Abstract
Bone has the potential for spontaneous healing. This process, however, often fails in patients with comorbidities. Tissue engineering combining functional cells, biomaterials and osteoinductive cues may provide alternative treatment strategies. We have recently demonstrated that stromal cell-derived factor-1β (SDF-1β) works in concert with bone morphogenetic protein-2 (BMP-2) to potentiate osteogenic differentiation of bone marrow-derived mesenchymal stem/stromal cells (BMSCs). Here, we test the hypothesis that SDF-1β overexpressed in Tet-Off-SDF-1β BMSCs, delivered on acellular dermal matrix (ADM), synergistically augments BMP-2-induced healing of critical-sized mouse calvarial defects. BMSC therapies alone showed limited bone healing, which was increased with co-delivery of BMP-2. This was further enhanced in Tet-Off-SDF-1β BMSCs + BMP-2. Only limited BMSC retention on ADM constructs was observed after 4 weeks in vivo, which was increased with BMP-2 co-delivery. In vitro cell proliferation studies showed that supplementing BMP-2 to Tet-Off BMSCs significantly increased the cell number during the first 24 h. Consequently, the increased cell numbers decreased the detectable BMP-2 levels in the medium, but increased cell-associated BMP-2. The data suggest that SDF-1β provides synergistic effects supporting BMP-2-induced, BMSC-mediated bone formation and appears suitable for optimization of bone augmentation in combination therapy protocols.
Keywords: BMSCs, SDF-1/CXCL12, BMP-2, stem cell transplantation, bone formation
1. Introduction
Bone is a dynamic and highly vascularized tissue that continues to remodel throughout life and has the innate capacity for healing upon damage. This regenerative process, however, often fails in patients with significant mechanical or metabolic restrictions or impaired health status, requiring surgical intervention to augment natural fracture repair (Mountziaris and Mikos, 2008). In these cases, autologous bone grafting remains the standard of care, as it provides a consistent clinical outcome. Autografts reliably integrate with host bone tissue and lack the immune- and disease-related complications of allogeneic or xenogeneic bone grafts (Bauer and Muschler, 2000). Nevertheless, its use is severely hampered by short supply and considerable donor site morbidity associated with the harvest (Sen and Miclau, 2007; Silber et al., 2003). The field of bone tissue engineering provides alternative treatment options to reduce problems associated with the current therapeutic strategies (Grabowski and Cornett, 2013). Three specific areas have emerged as components in the development of more effective treatment modalities: (a) multipotent stem/progenitor cells; (b) biomaterials; and (c) manipulations of osteogenic signalling pathways (Amini et al., 2012; Giannoudis et al., 2005).
Bone marrow-derived mesenchymal stem/stromal cells (BMSCs) hold great promise for regenerative therapies in the musculoskeletal system (recently reviewed in Ma et al., 2014; Steinert et al., 2012), due to their capacity for multipotent differentiation (Caplan, 1991, 2005, 2009; Caplan and Bruder, 2001). Further, BMSCs possess immunoregulatory functions, secrete paracrine factors and act as signalling centres to orchestrate the host response to injuries (Caplan and Dennis, 2006; Jones and Yang, 2011). For tissue-engineering applications, the synergistic combination of biomaterials and cell therapy is of great interest. In addition, osteoinductive cues can be incorporated to enhance the regenerative capacities of BMSCs (Janicki and Schmidmaier, 2011). Among these, bone morphogenetic protein-2 (BMP-2) is FDA-approved for procedures such as sinus augmentation and spinal fusion. However, side-effects and aberrant events, in particular with off-label use, have been linked to the high-dose commercial BMP-2 product and have evoked controversy (Carragee et al., 2011; Govender et al., 2002). Considerable efforts are being made to enhance our understanding of BMP biology to improve and expand clinical utility. One approach is the use of co-delivery strategies, using different growth factors, cytokines or chemokines to augment BMP-2-mediated healing.
Stromal cell-derived factor-1 (SDF-1/CXCL12) is a member of the CXC chemokine family (Zlotnik and Yoshie, 2000). SDF-1 and its cognate receptor (CXCR4) are expressed constitutively in various tissues (Bleul et al., 1996; Feng et al., 1996; Heesen et al., 1996). Among other signalling pathways, binding of SDF-1 to CXCR4 initiates the recruitment of regenerative cells to injury sites during the acute phase of bone repair (Granero-Molto et al., 2009; Kitaori et al., 2009; Otsuru et al., 2008). We and others have shown a direct regulatory role for SDF-1 signalling in BMP-2-induced osteogenic differentiation of mesenchymal cells in vitro (Herberg et al., 2013a; Hosogane et al., 2010; Zhu et al., 2007) and in vivo (Herberg et al., 2014c; Higashino et al., 2011; Ratanavaraporn et al., 2011; Wise et al., 2012; Zhu et al., 2011). We have also recently described genetically engineered BMSCs that conditionally overexpress SDF-1β using the tetracycline (Tet)-regulatory system (Tet-Off-SDF-1β BMSCs) (Herberg et al., 2013a). SDF-1β was selected over the more abundant splice variant SDF-1α, due to its greater resistance to C-terminal proteolytic cleavage and glycosaminoglycan-dependent stabilization on cell and extracellular surfaces (Davis et al., 2005; De La Luz et al., 2004; Herberg et al., 2013a; Marquez-Curtis et al., 2008). We showed that SDF-1β enhances mineralization and expression of key osteogenic markers, and modulates BMP-2 signal transduction in vitro (Herberg et al., 2013a). In addition, we recently reported that SDF-1β augments cell-mediated bone formation in a model of direct intramedullary tibial transplantation of Tet-Off-SDF-1β BMSCs following total body irradiation, providing in vivo proof-of-principle of the Tet-Off regulatory system (Herberg et al., 2015).
The objective of this study was to investigate the regenerative capacity of Tet-Off BMSCs + BMP-2 combination therapies relative to BMSC controls and BMP-2 therapy alone in a model of acute bone injury. Experimental treatments were soak-loaded on acellular dermal matrix (ADM) delivery scaffolds. We tested the hypothesis that SDF-1β, expressed at high levels in Tet-Off-SDF- 1β BMSCs, in combination with BMP-2, synergistically augments healing of critical-sized mouse calvarial defects.
2. Materials and methods
2.1. Animals
C57BL/6J male mice aged 8 weeks were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and maintained at the Laboratory Animal Services research facility at Georgia Regents University. All aspects of the research were conducted in accordance with the guidelines set by the Georgia Regents University Institutional Animal Care and Use Committee, following an approved Animal Use Protocol.
2.2. Isolation and culture of BMSCs
BMSCs were derived from 18 month-old male C57BL/6J mice at the Georgia Regents University Stem Cell Core Facility, as described previously (Herberg et al., 2013a, 2013b, 2014a; Zhang et al., 2008a, 2008b) (see supporting information, Supplementary methods). Animals were purchased from the National Institute on Aging (Bethesda, MD, USA) aged rodent colony. Wellisolated, multipotent, BMSCs (CD11b-, CD45R-, CD11cand PDCA-1-negative; Sca-1-positive) were subjected to retroviral-mediated transduction with ΔU3–GFP plasmid DNA, constructed in the replication-defective ΔU3nlsLacZ vector by inserting the full-length coding region of Gfp cDNA (Zhang et al., 2008b). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Cellgro, Mediatech, Manassas, VA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, USA) and used at 70–80% confluence.
2.3. Genetic modification of BMSCs for conditional expression of SDF-1β
BMSCs were transduced with retroviral Tet-Off expression vectors (Clontech Laboratories, Mountain View, CA, USA), using the sequential protocol of retrovirus production (Ory et al., 1996), two-step infection and selection to generate double-stable Tet-Off-SDF-1β and Tet-Off-EV (empty vector) BMSCs, as previously described (Herberg et al., 2013a, 2013b) (see supporting information, Supplementary methods). Tet-Off-SDF-1β BMSCs have been shown to overexpress ~30-fold SDF-1β mRNA and ~five-fold SDF-1β protein at 24 h compared to doxycycline (Dox)-suppressed controls (Herberg et al., 2013a, 2015). Genetically engineered Tet-Off BMSCs were maintained in normal proliferation medium comprising DMEM supplemented with 10% Tet–FBS (Clontech), 400 μg/ml G418 (MP Biomedicals, Solon, OH, USA) and 2.5 μg/ml puromycin (Sigma-Aldrich, St. Louis, MO, USA).
2.4. BMSCs retention on acellular dermal matrix in vitro
Ultrathin DermaMatrix™ acellular dermis (ADM; Synthes, West Chester, PA, USA) was cut to size using a standard 4.0-mm tissue punch (Niltex, York, PA, USA). Tet-Off-EV BMSCs (Herberg et al., 2013a) were expanded to ~70% confluence in normal proliferation medium in 150 cm2 tissue-culture flasks, lifted with trypsin–EDTA and resuspended at a density of 2.6 × 107 cells/ml in PBS. Sterile ADM discs (4.0 × 0.3 mm) were transferred to nontreated 24-well cell-culture plates and 3.8 μl Tet-Off-EV BMSCs (1.0 × 105 cells, passage 16) were soak-loaded onto the dermal surface. After 15 min of incubation, 1.0 ml normal proliferation medium was added. Cell retention was assessed after 1, 3, 6, 24, 72 and 168 h in vitro. Fluorescence microscopy images were captured using an inverted Carl Zeiss microscope with AxioVision Image Analysis software v 4.7.1.0 (Carl Zeiss, Thornwood, NY, USA). The Tet-Off-EV BMSCs’ strong inherent GFP expression allows viewing of the cells without the need for antibody staining of the entire ADM construct.
2.5. In vivo study design and experimental groups
Seventy animals were randomized into seven groups of 10 each (Table 1). We evaluated 1.0 × 105 Tet-Off-EV and Tet-Off-SDF-1β BMSCs (passage 16) (Herberg et al., 2013a), suspended in saline alone or co-delivered with 542.5 ng BMP-2 (recombinant human BMP-2; PeproTech, Rocky Hill, USA), soak-loaded (3.8 μl) onto the dermal surface of 4.0 × 0.3 mm pre-cut ADM discs. The smaller size of the ADM discs compared to the defect size was chosen to account for the minor swelling upon soak loading, and to enable secure placement of the matrix without significant shifting during the healing interval. Controls included sham surgery and ADM soak-loaded with saline/vehicle control or 542.5 ng BMP-2. In preliminary studies we determined that the recently reported BMP-2 dose of 108.5 ng loaded onto the ADM via an inkjet-based biopatterning approach (Herberg et al., 2014b) was insufficient to promote significant bone healing in this model when applied by soak loading. Therefore, the BMP-2 dose was increased five-fold (see supporting information, Figure S1). Furthermore, we have previously shown that Tet-Off-EV (± Dox) and Tet-Off-SDF-1β BMSCs (+ Dox) have comparable osteogenic capacities in vitro (Herberg et al., 2013a) and in vivo (Herberg et al., 2015), demonstrating the feasibility of the Tet-Off system to regulate SDF-1β overexpression. Hence, we did not include Tet-Off-EV and Tet-Off-SDF-1β BMSCs (+ Dox) controls.
Table 1.
Experimental groups, treatment doses and number of animals
| Group | Dose/ADM | n |
|---|---|---|
| Sham | – | 10 |
| Control | – | 10 |
| BMP-2 | 542.5 ng | 10 |
| Tet-Off-EV BMSCs | 1.0 × 105 cells | 10 |
| Tet-Off-EV BMSCs + BMP-2 | 1.0 × 105 cells + 542.5 ng | 10 |
| Tet-Off-SDF-1β BMSCs | 1.0 × 105 cells | 10 |
| Tet-Off-SDF-1β BMSCs + BMP-2 | 1.0 × 105 cells + 542.5 ng | 10 |
ADM, acellular dermal matrix; BMP-2, bone morphogenetic protein-2; BMSCs, bone marrow-derived mesenchymal stem/stromal cells; EV, empty vector; SDF-1β, stromal cell-derived factor 1β; Tet-Off, tetracycline-regulatory system.
2.6. Critical-sized calvarial defect model
The established critical-sized mouse calvarial defect model was performed as described previously (Cooper et al., 2010b; Herberg et al., 2014b; Hollinger and Kleinschmidt, 1990; Schmitz and Hollinger, 1986; Smith et al., 2012) (see supporting information, Supplementary methods). The experimental implants were prepared by aseptically soak loading the respective treatments (3.8 μl total volume) on the dermal side of the pre-cut ADM discs. In reference to the recommended protocol pertaining to clinical application of BMP-2 on a collagen sponge, the ADM constructs were allowed to air-dry for 15 min prior to implantation in the craniectomy defects, with the dermal surface approximating the dura mater. The skin was closed with 6 × 0 polypropylene sutures. The animals were euthanized at 4 weeks. The surgical sites were explanted and fixed for 24 h in 10% buffered formalin before preserving the specimens in 70% ethyl alcohol.
2.7. Radiographic analysis
Radiographic analysis was performed as previously described (Herberg et al., 2014b, 2014c). Calvarial specimens were placed in 100 mm cell culture dishes and radiographed using a digital imaging instrument (Faxitron X-Ray, Wheeling, IL, USA), following initial calibration. Percentage bone healing for each defect was estimated utilizing a 5.0 mm region of interest (ROI). Using ImageJ software v 1.47 (NIH, Washington, DC, USA), each 5.0 mm ROI was isolated and subjected to system default binary thresholding (white being new bone, black being defect or lack of bone). After thresholding, the ROI was analysed for the amount of new bone and percentage bone healing was calculated relative to the area of a 5.0 mm circle.
2.8. Micro-computed tomography
Micro-computed tomography (μCT) analysis was performed as described previously (Herberg et al., 2014b, 2014c). Calvarial specimens were scanned using an ex vivo μCT system (Skyscan 1174, Skyscan, Aartlesaar, Belgium). The scanner was equipped with a 50 kV, 800 μA X-ray tube and a 1.3 megapixel CCD coupled to a scintillator. Each sample was placed in a sample holder with the sagittal suture orientated parallel to the image plane and scanned in air using a 0.25 mm aluminium filter, 13 μm isotropic voxels, 1300 ms integration time, 0.5° rotation step, and frame averaging of 4. All samples were scanned within the same container, using the same scanning parameters. All scans were then reconstructed using NRecon software v 1.6.6.0 (Skyscan) with exactly the same reconstruction parameters. For 3D analysis (CTAn software v 1.12.0.0+, Skyscan), the greyscale was set at 50–140. This range allowed viewing of the normal bone architecture seen in the rawimages. All reconstructed images were adjusted to this greyscale before running the 3D analysis. Standard 3D morphometric parameters (Bouxsein et al., 2010) were determined in a ROI (5.0 mm circle, 50 cuts = 0.65 mm, total volume = 12.8 mm3) in all samples. Representative 3D images were created using CTvox software v 2.3.0 r810 (Skyscan).
2.9. Histological preparation and analysis
Calvarial specimens were decalcified in 0.25 M ethylene diamine tetra-acetic acid (EDTA), pH 7.4, for 7 days, with changes of the EDTA solution every other day as previously described (Herberg et al., 2014c). The specimens were washed, dehydrated in a graded series of ethyl alcohols (70–100%), cleared in xylene, embedded in paraffin parallel to the sagittal suture and sectioned at 7 μm thickness, using a microtome (Leica Microsystems, Buffalo Grove, IL, USA), prior to mounting on Frost Plus glass slides for histology. Serial coronal sections were stained using standard hematoxylin and eosin (H&E; Fisher Scientific, Kalamazoo, MI, USA), Masson’s trichrome (MTC; Fisher Scientific) and picrosirius red (PSR; Sigma-Aldrich) stains. Light microscopy images, employing linearly polarized light for PRS-stained sections, were captured using a Carl Zeiss microscope with AxioVision Image Analysis software v 4.7.1.0 (Carl Zeiss).
2.10. GFP signal intensity on ADM constructs
The GFP signal intensity as a measure of BMSC retention on the ADM constructs after 4 weeks in vivo was assessed in the explanted unprocessed surgical sites. Calvarial specimens were placed in 24-well plates and the amount of GFP signal in Tet-Off-EV and Tet-Off-SDF-1β BMSCloaded ADM constructs ± BMP-2 was determined by fluorescence microscopy, using an inverted Carl Zeiss microscope with AxioVision Image Analysis software v 4.7.1.0 (Carl Zeiss). The calvarial specimens were randomized and the GFP signal intensities quantified by two blinded independent investigators using 0 (no GFP)–3 (high GFP) arbitrary units scoring.
2.11. Immunohistochemistry
Paraffin sections, 7.0 μm thick, were deparaffinized in xylene, hydrated and permeabilized in 0.1% Triton X100 for 10 min, as previously described (Herberg et al., 2014a). Antigen retrieval was performed using Digest- All-3 solution (Invitrogen, Carlsbad, CA, USA). Nonspecific binding was blocked using 3% normal donkey serum (Jackson ImmunoResearch, West Grove, PA, USA) for 1 h at room temperature in a humidifying chamber. Serial sections were incubated with primary Rb (rabbit) anti-GFP antibody (1:300; Molecular Probes, Invitrogen) overnight at 4°C. For detection of immunopositive signals, sections were incubated with AlexaFluor® 488-conjugated secondary D (donkey) anti-Rb antibody (1:500; Jackson ImmunoResearch) for 2 h at roomtemperature in a humidifying chamber. To reduce autofluorescence stemming from the ADM, the sections were incubated with 0.1% Sudan Black B (Sigma-Aldrich) in 70%ethyl alcohol for 20 min, following an established protocol (Baschong et al., 2001). The sections were coverslipped using Vectashield mounting medium (Vector Laboratories Inc., Burlingame, CA, USA) containing 4′,6- diamidino-2-phenylindole (DAPI) nuclear stain. Fluorescence microscopy images were captured using a Carl Zeiss microscope with AxioVision Image Analysis software v 4.7.1.0 (Carl Zeiss).
2.12. Cell proliferation
Tet-Off BMSC proliferation was determined using a CellTiter 96® AQueous MTS Cell Proliferation Assay (Promega, Madison, WI, USA), according to the manufacturer’s recommendation. Tet-Off-EV and Tet-Off-SDF-1β BMSCs were plated in quadruplicate at a density of 4.0 × 103 cells/well in 96-well plates using normal proliferation medium. The following day, 100 μl fresh medium, alone or supplemented with 100 ng/ml BMP-2 (PeproTech), was added to each well. BMSCs were incubated for 1, 3 or 7 days, at which time points 20 μl/well MTS solution was added. Tet-Off BMSCs were incubated for 4 h and absorbance was read at 490 nm.
2.13. BMP-2 enzyme-linked immunosorbent assay
Levels of intracellular and secreted BMP-2 were analysed in diluted BMSC lysates and cell culture supernatants, using a Quantikine® enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s recommendations. Tet-Off-EV and Tet-Off-SDF-1β BMSCs were plated in triplicate at a density of 1.0 × 104 cells/cm2 in 12-well plates, using normal proliferation medium. The following day, 1 ml fresh medium, alone or supplemented with 100 ng/ml BMP-2 (PeproTech), was added to each well. BMSCs were incubated for 1, 3 or 7 days and supernatants were collected. Subsequently, Tet-Off BMSCs were washed with PBS before cell lysates were prepared in 150 μl Complete Lysis-M EDTA-free buffer containing protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA). Absorbance was read at 450 nm and BMP-2 protein expression was calculated using standard curves.
2.14. Statistical analysis
All data are expressed as mean ± SD. Radiographic bone healing was analysed using one-way analysis of variance (ANOVA). The 3D μCT bone morphometric parameters percentage bone volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp) were analysed using one-way ANOVA. Unpaired Student’s t-test and one-way ANOVA were employed for BMSC proliferation and BMP-2 expression analyses. In vitro experiments (n = 3–4) were performed twice independently. Tukey’s post hoc test was used to test for significant effects. The significance level was set at α = 0.05 and GraphPad Prism software v 5.0 (GraphPad Software, La Jolla, CA, USA) was used for all analyses.
3. Results
3.1. BMSC retention on ADM in vitro
We first determined the optimal dose for soak loading Tet-Off-EV BMSCs on pre-cut ADM discs in vitro. A dose range of 5.0 × 104–1.0 × 106 cells/ADM was used in a total loading volume of 3.8 μl (100% volume of the 4.0 × 0.3 mm ADM disc). Due to the physical limitations of the very thin and dense matrix, loading of Tet-Off-EV BMSCs at doses exceeding 1.0 × 105/ADM resulted in a significant amount of floating, non-adherent cells once the medium was added to the wells (data not shown). Therefore, in our system, the optimal BMSC loading density was defined as 1.0 × 105 cells/ADM. At that dose, the GFP-positive Tet-Off-EV BMSCs completely adhered to the centre of the dermal side of the ADM disc within 1 h, exhibiting predominantly rounded cell morphology (Figure 1A). The BMSCs started to spread out at 3–6 h (Figure 1B, C) and reached approximately 70% confluence by 24 h, with the typical fibroblast-like cell morphology (Figure 1D). Over the course of 3 days, the Tet-Off-EV BMSCs continued to proliferate (Figure 1E) and covered the entire ADM surface area by 7 days (Figure 1F). This suggested that the ADM allows for the soak-loaded Tet-Off BMSCs to adhere and proliferate in a relevant time frame without apparent cell loss.
Figure 1.

In vitro BMSC adherence, proliferation and retention on ADM. (A–F) Representative fluorescence micrographs of Tet-Off-EV BMSCs soak-loaded on ADM (dermal side) at 1.0 × 105 cells/matrix at 1, 3, 6, 24, 72 and 168 h: magnification ×4, bar = 500 μm; ×20, bar = 100 μm; green, GFP-positive BMSCs; arrows, ADM margins; n = 3/group
3.2. Radiographic and μCT analysis
Using the established critical-sized mouse calvarial defect, we next investigated the regenerative capacity of Tet-Off-EV or Tet-Off-SDF-1β BMSC-soak-loaded ADM constructs, with or without additional BMP-2 co-delivery. Quantitative analysis of the newly formed bone within the craniectomy defect revealed limited bone formation in the sham surgical and vehicle control-loaded ADM groups (Figure 2). A noticeable 1.6-fold increase in BMP-2-induced bone formation was observed relative to controls (Figure 2), which was significantly higher compared to defects treated with Tet-Off-EV (ap < 0.05) or Tet-Off-SDF-1β BMSCs (cp < 0.0001). Both BMSC therapy groups showed only limited healing, comparable to controls (Figure 2). Co-delivery of Tet-Off-EV BMSCs + BMP-2 did not result in increased bone formation relative to Tet-Off-EV BMSCs alone (Figure 2). In contrast, BMP-2 co-therapy significantly enhanced bone healing (#p < 0.05) in the Tet-Off-SDF-1β BMSC-treated group compared to Tet-Off-SDF-1β BMSCs alone (Figure 2). Importantly, high levels of SDF-1β in Tet-Off-SDF-1β BMSCs co-delivered with BMP-2 increased bone healing 1.4-fold relative to Tet-Off-EV BMSCs + BMP-2, with a strong trend towards significance (p = 0.056; Figure 2).
Figure 2.
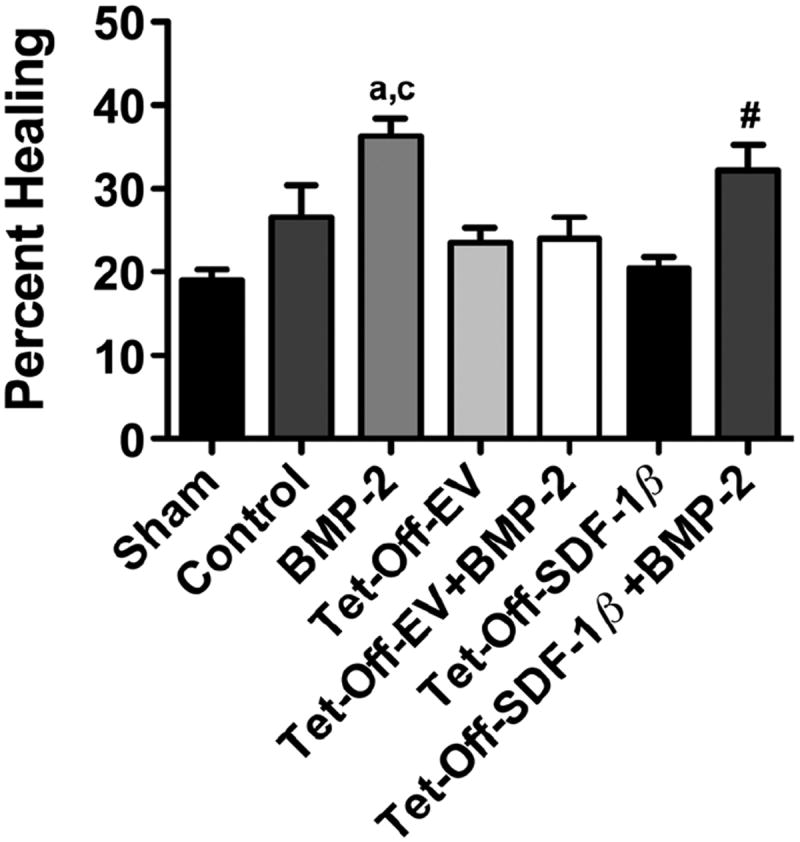
Radiographic analysis of bone healing within the craniectomy defects at 4 weeks post-transplantation. The ROI was defined as a 5.0 mm circle; the groups were: sham, vehicle control, BMP-2, Tet-Off-EV BMSCs, Tet-Off-EV BMSCs + BMP-2, Tet-Off-SDF-1β BMSCs or Tet-Off-SDF-1β BMSCs + BMP-2; ap < 0.05; cp < 0.0001 vs Tet-Off-EV or Tet-Off-SDF-1β BMSCs; #p < 0.05 vs Tet-Off-SDF-1β BMSCs; n = 10 animals/group
We next employed ex vivo μCT to further characterize bone formation in more detail. Representative 3D reconstructions at 4 weeks post-surgery are depicted in Figure 3. Untreated sham surgery defects showed no apparent bone formation. Significantly increased percentage bone volume (BV/TV) and trabecular number (Tb.N) were observed in the BMP-2-treated group compared to vehicle controls (**p < 0.01; Figure 4A, B). Furthermore, BMP-2 therapy significantly enhanced BV/TV and Tb.N relative to ADM soak-loaded with Tet-Off-EV (bp < 0.01, cp < 0.0001) or Tet-Off-SDF-1β BMSCs (cp < 0.0001). No differences in trabecular thickness (Tb.Th) were observed with BMP-2 treatment (Figure 4C). In contrast, BMP-2 therapy significantly reduced trabecular separation (Tb.Sp) compared to vehicle controls (***p < 0.0001) and Tet-Off-EV or Tet-Off-SDF-1β BMSCs (cp < 0.0001; Figure 4D). As seen with the radiographic analysis, both Tet-Off-EV and Tet-Off-SDF-1β BMSC therapy groups showed only limited bone formation, comparable to controls (Figure 4A–D). Co-delivery of Tet-Off-EV BMSCs + BMP-2 markedly increased BV/TV and Tb.N and decreased Tb.Sp (Figure 4A, B, D). Tb.Th was not affected by Tet-Off-EV BMSCs + BMP-2 co-therapy (Figure 4C). Tet-Off-SDF-1β BMSCs + BMP-2 co-delivery significantly enhanced BV/TV and Tb.N ($p < 0.05) compared to Tet-Off-SDF-1β BMSCs alone (Figure 4A, B). BMP-2 co-delivery also significantly reduced Tb.Sp compared to Tet-Off-SDF-1β BMSCs alone (%p < 0.0001; Figure 4D). No differences in Tb.Th were observed with Tet-Off-SDF- 1β BMSCs + BMP-2 co-therapy (Figure 4C). Notably, high levels of SDF-1β in Tet-Off-SDF-1β BMSCs co-delivered with BMP-2 increased BV/TV and Tb.N by 1.6- and 1.5-fold, respectively, compared to Tet-Off-EV BMSCs + BMP-2 (Figure 4A, B).
Figure 3.

Representative 3D reconstructions of critical-sized mouse calvarial defect μCT images at 4 weeks post-transplantation; transverse view of entire specimen with isolated 3D ROI underneath. (A) Schematic of ADM soak-loading procedure. (B) Untreated sham surgery. (C) Vehicle control. (D) BMP-2. (E) Tet-Off-EV BMSCs. (F) Tet-Off-EV BMSCs + BMP-2. (G) Tet-Off-SDF-1β BMSCs. (H) Tet-Off-SDF-1β BMSCs + BMP-2; n = 10 animals/group
Figure 4.

3D morphometric parameters of bone within the craniectomy defects at 4 weeks post-transplantation. The ROI was defined as follows: 5.0 mm circle, 50 cuts = 0.65 mm, total volume = 12.8 mm3. (A) BV/TV. (B) Tb.N. (C) Tb.Th. (D) Tb.Sp. The groups were: sham, vehicle control, BMP-2, Tet-Off-EV BMSCs, Tet-Off-EV BMSCs + BMP-2, Tet-Off-SDF-1β BMSCs or Tet-Off-SDF-1β BMSCs + BMP- 2; **p < 0.01, ***p < 0.0001 vs vehicle controls; bp < 0.01; cp < 0.0001 vs Tet-Off-EV or Tet-Off-SDF-1β BMSCs; #p < 0.05; $p < 0.01 vs Tet-Off-SDF-1β BMSCs; n = 10 animals/group
3.3. Histology
Qualitative histological analysis showed untreated sham surgery defects to have very limited bone formation emerging from the defect margins (see supporting information, Figure S2A1.1), comprised mostly of collagen type I-rich, strongly birefringent, osteoid, regenerated woven bone (W; see supporting information, Figure S2A1.2, 3) and lamellar bone (L) displaying weak birefringence (see supporting information, Figure S2A1.2, 3). Of note, the green-stained areas (PSR) of lamellar bone suggest tightly packed and highly aligned collagen molecules with polarization colours of longer wavelengths (Dayan et al., 1989). A thin fibrous tissue membrane comprised mostly of collagen type III, with no visible bone, covered the width of the defects (see supporting information, Figure S2A2/3.1–3). For vehicle controls, the ADM spanned the entire width of the defect, invested in cell-rich fibrovascular tissue resembling marrow, and limited bone originating from the defect margins (see supporting information, Figure S2B). The confined new bone tissue mostly comprised collagen type I-rich mineralized and non-mineralized osteoid, woven bone undergoing remodelling and lamellar bone (Figure 5A1-3.1-3). BMP-2 treated defects revealed robust bone formation. The internal cortical plates approximating the dura mater were frequently re-established, bridging the defect width. In contrast, the external cortical plates remained largely incomplete (Figure 5B1/3.1–3). Of note, frequent clusters of chondrocytes (ñ) in close proximity to newly formed bone suggested endochondral ossification (Figure 5B3.1). Sites receiving Tet-Off-EV or Tet-Off-SDF- 1β BMSCs showed limited bone emerging from the defect margins, comparable to controls (Figure 5C, E). BMSCs were not readily identifiable using standard histological stains (Figure 5C,E1-3.1-3). Tet-Off-EV BMSCs + BMP-2 co-treated defects revealed spatially restricted bone originating from the defect margins, comparable to Tet-Off-EV BMSCs alone (Figure 5D1.1-3). Both the internal and external cortical plates remained incomplete (Figure 5D2/3.1-3). Sites receiving Tet-Off-SDF-1β BMSCs + BMP-2 showed robust bone formation, comparable to BMP-2 therapy alone. The internal cortical plates were largely re-established; however, the external cortical plates remained incomplete (Figure 5F1/3.1-3). Again, clusters of chondrocytes (ñ) approximating the regenerated bone were observed (Figure 5F3.1).
Figure 5.

Representative H&E-, MTC- and PSR-stained photomicrographs of the critical-sized mouse calvarial defects at 4 weeks posttransplantation. Overview of three ROIs highlighting the defect margin (A-F1.1-3), the epidermal side of the ADM facing the periosteum (A-F1.2-3) and the dermal side approximating the dura mater (A-F1.3-3). (A) Vehicle control. (B) BMP-2. (C) Tet-Off-EV BMSCs. (D) Tet-Off-EV BMSCs + BMP-2. (E) Tet-Off-SDF-1β BMSCs. (F) Tet-Off-SDF-1β BMSCs + BMP-2; magnification ×20, bar = 100 μm; A-F1-3.1, H&E; A-GF1-3.2, MTC; A-F1-3.3, PSR linearly polarized light; L, lamellar bone; W, woven bone; n = 3 animals/group
3.4. GFP signal intensity and immunohistochemistry
We also assessed the GFP signal intensities in Tet-Off BMSC-loaded ADM constructs after 4 weeks in the unprocessed explanted calvarial specimens. Both Tet-Off-EV and Tet-Off-SDF-1β BMSC therapy groups showed only weak GFP signal, comparable between groups, suggesting limited retention of the soak-loaded BMSCs on the ADM in vivo (Figure 6B). This was in stark contrast to our previous in vitro findings (Figure 1). Co-delivery of Tet-Off-EV BMSCs + BMP-2 increased the GFP signal intensity 1.7-fold relative to Tet-Off-EV BMSCs alone, approaching significance (p = 0.079; Figure 6B). A significant 2.2-fold enhancement of the GFP signal intensity was observed for Tet-Off-SDF-1β BMSCs + BMP-2 co-therapy compared to Tet-Off-SDF-1β BMSCs alone (*p < 0.05; Figure 6B). The notable variability within groups stemmed from some specimens displaying medium to high GFP intensity, while others showed no signal at all. Qualitative immunohistochemistry confirmed these findings (Figure 6A).
Figure 6.
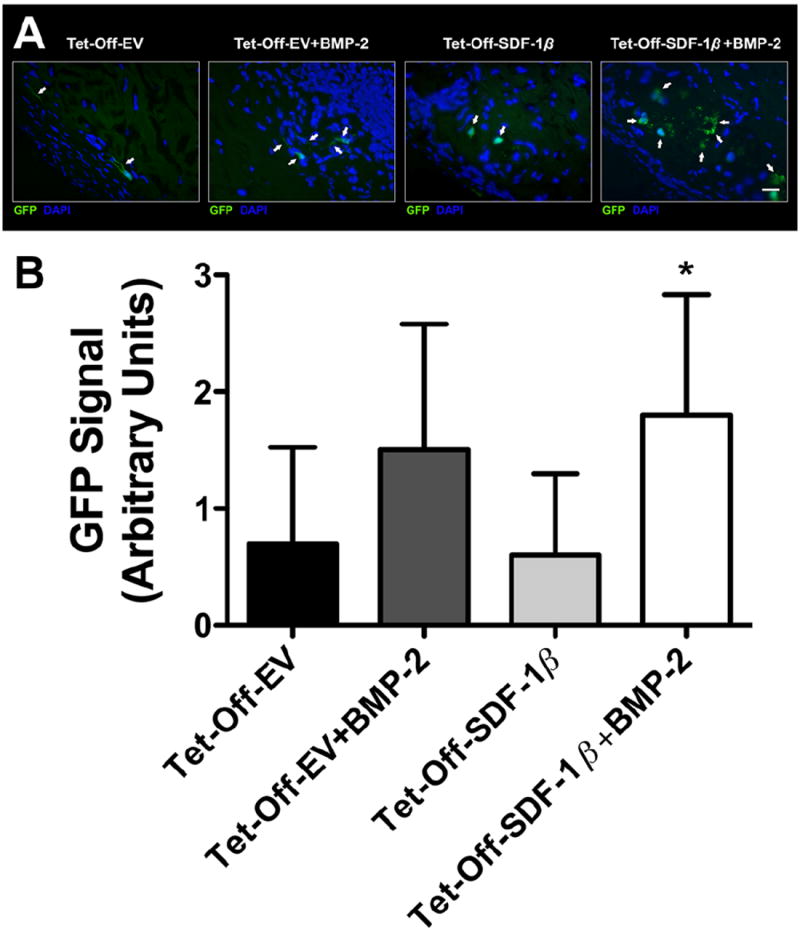
GFP signal intensity and immunohistochemistry of GFP-positive BMSCs invested in the ADM within the criticalsized mouse calvarial defects at 4 weeks post-transplantation. (A) Representative fluorescence micrographs; the groups were Tet-Off-EV BMSCs, Tet-Off-EV BMSCs + BMP-2, Tet-Off-SDF-1β BMSCs, or Tet-Off-SDF-1β BMSCs + BMP-2; magnification ×40, bar = 40 μm; green, GFP; blue, DAPI; arrows, GFP-positive BMSCs; n = 3 animals/group. (B) Quantitative analysis of GFP signal intensities, using 0 (no GFP) to 3 (high GFP) arbitrary units scoring; *p < 0.05 vs Tet-Off-SDF-1β BMSCs; n = 10 animals/group
3.5. Cell proliferation
To further investigate this phenomenon, cultures of Tet-Off-EV and Tet-Off-SDF-1β BMSCs were supplemented with 100 ng/ml BMP-2 and cell proliferation was quantified at 1, 3 and 7 days in vitro and compared to Tet-Off BMSCs alone. In our system, cell proliferation appeared to peak at 3 days, likely a result of BMSCs reaching confluence (Figure 7). Tet-Off-EV and Tet-Off- SDF-1β BMSCs showed comparable proliferation at 1, 3 and 7 days, confirming our previous data (Herberg et al., 2013b). Co-delivery of Tet-Off-EV BMSCs + BMP-2 significantly increased cell proliferation over the course of 7 days relative to Tet-Off-EV BMSCs alone (*p < 0.05, **p < 0.01, ***p < 0.0001; Figure 7). Interestingly, co-delivery of Tet-Off-SDF-1β BMSCs + BMP-2 had no apparent effect on cell proliferation compared to Tet-Off- SDF-1β BMSCs alone (Figure 7).
Figure 7.
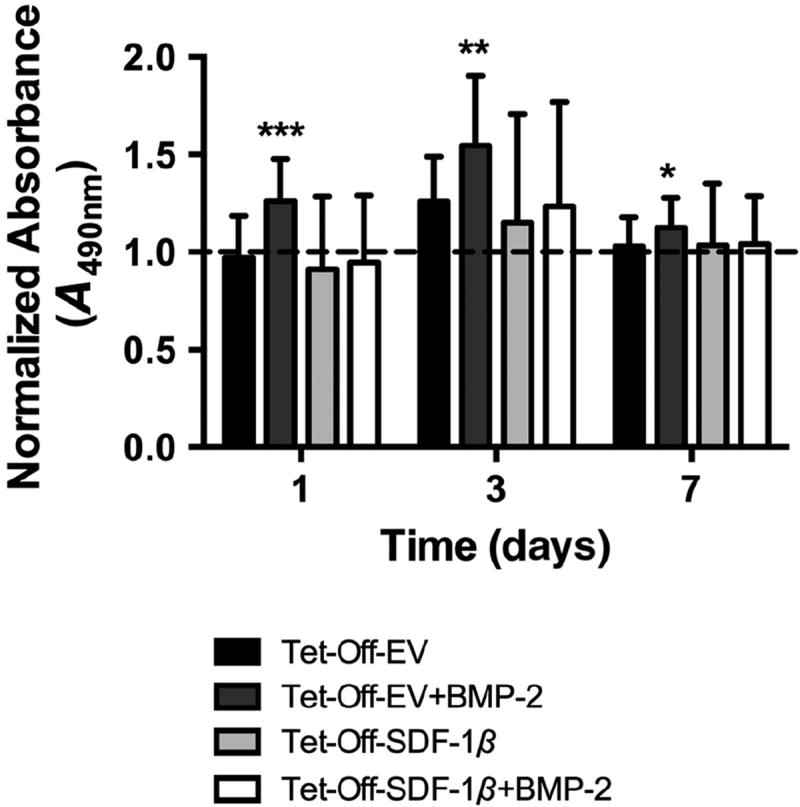
In vitro BMSC proliferation. Standard MTS colorimetric assay (normalized A490nm). The groups were Tet-Off-EV BMSCs, Tet-Off-EV BMSCs + BMP-2, Tet-Off-SDF-1β BMSCs or Tet-Off- SDF-1β BMSCs + BMP-2 (BMP-2 at 100 ng/ml); *p < 0.05, **p < 0.01, ***p < 0.0001 vs Tet-Off-EV BMSCs; n = 3/group
3.6. BMP-2 protein levels
The unexpected finding that co-delivery of BMP-2 and Tet-Off BMSCs reduced the osteoinductive capacity of BMP-2 stand-alone therapy at the same dose prompted the investigation of BMP-2 protein levels at 1, 3 and 7 days in vitro. Tet-Off-EV and Tet-Off-SDF-1β BMSCs were supplemented with 100 ng/ml BMP-2 and protein levels in the cell culture media were quantified and compared to medium controls. After 1 day, the addition of BMP-2 to Tet-Off-EV BMSCs significantly decreased the BMP-2 levels in the medium by 28.8% (**p < 0.01; Figure 8A), while supplementing BMP-2 toTet-Off-SDF-1β BMSCs significantly reduced the detectable BMP-2 levels by 16.6% (*p < 0.05; Figure 8A). This effect, however, appeared to subside by 3 and 7 days, respectively. In fact, a slight increase in the BMP-2 protein levels compared to medium controls was observed in both Tet-Off-EV and Tet-Off- SDF-1β BMSCs, albeit not significant (Figure 8A). The trends seen in cell culture media were in agreement with analysis of cell-associated BMP-2 levels (intracellular and/or bound to the extracellular surface). Cell lysates of Tet-Off-EV BMSCs in presence of BMP-2 showed 2.5-fold increased BMP-2 levels compared to BMSCs alone, while Tet-Off-SDF-1β BMSCs revealed a 2.7-fold increase relative to BMSCs alone after 1 day (*p < 0.05; Figure 8B). After 3 days, the addition of BMP-2 to Tet-Off-EV BMSCs had no enhancing effect on the detectable BMP-2 levels compared to Tet-Off-EV BMSCs alone (Figure 8B). This phenomenon appeared to hold up after 7 days in culture (Figure 8B). In contrast, the addition of BMP-2 to Tet-Off-SDF-1β BMSCs markedly increased the cell-associated BMP-2 levels at 3 and 7 days, with a trend towards significance (p = 0.093; Figure 8B). Collectively, the decrease of BMP-2 protein levels in the cell culture media correlated with an increase in cell-associated BMP-2 after 1 day. With further culture, Tet-Off-SDF-1β BMSCs appeared to respond to the BMP-2 treatment with continuous synthesis and secretion of BMP-2 protein, suggesting positive feedback. This was in agreement with our previous mRNA data (Herberg et al., 2013a).
Figure 8.

In vitro BMP-2 ELISA. Standard Quantikine® ELISA. (A) Normalized cell culture media; the groups were: normal proliferation medium + BMP-2, Tet-Off-EV BMSCs + BMP-2, or Tet-Off-SDF-1β BMSCs + BMP-2 (BMP-2 at 100 ng/ml); *p < 0.05; **p < 0.01 vs medium + BMP-2; n = 3/group. (B) Normalized BMSC lysates; the groups were: Tet-Off-EV BMSCs, Tet-Off-EV BMSCs + BMP-2, Tet-Off-SDF-1β BMSCs or Tet-Off-SDF-1β BMSCs + BMP-2 (BMP-2 at 100 ng/ml); *p < 0.05 vs Tet-Off-EV or Tet-Off-SDF-1β BMSCs; n = 3/group
4. Discussion
The aim of the present study was to investigate the healing of calvarial defects following implantation of Tet- Off-EV or Tet-Off-SDF-1β BMSCs + BMP-2 combination therapies soak-loaded on ADM. We tested the hypothesis that SDF-1β, expressed at high levels in Tet-Off-SDF-1β BMSCs, in combination with BMP-2 synergistically augments bone healing compared to co-delivery of Tet-Off-EV control BMSCs + BMP-2. Our data suggested that benchmark BMP-2 treatment promoted significant bone healing. Unexpectedly, both wild-type-like Tet-Off-EV BMSCs and Tet-Off-SDF-1β BMSCs soak-loaded on ADM failed to adequately promote healing. This was in stark contrast to our previous studies using a direct intramedullary tibial transplantation approach (Herberg et al., 2014a, 2015). Co-delivery of Tet-Off BMSCs + BMP-2 increased bone formation. Notably, healing of calvarial defects was significantly enhanced when Tet-Off- SDF-1β BMSCs were co-delivered with BMP-2, although it did not reach levels seen with standard BMP-2 therapy alone. Quantification of the GFP signal intensity in BMSC-loaded ADM constructs suggested only limited Tet-Off BMSC retention after 4 weeks in vivo, which was slightly increased with BMP-2 co-delivery in both groups. This likely interfered with bone healing and may have contributed to a reduction of bioavailable BMP-2. In vitro cell proliferation studies confirmed that the addition of BMP-2 toTet-Off-EV control BMSCs significantly increased the cell number during the first 24 h. In contrast, we have previously reported that the overexpression of SDF-1β drives BMSC osteogenic differentiation, not proliferation (Herberg et al., 2013a, 2013b). At the same time, both Tet-Off-EV and Tet-Off-SDF-1β BMSCs effectively decreased the detectable BMP-2 in the medium, but increased the cell-associated BMP-2 levels.
The emerging field of bone tissue engineering, with the triad principle of applying combinations of functionally active cells, supporting biomaterial scaffolds and osteoinductive growth factors, is suggested to provide promising treatment strategies to regenerate bone (Amini et al., 2012; Giannoudis et al., 2005). Here we utilized Tet-Off-SDF-1β BMSCs (Herberg et al., 2013a), genetically engineered to overexpress SDF-1β, in combination with BMP-2 as an osteoinductive cue, and delivered as combination therapies on ADM. SDF-1β was chosen over the more abundant α-splice variant, chiefly due to its greater resistance to proteolytic cleavage conferred by its additional four C-terminal amino acids (Davis et al., 2005; De La Luz Sierra et al., 2004), yet comparable general signalling properties, as the N-terminus is mainly responsible for receptor interactions (Crump et al., 1997; Janowski, 2009). This raises the possibility that SDF-1β may be well suited for local applications, particularly in bone injury sites, with increased inflammatory and proteolytic activity. BMP-2 has been shown both in vitro and in vivo to be important for osteogenesis, as it assists in the recruitment and differentiation of mesenchymal stem cells (Wozney, 1989). BMP-2 is simply supplemented to the culture medium in vitro, while sustained delivery of BMP-2 by means of appropriate biomaterials is needed to provide bioactive protein for an extended period of time in vivo (Li et al., 2006). To that end, the FDAapproved ADM delivery scaffold has excellent biocompatibility and possesses native binding affinities for growth factors such as BMP-2 (Cooper et al., 2010a). Our group has reported that ~80% of the BMP-2 applied using an inkjet-based biopatterning approach remained bound to the ADM after 24 h of incubation in standard cell culture medium at 37°C, with subsequent slow release of an additional ~10% over a period of 2 weeks (Cooper et al., 2010a). Clinically, BMP-2 is supplied by soakloading a high-concentration solution onto an absorbable collagen sponge (ACS), followed by air drying for 15 min prior to implantation. The retention of BMP-2 on ACS is generally reported as being rather limited. A recent study showed that only ~20% of the soak-loaded BMP-2 remains bound to the ACS after 6 days in vitro (Yang et al., 2012). Comparable brief retention times have been described in vivo using both ectopic and orthotopic bone formation models (Friess et al., 1999; Geiger et al., 2003). Our preliminary studies employing a low BMP-2 dose of 108.5 ng, which we recently found to significantly promote bone healing in this model when biopatterned on ADM (Herberg et al., 2014b), and a medium BMP-2 dose of 542.5 ng showed that this five-fold increase in dose only resulted in a ~two-fold increase in BV/TV (see supporting information, Figure S1). This suggests that soak-loading BMP-2 on ADM is inferior to biopatterning, and that the release kinetics of soak-loaded BMP-2 from ADM may be similar to ACS. Despite the strong dependency of the BMP-2 release kinetics on the mode of application, the allograft ADM (derived from donor harvested human skin) maintains the original dermal collagen matrix, making it an attractive candidate biomaterial for bone tissue engineering and stem cell therapy applications (Hodgkinson and Bayat, 2011; Kim and Evans, 2012).
The rationale for the use of SDF-1β in combination with the potent growth factor BMP-2 is based on previous studies from our group, suggesting that SDF-1β potentiates BMP-2 signalling in vitro (Herberg et al., 2013a) and in vivo across species (rat vs mouse) and delivery technologies (ACS vs ADM; soak-loaded vs biopatterned) (Herberg et al., 2014b, 2014c). Furthermore, others have reported that the CXCR4–SDF-1 axis acts as an endogenous signalling component necessary for bone formation (Higashino et al., 2011; Hosogane et al., 2010; Ratanavaraporn et al., 2011; Wise et al., 2012; Zhu et al., 2007, 2011). Together, these findings indicate proosteogenic effects of SDF-1 signalling independent of the splice variant engaging with the receptor. Our data obtained using radiographic, μCT and histological analyses confirmed that overexpression of SDF-1β in Tet-Off-SDF- 1β BMSCs [mRNA, ~30-fold; protein, ~5-fold (2060.6 pg/mg vs 390.5 pg/mg)] at 24 h (Herberg et al., 2013a, 2015) synergizes with BMP-2 to augment cell-mediated bone healing. Bone formation levels were higher compared to Tet-Off-EV BMSCs + BMP-2, yet did not reach levels seen with BMP-2 benchmark therapy. In vitro cell culture studies addressing this observation suggest that the addition of BMP-2 to Tet-Off BMSCs significantly increased the cell number during the early phase of culture, which was in agreement with previous reports (Su et al., 2013; Wang et al., 2010). At the same time, this effectively decreased the detectable BMP-2 protein levels in the cell culture medium, while it increased the levels of cellassociated BMP-2. High levels of SDF-1β in Tet-Off-SDF- 1β BMSCs appeared to work in concert with the BMP-2 treatment, causing the cells to continuously secrete BMP-2 protein over the course of 7 days, indicative of positive feedback consistent with our previous mRNA data (Herberg et al., 2013a). Collectively, these observations suggest that BMP-2 co-delivered with BMSCs may act in different ways.
First, it promotes cell proliferation while at the same time effectively reducing the bioavailable BMP-2 dose on the BMSC-loaded ADM constructs. This may possibly occur via rapid uptake by the co-transplanted BMSCs, limiting soluble BMP-2 available for chemotactic signalling and effectively reducing the local concentration of BMP-2. This initial drop in the therapeutic BMP-2 dose relative to BMP-2 alone may cause a delay in BMP-2 osteoinduction, reflected by a decrease in bone healing at 4 weeks. Second, in the presence of excess SDF-1β, osteogenic differentiation of endogenous and exogenous BMSCs is pushed rather than by proliferation. Additionally, it primes the cells for osteogenic differentiation, as previously noted (Herberg et al., 2013a, 2014c) and evidenced by positive feedback stimulation of BMP-2 protein expression. In wild-type-like Tet-Off-EV BMSCs this results in only marginally increased bone formation relative to BMSCs alone. In contrast, in Tet-Off-SDF-1β BMSCs expressing high levels of SDF-1β protein and consequently engaging the CXCR4 signalling axis, this leads to overall amplification of the osteoinductive properties of BMP-2, thereby confirming our previous in vitro (Herberg et al., 2013a) and in vivo findings (Herberg et al., 2014b, 2014c).
In conjunction with the potential reduction of the bioavailable BMP-2, we also observed limited retention of GFP-positive Tet-Off BMSCs in all groups after 4 weeks in vivo, suggesting that the transplanted BMSCs were largely removed from the ADM scaffold within the defect site over the course of the 4 week healing interval. This is in agreement with a growing body of evidence suggesting that very few regenerative cells may actually survive following transplantation (Dupont et al., 2010; Grayson et al., 2015). Various factors have been shown to contribute to cell loss in the delivery site, such as increased levels of proinflammatory cytokines/chemokines and cellular stress generating reactive oxygen species, and decreased vascularity and oxygen levels, potentially leading to cell death via activating apoptotic cascades (Rodrigues et al., 2010). Although BMP-2 co-therapy increased the number of GFP-positive Tet-Off BMSCs, with a bias toward greater enhancement in the presence of high levels of SDF-1β, probably due to synergistically stimulating cell proliferation, it was not sufficient to retain relevant numbers of therapeutic BMSCs in the defect site. In light of the benchmark BMP-2 therapy inducing robust bone formation, it appears likely that at 4 weeks these cellular effects were partially responsible for the observed reduction in therapeutic efficacy seen with the combined stem cell and growth factor therapies relative to the conventional BMP- 2 treatment. Furthermore, it raises the possibility that the ADM scaffold, while being well suited for therapeutic growth factor delivery, may be limited in capacity to serve as a BMSC carrier under these conditions.
Cell-seeding densities in comparable studies range from 1.5 × 105 to 2.0 × 106 cells/defect (Mohajeri et al., 2010; Park et al., 2013; Zou et al., 2011). Due to the physical limitations of the very thin and dense ADM scaffold, the BMSC loading density in our model was restricted to 1.0 × 105 cells/ADM. At that dose the ADM allows for the soak-loaded BMSCs to rapidly adhere and proliferate without apparent cell loss. We can only speculate as to why we observed discrepancies between in vitro and in vivo retention despite using the identical loading protocol. There are several differences between those two scenarios, which are difficult to simulate in a cell culture dish. After the initial 15 min incubation, the BMSC-loaded ADM constructs were simply overlaid with proliferation medium in vitro, with no further stress applied. Upon implantation of the BMSC-loaded ADM constructs in the calvarial defects, however, cells are facing a complex and less ideal environment in vivo. In addition to the aforementioned processes prohibiting long-term cell survival in the defect site, limited nutrient supply (dura mater side) and mechanical pressure upon surgical suture closure (periosteum side) may further interfere with the early phase of cell adherence and subsequent proliferation.
To that end, the concept of predifferentiation of the cells into the osteogenic lineage before in vivo implantation has been shown to be beneficial for enhancing osteogenesis in comparison with non-predifferentiated cells (Song et al., 2009; Thimm et al., 2011). However, the ideal osteogenic preculture time for cells in bone regeneration studies is controversial. Short-term osteogenic induction is likely insufficient for stimulating significant cell differentiation, whereas long-term induction can lead to apoptosis (Siebers et al., 2006). From a translational perspective, one-step surgical techniques, in which freshly isolated cells (without expansion) are used directly within the operating room, are recommended for clinical applications of cell-based bone tissue-engineering strategies (Farre-Guasch et al., 2013). This suggests that a balance is needed between the regenerative efficacy as a result of relatively longer preculture time, and the benefits that patients gain from a one-step surgical procedure. Future studies aim to investigate the effects of preculturing Tet-Off BMSCs upon soak-loading on ADM in both normal proliferation and osteogenic induction medium to better assess the regenerative capacity of BMSCs + BMP-2 co-delivery strategies.
5. Conclusion
In conclusion, our results show that SDF-1β, overexpressed in genetically engineered BMSCs, synergizes with BMP-2 to augment cell-mediated healing of critical-sized mouse calvarial defects. The discrepancy in bone formation observed between this combination therapy and conventional BMP-2 treatment at the same dose may stem from limited adherence and/or survival of transplanted BMSCs, or a metabolic reduction of bioavailable BMP-2 on the ADM scaffold in the presence of BMSCs. This relationship necessitates further investigation to determine the limitations and clinical utility of combination therapies following bone tissue-engineering principles.
Supplementary Material
Figure S1. Representative 3D reconstructions of critical-sized mouse calvarial defect μCT images at 4 weeks post-transplantation
Figure S2. Representative H&E-, MTC- and PSR-stained photomicrographs of critical-sized mouse calvarial defects at 4 weeks post-transplantation
Acknowledgments
The authors appreciate the technical support of Asma Daoudi, Georgia Regents University μCT Core Facility, and Donna Kumiski and Penny Roon, Georgia Regents University Histology Core Facility. This study was supported by the Musculoskeletal Transplant Foundation (grant to J.C.), the Institute for Regenerative and Reparative Medicine at Georgia Regents University (to J.C.) and the National Institute on Aging (Grant No. NIHNIA PO1-AG036675, to W.D.H.), and supported in part by the Department of Veterans Affairs (VA Merit Award No. 104462, to W.D.H.). The contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
This study was performed at Georgia Regents University.
Supporting information
The following supporting information may be found in the online version of this article:
Conflict of interest
The authors declare no conflicts of interest.
References
- Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM) J Histochem Cytochem. 2001;49:1565–1572. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10–27. [PubMed] [Google Scholar]
- Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Why are MSCs therapeutic? Newdata: newinsight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Cooper GM, Miller ED, Decesare GE, et al. Inkjet-based biopatterning of bone morphogenetic protein-2 to spatially control calvarial bone formation. Tissue Eng A. 2010a;16:1749–1759. doi: 10.1089/ten.tea.2009.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GM, Mooney MP, Gosain AK, et al. Testing the critical size in calvarial bone defects: revisiting the concept of a critical-size defect. Plast Reconstr Surg. 2010b;125:1685–1692. doi: 10.1097/PRS.0b013e3181cb63a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump MP, Gong JH, Loetscher P, et al. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DA, Singer KE, De La Luz SM, et al. Identification of carboxypeptidase N as an enzyme responsible for Cterminal cleavage of stromal cell-derived factor-1α in the circulation. Blood. 2005;105:4561–4568. doi: 10.1182/blood-2004-12-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan D, Hiss Y, Hirshberg A, et al. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry. 1989;93:27–29. doi: 10.1007/BF00266843. [DOI] [PubMed] [Google Scholar]
- De La Luz SM, Yang F, Narazaki M, et al. Differential processing of stromalderived factor-1α and stromal-derived factor-1β explains functional diversity. Blood. 2004;103:2452–2459. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- Dupont KM, Sharma K, Stevens HY, et al. Human stem cell delivery for treatment of large segmental bone defects. Proc Natl Acad Sci U S A. 2010;107:3305–3310. doi: 10.1073/pnas.0905444107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre-Guasch E, Prins HJ, Overman JR, et al. Human maxillary sinus floor elevation as a model for bone regeneration enabling the application of one-step surgical procedures. Tissue Eng B Rev. 2013;19:69–82. doi: 10.1089/ten.TEB.2012.0404. [DOI] [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, et al. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Friess W, Uludag H, Foskett S, et al. Characterization of absorbable collagen sponges as rhBMP-2 carriers. Int J Ppharmaceut. 1999;187:91–99. doi: 10.1016/s0378-5173(99)00174-x. [DOI] [PubMed] [Google Scholar]
- Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(suppl 3):S20–27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of 450 patients. J Bone Joint Surg Am. 2002;84A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Grabowski G, Cornett CA. Bone graft and bone graft substitutes in spine surgery: current concepts and controversies. J Am Acad Orthop Surg. 2013;21:51–60. doi: 10.5435/JAAOS-21-01-51. [DOI] [PubMed] [Google Scholar]
- Granero-Molto F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27:1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Bunnell BA, Martin E, et al. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol. 2015;11(3):140–150. doi: 10.1038/nrendo.2014.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesen M, Berman MA, Benson JD, et al. Cloning of the mouse fusin gene, homologue to a human HIV-1 co-factor. J Immunol. 1996;157:5455–5460. [PubMed] [Google Scholar]
- Herberg S, Fulzele S, Yang N, et al. Stromal cell-derived factor-1β potentiates bone morphogenetic protein-2-stimulated osteoinduction of genetically engineered bone marrow-derived mesenchymal stem cells in vitro. Tissue Eng A. 2013a;19:1–13. doi: 10.1089/ten.tea.2012.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg S, Kondrikova G, Hussein KA, et al. Mesenchymal stem cell expression of stromal cell-derived factor-1β augments bone formation in a model of local regenerative therapy. J Orthop Res. 2015;33:174–184. doi: 10.1002/jor.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg S, Kondrikova G, Hussein KA, et al. Total body irradiation is permissive for mesenchymal stem cell-mediated new bone formation following local transplantation. Tissue Eng A. 2014a;20:3212–3227. doi: 10.1089/ten.tea.2013.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg S, Kondrikova G, Periyasamy-Thandavan S, et al. Inkjet-based biopatterning of SDF-1β augments BMP- 2-induced repair of critical size calvarial bone defects in mice. Bone. 2014b;67:95–103. doi: 10.1016/j.bone.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg S, Shi X, Johnson MH, et al. Stromal cell-derived factor-1β mediates cell survival through enhancing autophagy in bone marrow-derived mesenchymal stem cells. PLoS One. 2013b;8:e58207. doi: 10.1371/journal.pone.0058207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg S, Susin C, Pelaez M, et al. Low-dose bone morphogenetic protein-2/stromal cell-derived factor-1β cotherapy induces bone regeneration in critical-size rat calvarial defects. Tissue Eng A. 2014c;20:1444–1453. doi: 10.1089/ten.tea.2013.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashino K, Viggeswarapu M, Bargouti M, et al. Stromal cell-derived factor-1 potentiates bone morphogenetic protein-2 induced bone formation. Tissue Eng A. 2011;17:523–530. doi: 10.1089/ten.tea.2010.0168. [DOI] [PubMed] [Google Scholar]
- Hodgkinson T, Bayat A. Dermal substitute-assisted healing: enhancing stem cell therapy with novel biomaterial design. Arch Dermatol Res. 2011;303:301–315. doi: 10.1007/s00403-011-1131-2. [DOI] [PubMed] [Google Scholar]
- Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990;1:60–68. doi: 10.1097/00001665-199001000-00011. [DOI] [PubMed] [Google Scholar]
- Hosogane N, Huang Z, Rawlins BA, et al. Stromal derived factor-1 regulates bone morphogenetic protein 2-induced osteogenic differentiation of primary mesenchymal stem cells. Int J Biochem Cell Biol. 2010;42:1132–1141. doi: 10.1016/j.biocel.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki P, Schmidmaier G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury. 2011;42(suppl 2):S77–81. doi: 10.1016/j.injury.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Janowski M. Functional diversity of SDF-1 splicing variants. Cell Adhes Migrat. 2009;3:243–249. doi: 10.4161/cam.3.3.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Yang X. Mesenchymal stem cells and bone regeneration: current status. Injury. 2011;42:562–568. doi: 10.1016/j.injury.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Evans GR. Applications of biomaterials in plastic surgery. Clin Plast Surg. 2012;39:359–376. doi: 10.1016/j.cps.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheumat. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- Li C, Vepari C, Jin HJ, et al. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Ma J, Both SK, Yang F, et al. Concise review: cell-based strategies in bone tissue engineering and regenerative medicine. Stem Cells Transl Med. 2014;3:98–107. doi: 10.5966/sctm.2013-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Curtis L, Jalili A, Deiteren K, et al. Carboxypeptidase M expressed by human bone marrow cells cleaves the C-terminal lysine of stromal cell-derived factor-1α: another player in hematopoietic stem/progenitor cell mobilization? Stem Cells. 2008;26:1211–1220. doi: 10.1634/stemcells.2007-0725. [DOI] [PubMed] [Google Scholar]
- Mohajeri S, Hosseinkhani H, Ebrahimi NG, et al. Proliferation and differentiation of mesenchymal stem cell on collagen sponge reinforced with polypropylene/polyethylene terephthalate blend fibers. Tissue Eng A. 2010;16:3821–3830. doi: 10.1089/ten.TEA.2009.0520. [DOI] [PubMed] [Google Scholar]
- Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng B Rev. 2008;14:179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci U S A. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuru S, Tamai K, Yamazaki T, et al. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–234. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- Park SY, Kim KH, Shin SY, et al. Dual delivery of rhPDGF-BB and bone marrow mesenchymal stromal cells expressing the BMP2 gene enhance bone formation in a critical-sized defect model. Tissue Eng A. 2013;19:2495–2505. doi: 10.1089/ten.tea.2012.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratanavaraporn J, Furuya H, Kohara H, et al. Synergistic effects of the dual release of stromal cell-derived factor-1 and bone morphogenetic protein-2 from hydrogels on bone regeneration. Biomaterials. 2011;32:2797–2811. doi: 10.1016/j.biomaterials.2010.12.052. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Therap. 2010;1:32. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial non-unions. Clin Orthop Rel Res. 1986;205:299–308. [PubMed] [Google Scholar]
- Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating non-unions? Injury. 2007;38(supp0l 1):S75–80. doi: 10.1016/j.injury.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Siebers MC, Walboomers XF, Leeuwenburgh SC, et al. The influence of the crystallinity of electrostatic spray depositionderived coatings on osteoblast-like cell behavior in vitro. J Biomed Mater Res A. 2006;78:258–267. doi: 10.1002/jbm.a.30700. [DOI] [PubMed] [Google Scholar]
- Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- Smith DM, Cray JJ, Jr, Weiss LE, et al. Precise control of osteogenesis for craniofacial defect repair: the role of direct osteoprogenitor contact in BMP-2-based bioprinting. Ann Plast Surg. 2012;69:485–488. doi: 10.1097/SAP.0b013e31824cfe64. [DOI] [PubMed] [Google Scholar]
- Song IH, Caplan AI, Dennis JE. In vitro dexamethasone pretreatment enhances bone formation of human mesenchymal stem cells in vivo. J Orthop Res. 2009;27:916–921. doi: 10.1002/jor.20838. [DOI] [PubMed] [Google Scholar]
- Steinert AF, Rackwitz L, Gilbert F, et al. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1:237–247. doi: 10.5966/sctm.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Xu H, Sun J, et al. Dual delivery of BMP-2 and bFGF from a new nanocomposite scaffold, loaded with vascular stents for large-size mandibular defect regeneration. Int J Mol Sci. 2013;14:12714–12728. doi: 10.3390/ijms140612714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm BW, Wust S, Hofmann S, et al. Initial cell pre-cultivation can maximize ECM mineralization by human mesenchymal stem cells on silk fibroin scaffolds. Acta Biomater. 2011;7:2218–2228. doi: 10.1016/j.actbio.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Wang L, Huang Y, Pan K, et al. Osteogenic responses to different concentrations/ratios of BMP-2 and bFGF in bone formation. Ann Biomed Eng. 2010;38:77–87. doi: 10.1007/s10439-009-9841-8. [DOI] [PubMed] [Google Scholar]
- Wise JK, Sumner DR, Virdi AS. Modulation of stromal cell-derived factor-1/CXC chemokine receptor 4 axis enhances rhBMP-2-induced ectopic bone formation. Tissue Eng A. 2012;18:860–869. doi: 10.1089/ten.tea.2011.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney JM. Bone morphogenetic proteins. Progr Growth Factor Res. 1989;1:267–280. doi: 10.1016/0955-2235(89)90015-x. [DOI] [PubMed] [Google Scholar]
- Yang HS, La WG, Cho YM, et al. Comparison between heparin-conjugated fibrin and collagen sponge as bone morphogenetic protein-2 carriers for bone regeneration. Exp Mol Med. 2012;44:350–355. doi: 10.3858/emm.2012.44.5.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ou G, Hamrick M, et al. Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J Bone Miner Res. 2008a;23:1118–1128. doi: 10.1359/JBMR.080304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yang N, Shi XM. Regulation of mesenchymal stem cell osteogenic differentiation by glucocorticoid-induced leucine zipper (GILZ) J Biol Chem. 2008b;283:4723–4729. doi: 10.1074/jbc.M704147200. [DOI] [PubMed] [Google Scholar]
- Zhu W, Boachie-Adjei O, Rawlins BA, et al. A novel regulatory role for stromalderived factor-1 signaling in bone morphogenic protein-2 osteogenic differentiation of mesenchymal C2C12 cells. J Biol Chem. 2007;282:18676–18685. doi: 10.1074/jbc.M610232200. [DOI] [PubMed] [Google Scholar]
- Zhu W, Liang G, Huang Z, et al. Conditional inactivation of the CXCR4 receptor in osteoprecursors reduces postnatal bone formation due to impaired osteoblast development. J Biol Chem. 2011;286:26794–26805. doi: 10.1074/jbc.M111.250985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Zou D, Zhang Z, Ye D, et al. Repair of critical-sized rat calvarial defects using genetically engineered bone marrow-derived mesenchymal stem cells overexpressing hypoxia-inducible factor-1α. Stem Cells. 2011;29:1380–1390. doi: 10.1002/stem.693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative 3D reconstructions of critical-sized mouse calvarial defect μCT images at 4 weeks post-transplantation
Figure S2. Representative H&E-, MTC- and PSR-stained photomicrographs of critical-sized mouse calvarial defects at 4 weeks post-transplantation


