Abstract
The blood–brain barrier (BBB) significantly reduces the delivery of many systemically administered agents to the central nervous system. Although temozolomide is the only chemotherapy to improve survival in patients with glioblastoma, its concentration in brain is only 20 % of that in blood. Regadenoson, an FDA approved adenosine receptor agonist used for cardiac stress testing, transiently disrupts rodent BBB allowing high molecular weight dextran (70 kD) to enter the brain. This study was conducted to determine if regadenoson could facilitate entry of temozolomide into normal rodent brain. Temozolomide (50 mg/kg) was administered by oral gavage to non-tumor bearing F344 rats. Two-thirds of the animals received a single dose of intravenous regadenoson 60–90 min later. All animals were sacrificed 120 or 360 min after temozolomide administration. Brain and plasma temozolomide concentrations were determined using HPLC/MS/MS. Brain temozolomide concentrations were significantly higher at 120 min when it was given with regadenoson versus alone (8.1 ± 2.7 and 5.1 ± 3.5 μg/g, P <0.05). A similar trend was noted in brain:plasma ratios (0.45 ± 0.08 and 0.29 ± 0.09, P < 0.05). Brain concentrations and brain:plasma ratios were not significantly different 360 min after temozolomide administration. No differences were seen in plasma temozolomide concentrations with or without regadenoson. These results suggest co-administration of regadenoson with temozolomide results in 60 % higher temozolomide levels in normal brain without affecting plasma concentrations. This novel approach to increasing intracranial concentrations of systemically administered agents has potential to improve the efficacy of chemotherapy in neuro-oncologic disorders.
Keywords: Blood–brain barrier, Regadenoson, Temozolomide, Brain tumor, Brain metastases, Pharmacology
Introduction
The blood–brain barrier (BBB) is an intricate barrier composed of a luminal negative charge, basal lamina, efflux pumps, and three distinct cell types: brain endothelial cells, pericytes, and astrocytic foot processes. Molecules that are small and lipophilic may easily traverse the BBB, while large (>180 Daltons) and/or hydrophilic particles require active transport, or receptor mediation [1]. The BBB integrity and degree of permeability is regulated by the brain capillary endothelial cells in response to astrocytic signals and to the strength of intercellular junctions [1–3]. Modulation of these paracellular properties could affect BBB integrity and improve drug penetration to the brain.
A normal BBB effectively restricts certain toxins and other blood borne substances from reaching the brain. Unfortunately, the BBB also makes it difficult for most systemically administered drugs to reach therapeutic concentrations within the central nervous system (CNS). This has limited the efficacy of numerous agents in the treatment of brain malignancies, infections, and other serious neurologic disorders. As a result, neuro-oncologists have used chemotherapy laden biodegradable polymers placed intra-operatively in the surgical resection cavities of patients with glioblastoma or intratumoral infusions of chemotherapy in an effort to improve drug delivery to the CNS [4–6]. In addition, efforts to transiently disrupt the BBB have been pursued for over three decades. This began in earnest in 1979, with the use of intra-arterial infusions of hypertonic mannitol to transiently decrease the integrity of tight junctions [7]. Hypertonic mannitol requires general anesthesia, hospitalizations, intra-arterial catheterization and intra-arterial chemotherapy which can be complicated by seizures, cerebrovascular events and other significant toxicities.
Alternate approaches to transiently disrupting the BBB in an outpatient setting involved the use of pharmacologic agents [8–10]. The bradykinin analog, lobradimil, was shown in preclinical studies to rapidly and transiently increase the permeability of the BBB [11]. However, when lobradimil was tested with systemically administered carboplatin in children with primary brain tumors the combination failed to result in improved response rates or time to disease progression [12]. In retrospect, these results were limited by the use carboplatin, which is not a very effective in this cancer, and by failure to measure intratumoral carboplatin levels with and without lobradimil. In addition, an adenosine agonist/analog has also been studied pre-clinically to transiently open the BBB [13]. Adenosine function is regulated by four structurally related G-protein coupled receptors: A1, A2A, A2B and A3 [14]. Specifically, A1 and A2A have high expression levels within the brain [13, 15, 16]. Regadenoson is an FDA approved selective A2A receptor agonist routinely used as a pharmacologic cardiac stress agent in patients who are unable to walk on a treadmill during outpatient cardiac stress testing [17]. In 2011, Carman et al. demonstrated that regadenoson increased BBB permeability to dextran (70 kD) in both mice and rats. The large dextran molecule was seen in the brain for up to 180 min following a single regadenoson injection [13]. Maximum brain penetration of dextran post regadenoson was seen 30 min after regadenoson administration. This transient BBB disruption was thought to be due to this agent’s ability to reduce the expression of several tight junction molecules including ZO-1, Claudin-5 and Occludin.
We sought to determine if regadenoson could increase chemotherapy concentrations in rodent brain with an intact BBB. We used temozolomide which is the most effective systemically delivered agent currently available for the treatment of glioblastoma. Although patients are not cured with this therapy, the 2 year survival of patients with newly diagnosed glioblastoma treated with radiation alone is 10 % and with combined radiation and temozolomide is 24 % [18]. Previous studies have shown that brain concentrations of this 194 dalton alkylating agent, which reaches peak serum levels 1–2 h after administration, are only 17–20 % of that in the blood [19, 20]. Temozolomide’s dose limiting toxicity of leukopenia and thrombo-cytopenia precludes the use of higher doses which theoretically could result in higher intratumoral concentrations. Thus, a safe, non-invasive, outpatient means to improve the delivery of systemically administered therapeutic agents to the brain, without increasing systemic toxicities, could be of significant impact in patients at risk for CNS metastases or with brain tumors, CNS infections, or other neurologic disorders.
Materials/methods
Chemicals and reagents
Regadenoson was purchased from Astellas Pharma US, Inc, Northbrook, IL. Temozolomide was purchased from Vivan Life Sciences, Mumbai, India. The HPLC internal standard for these studies was Temazepam which was purchased from Sigma-Aldrich Co, St. Louis, MO. All other chemicals and reagents were commercially available and of the highest grade.
Animal studies
Female F344 rats, weighing 150–170 g, were purchased from Harlan Bioproducts (Indianapolis, IN). All rats were housed in standard facilities and provided free access to rodent chow and Baltimore City water. The animal protocol was approved by the Johns Hopkins University Animal Care and Use Committee and the care and use of all study animals conformed to the National Institutes of Health rules Guide for the Care and Use of Laboratory Animals (National Academy of Sciences).
Thirty-six rats received temozolomide (50 mg/kg dissolved in water) by oral gavage (See Table 1). The rats were then assigned to one group (N = 18) which was sacrificed 120 min after the administration of temozolomide or a second group (N = 18) which was sacrificed 360 after temozolomide. Each cohort consisted of 18 animals in which 6 animals received temozolomide alone, regadenoson 60 min after temozolomide administration or regadenoson 90 min after temozolomide administration. Previous animal studies demonstrated regadenoson demonstrated maximum drug delivery to the brain 30 min after administration, thus we elected to administer regadenoson after temozolomide to result in maximum drug concentration of both drugs at 120 min [13]. For regadenoson injection, rats were placed into a small animal restrainer and received 0.0005 mg/kg of regadenoson by intravenous tail injection (Table 1). The dose of 0.0005 mg/kg is based on previous studies which demonstrated increased brain penetration of dextran with regadenoson at this dose [13]. Rats were sacrificed using a 0.4 mL intraperitoneal injection of the stock solution containing ketamine hydrochloride, 75 mg/mL (Ketathesia, Butler Animal Health Supply; Dublin, OH); xylazine 7.5 mg/mL (Lloyd Laboratories; Shenandoah, Iowa), and 14.25 % ethyl alcohol in 0.9 % NaCl. Blood was collected from the rats immediately after the ketamine was administered but prior to death by cardiac puncture. The blood was collected in a heparinized syringe and placed on ice. Within 30 min, all blood samples were centrifuged at 2500×g RPM for 10 min at room temperature. After centrifugation, the plasma supernatant (3 μL) was mixed with 8.5 % phosphoric acid (1 mL) and then transferred into cryovials and placed on ice until processed for analysis. After exsanguination, the whole brain was rapidly removed and flash frozen for subsequent analyses.
Table 1.
Animal conditions for n = 36 rats receiving temozolomide with and without regadenoson sacrificed 120 or 360 min from temozolomide administration
| No. animals | Treatment delivered | Regadenoson dose (mg/kg) | Time from temozolomide administration to sacrifice (min) |
|---|---|---|---|
| 6 | Temozolomide | 120 | |
| 6 | Temozolomide + Regadenoson at 60 min | 0.0005 | 120 |
| 6 | Temozolomide +Regadenoson at 90 min | 0.0005 | 120 |
| 6 | Temozolomide | 360 | |
| 6 | Temozolomide +Regadenoson at 60 min | 0.0005 | 360 |
| 6 | Temozolomide +Regadenoson at 90 min | 0.0005 | 360 |
Temozolomide analytical method and pharmacokinetics
Temozolomide was quantified in the plasma and brain samples. The whole brain was homogenized in 4 mL of 100 % methanol (v:v) prior to extraction. Homogenates were then centrifuged at 2500 RPM for 10 min at room temperature. Plasma and brain homogenate were further diluted in drug-free matrices prior to extraction. Temozolomide was extracted from 50 μL of sample (either acidified plasma or brain tissue extract) with 1 mL of 10 mg/mL temazepam in ethyl acetate. After each sample was vortexed and centrifuged, the supernatant of each sample was transferred into a glass tube and evaporated to dryness under nitrogen at 40 °C. Samples were reconstituted in 100 μL of 50 % acetonitrile:water (v:v) and 10 μL was injected into the UPLC. Separation was achieved with a Waters X-Terra MS C18 (3.5 μm × 150 mm × 2.1 mm, Milford, MA, USA) column at room temperature with acetonitrile/water mobile phase (30:70, v:v). The mobile phase used for the chromatographic separation was composed of 0.1 % (v:v) formic acid in water (mobile phase A) and 0.1 % (v:v) formic acid in acetonitrile (mobile phase B) with a flow rate of 0.3 mL/min. The initial mobile phase composition was 30 % mobile phase A and 70 % mobile phase B. From 0 to 0.5 min, mobile phase B was increased to 100 % and maintained until 2 min. From 2 to 2.1 min, the gradient decreased to 70 % mobile phase B and the conditions were maintained until 3 min to re-equilibrate the column for the next injection. The column effluent was monitored using an AB Sciex 5500 triple quadrapole™ 5500 mass-spectrometric detector (Applied Biosystems, Foster City, CA, USA) using electrospray ionization operating in positive mode. The spectrometer was programmed to monitor the following MRM transition 195.0 → 138.0 for temozolomide and 301.1 → 255.1 for the internal standard temazepam. Calibration curves for temozolomide were over the range of 5–1000 ng/mL with dilutions of up to 1:100 (v:v). The values for precision and accuracy for both plasma and brain homogenate samples during the in-study evaluation were within 15 %.
The plasma concentrations were calculated by raw data multiplied by a dilution factor of 100 or 50 for the 120 and 360 min samples, respectively. Temozolomide brain concentrations were calculated by raw data multiplied by a dilution factor based on each individual brain weight, additional methanol for tissue homogenization and a dilution factor of 10 or 5 for the 120 and 360 min samples, respectively.
Statistical analysis
The temozolomide concentrations were summarized using descriptive statistics. Differences in the temozolomide concentrations with and without regadenoson were evaluated statistically by use of Wilcoxon rank-sum test. The statistical analysis was done using JMP statistical Discovery Software version 11 (SAS Institute, Cary, NC). The a priori level of significance was P <0.05.
Results
Plasma temozolomide levels were studied with and without regadenoson to determine if this agent alters plasma temozolomide concentrations. As shown in Table 2, the mean plasma levels 120 min after temozolomide administration were not statistically significant between animals (16.1 μg/mL in the six animals that received temozolomide alone and 19.0 and 16.7 μg/mL in the twelve animals that received temozolomide and regadenoson 60 and 90 min post temozolomide respectively, P = 0.88). Similarly the plasma temozolomide concentrations in the 18 animals sacrificed 360 min after temozolomide ingestion were similar (5.0 μg/mL in the six animals that received temozolomide alone and 4.4 and 6.2 μg/mL in the twelve animals that received temozolomide and regadenoson 60 and 90 min post temozolomide respectively, P = 0.99) whether or not the animals received regadenoson.
Table 2.
Brain, plasma and brain:plasma temozolomide concentrations with and without regadenoson after oral administration of temozolomide
| Condition | Brain temozolomide concentration (μg/g) | Plasma temozolomide concentration (μg/mL) | Brain:plasma temozolomide concentration |
|---|---|---|---|
| Sacrifice at 120 min | |||
| Temozolomide | 5.1 ± 3.5 | 16.1 ± 7.7 | 0.29 ± 0.09 |
| Temozolomide + Regadenoson at 60 min | 8.7 ± 2.4* | 19.0 ± 5.5 | 0.46 ± 0.06* |
| Temozolomide +Regadenoson at 90 min | 7.4 ± 3.1* | 16.7 ± 3.8 | 0.43 ± 0.09* |
| Temozolomide + Regadenoson (all) | 8.1 ± 2.7* | 17.8 ± 4.7 | 0.45 ± 0.08* |
| Sacrifice at 360 min | |||
| Temozolomide | 1.4 ± 0.7 | 5.0 ± 2.5 | 0.27 ± 0.03 |
| Temozolomide +Regadenoson at 60 min | 1.7 ± 0.8 | 4.4 ± 2.6 | 0.51 ± 0.41 |
| Temozolomide +Regadenoson at 90 min | 2.2 ± 0.9 | 6.2 ± 2.8 | 0.37 ± 0.12 |
| Temozolomide +Regadenoson (all) | 1.9 ± 0.9 | 5.3 ± 2.7 | 0.44 ± 0.3 |
Results are presented as the mean ± SD
P < 0.05 comparing the temozolomide alone 120 min treatment group using the Wilcoxon rank-sum test
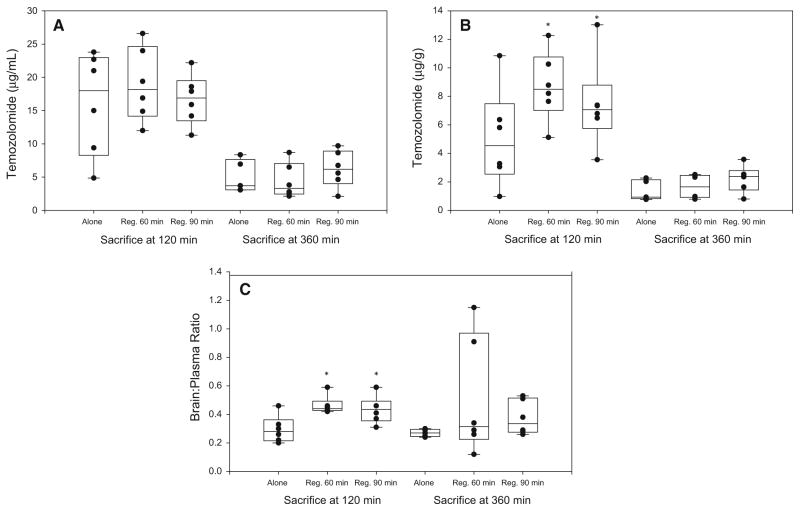
Concentrations of temozolomide in the brain were higher in the animals sacrificed at 120 min who received temozolomide and regadenoson (8.7 and 7.4 μg/g 60 and 90 min post temozolomide respectively), than in those treated with temozolomide alone (5.1 μg/g; P = 0.049) (Table 2). Although the differences at 360 min were in the same direction, the differences with regadenoson were not statistically significant at this later time point (P = 0.96). Similarly, an analysis of the mean brain to plasma temozolomide ratios of the animals studied 120 min from temozolomide administration revealed a significant increase in temozolomide penetration into the brain tissue. This was independent of whether the regadenoson was administered at 60 or 90 min following ingestion of temozolomide (Table 2). The mean temozolomide brain:-plasma ratio in these rats was 0.29 ± 0.09 after temozolomide alone and 0.46 ± 0.06 or 0.43 ± 0.09 after temozolomide with regadenoson at 60 min or at 90 min respectively. A similar increase in temozolomide brain:-plasma ratio was found in the animals studied at 360 min after temozolomide ingestion (0.27 ± 0.03 after temozolomide alone and 0.51 ± 0.41 and 0.37 ± 0.12 after temozolomide with regadenoson at 60 or 90 min respectively) (Fig. 1).
Fig. 1.
Lack of influence of regadenoson on temozolomide plasma concentrations (a) at 120 and 360 min from temozolomide administration. Increased temozolomide brain penetration seen 120 min but not 360 min from temozolomide administration (b). The temozolomide brain:plasma ratio (c) shows increased penetration into the brain at 120 and 360 min from temozolomide administration. The values are presented as the mean ± SD. *P < 0.05, compared temozolomide alone 120 min treatment group
Discussion
Clinicians have long been aware of the need for systemically administered agents with excellent penetration into the CNS for the optimal treatment of brain infections, brain tumors, and other serious neurologic illnesses. One commonly stated reason for the lack of progress and continued poor outcomes for patients with primary brain tumors has been the failure to achieve therapeutic drug concentrations within brain parenchyma. This problem has been recently highlighted in patients with systemic malignancies, such as breast and lung cancer, where systemic treatments have improved, but increasingly patients with excellent systemic responses are relapsing in the CNS. Thus, fatal CNS metastases could be prevented if these novel agents could potentially penetrate brain parenchyma.
Previous attempts to improve drug delivery to the CNS are well documented. Examples include placement of therapeutic agents on the other side of the BBB with chemotherapy impregnated wafers (Gliadel), convection enhanced delivery, and intra-arterial chemotherapy delivery [3, 4, 21]. In recent years, transient disruption of the BBB by pharmacologic and mechanical means have been evaluated with mannitol followed by intra-arterial administration of chemotherapy, the bradykinin analog lobradimil and use of focused ultrasound with microbubbles [1, 4, 12, 22]. Other than the implantation of the Gliadel wafer following gross total resection, none of the other approaches has been found to be practical, cost effective, therapeutically beneficial with durable effects or FDA approved [6].
In this modestly sized animal study we sought to expand upon the work of Carman et al. who demonstrated that regadenoson transiently increased BBB permeability to dextran (70 kD) in mice and rats maximally 30 min after Regadenoson administration [13]. Our goals were to determine if it would also improve delivery of a much smaller (194 daltons) alkylating agent to normal rat brain parenchyma. This was done by administering temozolomide alone to 33 % of the animals and giving the others temozolomide with regadenoson.
Additionally, this study is the first to explore the ability of regadenoson to improve the delivery of systemically administered chemotherapy to brain parenchyma. Previous studies have demonstrated the median Tmax of temozolomide, as measured by microdialysis in normal rat brains, to be 1.5–2 h after temozolomide ingestion [23, 24]. These observations greatly influenced our study design to evaluate temozolomide concentrations after 120 and 360 min. With a known temozolomide Cmax of 2 h, we were able to show statistically different brain concentrations and brain:plasma ratios post regadenoson 2 h after temozolomide administration. It was also previously shown that the effect of regadenoson on dextran passage through the BBB lasts for <180 min [13]. Overall, temozolomide concentrations in the brain were 60 % higher 120 min after temozolomide ingestion with regadenoson (P = 0.049) but was not statistically different at 360 min (P = 0.96), likely due to the transient effect of regadenoson. Importantly, the mean plasma concentrations of temozolomide with and without regadenoson demonstrated no differences at 120 min (P = 0.88) or 360 min (P = 0.99). This suggests that regadenoson might deliver more drug to the brain without adding to chemotherapy related systemic toxicities.
There are several shortcomings to this study. The first is that we were only able to measure temozolomide and not its active metabolite [5-(3-methyltriazen-1-yl)imidazole-4-carboxamide] (MTIC) [25]. Unfortunately, this metabolite is too unstable to allow for reliable sampling of MTIC concentrations in the brain and plasma of these animals and has a low systemic exposure (<2.2 %) compared to temozolomide [19, 26]. However, the molecular weight of MTIC is 168 Da making it plausible that if regadenoson can increase brain permeability for 194 Da temozolomide, MTIC could also be granted entry. In addition, we measured concentrations of temozolomide from the whole rat brain. These values do not provide concentrations in specific brain regions and could be contaminated by intravascular blood. Consequently, our temozolomide brain and brain to plasma concentration values are different than those obtained using microdialysis pharmacokinetics in humans and preclinical models [19, 20, 23].
We also studied only one dose of regadenoson in the experiments described in this manuscript. Carman et al. have previously studied the effect of different regadenoson doses and schedules on the entry of high molecular weight (MW 70,000 daltons) dextran in the brain of non-tumor containing rodents [13]. Their results suggest that altering the dose of this agent does not result in major changes in the intracranial dextran concentrations. In our experiments, we used the same dose that Carman et al. administered to achieve surprisingly high brain penetration of high molecular weight dextran hoping that this would increase the delivery of temozolomide (MW 140 daltons) to the normal brain. Our results demonstrate a modest but statistically significant increase in temozolomide in a small number of animals with normal brains. Given the previously published data, we do not believe that additional experiments with different dose schedules will add substantially to our findings.
Another potential criticism of this research effort is that we did not study animals with intracranial tumors. Our focus on using only animals without brain tumors was taken for two major reasons. First, high grade gliomas, which almost uniformly have contrast enhancing lesions on magnetic resonance imaging are already known to have a disrupted BBB as it is the entry of gadolinium containing contrast (such as Magnevist MW 938) which is seen on T1 weighted MRI scans. There is an abundance of data demonstrating that this region of the tumor is ‘‘open’’ to many diagnostic and therapeutic substances of high molecular weight. Examples include studies using Evan’s blue labeled albumin (MW 60,000 daltons) in animals with brain tumors, radiolabeled albumin (MW 60,000 daltons) used in the radionucleotide brain scans in humans before the availability of CT or MRI scans, and current studies using ferumoxytol, a high molecular weight contrast (MW 731,000 daltons), in patients with brain tumors [27–30]. As a result, the major challenge clinically is in opening regions of the brain where the BBB is much more intact. As animal models and human brain tumors have marked regional variation in enhancement, the most stringent test of an agent’s ability to open the BBB is in animals without known CNS pathology.
The second reason for using non-tumor bearing animals relates to the likely future use of transient BBB disruption in oncology. Currently, there are many patients with lung and breast cancer who respond to therapeutic agents systemically and subsequently progress only in the brain due to failure of these agents to penetrate an intact BBB. Thus a BBB disrupting agent that could be safely co-administered with standard chemotherapy might significantly reduce the incidence of CNS metastases. In order for this to be effective, it must be able to transiently affect the integrity of the BBB in a normal brain. Our data suggests that this may be possible with regadenoson.
The results of this study suggest that an FDA approved selective A2A receptor might significantly increase the penetration of systemically administered chemotherapy into brain parenchyma. This agent is safe and its administration in an oncology out-patient setting is feasible given that it is routinely administered to outpatients who are unable to exercise for cardiac stress testing. Future studies are planned to address if this approach will improve delivery for larger chemotherapy agents, monoclonal antibodies, compounds that are highly charged, or drugs that are targets for multiple efflux pumps. These pre-clinical observations must be moved to the clinical setting to determine if this is a useful approach to increasing drug delivery to the CNS in humans.
Acknowledgments
This work was supported in part by fellowship support T32GM066691 in Johns Hopkins Clinical pharmacology training program (Sadhana Jackson). The project described was supported by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (National Institutes of Health grants P30 CA006973 and UL1 TR 001079, and Shared Instrument Grant 1S10RR026824-01). Grant Number UL1 TR 001079 is from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health, and National Institutes of Health Roadmap for Medical Research.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- 1.Neuwelt EA. Mechanisms of disease: the blood–brain barrier. Neurosurgery. 2004;54:131–140. doi: 10.1227/01.neu.0000097715.11966.8e. Discussion 141–142. [DOI] [PubMed] [Google Scholar]
- 2.Pardridge WM. Blood–brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody. Expert Opin Drug Deliv. 2015;12:207–222. doi: 10.1517/17425247.2014.952627. [DOI] [PubMed] [Google Scholar]
- 3.Kroll RA, Neuwelt EA. Outwitting the blood–brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42:1083–1099. doi: 10.1097/00006123-199805000-00082. Discussion 1099–1100. [DOI] [PubMed] [Google Scholar]
- 4.Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15:2887–2893. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 5.White E, Bienemann A, Pugh J, Castrique E, Wyatt M, Taylor H, Cox A, McLeod C, Gill S. An evaluation of the safety and feasibility of convection-enhanced delivery of carboplatin into the white matter as a potential treatment for high-grade glioma. J Neurooncol. 2012;108:77–88. doi: 10.1007/s11060-012-0833-4. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhary SA, Ryken T, Newton HB. Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: a meta-analysis. J Neurooncol. 2015;122:367–382. doi: 10.1007/s11060-015-1724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuwelt EA, Frenkel EP, Diehl JT, Maravilla KR, Vu LH, Clark WK, Rapoport SI, Barnett PA, Hill SA, Lewis SE, et al. Osmotic blood–brain barrier disruption: a new means of increasing chemotherapeutic agent delivery. Trans Am Neurol Assoc. 1979;104:256–260. [PubMed] [Google Scholar]
- 8.Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41:44–48. doi: 10.1097/00006123-199707000-00011. Discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 9.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G The Polymer-brain Tumor Treatment Group. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 10.Kemper EM, Boogerd W, Thuis I, Beijnen JH, van Tellingen O. Modulation of the blood–brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours? Cancer Treat Rev. 2004;30:415–423. doi: 10.1016/j.ctrv.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Elliott PJ, Hayward NJ, Dean RL, Blunt DG, Bartus RT. Intravenous RMP-7 selectively increases uptake of carboplatin into rat brain tumors. Cancer Res. 1996;56:3998–4005. [PubMed] [Google Scholar]
- 12.Warren K, Jakacki R, Widemann B, Aikin A, Libucha M, Packer R, Vezina G, Reaman G, Shaw D, Krailo M, et al. Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: a report from the Children’s Oncology Group. Cancer Chemother Pharmacol. 2006;58:343–347. doi: 10.1007/s00280-005-0172-7. [DOI] [PubMed] [Google Scholar]
- 13.Carman AJ, Mills JH, Krenz A, Kim DG, Bynoe MS. Adenosine receptor signaling modulates permeability of the blood–brain barrier. J Neurosci. 2011;31:13272–13280. doi: 10.1523/JNEUROSCI.3337-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 15.Marala RB, Mustafa SJ. Immunological characterization of adenosine A2A receptors in human and porcine cardiovascular tissues. J Pharmacol Exp Ther. 1998;286:1051–1057. [PubMed] [Google Scholar]
- 16.Kassner A, Thornhill R. Measuring the integrity of the human blood–brain barrier using magnetic resonance imaging. Methods Mol Biol. 2011;686:229–245. doi: 10.1007/978-1-60761-938-3_10. [DOI] [PubMed] [Google Scholar]
- 17.Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, Lieu H, Mahmarian JJ, Olmsted A, Underwood SR, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14:645–658. doi: 10.1016/j.nuclcard.2007.06.114. [DOI] [PubMed] [Google Scholar]
- 18.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 19.Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15:7092–7098. doi: 10.1158/1078-0432.CCR-09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostermann S, Csajka C, Buclin T, Leyvraz S, Lejeune F, Decosterd LA, Stupp R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 21.Doolittle ND, Muldoon LL, Culp AY, Neuwelt EA. Delivery of chemotherapeutics across the blood–brain barrier: challenges and advances. Adv Pharmacol. 2014;71:203–243. doi: 10.1016/bs.apha.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood–brain barrier disruption. Ultrasonics. 2008;48:279–296. doi: 10.1016/j.ultras.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossman R, Tyler B, Rudek MA, Kim E, Zadnik P, Khan U, Blakeley JO, Pathak AP, Brem H. Microdialysis measurement of intratumoral temozolomide concentration after cediranib, a pan-VEGF receptor tyrosine kinase inhibitor, in a U87 glioma model. Cancer Chemother Pharmacol. 2013;72:93–100. doi: 10.1007/s00280-013-2172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q, Guo P, Wang X, Nuthalapati S, Gallo JM. Pre-clinical pharmacokinetic and pharmacodynamic evaluation of metronomic and conventional temozolomide dosing regimens. J Pharmacol Exp Ther. 2007;321:265–275. doi: 10.1124/jpet.106.118265. [DOI] [PubMed] [Google Scholar]
- 25.Denny BJ, Wheelhouse RT, Stevens MF, Tsang LL, Slack JA. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994;33:9045–9051. doi: 10.1021/bi00197a003. [DOI] [PubMed] [Google Scholar]
- 26.Rudek MA, Donehower RC, Statkevich P, Batra VK, Cutler DL, Baker SD. Temozolomide in patients with advanced cancer: phase I and pharmacokinetic study. Pharmacotherapy. 2004;24:16–25. doi: 10.1592/phco.24.1.16.34800. [DOI] [PubMed] [Google Scholar]
- 27.Tator CH, Morley TP, Olszewski J. A study of the factors responsible for the accumulation of radioactive iodinated human serum albumin (Rihsa) by intracranial tumours and other lesions. J Neurosurg. 1965;22:60–76. doi: 10.3171/jns.1965.22.1.0060. [DOI] [PubMed] [Google Scholar]
- 28.Neuwelt EA, Varallyay CG, Manninger S, Solymosi D, Haluska M, Hunt MA, Nesbit G, Stevens A, Jerosch-Herold M, Jacobs PM, et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery. 2007;60:601–611. doi: 10.1227/01.NEU.0000255350.71700.37. Discussion 611–612. [DOI] [PubMed] [Google Scholar]
- 29.Hossmann KA, Bothe HW, Bodsch W, Paschen W. Pathophysiological aspects of blood–brain barrier disturbances in experimental brain tumors and brain abscesses. Acta Neuropathol Suppl. 1983;8:89–102. doi: 10.1007/978-3-642-68970-3_8. [DOI] [PubMed] [Google Scholar]
- 30.Salcman M, Scott EW, Schepp RS, Knipp HC, Broadwell RD. Transplantable canine glioma model for use in experimental neuro-oncology. Neurosurgery. 1982;11:372–381. doi: 10.1227/00006123-198209000-00007. [DOI] [PubMed] [Google Scholar]