Abstract
Schizophrenia is a severe psychiatric disorder that is characterized by a wide array of symptoms and complex neuropathology. A well-characterized neurobiological feature of schizophrenia is abnormal synaptic plasticity, although the mechanisms underlying this are not fully understood. Numerous studies have demonstrated a link between proper functioning of the cytoskeleton and synaptic plasticity. The Arp2/3 complex is responsible for the nucleation of new actin filaments and elongation of existing actin filaments and is thus crucial to cytoskeletal dynamics, especially actin polymerization and organization. To determine if the Arp2/3 complex is abnormally expressed in schizophrenia, we measured the protein expression of Arp2 and Arp3, as well as Arp2/3 complex binding partners and associated proteins including cortactin, N-WASP, WAVE1, and Abi1 in superior temporal gyrus of paired schizophrenia and comparison subjects. No changes were found in Arp2, Arp3, N-WASP, WAVE1, or Abi1. However, all three isoforms of cortactin were decreased in schizophrenia. Specifically, the 62 kDa isoform was decreased 43%; the 71kDa isoform was decreased 32%; and the 58 kDa isoform was decreased 35%. Cortactin regulates branching of filamentous actin via its binding and activation of the Arp2/3 complex and is thus critical to the formation of stable actin networks. These findings contribute to a growing body of evidence implicating altered cytoskeletal dynamics in schizophrenia.
Keywords: synaptic plasticity, arp2/3 complex, dendritic spine, actin, cytoskeleton, postmortem, WASP
Introduction
Converging evidence has implicated synaptic dysfunction and degeneration as key components in the neuropathology of schizophrenia [1]. Synapses exhibit extensive morphological plasticity after receptor activation, and morphological change is facilitated by the dynamic actin cytoskeleton [2]. Altered morphology of dendritic spines has been documented in multiple brain regions and is one of the most well-characterized neurobiological changes in schizophrenia [3]. Our lab has previously reported abnormal expression of cytoskeletal regulators in schizophrenia brain, including decreased protein levels of duo, cell division cycle protein 42 (cdc42), and myristoylated alanine-rich C kinase substrate (MARCKS), as well as decreased phosphorylation of p21-activated kinase 1 (PAK1) and MARCKS [4, 5].
The actin-related protein-2/3 (Arp2/3) complex is a chief initiator of actin polymerization and governs the formation of branched actin networks [6]. Conditional knockout of the Arp2/3 complex has been shown to lead to loss of spine synapses, consistent with dendritic spine abnormalities reported in schizophrenia [3, 7]. The Arp2/3 complex consists of 7 subunits: actin-related protein 2 (Arp2), actin-related protein 3 (Arp3), and five other subunits [6]. The Arp2/3 complex is regulated by members of the Wiskcott-Aldrich syndrome protein (WASP) family of proteins, comprised of the WASP/neuronal-WASP (N-WASP) class of proteins and the Scar/WASP-family verprolin homology protein (Scar/WAVE) class of proteins [6]. Specifically, binding of WASP family proteins or cortactin, a key branched actin regulator and Src kinase substrate, activates the Arp2/3 complex [8, 9]. Interestingly, it has been shown in hippocampal neurons that, similar to knockout of the Arp2/3 complex, small interfering RNA (siRNA) knockdown of cortactin results in decreased dendritic spine density [10]. Abelson interactor 1 (Abi1) is an adaptor protein that binds the Wiskott–Aldrich syndrome protein (WASP)-family verprolin homologous protein 1 (WAVE1) and is critical for neurotypical dendritic spine morphology through interaction with Ca2+/calmodulin-dependent kinase IIα [11, 12].
Abnormal cytoskeletal dynamics, including disrupted actin polymerization and reorganization, have been implicated in the pathophysiology of schizophrenia. Disruption of signaling cascades converging upon the Arp2/3 complex, necessary for cytoskeletal regulation, may represent a possible mechanism underlying abnormal dendritic spine morphology in schizophrenia. In this study, we hypothesized that the Arp2/3 complex and/or its activators and associated proteins may be abnormally expressed in schizophrenia. To test our hypothesis, we performed western blot analysis to measure the protein expression of Arp2, Arp3, WAVE1, N-WASP, isoforms of cortactin, and Abi1 in postmortem superior temporal gyrus (STG) from schizophrenia and paired comparison subjects.
Methods
Subjects, Tissue Acquisition, and Sample Preparation
Samples of gray matter comprising the full cortical thickness of the left STG (Brodmann Area 22) were obtained from the Mount Sinai Medical Center brain collection. STG was studied as decreased volume of this cortical region has been found in schizophrenia, and the left STG has been linked to auditory hallucinations, disrupted thought processes, and impaired working memory in schizophrenia [13, 14, 15]. Schizophrenia subjects (N=13 : age= 77 ± 11 years; tissue pH = 6.5 ± 0.2; postmortem interval = 11.1 ± 6.6 hours; 6 males, 7 females) and comparison subjects (N = 13; age = 81 ± 7 years; tissue pH = 6.5 ± 0.3; postmortem interval = 12.8 ± 8.3 hours; 6 males, 7 females) were paired by age, sex, tissue pH, and postmortem interval (PMI). The diagnosis of schizophrenia was determined by two independent clinicians using DSM-III-R criteria. All patients had a history of psychotic symptoms prior to the age of 40 and at least 10 years of hospitalization. Patients were recruited prospectively and underwent extensive antemortem clinical assessment. Subject medical history was assessed for alcoholism, substance abuse, death by suicide, coma for more than 6 h before death, or evidence of neurodegenerative disorders, and subjects were excluded if any of these were present. Comparison subjects were assessed similarly and confirmed to be without neuropathological evidence of neurodegenerative disorders and history of psychiatric illness or substance abuse.
Brains were collected at autopsy, as previously described [16]. Briefly, each sample was snap frozen, pulverized into a powder with small amounts of liquid nitrogen, and stored at −80°C until use. Samples were subsequently reconstituted and homogenized in cold RIPA buffer [50mM Tris HCl (pH 7.4), 150mM NaCl, 0.5mM EGTA, 1mM EDTA, 1% Tritonx-100, 1% sodium deoxycholate, 0.5% SDS, deubiquitinase inhibitors, and a protease and phosphatase inhibitor tablet (Complete Mini and Phostop, respectively, Roche Diagnostics, Manheim, Germany)] using a Power Gen 125 homogenizer (Thermo Fisher Scientific, Waltham, MA). Homogenates were assayed for protein concentration using a BCA protein assay kit (Thermo Fisher Scientific) and stored at −80°C until use.
Antipsychotic Treated Rats
Animal studies and procedures were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Efforts were made to minimize the number of animals used and any unnecessary suffering. 20 house-paired male Sprague Dawley rats (Charles River, Wilmington, MA, USA; 250g) were intramuscularly injected every three weeks with either haloperidol decanoate in sesame oil (28.5 mg/kg, 10 rats) or sesame oil only (10 rats) for a total of 12 injections. Haloperidol decanoate is a first-generation, long-acting antipsychotic that is administered monthly to patients. The goal of this rat study was to emulate chronic antipsychotic treatment used in patients. The dose and duration of treatment was converted from patient-appropriate doses to rat-appropriate doses to recapitulate chronic treatment with typical antipsychotics, and has been previously been described [4, 5]. The animals were killed by decapitation, and the brains were immediately harvested, dissected, and stored at −80°C.
Western Blot Analysis
Each sample was diluted to a concentration of 1 μg/μl and denatured at 70°C for 10 minutes under reducing conditions. For each subject, 10 μg total protein per lane was loaded in duplicate into 4-12% gradient bis-tris polyacrylamide gels (Life Technologies, Grand Island, NY) and electrophoresed using the Novex Mini Cell NuPAGE system (Life Technologies).
After electrophoresis, proteins were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA) using a semi-dry transfer apparatus (Bio-Rad). Membranes were blocked with either Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) or 5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 1 hour at room temperature prior to incubation with primary antibody (conditions listed in Table 1). The membranes were washed with PBS containing 0.1% Tween-20 (PBST), and then probed with IR-Dye labeled secondary antibodies (LI-COR Biosciences). A final PBST wash was performed prior to scanning membranes using an Odyssey Infrared Imaging System (LI-COR Biosciences) at an intensity of 5.0 and resolution of 169 μm. Vasolin-containing protein (VCP; 110 kDa) was chosen as a loading control because it is ubiquitously expressed in human brain, and VCP was verified to be unchanged in schizophrenia consistent with prior reports [17].
Table 1.
Antibodies used for Western Blot Analysis
| Antibody | Species | Dilution | Buffer | Incubation | Company (Cat. #) |
|---|---|---|---|---|---|
| Arp2 | Rabbit | 1:500 | Li-Cor | 16hr 4°C | PTG (10922-1-AP) |
| Arp3 | Rabbit | 1:5000 | Li-Cor | 16hr 4°C | PTG (13822-1-AP) |
| N-WASP | Rabbit | 1:1000 | 5% BSA | 16hr 4°C | PTG (14306-1-AP) |
| WAVE1 | Rabbit | 1:3500 | 5% BSA | 16hr 4°C | Abcam (ab50356) |
| Cortactin | Rabbit | 1:1000 | 5% BSA | 16hr 4°C | PTG (11381-1-AP) |
| Abi1 | Rabbit | 1:1000 | Li-Cor | 16hr 4°C | Sigma Aldrich (A5106) |
| VCP | Mouse | 1:10000 | Li-Cor | 1hr RT | Abcam (ab11433-50) |
Abbreviations: vasolin-containing protein, VCP; room temperature, RT; Protein Tech Group, PTG; bovine serum albumin, BSA
Data Analysis
Image Studio 4.0 analytical software (LI-COR Biosciences) was used to measure the near-infrared fluorescence value for each target protein band of interest. For each subject, the mean of duplicate lanes for each protein was normalized to intralane VCP. Data were analyzed using Prism 6.04 software (GraphPad, La Jolla, CA). The D'Agostino-Pearson test was used to test for normality of distribution for each dependent variable. For experiments using human subjects, normally distributed data were analyzed using two-tailed paired Student’s t-tests, and for data not normally distributed, Wilcoxon matched-pair signed-rank tests were used. Data from rats were normally distributed and analyzed using two-tailed unpaired Student’s t-test. For all statistical analyses, α = 0.05.
Results
Cortactin expression is decreased in schizophrenia
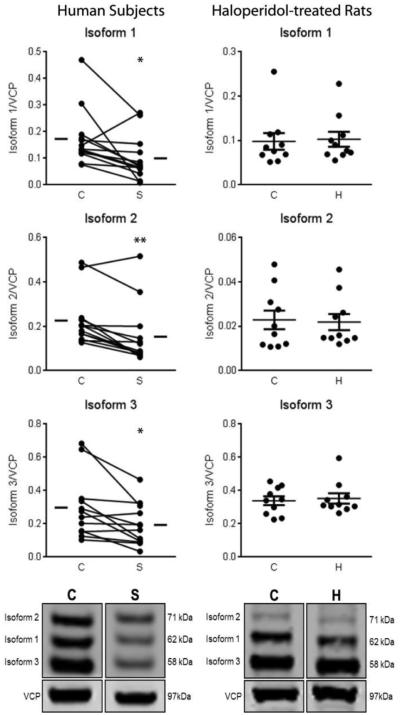
We measured the protein expression of Arp2, Arp3, N-WASP, WAVE1, Abi1, and cortactin in STG of paired schizophrenia and comparison subjects. The antibody used for cortactin recognized three unique isoforms, consistent with previous reports [18, 19]. We found decreased expression of all three isoforms of cortactin (Figure 1) in patients with schizophrenia, while none of the other proteins were different between groups (Arp2 t = 0.84; Arp3 t = 1.69; N-WASP t = 0.40; WAVE1 W = −25; Abi1 W = 45; Figure 2, Table 2). Cortactin isoform 1 (62kDa) was decreased by 43%; isoform 2 (71 kDa) was decreased by 32%; and isoform 3 was decreased by 35%. Correlation analysis of all significant dependent measures with age, pH, and PMI revealed no significant correlations between protein expression and any of these variables.
Figure 1.
Expression of isoforms of cortactin in STG from paired schizophrenia (S) and comparison (C) subjects (left) and in rats chronically treated with haloperidol decanoate (H) or vehicle (C) (right). Data are presented as mean signal intensity for the isoform of interest divided by the signal of intralane vasolin-containing protein (VCP). All three isoforms of cortactin are decreased in schizophrenia, but are unchanged in haloperidol-treated rats. p < 0.05*; p < 0.01**
Figure 2.
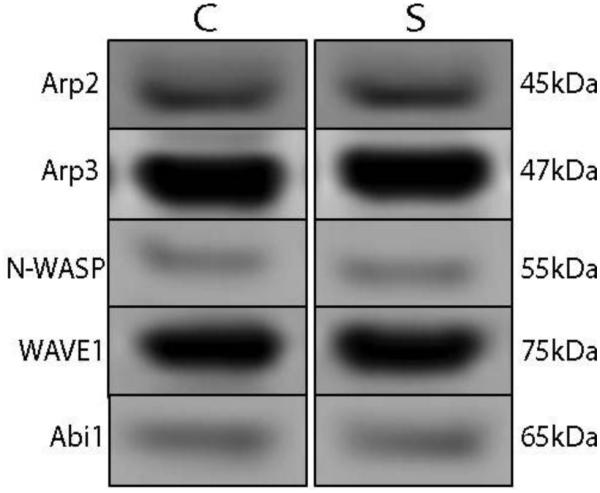
Representative images of immunoblots illustrating expression of Arp2, Arp3, N-WASP, WAVE1, and Abi1 in schizophrenia (S) and comparison (C) subjects. Expression of these proteins did not differ between subject groups.
Table 2.
Expression of Arp2/3 Complex Proteins in Schizophrenia
| Protein | Comparison | Schizophrenia | Test Statistic | p |
|---|---|---|---|---|
| Arp2 | 0.17±0.04 | 0.18±0.05 | t(12)=0.84 | n.s. |
| Arp3 | 2.62±0.60 | 2.42±0.70 | t(12)=1.69 | n.s. |
| N-WASP | 0.07±0.02 | 0.06±0.02 | t(12)=0.40 | n.s. |
| WAVE1 | 0.42±0.22 | 0.34±0.18 | W=−25 | n.s. |
| Abi1 | 0.22±0.09 | 0.26±0.11 | W=45 | n.s. |
| Cortactin Iso 1 (62 kDa) | 0.17±0.08 | 0.10±0.06 | t(12)=2.62 | 0.02 |
| Cortactin Iso 2 (71 kDa) | 0.23±0.11 | 0.16±0.12 | W=-85 | 0.001 |
| Cortactin Iso 3 (58 kDa) | 0.30±0.19 | 0.19±0.12 | t(11)=2.99 | 0.01 |
Data are averaged target protein expression normalized to intralane vasolin-containing protein (VCP) and presented as means ± standard deviation. Not significant, n.s.
Haloperidol does not alter the expression of cortactin in rat brain
To address the possibility that changes in expression of the three isoforms of cortactin were due to the schizophrenia subjects receiving antipsychotic treatment, we conducted parallel experiments in rats chronically treated with haloperidol decanoate. The protein expression of the three cortactin isoforms was not different between haloperidol and vehicle-treated rats (Figure 1).
Discussion
In this study, we assessed the expression of proteins directly involved in Arp2/3 complex-dependent actin polymerization in STG of schizophrenia and comparison subjects. We found decreased expression of all three isoforms of cortactin in schizophrenia, while there were no differences in expression of Arp2, Arp3, WAVE1, N-WASP, or Abi1 between groups. Expression of these cortactin isoforms was unchanged in the cortex of rats chronically treated with haloperidol.
The filamentous actin (F-actin) binding protein cortactin has been implicated in the progression of many cancers [20], though its role in neuropsychiatric illness is not well-defined. Cortactin plays a role in actin polymerization by binding and activating the Arp2/3 complex at its N-terminal acidic domain [18], and knockdown of cortactin has been shown to lead to diminished dendritic spine density and formation in vitro, potentially through interaction with Dock4 [10, 21]. Three isoforms of cortactin arising from two splice variants have been identified [18, 19]. Compared to isoforms 1 (62 kDa) and 2 (71 kDa), Isoform 3 (58kDa) exhibits decreased F-actin bundling activity, leading to reduction in Arp2/3 recruitment to F-actin and Arp2/3 complex-mediated actin polymerization [19]. Cortactin-mediated activation of the Arp2/3 complex differs from WASP or SCAR/WAVE-mediated activation, in that cortactin-mediated Arp2/3 activation moderately potentiates actin nucleation and leads to assembly of stable branched actin networks, while WASP or SCAR/WAVE-mediated activation strongly potentiates actin nucleation but leads to formation of unstable branched actin networks that quickly disassemble [22]. Our finding of decreased expression of all three isoforms of cortactin suggests decreased binding and subsequent activation of the Arp2/3 complex, which may lead to reduced assembly of stable actin networks in schizophrenia and hence contribute to abnormal dendritic spine morphology.
We did not find altered expression of Arp2 or Arp3, though the function of the Arp2/3 complex may be compromised in schizophrenia. For example, protein-protein interactions within the complex may be altered, diminishing the activity of the complex in nucleating F-actin. Recently, Arp2/3 knockout mice were found to exhibit many hallmark neurobiological traits of schizophrenia, including decreased spine formation, enhanced excitation of cortical neurons, and abnormal striatal output, suggesting dysfunction of the complex in this illness [23]. Phosphorylation of Arp2/3 complex activators may also be dysregulated. WAVE1 phosphorylation has been shown to inhibit Arp2/3 complex-dependent actin polymerization and decrease dendritic spine density [24], and N-WASP will only bind and activate the Arp2/3 complex upon phosphorylation at the Tyr-526 residue [25].
There are limitations to this study. The patients studied were elderly and in the late stages of this disorder, and these findings may not generalize to younger patients. Potential effects of chronic antipsychotic treatment on dependent measures are a concern in schizophrenia studies. We assessed the expression of the 3 isoforms of cortactin in frontal cortex of rats chronically treated with haloperidol and found no difference in these protein levels. Accordingly, it is unlikely that the abnormal protein expression we report here is due to medication effects, and more likely associated with the illness itself.
In conclusion, we found decreased expression of all three isoforms of cortactin in schizophrenia brain. This alteration may attenuate stable branched actin network formation and thereby contribute to the altered dendritic spine pathology observed in schizophrenia. As dendritic spines are the prime recipients of excitatory synaptic input, a decrease in dendritic spine density can impair synaptic plasticity, which may contribute to cognitive dysfunction associated with schizophrenia. These data implicate dysregulated cortactin-mediated Arp2/3 complex-dependent actin polymerization as a potential substrate of the pathophysiology of this illness.
Acknowledgments
Source of Funding: Funding was provided by NIH grants MH53327 (JMW), MH066392 (VH), and MH064673 (VH)
Footnotes
Conflicts of Interest: None of the authors have any conflicts to disclose.
References
- 1.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 2.Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- 3.Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;22:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubio MD, Haroutunian V, Meador-Woodruff JH. Abnormalities of the Duo/Ras-related C3 botulinum toxin substrate 1/p21-activated kinase 1 pathway drive myosin light chain phosphorylation in frontal cortex in schizophrenia. Biol Psychiatry. 2012;10:906–914. doi: 10.1016/j.biopsych.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinner AL, Haroutunian V, Meador-Woodruff JH. Alterations of the myristoylated, alanine-rich C kinase substrate (MARCKS) in prefrontal cortex in schizophrenia. Schizophr Res. 2014;154:36–41. doi: 10.1016/j.schres.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 7.Kim IH, Racz B, Wang H, Burianek L, Weinberg R, Yasuda R, et al. Disruption of Arp2/3 results in asymmetric structural plasticity of dendritic spines and progressive synaptic and behavioral abnormalities. J Neurosci. 2013;33:6081–6092. doi: 10.1523/JNEUROSCI.0035-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodal AA, Sokolova O, Robins DB, Daugherty KM, Hippenmeyer S, Riezman H, et al. Conformational changes in the Arp2/3 complex leading to actin nucleation. Nat Struct Mol Biol. 2005;12:26–31. doi: 10.1038/nsmb870. [DOI] [PubMed] [Google Scholar]
- 9.Kirkbride KC, Sung BH, Sinha S, Weaver AM. Cortactin: a multifunctional regulator of cellular invasiveness. Cell Adh Migr. 2011;5:187–198. doi: 10.4161/cam.5.2.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hering H, Sheng M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J Neurosci. 2003;23:11759–11769. doi: 10.1523/JNEUROSCI.23-37-11759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echarri A, Lai MJ, Robinson MR, Pendergast AM. Abl interactor 1 (Abi-1) wave-binding and SNARE domains regulate its nucleocytoplasmic shuttling, lamellipodium localization, and wave-1 levels. Mol Cell Biol. 2004;24:4979–4993. doi: 10.1128/MCB.24.11.4979-4993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park E, Chi S, Park D. Activity-dependent modulation of the interaction between CaMKIIα and Abi1 and its involvement in spine maturation. J Neurosci. 2012;32:13177–13188. doi: 10.1523/JNEUROSCI.2257-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;11:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- 14.Rajarethinam RP, DeQuardo JR, Nalepa R, Tandon R. Superior temporal gyrus in schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res. 2000;41:303–312. doi: 10.1016/s0920-9964(99)00083-3. [DOI] [PubMed] [Google Scholar]
- 15.Zierhut KC, Schulte-Kemna A, Kaufmann J, Steiner J, Bogerts B, Schiltz K. Distinct structural alterations independently contributing to working memory deficits and symptomatology in paranoid schizophrenia. Cortex. 2013;49:1063–1072. doi: 10.1016/j.cortex.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Rubio MD, Wood K, Haroutunian V, Meador-Woodruff JH. Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology. 2013;38:1910–1920. doi: 10.1038/npp.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller TM, Haroutunian V, Meador-Woodruff JH. N-Glycosylation of GABAA receptor subunits is altered in Schizophrenia. Neuropsychopharmacology. 2014;39:528–537. doi: 10.1038/npp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowieson NP, King G, Cookson D, Ross I, Huber T, Hume DA, et al. Cortactin Adopts a Globular Conformation and Bundles Actin into sheets. J Biol Chem. 2008;283:16187–16193. doi: 10.1074/jbc.M708917200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Rossum AG, de Graaf JH, Schuuring-Scholtes E, Kluin PM, Fan YX, Zhan X, et al. Alternative splicing of the actin binding domain of human cortactin affects cell migration. J Biol Chem. 2003;278:45672–45679. doi: 10.1074/jbc.M306688200. [DOI] [PubMed] [Google Scholar]
- 20.McGrath SM, Koleske AJ. Cortactin in cell migration and cancer at a glance. J Cell Sci. 2012;125:1621–1626. doi: 10.1242/jcs.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueda S, Negishi M, Katoh H. Rac GEF Dock4 interacts with cortactin to regulate dendritic spine formation. Mol Biol Cell. 2013;24:1602–1613. doi: 10.1091/mbc.E12-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uruno T, Liu J, Li Y, Smith N, Zhan X. Sequential interaction of actin-related proteins 2 and 3 (Arp2/3) complex with neural Wiscott-Aldrich Syndrome Protein (N-WASP) and Cortactin during branched actin filament network formation. J Biol Chem. 2003;278:26086–26093. doi: 10.1074/jbc.M301997200. [DOI] [PubMed] [Google Scholar]
- 23.Kim IH, Rossi MA, Aryal DK, Racz B, Kim N, Uezu A, et al. Spine pruning drives antipsychotic-sensitive locomotion via circuit control of striatal dopamine. Nat Neurosci. 2015;18:883–891. doi: 10.1038/nn.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- 25.Rajput C, Kini V, Smith M, Yazbeck P, Chavez A, Schmidt T, et al. Neural Wiskott-Aldrich syndrome protein (N-WASP)-mediated p120-catenin interaction with Arp2-Actin complex stabilizes endothelial adherens junctions. J Biol Chem. 2013;288:4241–4250. doi: 10.1074/jbc.M112.440396. [DOI] [PMC free article] [PubMed] [Google Scholar]