Abstract
Context
Patients with advanced illness are prescribed multiple medications in the last year of life, intensifying the risk of negative consequences related to polypharmacy.
Objectives
To describe the medication burden of patients near the end of life and identify potential areas for improvement in clinician prescribing practices.
Methods
This was a pre-specified secondary analysis of data from a prospective trial. Eligible participants were adults with less than 12 months estimated prognosis taking a statin medication for primary prevention of cardiovascular disease. Participants were enrolled from 15 sites, randomized to continue or discontinue statin medications, and followed for up to a year. Concomitant medications were recorded at least monthly from study enrollment through death. Prescribed medications were categorized by class and subclass. Descriptive statistics were calculated.
Results
On average, participants (N=244) were 74.3 years old (SD 11.5) and lived 264 days (SD 128); 47.5% of the patients had a primary diagnosis of malignant tumor. This population was exposed to medications across 51 classes, 192 subclasses and 423 individual medications. Patients took an average of 11.5 (SD 5) medications at the time of enrollment and 10.7 (SD 5) medications at death or study termination. The five most common classes of medications prescribed near the end of life were, anti-hypertensives, broncholytics/bronchodilators, laxatives, antidepressants, and gastric protection agents.
Conclusion
There is a significant medication burden placed on patients with advanced illness. Although most medications were prescribed for supportive care, we observed a high prevalence of medications for managing non-life threatening comorbidities.
Keywords: palliative care, end of life, polypharmacy
Introduction
Clinicians who provide care to patients near the end of life are challenged with managing the expanding medication portfolios of these patients. These medications include four types of interventions. First, medications for the primary prevention of disease (e.g., hypertension medications for prevention of strokes) are often continued despite changes in relative risk of these potential diseases to impact quality of life. Second, medications are often continued to control non-life threatening comorbidities present concomitant to life-limiting illness (e.g., warfarin for known atrial fibrillation). Third, disease-directed treatments may add to the total medication burden, such as oral tyrosine kinase inhibitors for advanced cancer or immunologic agents for neurologic conditions. Fourth, and most commonly actively managed by palliative care clinicians, are medications to address the symptom burden that increases along the trajectory of serious illness (e.g., laxatives to counter the constipating effect of many medications) (1,2). It is, therefore, common for patients to experience a gradual increase in the total medication burden near the end of life. For example, studies demonstrate that up to 20% of patients receiving palliative care services regularly take more than eight medications (1,3–5).
The imperative for palliative care clinicians to address the expanding portfolio of medications is highlighted by several common harms. For example, the complex interplay of concomitantly managing life-threatening illnesses (e.g., heart failure), non-life threatening comorbid conditions (e.g., hypertension, atrial fibrillation), and symptoms (e.g., dyspnea, pain) creates challenges for both the clinicians and patients. Also, as medication lists grow, the risk for drug-drug interactions (DDIs), drug-food interactions and other adverse drug events (ADEs) increases (6–8). Naturally, it becomes difficult for even experienced and attentive clinicians to not become overwhelmed with simultaneously managing multiple medications during brief clinical visits. Additionally, as medication lists grow, patients are faced with challenging medication administration regimens (e.g., four times daily dosing, nightly before bed dosing), increasing the risk for non-adherence. Further, patients with advanced illness frequently experience anorexia, swallowing difficulties, and early satiety, so taking multiple pills per day becomes significantly more difficult. Finally, patients and families are often burdened by high out-of-pocket costs associated with the use of multiple medications (9).
The Palliative Care Research Cooperative Group (PCRC) conducted a randomized clinical trial assessing the impact of discontinuing statins in patients near the end of life. This study demonstrated improved quality of life in patients with a life-limiting illness without an adverse impact on morbidity and mortality (10–13). Records of patient medication profiles were collected during the study. We aim to describe the breadth and depth of polypharmacy burden among patients within this clinical trial.
Methods
Overview
This study is an observational analysis of medication records from patients enrolled in a multi-center statin discontinuation clinical trial performed by the Palliative Care Research Cooperative (PCRC) Group (11). IRB approval was obtained for each site participating in the trial. A total of 381 patients were enrolled in the clinical trial that serves as the source of data for these analyses.
Patient Population
Patients eligible for this trial were those who had received a life-limiting diagnosis where at least one physician indicated he/she “would not be surprised if the patient died in the next year,” had a life expectancy greater than one month, had been taking statins for three months or more, and had recent deterioration in functional status. Outcomes, which were measured at baseline and at least monthly, included concomitant medications, quality of life, cardiovascular events, performance status, and symptoms.
Data Collection
As a part of this trial, the medications being taken by study participants were recorded at baseline and at weeks 2, 4, 8, 12, 16, 20, and 24 in order to document changes in medication use over the course of the trial. Patients for this secondary analysis were included if they had medication data from at least two time points, and were alive for at least 30 days after enrollment in the trial (N=244). Spelling mistakes were corrected and trade names were converted to generics. Medications were then coded as to class, subclass, and individual medications by one of the authors (M.J.M.) (Appendix 1). An expert panel of palliative care, oncology, geriatrics, and primary care physicians guided medication categorization. Medication classes were based on prior studies and the World Health Organization’s “Guidelines for Anatomic Therapeutic Class (ATC) Classification and Defined Daily Dose (DDD) Assignment” (3,14). Dosing and route of administration were not evaluated in this analysis. Because half of the study population was randomized to discontinue statins in accordance with the parent trial protocol, statin medication use is excluded from these analyses.
Calculations and Analysis
Data were analyzed using descriptive statistics. Noting parametric distribution of data during the data visualization step, we report means and frequencies.
Results
We included 244 patients who met inclusion criteria for this analysis. Mean (SD) age was 74.3 (11.5) years. One hundred ten patients (45.1%) were female. The most common life-limiting illness was malignant tumor (n=116, 47.5%); the mean (SD) Charlson Comorbidity Index (CCI) (15) was 4.8 (2.8). Eighty-seven patients (35.7%) were enrolled in hospice at baseline, and 132 (54.1%) participated in hospice at some point in their care. A total of 104 of the 244 patients in the cohort died during the study, with the mean (SD) length of time from study enrollment to death being 264.5 (128) days. The basic demographics of the patient cohort are depicted in Table 1.
Table 1.
Demographics of Patient Cohort
| Variable | N (%) |
|---|---|
| Age, mean (SD) | 74.3 (11.5) |
| Sex | |
| Male | 134 (54.9%) |
| Female | 110 (45.1%) |
| Primary Diagnosis | |
| Malignant Tumor | 116 (47.5%) |
| Malignant Lymphoma | 9 (3.7%) |
| Chronic obstructive pulmonary disease | 29 (11.9%) |
| Congestive heart failure | 20 (8.2%) |
| Dementia | 17 (7.0%) |
| Renal | 11 (4.5%) |
| Cerebrovascular | 4 (1.6%) |
| Leukemia | 1 (0.4%) |
| Other | 37 (15.2%) |
| Charlson Comorbidity Indexa | |
| Index Score, mean (SD) | 4.8 (2.8) |
| Hospice at Baseline: | |
| Yes | 87 (35.7%) |
| No | 157 (64.3%) |
The Charlson Comorbidity Index is a calculated measure that assesses the burden of comorbidity on a patient (15).
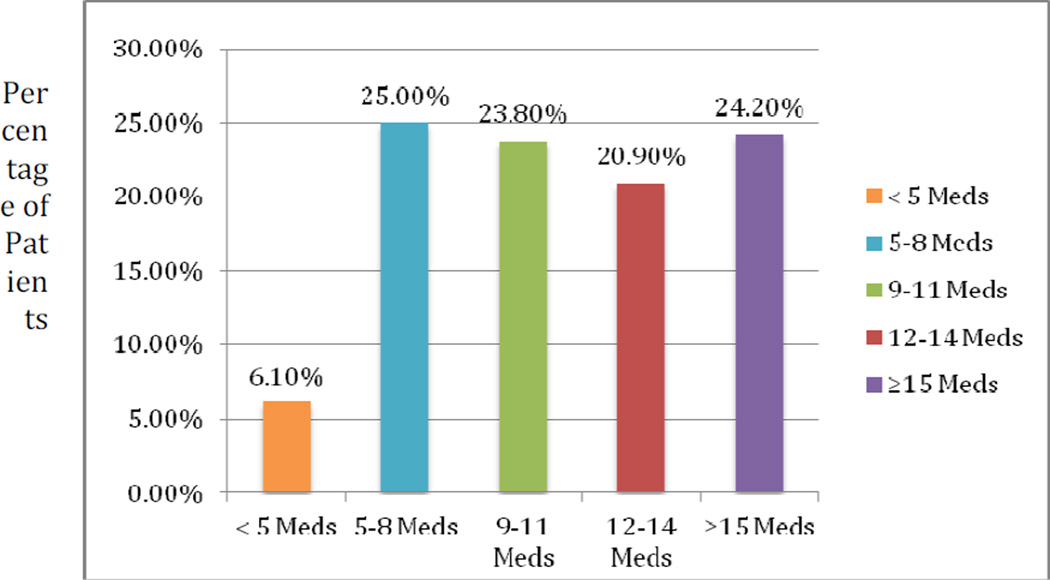
Patients took an average of 11.5 (SD 5) non-statin medications at the time of enrollment and 10.7 (SD 5) non-statin medications at death or study termination. There was not a significant change in the mean number of medications patients took over time. Less than 7% of patients were on five medications or fewer; 68% of patients were on more than eight medications, including 24.2% who were on 15 medications or more at baseline (Fig. 1). Eighty patients (32.8%) were on 15 medications or more at some point during the study period.
Fig. 1.
Medications per patient at baseline.
In total, 13,138 medications were recorded, which encompassed 51 classes, 192 subclasses and 423 different medications. The five most common classes of medications taken by these patients were antihypertensives, broncholytics/bronchodilators, laxatives, antidepressants, and gastric protection agents. These five medication classes accounted for 33.9% of all medications prescribed (Table 2). Of note, opioids were not among the most common classes prescribed to these patients and only 4.1% of medications prescribed were nonopioid analgesics. Vitamins and minerals were also among the top 10 most common classes of medications, with 4.1% of all medications being vitamins or minerals. There were 21 different antihypertensive medications prescribed to this patient population along with 31 different antibiotic medications, and 17 antineoplastic agents.
Table 2.
Most Common Medication Classes Prescribed to Patients With a Life-Limiting Illness:
| Class | Incidence |
|---|---|
| Anti-Hypertensives | 1222 (9.3%) |
| Broncholytics/Bronchodilators | 903 (6.9%) |
| Laxatives | 841 (6.4%) |
| Antidepressants | 749 (5.7%) |
| Gastric Protection | 734 (5.6%) |
| Anti-Inflammatory | 681 (5.2%) |
| Analgesic | 540 (4.1%) |
| Vitamins/Minerals | 531 (4.1%) |
| Diuretics | 518 (4.0%) |
| Antiemetic | 465 (3.5%) |
| Total Number of Medications Prescribed | 13,138 |
The five most common subclasses of medications among these patients were: nonsteroidal anti-inflammatory drugs (NSAIDs), short-acting strong opioids, proton-pump inhibitors (PPIs), osmotic laxatives, and beta-blockers (Appendix 2). The five most common medications were: aspirin, omeprazole, furosemide, acetaminophen, and senna (Appendix 3).
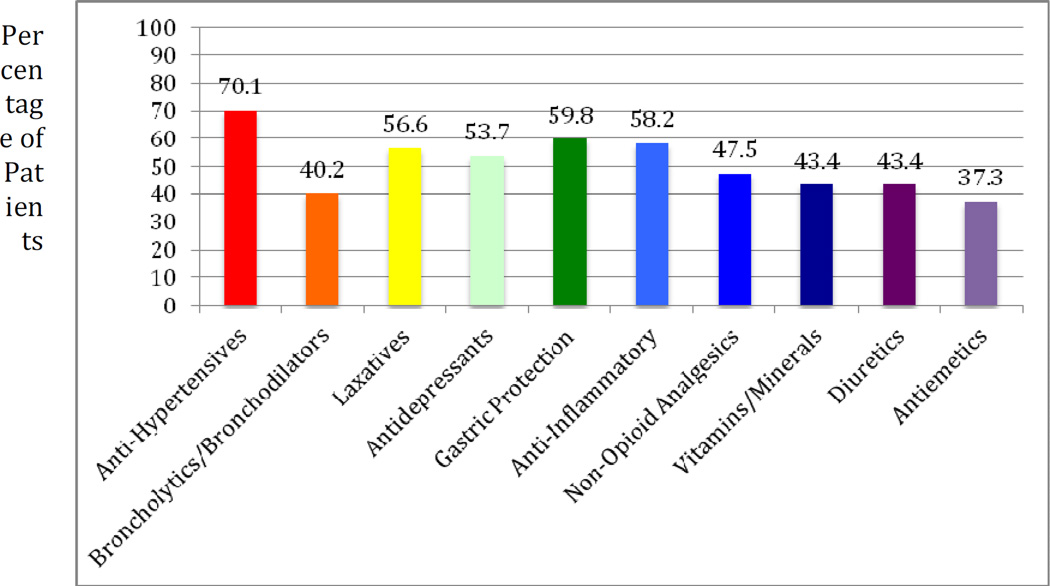
More than 50% of patients took a medication from each of the following classes: antihypertensives, laxatives, antidepressants, gastric protection agents, and anti-inflammatory agents (Fig. 2). While broncholytics/bronchodilators was the second most common class of medications, only 40.2% of patients were taking these. This is because of the fact that a large proportion of these patients were taking multiple broncholytics/bronchodilators; including combination drugs. Of note 12.3% of patients were taking antiarrhythmics including digoxin, amiodarone, and sotalol. There were also 15.2% who were taking neuroleptics and 11.9% receiving chemotherapy.
Fig. 2.
Percentage of patients taking the most common medication classes.
Discussion
We found a high burden of polypharmacy in patients near the end of life. The average number of medications taken per patient was 11.5; almost one-third of patients were on 15 medications or more during the study period. Importantly, these medications were all non-statin medications, which, according to study protocol, all patients were taking at the time of study enrollment. Therefore, the total burden of polypharmacy in each patient is actually higher.
A significant proportion of patients near the end of life were prescribed medications to control or prevent non-life threatening comorbidities. The most common class of medications prescribed in this particular cohort was antihypertensives. Aspirin and metoprolol were also very common. Also of note, opioids were not among the most common classes of medications, which counters the perception that pain medications comprise the majority of the pharmacologic toolbox used by palliative care clinicians.
There is no strict definition used in the literature to describe polypharmacy; a quantitative definition of polypharmacy can range from 5–10 concurrent medications (16). We recognize that even one medication inappropriately prescribed can be burdensome to the patient. For the purposes of our analysis, we used the more common definition of five or more medications (8,16). Close to 94% of patients in our cohort experienced polypharmacy using this definition. Previous studies demonstrated up to 20% of patients taking more than eight medications while our cohort had 68% with more than eight medications (1, 3–5).
One of the downstream implications of continued, high medication burden in patients near the end of life is significant risk of adverse events related to polypharmacy. Other studies have demonstrated a clear relationship between an increasing number of medications and increase in unintended ADEs (6,8). This relationship is not linear; in fact, one Italian study demonstrated a fourfold increase in the risk of an ADE as the medication burden increased by only three individual medications, from five to eight (17).
This is the first study that we are aware of that identifies the medications prescribed to palliative care patients in the United States. Our findings are similar to the polypharmacy burden near the end of life reported by others, however with several key differences. Three studies have previously assessed medication prescribed during end-of-life care (3,18,19). The first, Nauck et al., a representative survey of drugs in palliative care performed in Germany, demonstrated strong opioids as the most common class. This group also found a large percentage of patients taking antihypertensives at the end of life. In our study, opioids were not among the top ten most common classes, and antihypertensives were in fact the most common class. Kotlinska-Lemieszek et al. assessed the medication profiles of patients with advanced cancer who were also taking opioids for pain. They identified the most common drugs as PPIs and laxatives. While PPIs and laxatives were among the most common medications prescribed in our cohort, we saw a higher number of antihypertensives and bronchodilators/broncholytics. Our study demonstrated a large burden of medications for comorbidities, along with the medications for symptom alleviation. We also recorded a much higher number of overall medications prescribed, as the mean number of medications was 11.5 in our cohort compared to 4.8 in the Nauck et al. study, and 7.8 in the Kotlinska-Lemieszek study. The exception to this is a study conducted among U.S. hospice patients, which found an even higher number of medications (15.7) on average prescribed to patients (18).
We also found that the polypharmacy burden is higher than what others report in nonpalliative care settings. For patients 65 years or older, one study found more than 50% were taking, on average, five medications or more (20). Another study that evaluated over 3000 patients aged 57 to 85 years of age found that 29% were concurrently using five medications or more (21). In contrast, our study demonstrated more than 90% of patients were on more than five medications.
Despite this significant medication burden among patients near the end of life, there remains little guidance or recommendations regarding the appropriate discontinuation of medications near the end of life. A few resources are available that discuss the need for addressing polypharmacy in palliative care (22,23) but there are few randomized controlled trials that demonstrate effective medication discontinuation at the end of life. The results of the statin discontinuation study demonstrate, for example, the safety of stopping statin medications for primary prevention in patients with limited prognosis and supports the role for careful, patient-centered, collaborative conversations between patients and clinicians. Importantly, there are no guidelines or quality measures to date that guide the discontinuation of medications in patients for whom there may not be a benefit.
This study demonstrates that multiple medications are being prescribed to patients near the end of life and that many of these medications are being used to address chronic conditions and not address symptom alleviation. In order to improve prescribing practices near the end of life, it will be important for providers to more diligently reconcile medications with patients and identify unnecessary medications. One potential medication class that might be amenable to future studies is the effect of discontinuing antihypertensives among selected patients diagnosed with a life-limiting illness.
This study had several limitations. The study design excluded patients with less than one month to live, those who were not prescribed statins, and those for whom their physicians believed it was unsafe to discontinue a statin. Also, medication lists were recorded by phone or personal interactions with the patients or caregivers. This could have impacted the accuracy of the data. Dosages, frequency and administration route were not recorded; therefore, an assessment of the medication regimen complexity could not be assessed. Also, while we have the medication names, we do not have the indication for the prescription, which is important information when deciding whether a medicine should be continued or discontinued. Finally, this analysis excluded the medication that was the focus of the parent study, statins; therefore, the total number of medications for these patients is actually higher.
Conclusions
This study demonstrated that patients near the end of life are prescribed multiple medications. Consequently, clinicians caring for these patients must manage a highly diverse set of medications classes. Areas of future research need to assess changes in medication prescriptions as a patient approaches the end of life; more research is needed to identify which of these medications are beneficial, and harmful, in which segments of this population. Finally, developing an appropriateness guideline for medication discontinuation in the palliative care population is warranted.
Acknowledgments
This work was funded by the National Institute of Nursing Research (UC4-NR012584, U24-NR014637).
The authors thank Dr. Claudia Bausewein from The University of Munich and Dr. Michael Steinman from the University of California-San Francisco for their support and advice on the project.
Appendix 1
Medication Classes and Examples of Subclasses and Medications Used in a Cohort of Patients with a Life-Limiting Illness
| Classes: | Subclasses: | Examples: |
|---|---|---|
| Analgesic | Acetaminophen, Topical | Acetaminophen, Lidocaine patch |
| Antiarrythmics | Beta-Blocker, Inotrope | Sotalol, Verapamil, Digoxin |
| Anti-HTN | Beta-Blocker, ACE Inhibitor, ARB | Carvedilol, Losartan, Lisinopril |
| Antibiotics/Antivirals | Cephalosporin, Penicillins | Ceftriaxone, Ciprofloxacin, Ampicillin |
| Anticonvulsants/Anti Parkinsons | Benzodiazepines, Dopamine agonists, | Clonazepam, Carbidopa, Valproic Acid |
| Antidiarrheals | Anticholingergic, Antipropulsive | Diphenoxylate, Loperamide |
| Antidepressants | SNRI, SSRI, TCA | Venlafaxine, Fluoxetine, Amitriptyline |
| Antidementia | Cholinesterase Inhibitor, NMDA antagonist | Donepezil, Rivastigmine, Memantine |
| Antiemetics | 5HT3 antagonist, H1 blocker, | Ondansetron, Promethazine, Prochlorperazine |
| Antiinflammatory/Antiplatelet | NSAIDs, Platelet aggregation inhibitor | Aspirin, Celecoxib, Clopidogrel |
| Antimycotic | Azoles, Topical | Ketoconazole, Nystatin |
| Antipsychotics | Atypical, Typical | Quetiapine, Haloperidol |
| Antispasmodic | Anticholinergic | Dicyclomine |
| Antithrombolytics | tPA | Alteplase |
| Antithrombin | Direct Thrombin Inhibitors | Dabigatran |
| Anxiolytics/Sedative | Benzodiazapines, GABA analog | Lorazepam, Zolpidem |
| Bisphosphonates | Bisphosphonates | Alendronate, Zoledronic acid |
| BPH | 5 alpha reductase inhibitors, α-antagonists | Finasteride, Tamsulosin |
| Bronchiolytics/Bronchodilators | Anticholinergic, B2 agonist | Ipratropium, Albuterol, Montelukast |
| Chelating Agents | Phosphate Binder | Sevelamer |
| Chemotherapy/Antineoplastic Agents | Antimetabolites, EGFR Inhibitors, | Capecitabine, Erlotinib, Paclitaxel |
| Corticosteroids | Topical, Nasal, Systemic | Betamethasone, Fluticasone, Dexamethasone |
| Dermatologic | Emollients, Retinoids | Ammonium lactate, Tretinoin |
| Diuretic | Loop, Potassium sparing, Thiazide | Furosemide, Spironolactone, Hydrochlorothiazide |
| Drugs used for dependence | Opioid | Methadone |
| Erectile Dysfunction | PPD5 inhibitors | Sildenafil, Vardenefil |
| Emollients | Cicatrizants | Collagenase |
| Enzymes | Digestive | Pancrelipase, Pancreatin |
| Gastric Anti-inflammatory | Aminosalicylates | Mesalamine |
| Gastric Protection | H2 blocker, Proton pump inhibitor | Aluminum Hydroxide, Ranitidine, Omeprazole |
| Gout | Neutrophil migration inhibitor, Xanthine Oxidase Inhibitors | Allopurinol, Colchicine |
| Heparins | Antithrombin inhibitor, Low Molecular Weight Heparin (LMWH) | Heparin, Enoxaparin |
| Hormones | Estrogens, Testosterones | Megestrol, Depo-testosterone |
| Hormone Antagonists | Aromatase inhibitor, SERMs | Anastrozole, Raloxifene |
| Immunostimulants | Colony Stimulating Factor | Filgrastrim |
| Immunosuppresants | Calcineurin Inhibitor | Cyclosporin, Thalidomide |
| Insulins | Intermediate, Long acting, Short acting | Insulin NPH, Insulin glargine, Insulin lispro |
| Laxatives | Osmotic, Opioid antagonists | Lactulose, Senna, Docusate |
| Lipid Modifying Agents | Bile Acid Sequestrants, Fibrates, Niacin | Cholestyramine, Fenofibrate, Niacin |
| Muscle Relaxants | Central Acting | Baclofen, Cyclobenzaprine |
| Ophthalmic | Adrenergic agonist, Beta blocker, | Pilocarpine, Timolol, Latanoprost |
| Oral Anticoagulants | Warfarin | Warfarin |
| Oral Antidiabetics | Biguanides, Sulfonylureas | Metformin, Sitagliptin, Glipizide |
| Other Hormones | Desmopressin, Erythropoietin, Thyroid | Desmopressin, Darbepoetin, Levothyroxine |
| Mucolytics and Antihistamine | Expectorant, H1 blocker | Dextromethorphan, Cetirizine |
| Psychostimulants | Nootropics, Sympathomimetics | Modafinil, Methylphenidate |
| Smoking Cessation | Transdermal | Nicotine patch |
| Strong Opioids | Short acting, Long acting | Morphine, Oxycodone, Oxycontin |
| Weak Opioids | Central acting, Opioid+Acetaminophen | Codeine, Hydrocodone/Acetaminophen |
| Vitamins/Minerals | Vitamins, Minerals | Vitamin B12, Vitamin D, Potassium chloride |
| Various | Saline, Herbal supplements, Probiotics | Saline, Herbal supplement, Lactobacillus |
Appendix 2
Most Common Medication Subclasses Prescribed to Patients with a Life-Limiting Illness
| Subclass | Incidence |
|---|---|
| NSAIDs | 643 (4.9%) |
| Short Acting Strong Opioids | 620 (4.8%) |
| PPI | 534 (4.1%) |
| Osmotic Laxatives | 517 (4.0%) |
| Beta Blocker | 498 (3.8%) |
| Acetaminophen | 375 (2.9%) |
| Loop Diuretics | 375 (2.9%) |
| Benzodiazepines | 335 (2.6%) |
| SSRI | 319 (2.4%) |
| GABA analog | 291 (2.2%) |
Appendix 3
Most Common Medications Prescribed to Patients with a Life-Limiting Illness
| Medication | Incidence |
|---|---|
| Aspirin | 505(3.8%) |
| Omeprazole | 310 (2.4%) |
| Furosemide | 308(2.3%) |
| Acetaminophen | 304 (2.3%) |
| Senna | 282 (2.1%) |
| Levothyroxine | 239 (1.8%) |
| Albuterol | 229 (1.7%) |
| Metoprolol | 226 (1.7%) |
| Lorazepam | 213 (1.6%) |
| Docusate | 205 (1.6%) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflicts of interest.
References
- 1.Currow DC, Stevenson JP, Abernethy AP, Plummer J, Shelby-James TM. Prescribing in palliative care as death approaches. J Am Geriatr Soc. 2007;55:590–595. doi: 10.1111/j.1532-5415.2007.01124.x. [DOI] [PubMed] [Google Scholar]
- 2.Mamun K, Lien CT, Goh-Tan CY, Ang WS. Polypharmacy and inappropriate medication use in Singapore nursing homes. Ann Acad Med Singapore. 2004;33:49–52. [PubMed] [Google Scholar]
- 3.Nauck F, Ostgathe C, Klaschik E, et al. Drugs in palliative care: results from a representative survey in Germany. Palliat Med. 2004;18:100–107. doi: 10.1191/0269216304pm852oa. [DOI] [PubMed] [Google Scholar]
- 4.Wilcock A, Thomas J, Frisby J, et al. Potential for drug interactions involving cytochrome P450 in patients attending palliative day care centres: a multicentre audit. Br J Clin Pharmacol. 2005;60:326–329. doi: 10.1111/j.1365-2125.2005.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeppetella G. How do terminally ill patients at home take their medication? Palliat Med. 1999;13:469–475. doi: 10.1191/026921699675653923. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg RM, Mabee J, Chan L, Wong S. Drug-drug and drug-disease interactions in the ED: analysis of a high-risk population. Am J Emerg Med. 1996;14:447–450. doi: 10.1016/S0735-6757(96)90147-3. [DOI] [PubMed] [Google Scholar]
- 7.Steinhauser KE, Christakis NA, Clipp EC, et al. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284:2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 8.Weng MC, Tsai CF, Sheu KL, et al. The impact of number of drugs prescribed on the risk of potentially inappropriate medication among outpatient older adults with chronic diseases. QJM. 2013;106:1009–1015. doi: 10.1093/qjmed/hct141. [DOI] [PubMed] [Google Scholar]
- 9.Kelley AS, McGarry K, Fahle S, et al. Out-of-pocket spending in the last five years of life. J Gen Intern Med. 2013;28:304–309. doi: 10.1007/s11606-012-2199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abernethy APKJ, Blatchford PJ. Managing comorbidities in oncology: a multisite randomized controlled trial of continuing versus discontinuing statins in the setting of life-limiting illness [abstract] J Clin Oncol. 2014;32(5) Suppl abstr LBA9514. [Google Scholar]
- 11.Kutner JS, Blatchford PJ, Taylor DH, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med. 2015;175:691–700. doi: 10.1001/jamainternmed.2015.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abernethy AP, Aziz NM, Basch E, et al. A strategy to advance the evidence base in palliative medicine: formation of a palliative care research cooperative group. J Palliat Med. 2010;13:1407–1413. doi: 10.1089/jpm.2010.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leblanc TW, Kutner JS, Ko D, et al. Developing the evidence base for palliative care: formation of the palliative care research cooperative and its first trial. Hosp Pract. 2010;38:137–143. doi: 10.3810/hp.2010.06.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Geneva: World Health Organization; 2013. Guidelines for ATC classification and DDD assignment. [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Ferner RE, Aronson JK. Communicating information about drug safety. BMJ. 2006;333:143–145. doi: 10.1136/bmj.333.7559.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onder G, Petrovic M, Tangiisuran B, et al. Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: the GerontoNet ADR risk score. Arch Intern Med. 2010;170:1142–1148. doi: 10.1001/archinternmed.2010.153. [DOI] [PubMed] [Google Scholar]
- 18.Kotlinska-Lemieszek A, Paulsen O, Kaasa S, Klepstad P. Polypharmacy in patients with advanced cancer and pain: a european cross-sectional study of 2282 patients. J Pain Symptom Manage. 2014;48:1145–1159. doi: 10.1016/j.jpainsymman.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Sera L, McPherson ML, Holmes HM. Commonly prescribed medications in a population of hospice patients. Am J Hosp Palliat Care. 2014;31:126–131. doi: 10.1177/1049909113476132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 21.Qato DM, Alexander GC, Conti RM, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300:2867–2878. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes HM. Rational prescribing for patients with a reduced life expectancy. Clin Pharmacol Ther. 2009;85:103–107. doi: 10.1038/clpt.2008.211. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson J, Abernethy AP, Miller C, Currow DC. Managing comorbidities in patients at the end of life. BMJ. 2004;329:909–912. doi: 10.1136/bmj.329.7471.909. [DOI] [PMC free article] [PubMed] [Google Scholar]