detection of ischemic stroke, because ischemic neuronal injury results in restricted diffusion of protons as water diffuses across disrupted cellular membranes. The apparent diffusion coefficient (ADC) is calculated by subtracting T2 signal from DWI sequences and it has shown promise in detecting early ischemic injury after CA (61, 64). ADC is a quantitative measure which varies with progression of ischemia (65). Reduction of ADC, when it is more than 31%, is associated with poor outcome in comatose CA patients, while when it is below 27% it is associated with favorable outcome (66), however the reduction percentage may be subject to variation due to the limited sample size (n=20 with 3 patients receiving hypothermia), as well as being dependent on the timing of imaging (65). Recent study suggested that DWI has high sensitivity for predicting poor outcome (98.5%) in CA patients, but has low specificity of 46.2%. This finding may be due to the fact that DWI abnormalities at the time of imaging return to normal at later time periods, as well as the qualitative evaluation nature of the study with kappa as 0.49 indicating moderate agreement between two observers, which could account for lower specificity (67). Another study showed that quantitative ADC values increased the specificity for predicting poor outcome to 100%, while maintaining high sensitivity (64). In this study, threshold ADC values helped differentiate survivors from non-survivors and provided further information about the degree of neurologic impairment in survivors. DWI is only accurate 2–5 days after CA (64, 68), however, this limits the usefulness of MRI compared to other prognostic tests (Figure S2).
Standard T2-weighted MRI is not sensitive enough to accurately detect early ischemia after CA (69). Predicting long-term functional recovery for patients who have awakened from coma based on MRI in conjunction with other neurophysiologic and biochemical predictors has shown some promise (8, 48). In addition to the scalar ADC value, new quantitative indices based on diffusion-tensor imaging (DTI) can measure white matter integrity (70). DTI may be a more accurate predictor for long-term functional outcome than ADC and its potential should be further explored in larger cohort studies. DTI and ADC sequences may eventually assist families and providers in making decisions regarding treatment of comatose patients within the first 3–5 days after CA.
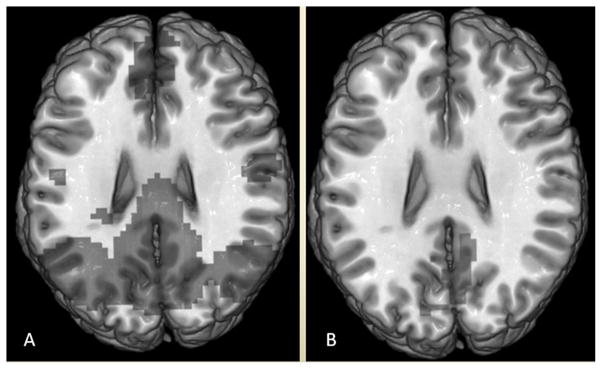
Novel imaging markers based on functional MRI (fMRI), which measures fluctuations in brain perfusion as an indirect measure of connectivity between different brain regions, are being studied as predictors of neurological outcomes in CA survivors. Measurement of the blood oxygenation level dependent (BOLD) signal has been found to have important correlations with metabolic activity (71) and brain function can be evaluated by quantifying the BOLD signal in fMRI. Coma and other disorders of consciousness have been shown to disrupt the BOLD signal (72), which may help researchers better understand brain injury after CA. This discovery may help investigate level of consciousness and predict the degree of neurological recovery after CA. Using resting state fMRI, a default mode network has been identified that includes the anterior and posterior cingulate, precuneus, and temporal-parietal junction (73). Disruption of connectivity within this network has been demonstrated in chronic disorders of consciousness and pharmacological anesthesia (74). Two recent small studies showed disruption of default mode network connectivity in patients with poor outcomes after CA and preserved connectivity in patients who recovered consciousness after CA (73, 75) (Figure 3). Despite promising results, further validation of resting state fMRI is required before it can be used for prognostication in routine clinical practice. However, fMRI may have a role as a complementary technique to clinical examination and electrophysiological testing with SSEP and EEG (76).
Figure 3.
Resting state fMRI in cardiac arrest survivors showing preserved functional connectivity with the default mode network in patients with good outcomes (A) and severely diminished connectivity in those with poor outcomes (B).
Positron Emission Tomography (PET)
Unlike MRI and CT, which show structural changes, PET visualizes alterations in brain metabolism. Reduced glucose metabolism has been associated with global brain injury following ischemia (77). In PET studies of vegetative state and comatose patients, glucose consumption alterations represented not just functional inactivation, but irreversible brain injury (78). Other studies tested whether reduced cerebral blood flow and oxygen metabolism on PET is specific for poor outcomes one week after CA, but results suggested that subsequent recovery was possible whether or not global energy metabolism was restored (79). A useful measure for monitoring oxygen metabolism is the cerebral metabolic rate of oxygen (cMRO2) derived from PET scans. Following ischemia, cMRO2 had significantly reduced values, possibly as a result of secondary brain injury (80). The same trend of suppressed oxygen metabolism in patients with poor outcome was predicted by measuring the oxygen extraction fraction (OEF) at the end of TH (83). OEF was significantly lower in patients with poor outcome compared to patients with favorable outcome.
There are only a limited number of studies testing PET as a predictive tool for neurological outcomes and the existing studies are marred by small sample size, concern for self-fulfilling prognostication of poor outcomes, and lack of comparison with other prognostic methods. Another important limitation of PET is that it is very expensive, not readily available in hospitals, and often hard to interpret if no CT data is available. Neuroimaging is increasingly being used as part of a multimodal strategy for prognostication in conjunction with other prognostic markers. Nevertheless, the results derived from studies using neuroimaging modalities should be treated cautiously, because of possible selection bias, high rates of self-fulfilling prophesies and small sample groups.
Multimodal Approaches to Prognostication
As no single test is perfect for prognostication after cardiac arrest, using a multimodal approach avoids the problems with the reduced sensitivity of individual tests and increase the regions of confidence when multiple tests are used. Previous meta-analyses have concurred with the idea that combining modalities increased area under receiver operating characteristics (ROC) curves and achieved a PPV of 100% for poor outcome (50), as well as reduce the risk of false positives (27) (Table 1).
Table 1.
Existing Multimodal Approaches for Prognostication of Poor Outcome
| Research Group | Variables | About this test |
|---|---|---|
| C. Sandroni et al.2014 (84) | A combination of:
|
The most robust predictors, (pupillary relexes and corneal reflexes) and bilaterally absent SSEP N20 waves are evaluated. If none of the signs above, are present, the second set of signs is used, but precision is lower. At least 2 predictors should be present for prognostication. |
| M. Oddo, et al. 2014 | A combination of:
|
Accuracy of markers: Sensitivity 0.49; Specificity 1.00;
PPV 1.00; NPV 0.62 Addition of SSEP did not improve prognostic accuracy. First test that found an optimal combination of markers to test for poor prognosis. |
| T. Cronberg et al. 2013 |
|
Multivariable test for allowing discontinuation of life support |
| F. Thömke et al. 2013 (83) |
|
At least one clinical and one electrophysiological factor and/or significantly elevated NSE concentration should be present to indicate poor outcome |
| C. Sandroni et al 2013 |
|
Meta-analysis of existing literature. Primary limitation of the tests of poor outcome was the lack of blinding between studies and use of one predictor to support withdrawal from life support |
| H. Friberg et. al 2013 (84) |
|
Daily clinical examination and continuous EEG monitoring
is critical for making a decision on level of care, while SSEP, NSE, and MRI are
recommended but not strictly required. Decision making should happen at least after 72 hours after normothermia has been achieved. |
In all comatose patients, conventional EEG is performed 12–36 hours after rewarming
Unless otherwise stated, “hours” means to hours passed since cardiac arrest.
Abbreviations: ROSC = return of spontaneous circulation; SSEP= somatosensory evoked potentials; GW/WM=grey to white matter ratio; EEG = electroencephalography; NSE = neuron serum enolase; PPV = positive predictive value; NPV = negative predictive value; PR = pupillary reflexes; CR = corneal reflexes; BS = burst suppression; aEEG = amplitude integrated EEG; CT= computer tomography; MRI= magnetic resonance imaging
Although there is some inconsistency as to which parameters to include in a multimodal algorithm, it has been shown that EEG markers, in conjunction with neurological examination, can predict outcomes with relatively high specificity and sensitivity, but sedation should be taken into consideration. A new study identified predictors of favorable outcome to be: interval to first CPR <5 minutes, initial rhythm of ventricular fibrillation or ventricular tachycardia, interval from CA to ROSC <30 minutes, and recovery of pupillary light reflexes while in the Emergency Department (31). This approach highlights the use of a multimodal strategy for identification of patients who would benefit from targeted temperature management. NSE levels had previously been recommended (7), but, in patients treated with TH, the prognostic accuracy is only acceptable in combination with other tests (59, 61). Oddo et al. studied the neurologic examination, EEG reactivity during TH, and NSE levels as a multimodal predictive algorithm (50). In this study, EEG reactivity was the single most reliable marker with a sensitivity of 74%, but the addition of NSE levels and clinical examination improved the FPR. SSEP is still considered a cornerstone of most prognostic algorithms in CA patients treated with TH (2, 50), but some studies have shown that it does not further increase prognostic accuracy (50). Many authors now recommend delaying SSEP until 48–72 hours post-CA (9) or later (15). Recently, there has been a resurgence of interest in using neuroimaging – particularly CT and MRI-derived parameters – to provide further prognostic insights (2, 9, 63). A study comparing NSE levels, SSEP, and CT GWR showed that GWR <1.16 can improve the sensitivity for poor outcome prediction when 2 or more modalities out of 3 are used (59). Another case report also suggested that there is an association between neuroimaging and neurophysiologic methods when stimulation to the median nerve is used for prognostic testing (76). Although CT and MRI require more prospective studies before routine clinical use, they hold great potential in resolving dilemmas in predicting neurological outcome when other methods are inconclusive. The delayed timing of MRI (48–108 hours after NT), however, may limit this test to visualizing injury and supporting an already existing prognosis.
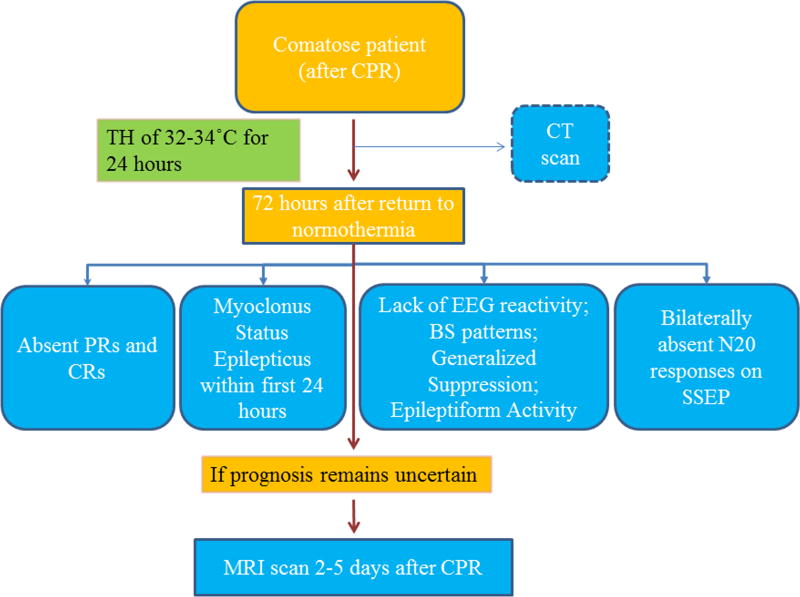
In order to avoid premature withdrawal of life support, a multimodal approach has to be applied and definitive prognostication should be delayed for 72 hours after rewarming. Some authors even suggest that further delay should be utilized to await awakening of patients who have uncertain prognosis based on clinical, neurophysiological, biochemical, and neuroimaging markers (32). This suggestion is consistent with findings that reveal that late awakening is possible and favorable neurological recovery is not uncommon even when awakening is delayed (9, 32). A multimimodal approach with three out of four markers selected was based on what are believed to be the most reliable prognosticators from literature. We suggest prognostication at least 72 hours after normothermia as a way to avoid the confounding effects of sedation and to give more time to the patient in the case of later awakening.
Conclusion
There is a strong need for an accurate multimodal prognostic approach that is viable in patients treated with TH and gives sufficient time for recovery in comatose patients. We recommend an approach in which at least three out of the listed markers are present, before making a decision regarding further treatment or its discontinuation (Table 2, Figure 4). We recommend use of a multimodal prognostic approach for poor outcome in order to avoid withdrawal of life support based heavily on a single marker.
Table 2.
Multimodal Approach for Poor Prognosis
| Category | Timing | Markers | |
| Clinical Neurologic Examination | 72 hours after rewarming | Absent PLR, or Absent CR, or Myoclonus status epilepticus within the first 24 hours |
|
| Electroencephalography | Continuous monitoring starting at TH and up to 72 hours after normothermia | BS pattern, or Generalized suppression, or Epileptiform activity on a flat EEG background, or Absence of EEG reactivity | |
| Somatosensory evoked Potentials | 24–72 hours after CA | Bilaterally absent N20 potentials | |
| Neuroimaging: | DWI-MRI | 2–5 days after CA | Qualitative analysis of DWI abnormalities Reduction of ADC by >31% |
| DTI-MRI | 7 days after CA | Fractional anisotropy value 0.44 on DTI (70) | |
| CT | Within 48 hours | Cerebral edema and GWR <1.16 | |
Note*: Biomarkers, such as NSE levels and S-100B, could be used to support a prognosis based on some of the markers above (61), but their use is not recommended because of the variability in their values (14, 35) and limited specificity (2, 37).
Note**:Other neuroimaging methods such as fMRI, CT, and PET can be useful to detect the degree of damage in functional connectivity (73, 75), metabolic abnormalities as a result of ischemia (80), and identify other potential causes for coma (40). However, their use is cautioned against because bigger cohort studies have to be performed before they can be used in routine practice.
Abbreviations: CA = cardiac arrest; PLR = pupillary light reflexes; CR= corneal reflexes; TH= therapeutic hypothermia; BS = burst suppression patterns; EEG = electroencephalography; DWI-MRI = diffusion weighted imaging magnetic resonance imaging; ADC = apparent diffusion coefficient; DTI= diffusion-tensor imaging; CT = computer tomography; GWR= gray/white matter ratio
Figure 4.
This is an example of a multimodal approach to negative prognostication. If three out of the four listed predictors are present, poor outcome is very likely (CPC 3–5). If the predictors give inconclusive results at the 72 hours, more time should be allotted for further neuroimaging tests such as DWI-MRI unless awakening has not occurred earlier. Discontinuation of life support should be carefully considered if neuroimaging tests suggest an unfavorable outcome.
The need to establish robust biomarkers that can reliably predict the degree of neurological injury has been addressed in recent years, as costs of ICU care have risen. SSEP, qEEG, and neuroimaging methods hold promise for developing more reliable and objective prognostic tools that lessen the risk of errors. More prospective studies with larger patient groups are still required in order to eliminate uncertainties created by early withdrawal of life support in many retrospective studies. More importantly, some existing studies often use the studied variables in the decision to withdraw care, falling prey to the self-fulfilling prophecy, and tests are often applied only to selected patients at inconsistent time points. An ideal study would have pre-designed tests that will be applied to all patients uniformly and that would not factor into the decision to withdraw life support. Other potential issues to be resolved also include standardization of the timing of prognostic algorithms, development of an optimal multimodal algorithm that can be executed in most clinical settings, and verifying the cut-off values of some prognostic parameters in patients treated with TH. The quality of evidence, especially when considering the value of neuroimaging results, should be treated carefully because selective bias could be present regarding the choice of test, and consequently self-fulfilling prophecy risk.
While the accuracy of many prognostic tests in CA patients treated with TH has been called into question, absent pupillary light reflexes at 72 hours, MSE, and bilaterally absent cortical N20 potentials on SSEP are still considered reliable methods for early prediction of poor outcomes. Biochemical markers, particularly serum NSE, should be treated with caution in TH-treated patients and should only be used as one component of a multimodal prognostic algorithm. CT and newer MRI neuroimaging modalities may ultimately provide further insight but they still require additional study before they can be recommended for routine clinical practice. Until further studies are available, a multimodal prognostic algorithm that combines the clinical exam, SSEP, and EEG for assessment of reactivity is most pragmatic, especially in TH-treated patients. Although each of the different tests mentioned above has individual strengths, a single one should never be used alone for prognostication. All tests should be treated with caution and should only be used as one component of a multimodal prognostic algorithm, as life and death decisions are not always so algorithmic given the complexity of family input into shared decision-making. Different studies use different definitions of accuracy, and often cite high specificity which has been gained at the expense of low sensitivity. It is worth recognizing that the decision making process is a difficult task and relies on the input not only of medical doctors, but also on the family members of the patient who would bear the biggest financial and psychological cost of the decision.
Supplementary Material
Figure 1S: Burst-suppression EEG pattern in a comatose patient resuscitated from cardiac arrest.
Figure 2S: FLAIR (A), DWI (B), and ADC (C) MRI sequences from a comatose patient resuscitated from cardiac arrest. These images were acquired a week after resuscitation and demonstrate hyperintensity of the cortical ribbon on the FLAIR and DWI sequences but high ADC values consistent with subacute ischemic injury.
Acknowledgments
Financial support used for the study: The work was supported by R01HL118084 from NIH (to XJ) and 09SDG2110140 from American Heart Association (to XJ). Dr. Jia was supported in partial by Maryland Stem Cell Research Fund (2013-MSCRFE-146-00) (to XJ).
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Fugate JE, Wijdicks EFM, Mandrekar J, Claassen DO, Manno EM, White RD, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Annals of neurology. 2010;68(6):907–14. doi: 10.1002/ana.22133. [DOI] [PubMed] [Google Scholar]
- 3.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452–83. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 4.Yenari Ma, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nature reviews Neuroscience. 2012;13(4):267–78. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- 5.Storm C, Steffen I, Schefold JC, Krueger A, Oppert M, Jörres A, et al. Mild therapeutic hypothermia shortens intensive care unit stay of survivors after out-of-hospital cardiac arrest compared to historical controls. Crit Care. 2008;12(3):R78. doi: 10.1186/cc6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010;81(10):1305–52. doi: 10.1016/j.resuscitation.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S Quality Standards Subcommittee of the American Academy of N. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203–10. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 8.Oddo M, Rossetti AO. Predicting neurological outcome after cardiac arrest. Current opinion in critical care. 2011;17(3):254–9. doi: 10.1097/MCC.0b013e328344f2ae. [DOI] [PubMed] [Google Scholar]
- 9.Greer DM. Unexpected good recovery in a comatose post-cardiac arrest patient with poor prognostic features. Resuscitation. 2013;84(6):e81–2. doi: 10.1016/j.resuscitation.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Bouwes A, Binnekade JM, Kuiper Ma, Bosch FH, Zandstra DF, Toornvliet AC, et al. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Annals of neurology. 2012;71(2):206–12. doi: 10.1002/ana.22632. [DOI] [PubMed] [Google Scholar]
- 11.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Annals of neurology. 2010;67(3):301–7. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
- 12.Al Thenayan E, Savard M, Sharpe M, Norton L, Young B. Predictors of poor neurologic outcome after induced mild hypothermia following cardiac arrest. Neurology. 2008;71(19):1535–7. doi: 10.1212/01.wnl.0000334205.81148.31. [DOI] [PubMed] [Google Scholar]
- 13.Leithner C, Ploner CJ, Hasper D, Storm C. Does hypothermia influence the predictive value of bilateral absent N20 after cardiac arrest? Neurology. 2010;74(12):965–9. doi: 10.1212/WNL.0b013e3181d5a631. [DOI] [PubMed] [Google Scholar]
- 14.Steffen IG, Hasper D, Ploner CJ, Schefold JC, Dietz E, Martens F, et al. Mild therapeutic hypothermia alters neuron specific enolase as an outcome predictor after resuscitation: 97 prospective hypothermia patients compared to 133 historical non-hypothermia patients. Crit Care. 2010;14(2):R69. doi: 10.1186/cc8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golan E, Barrett K, Alali AS, Duggal A, Jichici D, Pinto R, et al. Predicting Neurologic Outcome After Targeted Temperature Management for Cardiac Arrest: Systematic Review and Meta-Analysis. Critical care medicine. 2014;42(8):1919–1930. doi: 10.1097/CCM.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 16.Greer DM, Yang J, Scripko PD, Sims JR, Cash S, Wu O, et al. Clinical examination for prognostication in comatose cardiac arrest patients. Resuscitation. 2013;84(11):1546–51. doi: 10.1016/j.resuscitation.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samaniego EA, Mlynash M, Caulfield AF, Eyngorn I, Wijman AC. Sedation Confounds Outcome Prediction in Cardiac Arrest Survivors Treated with Hypothermia. Neurocritical Care. 2011;15(1):113–9. doi: 10.1007/s12028-010-9412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Critical care medicine. 2009;37(7):S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 19.Leary M, Grossestreuer AV, Iannacone S, Gonzalez M, Shofer FS, Povey C, et al. Pyrexia and neurologic outcomes after therapeutic hypothermia for cardiac arrest. Resuscitation. 2013;84(8):1056–61. doi: 10.1016/j.resuscitation.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. The New England journal of medicine. 2013;369(23):2197–206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 21.Phelps R, Dumas F, Maynard C, Silver J, Rea T. Cerebral Performance Category and long-term prognosis following out-of-hospital cardiac arrest. Critical care medicine. 2013;41(5):1252–7. doi: 10.1097/CCM.0b013e31827ca975. [DOI] [PubMed] [Google Scholar]
- 22.Hsu CH, Li J, Cinousis MJ, Sheak KR, Gaieski DF, Abella BS, et al. Cerebral Performance Category at Hospital Discharge Predicts Long-Term Survival of Cardiac Arrest Survivors Receiving Targeted Temperature Management. Critical Care Medicine. 2014;42(12):2575–2581. doi: 10.1097/CCM.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong W, Jia X. Is Neurologic Prognostication After Hypothermia Ready for Primetime? Critical care medicine. 2014;42(12):2644–2645. doi: 10.1097/CCM.0000000000000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cronberg T, Horn J, Kuiper Ma, Friberg H, Nielsen N. A structured approach to neurologic prognostication in clinical cardiac arrest trials. Scandinavian journal of trauma, resuscitation and emergency medicine. 2013;21(1):45. doi: 10.1186/1757-7241-21-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dragancea I, Rundgren M, Englund E, Friberg H, Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation. 2013;84(3):337–42. doi: 10.1016/j.resuscitation.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Kamps MJa, Horn J, Oddo M, Fugate JE, Storm C, Cronberg T, et al. Prognostication of neurologic outcome in cardiac arrest patients after mild therapeutic hypothermia: a meta-analysis of the current literature. Intensive care medicine. 2013;39(10):1671–82. doi: 10.1007/s00134-013-3004-y. [DOI] [PubMed] [Google Scholar]
- 27.Sandroni C, Cavallaro F, Callaway CW, D’Arrigo S, Sanna T, Kuiper Ma, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 2: Patients treated with therapeutic hypothermia. Resuscitation. 2013;84(10):1324–38. doi: 10.1016/j.resuscitation.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Lucas JM, Cocchi MN, Salciccioli J, Stanbridge JA, Geocadin RG, Herman ST, et al. Neurologic recovery after therapeutic hypothermia in patients with post-cardiac arrest myoclonus. Resuscitation. 2012;83(2):265–9. doi: 10.1016/j.resuscitation.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Rothstein TL. The role of evoked potentials in anoxic-ischemic coma and severe brain trauma. J Clin Neurophysiol. 2000;17(5):486–97. doi: 10.1097/00004691-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez Ra, Bussière M, Froeschl M, Nathan HJ. Auditory-evoked potentials during coma: do they improve our prediction of awakening in comatose patients? Journal of critical care. 2014;29(1):93–100. doi: 10.1016/j.jcrc.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Okada K, Ohde S, Otani N, Sera T, Mochizuki T, Aoki M, et al. Prediction protocol for neurological outcome for survivors of out-of-hospital cardiac arrest treated with targeted temperature management. Resuscitation. 2012;83(6):734–9. doi: 10.1016/j.resuscitation.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 32.Gold B, Puertas L, Davis SP, Metzger A, Yannopoulos D, Oakes Da, et al. Awakening after cardiac arrest and post resuscitation hypothermia: are we pulling the plug too early? Resuscitation. 2014;85(2):211–4. doi: 10.1016/j.resuscitation.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Wojtczak-Soska K, Lelonek M. S-100B protein: An early prognostic marker after cardiac arrest. Cardiology journal. 2010;17(5):532–6. [PubMed] [Google Scholar]
- 34.Shinozaki K, Oda S, Sadahiro T, Nakamura M, Abe R, Nakada T-A, et al. Serum S-100B is superior to neuron-specific enolase as an early prognostic biomarker for neurological outcome following cardiopulmonary resuscitation. Resuscitation. 2009;80(8):870–5. doi: 10.1016/j.resuscitation.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Almaraz AC, Bobrow BJ, Wingerchuk DM, Wellik KE, Demaerschalk BM. Serum neuron specific enolase to predict neurological outcome after cardiopulmonary resuscitation: a critically appraised topic. The neurologist. 2009;15(1):44–8. doi: 10.1097/NRL.0b013e318191f810. [DOI] [PubMed] [Google Scholar]
- 36.Mörtberg E, Zetterberg H, Nordmark J, Blennow K, Rosengren L, Rubertsson S. S-100B is superior to NSE, BDNF and GFAP in predicting outcome of resuscitation from cardiac arrest with hypothermia treatment. Resuscitation. 2011;82(1):26–31. doi: 10.1016/j.resuscitation.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Samaniego EA, Mlynash M, Caulfield AF, Eyngorn I, Wijman AC. Sedation Confounds Outcome Prediction in Cardiac Arrest Survivors Treated with Hypothermia. Neurocritical Care. 2011;15(1):113–9. doi: 10.1007/s12028-010-9412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiainen M, Roine RO, Pettilä V, Takkunen O. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34(12):2881–2886. doi: 10.1161/01.STR.0000103320.90706.35. [DOI] [PubMed] [Google Scholar]
- 39.Huntgeburth M, Adler C, Rosenkranz S, Zobel C, Haupt WF, Dohmen C, et al. Changes in Neuron-Specific Enolase are More Suitable Than Its Absolute Serum Levels for the Prediction of Neurologic Outcome in Hypothermia-Treated Patients with Out-of-Hospital Cardiac Arrest. Neurocrit Care. 2014;20(3):358–366. doi: 10.1007/s12028-013-9848-8. [DOI] [PubMed] [Google Scholar]
- 40.Cocchi MN, Lucas JM, Salciccioli J, Carney E, Herman S, Zimetbaum P, et al. The role of cranial computed tomography in the immediate post-cardiac arrest period. Internal and emergency medicine. 2010;5(6):533–8. doi: 10.1007/s11739-010-0403-8. [DOI] [PubMed] [Google Scholar]
- 41.Cho YM, Lim YS, Yang HJ, Park WB, Cho JS, Kim JJ, et al. Blood ammonia is a predictive biomarker of neurologic outcome in cardiac arrest patients treated with therapeutic hypothermia. The American journal of emergency medicine. 2012;30(8):1395–401. doi: 10.1016/j.ajem.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Lee TR, Kang MJ, Cha WC, Shin TG, Sim MS, Jo IJ, et al. Better lactate clearance associated with good neurologic outcome in survivors who treated with therapeutic hypothermia after out-of-hospital cardiac arrest. Critical care. 2013;17(5):R260. doi: 10.1186/cc13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Starodub R, Abella BS, Grossestreuer AV, Shofer FS, Perman SM, Leary M, et al. Association of serum lactate and survival outcomes in patients undergoing therapeutic hypothermia after cardiac arrest. Resuscitation. 2013;84(8):1078–82. doi: 10.1016/j.resuscitation.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Thenayan EAL, Savard M, Sharpe MD, Norton L, Young B. Electroencephalogram for prognosis after cardiac arrest. Journal of critical care. 2010;25(2):300–4. doi: 10.1016/j.jcrc.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 45.Rossetti AO, Urbano LA, Delodder F, Kaplan PW, Oddo M. Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care. 2010;14(5):R173. doi: 10.1186/cc9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rundgren M, Westhall E, Cronberg T, Rosén I, Friberg H. Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Critical care medicine. 2010;38(9):1838–44. doi: 10.1097/CCM.0b013e3181eaa1e7. [DOI] [PubMed] [Google Scholar]
- 47.Rundgren M, Rosén I, Friberg H. Amplitude-integrated EEG (aEEG) predicts outcome after cardiac arrest and induced hypothermia. Intensive care medicine. 2006;32(6):836–42. doi: 10.1007/s00134-006-0178-6. [DOI] [PubMed] [Google Scholar]
- 48.Shah DK, Wusthoff CJ, Clarke P, Wyatt JS, Ramaiah SM, Dias RJ, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Archives of disease in childhood Fetal and neonatal edition. 2014;99(3):F219–24. doi: 10.1136/archdischild-2013-305206. [DOI] [PubMed] [Google Scholar]
- 49.Crepeau AZ, Rabinstein Aa, Fugate JE, Mandrekar J, Wijdicks EF, White RD, et al. Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology. 2013;80(4):339–44. doi: 10.1212/WNL.0b013e31827f089d. [DOI] [PubMed] [Google Scholar]
- 50.Oddo M, Rossetti AO. Early Multimodal Outcome Prediction After Cardiac Arrest in Patients Treated With Hypothermia. Critical care medicine. 2014;42(6):1340–1347. doi: 10.1097/CCM.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 51.Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJaM. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Critical care medicine. 2012;40(10):2867–75. doi: 10.1097/CCM.0b013e31825b94f0. [DOI] [PubMed] [Google Scholar]
- 52.Lucas JM, Cocchi MN, Salciccioli J, Stanbridge Ja, Geocadin RG, Herman ST, et al. Neurologic recovery after therapeutic hypothermia in patients with post-cardiac arrest myoclonus. Resuscitation. 2012;83(2):265–9. doi: 10.1016/j.resuscitation.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 53.Tjepkema-Cloostermans MC, van Meulen FB, Meinsma G, van Putten MJ. A Cerebral Recovery Index (CRI) for early prognosis in patients after cardiac arrest. Crit Care. 2013;17(5):R252. doi: 10.1186/cc13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia X, Koenig Ma, Shin H-C, Zhen G, Pardo Ca, Hanley DF, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008;76(3):431–42. doi: 10.1016/j.resuscitation.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia X, Koenig Ma, Nickl R, Zhen G, Thakor NV, Geocadin RG. Early electrophysiologic markers predict functional outcome associated with temperature manipulation after cardiac arrest in rats. Critical care medicine. 2008;36(6):1909–16. doi: 10.1097/CCM.0b013e3181760eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howell K, Grill E, Klein A-M, Straube A, Bender A. Rehabilitation outcome of anoxic-ischaemic encephalopathy survivors with prolonged disorders of consciousness. Resuscitation. 2013;84(10):1409–15. doi: 10.1016/j.resuscitation.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 57.Madhok J, Maybhate A, Xiong W, Koenig MA, Geocadin RG, Jia X, et al. Quantitative assessment of somatosensory-evoked potentials after cardiac arrest in rats: prognostication of functional outcomes. Critical care medicine. 2010;38(8):1709–17. doi: 10.1097/CCM.0b013e3181e7dd29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong W, Koenig MA, Madhok J, Jia X, Puttgen HA, Thakor NV, et al. Evolution of Somatosensory Evoked Potentials after Cardiac Arrest induced hypoxic-ischemic injury. Resuscitation. 2010;81(7):893–7. doi: 10.1016/j.resuscitation.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheel M, Storm C, Gentsch A, Nee J, Luckenbach F, Ploner CJ, et al. The prognostic value of gray-white-matter ratio in cardiac arrest patients treated with hypothermia. Scandinavian journal of trauma, resuscitation and emergency medicine. 2013;21(1):23. doi: 10.1186/1757-7241-21-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torbey MT, Selim M, Knorr J, Bigelow C, Recht L. Quantitative analysis of the loss of distinction between gray and white matter in comatose patients after cardiac arrest. Stroke; a journal of cerebral circulation. 2000;31(9):2163–7. doi: 10.1161/01.str.31.9.2163. [DOI] [PubMed] [Google Scholar]
- 61.Cronberg T, Rundgren M, Westhall E, Englund E, Siemund R, Rosén I, et al. Neuron-specific enolase correlates with other prognostic markers after cardiac arrest. Neurology. 2011;77(7):623–30. doi: 10.1212/WNL.0b013e31822a276d. [DOI] [PubMed] [Google Scholar]
- 62.Torbey MT, Geocadin R, Bhardwaj A. Brain arrest neurological outcome scale (BrANOS): predicting mortality and severe disability following cardiac arrest. Resuscitation. 2004;63(1):55–63. doi: 10.1016/j.resuscitation.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 63.Wu O, Batista LM, Lima FO, Vangel MG, Furie KL, Greer DM. Predicting clinical outcome in comatose cardiac arrest patients using early noncontrast computed tomography. Stroke; a journal of cerebral circulation. 2011;42(4):985–92. doi: 10.1161/STROKEAHA.110.594879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wijman CAC, Mlynash M, Caulfield AF, Hsia AW, Eyngorn I, Bammer R, et al. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Annals of neurology. 2009;65(4):394–402. doi: 10.1002/ana.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu O, Sorensen AG, Benner T, Singhal AB, Furie KL, Greer DM. Comatose patients with cardiac arrest: predicting clinical outcome with diffusion-weighted MR imaging. Radiology. 2009;252(1):173–81. doi: 10.1148/radiol.2521081232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oualha M, Gatterre P, Boddaert N, Dupic L, De Saint Blanquat L, Hubert P, et al. Early diffusion-weighted magnetic resonance imaging in children after cardiac arrest may provide valuable prognostic information on clinical outcome. Intensive care medicine. 2013;39(7):1306–12. doi: 10.1007/s00134-013-2930-z. [DOI] [PubMed] [Google Scholar]
- 67.Greer D, Scripko P, Bartscher J, Sims J, Camargo E, Singhal A, et al. Clinical MRI interpretation for outcome prediction in cardiac arrest. Neurocritical care. 2012;17(2):240–4. doi: 10.1007/s12028-012-9716-y. [DOI] [PubMed] [Google Scholar]
- 68.Jarnum H, Knutsson L, Rundgren M, Siemund R, Englund E, Friberg H, et al. Diffusion and perfusion MRI of the brain in comatose patients treated with mild hypothermia after cardiac arrest: a prospective observational study. Resuscitation. 2009;80(4):425–30. doi: 10.1016/j.resuscitation.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 69.Heradstveit BE, Larsson E-M, Skeidsvoll H, Hammersborg S-M, Wentzel-Larsen T, Guttormsen AB, et al. Repeated magnetic resonance imaging and cerebral performance after cardiac arrest--a pilot study. Resuscitation. 2011;82(5):549–55. doi: 10.1016/j.resuscitation.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 70.Luyt C-E, Galanaud D, Perlbarg V, Vanhaudenhuyse A, Stevens RD, Gupta R, et al. Diffusion tensor imaging to predict long-term outcome after cardiac arrest: a bicentric pilot study. Anesthesiology. 2012;117(6):1311–21. doi: 10.1097/ALN.0b013e318275148c. [DOI] [PubMed] [Google Scholar]
- 71.Schmitz B, Bock C, Hoehn-Berlage M, Kerskens CM, Böttiger BW, Hossmann Ka. Recovery of the rodent brain after cardiac arrest: a functional MRI study. Magn Reson Med. 1998;39(5):783–8. doi: 10.1002/mrm.1910390516. [DOI] [PubMed] [Google Scholar]
- 72.Gofton TE, Chouinard PA, Young GB, Bihari F, Nicolle MW, Lee DH, et al. Functional MRI study of the primary somatosensory cortex in comatose survivors of cardiac arrest. Exp Neurol. 2009;217(2):320–7. doi: 10.1016/j.expneurol.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 73.Koenig MA, Holt JL, Ernst T, Buchthal SD, Nakagawa K, Stenger VA, et al. MRI default mode network connectivity is associated with functional outcome after cardiopulmonary arrest. Neurocritical Care. 2014;20(3):348–57. doi: 10.1007/s12028-014-9953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJF, Bruno MA, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133(Pt 1):161–71. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Norton L, Hutchison RM, Young GB, Lee DH, Sharpe MD, Mirsattari SM. Disruptions of functional connectivity in the default mode network of comatose patients. Neurology. 2012;78(3):175–81. doi: 10.1212/WNL.0b013e31823fcd61. [DOI] [PubMed] [Google Scholar]
- 76.Zanatta P, Messerotti Benvenuti S, Baldanzi F, Bendini M, Saccavini M, Tamari W, et al. Pain-related somatosensory evoked potentials and functional brain magnetic resonance in the evaluation of neurologic recovery after cardiac arrest: a case study of three patients. Scandinavian journal of trauma, resuscitation and emergency medicine. 2012;20(1):22. doi: 10.1186/1757-7241-20-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rudolf J, Ghaemi M, Haupt WF, Szelies B, Heiss WD. Cerebral glucose metabolism in acute and persistent vegetative state. J Neurosurg Anesthesiol. 1999;11(1):17–24. doi: 10.1097/00008506-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 78.Rudolf J, Sobesky J, Ghaemi M, Heiss W-D. The correlation between cerebral glucose metabolism and benzodiazepine receptor density in the acute vegetative state. Eur J Neurol. 2002;9(6):671–7. doi: 10.1046/j.1468-1331.2002.00468.x. [DOI] [PubMed] [Google Scholar]
- 79.Edgren E, Enblad P, Grenvik Å, Lilja A, Valind S, Wiklund L, et al. Cerebral blood flow and metabolism after cardiopulmonary resuscitation. A pathophysiologic and prognostic positron emission tomography pilot study. Resuscitation. 2003;57(2):161–70. doi: 10.1016/s0300-9572(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 80.Mörtberg E, Cumming P, Wiklund L, Rubertsson S. Cerebral metabolic rate of oxygen (CMRO2) in pig brain determined by PET after resuscitation from cardiac arrest. Resuscitation. 2009;80(6):701–6. doi: 10.1016/j.resuscitation.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Nakamura T, Kuroda Y, Torigoe N, Abe Y, Yamashita S, Kawakita K, et al. Cerebral metabolism monitoring during hypothermia following resuscitation from cardiopulmonary arrest. Acta neurochirurgica Supplement. 2008;102:203–6. doi: 10.1007/978-3-211-85578-2_40. [DOI] [PubMed] [Google Scholar]
- 82.Sandroni C, Cariou A, Cavallaro F, Cronberg T, Friberg H, Hoedemaekers C, et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation. 2014;85(12):1779–89. doi: 10.1016/j.resuscitation.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 83.Thomke F. Assessing prognosis following cardiopulmonary resuscitation and therapeutic hypothermia-a critical discussion of recent studies. Deutsches Arzteblatt international. 2013;110(9):137–43. doi: 10.3238/arztebl.2013.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Friberg H, Rundgren M, Westhall E, Nielsen N, Cronberg T. Continuous evaluation of neurological prognosis after cardiac arrest. Acta anaesthesiologica Scandinavica. 2013;57(1):6–15. doi: 10.1111/j.1399-6576.2012.02736.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1S: Burst-suppression EEG pattern in a comatose patient resuscitated from cardiac arrest.
Figure 2S: FLAIR (A), DWI (B), and ADC (C) MRI sequences from a comatose patient resuscitated from cardiac arrest. These images were acquired a week after resuscitation and demonstrate hyperintensity of the cortical ribbon on the FLAIR and DWI sequences but high ADC values consistent with subacute ischemic injury.