Abstract
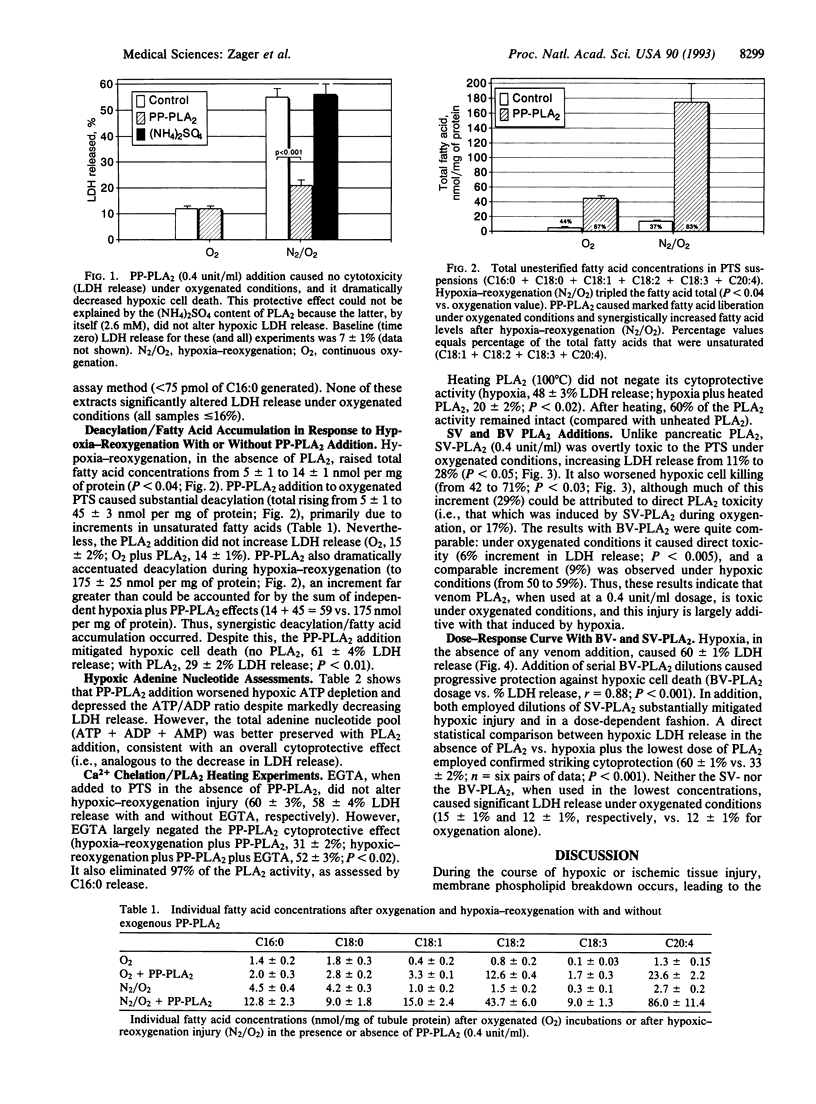
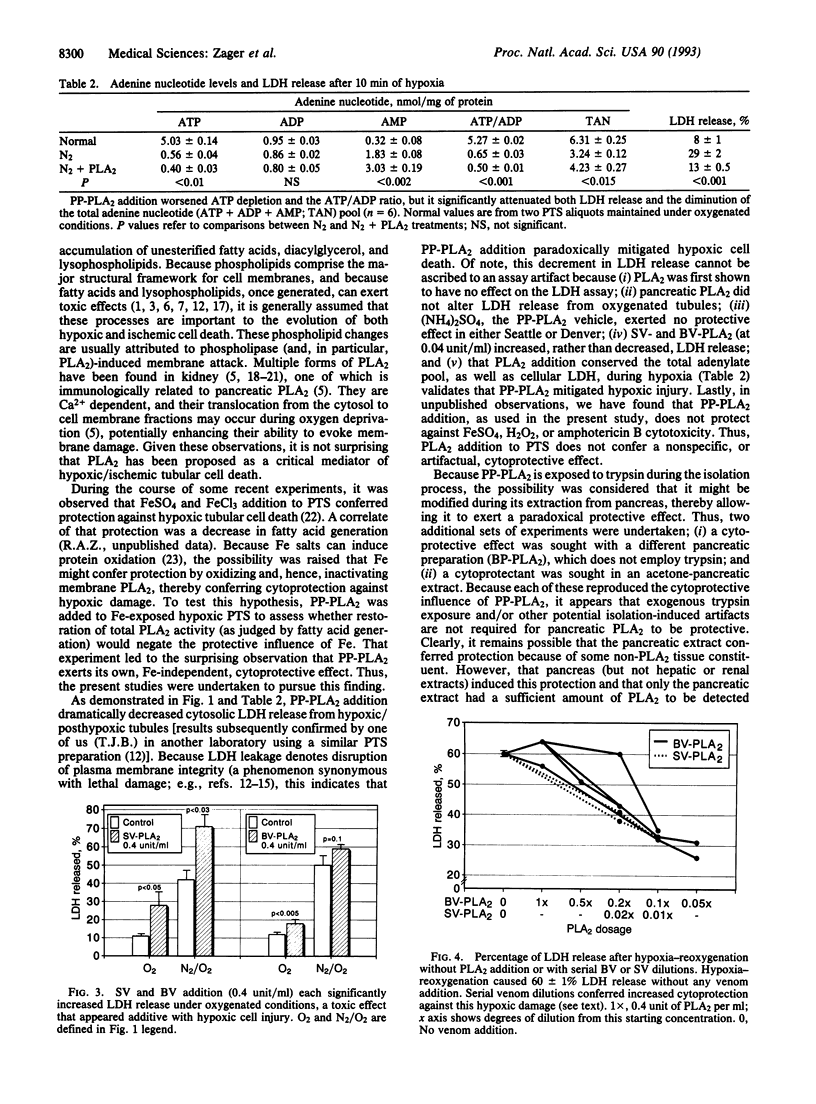
During hypoxic or ischemic renal tubular injury, phospholipase A2 (PLA2) induces membrane deacylation, causing fatty acid accumulation and phospholipid breakdown. Because these changes can compromise cellular integrity, PLA2 activity has been widely proposed as a critical mediator of hypoxic renal tubular injury and, hence, of ischemic acute renal failure. To explore this hypothesis, isolated rat proximal tubules were subjected to continuous oxygenation or to hypoxic injury with or without exogenous PLA2 addition (porcine or bovine pancreatic PLA2; bee or snake venom PLA2). Cell death was quantified by lactic dehydrogenase (LDH) release. Pancreatic PLA2 (0.4 unit/ml) caused no LDH release under oxygenated conditions, and it dramatically attenuated hypoxic cell death (e.g., no PLA2, 55 +/- 3% LDH release; porcine pancreatic PLA2, 22 +/- 1% LDH release; P < 0.001). Bee and snake venom PLA2 (0.4 unit/ml) were directly toxic to tubules under oxygenated conditions, and this injury was additive with that induced by hypoxia. However, when these venoms were serially diluted (removing their overt toxicity), they, too, mitigated hypoxic cell death (LDH release with PLA2, 33 +/- 2%; without PLA2, 60 +/- 1% LDH release; P < 0.001). PLA2-mediated cytoprotection was Ca2+ dependent (negated by Ca2+ chelation), and it was expressed despite worsening hypoxia-associated membrane deacylation/fatty acid accumulation (12 times) and ATP depletion. These results indicate that PLA2 activity can exert both beneficial and deleterious effects on O2-deprived renal tubules, the net result of which can be a salvaging of cells from hypoxic cell death.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonventre J. V., Cheung J. Y. Effects of metabolic acidosis on viability of cells exposed to anoxia. Am J Physiol. 1985 Jul;249(1 Pt 1):C149–C159. doi: 10.1152/ajpcell.1985.249.1.C149. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V. Phospholipase A2 and signal transduction. J Am Soc Nephrol. 1992 Aug;3(2):128–150. doi: 10.1681/ASN.V32128. [DOI] [PubMed] [Google Scholar]
- Chiariello M., Ambrosio G., Cappelli-Bigazzi M., Nevola E., Perrone-Filardi P., Marone G., Condorelli M. Inhibition of ischemia-induced phospholipase activation by quinacrine protects jeopardized myocardium in rats with coronary artery occlusion. J Pharmacol Exp Ther. 1987 May;241(2):560–568. [PubMed] [Google Scholar]
- Clemens J. A., Ho P. P., Panetta J. A. LY178002 reduces rat brain damage after transient global forebrain ischemia. Stroke. 1991 Aug;22(8):1048–1052. doi: 10.1161/01.str.22.8.1048. [DOI] [PubMed] [Google Scholar]
- Gronich J. H., Bonventre J. V., Nemenoff R. A. Purification of a high-molecular-mass form of phospholipase A2 from rat kidney activated at physiological calcium concentrations. Biochem J. 1990 Oct 1;271(1):37–43. doi: 10.1042/bj2710037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes H. D., Nguyen V. D., Cieslinski D. A., Messana J. M. The role of free fatty acids in hypoxia-induced injury to renal proximal tubule cells. Am J Physiol. 1989 Apr;256(4 Pt 2):F688–F696. doi: 10.1152/ajprenal.1989.256.4.F688. [DOI] [PubMed] [Google Scholar]
- Matthys E., Patel Y., Kreisberg J., Stewart J. H., Venkatachalam M. Lipid alterations induced by renal ischemia: pathogenic factor in membrane damage. Kidney Int. 1984 Aug;26(2):153–161. doi: 10.1038/ki.1984.149. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Nemenoff R. A., Gronich J. H., Bonventre J. V. Subcellular characteristics of phospholipase A2 activity in the rat kidney. Enhanced cytosolic, mitochondrial, and microsomal phospholipase A2 enzymatic activity after renal ischemia and reperfusion. J Clin Invest. 1991 May;87(5):1810–1818. doi: 10.1172/JCI115202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V. D., Cieslinski D. A., Humes H. D. Importance of adenosine triphosphate in phospholipase A2-induced rabbit renal proximal tubule cell injury. J Clin Invest. 1988 Sep;82(3):1098–1105. doi: 10.1172/JCI113666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlock G. S., Southard J. H., Lutz M. F., Belzer J. P., Belzer F. O. Effects of mannitol and chlorpromazine pretreatment of rabbits on kidney mitochondria following in vivo ischemia and reflow. Life Sci. 1981 Dec 21;29(25):2667–2672. doi: 10.1016/0024-3205(81)90642-1. [DOI] [PubMed] [Google Scholar]
- Portilla D., Mandel L. J., Bar-Sagi D., Millington D. S. Anoxia induces phospholipase A2 activation in rabbit renal proximal tubules. Am J Physiol. 1992 Mar;262(3 Pt 2):F354–F360. doi: 10.1152/ajprenal.1992.262.3.F354. [DOI] [PubMed] [Google Scholar]
- Stocchi V., Cucchiarini L., Canestrari F., Piacentini M. P., Fornaini G. A very fast ion-pair reversed-phase HPLC method for the separation of the most significant nucleotides and their degradation products in human red blood cells. Anal Biochem. 1987 Nov 15;167(1):181–190. doi: 10.1016/0003-2697(87)90150-3. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Kreisberg J. I., Stein J. H., Lifschitz M. D. Salvage of ischemic cells by impermeant solute and adenosinetriphosphate. Lab Invest. 1983 Jul;49(1):1–3. [PubMed] [Google Scholar]
- Venkatachalam M. A., Patel Y. J., Kreisberg J. I., Weinberg J. M. Energy thresholds that determine membrane integrity and injury in a renal epithelial cell line (LLC-PK1). Relationships to phospholipid degradation and unesterified fatty acid accumulation. J Clin Invest. 1988 Mar;81(3):745–758. doi: 10.1172/JCI113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M. The cell biology of ischemic renal injury. Kidney Int. 1991 Mar;39(3):476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- Wetzels J. F., Wang X., Gengaro P. E., Nemenoff R. A., Burke T. J., Schrier R. W. Glycine protection against hypoxic but not phospholipase A2-induced injury in rat proximal tubules. Am J Physiol. 1993 Jan;264(1 Pt 2):F94–F99. doi: 10.1152/ajprenal.1993.264.1.F94. [DOI] [PubMed] [Google Scholar]
- Zager R. A. Combined mannitol and deferoxamine therapy for myohemoglobinuric renal injury and oxidant tubular stress. Mechanistic and therapeutic implications. J Clin Invest. 1992 Sep;90(3):711–719. doi: 10.1172/JCI115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager R. A., Gmur D. J., Bredl C. R., Eng M. J. Temperature effects on ischemic and hypoxic renal proximal tubular injury. Lab Invest. 1991 Jun;64(6):766–776. [PubMed] [Google Scholar]
- Zager R. A., Schimpf B. A., Bredl C. R., Gmur D. J. Inorganic iron effects on in vitro hypoxic proximal renal tubular cell injury. J Clin Invest. 1993 Feb;91(2):702–708. doi: 10.1172/JCI116251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager R. A., Schimpf B. A., Gmur D. J. Physiological pH. Effects on posthypoxic proximal tubular injury. Circ Res. 1993 Apr;72(4):837–846. doi: 10.1161/01.res.72.4.837. [DOI] [PubMed] [Google Scholar]



