Abstract
Uterine leiomyomata (UL) have a substantial impact on women's health, but relatively few studies have identified opportunities for primary prevention of these neoplasms. Most established risk factors are not modifiable, including premenopausal age, African ancestry, age at menarche, and childbearing history. The main challenge in studying UL is that a large proportion of tumors are asymptomatic. Herein, we review the epidemiology of UL from published studies to date. We highlight the advantages of ultrasound screening studies and the ways in which their innovative methods have helped clarify the etiology of disease. We conclude with a discussion of promising new hypotheses.
I. Introduction
Uterine leiomyomata (UL), commonly called “fibroids,” are benign neoplasms of uterine smooth muscle. Although they are often asymptomatic, UL can cause excessive menstrual bleeding, pelvic pain, and other symptoms that seriously affect a woman’s quality of life. Symptomatic UL may require medical or surgical intervention and increased medical utilization; in the U.S., they account for nearly 30% of all hysterectomies among women ages 18–44 years [1] and $9.4 billion in annual health care costs [2]. From 1993 to 2003, inpatient admissions for UL in U.S. hospitals increased by more than 20% [3], and UL remain the most common diagnosis among inpatient hospitalizations for gynecologic conditions in women 15–54 years of age [4]. During 1997–2005, the percentage of hysterectomies due to UL decreased from 31.4% to 26.9% [1]. However, rates of alternative surgeries, such as myomectomy and uterine artery embolization, have increased during the same period [3, 5, 6]. Despite their substantial impact on gynecologic morbidity, relatively little is known about the etiology of UL.
II. Issues in the Design of Epidemiologic Studies
A. Symptomatology
Heavy menstrual bleeding and pelvic pressure are the primary symptoms associated with UL [7]. Other symptoms include infertility, increased urinary frequency or incontinence, constipation, abdominal bloating, dyspareunia, and fatigue (due to anemia from heavy bleeding) [8, 9]. The spectrum and severity of symptoms often depends on the size, location, and number of tumors in the uterus. In an ultrasound-screening study (NIEHS Uterine Fibroid Study, UFS) of a randomly-selected sample of 1,349 women aged 35–49 years enrolled in an urban health plan, risk of self-reported heavy bleeding increased with increasing tumor size [24]. Studies that use hysteroscopy to examine the uterine cavity have found that UL that distort the cavity (Class 0, I or II submucosal UL) are more closely associated with anemia than other types of UL, even though self-reported bleeding scores do not differ [10, 11].
A large proportion of UL are diagnosed in the absence of symptoms. For example, in two prospective cohort studies of women who reported clinically-diagnosed UL, between 29% [12] and 33% [13] reported that their UL were found incidentally at the time of a routine pelvic exam or screening for another medical condition. These observations support findings from the UFS, in which 51% of premenopausal women without a clinical diagnosis of UL had ultrasound evidence of UL [14].
B. Methods of Diagnosis
Information on new UL diagnoses may be obtained via self-report, hospital or clinical records, or automated hospital discharge and ambulatory care databases. Ideally, original diagnostic imaging, surgical, and pathology reports are reviewed to validate the diagnosis. For example, self-reported diagnoses were accurate in 93% and 96%, respectively, of women who agreed to release their medical records in a randomly-selected subsample of respondents from two large prospective cohort studies [12, 13].
Ultrasound screening studies are becoming increasingly more common. Ultrasound can confirm UL diagnosis and decrease misclassification among the controls who may have asymptomatic UL. These studies provide the most valid information regarding the prevalence of UL [9, 14–18]. With universal ultrasound screening to classify UL, exposures could be compared not only between symptomatic cases and asymptomatic controls, but also between cases and those controls with uteri free of occult UL, and between controls with and without occult UL.
Abdominal [19] or transvaginal [20, 21] ultrasound provides reasonably sensitive, minimally-invasive confirmation of a suspected UL diagnosis relative to histologic evidence [19, 21]. However, ultrasound can be a costly addition to a prospective study and the sensitivity of ultrasound wanes when the uterus is enlarged (e.g., pregnancy) or there are multiple UL in the uterus. Ultrasound has a limited ability to detect tumors that are <0.5 cm [17] makes it difficult to determine the exact onset of disease.
Diagnosis of UL by magnetic resonance imaging (MRI) is more expensive, but has better accuracy than ultrasound for mapping UL, in particular for larger uteri or multiple UL [21]. Only a subset of women with a radiologic diagnosis will ultimately proceed to surgery and histologic confirmation (gold standard). Operative and pathology reports from surgical procedures provide the most precise information regarding the size, location, and number of tumors. However, women who present for hysterectomy generally have failed medical or non-surgical management and represent the symptomatic end of the disease spectrum. Thus, epidemiologic studies that use only hysterectomy cases may identify risk factors operating relatively late in the growth and development of the tumors. Identifying the etiology of UL or options for early intervention is more difficult when the UL have grown to a large size, as they have likely been there for many years.
If systematic pelvic imaging is not possible, epidemiologic studies can reduce the impact of bias by including cases newly-diagnosed by ultrasound (or MRI) in addition to those that are confirmed histologically, though this approach will still result in misclassification among controls. Another approach to reducing disease misclassification involves restricting the case definition to self-reported diagnoses of early-onset UL [22, 23] because a lower proportion of younger cases will be misclassified as noncases [14].
UL are often associated with other gynecologic diseases such as endometriosis. Some UL cases will be identified incidentally during examinations prompted by gynecologic symptoms caused by these other conditions. Incidental diagnoses are inevitable because it is difficult to determine whether the symptoms are due to UL or to other pathologic conditions. However, if a characteristic is not related to UL, but is related to a condition that often coexists with these tumors (e.g., adenomyosis), a spurious association with UL could result. To reduce the potential impact of comorbid diagnoses, cases should not be selected preferentially from specialty clinics that treat gynecologic, urologic, or infertility conditions [11, 24].
III. Frequency of UL and Demographic Patterns
Prevalence and Incidence
Given the high frequency of asymptomatic UL, the most valid estimates of their prevalence and incidence come from epidemiologic studies that use universal ultrasound screening [9, 14, 15, 17, 25, 26]. However, the best measures of disease burden and healthcare expenditures come from studies of hospital discharge data or self-reported rates of clinical diagnoses. Incidence rates of UL diagnoses in U.S. populations are based largely on data from national hospital discharge studies [4, 27], nationally-representative studies [28], and large prospective cohort studies [12, 13, 29]. In these studies, rates vary according to case definition, ranging from 12.8 per 1,000 person-years for all diagnoses (by pelvic exam, ultrasound, or hysterectomy) to approximately 2.0 per 1,000 person-years for hysterectomy-confirmed cases. During 1998 through 2005, the rate of hospitalizations for UL among women aged 15–54 years was 2.8 cases per 1,000 person-years (Table 1). The vast majority of hospitalizations for UL involved a surgical procedure (94.4%), most commonly hysterectomy (79.2%) [4].
Table 1.
Estimated Number and Rate (per 10,000 person-years) of Inpatient Hospitalizations for Uterine Leiomyomata by Age Group among US Women, Nationwide Inpatient Sample, 1998–2005 (Source: Whiteman et al. 2010)[4]
| Age | N | Rate* (SE) |
|---|---|---|
| 15–24 | 4,831 | 0.3 (0.01) |
| 25–29 | 31,623 | 4.1 (0.1) |
| 30–34 | 125,360 | 15.3 (0.3) |
| 35–39 | 315,975 | 35.9 (0.6) |
| 40–44 | 546,786 | 59.9 (0.8) |
| 45–49 | 532,405 | 62.7 (0.8) |
| 50–54 | 238,493 | 31.8 (0.5) |
| Total | 1,795,473 | 27.5 (0.4) |
1. Age
UL tend to increase with age through the reproductive years and decline in the postmenopausal years [4, 13, 27, 29–31]. During 1998–2005, rates of inpatient hospitalization for UL in the U.S. increased steadily by age until reaching a peak among women aged 45–49 years (6.3 per 1,000 person-years), and then declined among women aged 50–54 years (3.2 per 1,000 person-years) (Table 1) [4]. Age-specific prevalence estimates for UL, regardless of symptomatology, come from five ultrasound-screening studies (Figure 1). They also show an increase in prevalence of UL with increasing premenopausal age.
Figure 1.
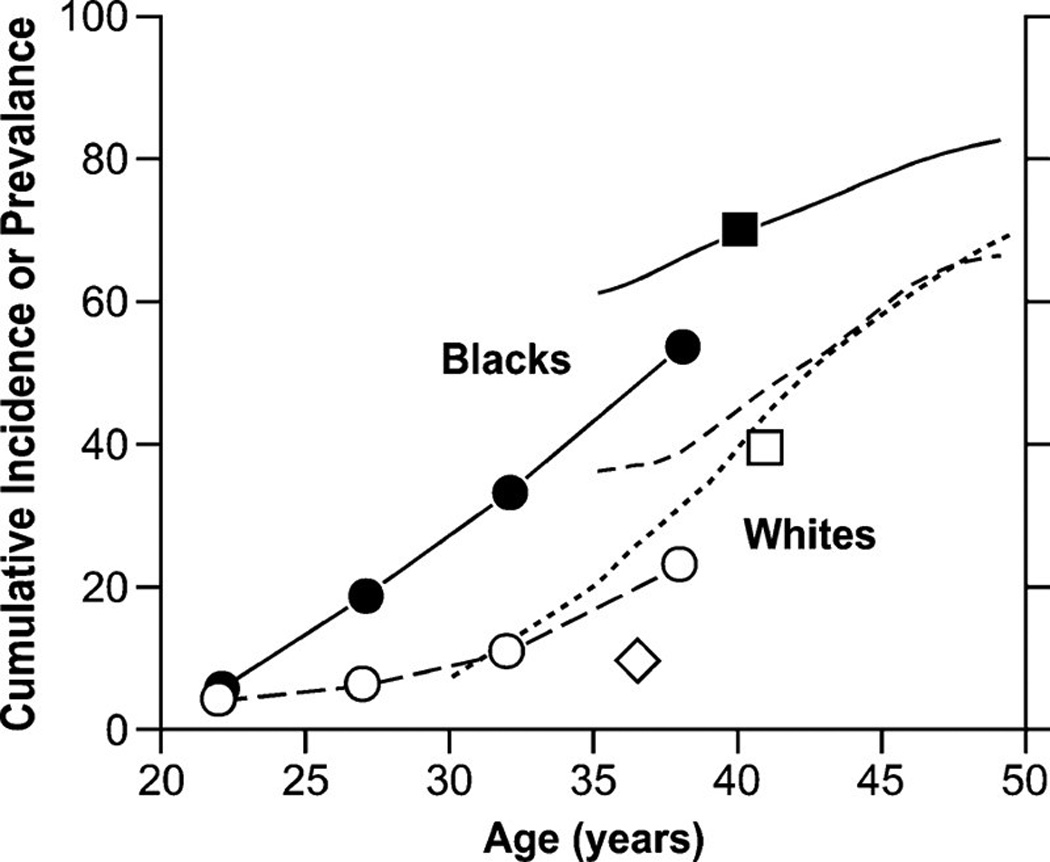
Age-specific cumulative incidence or prevalence estimates of uterine leiomyomata based on ultrasound-screening studies. The closed and open circles are prevalence data averaged over the age ranges shown for black and white women participating in Right from the Start [82], a community-based pregnancy study that screens for leiomyomata at ~7 weeks of gestation. The solid line and line of long dashes are cumulative incidence data from the UFS [14] a study of 35- to 49-year-old health plan members whose case status was based on either ultrasound screening for uterine leiomyomata (premenopausal women) or on prior diagnosis of uterine leiomyomata (postmenopausal women). The line of short dashes is cumulative incidence data from the low-exposed group of potentially dioxin-exposed women (Seveso, Italy) [9, 26, 87], 30- to 50-year-olds whose case status was based on either ultrasound screening for uterine leiomyomata (premenopausal women) or on prior diagnosis of uterine leiomyomata (postmenopausal women). The squares are average cumulative incidence data for samples of 33- to 46-year-old black and white participants in the Coronary Artery Risk Development in Young Adults study [25], a population-based study of cardiovascular disease. The diamond is the average prevalence of uterine leiomyomata for a group of 33- to 40-year-old representative Swedish women who had ultrasound screening for uterine leiomyomata [15].
2. Race
Incidence of UL is 2–3 times greater among black than white women [13, 14, 28], after adjustment for age and other risk factors. The higher incidence among black women is evident at nearly all ages [13, 27]. Rates of hospitalization for UL are about 5.3 per 1,000 person-years for black women and 2.4 per 1,000 person-years for white women [27]. The UFS was the first definitive study to confirm the black-white difference in incidence, showing that the cumulative incidence by age 50 (measure of lifetime risk) was nearly 70% for white women and >80% for black women [14]. Black women had nearly three times the age-specific cumulative incidence of white women. When restricted to clinically-significant UL (≥9-week gestation uterus, at least one >4 cm tumor, or at least one submucosal leiomyoma), estimates were 50% for black women and 25% for white women [14].
Ultrasound data on younger women come from an early-pregnancy screening study [17], demonstrating an age at onset about ten years earlier among black than white women. The cumulative incidence of UL steadily and rapidly increases for 10 years beginning at about age 25 years for black women and about age 35 years for white women (Figure 1). Similarly, an ultrasound screening study of asymptomatic women 18 to 30 years found a prevalence of 26% among black women and 7% among white women [13]. Other findings also support a black-white difference in age at onset and disease severity. Among women undergoing hysterectomy for UL, mean ages at first diagnosis and hysterectomy were lower among black women than white women [8, 32], and black women had longer hospital stays and higher medical costs per day [3].
The reason for the more frequent and earlier diagnoses of UL among black women is unclear. The excess does not seem to be attributable to differences in the types and severity of symptoms, use of health care, or prevalence of putative risk factors [8, 13]. In both the Nurses’ Health Study (NHS II) and UFS, adjustment for several known or suspected risk factors did not substantially attenuate the three-fold difference in risk between black and white women [13, 14]. The observation that black women have more UL, larger tumors, and heavier uterine weight than white women [8, 13, 14] suggests a genetic basis for the racial difference. However, yet-to-be identified or elucidated risk factors such as vitamin D deficiency, reproductive tract infections, psychosocial stessors, or other environmental factors cannot be ruled out as possible explanations.
IV. Etiologic Hypotheses and Risk Factors
A. Etiologic Hypotheses
UL are thought to arise from an initiating event related to three pathways: 1) sex steroid hormones, 2) disordered wound healing, and 3) genetic abnormalities [33]. The initiating event likely causes a myometrial cell to acquire a somatic genetic or epigenetic change that confers increased sensitivity to growth factors or hormones [34, 35]. An expansion and growth phase follows, during which cloning of the myometrial cell is accompanied by stimulation of the extracellular environment that provides structure and increased mass to the tumor [35]. Factors involved in the initiation of UL are being studied using animal models [36–39]. A large prospective study of African-American women aged 23–34 years in Detroit is on-going to study risk factors and exposures present before UL diagnosis (screened with ultrasound) [40, 41].
1. Estrogen and Progesterone
UL occur during the reproductive years and tend to regress after menopause, signifying that estrogen and progesterone play prominent roles in promoting growth [33]. When compared with normal myometrium, smooth muscle cells in UL exhibit increased expression of steroid hormone receptors, growth factors, and growth factor receptors, most of which are regulated by estrogen [35, 42]. While circulating sex steroid hormones are no different in women with and without UL [43], tissue concentrations of estrogens are higher in leiomyoma tissue. A contributing factor to this elevation is aromatase, which converts testosterone and androstenedione to estrogens. Aromatase [44] and estrogen receptor [45] expression is higher in leiomyoma tissue than myometrium. Because of these findings, aromatase inhibitors or selective estrogen receptor modulators are under investigation as therapeutic options [46, 47]. In an Eker rat model, estrogen stimulated UL growth while estrogen antagonists, such as Tamoxifen, inhibited growth. Environmental compounds that can interact with estrogen receptors are also being studied [22, 26, 48, 49]. Clinical studies of UL treated with gonadotropin-releasing hormone (GnRH) agonists provide parallel data from humans. GnRH agonists create a temporary hypoestrogenic state by reducing biologically active gonadotropin secretions of the pituitary gland, which markedly reduces UL size [50].
Progesterone has recently become a focus of research in UL development and growth [51], and may be the primary hormone stimulating UL growth [52, 53]. In in vivo models, progesterone and the progesterone receptor directly induced growth, likely through the production of extracellular matrix via down-regulation of a tumor suppressor [53–55]. During the secretory phase of the menstrual cycle when progesterone is highest, anti-apoptotic proto-oncogene bcl-2 activity in UL is highest [56–58]. Further, the proliferative activity of UL appears to be higher with medroxyprogesterone acetate (MPA) use compared with other combined oral contraceptive or no hormonal contraception use [59]. UL proliferation is higher in postemenopausal women receiving combined estrogen and progesterone therapy compared with estrogen therapy alone [57]. A role of progesterone in UL growth also is supported by most studies of UL treated with progesterone inhibitors. The progesterone receptor antagonist, mifepristone, causes UL shrinkage in a dose-response fashion [60]; however, therapeutic trials have been limited by adverse endometrial changes [61]. Progesterone receptor modulators, which have mixed agonist/antagonist effects, reduce UL symptoms in clinical trials [62]. Ulipristal acetate, a selective progesterone receptor modulator, has been shown to effectively reduce UL size and control UL-related symptoms with similar efficacy to GnRH agonists, and with less hot flashes [63, 64]. This medication is approved in Europe and Canada for preoperative treatment of UL. Repeated 12-week courses were shown to be effective and safe leading to the potential for preventative medical therapy for UL [65].
2. Growth Factors & Disordered Wound Healing
The expression of various growth factors is higher in UL than normal myometrium. Vascular endothelial growth factor-A (VEGF-A) has been well-studied because of its role in angiogenesis, which is important for tumor growth and cell proliferation. VEGF expression is influenced by estrogen and progesterone and is higher in UL than myometrium [43]. Fibroblast growth factor has effects on endothelium, smooth muscle cells, and fibroblasts and may be preferentially expressed in extracellular matrix [43]. Similarly, insulin-like growth factor-1 (IGF-1) is higher in leiomyoma tissue and may be regulated by estrogen and autocrine control [35]. IGF-1 stimulates leiomyocyte proliferation, but elevated circulating levels are not associated with tumor prevalence [66].
One of the most studied growth factors is transforming growth factor-beta (TGF-β), which induces extracellular matrix formation in UL through several signaling pathways [67–69]. The TGF-β pathway also plays a role in disordered wound healing which may lead to tumorigenesis [68, 70]. Myometrial injury causes growth factor changes that increase cellular proliferation, decrease apoptosis, and increase extracellular matrix production [71]. Fibrous thickening of tissue (myometrial hyperplasia) is associated with seedling leiomyomas, a leiomyoma precursor [72]. The inhibition of TGF-β pathways appears to be effective in decreasing growth and bulk of extracellular matrix; current investigations into these pathways may elucidate potential preventive factors.
3. Genetic Factors
The well-documented racial disparity in UL prevalence and the increased familial aggregation of UL indicate that genetic factors may underlie UL formation. Pathology data show nonrandom chromosomal abnormalities, especially in larger tumors, related to mutations in cell growth regulation. Clonal expansion of leiomyoma cells precedes these chromosomal anomalies, indicating that chromosomal anomalies are an effect of growth [73]. A detailed review of studies pertaining to UL genetics appears in a subsequent chapter of this monograph.
B. Risk Factors for UL Development
In this section, we summarize the results of epidemiologic studies of risk factors for UL, focusing on the etiologic factors mentioned above: estrogens, progesterone, growth factors, and genetic factors. Most evidence regarding risk factors comes from twenty-two studies based on surgical, clinical, or ultrasound diagnoses (Table 2).
Table 2.
Characteristics of Published Epidemiologic Studies of Uterine Leiomyomata
| Study type | Name of study (or first author) | Location | Enrollment date |
End of follow-up |
Age at enrollment |
Basis of case ascertainment |
Cases | Person-years (p- y) or controls |
|---|---|---|---|---|---|---|---|---|
| Cohort | Walnut Creek Contraceptive Study | U.S. | 1968–1972 | 1977 | 18–54 | Hospital discharge | unknown | 107,165a |
| Nurses’ Health Study (NHS) II | U.S. | 1989 | 2003f | 25–42 | Self-reportb | 9,847 | 1,163,439 p-y | |
| OC Study of the Royal College of General Practitioners | U.K. | 1968–1969 | 1971 (?) | 15–45 | Physician report | 168 | 87,000 p-ya | |
| Oxford Family Planning Association study | U.K. | 1968–1974 | 1985 | 29–35 | Path report | 538 | 198,653 p-y | |
| Black Women’s Health Study (BWHS) | U.S | 1997 | 2009f | 23–50 | Self-reportb | 6,627 | 185,013 p-y | |
| DES Collaborative Follow-up Study | U.S. | 1994 | 1997 | 18–61 | Path report | 85 | 112,229 p-y | |
| California Teachers’ Study (CTS) | U.S. | 1995–1996 | 2006 | 22–90 | Hospital discharge | 1,790 | 795,882 p-y | |
| Seveso Women’s Health Study | Italy | 1996–1998 | 2007f | 20–60 | Ultrasound | 96 | 539 | |
| Right From the Start | U.S. | 2001–2007 | 2001–2008 | ≥18 | Ultrasound | 458 | 3,813 | |
| NIEHS Fibroid Growth Study (FGS) | U.S. | 2001 | 2004 | 24–54 | MRI | 72 | 72c | |
| Study of Environment Lifestyle and Fibroids (SELF) | U.S. | 2012 | 2017 | 23–34 | U/S | ~330 | ~6,500 | |
| Case-Control | Group Health Cooperative of Puget Sound Study | U.S. | 1985–1987 | — | 40–64 | Path report | 345 | 749 |
| Parazini et al. | Italy | 1986–1993 | — | ≤58 | Path report | 843d | 1,557 | |
| Lumbiganon et al. | Thailand | 1991–1993 | — | ≤60 | Path report | 901 | 2,709 | |
| Cancer and Steroid Hormone Study et al. | U.S. | 1980–1982 | — | 20–54 | Self-reporte | 201 | 1,503 | |
| Chen et al. | U.S. | 1978–1987 | — | 17–44 | Self-report or lap | 317 | 2,854 | |
| Baltimore Women’s Health Study | U.S. | 1990–1993 | — | 18–55 | U/S or path report | 318 | 394 | |
| Uterine Leiomyomata Epidemiology Project (TULEP) | U.S. | 1995–1998 | — | 25–59 | U/S or surgery | 647 | 637 | |
| Cross-sectional | NIEHS Uterine Fibroid Study (UFS) | U.S. | 1996–1999 | — | 35–49 | U/S | 816 | 373 |
| Italian Menopause Study | Italy | 1997–2003 | — | ? | Pelvic exam or U/S | 2,239 | 85,967 | |
| NIEHS Sister Study | U.S. | 2004–2007 | — | 35–59 | Self-reporte | 1,526 | 18,446 | |
| National Health and Nutrition Examination Survey | U.S. | 1971–1975 | 2004f | 25–49 | Hospital discharge | unknown | Unknown |
MRI = magnetic resonance imaging, U/S = ultrasound, path = pathology, lap = laparoscopy.
Estimated from information provided in published manuscript(s).
Self-reported diagnosis by ultrasound or surgery. In a random sample of cases, self-reports were confirmed by medical record (NHS II: 93%; BWHS 96%).
Serial MRIs were measured on same tumors at regular intervals in time, with cases serving as their own controls.
The numbers of cases and controls interviewed through 1993. Earlier reports were based on smaller numbers of cases and controls.
The self-report of uterine leiomyomata diagnosis was not validated.
Study is ongoing. End of observation reflects year of last follow-up from most recent publication.
1. Markers of Endogenous Hormone Levels
Menarche, Menstrual Patterns, and Menopause
Women with early onset of menses or late onset of menopause will, on the average, have increased lifetime exposure to ovulatory cycles. Because mitotic activity in the myometrium is greatest during the luteal phase of the menstrual cycle [74], a longer history of cycling would be expected to increase UL risk. In support of this, most studies have shown that UL risk increases with earlier age at menarche [22, 29, 75–81]. No studies have investigated the relation between late age at menopause and risk for UL. However, the NIEHS Fibroid Growth Study (FGS) compared UL growth rates in women approaching menopause and found that growth rates declined with premenopausal age among white women, but not black women [82]. Among women 45 years and older, the UL growth rate per 6 months was 2% for white women and 15% for black women. After menopause, women have a lower risk of developing UL [29, 30, 76, 83–85]; pathologic studies of hysterectomy specimens found a reduction both in the size and number of UL in postmenopausal compared with premenopausal women [86]. However, the same proportion of premenopausal and postmenopausal women had physical evidence of UL [86]. The relationship between UL and menstrual cycle patterns is less clear. In the NHS II, irregular menstrual cycles and longer menstrual cycle length were associated with decreased UL risk [80], but no such associations were found in previous studies [79, 87, 88].
Parity
Having a child has been associated with a decreased risk of developing UL in many studies [75, 77, 78, 80, 85, 88, 89]. The reduction in risk ranges from 20–50% when comparing parous with nulliparous women, and risk appears to decrease with a higher number of children in most [30, 75, 77, 80, 85, 89] but not all [78] studies. Spontaneous abortions or incomplete pregnancies appear to be unrelated to risk [28, 78, 88, 89]. Women with infertility are more likely to have UL [29, 77, 89], particularly women with infertility at younger ages (<25 years) [78, 79]. Even when controlling for infertility, multiparity is associated with a reduced risk of UL [89, 90, 92].
Older age at first term birth has been associated with a lower risk of UL in four [29, 76, 78, 80] of eight studies [28, 30, 75, 89]. Studies more consistently show that the longer the time since the last birth, the higher the risk of UL [29, 30, 75, 77, 78, 80, 88], although the association is not linear in all studies [78]. In the UFS, age at birth in the mid-reproductive years (25–29 years) was most protective against UL [90].
Direct protective effects for parity were observed in experimental data from the Eker rat [37]. Although little is known about the mechanism, several theories exist such as altered endocrine profiles following a first or second pregnancy [91–93], especially if initiated late in reproductive life. Similarly, pregnancy may lead to a reduction in estrogen receptor levels in myometrial tissue [74]. Alternatively, childbearing may counteract UL development through nonhormonal mechanisms. For example, reductions in collagen content and smooth muscle cell cytoplasm during the postpartum period could eliminate or reduce the size of UL [24], and ischemia during parturition and uterine remodeling could preferentially eliminate UL due to differences in vascularity compared with myometrium [94, 95]. The inverse associations observed with later age at first birth and shorter time since last birth are consistent with a nonhormonal hypothesis of pregnancy-related elimination of UL [90]. A study that systematically screened for UL in early pregnancy and postpartum was designed to test this hypothesis [18]. Among the 171 women with a single leiomyoma in early-pregnancy, 36% of tumors were eliminated by the time of the ultrasound screen 3–6 months postpartum; tumors that were not eliminated shrank on average. The degree of elimination and shrinkage was much greater than would be expected based on data from nonpregnant women, and both small and large tumors were eliminated [18]. Breastfeeding, which suppresses ovulation and ovarian hormone production, did not mediate the pregnancy-associated elimination of UL [96]. These results are consistent with epidemiologic studies that show little, if any, protective effect of breastfeeding on UL after adjustment for parity [75, 78, 80, 84].
Anthropometric Characteristics
Body mass index (BMI, kg/m2) has been associated with a modest increased risk of UL in several [29, 30, 75, 85, 89, 97–100] but not all [76, 84, 88] studies. In the UFS, a positive association was observed among black but not white women [101]. While some studies have shown a positive linear relation between BMI and UL risk [30, 97], most positive studies have found a non-linear association, with risk increasing up through the overweight categories, and then decreasing slightly among the heaviest or obese women [75, 83, 98, 100, 101]. BMI in adolescence and young adulthood has not been associated with UL risk [22, 97, 98, 100].
The peripheral conversion of androgens to estrogens that occurs in adipose tissue is unlikely to explain an association between BMI and UL because the vast majority of circulating estrogens in premenopausal women comes from the ovaries [102]. However, higher BMI is correlated with lower circulating levels of sex hormone binding globulin, potentially increasing the bioavailability of circulating estrogens and androgens in overweight and obese women [102]. Obesity-related anovulation [103] could counteract this effect by decreasing progesterone levels, thereby explaining the non-linear pattern in risk. The observed BMI association also could be due to detection bias, as pelvic examinations are less effective among obese women.
U.S. prospective cohort studies have consistently shown an association between gaining weight during adulthood and increased UL risk [29, 97, 98, 100]. The CTS found 16% and 23% increased risks of surgically-treated UL associated with weight gain of 10–20kg and >=20kg respectively compared with women who gained <10kg. For women who maintained weight or lost weight, there was a 13% reduced risk of UL compared with women who gained <10kg [29]. Central adiposity as measured by waist circumference or the waist-to-hip ratio was not associated with UL risk in the BWHS [98] or the UFS (unpublished data), but waist-to-hip ratio was weakly positively associated with risk in the NHS II [100]. No association has been found between height and risk of UL diagnosed by ultrasound or surgery [22, 97, 98, 100].
Physical Activity
The data are more ambiguous on physical activity, but lean towards a protective effect of exercise. In a study of former college athletes and nonathletes surveyed about their history of benign gynecologic diseases, nonathletes were more likely to report a history of benign uterine tumors [104]. No association was observed between lifetime moderate and strenuous physical activity and surgically-treated UL in the CTS [29]. By contrast, the UFS reported an inverse association between regular exercise and UL risk in both black and white women: UL was 40% lower comparing the highest (≥7 hours/week) versus lowest category (<2 hours/week) of physical activity, and a dose-response relation was observed [101]. The effect may be mediated by lower luteal phase estrogens in premenopausal women with greater physical activity [116].
Cigarette Smoking
Early studies suggested an inverse relation between UL and smoking [29, 30, 75, 76, 105], with risk reduction ranging from 20–50% lower among current or ever smokers relative to never smokers. However, more recent case-control [79, 88] and prospective cohort studies [23, 97] find no such association. In the UFS, there was a positive relation of smoking with diffuse UL but not with submucosal or intramural/subserosal UL [81]. Tobacco components may inhibit aromatase [106] and shift estradiol metabolism toward less potent forms of estrogen [107, 108]. Conversely, components of cigarette smoke may also exert estrogen-related effects on the uterus that could promote cell proliferation [109].
Alcohol and Caffeine
Three [13, 23, 110] out of the four studies that have investigated the relation of alcohol intake to UL risk report modest positive associations [13, 23, 110, 111]. Alcohol consumption is associated with higher endogenous levels of estradiol and estrone in some studies [112, 113]. Although caffeine was not associated with UL in a case-control study [111], the BWHS found an increased risk of UL among the heaviest consumers of coffee (≥3 cups/day) and caffeine (≥500 mg/day) aged <35 years [23]. Coffee and caffeine consumption are associated with increased levels of early follicular phase estradiol [114] and may enhance sex steroid production [115].
Dietary Factors
A plant-based diet has been hypothesized to decrease risk of UL by reducing the bioavailability of endogenous hormones [116, 117]. An Italian case-control study of surgically-confirmed cases examined risk of UL in relation to diet, finding inverse associations with greater intake of fruits and vegetables and positive associations with greater intake of red meat and ham [111]. Subsequently, several studies on diet and UL have been published in which validated food frequency questionnaires or nutrient biomarkers were used. The BWHS found that higher intakes of fruits and vegetables was associated with a reduced risk of UL [118]. In a subset of participants from The Uterine Leiomyomata Epidemiology Project (TULEP), urinary isoflavones and lignans were measured as biomarkers of soy intake [119]. Because soy exhibits antiestrogenic activity among those with high levels of endogenous estrogens, the authors hypothesized that soy intake would reduce UL risk. The study showed no association with isoflavones, consistent with two subsequent studies of soy intake (measured by food frequency questionnaire) and UL risk [110, 120], even in populations where soy intake was high [110]. Lignans, found in fruits and vegetables, were associated with a lower risk of UL in TULEP, consistent with results from the Italian case-control study [111] and the BWHS [118].
Although experimental animal data show a protective effect of dietary supplementation with lycopene—a carotenoid found in tomato products and other fruits and vegetables—on incidence of UL, epidemiologic data from the NHS II and BWHS show no associations between intake of lycopene, or any other carotenoids, and risk of UL [36, 118, 121, 122].
The BWHS provided the first epidemiologic evidence of reduced UL risk associated with dairy consumption [120]. UL risk was 30% lower among women consuming ≥4 versus <1 serving/day of total dairy (95% CI: 0.58–0.86; P-trend <0.001). Results were similar for high- and low-fat dairy. Dietary calcium, phosphorus, and calcium-to-phosphorus ratio (a marker of bioavailable calcium) were also inversely associated with risk [120].
In the BWHS, dietary intakes of total fat and fat subtypes were not associated with UL risk overall, although statistically significant associations were observed for specific saturated (inverse) and monounsaturated and polyunsaturated (positive) fatty acids. With respect to polyunsaturated fats, the incidence rate ratio (IRR) for the highest versus lowest quintiles of marine fatty acid intake [the sum of omega-3 (n-3) polyunsaturated fatty acids eicosapentanoic acid, docosapentaenoic acid, and docosahexaenoic acid] was 1.18 (95% CI: 1.05, 1.34; P-trend=0.005). The IRR for the highest versus lowest categories of dark-meat fish consumption was 1.13 (95% CI: 1.00, 1.28) [123].
Luteinizing Hormone (LH)
The UFS found an increased risk of UL with increasing LH level [124], with a stronger association for larger tumors. LH shares a receptor with human chorionic gonadotropin, a hormone that stimulates uterine growth during early pregnancy.
IGF-1, Diabetes, and Polycystic Ovary Syndrome (PCOS)
In vitro studies have found a link between IGF-1 and leiomyoma cell proliferation and gene expression. IGF-1 stimulates UL cell proliferation in culture [125, 126]. Studies of human UL cells have found increased IGF-1 gene expression [127–129] and protein levels [130, 131] relative to normal myometrial cells. However, the UFS showed no association between plasma levels of IGF-1 and UL risk [66]. BWHS investigators hypothesized that high dietary glycemic index (GI) and glycemic load (GL) would increase UL risk by increasing endogenous concentrations of IGF-1 or estrogen bioavailability [132]. However, dietary GI was not appreciably associated with risk of UL overall, and only small positive associations were observed for GL among women aged <35 years (IRR for highest versus lowest quintile: 1.18; 95% CI 1.02–1.37).
Hyperinsulinemia and diabetes have been hypothesized to protect against UL risk via localized vascular dysfunction, given that UL have less vascularization than normal myometrium [133], and systemic vascular dysfunction can inhibit tumor development. In support of this, the UFS found an association of both high insulin and diabetes with a lower UL risk among black women [66]. Diabetes was also inversely associated with UL risk in the BWHS [134] and CTS [29]. Interestingly, PCOS, despite its association with hyperinsulinemia, was associated with a 65% increased risk of UL in the BWHS [134]. Other mechanisms by which PCOS could influence UL include increased levels of LH or unopposed estrogens [124].
Stress
The role of stress in the etiology of UL has only recently been studied [135]. In addition to influencing health-related behaviors that promote UL (e.g., physical inactivity, heavy alcohol consumption), stress can down-regulate the hypothalamic/pituitary synthesis of ovarian hormones, but in some circumstances up-regulate adrenal progesterone [135–137]. Granulosa cell tissue culture studies also show increased rather than decreased ovarian steroid secretion following exposure to stress hormones [138], and various growth factors, cytokines, and matrix metalloproteinases can be up-regulated by stress hormones [136, 137].
In the BWHS, two measures of perceived racism were positively associated with UL, and weaker associations were found among women with higher coping skills [139]. In a cross-sectional study, a higher risk of UL was found with greater number of major life events and “stress intensity” [140]. The NHS II found a higher incidence of UL among women who experienced early-life abuse [141]; among women reporting an emotionally supportive relationship in childhood, the risk was lower, suggesting that social and emotional support may buffer the impact of stress on risk.
2. Use of Exogenous Hormones
Oral Contraceptives
Although the association between oral contraceptive (OC) use and UL has been studied extensively, no clear patterns have emerged. Studies have shown reduced [30, 75], similar [29, 76, 78, 80, 84, 85, 88, 142, 143], and increased [144] risks of UL among ever users of OCs relative to never users. Results regarding status of use (i.e., current versus former use) and duration of use are similarly inconsistent [29, 30, 76–78, 142–145]. Risk is not associated with time since most recent OC use [30, 78, 142], but two studies observed an increased risk (20–29%) among women who initiated OCs prior to 17 years of age compared with never users [77, 78]. Because early initiation of OCs may serve as a marker for early sexual activity and exposure to sexually transmitted infections [146], these data may support an infectious etiology. OCs may also be prescribed to women with other pelvic disease (e.g., endometriosis or dysmenorrhea), thereby increasing the opportunity for incidental detection of UL. A case-control study found that use of OCs containing progestins with estrogenic properties was more common in cases than in controls [30]. However, the BWHS found no association of UL with estrogenic and progestational potency, type of progestin, or estrogen formulation (monophasic vs. biphasic/triphasic) [78].
Depot Medroxyprogesterone Acetate (DMPA)
A study in Thailand showed a strong inverse association (OR=0.4, 95% CI 0.3–0.5) between a history of DMPA use and risk of surgically-confirmed UL [75]. Risk declined with increasing duration of use, such that use for more than 5 years was associated with a 90% lower risk of UL; the inverse association weakened with increasing time since last use. The BWHS replicated this association, reporting a 40% reduced risk (95% CI 0.4–0.9) comparing current users of progestin-only injectables with non-users of hormonal contraception [78]. A cross-sectional analysis from the Study of Environment Lifestyle and Fibroids (SELF), an ultrasound screening study of young African American women, also found an inverse association between DMPA and UL (adjusted RR: 0.8, 95% CI: 0.6, 0.9) [40]. The effect of DMPA is likely mediated through the lower estradiol concentrations which resemble those of postmenopausal women; in addition, estradiol suppression increases with longer duration of DMPA use [147].
Postmenopausal Hormone Use
Use of exogenous hormones after menopause is associated with a higher risk of UL diagnosis in most studies. In both prospective and case-control studies, the risk of surgically-confirmed UL was increased up to 6-fold in women using estrogen or combined estrogen-progestin therapy compared with non-users [155, 88, 46, 44].
Diethylstilbestrol (DES)
Prenatal exposure to DES has been shown to cause long-term changes in estrogen-related gene expression [160] and endogenous hormones of premenopausal women [148]; thus, an association with increased risk of UL is plausible. Although an association has been found in laboratory rodents [149, 150], the epidemiologic data are conflicting, possibly because prenatal exposure to DES is difficult to assess and such studies are prone to recall bias. One prospective cohort study, which used medical records to document exposure found no association between prenatal DES exposure and UL [48]. A second study found a 21% increased risk of UL among women who self-reported exposure to DES in the first trimester [151]. Two cross-sectional studies found a positive association between self-reported prenatal DES exposure and UL risk [22, 152]; one found that only “probable,” but not “definite,” prenatal DES exposure was associated with UL risk, suggesting that recall bias could explain these results. To minimize the influence of reporting bias, future studies should seek medical documentation of DES exposure.
3. Growth Factors and Wound Healing
Infection and Uterine Injury
The role of infection or inflammation as an etiologic factor was first proposed in the 1930s [153, 154]. Studies show that inflammation increases the production of extracellular matrix and decreases apoptosis in UL [68, 155], but epidemiologic studies have been inconclusive. Although 2 studies showed non-significant increases with Chlamydia [156], a recent cross-sectional ultrasound-based study found an inverse association with Chlamydia and no association with other reproductive tract infections [41, 157]. Prospective studies are needed to clarify the temporal relationship between exposure and disease.
Having an abnormal Pap test has been associated with a decreased risk of UL in cross-sectional studies from 2 different populations and a case-control study [41, 95]. Among young African-American women, the risk of UL was 39% lower if a cervical procedure was required [41]. HPV is presumably a factor in that association, and a protective pathway has been proposed.
It has been postulated that uterine injury results in UL due to disordered wound healing, similar to keloids [68]. Keloids and hypertrophic scars, which result from skin injury have similar extracellular matrix and collagen features and are more frequent in African-Americans [158, 159]. In a cross-sectional analysis of African American women in the SELF study, self-reported keloids and hypertrophic scars were not associated with ultrasound-screened UL [160]. In an insurance database in Taiwan, keloids were rare but were associated with 2-fold increase in UL compared with women who did not have a diagnostic code for UL [161].
4. Genetics
Several studies have found evidence of genetic predisposition to UL including familial aggregation studies [162, 163], twin studies [164, 165], and genetic linkage studies in families with syndromes that are associated with UL [166–168]. Twin studies show a strong element of heritability in women undergoing hysterectomy [164, 165], with the concordance rate for hysterectomy for all indications in monozygotic twins being twice that of dizygotic twins [164]. Having first-degree affected relatives increases UL risk [162, 169].
Genetic linkage analyses have identified genes that cause rare familial forms of UL. The most clinically important syndrome is hereditary leiomyomatosis and renal cell carcinoma (HLRCC) [170], an autosomal dominant disease caused by genetic mutations in the fumarate hydratase (FH) gene [171–173]. Several mutations in the FH gene exist, all leading to an absent, truncated, or nonfunctional protein [172, 174], which causes loss of tumor suppression. Signs of HLRCC include UL and a family history of cutaneous leiomyomata or papillary renal cell carcinoma [171], and an increased risk of uterine sarcomas at an early age. The association of FH mutations with nonsyndromic UL has been shown consistently in white women [172, 174–182], but not black women [179]. While large linkage studies offer the promise of finding common genetic variants of strong effect (>3–4-fold increase risk per copy) [183–185], they have low power to find genetic variants of weaker effect (<2.5-fold increased risk per copy).
Association studies are beginning to identify common DNA polymorphisms (i.e., small sequence variations with an allele frequency of >1% in a given population) that influence risk of UL. Most studies of genetic polymorphisms in relation to UL have focused on genes involved in steroidogenesis because studies suggest endogenous sex hormones are important in the development and progression of disease. However, candidate gene studies have produced inconsistent findings, which may be attributable to small sample size and inadequately-characterized study populations [186]. The first ever genome-wide association study (GWAS) of UL was conducted in a Japanese population [187], finding three significant loci on chromosomes 10q24.33, 22q13.1 and 11p15.5. A subsequent GWAS in women of European descent found that SNPs in the FASN gene were associated with UL, but did not replicate the Japanese GWAS findings [188], nor did the BWHS among African Americans [189]. However, the BioVU and Right From the Start cohorts replicated two SNP associations from the Japanese GWAS among European Americans (blocked early in transport 1 homolog (BET1L) rs2280543, and trinucleotide repeat containing 6B (TNRC6B) rs12484776) [190, 191]. In the BWHS, admixture mapping analyses showed suggestive evidence of association at chromosomes 2, 4, and 10 (2q37, 4p16.1, and 10q26) [189], with the region in chromosome 2 being replicated in the UFS [192].
5. Other Potential Risk Factors
Demographic Characteristics
Women diagnosed with UL are more likely to be married, have more years of education, and have an occupation categorized as “professional” [12, 13, 75, 83]. These associations could be explained by greater access to medical care and opportunity for UL detection.
High Blood Pressure
A positive association was found between high blood pressure and UL in several epidemiologic reports based on cross-sectional [193, 194], case-control [99, 156], and prospective cohort [29, 195–198] study designs. In a population-based case-control study, hypertension was not higher preoperatively among women undergoing hysterectomy for UL compared with aged-matched referents [199]. However, all but two of these studies [156, 196] were limited to surgical cases of UL. In the NHS II [196], the association between self-reported blood pressure and risk of UL appeared to be strongest for surgical cases (N=1,661). In the BWHS, physician-diagnosed hypertension was associated with UL confirmed by hysterectomy but not by ultrasound or other surgery [197]. Whether the association is due to detection bias or shared etiology is unclear.
Environmental Contaminants
Environmental exposures may affect UL risk via multiple mechanisms, including endocrine disruption. In a cross-sectional study, no association was found between blood levels of lead, mercury, and cadmium, and self-reported UL history [200]. High serum levels of dioxin measured after a chemical explosion in Seveso, Italy were associated with reduced UL risk [26]. The inverse association may be explained by dioxin’s antiestrogen effects and its ability to limit extracellular matrix production via TGF-β pathways [201]. Phthalates, ubiquitous chemicals found in consumer products, are reproductive toxicants in animals [202]. Of the four epidemiologic studies that have assessed phthalates in relation to UL [203–206], two showed an increased risk with higher exposure to some phthalates [204, 205]. In another study, self-reported UL were positively associated with serum polychlorinated biphenyl (PCB) levels and groupings of estrogenic, antiestrogenic, and dioxin-like PCBs [207]. Two subsequent studies also found a higher UL prevalence among those with greater exposure to PCBs [207–209]. Urinary levels of bisphenol A, which also has endocrine-disrupting properties, have been positively associated with UL prevalence in three studies [210–212] but not a fourth study [206]. Existing studies are limited by small sample size [203, 204, 209–212], uncertain temporality due to the use of cross-sectional [205] or case-control designs [203, 204, 206, 208–210], and suboptimal measurement of environmental chemicals [203, 211] and UL [204, 205, 207–212].
6. Predictors of Therapeutic Intervention
Few data have been reported on the frequency and predictors of surgical or pharmacologic interventions among women diagnosed with UL. In a study confined to women whose uteri were ≥8 weeks gestation, 25% of cases had surgery within one year of diagnosis [213]. In a study of cases with a broader range of uterine sizes, approximately 26% had surgery within 5 years; risk of progressing to hysterectomy was inversely related to recommended therapeutic use of OCs or progestins [214]. The 3-year cumulative incidence for hysterectomy was 83% among women aged >35 years who had uteri >8 weeks size, and who had at least one of the other characteristics related to increased risk of hysterectomy [214]. In the FGS, 44% of black women and 40% of white women chose treatment during the study. Symptoms of heavy bleeding and pain were related to choosing intervention, while size and number of UL were not related [215].
Few studies have investigated the predictors of choosing a uterine-sparing procedure over a hysterectomy, although geography has been shown to play a role [216]. In a national survey of women with UL, African American women were nearly 3-fold more likely to value treatment options that preserved fertility than white women [217]. Overall in this cohort, 79% valued less invasive therapy, which was stronger among the younger women [218].
VI. Summary and Directions for Future Research
UL are a common cause of reproductive health problems and medical care utilization. Clarifying the etiology of UL initiation and growth is essential to learning about pathways for primary and secondary prevention. While estrogen and progesterone are almost certainly involved in UL pathogenesis, the in vivo mechanisms through which these hormones act are not well understood. Few modifiable risk factors have been identified. Further research incorporating the steroid hormone and extracellular matrix production pathways is needed.
Epidemiologic studies that evaluate molecular pathways by incorporating biologic measures of exposure and concrete disease assessment will be essential to making stronger inferences regarding specific risk factors and mechanisms. Although greater knowledge about tumor onset may provide strategies for primary prevention, identifying risk factors for tumor growth and symptoms may decrease the adverse effects and public health burden of these tumors. It is valuable to stratify cases according to ultrasonographic or molecular characteristics to identify UL subtypes that are associated with particular exposures.
Alternative etiologic hypotheses based on epidemiologic associations need to be explored. For example, similarities between smooth muscle cell proliferation in UL and atheromas, and reported associations between hypertension and surgically-diagnosed UL, suggest the potential for shared etiologic mechanisms with coronary heart disease. The occurrence of leiomyomata outside of the uterus in patients immunocompromised due to HIV infection suggests possible roles for infectious agents or immunological factors. These hypotheses should be assessed with direct testing for infection or inflammation and screening for UL onset. Additional epidemiologic studies of nutritional factors prior to known tumor onset would be informative, particularly factors that can influence the endogenous hormonal millieu. In theory, promising advances also might result from studies that seek to explain behavioral, social, and biologic factors that underlie differences in UL development and growth among women of different ethnic origins. For example, differences in vitamin D status should be explored given intriguing preliminary evidence of an association [219–223].
New cohorts or those of young women currently followed for other reasons should be pursued. For example, the prospective study of UL among young African-American women from the Detroit area (SELF) was initiated in 2012 and will provide invaluable information (PI: Baird), specifically the extent to which vitamin D deficiency influences UL risk.
Finally, studies focused on medical utilization and clinical outcomes are needed to identify characteristics of patients and their providers that influence use of different treatments for UL at different stages of development. A national UL registry will assess factors that affect treatment choices as well as outcomes of treatment options (clinicaltrials.gov NCT02260752). Studies should attempt to determine the extent to which patient characteristics, ultrasonographic features, and physiologic measures are related to UL progression from clinical diagnosis to surgical therapy, particularly hysterectomy. For example, one study suggested that increased tumor vascularity, as measured by Doppler ultrasonography, is strongly associated with increased tumor growth [224]. Similar studies, augmented by analyses of molecular features of excised UL, may help define predictors of recurrence risk following myomectomy. Results from these studies could provide patients and health care providers with information that will improve the management of UL.
Acknowledgments
We are grateful to Drs. Donna D. Baird, Elizabeth A. Stewart, Stephen M. Schwartz, and Lynn M. Marshall for their helpful feedback on this work.
REFERENCES
- 1.Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit. 2008;14(1):CR24–CR31. [PubMed] [Google Scholar]
- 2.Cardozo ER, et al. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211 e1–211 e9. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker ER. National trends and determinants of hospitalization costs and lengths-of-stay for uterine fibroids procedures. Journal of Health Care Finance. 2007;33(3):1–16. [PubMed] [Google Scholar]
- 4.Whiteman MK, et al. Inpatient hospitalization for gynecologic disorders in the United States. Am J Obstet Gynecol. 2010;202(6):541 e1–541 e6. doi: 10.1016/j.ajog.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Becker ER, et al. Inpatient surgical treatment patterns for patients with uterine fibroids in the United States, 1998–2002. J Natl Med Assoc. 2005;97(10):1336–1342. [PMC free article] [PubMed] [Google Scholar]
- 6.Viswanathan M, et al. Management of uterine fibroids: an update of the evidence. Evid Rep Technol Assess (Full Rep) 2007;154:1–122. [PMC free article] [PubMed] [Google Scholar]
- 7.Wegienka G, et al. Self-reported heavy bleeding associated with uterine leiomyomata. Obstet Gynecol. 2003;101(3):431–437. doi: 10.1016/s0029-7844(02)03121-6. [DOI] [PubMed] [Google Scholar]
- 8.Kjerulff KH, et al. Uterine leiomyomas: racial differences in severity, symptoms, and age at diagnosis. J Reprod Med. 1996;41(7):483–490. [PubMed] [Google Scholar]
- 9.Lippman SA, et al. Uterine fibroids and gynecologic pain symptoms in a population-based study. Fertil Steril. 2003;80(6):1488–1494. doi: 10.1016/s0015-0282(03)02207-6. [DOI] [PubMed] [Google Scholar]
- 10.Puri K, et al. Submucosal fibroids and the relation to heavy menstrual bleeding and anemia. Am J Obstet Gynecol. 2014;210(1):38 e1–38 e7. doi: 10.1016/j.ajog.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JH, et al. Impact of submucous myoma on the severity of anemia. Fertil Steril. 2011;95(5):1769–1772. e1. doi: 10.1016/j.fertnstert.2011.01.142. [DOI] [PubMed] [Google Scholar]
- 12.Wise LA, et al. Age-specific incidence rates for self-reported uterine leiomyomata in the Black Women's Health Study. Obstet Gynecol. 2005;105(3):563–568. doi: 10.1097/01.AOG.0000154161.03418.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall LM, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90(6):967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 14.Baird DD, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 15.Borgfeldt C, Andolf E. Transvaginal ultrasonographic findings in the uterus and endometrium: low prevalence of leiomyoma in a random sample of women age 25–40 years. Acta Obstet Gynecol Scand. 2000;79:202–207. [PubMed] [Google Scholar]
- 16.Baird DD, et al. Short-term change in growth of uterine leiomyoma: tumor growth spurts. Fertil Steril. 2011;95(1):242–246. doi: 10.1016/j.fertnstert.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laughlin SK, et al. Prevalence of Uterine Leiomyomas in the First Trimester of Pregnancy: An Ultrasound-Screening Study. Obstetrics & Gynecology. 2009;113(3):630–635. doi: 10.1097/AOG.0b013e318197bbaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laughlin SK, et al. Pregnancy-related fibroid reduction. Fertil Steril. 2010;94(6):2421–2423. doi: 10.1016/j.fertnstert.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loutradis D, et al. The validity of gynecological ultrasonography. Gynecol Obstet Invest. 1990;29(1):47–50. doi: 10.1159/000293299. [DOI] [PubMed] [Google Scholar]
- 20.Fedele L, et al. Transvaginal ultrasonography versus hysteroscopy in the diagnosis of uterine submucous myomas. Obstet Gynecol. 1991;77(5):745–748. [PubMed] [Google Scholar]
- 21.Dueholm M, et al. Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol. 2002;186(3):409–415. doi: 10.1067/mob.2002.121725. [DOI] [PubMed] [Google Scholar]
- 22.D'Aloisio AA, et al. Association of intrauterine and early-life exposures with diagnosis of uterine leiomyomata by 35 years of age in the sister study. Environ Health Perspect. 2010;118(3):375–381. doi: 10.1289/ehp.0901423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise LA, et al. Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women's Health Study. Hum Reprod. 2004;19(8):1746–1754. doi: 10.1093/humrep/deh309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz SM, Marshall LM, Baird DD. Epidemiologic contributions to understanding the etiology of uterine leiomyomata. Environ Health Perspect. 2000;108(Suppl 5):821–827. doi: 10.1289/ehp.00108s5821. [DOI] [PubMed] [Google Scholar]
- 25.Bower JK, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99(2):300–307. doi: 10.2105/AJPH.2008.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eskenazi B, et al. Serum dioxin concentrations and risk of uterine leiomyoma in the Seveso Women's Health Study. Am J Epidemiol. 2007;166(1):79–87. doi: 10.1093/aje/kwm048. [DOI] [PubMed] [Google Scholar]
- 27.Velebil P, et al. Rate of hospitalization for gynecologic disorders among reproductive-age women in the United States. Obstet Gynecol. 1995;86(5):764–769. doi: 10.1016/0029-7844(95)00252-M. [DOI] [PubMed] [Google Scholar]
- 28.Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6(3):309–316. doi: 10.1089/jwh.1997.6.309. [DOI] [PubMed] [Google Scholar]
- 29.Templeman C, et al. Risk factors for surgically removed fibroids in a large cohort of teachers. Fertil Steril. 2009;92(4):1436–1446. doi: 10.1016/j.fertnstert.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross RK, et al. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J Clin Res Ed. 1986;293(6543):359–362. doi: 10.1136/bmj.293.6543.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed SD, et al. Postmenopausal estrogen and progestogen therapy and the risk of uterine leiomyomas. Menopause. 2004;11(2):214–222. doi: 10.1097/01.gme.0000082297.18134.51. [DOI] [PubMed] [Google Scholar]
- 32.Kjerulff KH, Langenberg P, Guzinski GM. The socioeconomic correlates of hysterectomies in the United States. Am J Public Health. 1993;83(1):106–108. doi: 10.2105/ajph.83.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111(8):1037–1054. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asada H, et al. Potential link between estrogen receptor-alpha gene hypomethylation and uterine fibroid formation. Mol Hum Reprod. 2008;14(9):539–545. doi: 10.1093/molehr/gan045. [DOI] [PubMed] [Google Scholar]
- 35.Andersen A, Barbieri RL. Abnormal gene expression in uterine leiomyomas. J Soc Gynecol Invest. 1995;2(5):663–672. doi: 10.1016/1071-5576(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 36.Sahin K, et al. Lycopene supplementation prevents the development of spontaneous smooth muscle tumors of the oviduct in Japanese quail. Nutr Cancer. 2004;50(2):181–189. doi: 10.1207/s15327914nc5002_8. [DOI] [PubMed] [Google Scholar]
- 37.Walker CL, et al. Protective effect of pregnancy for development of uterine leiomyoma. Carcinogenesis. 2001;22(12):2049–2052. doi: 10.1093/carcin/22.12.2049. [DOI] [PubMed] [Google Scholar]
- 38.Munday JS, Stedman NL. Uterine leiomyomas in two Vietnamese pot-bellied pigs (Sus scrofa) Veterinary Pathology. 2002;39(5):580–583. doi: 10.1354/vp.39-5-580. [DOI] [PubMed] [Google Scholar]
- 39.Blin C, et al. Functional and growth properties of a myometrial cell line derived from transgenic mice: effects of estradiol and antiestrogens. Endocrinology. 1996;137(6):2246–2253. doi: 10.1210/endo.137.6.8641172. [DOI] [PubMed] [Google Scholar]
- 40.Harmon QE, Baird DD. Use of depot medroxyprogesterone acetate and prevalent leiomyoma in young African American women. Hum Reprod. 2015;30(6):1499–1504. doi: 10.1093/humrep/dev069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore KR, et al. Cervical neoplasia-related factors and decreased prevalence of uterine fibroids among a cohort of African American women. Fertil Steril. 2014;101(1):208–214. doi: 10.1016/j.fertnstert.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersen J. Growth factors and cytokines in uterine leiomyomas. Seminar Reprod Endocrinol. 1996;14(3):269–282. doi: 10.1055/s-2007-1016336. [DOI] [PubMed] [Google Scholar]
- 43.Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):571–588. doi: 10.1016/j.bpobgyn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Bulun SE, et al. Aromatase in endometriosis and uterine leiomyomata. J Steroid Biochem Mol Biol. 2005;95(1–5):57–62. doi: 10.1016/j.jsbmb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Sadan O, et al. Oestrogen and progesterone receptor concentrations in leiomyoma and normal myometrium. Ann Clin Biochem. 1987;24(Pt 3):263–267. doi: 10.1177/000456328702400304. [DOI] [PubMed] [Google Scholar]
- 46.Parsanezhad ME, et al. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril. 93(1):192–198. doi: 10.1016/j.fertnstert.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 47.Wu T, Chen X, Xie L. Selective estrogen receptor modulators (SERMs) for uterine leiomyomas. Cochrane Database Syst Rev. 2007;(4):CD005287. doi: 10.1002/14651858.CD005287.pub3. [DOI] [PubMed] [Google Scholar]
- 48.Wise LA, et al. Risk of benign gynecologic tumors in relation to prenatal diethylstilbestrol exposure. Obstet Gynecol. 2005;105(1):167–173. doi: 10.1097/01.AOG.0000147839.74848.7c. [DOI] [PubMed] [Google Scholar]
- 49.Di X, et al. A low concentration of genistein induces estrogen receptor-alpha and insulin-like growth factor-I receptor interactions and proliferation in uterine leiomyoma cells. Hum Reprod. 2008;23(8):1873–1883. doi: 10.1093/humrep/den087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman AJ, et al. Long-term medical therapy for leiomyomata uteri: a prospective, randomized study of leuprolide acetate depot plus either oestrogen-progestin or progestin 'add-back' for 2 years. Hum Reprod. 1994;9(9):1618–1625. doi: 10.1093/oxfordjournals.humrep.a138762. [DOI] [PubMed] [Google Scholar]
- 51.Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(Suppl 5):791–793. doi: 10.1289/ehp.00108s5791. [DOI] [PubMed] [Google Scholar]
- 52.Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells, and genetic contribution. Curr Opin Obstet Gynecol. 2015;27(4):276–283. doi: 10.1097/GCO.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moravek MB, et al. Ovarian steroids, stem cells and uterine leiomyoma: therapeutic implications. Hum Reprod Update. 2015;21(1):1–12. doi: 10.1093/humupd/dmu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiang W, et al. Down-regulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology. 2014;155(3):663–669. doi: 10.1210/en.2013-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishikawa H, et al. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433–2442. doi: 10.1210/en.2009-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawaguchi K, et al. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989;160(3):637–641. doi: 10.1016/s0002-9378(89)80046-8. [DOI] [PubMed] [Google Scholar]
- 57.Lamminen S, et al. Proliferative activity of human uterine leiomyoma cells as measured by automatic image analysis. Gynecol Obstet Invest. 1992;34(2):111–114. doi: 10.1159/000292738. [DOI] [PubMed] [Google Scholar]
- 58.Matsuo H, Maruo T, Samoto T. Increased expression of bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab. 1997;82(1):293–299. doi: 10.1210/jcem.82.1.3650. [DOI] [PubMed] [Google Scholar]
- 59.Tiltman A. The effect of progestins on the mitotic activity of uterine fibromyomas. Int J Gynecol Path. 1985;4:89–96. doi: 10.1097/00004347-198506000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Murphy AA, et al. Regression of uterine leiomyomata to the antiprogesterone RU486: dose-response effect. Fertil Steril. 1995;64(1):187–190. [PubMed] [Google Scholar]
- 61.Bagaria M, et al. Low-dose mifepristone in treatment of uterine leiomyoma: a randomised double-blind placebo-controlled clinical trial. Aust N Z J Obstet Gynaecol. 2009;49(1):77–83. doi: 10.1111/j.1479-828X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 62.Spitz IM. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol. 2009;21(4):318–324. doi: 10.1097/GCO.0b013e32832e07e8. [DOI] [PubMed] [Google Scholar]
- 63.Donnez J, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366(5):421–432. doi: 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]
- 64.Donnez J, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 65.Donnez J, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103(2):519–527. doi: 10.1016/j.fertnstert.2014.10.038. e3. [DOI] [PubMed] [Google Scholar]
- 66.Baird DD, et al. Uterine leiomyomata in relation to insulin-like growth factor-I, insulin, and diabetes. Epidemiology. 2009;20(4):604–610. doi: 10.1097/EDE.0b013e31819d8d3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010;28(3):180–203. doi: 10.1055/s-0030-1251476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195(2):415–420. doi: 10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arslan AA, et al. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod. 2005;20(4):852–863. doi: 10.1093/humrep/deh698. [DOI] [PubMed] [Google Scholar]
- 70.Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab. 2001;86(2):913–920. doi: 10.1210/jcem.86.2.7237. [DOI] [PubMed] [Google Scholar]
- 71.Malik M, et al. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Semin Reprod Med. 2010;28(3):169–179. doi: 10.1055/s-0030-1251475. [DOI] [PubMed] [Google Scholar]
- 72.Cramer SF, et al. Association of seedling myomas with myometrial hyperplasia. Hum Pathol. 2009;40(2):218–225. doi: 10.1016/j.humpath.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Mashal RD, et al. Analysis of androgen receptor DNA reveals the independent clonal origins of uterine leiomyomata and the secondary nature of cytogenetic aberrations in the development of leiomyomata. Genes Chromosomes Cancer. 1994;11(1):1–6. doi: 10.1002/gcc.2870110102. [DOI] [PubMed] [Google Scholar]
- 74.Kawaguchi K, et al. Immunohistochemical analysis of oestrogen receptors, progesterone receptors and Ki-67 in leiomyoma and myometrium during the menstrual cycle and pregnancy. Virchows Arch A Pathol Anat Histopathol. 1991;419(4):309–315. doi: 10.1007/BF01606522. [DOI] [PubMed] [Google Scholar]
- 75.Lumbiganon P, et al. Protective effect of depot-medroxyprogesterone acetate on surgically treated uterine leiomyomas: a multicentre case-control study. Br J Obstet Gynaecol. 1996;103(9):909–914. doi: 10.1111/j.1471-0528.1996.tb09911.x. [DOI] [PubMed] [Google Scholar]
- 76.Romieu I, Walker AM, Jick S. Determinants of uterine fibroids. Post Mark Surveill. 1991;5:119–133. [Google Scholar]
- 77.Marshall LM, et al. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70(3):432–439. doi: 10.1016/s0015-0282(98)00208-8. [DOI] [PubMed] [Google Scholar]
- 78.Wise LA, et al. Reproductive factors, hormonal contraception and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol. 2004;159(2):113–123. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faerstein E, Szklo M, Rosenshein N. Risk factors for uterine leiomyoma: a practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. American Journal of Epidemiology. 2001;153(1):1–10. doi: 10.1093/aje/153.1.1. [DOI] [PubMed] [Google Scholar]
- 80.Terry KL, et al. Reproductive characteristics and risk of uterine leiomyomata. Fertil Steril. 2010;94(7):2703–2707. doi: 10.1016/j.fertnstert.2010.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dragomir AD, et al. Potential risk factors associated with subtypes of uterine leiomyomata. Reprod Sci. 2010;17(11):1029–1035. doi: 10.1177/1933719110376979. [DOI] [PubMed] [Google Scholar]
- 82.Peddada SD, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A. 2008;105(50):19887–19892. doi: 10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parazzini F, et al. Epidemiologic characteristics of women with uterine fibroids: a case-control study. Obstet Gynecol. 1988;72(6):853–857. doi: 10.1097/00006250-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Samadi AR, et al. Risk factors for self-reported uterine fibroids: a case-control study. Am J Public Health. 1996;86(6):858–862. doi: 10.2105/ajph.86.6.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parazzini F. Risk factors for clinically diagnosed uterine fibroids in women around menopause. Maturitas. 2006;55(2):174–179. doi: 10.1016/j.maturitas.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 86.Cramer SF, Patel A. The frequency of uterine leiomyoma. Am J Clin Pathol. 1990;94:435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 87.Marino JL, et al. Uterine leiomyoma and menstrual cycle characteristics in a population-based cohort study. Hum Reprod. 2004;19(10):2350–2355. doi: 10.1093/humrep/deh407. [DOI] [PubMed] [Google Scholar]
- 88.Chen CR, et al. Risk factors for uterine fibroids among women undergoing tubal sterilization. American Journal of Epidemiology. 2001;153(1):20–26. doi: 10.1093/aje/153.1.20. [DOI] [PubMed] [Google Scholar]
- 89.Parazzini F, et al. Reproductive factors and risk of uterine fibroids. Epidemiology. 1996;7(4):440–442. doi: 10.1097/00001648-199607000-00018. [DOI] [PubMed] [Google Scholar]
- 90.Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14:247–250. doi: 10.1097/01.EDE.0000054360.61254.27. [DOI] [PubMed] [Google Scholar]
- 91.Bernstein L, et al. Estrogen and sex hormone-binding globulin levels in nulliparous and parous women. J Natl Cancer Inst. 1985;74(4):741–745. [PubMed] [Google Scholar]
- 92.Dorgan JF, et al. Relationships of age and reproductive characteristics with plasma estrogen and androgens in premenopausal women. Cancer Epidemiol Biomarkers Prev. 1995;4(4):381–386. [PubMed] [Google Scholar]
- 93.Musey VC, et al. Long term effects of a first pregnancy on the hormonal environment: estrogens and androgens. J Clin Endocrinol Metab. 1987;64(1):111–118. doi: 10.1210/jcem-64-1-111. [DOI] [PubMed] [Google Scholar]
- 94.Burbank F. Childbirth and myoma treatment by uterine artery occlusion: do they share a common biology? Journal of the American Association of Gynecologic Laparoscopists. 2004;11(2):138–152. doi: 10.1016/s1074-3804(05)60189-2. [DOI] [PubMed] [Google Scholar]
- 95.Laughlin SK, Schroeder JC, Baird DD. New directions in the epidemiology of uterine fibroids. Semin Reprod Med. 2010;28(3):204–217. doi: 10.1055/s-0030-1251477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laughlin SK, Hartmann KE, Baird DD. Postpartum factors and natural fibroid regression. Am J Obstet Gynecol. 2011;204(6):496.e1–496.e6. doi: 10.1016/j.ajog.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marshall LM, et al. Risk of uterine leiomyomata among premenopausal women in relation to body size and cigarette smoking. Epidemiology. 1998;9(5):511–517. [PubMed] [Google Scholar]
- 98.Wise LA, et al. Influence of body size and body fat distribution on risk of uterine leiomyomata in U.S. black women. Epidemiology. 2005;16(3):346–354. doi: 10.1097/01.ede.0000158742.11877.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takeda T, et al. Relationship between metabolic syndrome and uterine leiomyomas: a case-control study. Gynecol Obstet Invest. 2008;66(1):14–17. doi: 10.1159/000114250. [DOI] [PubMed] [Google Scholar]
- 100.Terry KL, et al. Anthropometric Characteristics and Risk of Uterine Leiomyoma. Epidemiology. 2007;18(6):758–763. doi: 10.1097/EDE.0b013e3181567eed. [DOI] [PubMed] [Google Scholar]
- 101.Baird DD, et al. Association of physical activity with development of uterine leiomyoma. Am J Epidemiol. 2007;165(2):157–163. doi: 10.1093/aje/kwj363. [DOI] [PubMed] [Google Scholar]
- 102.Azziz R. Reproductive endocrinologic alterations in female asymptomatic obesity. Fertil Steril. 1989;52(5):703–725. doi: 10.1016/s0015-0282(16)61020-8. [DOI] [PubMed] [Google Scholar]
- 103.Pasquali R, Gambineri A. Metabolic effects of obesity on reproduction. Reprod Biomed Online. 2006;12(5):542–551. doi: 10.1016/s1472-6483(10)61179-0. [DOI] [PubMed] [Google Scholar]
- 104.Wyshak G, et al. Lower prevalence of benign diseases of the breast and benign tumours of the reproductive system among former college athletes compared to non-athletes. Br J Cancer. 1986;54(5):841–845. doi: 10.1038/bjc.1986.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parazzini F, et al. Uterine myomas and smoking. Results from an Italian study. J Reprod Med. 1996;41(5):316–320. [PubMed] [Google Scholar]
- 106.Barbieri RL, McShane PM, Ryan KJ. Constituents of cigarette smoke inhibit human granulosa cell aromatase. Fertil Steril. 1986;46:232–236. [PubMed] [Google Scholar]
- 107.Michnovicz JJ, et al. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med. 1986;315:1305–1309. doi: 10.1056/NEJM198611203152101. [DOI] [PubMed] [Google Scholar]
- 108.Bradlow L. In: Variations in estrogen metabolism, in Steroid Contraceptives and Women's Response. Snow R, Hall P, editors. New York: Plenum Press; 1994. pp. 171–178. [Google Scholar]
- 109.Ohtake F, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423(6939):545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 110.Nagata C, et al. Association of intakes of fat, dietary fibre, soya isoflavones and alcohol with uterine fibroids in Japanese women. Br J Nutr. 2009;101(10):1427–1431. doi: 10.1017/s0007114508083566. [DOI] [PubMed] [Google Scholar]
- 111.Chiaffarino F, et al. Diet and uterine myomas. Obstet Gynecol. 1999;94(3):395–398. doi: 10.1016/s0029-7844(99)00305-1. [DOI] [PubMed] [Google Scholar]
- 112.Katsouyami K, Boyle P, Trichopoulos D. Diet and urine estrogens among postmenopausal women. Oncology. 1991;48:490–494. doi: 10.1159/000226987. [DOI] [PubMed] [Google Scholar]
- 113.Reichman ME, et al. Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J Natl Cancer Inst. 1993;85:722–727. doi: 10.1093/jnci/85.9.722. [DOI] [PubMed] [Google Scholar]
- 114.Lucero J, et al. Early follicular phase hormone levels in relation to patterns of alcohol, tobacco, and coffee use. Fertil Steril. 2001;76(4):723–729. doi: 10.1016/s0015-0282(01)02005-2. [DOI] [PubMed] [Google Scholar]
- 115.Leonard TK, Watson RR, Mohs ME. The effects of caffeine on various systems: a review. J Am Diet Assoc. 1987;87:1048–1053. [PubMed] [Google Scholar]
- 116.Michnovicz JJ, Bradlow HL. Dietary and pharmacological control of estradiol metabolism in humans. Ann N Y Acad Sci. 1990;595:291–299. doi: 10.1111/j.1749-6632.1990.tb34303.x. [DOI] [PubMed] [Google Scholar]
- 117.Longcope C. In: Diet and estrogen metabolism, in Steroid Contraceptives and Women's Response. Snow R, Hall P, editors. New York: Plenum Press; 1994. pp. 143–152. [Google Scholar]
- 118.Wise LA, et al. Intake of fruit, vegetables, and carotenoids in relation to risk of uterine leiomyomata. Am J Clin Nutr. 2011;94(6):1620–1631. doi: 10.3945/ajcn.111.016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Atkinson C, et al. Lignan and isoflavone excretion in relation to uterine fibroids: a case-control study of young to middle-aged women in the United States. Am J Clin Nutr. 2006;84(3):587–593. doi: 10.1093/ajcn/84.3.587. [DOI] [PubMed] [Google Scholar]
- 120.Wise LA, et al. A prospective study of dairy intake and risk of uterine leiomyomata. Am J Epidemiol. 2010;171(2):221–232. doi: 10.1093/aje/kwp355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Terry KL, et al. Lycopene and other carotenoid intake in relation to risk of uterine leiomyomata. Am J Obstet Gynecol. 2008;198(1):37 e1–37 e8. doi: 10.1016/j.ajog.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sahin K, et al. Dietary tomato powder supplementation in the prevention of leiomyoma of the oviduct in the Japanese quail. Nutr Cancer. 2007;59(1):70–75. doi: 10.1080/01635580701365076. [DOI] [PubMed] [Google Scholar]
- 123.Wise LA, et al. A prospective study of dietary fat and risk of uterine leiomyomata. Am J Clin Nutr. 2014;99(5):1105–1116. doi: 10.3945/ajcn.113.073635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baird DD, Kesner JS, Dunson DB. Luteinizing hormone in premenopausal women may stimulate uterine leiomyomata development. J Soc Gynecol Investig. 2006;13(2):130–135. doi: 10.1016/j.jsgi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 125.Van der Ven LT, et al. Modulation of insulin-like growth factor (IGF) action by IGF-binding proteins in normal, benign, and malignant smooth muscle tissues. J Clin Endocrinol Metab. 1996;81(10):3629–3635. doi: 10.1210/jcem.81.10.8855813. [DOI] [PubMed] [Google Scholar]
- 126.Strawn EY, et al. Insulin-like growth factor I promotes leiomyoma cell growth in vitro. American Journal of Obstetrics and Gynecology. 1995;172(6):1837–1844. doi: 10.1016/0002-9378(95)91420-x. [DOI] [PubMed] [Google Scholar]
- 127.Boehm KD, et al. Expression of the insulin-like and platelet-derived growth factor genes in human uterine tissues. Mol Reprod Dev. 1990;27(2):93–101. doi: 10.1002/mrd.1080270203. [DOI] [PubMed] [Google Scholar]
- 128.Giudice LC, et al. Insulin-like growth factor (IGF), IGF binding protein (IGFBP), and IGF receptor gene expression and IGFBP synthesis in human uterine leiomyomata. Hum Reprod. 1993;8(11):1796–1806. doi: 10.1093/oxfordjournals.humrep.a137937. [DOI] [PubMed] [Google Scholar]
- 129.Englund K, et al. Gene expression and tissue concentrations of IGF-I in human myometrium and fibroids under different hormonal conditions. Mol Hum Reprod. 2000;6(10):915–920. doi: 10.1093/molehr/6.10.915. [DOI] [PubMed] [Google Scholar]
- 130.Dixon D, He H, Haseman JK. Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environmental Health Perspectives. 2000:795–802. doi: 10.1289/ehp.00108s5795. [DOI] [PubMed] [Google Scholar]
- 131.Wolanska M, Bankowski E. An accumulation of insulin-like growth factor I (IGF-I) in human myometrium and uterine leiomyomas in various stages of tumour growth. Eur Cytokine Netw. 2004;15(4):359–363. [PubMed] [Google Scholar]
- 132.Radin RG, et al. Dietary glycemic index and load in relation to risk of uterine leiomyomata in the Black Women's Health Study. Am J Clin Nutr. 2010;91(5):1281–1288. doi: 10.3945/ajcn.2009.28698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093. doi: 10.1093/humrep/deg213. [DOI] [PubMed] [Google Scholar]
- 134.Wise LA, et al. Polycystic ovary syndrome and risk of uterine leiomyomata. Fertil Steril. 2007;87(5):1108–1115. doi: 10.1016/j.fertnstert.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baird D, Wise LA. Childhood abuse and fibroids. Epidemiology. 2011;22(1):15–17. doi: 10.1097/EDE.0b013e3181fe1fbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ferin M. Clinical review 105: Stress and the reproductive cycle. J Clin Endocrin Metab. 1999;84(6):1768–1774. doi: 10.1210/jcem.84.6.5367. [DOI] [PubMed] [Google Scholar]
- 137.Puder JJ, et al. Stimulatory effects of stress on gonadotropin secretion in estrogen-treated women. J Clin Endocrin Metab. 2000;85(6):2184–2188. doi: 10.1210/jcem.85.6.6629. [DOI] [PubMed] [Google Scholar]
- 138.Kornya L, et al. Modulatory effect of acetylcholine on gonadotropin-stimulated human granulosa cell steroid secretion. Gynecol Obstet Invest. 2001;52(2):104–107. doi: 10.1159/000052952. [DOI] [PubMed] [Google Scholar]
- 139.Wise LA, et al. Perceived racial discrimination and risk of uterine leiomyomata. Epidemiology. 2007;18(6):747–757. doi: 10.1097/EDE.0b013e3181567e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vines AI, Ta M, Esserman DA. The Association Between Self-Reported Major Life Events and the Presence of Uterine Fibroids. Women's Health Issues. 2010;20(4):294–298. doi: 10.1016/j.whi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boynton-Jarrett R, et al. Abuse in Childhood and Risk of Uterine Leiomyoma: The Role of Emotional Support in Biologic Resilience. Epidemiology. 2011;22(1):6–14. doi: 10.1097/EDE.0b013e3181ffb172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Parazzini F, et al. Oral contraceptive use and risk of uterine fibroids. Obstet Gynecol. 1992;79(3):430–433. doi: 10.1097/00006250-199203000-00021. [DOI] [PubMed] [Google Scholar]
- 143.Chiaffarino F, et al. Use of oral contraceptives and uterine fibroids: results from a case-control study. Br J Obstet Gynaecol. 1999;106(8):857–860. doi: 10.1111/j.1471-0528.1999.tb08409.x. [DOI] [PubMed] [Google Scholar]
- 144.Ramcharan S, Pelligrin FA, Ray R, et al. The Walnut Creek Contraceptive Drug Study. A prospective study of the side effects of oral contraceptives. Center Popul Res Monogr. 1981:69–74. [PubMed] [Google Scholar]
- 145.Royal College of General Practitioners. London: Pittman Medical; 1974. Oral Contraceptives and Health: An Interim Report from the Oral Contraception Study of the Royal College of General Practitioners. [Google Scholar]
- 146.Winter L, Goldy AS, Baer C. Prevalence and epidemiologic correlates of Chlamydia trachomatis in rural and urban populations. Sexually Transmitted Diseases. 1990;17(1):30–36. [PubMed] [Google Scholar]
- 147.Clark MK, et al. Magnitude and variability of sequential estradiol and progesterone concentrations in women using depot medroxyprogesterone acetate for contraception. Fertil Steril. 2001;75(5):871–877. doi: 10.1016/s0015-0282(01)01748-4. [DOI] [PubMed] [Google Scholar]
- 148.Wise LA, et al. Prenatal diethylstilbestrol exposure and reproductive hormones in premenopausal women. J Dev Orig Health Dis. 2015;6(3):208–216. doi: 10.1017/S2040174415000082. [DOI] [PubMed] [Google Scholar]
- 149.Newbold R. Cellular and molecular effects of developmental exposure to diethylstilbestrol: implications for other environmental estrogens. Environmental Health Perspectives. 1995;103(Suppl 7):83–87. doi: 10.1289/ehp.95103s783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Greathouse KL, et al. Identification of uterine leiomyoma genes developmentally reprogrammed by neonatal exposure to diethylstilbestrol. Reprod Sci. 2008;15(8):765–778. doi: 10.1177/1933719108322440. [DOI] [PubMed] [Google Scholar]
- 151.Mahalingaiah S, et al. Prenatal Diethylstilbestrol Exposure and Risk of Uterine Leiomyomata in the Nurses' Health Study II. Am J Epidemiol. 2013;179(2):186–191. doi: 10.1093/aje/kwt250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baird DD, Newbold R. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod Toxicol. 2005;20(1):81–84. doi: 10.1016/j.reprotox.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 153.Witherspoon JT. A possible cause of uterine fibroids. Endocrinology (Baltimore) 1933;17:703–708. [Google Scholar]
- 154.Witherspoon JT, Butler VW. The etiology of uterine fibroids. Surg Gynecol Obstet. 1934;58:57–61. [Google Scholar]
- 155.Cramer SF, et al. Myometrial hyperplasia in pediatric, adolescent, and young adult uteri. J Pediatr Adolesc Gynecol. 2003;16(5):301–306. doi: 10.1016/s1083-3188(03)00158-x. [DOI] [PubMed] [Google Scholar]
- 156.Faerstein E, Szklo M, Rosenshein NB. Risk factors for uterine leiomyoma: a practice-based case-control study. II. Atherogenic risk factors and potential sources of uterine irritation. American Journal of Epidemiology. 2001;153(1):11–19. doi: 10.1093/aje/153.1.11. [DOI] [PubMed] [Google Scholar]
- 157.Moore KR, et al. Self-Reported Reproductive Tract Infections and Ultrasound Diagnosed Uterine Fibroids in African-American Women. J Womens Health (Larchmt) 2015 doi: 10.1089/jwh.2014.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carrino DA, et al. Proteoglycans of uterine fibroids and keloid scars: similarity in their proteoglycan composition. Biochem J. 2012;443(2):361–368. doi: 10.1042/BJ20111996. [DOI] [PubMed] [Google Scholar]
- 159.Catherino WH, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40(3):204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Harmon QE, Laughlin SK, Baird DD. Keloids and ultrasound detected fibroids in young African American women. PLoS One. 2013;8(12):e84737. doi: 10.1371/journal.pone.0084737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun LM, Wang KH, Lee YC. Keloid incidence in Asian people and its comorbidity with other fibrosis-related diseases: a nationwide population-based study. Arch Dermatol Res. 2014;306(9):803–808. doi: 10.1007/s00403-014-1491-5. [DOI] [PubMed] [Google Scholar]
- 162.Vikhlyaeva EM, Khodzhaeva ZS, Fantschenko ND. Familial predisposition to uterine leiomyomas. Int J Gynaecol Obstet. 1995;51(2):127–131. doi: 10.1016/0020-7292(95)02533-i. [DOI] [PubMed] [Google Scholar]
- 163.Kurbanova MKH, Koroleva AG, Sergeev AS. Genetic-epidemiologic analysis of uterine myoma: assessment of repeated risk. Genetika. 1989;25(10):1896–1898. [PubMed] [Google Scholar]
- 164.Treloar SA, et al. Pathways to hysterectomy: insights from longitudinal twin research. Am J Obstet Gynecol. 1992;167(1):82–88. doi: 10.1016/s0002-9378(11)91632-9. [DOI] [PubMed] [Google Scholar]
- 165.Luoto R, Kaprio J, Rutanen EM, Taipale P, Perola M, Koskenvuo M. Heritability and risk factors of uterine fibroids--the Finnish Twin Cohort study. Maturitas. 2000;37(1):15–26. doi: 10.1016/s0378-5122(00)00160-2. [DOI] [PubMed] [Google Scholar]
- 166.Reed WB, Walker R, Horowitz R. Cutaneous leiomyomata with uterine leiomyomata. Acta Derm Venereol. 1973;53(5):409–416. [PubMed] [Google Scholar]
- 167.Garcia Muret MP, et al. Familial leiomyomatosis cutis et uteri (Reed's syndrome) Arch Derm Res. 1988;280(Suppl):S29–S32. [PubMed] [Google Scholar]
- 168.Launonen V, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci USA. 2001;98(6):3387–3392. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Van Voorhis BJ, Romitti PA, Jones MP. Family history as a risk factor for development of uterine leiomyomas. Results of a pilot study. J Reprod Med. 2002;47(8):663–669. [PubMed] [Google Scholar]
- 170.Kiuru M, et al. Familial cutaneous leiomyomatosis is a two-hit condition associated with renal cell cancer of characteristic histopathology. Am J Pathol. 2001;159:825–829. doi: 10.1016/S0002-9440(10)61757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stewart EA, Morton CC. The Genetics of Uterine Leiomyomata: What Clinicians Need to Know. Obstet Gynecol. 2006;107(4):917–921. doi: 10.1097/01.AOG.0000206161.84965.0b. [DOI] [PubMed] [Google Scholar]
- 172.Alam NA, et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet. 2003;12(11):1241–1252. doi: 10.1093/hmg/ddg148. [DOI] [PubMed] [Google Scholar]
- 173.Colgan TJ, et al. Pathologic features of uteri and leiomyomas following uterine artery embolization for leiomyomas. Am J Surg Pathol. 2003;27:167–177. doi: 10.1097/00000478-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 174.Tomlinson IP, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30(4):406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 175.Martinez-Mir A, et al. Germline fumarate hydratase mutations in families with multiple cutaneous and uterine leiomyomata. J Invest Dermatol. 2003;121(4):741–744. doi: 10.1046/j.1523-1747.2003.12499.x. [DOI] [PubMed] [Google Scholar]
- 176.Lehtonen R, et al. Biallelic inactivation of fumarate hydratase (FH) occurs in nonsyndromic uterine leiomyomas but is rare in other tumors. Am J Pathol. 2004;164(1):17–22. doi: 10.1016/S0002-9440(10)63091-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kiuru M, et al. Few FH mutations in sporadic counterparts of tumor types observed in hereditary leiomyomatosis and renal cell cancer families. Cancer Res. 2002;62(16):4554–4557. [PubMed] [Google Scholar]
- 178.Barker KT, et al. Low frequency of somatic mutations in the FH/multiple cutaneous leiomyomatosis gene in sporadic leiomyosarcomas and uterine leiomyomas. Br J Cancer. 2002;87(4):446–448. doi: 10.1038/sj.bjc.6600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gross KL, et al. Involvement of fumarate hydratase in nonsyndromic uterine leiomyomas: genetic linkage analysis and FISH studies. Genes Chrom Cancer. 2004;41(3):183–190. doi: 10.1002/gcc.20079. [DOI] [PubMed] [Google Scholar]
- 180.Chuang GS, et al. Germline fumarate hydratase mutations and evidence for a founder mutation underlying multiple cutaneous and uterine leiomyomata. J Am Acad Dermatol. 2005;52(3 Pt 1):410–416. doi: 10.1016/j.jaad.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 181.Shveiky D, et al. Family history of uterine fibroids associated with low level of fumarate hydratase in leiomyomata cells. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):234–235. doi: 10.1016/j.ejogrb.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 182.Stewart L, et al. Association of germline mutations in the fumarate hydratase gene and uterine fibroids in women with hereditary leiomyomatosis and renal cell cancer. Archives of Dermatology. 2008;144(12):1584–1592. doi: 10.1001/archdermatol.2008.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gross K, Morton C, Stewart EA. Finding genes for uterine fibroids [abstract] Obstet Gynecol. 2000;95(4 suppl):S60. [Google Scholar]
- 184.Huyck KL, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. American Journal of Obstetrics and Gynecology. 2008;198(2):168.e1–168.e9. doi: 10.1016/j.ajog.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273(5281):1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- 186.Wise LA, Laughlin-Tommaso SK. Uterine Leiomyomata. In: Goldman MB, Troisi R, Rexrode KM, editors. Women And Health. San Diego, CA: Academic Press; 2013. pp. 285–306. [Google Scholar]
- 187.Cha PC, et al. A genome-wide association study identifies three loci associated with susceptibility to uterine fibroids. Nat Genet. 2011;43(5):447–450. doi: 10.1038/ng.805. [DOI] [PubMed] [Google Scholar]
- 188.Eggert SL, et al. Genome-wide linkage and association analyses implicate FASN in predisposition to Uterine Leiomyomata. Am J Hum Genet. 2012;91(4):621–628. doi: 10.1016/j.ajhg.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wise LA, et al. African ancestry and genetic risk for uterine leiomyomata. Am J Epidemiol. 2012;176(12):1159–1168. doi: 10.1093/aje/kws276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Edwards TL, Hartmann KE, Velez Edwards DR. Variants in BET1L and TNRC6B associate with increasing fibroid volume and fibroid type among European Americans. Hum Genet. 2013 doi: 10.1007/s00439-013-1340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Edwards TL, et al. BET1L and TNRC6B associate with uterine fibroid risk among European Americans. Hum Genet. 2013;132(8):943–953. doi: 10.1007/s00439-013-1306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang K, Wiener H, Aissani B. Admixture mapping of genetic variants for uterine fibroids. J Hum Genet. 2015 doi: 10.1038/jhg.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Luoto R, Rutanen EM, Auvinen A. Fibroids and hypertension - A cross-sectional study of women undergoing hysterectomy. Journal of Reproductive Medicine. 2001;46(4):359–364. [PubMed] [Google Scholar]
- 194.Silver MA, et al. Systemic hypertension among women with uterine leiomyomata: potential final common pathways of target end-organ remodeling. J Clin Hypertens (Greenwich) 2005;7(11):664–668. doi: 10.1111/j.1524-6175.2005.04384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Settnes A, Andreasen AH, Jorgensen T. Hypertension is associated with an increased risk for hysterectomy: a Danish cohort study. Eur J Obstet Gynecol Reprod Biol. 2005;122(2):218–224. doi: 10.1016/j.ejogrb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 196.Boynton-Jarrett R, et al. A prospective study of hypertension and risk of uterine leiomyomata. American Journal of Epidemiology. 2005;161(7):628–638. doi: 10.1093/aje/kwi072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Radin RG, et al. Hypertension and risk of uterine leiomyomata in US black women. Hum Reprod. 2012;27(5):1504–1509. doi: 10.1093/humrep/des046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haan YC, et al. Hypertension risk in Dutch women with symptomatic uterine fibroids. Am J Hypertens. 2015;28(4):487–492. doi: 10.1093/ajh/hpu183. [DOI] [PubMed] [Google Scholar]
- 199.Laughlin-Tommaso SK, et al. Cardiovascular risk factors and diseases in women undergoing hysterectomy with ovarian conservation. Menopause. 2015 doi: 10.1097/GME.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jackson LW, Zullo MD, Goldberg JM. The association between heavy metals, endometriosis and uterine myomas among premenopausal women: National Health and Nutrition Examination Survey 1999–2002. Hum. Reprod. 2008;23(3):679–687. doi: 10.1093/humrep/dem394. [DOI] [PubMed] [Google Scholar]
- 201.Gomez-Duran A, et al. Fitting a xenobiotic receptor into cell homeostasis: how the dioxin receptor interacts with TGFbeta signaling. Biochemical Pharmacology. 2009;77(4):700–712. doi: 10.1016/j.bcp.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 202.Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62(11):806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Luisi S, et al. Low serum concentrations of di-(2-ethylhexyl)phthalate in women with uterine fibromatosis. Gynecol Endocrinol. 2006;22(2):92–95. doi: 10.1080/09513590600551312. [DOI] [PubMed] [Google Scholar]
- 204.Huang PC, et al. Association between phthalate exposure and glutathione S-transferase M1 polymorphism in adenomyosis, leiomyoma and endometriosis. Hum Reprod. 2010;25(4):986–994. doi: 10.1093/humrep/deq015. [DOI] [PubMed] [Google Scholar]
- 205.Weuve J, et al. Association of Exposure to Phthalates with Endometriosis and Uterine Leiomyomata: Findings from NHANES, 1999–2004. Environ Health Perspect. 2010;118(6):825–832. doi: 10.1289/ehp.0901543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pollack AZ, et al. Bisphenol A, benzophenone-type ultraviolet filters, and phthalates in relation to uterine leiomyoma. Environ Res. 2015;137:101–107. doi: 10.1016/j.envres.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lambertino A, et al. Uterine leiomyomata in a cohort of Great Lakes sport fish consumers. Environ Res. 2011;111(4):565–572. doi: 10.1016/j.envres.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Qin Y, et al. Persistent organic pollutants and heavy metals in adipose tissues of patients with uterine leiomyomas and the association of these pollutants with seafood diet, BMI, and age. Environmental Science and Pollution Research. 2010;17(1):229–240. [Google Scholar]
- 209.Trabert B, et al. Persistent organic pollutants (POPs) and fibroids: results from the ENDO study. J Expos Sci Environ Epidemiol. 2014 doi: 10.1038/jes.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shen Y, et al. Measurement of phenolic environmental estrogens in women with uterine leiomyoma. PLoS One. 2013;8(11):e79838. doi: 10.1371/journal.pone.0079838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Han MS, et al. Bisphenol-A Concentrations from Leiomyoma Patients by LC/MS. Toxicol Res. 2011;27(1):49–52. doi: 10.5487/TR.2011.27.1.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhou F, et al. Measurement of phenolic environmental estrogens in human urine samples by HPLC-MS/MS and primary discussion the possible linkage with uterine leiomyoma. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;938:80–85. doi: 10.1016/j.jchromb.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 213.Carlson KJ, Miller BA, Fowler FJ. The Maine Women's Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83(4):566–572. doi: 10.1097/00006250-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 214.Weber AM, et al. Uterine myomas and factors associated with hysterectomy in premenopausal women. Am J Obstet Gynecol. 1997;176(6):1213–1217. doi: 10.1016/s0002-9378(97)70337-5. discussion 1217-9. [DOI] [PubMed] [Google Scholar]
- 215.Davis BJ, et al. The fibroid growth study: determinants of therapeutic intervention. J Womens Health (Larchmt) 2009;18(5):725–732. doi: 10.1089/jwh.2008.0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wu JM, et al. Hysterectomy Rates in the United States, 2003. Obstet Gynecol. 2007;110(5):1091–1095. doi: 10.1097/01.AOG.0000285997.38553.4b. [DOI] [PubMed] [Google Scholar]
- 217.Stewart EA, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larchmt) 2013;22(10):807–816. doi: 10.1089/jwh.2013.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Borah BJ, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319 e1–319 e20. doi: 10.1016/j.ajog.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Baird DD. Hypothesis: vitamin D protects against uterine fibroid development [Abstract] Ann Epidemiol. 2008;18(9):710. [Google Scholar]
- 220.Blauer M, et al. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril. 2009;91(5):1919–1925. doi: 10.1016/j.fertnstert.2008.02.136. [DOI] [PubMed] [Google Scholar]
- 221.Sharan C, et al. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril. 2011;95(1):247–253. doi: 10.1016/j.fertnstert.2010.07.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 Reduces TGF-{beta}3-Induced Fibrosis-Related Gene Expression in Human Uterine Leiomyoma Cells. J Clin Endocrinol Metab. 2011;96(4):E754–E762. doi: 10.1210/jc.2010-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wise LA, et al. Polymorphisms in vitamin D–related genes and risk of uterine leiomyomata. Fertility and Sterility. 2014;102:503–510. doi: 10.1016/j.fertnstert.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tsuda H, et al. Clinical predictors in the natural history of uterine leiomyoma: preliminary study. Journal of Ultrasound in Medicine. 1998;17(1):17–20. doi: 10.7863/jum.1998.17.1.17. [DOI] [PubMed] [Google Scholar]


