Abstract
Tumor cells evade immune destruction, at least partially, by upregulating inhibitory signals to limit effector T cell activation. Programmed death 1 (PD-1) is one of the most critical co-inhibitory molecules limiting the T-cell antitumor response. PD-1 and its ligands, PD-L1 and PD-L2, are overexpressed by various types of tumors as well as reactive cells in the tumor microenvironment. A growing body of evidence has shown the clinical efficiency and minimal toxicity of PD-1 pathway inhibitors in patients with solid tumors, but the role of these inhibitors in lymphoid malignancies is much less well studied. In this review, we review the pathologic role of the PD-1 pathway in most common lymphoid malignancies and we organize the clinical data from clinical trials of PD-1 pathway inhibitors. Several anti–PD-1 regimens have shown encouraging therapeutic effects in patients with relapsed/refractory Hodgkin lymphoma, follicular lymphoma, and diffuse large B cell lymphoma. Additional progress is needed to foster an improved understanding of the role of anti–PD-1 therapy in reconstituting antitumor immunity in patients with lymphoid malignancies. Upcoming trials will explore the clinical efficiency of combining PD-1 pathway inhibitors and various agents with diverse mechanisms of action and create more therapeutic possibilities for afflicted patients.
Keywords: PD-1, PD-L1, PD-L2, lymphoid malignancies, pidilizumab, nivolumab, pembrolizumab
1. Introduction
The immune system has the critical function of balancing pathogen eradication while also limiting self-reactivity to avoid collateral tissue damage. This highly organized process is controlled by complex interactions between antigen-presenting cells (APCs) and T cells and determines the intensity and eventual outcome for each immune response. In the context of chronic infection or cancer, failure to eliminate antigens results in progressive loss of T cell function marked by sustained overexpression of multiple inhibitory receptors, indicating an evasive mechanism co-opted by tumor cells to avoid immune destruction [1]. The observation that blocking these critical immune checkpoints led to tumor regression in mouse models has promoted further clinical investigations [2–4].
Programmed death-1 (PD-1), also known as PDCD1 or CD279, is a key regulator of tumor immune escape. PD-1 delivers a co-inhibitory signal upon binding to either of its two ligands, PD-L1 (CD274, B7-H1) or PD-L2 (PDCD1LG2, CD273, B7-DC) [5]. Blockade of the PD-1/PD-L pathway has shown great promise in the treatment of patients with various tumors, including advanced non–small cell lung cancer; prostate renal cell and colorectal carcinoma; and especially melanoma [6,7]. This review focuses on PD-1/PD-L pathway dysfunction associated with lymphoid malignancies as well as the preclinical rationale and clinical application of antibodies against PD-1 or its ligands for patients with diverse lymphoid malignancies.
2. Structural features of PD-1 and its ligands
PD-1 is encoded by PDCD1, located on chromosome 2q37.3. PD-L1 and PD-L2 are encoded by CD274 and the adjacent PDCD1LG2, respectively, both on chromosome 9p24.1. PD-1 exists as a monomer on the cell surface of activated T cells, B cells, natural killer T cells, monocytes, and some dendritic cell subsets [5]. PD-1 is a 55-kDa type I transmembrane protein that belongs to the immunoglobulin superfamily. Structurally, PD-1 is composed of an extracellular IgV domain, a transmembrane domain, and an intracellular cytoplasmic tail that contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM). Both of these motifs can be phosphorylated upon PD-1 engagement and can then recruit Src homology region 2 domain-containing phosphatase-1 (SHP-1) and SHP-2, although ITSM seems to play the primary role in recruitment [8,9]. In contrast, the 40 kDa PD-L1 and 25 kDa PD-L2 contain extracellular IgV and IgC domains and a transmembrane domain, but lack an identifiable intracellular signaling domain [10]. PD-L1 and PD-L2 share 37% identity, but differ in their affinity for PD-1 and their expression patterns. PD-L2 has a 3-fold higher affinity [11] and is inducibly expressed on activated dendritic cells and some macrophages, whereas PD-L1 is widely expressed by both hematopoietic and non-hematopoietic cells [5].
PD-1 uses its IgV domain β-sheet to bind to the front β-sheets of PD-L1 and PD-L2, forming a surface that resembles the antigen-binding surface of antibodies and T cell receptors [12]. Six of 14 amino acids involved in binding PD-1 are fully conserved between the two ligands, suggesting their critical role in receptor recognition by both PD-L1 and PD-L2. Of note, disruption of W110, an amino acid that uniquely located only in the core of the binding interface of PD-L2, leads to significantly reduced PD-1 binding. Consequently, W110 is assumed to be an important determinant of the higher affinity of PD-L2 for PD-1 [13].
3. Inhibitory effects of PD-1 and its ligands on the immune system
3.1 Molecular mechanism of PD-1 signaling in T cells and B cells
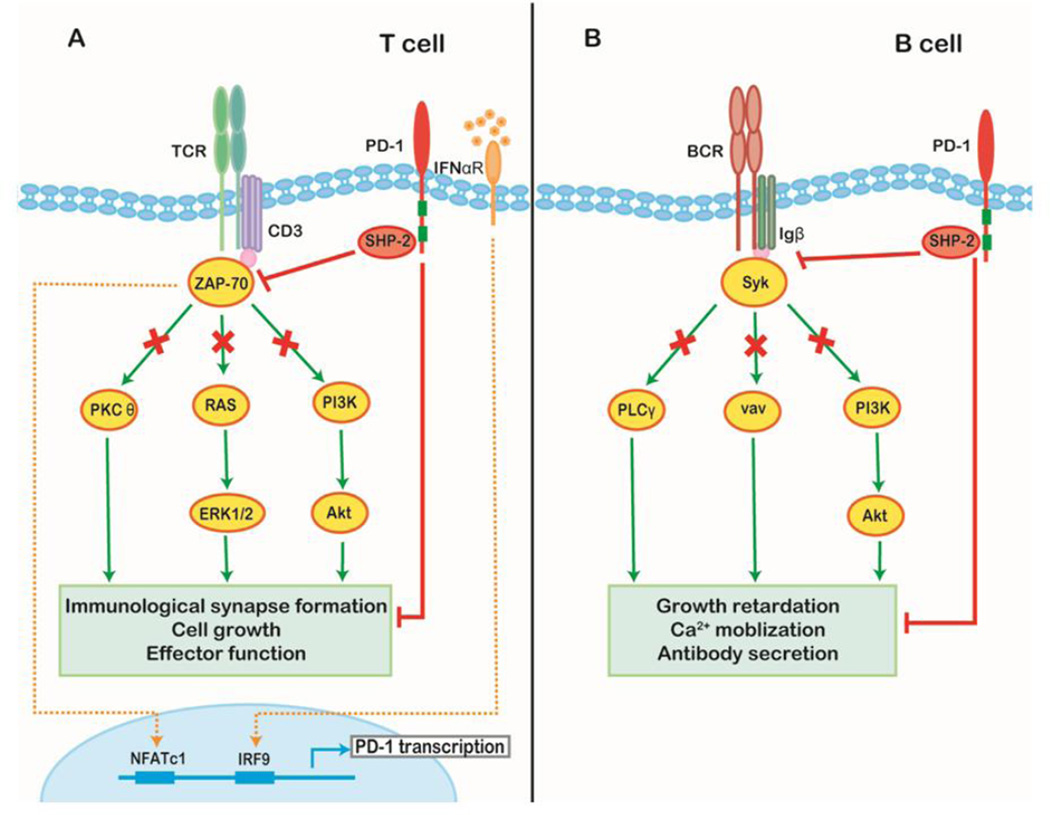
Upon T-cell receptor (TCR) engagement, PD-1 is transcriptionally activated in an NFATc1-dependent manner [14]. In chronically activated T cells, such transcriptional activity can be prolonged by interferon-α (IFN-α) stimulation to enable the constitutive expression of PD-1 by T cells [15]. In the early activation phase, PD-1 translocates from a random membrane location to the immunological synapse and accumulates at the central supramolecular activation cluster (c-SMAC) region upon ligand binding. SHP2, and to a lesser degree SHP1, are recruited immediately to the ITSM, directly dephosphorylate proximal TCR signaling molecules; SHP1 and SHP2 also block CD28-induced co-stimulation indirectly by driving CD28 away, but not excluding it from, the c-SMAC [16,17]. PD-1 ligation also directly inhibits phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and the downstream activity of Akt which rely on the integrity of ITSM [18]. Recently, PD-1 has been shown to increase phosphatase and tensin homologue (PTEN) activity through disruption of casein kinase 2 (CK2)-mediated phosphorylation, thereby suggesting a possible alternative means of suppressing the PI3K/Akt axis since PTEN is a crucial cellular phosphatase inhibitor of PI3K [19]. Moreover, PD-1 signaling also suppresses Ras-ERK1/2 mediated cell growth [20]. Collectively, activation of the PD-1 pathway antagonizes TCR signaling and ultimately decreases the induction of cytokines and survival proteins, leading to T cell exhaustion (Figure 1A).
Figure 1. Downstream molecular pathway of PD-1 signaling.
(A) TCR engagement initiates PD-1 transcription through NFATc1 binding at the 5’ promoter region of PD-1. Transcriptional activity is prolonged by sustained stimulation of interferon-α in the context of chronic infection or tumor through association of IFN-responsive factor 9 (IRF9) with the IFN stimulation response element within the PD-1 promoter. Upon PD-1 engagement, SHP1/2 are recruited and dephosphorylate downstream members of the TCR signaling pathway (e.g. CD3δ and ZAP70) disrupting the normal TCR response as well as inhibiting PKCθ, RAS-Erk, and PI3K–Akt signaling. Consequently, PD-1 activation reduces the stability of the immunological synapse as well as the level of T cell survival proteins and leads to impaired cell growth and effector function. (B) In B cells, the recruitment of SHP-2 reduces the tyrosine phosphorylation levels of key signal transducers including the Igβ, Syk, PLCγ, vav, and PI3K pathways, thus suppressing BCR-mediated growth retardation, Ca2+ mobilization, and antibody secretion.
PD-1 is also a negative regulator of B cells. Co-ligation of PD-1 with the B cell receptor (BCR) recruits SHP2 and subsequently attenuates the tyrosine phosphorylation of key signal transducers including Igβ and spleen tyrosine kinase (Syk), phospholipase C-gamma 2 (PLCγ2), PI3K, and vav leading to inhibition of BCR signaling [9]. Accordingly, PD-1 blockade can improve B cell responsiveness towards antigens [21]. PD-1 signaling activation is also responsible for the suppression of B-1b cell proliferation and overall B cell antibody production in response to T cell-independent type 2 antigens in normal mice (Figure 1B) [22].
3.2 Co-inhibitory network of PD-1 signaling in immunity
The phenotype of PD-1 knockout mice is characterized by late-onset, organ-specific autoimmunity, highlighting the role of PD-1 in induction and maintenance of immune tolerance [23,24]. PD-1 ligation provides inhibitory signals that prevent self-reactive effector T cells from attacking normal tissues and regulates both central and peripheral tolerance [5,25].
Besides its inhibition of T cell survival, proliferation, and cytokine production, PD-1 signaling is assumed to shorten the duration of T cell–APC contact, which is required for the stable formation of immunological synapses [16,26]. However, this model has been challenged because a more rapid detachment of T cells from APCs was observed upon PD-1 blockade in an LCMV infection mouse model [27], implying additional complexity of the PD-1 pathway.
During the immune response, PD-L1 levels on APCs are elevated upon the secretion of inflammatory cytokines by activated T cells and natural killer (NK) cells [28,29]. Notably, PD-L1 also can bind to CD80 (B7-1), a ligand of CD28, thereby competitively antagonizing the costimulatory signal delivered by CD28-CD80 binding and further diminishing TCR signaling [30,31]. Moreover, PD-L1 may deliver reverse signals into its host cells. One group has reported that engaging PD-L1 on bone marrow–derived dendritic cells with soluble PD-1 can induce indoleamine 2,3-dioxygenase (IDO)-independent IL-10 production and dendritic cell inactivation [32]. Similar reverse signaling was also observed in PD-L1+ T cells and PD-L2+ dendritic cells [33,34]. Further studies are needed to confirm these observations.
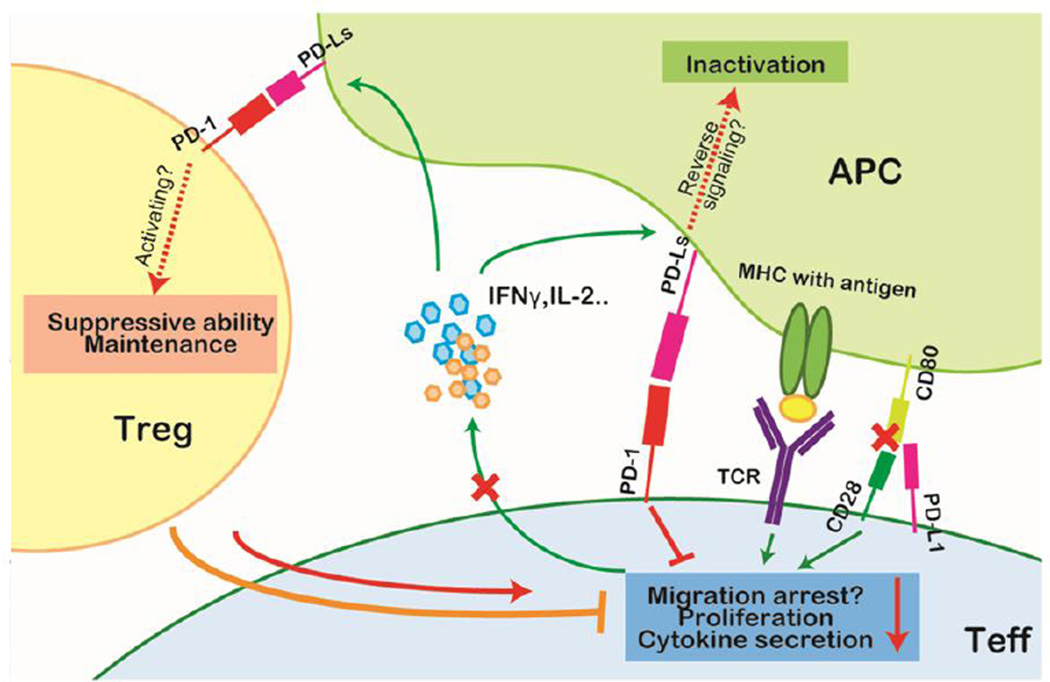
The PD-1–PD-L1 interaction has been reported to play a critical role in the development and maintenance of T regulatory (Treg) cells. Francisco et al. demonstrated that PD-L1 promotes the conversion of naïve T cells into Treg cells by synergizing with TGF-β in vitro and that mice with PD-L1 deficiency have remarkable reductions of Treg cells [35]. However, Franceschini and colleagues showed somewhat contrary results, finding that the expansion of Treg cells was correlated inversely with PD-1 expression in patients chronically infected with hepatitis C virus (HCV) and that PD-L1 blockade can facilitate Treg cell proliferation in vitro [36]. A recent report suggested that the apparent contradictory effects of PD-1 signaling may be attributed to the different status of Treg cells during infection; PD-1 ligation activates Treg cells at the initial stage of infection, but also induces Treg cell apoptosis after sustained TCR stimulus (Figure 2) [37].
Figure 2. PD-1 signaling in the cellular immune response.
In the cellular immune response, IFN-γ serves as a measure of T-cell activation. Upon initial TCR ligation, T cells secrete IFN-γ which induces PD-L1 upregulation on antigen-presenting cells (APCs). Activated T cells highly express PD-1. The activation of the PD-1 signaling pathway negatively regulates effector T (Teff) cells by limiting cytokine secretion and proliferation, and possibly affects the retention time on APCs to execute cytotoxic activity. PD-L1 also can competitively bind to CD80 and inhibit the costimulation effect of CD28. Moreover, although controversial, PD-1 signaling is presumed to promote the maintenance and suppressive ability of regulatory T (Treg) cells and thus further strengthen the inhibition of effector function. Additionally, reverse signaling through PD-Ls may deliver negative signals to the APCs.
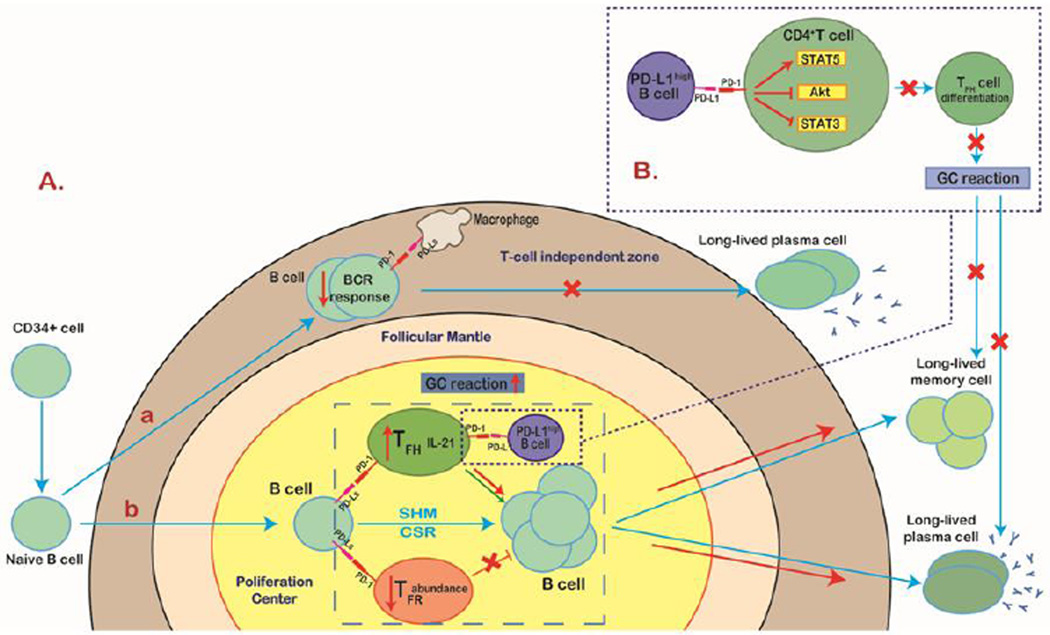
PD-1 regulates antibody responses. PD-1 signaling has been shown to inhibit the T cell– independent humoral response through downregulation of the BCR response in mice [22]. Nevertheless, PD-1 signaling mediates T cell–dependent humoral immunity by a completely different mechanism. In germinal centers (GCs), PD-1 is highly expressed by follicular helper T (TFH) cells and follicular regulatory T (TFR) cells, whereas PD-L1 and PD-L2 are inducibly upregulated on GC B cells and memory B cells during the primary response. PD-1–deficient mice showed greater numbers of TFR cells and qualitatively deficient TFH cells that secreted less IL-21, a cytokine essential for GC formation as well as B cell proliferation and differentiation into memory B cells and plasma cells [38–40]. Additionally, a recent report identified a specific subset of B regulatory cells that inhibits TFH cell function through elevated expression of PD-L1, further indicating the complexity of the role of PD-1 signaling in the humoral immune response (Figure 3) [41].
Figure 3. PD-1 signaling in the humoral immune response.
A (a) PD-1 is highly expressed on naïve B cells. In T cell–independent humoral immunity, activation of PD-1 signaling inhibits the B cell receptor response and therefore suppresses B1 cell expansion and subsequent long-lived IgG production. (b) In sharp contrast with the innate-like response, PD-1 signaling acts as a promoter, at least partially, for T cell–dependent humoral immunity. Both follicular helper T (TFH) and follicular regulatory T (TFR) cells express PD-1. TFH cells, with a phenotype of CXCR5+PD-1hiFoxp3−, are specialized for helping germinal center (GC) reaction, whereas TFR cells, displaying CXCR5+PD-1hiFoxp3+, potently inhibit TFH cell function. In the proliferation center, the ligation of PD-1 with PD-Ls expressed on B cells profoundly reduces the abundance of TFR cells and increases the level of IL-21 produced by TFH cells, leading to an enhanced GC reaction and the formation of long-lived plasma cells and memory cells. (B) However, a specific B cell subset that highly expresses PD-L1 can suppress TFH cell function in a PD-L1–dependent manner. PD-L1high B cells restrict CD4+ T cell differentiation to TFH cells by upregulating STAT5 and synchronous inhibition of Akt and STAT3 and therefore downregulate the GC reaction and reduce T cell–dependent humoral immunity.
PD-1 expression can be induced on NK cells after prolonged exposure to antigens during chronic infection or tumor [42, 43]. Benson et al. observed a reduction of NK cell migration and deterioration of cytotoxicity towards PD-L1–bearing tumor cells upon PD-1 overexpression, both of which were reversed by PD-1 blockade [43]. In addition, the existence of PD-1 microclusters at the NK immunological synapse has been confirmed [44].
4. Expression and clinical significance of PD-1 and its ligands on lymphoid malignancies
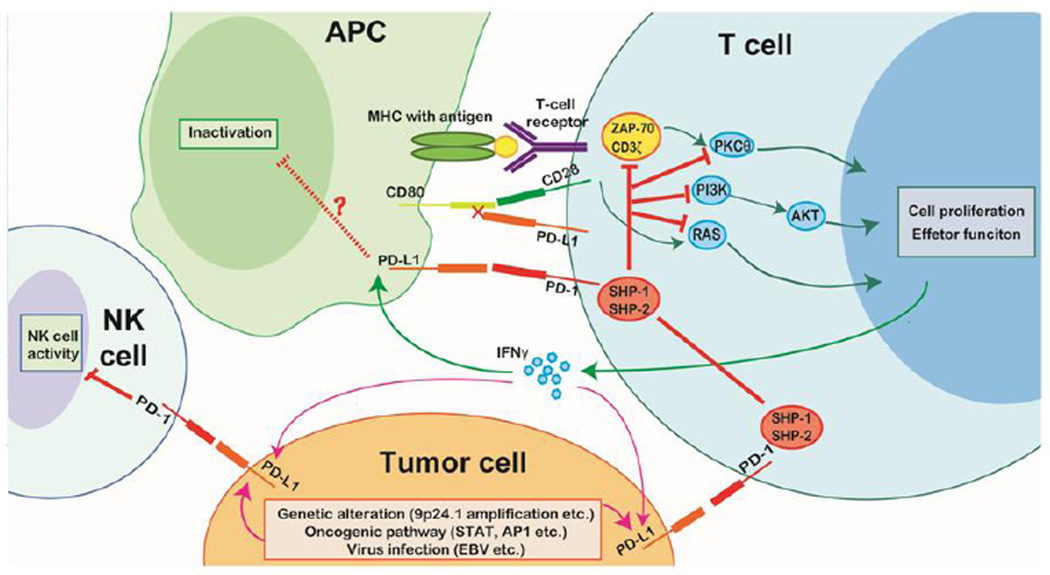
The interaction of PD-1 and its ligands plays a pivotal role in the immune suppression associated with tumors. Persistent stimulation from tumor antigens induces PD-1 overexpression by tumor-infiltrating lymphocytes, whereas expression of PD-Ls is characteristic of malignant cells and various tumor-infiltrating APCs. In lymphoid malignancies, diverse mechanisms contribute to the constitutive expression of PD-Ls by tumor cells, including genetic alterations, transcriptional activation by certain oncogenic signaling pathways, viral infection, and induction by inflammatory cytokines [1,45,46]. Binding of PD-Ls to PD-1 on T cells functionally silences the activation of tumor-associated T cells and leads to impaired cell survival and effector function, producing a tumor-permissive microenvironment (Figure 4) [47].
Figure 4. PD-1 signaling in the presence of tumor cells.
Tumor cells typically overexpress PD-L1 due to intrinsic factors (genetic alterations, activation of certain signaling pathways, and viral infection) and extrinsic factors (cytokines, such as IFN-γ, secreted by T cells attempting to destroy tumor cells). Interactions of tumor cell PD-L1 and PD-1 on immune cells of the tumor microenvironment (T cells and NK cells) induce immune cell exhaustion and result in failure to activate antitumor immunity.
4.1 Hodgkin lymphoma
In most cases of classical Hodgkin lymphoma (cHL), Reed-Sternberg and Hodgkin (RS-H) cells show strong membranous expression of PD-L1. Approximately 40% of patients of with nodular sclerosis cHL have amplification of chromosome 9p24.1, a region that includes PD-L1 and PD-L2; this amplification explains the overexpression of PD-Ls at the transcriptional and protein levels. Additionally, another gene located on 9p24.1 is also amplified, Janus kinase 2 (JAK2), which can transcriptionally activate the candidate STAT-responsive element within the PD-L1 and PD-L2 promoter regions through JAK2-signal transducer and activator of transcription (JAK2-STAT) signaling, thereby further promoting overexpression of PD-Ls [45,48].
Alternative mechanisms driving PD-L1 expression by RS-H cells in cHL include Epstein-Barr virus (EBV) infection and constitutive activator protein 1 (AP-1) activity [46]. Two AP-1 components, JUNB and cJUN, are overexpressed constitutively by RS-H cells [49]; binding of either can activate the AP-1–responsive enhancer element located on intron 1 of PD-L1 and enhance PD-L1 transcription in an AP-1–dependent manner. In contrast, there is no such enhancer for PD-L2, although PD-L2 is also upregulated in cHL cells [46]. Clinically, an increased level of PD-1+ infiltrating lymphocytes (more than 23 cells/mm2) has been found to be a stage-independent negative prognostic factor of overall survival for patients with cHL [50].
Much less data are available for nodular lymphocyte-predominant HL. In this disease, tumor-infiltrating T cells consistently express PD-1. In fact, PD-1+ T-cells form rosettes around the neoplastic cells and this is a typical feature of nodular lymphocyte-predominant HL [51].
4.2 Diffuse large B-cell lymphoma
Rare cases of diffuse large B-cell lymphoma (DLBCL),-not otherwise specified, are positive for PD-Ls and expression is largely restricted to activated B cell-like DLBCL [52,53]. In addition, the level of soluble PD-L1 is remarkably elevated in the plasma of DLBCL patients compared with healthy controls independent of PD-L1 expression by the lymphoma cells [54,55]. Increased soluble PD-L1 may be secreted by tumor-infiltrating non-malignant cells after pro-inflammatory cytokine stimulation as a feedback mechanism in response to inflammation [4,56]. In a prospective trial including 288 patients with newly diagnosed DLBCL who were treated with either rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or R-high dose therapy, investigators found that a soluble PD-L1 level in plasma of greater than 1.52 ng/ml at time of diagnosis was associated with an inferior overall survival in patients less than 60 years old with an age-adjusted International Prognostic Index score greater than or equal to 2. These investigators also observed dynamic changes in soluble PD-L1 levels that decreased dramatically in patients who achieved complete remission (CR), indicating a treatment-related immunological effect on both the lymphoma cells and the surrounding microenvironment [55].
Primary mediastinal large B-cell lymphoma (PMBCL) is a specific subtype of DLBCL that can resemble cHL in its clinical features and histologic features of cHL and PMBCL can overlap in some cases. Approximately 70% of PMBCLs harbor amplification of chromosome 9p24.1, thereby overexpressing PD-Ls as do cHL [45,48,57]. A recent report demonstrated that translocations involving chromosome 9p24.1 (the site of the PD-L locus) occur in approximately 20% patients with PMBCL. In translocated cases, PD-L2 transcript levels are significantly higher than in cases in which are 9p24.1 neutral, gained, or even amplified; PD-L1 transcript levels also are higher, but to a lesser extent [58].
Robust PD-L1 expression has been reported in several other DLBCL subtypes, including T cell/histiocyte-rich B cell lymphoma, EBV-associated DLBCL, plasmablastic lymphoma, HHV8-associated primary effusion lymphoma, and primary central nervous system DLBCL [52,59]. To date, only small series of patients with these tumors have been analyzed.
4.3 Follicular lymphoma
Follicular lymphoma (FL) is derived from GC B cells that are in close contact with PD-1+ follicular T cells. Using immunohistochemistry, Yang et al. observed that CD4+PD-1high T cells reside predominantly within lymph node follicles and resemble TFH cells that support B cell growth, whereas CD4+PD-1low T cells are located mainly in the interfollicular areas and display an exhausted phenotype with impaired cytokine production and signal transduction [60]. In contrast, Myklebust et al. reported that the cytokine signaling deficit was restricted in CD4+ PD-1high tumor-infiltrating T cells that contained both TFH and non-TFH cells and displayed functional changes similar to those in exhausted T cells by phospho-flow cytometry [61]. In aggregate, these results suggest the existence of diverse PD-1+ tumor-infiltrating T cell subsets with distinctive functions in the development of FL.
The correlation of intratumoral PD-1+ T cells with the clinical outcome of patients with FL has been controversial. Some immunohistochemistry studies have shown that a greater number of PD-1+ cells is associated with a better outcome independently of FL International Prognostic Index status and that patients with PD-1+ cells less than or equal to 5% showed a worse prognosis and greater risk of high-grade transformation [62,63]. However, other investigators have observed that the number of PD-1+ cells independently associated with poor prognosis [64]. One recent report suggested that prognostic groups can be subdivided according to the intensity of PD-1 expression and that only the amount of dimly stained cells correlates with disease outcome [60]. Overall, considering the different methods and heterogeneity of patients in these studies, the precise function of the PD-1 signaling pathway in FL and its prognostic value remain to be elucidated in a large series of patients.
4.4 Chronic lymphocytic leukemia
Chronic lymphocytic leukemia (CLL) relies on a tumor-supportive microenvironment [65]. The development of CLL is correlates significantly with increased numbers of CD8+ T cells; these cells highly express PD-1 and are skewed toward terminal differentiation. PD1+ T cells are in close contact with PD-L1+ CLL cells within the proliferation center and are exhausted [66]. In line with this finding, treating Eµ-TCL1 mice with PD-L1-blocking antibodies can re-activate antitumor immunity and prevent CLL development [67]. Riches et al. demonstrated that CLL-specific T cells, despite being exhausted, retain the capacity to produce IFN-γ and TNF-α, both of which have been shown to promote CLL as well as PD-L1 expression [68]; others have found contradictory results [66]. One possible explanation for these conflicting results is the heterogeneity of PD-1+ T cells within CLL. Indeed, in vivo experiments using the Eµ-TCL1 mouse model suggested that CLL-induced increased T-cell proliferation favors the PD-1high subset over the PD-1low subset and that IFN-γ ratios are higher in PD-1high than in PD-1low cells. Moreover, the distribution of PD-L1 expression on CLL cells seems to be tissue specific, with the highest frequency found in the spleen, which might offer an explanation for the tumor-protecting capability of microenvironmental niches [69]. Clinically, expansion of the CD8+PD1+ T cells has been associated with a more aggressive clinical course in CLL patients [70].
4.5 Mature T-cell lymphomas
Angioimmunoblastic T-cell lymphoma and approximately 20% of peripheral T-cell lymphomas not otherwise specified are presumed to originate from TFH cells and thus express the GC T cell marker PD-1 [71,72]. Cutaneous proliferations of T cells may express markers of TFH cells. Accordingly, PD-1 is expressed by the tumor cells of patients with Sézary syndrome [73], primary cutaneous CD4+ small/medium T-cell lymphoma [74], and a rare type of CD4/CD8 double-negative mycosis fungoides [75]. Additionally, PD-L1 is overexpressed by anaplastic lymphoma kinase positive (ALK+) anaplastic large cell lymphoma cells in a chimeric nucleophosmin/ALK-STAT3–dependent manner [76].
4.6 Virus-associated lymphomas
Viral infection induces PD-1 signaling–related immune tolerance by directly upregulating PD-L1 expression on tumor cells. Green et al. reported that LMP-1, an EBV-encoded antigen, was able to activate the promoter and enhancer of PD-L1 by enhancing JAK3-STAT5 signaling and AP-1 activation, respectively. This observation was further confirmed by the promotion of PD-L1 expression by B cells after EBV transformation and the synchronous activity of the PD-L1 promoter and enhancer [31]. EBV-associated lymphomas uniformly overexpress PD-L1, including EBV+ DLBCL, cHL, post-transplant lymphoproliferative disorders, extranodal nasal-type NK/T-cell lymphoma, and angioimmunoblastic T-cell lymphoma. Burkitt lymphoma is the exception to the rule owing to its type I latency pattern of infection with absence of LMP-1 expression [52,77].
Viral infection also upregulates PD-1 expression on tumor-infiltrating T cells. Ni et al. observed increased numbers of CD4+CD25+ and CD8+CD25+ Treg cells with PD-1 overexpression in the peripheral blood of patients with HCV-associated lymphomas compared with patients without HCV infection and healthy controls [78]. Similarly, increased levels of PD-1 can be found on human T-cell leukemia virus-1 (HTLV-1)–specific CD8+ T cells in patients with adult T-cell leukemia/lymphoma [63]. Interestingly, in these studies, PD-1 expression was significantly higher in cells of virus-related lymphomas compared with benign virally infected cells, reflecting a higher load of virus-associated antigens on the tumor cells [63].
The expression of components of the PD-1 signaling pathway in different lymphoid malignancies, according to the 2016 World Health Organization classification, is summarized in Table 1.
Table 1.
The expression of components of PD-1 signalling pathway in different lymphoid malignancies
| Lymphoma subtype |
Postulated normal counterpart |
Clinical features |
Immuno phenotype |
Tumor cells | Tumor microenvironment |
Clinical significance |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| PD 1 |
PD-L1 | PD-L2 | PD 1 |
PD L1 |
PD L2 |
|||||
| Mature B-cell neoplasms | ||||||||||
| Small lymphocytic lymphoma/ chronic lymphocytic leukemia |
Naïve or memory B-cells |
|
CD5+ CD10 CD20+/− CD23+ |
+ | +/− | − | + | .. | .. | ↑PD-1+ CD8(+) T lymphocytes : more advanced stage of disease |
| Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue |
Post germinal center, marginal zone B-cell |
|
CD5− CD10− CD20+ CD23− |
− | − | .. | +/− | .. | .. | |
| Nodal marginal zone Lymphoma |
Post-germinal center marginal B-cell |
|
CD5− CD10− CD20+ BCL2+ BCL6− |
− | − | .. | .. | .. | .. | |
| Follicular lymphoma | Germinal center B-cell |
|
CD5− CD10+ CD20+ BCL2+ BCL6+ |
III + I/II - |
− | − | + | + | .. | Controversy |
| Mantle cell Lymphoma |
Peripheral B-cell of inner mantle zone |
|
CD5+ CD10+ CD20+ FMC7+ CCND1+ |
− | +/− | − | − | .. | .. | |
| Diffuse large B-cell lymphomas, not other specified |
Peripheral B-cells of either germinal center or post germinal center origin |
|
CD5− CD10+/− CD20+ CD22+ |
− | GCB - Non- GCB + |
.. | +/− | .. | .. | ↑sPD-1 associated with inferior prognosis in young high risk patients |
| T-cell/histiocyte-rich B-cell lymphoma |
Germinal center B cells |
|
CD20+ BCL-6+ EMA+ BCL-2+ |
− | + | .. | + | + | .. | .. |
| Primary central nervous system lymphoma |
Activated (late germinal center) B-cell |
|
CD20+ CD10+/− BCL-6+ MUM- 1+ BCL-2+/− |
+/− | +/− | .. | + | +/− | .. | .. |
| EBV-positive DLBCL of Elderly |
Mature B lymphocyte, transformed by EBV |
Aggressive
|
CD20+ CD10− BCL-6− MUM- 1+ LMP1+ EBNA-2+ |
− | + | .. | .. | .. | .. | |
| Primary mediastina large B-cell lymphoma |
Thymic medullary, asteroid, (AID- positive), B-cell |
|
CD20+ CD10+/− CD30+ CD15+/− |
− | + | + | + | + | .. | |
| Plasmablastic Lymphoma |
Plasmablast |
|
CD38+ CD138+ CD20+/− CD56+/− |
.. | + | .. | .. | .. | .. | |
| Primary effusion Lymphoma |
Post-germinal center B-cell |
|
Lack pan-B-cell markers, BCL- 6-, LANA+ |
.. | + | .. | .. | .. | .. | |
| Burkitt lymphoma | Germinal center B- cell |
|
CD20+ CD10+ BCL6+ BCL2+/− TdT− |
− | − | − | .. | .. | .. | |
| Mature T- and NK-cell neoplasms | ||||||||||
| Adult T-cell leukaemia/lymphoma |
CD4+CD25+ FOXP3+ Treg |
|
CD4+ CD7+/− CD8+/− CD25+ CD30− FOXP3+ |
+ | +/− | .. | .. | + | .. | |
| Extranodal NK/T-cell Lymphoma |
Activated NK cells and cytotoxic T cells |
|
CD2+ CD56+ EBER+ CD3: surface- cytoplasmic+ |
− | + | .. | + | + | .. | |
| Mycosis fungoides | Mature skin homing CD4+ T cell |
|
CD2+ CD3+ CD4+CD5+ CD7+/− CD8− CLA+ |
+/− | + | .. | .. | .. | .. | |
| Sézary syndrome | Mature epidermotropic skin homing CD4+ T cell |
|
CD2+ CD3+ CD5+ CD7+/ CD4+CD8− TCRβ+ CCR4+ |
+ | .. | .. | .. | .. | .. | |
| Angioimmunoblastic T-cell lymphoma |
germinal center TFH |
|
CD4+ CD8+/− CD10+ BCL-6+ PD-1+ CXCL13+ |
+ | +/− | .. | .. | + | .. | |
| Anaplastic large cell Lymphoma |
Activated mature cytotoxic T cell. |
|
CD4−CD8+/− CD30+ ALK-1+/− |
+/− | + | .. | .. | .. | .. | |
| Hodgkin lymphoma | ||||||||||
| Classical Hodgkin Lymphoma |
Germinal center B- cell |
|
CD30+ CD15+ CD20+/− PAX5+ |
− | + | + | + | + | − | ↑PD-1+>23 cells/mm2 stage- independent negative prognostic factor for OS |
| Nodular lymphocyte predominant Hodgkin lymphoma |
Germinal center B- cell |
|
CD15− CD30− CD20+ BCL-6+ AID+ |
− | +/− | .. | + | .. | .. | |
Abbreviations: PD-1: programmed cell death-1; PD-L: programmed cell death ligand; +: expressed; -: not expressed; +/−: discordant result; ..: not reported.
5. Targeting PD-1 and its ligand pathways
Preclinical studies of PD-1 signaling pathway blockade have shown promising clinical efficiency in a broad variety of tumors. Anti–PD-1 therapy helps to blunt the inhibition of tumor-specific immune response by enhancing T-cell activity, restoring NK cell activity, and inducing PD-L1+ B-cell antibody production, thus benefiting patients with pre-existing antitumor immunity [29]. PD-1 inhibitors may be particularly attractive for patients with hematologic malignancies because the activation of adoptive immunity has long been proven effective in the form of allogeneic stem cell transplantation [79]. Diverse antibodies targeting the PD-1 pathway, by blockade of PD-1 or PD-L1, are under clinical evaluation (Table 2). Here, we focus on agents that have been used for the treatment of patients with lymphoid malignancies (Table 3).
Table 2.
Agents of PD-1, its ligands and clinical trials
| Target | Symbol | Type of construction | Developed by |
|---|---|---|---|
| PD-1 | Nivolumab* (BMS-936558, MDX- 1106, ONO-4538) |
Fully human IgG4 mAb (Whole antibody) | Bristol-Myers Squibb |
| Pembrolizumab# (lambrolizumab, MK-3475) |
Humanized IgG4 mAb (Whole antibody) | Merck | |
| Pidilizumab (CT-011) | Humanized (from mouse) IgG1 mAb (Whole antibody) | CureTech Ltd | |
| AMP-224 | Extracellular domain of PD-L2 and the Fc region of human IgG(fusion protein) |
Amplimmune/AstraZeneca | |
| AMP-110 | Monoclonal antibody | Amplimmune/AstraZeneca | |
| MEDI0680 (AMP-514) | Monoclonal antibody | Amplimmune/AstraZeneca | |
| PD-L1 | MPDL3280A (RG7446) | IgG1 mAb to PDL1 with an engineered Fc domain(Whole antibody) |
Genentech/Roche |
| BMS-936559 (MDX-1105) | Fully human IgG4 mAb (Whole antibody) | Bristol-Myers Squibb | |
| MEDI4736 | IgG1 mAb to PDL1 with an engineered Fc domain (Whole antibody) |
MedImmune/AstraZeneca | |
| MSB0010718C | Fully human IgG1 mAb | Merck KGaA |
Japan and U.S.A both approves Nivolumab (Opdivo®) for advanced melanoma.
FDA of U.S.A approves Pembrolizumab (Keytruda®) for advanced melanoma.
Table 3.
Completed Clinical Trials of anti-PD-1 and PD-L1 antibodies in patients with lymphoid malignancies
| Agent tested(Phase) |
Patients (No.) | Treatment arms | Response rates | Response duration | Adverse Events No. of patients |
|---|---|---|---|---|---|
| Pidilizumab(I) | Advanced stage of CLL (3), AML (7), NHL (4), HL (1) and MM (1) following chemotherapy and/or SCT. |
Pidilizumab five dose groups of 0.2, 0.6, 1.5, 3, and 6 mg/kg×1 cycles |
Clinical benefit: ORR: 33%(5/15) 1 CR(FL) 4 SD(2 CLL; 1 HL; 1 MM) 1 MR(AML) |
Mean survival time : 25±27 weeks Survival time: 1.7∼77 weeks. Averaging survival time of response patients: > 60 weeks |
All AEs: grade 1/2. Related to treatment 1 weakness, 1 flushing Other: 2 diarrhea, 1 rash, 1 urine infection, 1 pain, 1 back pain, 1 shortness of breath, 1 blurred vision, 1 pressure wound, |
| Pidilizumab (II) | Sixty-six patients after AHSCT from 30 to 90 days: de novo DLBCL (49); PMBCL (4); transformed indolent B- NHL (13) |
Pidilizumab 1.5 mg/kg every 42 days×3 cycles |
Measurable patients: ORR: 51% (CR:34%; PR: 17%) SD: 37% PD:11% PET-CT positive patients: ORR:33% SD:44% |
At 16 months: Measurable patients: PFS: 72% (90% CI, 60% to 82%) OS: 85% (90% CI, 74% to 92% PET-CT positive patients: PFS: 70%(90% CI, 51% to 82%) |
613 AEs occurred in 69 of patients in all 72 patients Related to treatment: 135 AEs The most common AEs Grade 3 to 4 14 neutropenia, 6 thrombocytopenia Grade 2 7 neutropenia, 5 URTI Grade 1 16 fatigue, 11 cough |
| Pidilizumab(II) | Adult patients with rituximab-sensitive follicular lymphoma relapsing after one to four previous therapies(32) |
Pidilizumab: All: 3 mg/kg q 4 weeks × 4 times Optional: stable disease or better plus 8 times q 4 weeks Rituximab: 375 mg/m2 q w × 4 weeks, starting 17 days after the first pidilizumab iv. |
Median follow up 15·4 months ORR: 66% CR: 52% PR: 14% Measurable tumor regression: 86% |
MTR: All: 88 days (53 to 392 days). Delayed responsers:6/29(21%)>4m Median PFS all patients: 18·8 m (95% CI 14·7 to not reached) Responders: not reached (95% CI 18·8 to not reached) Response duration: 20.2 m (95% CI 13·9 to not reached) |
No autoimmune or treatment-related AEs of grade 3 or 4. The most common AEs: Grade 2: 5 respiratory infection Grade 1: 14 anemia; 13 fatigue |
| Nivolumab(I) | Adult patients with relapsed or refractory Hodgkin’s lymphoma(23) |
Nivolumab: 3mg/kg every 2 weeks until CR, PD, or excessive toxic effects. |
Median follow up 24 months ORR: 87% CR: 17% PR: 70% SD: 13% |
PFS: 86% Median overall survival: NR Median duration: 10 m (0 to 18.75 m) |
Any grade AEs: 22 (96%) Grade 3: 12 (52%) Drug-related AEs: 18 (78%) The most common AEs: 5(22%) rash, 4 (17%) ↓ platelet, 3(13%) fatigue; Drug-related grade 3 AEs: 5 patients (22%): 1. pancreatitis, pneumonitis, stomatitis, colitis, gastroenteritis; 2. ↑lipase; 3. MPN, thrombocytopenia, ↓ lymphocyte, leukopenia. |
Abbreviations: AML: Acute Myeloid Leukemia; CML: Chronic Lymphocytic Leukemia; NHL: non-Hodgkin’s lymphoma; HL: Hodgkin’s lymphoma; MM: multiple myeloma; MPN: myelodysplastic syndrome; SCT: stem cell transplantation; AHSCT: autologous hematopoietic stem-cell transplantation; CR, complete response; PR: partial responses; SD, stable disease; MR: minimal response; NR: not reported; ORR: overall response rate; OR: objective response; MTR: median time to response; AE: adverse events; irAEs: immune-related adverse events; URTI: upper respiratory tract infection;
5.1 Pidilizumab
Pidilizumab (CT-011), a humanized IgG-1 kappa recombinant monoclonal antibody, was the first PD-1 inhibitor used for hematologic malignancies. One phase I, single arm, open-label study of pidilizumab enrolled 17 patients with advanced hematologic malignancies, including eight with acute myeloid leukemia, four with non-Hodgkin lymphoma (NHL; 1 FL, 2 DLBCL, 1 anaplastic large cell lymphoma), three with CLL, one with HL, and one with multiple myeloma. All patients had relapsed disease after failure of multiple lines of chemotherapy, including six patients who had received allogeneic stem cell transplants and three who had received autologous stem cell transplants. Patients received escalating doses of pidilizumab ranging from 0.2 to 6.0 mg/kg in a single intravenous infusion. No dose-limiting toxicity or maximum tolerated dose was determined. Despite receiving only a single dose of pidilizumab, one FL patient achieved CR with prolonged remission of greater than 68 weeks. Sustained elevation in peripheral blood CD4+ lymphocyte levels following treatment was observed in 15 of 17 patients from 24 hours after infusion to up to 21 days [80].
On the basis of the encouraging data from this phase I trial, a phase II trial was conducted focused on patients with recurrent DLBCL who had received autologous hematopoietic stem cell transplantation (AHSCT). The rationale of the protocol was to utilize the synergistic effect of breaking immune tolerance through PD-1 blockade to reconstitute antitumor immunity after AHSCT. Patients with DLBCL, PMBCL, and transformed indolent B-NHL who had undergone AHSCT were eligible. Patients received pidilizumab intravenously at a dose of 1.5 mg/kg every 42 days for three cycles, beginning 30 to 90 days after AHSCT. CT scans were performed at screening, before the second and third cycles, and at 30, 44, and 69 weeks after the first administration of pidilizumab. Of 72 patients who were enrolled, 60 finished all three cycles. Among the 66 patients eligible for analysis, 35 had measurable disease by CT imaging and nine had positive results on PET scan at the time of post-AHSCT restaging. The median time to response was 30 weeks (6–69 weeks). The overall 16-month progression-free survival (PFS) was 72% (90% CI, 60%–82%), exceeding the prespecified 16-month PFS of 69%. The 16-month overall survival was 85% (90% CI, 74%–92%). Notably, among the 35 patients with residual disease after AHSCT, the overall response rate was 51%, with 12 (34%) CRs and six (17%) PRs. However, the overall response rate dropped to 33% for the nine patients with positive PET scan results. The most frequently observed grade 3 to 4 adverse effects (AEs) were neutropenia and thrombocytopenia in 19% and 8% of patients, respectively. No significant autoimmune toxicity, infusion reactions, or treatment-related mortality was observed. Treatment with pidilizumab resulted in a significant increase in the absolute number of PD-L1–bearing activated helper T cells and monocytes [79].
Another phase II trial was conducted in patients with relapsed, grade 1–2 FL treated with pidilizumab in combination with rituximab, in the hope of achieving synergistic effects via activation of both the innate (NK cells) and adaptive (T cells) immune system [81]. Pidilizumab was administered at 3 mg/kg intravenously every 4 weeks for four infusions, plus eight optional infusions every 4 weeks for patients with stable disease or better. Rituximab was given at 375 mg/m2 intravenously weekly for 4 weeks starting 17 days after the first dose of pidilizumab. Assessments were performed at enrollment, after the second and fourth infusions of pidilizumab, and every 12 weeks thereafter for 2 years or until relapse. There were 29 evaluable patients with a median follow-up of 15.4 months; 19 (66%) responded to the therapy among which 15 (52%) achieved CR and four (14%) achieved PR. The median PFS for responders had not been reached by the time of the analysis (95% CI 18.8 months–not reached) which compared favorably with the 17.8 months reported with responders to rituximab monotherapy retreatment [82]. The median time to response was 88 days (range 53–392 days); six patients (21%) had a delayed initial response after 4 months or more. The treatment was well tolerated without any autoimmune or treatment-related AE of grade 3 or 4.
In this study, the baseline expression of PD-L1 was significantly higher in peripheral blood CD4+ cells, CD8+ cells, and CD14+ cells of responders than that of non-responders. Gene expression profiling data suggested that baseline signatures of genes upregulated during T cell activation or repressed in regulatory T cells were predictive of longer PFS. They also showed that signatures of genes more highly expressed by effector T cells compared with TFH in sorted CD4+ T cells predicted more tumor shrinkage and longer PFS. After 14 days of infusion, the absolute numbers of CD4+ effector and memory T cells increased, as did the activating receptor KLRK1 (killer cell lectin-like receptor subfamily K, member 1) on NK cells. Similarly, increased expression of T cell activation signatures after treatment was associated with longer PFS.
5.2 Nivolumab
Nivolumab (BMS-936558, MDX-1106, ONO-4538) is a fully humanized IgG4 blocking monoclonal antibody against PD-1 that has been approved by the US Food and Drug Administration for the treatment of unresectable primary and metastatic melanoma and squamous non–small cell lung cancer.
Classical HL may be a promising target for anti-PD1 therapy because PD-L1 is overexpressed by RS-H cells and the extensive but ineffective inflammatory immune cell infiltration in the tumor microenvironment [45,46,48]. Nivolumab was tested in a phase I trial in patients with relapsed or refractory HL. The trial enrolled 23 heavily pretreated patients, of whom 87% had received at least three previous regimens. Among all patients in the trial, 78% had received brentuximab vedotin and 78% had undergone AHSCT. Nivolumab was administered at 3 mg/kg every 2 weeks until the patient experienced CR, PD or excessive toxic effects. Patients were evaluated for treatment efficacy at 4, 8, 16, and 24 weeks and every 16 weeks thereafter. Twenty (87%) patients had objective responses including four (17%) who achieved CRs. The median PFS at 24 weeks was 86% (95% CI 62–95%). With a median follow-up of 40 weeks median OS was not reached. Sixty percent of responders had an initial response within 8 weeks, whereas other patients had a delayed response up to 39 weeks. Grade 3 or 4 AEs occurred in 12 (52%) patients, five of which were reported as drug related, including one decreased lymphocyte count, one increased serum lipase level, one stomatitis, one myelodysplastic syndrome, and one pancreatitis. Notably, increased copy numbers of PD-L1 and PD-L2 with protein overexpression were observed in all 10 patient samples that underwent FISH and immunochemical testing in this study [83].
Nivolumab was also evaluated in a cohort of patients with relapsed or refractory lymphoid malignancies. This phase I clinical trial enrolled 82 patients, including 29 with B-NHL, two with PMBCL, and 23 with T-NHL. Sixty-nine percent of B-NHL patients and 78% of T-NHL patients were pretreated extensively, including prior brentuximab vedotin treatment in 7% of B-NHL patients and 26% of T-NHL patients, and AHSCT in 14% of B-NHL patients and 9% of T-NHL patients. Nivolumab was administered using a dose-escalation design (1 mg/kg and 3 mg/kg) every 2 weeks for up to 2 years. Four (36%) patients with DLBCL, four (40%) with FL, two (15%) with mycosis fungoides, and two (40%) with peripheral T-cell lymphoma responded to the therapy, among whom one patient (9%) with DBLCL and one (10%) with FL achieved CR. Drug-related AEs occurred in 72% and 65% of B-NHL and T-NHL patients, respectively [84].
5.3 Pembrolizumab
Pembrolizumab (lambrolizumab, MK-3475), a humanized IgG-4 kappa isotype monoclonal antibody, is the second anti–PD-1 drug approved by the US Food and Drug Administration for the treatment of patients with unresectable and metastatic melanoma. In a phase I clinical trial of 15 patients with relapsed or refractory cHL whose disease had failed brentuximab vedotin therapy. Pembrolizumab was given at 10 mg/kg every 2 weeks. The preliminary results at 12 weeks showed that eight (53%) patients had objective responses including three (20%) who had CRs. The treatment was safe, with only one grade 3 AE. The trial is still ongoing [85].
In addition to anti-PD-1 agents, several antibodies targeting PD-L1 are also under clinical evaluation in patients with solid tumors and hematologic malignancies, including BMS935559 (MDX-1105), MPDL3280A, MEDI4736, and MSB0010718C (Table 4).
Table 4.
Ongoing studies of anti-PD-1 and PD-L1 antibodies in patients with lymphoid malignancies
| Agent | ClinicalTrial.gov ID | Phase | Additional Therapy | Disease |
|---|---|---|---|---|
| Pembrolizumab | NCT02362997 | II | None | R/R DLBCL or cHL prior to AHSCT |
| Pembrolizumab | NCT02408042 | Ib/II | DLBCL: RICE; cHL: ICE + Pembrolizumab or brentuximab vedotin |
R/R DLBCL or cHL |
| Pembrolizumab | NCT02332668 | I/II | None | R/R lymphoma patient less than 18 years old |
| Pembrolizumab | NCT01953692 | I | None | R/R HL, PMBCL, MM, PD-L1+ NHL, DLBCL or FL |
| Pembrolizumab | NCT02332980 | II | None | R/R CLL or other low grade B-NHL |
| Pembrolizumab | NCT02362035 | Ib/II | ACP-196 | NHL, MM, CLL, RS or WM |
| Pembrolizumab | NCT02243579 | II | None | Newly diagnosed MF or SS (Except for stage IA), Recurrent MF or SS |
| Nivolumab | NCT02038946 | II | None | Refractory FL without evidence of transformation |
| Nivolumab | NCT02038933 | II | None | R/R DLBCL failed or not fit for AHSCT |
| Nivolumab | NCT02304458 | I/II | Ipilimumab | Refractory HL or NHL between 12 months to 30 years old |
| Nivolumab | NCT02253992 | I/II | Urelumab | Previously treated advanced B-NHL |
| Nivolumab | NCT01592370 | I | Ipilimumab/lirilumab | R/R NHL, HL or MM |
| Nivolumab | NCT02181738 | II | None | R/R HL post AHSCT |
| Nivolumab | NCT02327078 | I/II | INCB24360 | R/R HL or NHL |
| Nivolumab | NCT02329847 | I/II | Ibrutinib | R/R B-NHL or CLL |
| MPDL3280A | NCT02220842 | I | Obinutuzumab | R/R FL or DLBCL |
| MPDL3280A | NCT01375842 | I | None | advanced hematologic malignancies |
| MEDI4736 | NCT02401048 | Ib/II | Ibrutinib | R/R FL or DLBCL |
| MEDI4736 | NCT02205333 | Ib/II | Rituximab | Refractory DLBCL |
| MEDI0680 (AMP-514) | NCT02271945 | Ib/II | MEDI-551 | R/R DLBCL, MCL or Grade 3B FL |
Abbreviations: FL: Follicular Lymphoma; DLBCL: Diffuse Large B-Cell Lymphoma; MM: Multiple myeloma; WM: Waldenström’s macroglobulinemia; RS: Richter Syndrome; MF: Mycosis fungoides; SS: Sezary syndrome; MCL: Mantle cell lymphoma; cHL: Classic Hodgkin’s lymphoma; PMLBCL: Primary mediastinal large B cell lymphoma; NHL: non-Hodgkin’s lymphoma; AHSCT: Autologous hematopoietic stem cell transplantation; ICE: Ifosfamide, carboplatin, and etoposide; RICE: Rituximab, ifosfamide, carboplatin, and etoposide;
6. Immune-related adverse events
Although PD-1 pathway blockade can lead to significant antitumor benefits, the synchronous general immunologic enhancement may give rise to a unique set of toxicities that have been designated as immune-related AEs (irAEs). The onset of irAEs is mainly due to the elevated inflammatory cytokines released by activated T cells that infiltrate the affected areas [86,87]. In a study by the National Cancer Institute, 62% of patients with metastatic melanoma who received ipilimumab, an anti-CTLA-4 agent, experienced irAEs [88]. The irAEs observed in PD-1 pathway blockade had no apparent dose-limiting toxicities and were less severe compared with those in anti-CTLA-4 trials. In PD-1 pathway blockade, the irAEs most commonly seen have been colitis/diarrhea, dermatitis, hepatitis, and endocrinopathies [89]. Reported irAEs in the aforementioned trials included diplopia, rash, mucosal inflammation, pneumonitis, stomatitis, pancreatitis, and enteritis. Fatigue, respiratory infection, cough, diarrhea, hyperglycemia, renal failure, pain, and urinary infection were also observed. However, it would be difficult to attribute all these adverse events to irAEs [79–81,83–85]. IrAEs can be life threatening, early recognition of irAEs and initiation of treatment are critical. Oral or intravenous corticosteroids are often used to control irAEs by temporary immunosuppression. Other options include TNF-α antagonists and mycophenolate mofetil [89]. The use of immunosuppressive treatment does not seem to compromise the antitumor effect, and no solid evidence so far has correlated the occurrence of irAEs with the clinical efficacy of checkpoint blockade [89,90].
7. Biomarkers
The efficacy of PD-1 blockade regimens seems to vary among different types of lymphoma, as a relatively small dose can induce a sustained CR in patients with certain types of indolent lymphoma whereas patients with other types of lymphoma barely benefit [80,84]. A better understanding of which patients benefit from PD-1 blockade would surely help enhance clinical efficiency and reduce unnecessary overuse. Although indicators of pre-existing immunity, such as expression of PD-L1 on tumor cells and expression of PD-1 by tumor-infiltrating T cells, have been associated with better response rates for anti–PD-1 treatment in solid tumors [91], no definitive predictive biomarkers have been proposed, likely due to highly dynamic changes in the tumor microenvironment.
Little is known about predicting response to PD-1 pathway blockade in lymphoid malignancies. Pretreatment PD-L1 expression on peripheral blood CD4+ cells, CD8+ cells, and CD14+ cells seemed to be a positive biomarker for clinical response in phase II trials of patients with relapsed/refractory FL treated with pidilizumab and rituximab [81]. Likewise, a significant increase in the absolute number of PD-L1–bearing activated helper T cells and monocytes after pidilizumab administration has been observed in patients with DLBCL after AHSCT [79]. Nevertheless, PD-L1 expression is not an absolute indicator for PD-1 blockade therapy because patients without PD-L1 expression can still have impressive clinical responses. Moreover, methodological considerations of PD-L1 assessment, such as the positive cut-off value and whether staining the tumor cells or infiltrating immune cells is relevant, remain to be determined [92]. Further investigation regarding PD-L1 and its interactions with other possible prognostic factors is needed.
8. Evaluation criteria of therapeutic response
Early experience suggests that immune checkpoint blockade agents, including anti–PD-1 and anti–CTLA-4 antibodies, are characterized by long median times before patients respond. Patients may require up to 1 year or more to achieve an objective response, although the response is durable and may exist even after withdrawal of drug [1,80,92]. Accordingly, in trials of PD-1 blockade in lymphoma patients, a considerable number of responders and even patients with stable disease have benefited with prolonged survival and remarkable reduction of tumor lesions. However, response may be obscured by early or simultaneously progressing lesions within the same patient during treatment [93]. Therefore, Wolchok et al. proposed a set of alternative evaluation criteria, specific for immunologic checkpoint blockade, called the immune-related response criteria. The immune-related response criteria allow new lesions to be included as part of the total tumor burden without being immediately considered as progressive disease, in order to avoid premature treatment withdrawal [94]. However, the immune-related response criteria are based mainly on experience using anti–CTLA-4 agents on solid tumors and are still undergoing revision and validation. All data from the clinical trials described above were evaluated by the traditional revised response evaluation criteria in solid tumors (RECIST) criteria adapted for lymphoma. An adequate evaluation system is required to better address the role of PD-1 blockade and other antibodies targeting immune checkpoint molecules in the future.
9. Opportunities for combinational therapy
9.1 Dual checkpoint blockade
As mentioned above, CTLA-4 wass the first recognized immune checkpoint molecule to play a non-redundant role with PD-1 in immune regulation. Unlike PD-1 which acts mainly as a brake on T cell effector function at a later stage of T cell activation, CTLA-4 is believed to be upregulated earlier in T cell activation, by directly blocking the costimulatory signal of CD28, and exhibits its inhibitory effect through a different mechanism [18,95]. Dual checkpoint blockade was therefore designed to synergistically remove co-inhibition signals and thus enhance antitumor immunity. Superior antitumor activity has been shown in patients with solid tumors by combining anti–CTLA-4 and anti–PD-1 agents [96,97]. Larkin et al. demonstrated a favorable median PFS of 11.5 months in previously untreated melanoma patients with nivolumab plus ipilimumab (an anti–CTLA-4 antibody) compared with 2.9 months with only ipilimumab and 6.9 months with only nivolumab. Two clinical trials of dual checkpoint blockade with nivolumab and ipilimumab for patients with relapsed/refractory NHL, HL, or multiple myeloma are currently recruiting (NCT02304458, NCT01592370) (Table 4). Of note, a higher occurrence of irAEs has been observed during combined checkpoint blockade due to the significant activation of host immunoreaction [89].
9.2 In combination with immune modulators
The combination of immune checkpoint blockade with other immune modulators may also act in a synergistic manner. Ibrutinib is aBruton tyrosine kinase (BTK) inhibitor approved by the US Food and Drug Administration for the treatment of some types of B-cell malignancy. Ibrutinib also inhibits interleukin-2–inducible T-cell kinase (ITK), an essential enzyme for Th2 T cells and therefore ibrutinib has been purported to have an immunomodulatory effect by skewing a shift of the T-cell immune responses to a Th1 T cell bias [98]. Recently, Sagiv-Barfi et al. reported a remarkable therapeutic outcome following the combined use of anti–PD-L1 and ibrutinib in a mouse model of lymphoma that was intrinsically insensitive to ibrutinib monotherapy [99]. The antitumor effect induced by the combination depended on augmentation of host T-cell immune response rather than BTK expression on tumor cells, suggesting a wide spectrum of indications besides B-cell malignancies. Clinical trials designed to test the efficacy of this and similar combinations on patients with relapsed/refractory NHL and HL are ongoing (NCT02401048, NCT02329847, and NCT02362035) (Table 4).
Antibodies that operate as agonists of costimulatory T-cell and NK-cell receptors also show promise as agents to be tested in combination with immune checkpoint blocking agents [100]. CD137 (4-1BB), CD134 (OX40), and glucocorticoid-induced TNFR (GITR; CD357) are members of the TNF receptor family and specialize in delivering a costimulatory signal on the surface in T cells and NK cells [101]. Considering that anti–PD-1 therapies can only partially restore the function of exhausted T cells, combinations of agonists specific for these molecules with agents targeting PD-1 have been evaluated in animal models [102]. The combination has shown promising antitumor effect by further forcing exhausted CD8+ T cells to exit quiescence and possibly enhance NK cell–mediated cytotoxicity [103,104]. Clinical trials combining anti– PD-1 therapy with urelumab (targeting CD137) or lirilumab (an anti-KIR antibody) are now available for patients with lymphoid malignancies (NCT02253992 and NCT01592370) (Table 4).
Indoleamine 2,3-dioxygenase (IDO) is a direct inhibitor of immune response and promotes differentiation of Treg cells [105]. Pro-inflammatory stimuli such as IFN-γ, can induce IDO potentially counteracting the effectiveness of antitumor therapy [106]. Upregulation of IDO has been assumed to be a possible mechanism of immune checkpoint blockade resistance. Dual blockade of IDO and CTLA-4 led to enhanced infiltration of tumor-specific effector T cells and tumor growth retardation in a mouse model of melanoma. Similar effects have also been observed in IDO-deficient mice treated with anti–PD-1 therapy [107]. Clinical trials evaluating combinations of INCB024360 (a small molecule inhibitor of IDO) and nivolumab are currently underway for patients with relapsed/refractory HL or NHL (NCT02327078) (Table 4).
9.3 Targeted therapy
The combination of agents that prevent immune escape with genetic or cell-target therapies may prove particularly effective in selected patients. Treatment using pidilizumab and rituximab has achieved a high overall response rate and good tolerance in patients with relapsed FL [81]. Combinations with other novel antibodies specific for diverse targets are in clinical evaluation for patients with lymphoid malignancies (NCT02220842, NCT02205333, NCT02271945, and NCT02408042) (Table 4).
Another area of active exploration includes the combination of PD-1 inhibitors with traditional cytotoxic therapies such as chemotherapy and radiotherapy. Preclinical mouse model studies have shown that traditional cytotoxic therapies induce changes that affect the antitumor immune response and may partner favorably with immunotherapies [108]. Further studies will evaluate the efficiency and safety profile of traditional cytotoxic therapies in combination with PD-1 blockade agents (NCT02408042) (Table 4).
9.4 Other approaches
The combination of PD-1 signaling inhibitors with vaccines is an attractive treatment approach, as is the application of PD-1 signaling blockade after allogeneic stem cell transplantation. Tumor-specific vaccines help to enhance tumor antigen presentation and therefore facilitate immune response in the microenvironment [109]. Concurrent peptide vaccination with immune checkpoint blockade has led to increased CTL responses in mouse models and in some patients with solid tumors [110]. Dendritic cell fusion vaccines after autologous transplantation in patients with multiple myeloma (NCT01067287) and acute myeloid leukemia (NCT01096602) are under investigation. Although anti–PD-1 therapy may aggravate graft-versus-host disease, it can also enhance the graft-versus-lymphoma effect. Koestner et al. observed restoration of graft-versus-lymphoma effect without triggering graft-versus-host disease by PD-L1 blockade in mouse models [111]. However, the optimal dose and timing for drug administration are still under preliminary study [112].
10. Concluding remarks
Although the diverse genomic landscape of each tumor creates challenges for tumor cell targeted therapies, neoantigens derived from tumor-specific mutations are potential targets for immune recognition and elimination. PD-1 pathway inhibitors are designed to unleash host immune cells to exert a tumor-specific cytotoxic effect and these inhibitors have shown encouraging therapeutic effects with minimal toxicity in patients with multiple cancer types, including lymphoid malignancies. In particular, the global activation of effector T cells induced by PD-1 blockade may overcome the development of tumor resistance and benefit patients, including those who have been heavily pretreated. Additionally, anti–PD-1 therapy may be of interest in the treatment of virus-associated lymphomas. However, many questions remain regarding the identification of biologic predictors and the establishment of criteria for evaluating clinical response. Despite the preliminary results obtained from clinical trials for relapsed/refractory DLBCL, FL, and HL, it is currently unknown whether PD-1 pathway inhibitors would be effective in other types of lymphoid malignancies or as first-line therapy. On the other hand, the multitude of available agents with various mechanisms of action creates diverse possibilities for combination therapy; each combination is an opportunity to improve the therapeutic response for various types of lymphoid malignancies. Further knowledge of the reconstitution of antitumor immunity among different histologic tumor types and inter-patient variability is needed to shed light upon the rationale for combination regimens. PD-1 pathway modulation has a promising future for the treatment of patients with lymphoid malignancies.
Highlights.
PD-1 and its ligands are overexpressed by various types of tumors and microenvironment-supporting cells.
Therapeutic targeting of PD-1 signaling has shown promising antitumor efficacy.
Acknowledgements
This study was supported by the National Cancer Institute/National Institutes of Health (R01CA138688 and 1RC1CA146299 to KHY). YX is a recipient of a hematology and oncology scholarship award. KHY is supported by The University of Texas MD Anderson Cancer Center Lymphoma Moonshot Program, Institutional Research and Development Fund, an Institutional Research Grant Award, an MD Anderson Cancer Center Lymphoma Specialized Programs on Research Excellence (SPORE) Research Development Program Award, an MD Anderson Cancer Center Myeloma SPORE Research Development Program Award, and a Gundersen Lutheran Medical Foundation Award and is partially supported by the National Cancer Institute/National Institutes of Health (P50CA136411 and P50CA142509) and by MD Anderson’s Cancer Center Support Grant CA016672.
Disclosure/conflicts of interest
KHY receives research support from Roche Molecular System, Gilead Science Pharmaceutical, Seattle Genetics, Dai Sanyo Pharmaceutical, Adaptive Biotechnology, and HTG Molecular Diagnostics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 3.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 5.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 9.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 11.Youngnak P, Kozono Y, Kozono H, Iwai H, Otsuki N, Jin H, Omura K, Yagita H, Pardoll DM, Chen L, Azuma M. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307:672–677. doi: 10.1016/s0006-291x(03)01257-9. [DOI] [PubMed] [Google Scholar]
- 12.Lin DY, Tanaka Y, Iwasaki M, Gittis AG, Su HP, Mikami B, Okazaki T, Honjo T, Minato N, Garboczi DN. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci U S A. 2008;105:3011–3016. doi: 10.1073/pnas.0712278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazar-Molnar E, Yan Q, Cao E, Ramagopal U, Nathenson SG, Almo SC. Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc Natl Acad Sci U S A. 2008;105:10483–10488. doi: 10.1073/pnas.0804453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, Honjo T. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol. 2011;186:2772–2779. doi: 10.4049/jimmunol.1003208. [DOI] [PubMed] [Google Scholar]
- 16.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, Chaudhary D. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 18.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patsoukis N, Li L, Sari D, Petkova V, Boussiotis VA. PD-1 increases PTEN phosphatase activity while decreasing PTEN protein stability by inhibiting casein kinase 2. Mol Cell Biol. 2013;33:3091–3098. doi: 10.1128/MCB.00319-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholas KJ, Zern EK, Barnett L, Smith RM, Lorey SL, Copeland CA, Sadagopal S, Kalams SA. B cell responses to HIV antigen are a potent correlate of viremia in HIV-1 infection and improve with PD-1 blockade. PLoS One. 2013;8:e84185. doi: 10.1371/journal.pone.0084185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol. 2011;187:5183–5195. doi: 10.4049/jimmunol.1101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 24.Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94:25–39. doi: 10.1189/jlb.1212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zinselmeyer BH, Heydari S, Sacristan C, Nayak D, Cammer M, Herz J, Cheng X, Davis SJ, Dustin ML, McGavern DB. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med. 2013;210:757–774. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L, Li N. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846–857. doi: 10.1038/cr.2008.80. [DOI] [PubMed] [Google Scholar]
- 29.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, Liu Y, Strome SE, Chen L, Tamada K. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuipers H, Muskens F, Willart M, Hijdra D, van Assema FB, Coyle AJ, Hoogsteden HC, Lambrecht BN. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur J Immunol. 2006;36:2472–2482. doi: 10.1002/eji.200635978. [DOI] [PubMed] [Google Scholar]
- 33.Van Keulen VP, Ciric B, Radhakrishnan S, Heckman KL, Mitsunaga Y, Iijima K, Kita H, Rodriguez M, Pease LR. Immunomodulation using the recombinant monoclonal human B7-DC cross-linking antibody rHIgM12. Clin Exp Immunol. 2006;143:314–321. doi: 10.1111/j.1365-2249.2005.02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong H, Strome SE, Matteson EL, Moder KG, Flies DB, Zhu G, Tamura H, Driscoll CL, Chen L. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest. 2003;111:363–370. doi: 10.1172/JCI16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, Mondelli MU, Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119:551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park HJ, Park JS, Jeong YH, Son J, Ban YH, Lee BH, Chen L, Chang J, Chung DH, Choi I, Ha SJ. PD-1 Upregulated on Regulatory T Cells during Chronic Virus Infection Enhances the Suppression of CD8+ T Cell Immune Response via the Interaction with PD-L1 Expressed on CD8+ T Cells. J Immunol. 2015;194:5801–5811. doi: 10.4049/jimmunol.1401936. [DOI] [PubMed] [Google Scholar]
- 38.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, Kato LM, Fagarasan S. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336:485–489. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 41.Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun. 2015;6:5997. doi: 10.1038/ncomms6997. [DOI] [PubMed] [Google Scholar]
- 42.Norris S, Coleman A, Kuri-Cervantes L, Bower M, Nelson M, Goodier MR. PD-1 expression on natural killer cells and CD8(+) T cells during chronic HIV-1 infection. Viral Immunol. 2012;25:329–332. doi: 10.1089/vim.2011.0096. [DOI] [PubMed] [Google Scholar]
- 43.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, Greenfield CN, Porcu P, Devine SM, Rotem-Yehudar R, Lozanski G, Byrd JC, Caligiuri MA. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jang JH, Huang Y, Zheng P, Jo MC, Bertolet G, Zhu MX, Qin L, Liu D. Imaging of Cell-Cell Communication in a Vertical Orientation Reveals High-Resolution Structure of Immunological Synapse and Novel PD-1 Dynamics. J Immunol. 2015;195:1320–1330. doi: 10.4049/jimmunol.1403143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O’Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR, Kutok JL, Shipp MA. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O’Donnell E, Neuberg D, Shipp MA. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18:1611–1618. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honda T, Egen JG, Lammermann T, Kastenmuller W, Torabi-Parizi P, Germain RN. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity. 2014;40:235–247. doi: 10.1016/j.immuni.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hao Y, Chapuy B, Monti S, Sun HH, Rodig SJ, Shipp MA. Selective JAK2 inhibition specifically decreases Hodgkin lymphoma and mediastinal large B-cell lymphoma growth in vitro and in vivo. Clin Cancer Res. 2014;20:2674–2683. doi: 10.1158/1078-0432.CCR-13-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathas S, Hinz M, Anagnostopoulos I, Krappmann D, Lietz A, Jundt F, Bommert K, Mechta-Grigoriou F, Stein H, Dorken B, Scheidereit C. Aberrantly expressed c-Jun and JunB are a hallmark of Hodgkin lymphoma cells, stimulate proliferation and synergize with NF-kappa B. EMBO J. 2002;21:4104–4113. doi: 10.1093/emboj/cdf389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muenst S, Hoeller S, Dirnhofer S, Tzankov A. Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol. 2009;40:1715–1722. doi: 10.1016/j.humpath.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 51.Nam-Cha SH, Roncador G, Sanchez-Verde L, Montes-Moreno S, Acevedo A, Dominguez-Franjo P, Piris MA. PD-1, a follicular T-cell marker useful for recognizing nodular lymphocyte-predominant Hodgkin lymphoma. Am J Surg Pathol. 2008;32:1252–1257. doi: 10.1097/PAS.0b013e318165b0d6. [DOI] [PubMed] [Google Scholar]
- 52.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA, Rodig SJ. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17:4232–4244. doi: 10.1158/1078-0432.CCR-10-2660. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine. 2011;56:231–238. doi: 10.1016/j.cyto.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, Le Gouill S, Haioun C, Tarte K, Lamy T, Milpied N, Fest T. S. Groupe Ouest-Est des Leucemies et Autres Maladies du, S. Groupe Ouest-Est des Leucemies et Autres Maladies du, High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28:2367–2375. doi: 10.1038/leu.2014.137. [DOI] [PubMed] [Google Scholar]
- 56.Wolfle SJ, Strebovsky J, Bartz H, Sahr A, Arnold C, Kaiser C, Dalpke AH, Heeg K. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41:413–424. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- 57.Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, Chan WC, Zhao T, Haioun C, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Campo E, Montserrat E, Lopez-Guillermo A, Ott G, Muller-Hermelink HK, Connors JM, Braziel R, Grogan TM, Fisher RI, Miller TP, LeBlanc M, Chiorazzi M, Zhao H, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Staudt LM. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Twa DD, Chan FC, Ben-Neriah S, Woolcock BW, Mottok A, Tan KL, Slack GW, Gunawardana J, Lim RS, McPherson AW, Kridel R, Telenius A, Scott DW, Savage KJ, Shah SP, Gascoyne RD, Steidl C. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123:2062–2065. doi: 10.1182/blood-2013-10-535443. [DOI] [PubMed] [Google Scholar]
- 59.Berghoff AS, Ricken G, Widhalm G, Rajky O, Hainfellner JA, Birner P, Raderer M, Preusser M. PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL) Clin Neuropathol. 2014;33:42–49. doi: 10.5414/np300698. [DOI] [PubMed] [Google Scholar]
- 60.Yang ZZ, Grote DM, Ziesmer SC, Xiu B, Novak AJ, Ansell SM. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J. 2015;5:e281. doi: 10.1038/bcj.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myklebust JH, Irish JM, Brody J, Czerwinski DK, Houot R, Kohrt HE, Timmerman J, Said J, Green MR, Delabie J, Kolstad A, Alizadeh AA, Levy R. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. 2013;121:1367–1376. doi: 10.1182/blood-2012-04-421826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carreras J, Lopez-Guillermo A, Roncador G, Villamor N, Colomo L, Martinez A, Hamoudi R, Howat WJ, Montserrat E, Campo E. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol. 2009;27:1470–1476. doi: 10.1200/JCO.2008.18.0513. [DOI] [PubMed] [Google Scholar]
- 63.Wahlin BE, Aggarwal M, Montes-Moreno S, Gonzalez LF, Roncador G, Sanchez-Verde L, Christensson B, Sander B, Kimby E. A unifying microenvironment model in follicular lymphoma: outcome is predicted by programmed death-1--positive, regulatory, cytotoxic, and helper T cells and macrophages. Clin Cancer Res. 2010;16:637–650. doi: 10.1158/1078-0432.CCR-09-2487. [DOI] [PubMed] [Google Scholar]
- 64.Richendollar BG, Pohlman B, Elson P, Hsi ED. Follicular programmed death 1-positive lymphocytes in the tumor microenvironment are an independent prognostic factor in follicular lymphoma. Hum Pathol. 2011;42:552–557. doi: 10.1016/j.humpath.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 65.Riches JC, Gribben JG. Understanding the immunodeficiency in chronic lymphocytic leukemia: potential clinical implications. Hematol Oncol Clin North Am. 2013;27:207–235. doi: 10.1016/j.hoc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 66.Brusa D, Serra S, Coscia M, Rossi D, D’Arena G, Laurenti L, Jaksic O, Fedele G, Inghirami G, Gaidano G, Malavasi F, Deaglio S. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica. 2013;98:953–963. doi: 10.3324/haematol.2012.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McClanahan F, Hanna B, Miller S, Clear AJ, Lichter P, Gribben JGM. Seiffert, PD-L1 Checkpoint Blockade Prevents Immune Dysfunction and Leukemia Development in a Mouse Model of Chronic Lymphocytic Leukemia. Blood. 2015 doi: 10.1182/blood-2015-01-622936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, Ramsay AG, Gribben JG. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–1621. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McClanahan F, Riches JC, Miller S, Day WP, Kotsiou E, Neuberg D, Croce CM, Capasso M, Gribben JG. Mechanisms of PD-L1/PD-1 mediated CD8 T-cell dysfunction in the context of aging-related immune defects in the Emu-TCL1 CLL mouse model. Blood. 2015 doi: 10.1182/blood-2015-02-626754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nunes C, Wong R, Mason M, Fegan C, Man S, Pepper C. Expansion of a CD8(+)PD-1(+) replicative senescence phenotype in early stage CLL patients is associated with inverted CD4:CD8 ratios and disease progression. Clin Cancer Res. 2012;18:678–687. doi: 10.1158/1078-0432.CCR-11-2630. [DOI] [PubMed] [Google Scholar]
- 71.de Leval L, Rickman DS, Thielen C, Reynies A, Huang YL, Delsol G, Lamant L, Leroy K, Briere J, Molina T, Berger F, Gisselbrecht C, Xerri L, Gaulard P. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952–4963. doi: 10.1182/blood-2006-10-055145. [DOI] [PubMed] [Google Scholar]
- 72.Roncador G, Garcia Verdes-Montenegro JF, Tedoldi S, Paterson JC, Klapper W, Ballabio E, Maestre L, Pileri S, Hansmann ML, Piris MA, Mason DY, Marafioti T. Expression of two markers of germinal center T cells (SAP and PD-1) in angioimmunoblastic T-cell lymphoma. Haematologica. 2007;92:1059–1066. doi: 10.3324/haematol.10864. [DOI] [PubMed] [Google Scholar]
- 73.Cetinozman F, Jansen PM, Vermeer MH, Willemze R. Differential expression of programmed death-1 (PD-1) in Sezary syndrome and mycosis fungoides. Arch Dermatol. 2012;148:1379–1385. doi: 10.1001/archdermatol.2012.2089. [DOI] [PubMed] [Google Scholar]
- 74.Cetinozman F, Jansen PM, Willemze R. Expression of programmed death-1 in primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoma, cutaneous pseudo-T-cell lymphoma, and other types of cutaneous T-cell lymphoma. Am J Surg Pathol. 2012;36:109–116. doi: 10.1097/PAS.0b013e318230df87. [DOI] [PubMed] [Google Scholar]
- 75.Kempf W, Kazakov DV, Cipolat C, Kutzner H, Roncador G, Tomasini D. CD4/CD8 double negative mycosis fungoides with PD-1 (CD279) expression--a disease of follicular helper T-cells? Am J Dermatopathol. 2012;34:757–761. doi: 10.1097/DAD.0b013e31825b26d1. [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto R, Nishikori M, Tashima M, Sakai T, Ichinohe T, Takaori-Kondo A, Ohmori K, Uchiyama T. B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci. 2009;100:2093–2100. doi: 10.1111/j.1349-7006.2009.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paydas S, Bagir E, Seydaoglu G, Ercolak V, Ergin M. Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol. 2015 doi: 10.1007/s00277-015-2403-2. [DOI] [PubMed] [Google Scholar]
- 78.Ni L, Ma CJ, Zhang Y, Nandakumar S, Zhang CL, Wu XY, Borthwick T, Hamati A, Chen XY, Kumaraguru U, Moorman JP, Yao ZQ. PD-1 modulates regulatory T cells and suppresses T-cell responses in HCV-associated lymphoma. Immunol Cell Biol. 2011;89:535–539. doi: 10.1038/icb.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, Kaminski MS, Holland HK, Winter JN, Mason JR, Fay JW, Rizzieri DA, Hosing CM, Ball ED, Uberti JP, Lazarus HM, Mapara MY, Gregory SA, Timmerman JM, Andorsky D, Or R, Waller EK, Rotem-Yehudar R, Gordon LI. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31:4199–4206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 81.Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, Romaguera J, Hagemeister F, Fanale M, Samaniego F, Feng L, Baladandayuthapani V, Wang Z, Ma W, Gao Y, Wallace M, Vence LM, Radvanyi L, Muzzafar T, Rotem-Yehudar R, Davis RE, Neelapu SS. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coiffier B, Osmanov EA, Hong X, Scheliga A, Mayer J, Offner F, Rule S, Teixeira A, Walewski J, de Vos S, Crump M, Shpilberg O, Esseltine DL, Zhu E, Enny C, Theocharous P, van de Velde H, Elsayed YA, Zinzani PL, investigators LYMs. Bortezomib plus rituximab versus rituximab alone in patients with relapsed, rituximab-naive or rituximab-sensitive, follicular lymphoma: a randomised phase 3 trial. Lancet Oncol. 2011;12:773–784. doi: 10.1016/S1470-2045(11)70150-4. [DOI] [PubMed] [Google Scholar]
- 83.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ, Lebovic D, Dhodapkar MV, Avigan D, Chapuy B, Ligon AH, Rodig SJ, Cattry D, Zhu LL, Grosso JF, Kim SY, Shipp MA, Borrello I, Timmerman J. Preliminary Results of a Phase I Study of Nivolumab (BMS-936558) in Patients with Relapsed or Refractory Lymphoid Malignancies. Blood. 2014;124 Abstr 291. [Google Scholar]
- 85.Moskowitz CH, Ribrag V, Michot JM, Martinelli G, Zinzani PL, Gutierrez M, De Maeyer G, Jacob AG, Giallella K, Anderson JW, Derosier M, Wang J, Yang ZJ, Rubin E, Rose S, Shipp MA, Armand P. PD-1 Blockade with the Monoclonal Antibody Pembrolizumab (MK-3475) in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Preliminary Results from a Phase 1b Study (KEYNOTE-013) Blood. 2014;124 Abstr 290. [Google Scholar]
- 86.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnston RL, Lutzky J, Chodhry A, Barkin JS. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig Dis Sci. 2009;54:2538–2540. doi: 10.1007/s10620-008-0641-z. [DOI] [PubMed] [Google Scholar]
- 88.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015;35:76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- 90.Hinrichs CS, Palmer DC, Rosenberg SA, Restifo NP. Glucocorticoids do not inhibit antitumor activity of activated CD8+ T cells. J Immunother. 2005;28:517–524. doi: 10.1097/01.cji.0000177999.95831.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. [PMC free article] [PubMed] [Google Scholar]
- 94.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 95.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 96.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, Hessler JD, Liu TM, Chang BY, Larkin KM, Stefanovski MR, Chappell DL, Frissora FW, Smith LL, Smucker KA, Flynn JM, Jones JA, Andritsos LA, Maddocks K, Lehman AM, Furman R, Sharman J, Mishra A, Caligiuri MA, Satoskar AR, Buggy JJ, Muthusamy N, Johnson AJ, Byrd JC. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A. 2015;112:E966–E972. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aranda F, Vacchelli E, Eggermont A, Galon J, Fridman WH, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Immunostimulatory monoclonal antibodies in cancer therapy. Oncoimmunology. 2014;3:e27297. doi: 10.4161/onci.27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19:1044–1053. doi: 10.1158/1078-0432.CCR-12-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shindo Y, Yoshimura K, Kuramasu A, Watanabe Y, Ito H, Kondo T, Oga A, Ito H, Yoshino S, Hazama S, Tamada K, Yagita H, Oka M. Combination immunotherapy with 4-1BB activation and PD-1 blockade enhances antitumor efficacy in a mouse model of subcutaneous tumor. Anticancer Res. 2015;35:129–136. [PubMed] [Google Scholar]
- 103.Buchan SL, Manzo T, Flutter B, Rogel A, Edwards N, Zhang L, Sivakumaran S, Ghorashian S, Carpenter B, Bennett CL, Freeman GJ, Sykes M, Croft M, Al-Shamkhani A, Chakraverty R. OX40- and CD27-mediated costimulation synergizes with anti-PD-L1 blockade by forcing exhausted CD8+ T cells to exit quiescence. J Immunol. 2015;194:125–133. doi: 10.4049/jimmunol.1401644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Colevas AD, Weng WK, Clarke MF, Carlson RW, Stockdale FE, Mollick JA, Chen L, Levy R. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Belladonna ML, Grohmann U, Guidetti P, Volpi C, Bianchi R, Fioretti MC, Schwarcz R, Fallarino F, Puccetti P. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol. 2006;177:130–137. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- 107.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, Sian S, Nichol G, Davis T, Keler T, Yellin M, Weber J. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 111.Koestner W, Hapke M, Herbst J, Klein C, Welte K, Fruehauf J, Flatley A, Vignali DA, Hardtke-Wolenski M, Jaeckel E, Blazar BR, Sauer MG. PD-L1 blockade effectively restores strong graft-versus-leukemia effects without graft-versus-host disease after delayed adoptive transfer of T-cell receptor gene-engineered allogeneic CD8+ T cells. Blood. 2011;117:1030–1041. doi: 10.1182/blood-2010-04-283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davids MS, Kim HT, Costello CL, Avigan D, Chen YB, Armand P, Alyea EP, Hedlund J, McSweeney PA, Liguori R, Ritz J, Ball ED, Bashey A, Soiffer RJ. A Multicenter Phase I Study of CTLA-4 Blockade with Ipilimumab for Relapsed Hematologic Malignancies after Allogeneic Hematopoietic Cell Transplantation. Blood. 2014;124 Abstr 3964. [Google Scholar]