Abstract
We examined relationships between regional brain shrinkage and changes in cognitive performance, while taking into account the influence of age, vascular risk, Apolipoprotein E variant and socioeconomic status. Regional brain volumes and cognitive performance were assessed in 167 healthy adults (age 19-79 at baseline), 90 of whom returned for the follow-up after two years. Brain volumes were measured in six regions of interest (ROIs): lateral prefrontal cortex (LPFC), prefrontal white matter (PFw), hippocampus (Hc), parahippocampal gyrus (PhG), cerebellar hemispheres (CbH), and primary visual cortex (VC), and cognitive performance was evaluated in three domains: episodic memory (EM), fluid intelligence (Gf), and vocabulary (V). Average volume loss was observed in Hc, PhG and CbH, but reliable individual differences were noted in all examined ROIs. Average positive change was observed in EM and V performance but not in Gf scores, yet only the last evidenced individual differences in change. We observed reciprocal influences among neuroanatomical and cognitive variables. Larger brain volumes at baseline predicted greater individual gains in Gf, but differences in LPFC volume change were in part explained by baseline level of cognitive performance. In one region (PFw), individual change in volume was coupled with change in Gf. Larger initial brain volumes did not predict slower shrinkage. The results underscore the complex role of brain maintenance and cognitive reserve in adult development.
Keywords: memory, fluid abilities, volume, longitudinal, MRI, prefrontal cortex, white matter
1. Introduction
Human aging is accompanied by shrinkage of the brain parenchyma (Kemper, 1994; Raz & Kennedy, 2009) and declines in cognitive performance (Cattell, 1943; Salthouse, 2010). However the trajectories of age-related change vary among individuals (Raz, Ghisletta, Rodrigue, Kennedy, & Lindenberger, 2010), brain regions (Fjell et al., 2009; Persson et al., 2014; Raz et al., 2005; Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003) and cognitive domains (de Frias, Lövdén, Lindenberger, & Nilsson, 2007; Ghisletta & Lindenberger, 2003; Rabbitt, 1993).
Although the observed pattern of age-related change may in part depend on the sample characteristics and the time window of longitudinal assessment, age-related changes in the brain and cognition follow, on average, a reasonably consistent pattern. Brain shrinkage appears especially significant in medial temporal lobe (MTL) structures and tertiary association cortices, i.e., regions that are particularly important for support of age-sensitive cognitive functions. In contrast, sensory cortical regions, such as the visual cortex, evidence lesser age-related change (see Raz & Kennedy, 2009 for a review). Aging is characterized by a complex pattern of cognitive stability, growth and decline, and studies conducted over the past eight decades suggest significant heterogeneity of age-related change across cognitive domains (Cattell, 1943; Ghisletta, Rabbitt, Lunn, & Lindenberger, 2012; Miles, 1934; Rabbitt, 1993).
Cattell (1943), in refining Spearman’s (1904) concept of general intelligence, postulated the existence of two broad types of abilities: fluid and crystallized. Fluid abilities have been viewed as rapidly growing until mid-twenties, stabilizing thereafter, steadily declining into the seventh decade of life, and accelerating their decline into senium (Cattell, 1943). In contrast, crystallized abilities continue to improve throughout childhood and adulthood, with gradual declines becoming apparent only during the latest part of the lifespan (Cattell, 1943; Finkel, Reynolds, McArdle, Gatz, & Pedersen, 2003; Flicker, Ferris, & Reisberg, 1993; Ghisletta & Lindenberger, 2003; Horn & Cattell, 1967; McArdle, Ferrer-Caja, Hamagami, & Woodcock, 2002; Rabbitt, 1993; Rabbitt et al., 2004). Research conducted after Cattell’s initial proposal suggests a more refined structure of abilities and their response to aging, with the rates of change varying across cognitive domains. Working memory, episodic memory, processing speed and spatial reasoning exhibit particular sensitivity to aging, whereas vocabulary and verbal comprehension are spared until the end of life (Flicker et al., 1993; Ghisletta et al., 2012; Hultsch, Hertzog, Small, McDonald-Miszczak, & Dixon, 1992; Rabbitt et al., 2004; Small et al., 2011a).
In light of the surveyed findings in the brain and cognitive aging, it is plausible that brain changes play an important role in cognitive declines. Indeed, devolution of cognitive performance into dementia appears to follow prolonged deterioration of structural and functional characteristics of relevant brain structures (Mortamais, Artero, & Ritchie, 2013; Mungas et al., 2002; Tosto, Zimmerman, Carmichael, & Brickman, 2014). However, the associations between normal brain aging and cognitive development in late adulthood are not unequivocally established. One of the most prominent reasons for inconsistency is the reliance on cross-sectional design in the majority of investigations (but see Cohen, Small, Lalonde, Friz, & Sunderland, 2001; Kramer et al., 2007; Raz et al., 2008; Rusinek et al., 2003). Cross-sectional methodology precludes gauging individual differences in change of the brain and cognition and examining the associations between the two (McArdle & Nesselroade, 1994). Moreover, cross-sectional design impedes evaluation of neural mediators of cognitive decline in age-heterogeneous samples and is ineffectual for generating hypotheses about brain-cognition relationships over time (Hofer & Sliwinski, 2001; Lindenberger, von Oertzen, Ghisletta, & Hertzog, 2011; Maxwell & Cole, 2007; Raz et al., 2013). Longitudinal studies of non-demented adults show that global deterioration of the brain expressed in ventricular expansion (Grimm, An, McArdle, Zonderman, & Resnick, 2012; McArdle et al., 2004) as well as regional changes such as shrinkage of the hippocampus (Kramer et al., 2007; Rusinek et al., 2003) lead to declines in episodic memory.
It is important to emphasize, however, that associations between brain and cognition may not be unidirectional (see Salthouse, 2011 for a relevant discussion). Indeed, whereas among older adults smaller gross brain volume has been linked to decline in fluid intelligence (Rabbitt et al., 2008), variations in prefrontal volume are related to age differences in fluid reasoning and in more specific cognitive functions such as strategic control of episodic memory (Euston, Gruber, & McNaughton, 2012; Kane & Engle, 2002; Rajah & D’Esposito, 2005). Functional and structural imaging studies reveal the importance of the cerebellar cortices in multiple cognitive operations (Stoodley, 2012). Longitudinal evidence further links higher fluid intelligence and episodic memory scores to lesser shrinkage of the MTL (Borghesani et al., 2012; Raz et al., 2008; Rodrigue & Raz, 2004), and higher general cognitive ability measured in youth predicts larger brain volumes in old age (Royle et al., 2013). Thus, assessment of bidirectional influences between the brain and cognition is necessary for understanding neural substrates of cognitive aging.
Chronological age is associated with multiple factors that shape individual trajectories of age-related change in the brain and cognition, and these factors have been hypothesized to distinguish between successful and typical aging (Rowe & Kahn, 1987). The concept of brain reserve has been advanced to explain individual differences in resilience to trauma and neurodegeneration by greater initial number of neurons and synapses (Katzman et al., 1988) as well as greater gross brain volume (Satz, 1993). Numerous cardiovascular and pro-inflammatory risk factors have been implicated in exacerbating the neural and cognitive declines observed in normal aging (Anstey & Christensen, 2000; Bettcher & Kramer, 2014; Convit, Wolf, Tarshish, & De Leon, 2003; Ghisletta et al., 2014; Persson et al., 2014; Raz et al., 2005, 2010,2013; Whalley, Deary, Appleton, & Starr, 2004).
In addition, socio-economic status (SES), which reflects multiple inter-related variables such as individual and parental education, income, and occupational attainment, has been proposed as a significant mediator of individual differences in cognitive change throughout the lifespan (Renner, Hankonen, Ghisletta, & Absetz, 2012; Tucker & Stern, 2011; Vance, 2012; Whalley et al., 2004). The importance of SES-related characteristics for successful aging has been emphasized in developing a concept of cognitive reserve, and positing that higher educational and professional attainment early in life serve as a buffer against age-related cognitive declines (Stern, 2002). Although the extant literature does not present a consensus on the effects of these modifiers of aging, it is important to consider them while studying the relationship between brain shrinkage and cognitive change in healthy adults.
Our main objective in this research was to examine the relationship between previously reported regional heterogeneity of brain shrinkage (Persson et al., 2014) and change in performance in age-sensitive cognitive domains. In this hypotheses-driven study, we selected brain regions of interest (ROI) based on their theoretical and empirical relevance for the studied cognitive domains. The selection included ROIs with known relevance to age-sensitive cognitive functions: lateral prefrontal cortex (LPFC), prefrontal white matter (PFw), hippocampus (Hc), parahippocampal gyrus (PhG) and the cerebellar hemispheres (CbH), as well as a control region, primary visual cortex (VC), which we expected to show lesser age-related change and lesser relevance to age-sensitive cognitive skills (Buckner, 2013; Cabeza & Nyberg, 2000; Small, Schobel, Buxton, Witter, & Barnes, 2011b).
We chose two age-sensitive cognitive constructs, episodic memory (EM) and fluid ability (Gf), and one construct, vocabulary (V), which represents a crystallized ability that is relatively unaffected by aging. To examine changes in regional brain volumes and cognitive performance, we used latent change score models to assess mean change and variance in change. Further, we evaluated bidirectional lags between baseline levels and subsequent changes in brain volumes and cognitive performance scores. Finally, we tested the role of putative modifiers of change in the brain and cognition by adding cardiovascular risk, genetic variant associated with dementia risk, and socioeconomic status in addition to chronological age as determinants of individual differences in neuroanatomical and cognitive changes.
2 Methods
2.1. Participants
The data were collected in a major metropolitan area in the Midwestern USA. Volunteer participants across the adult lifespan were recruited through media advertisements and flyers. Persons who reported a history of cardiovascular, neurological or psychiatric disease, head trauma with loss of consciousness in excess of five minutes, thyroid dysfunction, diabetes mellitus, or history of drug and alcohol abuse, were excluded from the study. Participants with a reported diagnosis of hypertension who were taking prescription medication (e.g., beta-blockers, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors or potassium-sparing diuretics) were included in the study. Participants who reported taking anti-seizure medication, anxiolytics or antidepressants were excluded. Persons suffering from claustrophobia were advised not to participate in the study.
All participants were screened for dementia using the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975) with a cut-off of 26 (87%) correct responses, and for depression using the Center for Epidemiological Studies Depression Inventory (CES-D; Radloff, 1977), with a cut-off score of 15. All participants were right-hand dominant, as indicated by a score above 75% on the Edinburgh Handedness Questionnaire (Oldfield, 1971). One hundred sixty-seven persons were recruited for the study and assessed at baseline; 90 of them returned for cognitive and MRI evaluation. The average interval between assessments was two years and 24 days. A detailed description of the recruitment and attrition can be found in Persson et al. (2014; Figure 1). Descriptive statistics for demographic indicators and blood markers are presented in Table 1.
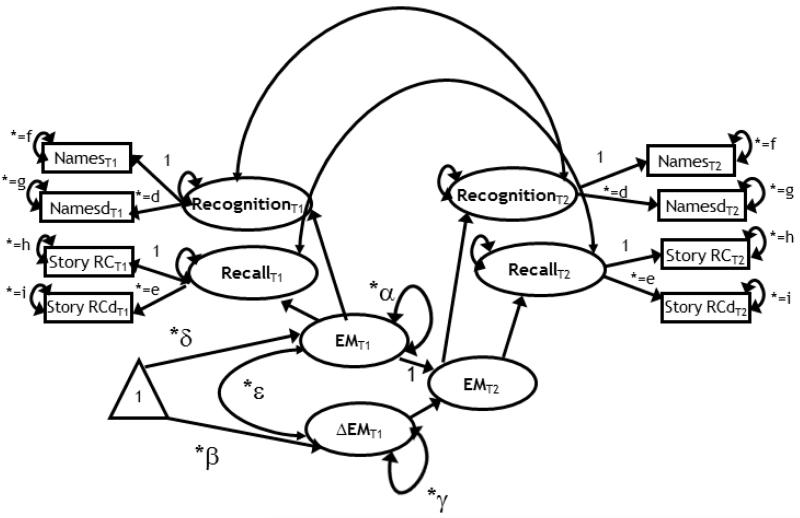
Figure 1.
A latent change score model for the assessment of two-occasion changes in episodic memory (EM). Squares represent observed variables, circles are latent variables. The triangle indicates that the model contains means. Free parameters are marked by an asterisk. Parameters with equal sign and the same subscript are constrained to be equal to each other. Change is modeled in a second order latent variable (EM), reflected by two subordinated latent variables: recall and recognition. Notations: δ = mean at baseline; α = variance at baseline; β = mean change, γ = variance in change; ε = covariance between individual differences in EM at baseline and individual differences in changes between baseline and follow-up. The model contains eight observed variables and nine free parameters, with 21 degrees of freedom.
Table 1.
Descriptive Statistics for Demographic Indicators and Blood Biomarkers at Baseline.
| N | Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|---|
| Age (years) | 167 | 19 | 79 | 52.80 | 15.71 |
| Systolic Pressure (mm Hg) | 167 | 91.33 | 156.67 | 122.35 | 12.97 |
| Diastolic Pressure (mm Hg) | 167 | 60 | 106.67 | 75.38 | 7.48 |
| Body-Mass Index (BMI), kg/m2 | 166 | 17.27 | 42.32 | 27.25 | 5.54 |
| Fasting Glucose, mg/dl | 158 | 65 | 115 | 86.75 | 9.03 |
| Low Density Lipoprotein (LDL), mg/dl | 164 | 41 | 214 | 116.24 | 34.43 |
| Education (years) | 167 | 12 | 20 | 15.41 | 2.30 |
| Maternal Education (years) | 150 | 3 | 21 | 12.47 | 3.22 |
| Paternal Education (years) | 144 | 0 | 21 | 12.28 | 4.20 |
Note. SD = standard deviation; mm Hg = millimeter of mercury; kg = kilogram; m = meter; mg/dl = milligrams per deciliter.
2.2. MRI Protocol
All images were acquired on the same 4-Tesla MRI system (Bruker Biospin, Ettlingen, Germany) with an 8-channel RF coil. For volume measurements, we acquired magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted images in the coronal plane. Image acquisition parameters were as follows: echo time (TE) = 4.38 ms, repetition time (TR) =1600 ms, inversion time (TI) = 800 ms, field of view (FOV) = 256 × 256 mm2, in-plane size = .67 × .67 mm2, slice thickness (ST) = 1.34 mm, matrix size = 384 × 384, flip angle (FA) = 8°, and GRAPPA acceleration factor = 2. In addition, fluid-attenuated inversion recovery (FLAIR) images were acquired in the transverse plane with the following parameters: TR = 8440 ms, TE = 112 ms, TI = 2200 ms, FA = 150°, FOV = 256 × 256 mm2, in plane resolution = 1.0 × 1.0 mm2, ST = 2 mm, matrix size = 256 × 256, number of slices = 50. The FLAIR images were used to assist in resolving potential incidental findings by a radiologist.
2.3. MRI image processing
In post-processing, we followed procedures described in our previous studies (e.g., Raz et al., 2004a). All image manipulations and measurements were conducted with Analyze 10.0 software (Biomedical Imaging Resource, Mayo Clinic College of Medicine). To correct for asymmetrical positioning of the head in the scanner, images were rotated to adjust for variation in head pitch, tilt and rotation. All images were processed by the same operators (at least two tracers per ROI) who attained reliability of at least 0.90 as measured by the intra-class correlation computed under an assumption of random raters through their training program (equation 2; Shrout & Fleiss, 1979). The images acquired on both occasions were coded and ordered in a randomized fashion within each individual. The tracers were blind to the time of acquisition of the specific images and the demographic characteristics of the participants.
A detailed description of segmentation rules for all structures of interest has been published elsewhere (Persson et al., 2014; Raz et al., 2004a, 2010). Prior to statistical analyses, all regional volumes were adjusted for the volume of the intracranial vault (ICV) through analysis of covariance (ANCOVA; Jack et al., 1989). For all ROIs, the assumptions of ANCOVA were tested, and if the slopes of ROI regression on ICV varied between the sexes, separate adjustments were performed for men and women.
2.4. Cognitive measures
2.4.1. Fluid Intelligence (Gf)
The Culture Fair Intelligence Test (CFIT; Cattell & Cattell, 1960) was used as a proxy for fluid ability. Although CFIT score is a single indicator, it combines four subtests (10 to 14 items each) that tap into various abilities believed to constitute fluid reasoning, such as manipulation of symbols in short-term memory, derivation of rules and eduction of relationships. Besides being one of the most commonly used tests of fluid reasoning, CFIT is also known for its sensitivity to aging (Rabbitt et al., 2004). The version used here has produced reliable negative correlations with age and various indices of brain structural integrity as well as associations with genetic variants in multiple samples tested in our lab (e.g., Raz et al., 1990; 2008; 2009).
2.4.2. Vocabulary
Word knowledge was measured by vocabulary tests generated from the ETS Kit of factor-referenced cognitive tests (Ekström, French, Harman, & Dermen, 1976). The items from V-1 through V-5 ETS subtests were culled for non-English and archaic words, and the remaining pool of items was randomly distributed among 6 lists of 26 items, with each list divided into two parts of 13 items each. The tests assess knowledge of word meaning in four- and five-alternative, multiple-choice format, and have a rated difficulty of 7-16th grade comprehension level. For each word, the participant was instructed to choose the synonym. When the participant was uncertain of the correct answer, the instruction was to not guess and to refrain from answering the item. The index of performance was the number of correct responses minus a quarter of the number incorrect (a penalty for guessing). At baseline, both parts of the newly created lists 1 and 2 were used. At follow-up, both parts of list 1 were repeated, and both parts of the list 3 were administered. Thus, the vocabulary score at follow-up reflects performance on one repeated list and one parallel list. The time limit was 4 min per page. Participants were instructed to ignore the time limit, but separate timed and untimed scores were generated. Most participants were able to complete the task during timed conditions.
2.4.3. Episodic Memory
Episodic memory was assessed by tests of recognition (associative memory) and story recall with immediate and delayed testing.
2.4.3.1 Name-picture associative memory
Associative memory was assessed by Memory for Names subtest of the Woodcock-Johnson Psychoeducational Battery – Revised (Woodcock & Johnson, 1989). The test was presented in a booklet form and was administered following the standardized procedures. For each trial in the immediate testing phase, participants viewed a line drawing of a novel stimulus (“space creature”), were read the creature’s nonsense name, and were asked to point to the creature named. Immediately following this, participants were presented with an array of nine space creatures (one target and eight distracters) and instructed to point to the creature named by the examiner. During this phase, participants were tested first on the creature that was just learned, followed by the previously learned creatures, presented in a pseudo-random order. In this way, the task was a progressive learning task. During the immediate recall, each incorrect response was corrected by the examiner. After a 20-min delay, the space creatures were displayed again as an array of nine creatures, and participants were required to point at the creature named by the examiner. All creatures were tested a total of three times, and participants did not receive feedback on incorrect responses. Total number of correct matches at the immediate and the delayed recall conditions served as performance indices.
2.4.3.2. Story recall
Free recall of a prose story was assessed by the Logical Memory (LM) subtest from the Wechsler Memory Scale-Revised (Wechsler, 1987). An experimenter read two short stories out loud to participants. Following each story, the experimenter asked the participant to recall the story in as much detail as possible. After a 20-min delay, participants were asked to repeat the stories as close to the original wording as they could. If a participant was unable to recall a story at all, a simple cue was provided. The maximum score correct was 50, following the standardized scoring protocol.
2.5. Metabolic and vascular risk biomarkers
For assessment of circulating biomarkers, blood was collected after an overnight fast. A nurse-phlebotomist drew 30 cm3 of venous blood from the medial cubital vein of a participant, who was comfortably seated in a quiet room.
2.5.1. Fasting glucose
Following a 12-h overnight fast, whole blood glucose levels were assessed by standard enzymatic glucose oxidase method, and persons with blood glucose levels above 126 mg/dl (<7.0 mmol/l), a cut-off recommended for diabetes diagnosis, were excluded. Due to laboratory errors, seven samples of glucose were discarded. Two participants evidenced extreme values of blood glucose exceeding the cut-off, due to either failure to follow fasting instructions or possible diabetes. These observations were considered missing.
2.5.2 Lipid panel
A standard lipids panel used the direct cholesterol oxidase/cholesterol esterase method to assess triglycerides (TG), total cholesterol, as well as high- and low-density lipoprotein (HDL and LDL) cholesterol. Due to laboratory errors, two samples were lost and one additional sample showed unrealistically high level of LDL. These observations were considered missing for further analyses.
2.5.3. Blood pressure
Blood pressure was measured on three separate days by a mercury sphygmomanometer (BMS 12-S25) using a standard blood pressure cuff (Omron Professional) on the left arm with participants seated and their forearm positioned on the table. The mean systolic and diastolic pressure across the measurement occasions was computed for each participant. Pulse pressure was calculated as the difference between systolic and diastolic blood pressure.
2.5.4. Body Mass Index
A trained technician measured body height and weight for later calculation of Body Mass Index (BMI). BMI was calculated as a ratio of weight in kg to squared height (m2). BMI was not obtained on one participant.
2.5.5. APOE ε variants (rs429358 and rs7412)
APOE polymorphisms (rs429358 and rs7412) were pre-amplified with forward 5’-CAATGCTACCGAGTTTTCTTCC-3’ and reverse primers 5’-TTCAGATTCTTCACAGATGCGTA-3’ in a 25 μl reaction containing 2.5 mmol MgCl2, 0.5 μmol of the primers, 1.25 U AmpliTaq Gold polymerase, and 200 μmol dTTPs. The mixture was denatured at 95°C for 10 minutes and amplification achieved by 15 cycles of 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute, followed by a final extension at 72°C for 10 minutes. One μl of this reaction was subsequently used for rs429358 and rs7412 5’-nuclease assays under standard conditions. The primers and probes for the rs429358 assay were 5’-GCGGGCACGGCTGT-3’, 5’-GCTTGCGCAGGTGGGA-3’, VIC-CATGGAGGACGTGTGC-NFQ and FAM-ATGGAGGACGTGCGC-NFQ. The primers and probes for the rs7412 assay were 5’-TCCGCGATGCCGATGAC-3’, 5’-CCCCGGCCTGGTACAC-3’, VIC-CAGGCGCTTCTGC-NFQ and FAM-CAGGCACTTCGC-NFQ.
Genetic samples were obtained for 145 of the 167 total participants with a separate consent form procedure. APOE genotyping yielded 18 APOE ε2/ε3, 5 ε2/ε4, 81 ε3/ε3, 38 ε3/ε4, 3 ε4/ε4, and no ε2/ε2 genotypes. Because of the small number of APOE ε4 homozygotes (n = 3), carriers of one or two copies of the APOE ε4 (n = 46) were compared with 99 persons who had no ε4 allele. Allelic frequency met the assumption of the Hardy-Weinberg equilibrium: χ2 = .01, p = .89. Frequency of APOE ε4 did not differ between Caucasian and African-American participants: χ2= .29, p = .59. There were no age differences between the allelic variant groups (t = −.85, p = .39), nor did they differ in years of education (t = −.08, p = .93), or MMSE (Mann-Whitney U = 2402, Z = −.93, p = .35).
2.5.6. Demographics
To capture the influence of childhood socioeconomic disparities that may affect brain and behavior beyond the current occupation and level of education (Noble et al., 2015) in a sample comprised of highly educated individuals, we used parental education as an indicator of SES beyond individual attainment. Self-reported years of maternal and paternal education were used as indicators in a common factor model to specify a SES latent variable. The scale refers to the number of years spent in a formal educational setting. Maternal education was missing for 17 persons and paternal for 23 persons. The reasons for missingness were not stated in most cases, except in one case of adoption. In the other missing cases, the participant indicated lack of knowledge or left the answer space blank.
2.6. Statistical analyses
2.6.1. Univariate Latent Change Score Models
A series of latent change score models (LCSM) were fitted to the data. In the univariate specification, LCSMs describe the sample average trajectory of the variable of interest assessed on two occasions, as well as heterogeneity in this trajectory due to individual differences that deviate from the sample average. The trajectory is defined by the level at the first occasion and by the change occurring thereafter between the two occasions. Individuals can thus deviate from the sample average initial level and from the average amount of change. Therefore, the model estimates both a mean and a variance for the initial level and for the change, as well as the covariance level-change (McArdle & Nesselroade, 1994). If multiple indicators of a same underlying construct are assessed on both occasions, a latent variable (common factor) can be defined and the LCSM can describe the initial level and change in the factor. This assures that the change estimation is reliable and not contaminated by measurement error as are the difference scores between measured indicators (Cronbach & Furby, 1970). Change is modeled in the latent difference score according to the following equation:
| (1) |
The latent change score is described by Δy(t)n, where t denotes time point (a measurement occasion), and n corresponds to a participant. For instance, the latent score at time point two of participant n (y(2)n) is constructed as the unit weighted sum of the latent score at baseline (y(1)n), and a latent score (Δy(2)n), which represents change that occurred between baseline (time 1) and follow-up (time 2) (McArdle, 2009). The LCSM then estimates both the sample mean and variance of the latent change score Δy(2), defined as y(2)n - y(1)n, where the mean represents the sample average change between the measurement occasions, and the variance represents individual differences in change.
2.6.2. Bivariate LCSMs
The univariate LCSM can be expanded to study the change of two variables simultaneously and their reciprocal influences. In particular, cross-lag effects can be integrated to examine if change in one domain can be determined by initial level in the other domain, and vice versa. Hence, the bivariate LCSM allows estimating the influence of one variable on change of the other variable. The equations of the bivariate LCSM can be written as follows (Grimm et al., 2012):
| (2a) |
| (2b) |
In these equations, Δy(t) denotes the change for y between times (t-1) and (t) (i.e., Δy(2)n = y(2)n - y(1)n). Equation 2a posits that the change in y is influenced by both y and x at the previous time point (t-1) and a constant αy. Hence, β represents the auto-proportion (effect of y on its own change), and y the influence from the other variable x on change in y (the cross-lag effect). By comparing the strengths of γx and γy it is possible to detect whether one variable influences more strongly the change in the other variable, despite the control for the auto-proportions βx and βy. In other words, this model allows estimating whether within a system of two variables changing over time, one leads and the other lags. A more detailed description of the bivariate LCSM can be found elsewhere (e.g., Grimm et al., 2012; McArdle & Nesselroade, 1994).
2.6.3. Modeling procedure
The analyses of brain-cognition relations proceeded in three steps. Including all the effects in one multivariate model was not feasible because the number of parameters in such a model would have exceeded the number of participants and hence could not be estimated. We therefore first fitted univariate LCSMs, composed of measurement models to reflect longitudinal change over three cognitive domains: EM, Gf and V, and over six ROIs: LPFC, PFw, Hc, PhG, CbH and VC. The common factors for the mentioned cognitive domains were represented by manifest variables as outlined above. Each ROI was represented by a latent variable reflected by bi-hemispheric volume measurements. We analyzed the data under the assumption of measurement equivalence and imposed equality constraints on the factor loadings for both occasions. We tested measurement invariance of each ROI and in all cases it was confirmed. The models were specified to estimate the average amount of change of the sample and the variance in change. This preliminary set of analyses is particularly important to determine which cognitive ability or ROI evidences heterogeneity (variance) in change.
At the second step of the analyses, we evaluated a series of bivariate LCSMs, each of which included one of the three latent cognitive abilities and one of the six latent regional volumes. In these models, we assessed change in each variable of interest pertaining to the brain and cognition, while taking into account the influence of their baseline values, chronological age, SES, vascular risk (VR), and APOE ε4 variant on the bivariate relationships between brain and cognitive domains. All covariates were entered simultaneously. We defined both VR and SES by latent variables that reflected various measures of vascular health and years of maternal and paternal education, respectively.
For all models, we assessed statistical fit of the models with the Comparative Fit Index (CFI), the Standardized Root Mean Square Residual (SRMR), and the Root-Mean-Square Error of Approximation (RMSEA), in addition to the χ2 statistic with its degrees of freedom (df). We used conventional criteria to consider a model as fitting well to the data: CFI > .95, SRMR < .08, and RMSEA < .08 (Browne & Cudeck, 1993; Hu & Bentler, 1998; 1999).
We assumed that the data were missing at random, and parameter estimates were obtained using full information maximum likelihood estimation (FIML; e.g., Little, 1995; McArdle & Nesselroade, 1994). Under the MAR assumption, missingness depends on the variables that are included in the models and possible differences in change patterns between participants with full data and drop-outs are minimized, if not overcome. FIML is routinely used in longitudinal studies in cognitive aging and development including analyses of brain volume changes in this sample (e.g., McArdle et al., 2002; Persson et al., 2014), and is superior to other commonly used procedures, such as list-wise deletion. Notably, restriction of a longitudinal sample to repeated assessment cases results in selective and biased set of estimates (McArdle & Nesselroade, 1994), and this problem can be avoided with FIML.
In the previous analyses on this sample (Persson et al., 2014), we examined the variables typically sensitive to attrition (Lindenberger, Singer & Baltes, 2002). The potential confounders of attrition included age, education, MMSE scores, and diagnosis of hypertension. We found that only age was an important predictor of attrition (see Persson et al., 2014 for details), and its influence has been taken into account by introducing baseline age in the models (see Supplementary Figure 1 for age distribution at baseline and follow-up). In this study, we assumed that the probability of not having data at time 2 was associated with participants’ cognitive scores, their brain volume, and the covariates (age, APOEε4 status, VR, and SES). We corrected for the False Discovery Rate (FDR) using the Benjamini and Hochberg (1995) method, with the critical level denoted α’ and the nominal significance level of α = .05. For each model, α’ depended on the number of parameters estimated and thus varied across models.
3. Results
3.1. Regional Volumes and Cognitive Indicators: Descriptive Statistics and Correlations
The correlations among regional volumes ranged between r = .198, for the VC and Hc at baseline to r = .845 for the PFw and LPFC at follow-up. Associations among cognitive factors were moderate at both measurement occasions and ranged between r = .230 for V and EM at baseline to r = .319 for Gf and EM at follow-up. The associations between Gf and all regional volumes were positive and moderate at both occasions, whereas the correlations between EM factor and regional volumes were positive but small. No associations between V and regional volumes were noted at either occasion. Correlations between measurement occasions (stability coefficients) for regional volumes were very high (range from r = .898 for PhG to r = .979 for PFw, with the striking exception of VC that showed poor stability of r = .299). For the cognitive factors, stability coefficients were uniformly high: from r = .785 for Gf to r = 1.000 for V. The perfect stability of vocabulary scores was attained despite them being derived from two scores, only one of which was repeated at follow-up. Descriptive statistics for the ROIs and cognitive scores are presented in Supplementary Material, Table S1, and a full zero-order correlation matrix is presented in Table S2. One observation was excluded due to its excessive influence that induced nonlinearity in the bivariate distribution of change scores in LPFC, and PFw volumes and fluid intelligence. That observation was also defined as a univariate outlier according to Turkey’s outlier labeling rule (Hoaglin & Iglewicz, 1987), as described in previous work on this sample (Persson et al., 2014).
3.2. Common factor Models for Covariates
Vascular risk (VR) was modeled as a latent variable comprising four indicators measured at baseline: BMI, fasting glucose, pulse pressure, and LDL cholesterol, all with standardized loadings greater than .30. The model fit the data very well: CFI = 1.00, SRMR = .01, RMSEA = .00, (confidence intervals (CI) 90 %, .00 - .08), χ2(2) = 0.356, p = .83.
A common SES factor was constructed from two manifest indicators: maternal and paternal education, which correlated r = .69, p < .001. For identification, we kept the indicators parallel. The model exhibited a very good fit: CFI = 1.000, SRMR = .02, RMSEA = .00, (CI 90%, .00 - .20), χ2 (1) = 0.73, p = .39.
3.3. Modeling Change: Univariate Latent Change Score Models
3.3.1. Regional Brain Volumes
Change in regional brain volumes was evaluated with the LCSM, in which each regional volume was represented by a latent factor formed by two manifest measures—the two hemispheric volumes adjusted for ICV. These analyses are identical to the ones presented in our recent report (Persson et al., 2014: Table 3), in which a more detailed account of the results can be found. In short, mean volumes of three ROIs (Hc, PhG, and CbH) changed over time and all regional volumes exhibited significant individual differences in change. In one region, CbH, the magnitude of change was related to the baseline volume: larger volume was associated with greater negative change (shrinkage) over time. The results are presented in Table S3.
Table 3.
Prediction of Change in Regional Volumes from Baseline Cognitive Performance after Controlling for Covariates.
| EM at Baseline | V at Baseline | Gf at Baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ROI | Change predicted | p | α ′ | Change predicted | p | α ′ | Change predicted | p | α ′ |
| LPFC | .631(.254)* | .013 | .017 | .381 (.177) | .130 | .398(.167) | .051 | .017 | |
| PFw | .331(.199 ) | .097 | .329 (.170 ) | .052 | .017 | .149(.168) | .375 | ||
| Hc | .052 (.177) | .771 | −.116 (.175) | .509 | −.160(.158) | .311 | |||
| PhG | .267(.198) | .178 | .002(.185) | .992 | .077(.177) | .664 | |||
| CbH | −.216.167) | .195 | −.232 (146) | .113 | −.155(.133) | .245 | |||
| VC | −.179(.155) | .249 | −.076(.154) | .621 | .250 (.138) | .070 | |||
Note. The results are from the models fitted separately for each cognitive construct, with all covariates (age, SES, APOEε4, and VR), statistically controlled.
Abbreviations: ROI = Region of Interest volumes; LPFC = Lateral Prefrontal Cortex; PFw = Prefrontal subcortical white mater; Hc = Hippocampus; PhG = Parahippocampal Gyrus; CbH = Cerebellar hemispheres; VC = (Primary) Visual Cortex; EM = Episodic Memory; V = Vocabulary; Gf = Fluid Intelligence. Δ prefix indicates change. All volumes were divided by 1000 prior to analyses (to avoid estimation and scaling problems). Controlling for age at baseline, socioeconomic status; APOE s4 and vascular risk. Probabilities are adjusted for false discovery rate using Benjamini-Hochberg correction (α′), with a nominal α = .05.
3.3.2. Cognitive Performance
Change in three cognitive constructs was examined in separate LCSMs, one of which is presented in Figure 1 for illustration of the model for EM. The models included cognitive constructs (latent common factors) derived via confirmatory factor analyses from the manifest indicators listed above. The loadings that were made equal across the two occasions to satisfy the assumption of measurement equivalence were substantial and highly significant. All models fit the data well, with indices of fit for EM: CFI = .99, SRMR = .033, RMSEA = .050 (90% CI, .00 - 0.09), and χ2(19) = 26.97, p = .11; for Gf - CFI = 1.00, SRMR =.02, RMSEA = .000 (90% CI, .000 - .189), and χ2(1) = 0.63, p = 0.42 and for V - CFI = 1.00, SRMR = .03, RMSEA = .03 (90% CI, .00 - .10), and χ2(7) = 8.41, p = .29.
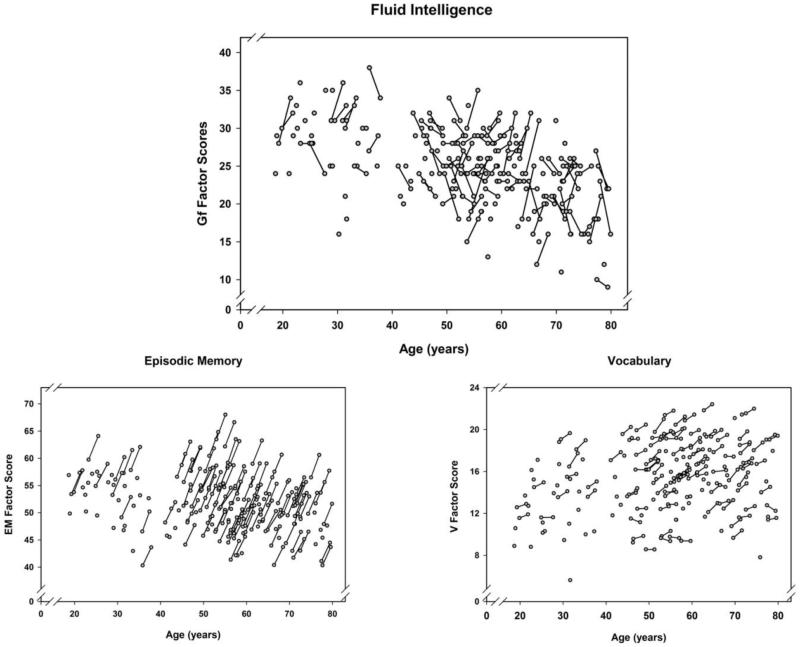
The results of the LCSM analyses revealed positive mean changes, but no variance in change in two cognitive constructs – EM and V. In contrast, Gf exhibited no average change but showed significant individual differences in change over time, displaying a mixture of gains, stability and declines. Moreover, higher Gf baseline scores were associated with smaller gains or greater declines over time (see Table S5). Individual factor scores obtained on two measurement occasions are presented in Figure 2 (a-c), with substantial individual differences in Gf slopes clearly visible.
Figure 2.
Longitudinal plots showing individual changes between baseline and follow-up for all participants. A. Fluid intelligence scores, Gf. B. Episodic memory scores, EM. C. Vocabulary scores, V. The factor scores are estimated from the LCSMs (expected values).
3.4. Bivariate Latent Change Score Models
We evaluated 18 bivariate LCSMs, modeling the relationships between each of the six regional volumes and each of the three cognitive abilities. Changes in volume and in cognitive ability were predicted by respective baseline values (see Statistical Analyses). We first report the analyses of change in brain volumes and cognition and associations between them (Tables 2-3). In these analyses, the direction of the lags was selected based on the presence of significant variance in change in the univariate LCSMs described in the previous step (see Table S3). To examine the influence of age, SES, VR, and APOE ε4 variant we added these covariates simultaneously (Tables S5-S7). The goal of these analyses was to explain the observed individual differences in change. In the LCSMs, the covariates were stipulated to predict baseline levels as well as changes in brain and cognition. As we found no effects of sex (all p’s > .05), we excluded that covariate from further analyses for the sake of model parsimony.
Table 2.
Prediction of Change in Fluid Intelligence from Baseline Regional Brain Volumes and Correlations between Fluid Intelligence and Regional Shrinkage, Adjusted for Covariates.
| ΔGf | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| ROI | Change predicted From Baseline |
p | α ′ | Change-Change Correlation |
p | α ′ |
| LPFC | .129 (.139) | .353 | .084 (.171) | .623. | ||
| PFw | .214 (.080)* | .008 | .017 | .346 (.121)* | .004 | .017 |
| Hc | .206 (.061)* | .001 | .017 | −.151 (.136) | .266 | |
| PhG | .228 (.078)* | .003 | .017 | −.005 (.150) | .974 | |
| CbH | .227 (.067)* | .001 | .017 | −.060 (.093) | .520 | |
| VC | .102 (.096) | .288 | .033 (.123) | .788 | ||
Note. The results are from the models fitted separately for each cognitive construct, with all covariates used for statistical control: age at baseline, socioeconomic status, APOE ε4 allele, and vascular risk.
Abbreviations: ROI = Region of Interest volumes; LPFC = Lateral Prefrontal Cortex; PFw = Prefrontal subcortical white mater; Hc = Hippocampus; PhG = Parahippocampal Gyrus; CbH = Cerebellar hemispheres; VC = (Primary) Visual Cortex; Gf = Fluid Intelligence. Δ prefix indicates change. All volumes were divided by 1000 prior to analyses (to avoid estimation and scaling problems). Probabilities are adjusted for false discovery rate using Benjamini-Hochberg correction (α′), with a nominal α=.05.
3.4.1. Effects of Covariates on Brain Volumes and Cognitive Performance
The analyses of cross-sectional associations (see Table S4 for details) show that at baseline, advanced age was associated with smaller volumes of all ROIs, independently of SES, VR, and APOE ε4 status. Older age was associated with lower memory, lower fluid intelligence scores and better vocabulary performance. SES was positively related to baseline vocabulary scores and fluid intelligence, but not to episodic memory. Of all ROIs measured at baseline, only the volume of Hc was associated with SES, and only in one of the three models that included this ROI. When considered with the other covariates, neither VR nor APOE ε4 variant displayed significant independent associations with any of the baseline volumes or cognitive factors.
The analyses of bivariate models for Gf and regional volumes revealed that in the model that included CbH, younger age and higher SES were associated with greater gains in Gf. No independent significant effects of age, SES, VR, or APOE ε4 variant on the rate of shrinkage of any ROI were observed, although trends for VR and APOE ε4 were noticed (see Tables S5-S7 for results). However, in at least one model (with CbH and Gf as target variables) the combined influence of baseline age, SES, VR, or APOE ε4 was significant: R2 = .551 (Table S5), with the direction of change suggesting faster shrinkage for older participants, carriers of the APOE ε4 allele and persons with lower SES.
3.4.2 Relationships among baseline brain volumes and cognitive variables and change therein in the presence of the covariates
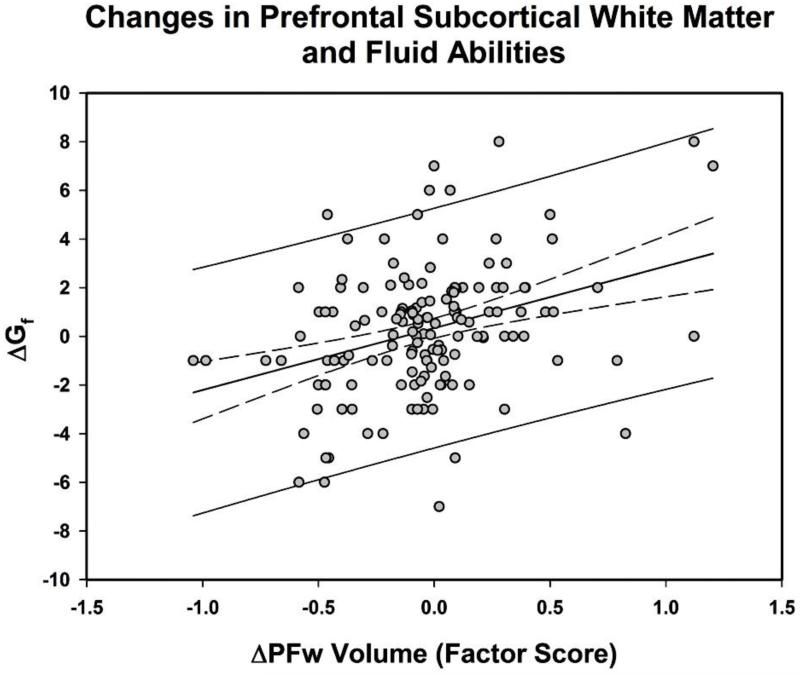
The results pertaining to relationships between change in Gf and baseline brain volumes as well as associations between Gf and regional shrinkage are summarized in Table 2. Larger volumes of four regions (PFw, Hc, PhG, and CbH) measured at baseline predicted positive change in Gf. Only in one region (PFw) lesser shrinkage was associated with greater gains in Gf scores, r = .346, Table 2, Figure 3.
Figure 3.
Coupling of changes in prefrontal subcortical white matter volume (ΔPFw) and fluid intelligence (ΔGf) (expected values). Dashed lines represent 95% confidence limits; solid lines upper and lower 95% observational prediction limits.
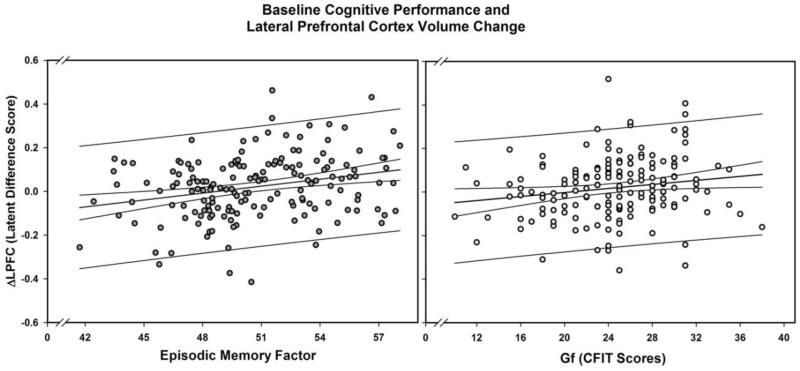
Baseline levels of EM predicted shrinkage of LPFC (p = .013, α’= .017), with other cognitive variables showing a trend (Gf p = .051) and a non-significant difference (V p = .13) in the same direction. No other regional volume showed similar direction of prediction for any cognitive construct (see Table 3 and Figure 4).
Figure 4.
Baseline cognitive performance predicts lateral prefrontal cortex shrinkage: Episodic memory (EM) and Fluid Ability (Gf). Latent change scores (expected values) of the lateral prefrontal cortex volume factor (ΔLPFC) are derived from the bivariate models for EM and Gf, respectively. Dashed lines represent 95% confidence limits; solid lines upper and lower 95% observational prediction limits.
To evaluate curvilinear age trends, we added baseline age squared to the models, adjusting for linear age and covariates effects. This addition affected none of the covariate effects (all p< .017), except for the association between baseline cerebellar volume and change in fluid intelligence scores (p = 0.053). After adding the quadratic age term to the model, we found that higher baseline CFIT scores predicted lesser change in LPFC volume (p = .023, up from p = .051 in the linear-only model). Although this effect was strengthened, it still did not reach the adjusted significance level of .017. Nonetheless, this marginally significant result suggests that the effect may be attenuated by a relatively high commonality between socioeconomic status and fluid intelligence resulting in suppression of the relationship between brain and intelligence. No other quadratic effects were noted beyond previously reported curvilinear effects on regional brain volumes (Persson et al., 2014).
4. Discussion
In a sample of healthy adults, we observed multiple associations between baseline values and change in both regional brain volumes and cognitive performance. Only one of the examined cognitive constructs, fluid intelligence, evidenced significant variance in change over time. Individual differences in change in Gf were positively associated with baseline volumes of several brain regions and with change in prefrontal white matter. Notably, the brain-cognition relationship was not unidirectional: better baseline memory performance predicted lesser shrinkage of the lateral prefrontal cortices, with baseline fluid intelligence showing a trend in the same direction.
Prediction of change in fluid intelligence from baseline brain volumes is in accord with a report of a similar relationship between total brain volume at follow-up and long-term changes in fluid intelligence performance assessed by the same Cattell Culture Fair Intelligence Test as the one used in the current study (Raz et al., 2008), but not on tests of episodic memory (Rabbitt et al., 2008). We did not replicate, however, the associations between shrinkage of the MTL structures (the hippocampus and the parahippocampal gyrus, which includes entorhinal cortex) and fluid intelligence scores observed in one of our previous studies (Raz et al., 2008). Although our results agree with the reports of MTL shrinkage even in persons who are at very low risk for dementia (Fjell, McEvoy, Holland, Dale, & Walhovd, 2013), within the examined period, hippocampal shrinkage was unrelated to changes in cognitive performance. It must be noted, that because the identical cognitive tests were used at both assessment occasions, the observed change might be underestimated by benefit from repeated exposure.
4.1. Cross-sectional associations between regional volumes and cognitive performance
In addition to the novel longitudinal findings, we replicated cross-sectional age-related differences in brain structure and cognition that have been widely reported in the extant literature (Cattell, 1943; Horn & Cattell, 1967; Raz & Kennedy, 2009). All regional volumes were greater in younger participants than in their older peers, with the greatest differences observed in the lateral prefrontal cortex and the smallest in the primary visual cortex, as often reported in the literature (see Raz & Kennedy, 2009; Raz, 2000 for reviews). Relatively small age differences observed in the hippocampal volume may provide additional support for the notion of prefrontal, rather than MTL, shrinkage being a hallmark of healthy aging (Raz, 2000). Older adults had lower fluid intelligence and episodic memory scores but better vocabulary in comparison to younger counterparts, which are oft-replicated findings (Horn & Cattell, 1967). We observed moderate correlations between all regional volumes and fluid intelligence scores, in accord with the prevalent findings (McDaniel, 2005), but found no correlations between MTL volumes and memory. The latter was consistent with mixed findings in the literature (van Petten, 2004). In contrast to some reports concerning healthy adults (Raz et al., 2013; Yates, Sweat, Yau, Turchiano, & Convit, 2012), we observed no cross-sectional associations of brain or cognition with vascular factors.
4.2. Effects of Covariates on Brain Volumes and Cognitive Performance
The covariates examined in this study – age, SES, APOEε4 variant and VR – had only limited influence on changes in the brain and cognition. The effects of age and SES on regional brain volumes (hippocampus, in one instance), and cognition (fluid intelligence) were observed only in one or two models and we found no specific effects of vascular risk or APOEε4. However, in combination, risk-relevant covariates (older age, lower SES, APOE ε4 allele and vascular risk) were associated with significantly greater individual variance in change (shrinkage, stability or growth) of at least one prominent region – lateral prefrontal cortex. Such cumulative influence of multiple inter-related risk factors rather than the independent effects of the latter may be a plausible scenario in a sample selected for better than typical health. Genetic (APOE ε4) and physiological (e.g., hypertension or hyperlipidemia) vascular risk factors can act in synergy with older age (Bender and Raz, 2012; de Frias, Schaie, & Willis, 2014; Persson, Lavebratt, & Wahlin, 2013) but the small proportions of carriers of the risk-related allelic variant and persons with vascular risk in the current study precluded testing such moderator effects.
We observed the association between SES indexed by parental education and the baseline volume of the hippocampus but not with other regional volumes or volume change over time. This finding is in accord with a recent reports suggesting that earlier parental markers of socioeconomic status may influence brain morphology (Noble et al., 2015), and that childhood SES exerts long-term effects on hippocampal size, even after accounting for childhood cognitive ability, adult SES and educational attainment (Staff et al., 2012). Caution should be exercised, however, in interpreting the SES-related findings in this study, as only two valid but limited indicators of SES (parental education) were available, while others, such as parental income or occupation were missing. Associations of regional brain volumes or cognition with vascular and metabolic risk indicators may be more prominent in samples with manifest pathologies (e.g., Whitmer, Gunderson, Quesenberry, Zhou, & Yaffe, 2007). Screening of the sample for multiple pathological conditions could have removed additional risk factors that were not explicitly considered in screening. For example, screening for depression could have dampened the contribution of vascular risk and SES. Cardiovascular risk and depression are confounded with cumulative stress experience, which in turn correlate with SES and have been linked to cognitive deficits and reduced hippocampal volume (Lupien, Fiocco, Wan, & Maheu, 2005). We observed no selective effects of vascular and genetic risk factors on brain shrinkage and cognition. While interpreting these findings in the context of the extant literature, it is important to consider the health characteristics of the participants employed in our study. There may be several reasons for not replicating previously observed associations between shrinkage of the MTL regions and vascular risk (Korf, White, Scheltens, & Launer, 2004; Raz et al., 2005; Yates et al., 2012). For example, by excluding persons with elevated depression scores, history of cardiovascular disease and diabetes, we might have significantly limited the range of vascular risk indicators, which are elevated in all of these conditions and in older adults may operate in synergy with depression (Rao, 2000).
Another factor commonly cited as a plausible modifier of cognitive and brain aging is hormonal status of pre- and post-menopausal women (Erickson et al., 2005; Raz, Rodrigue, Kennedy, & Acker, 2004b; Resnick & Maki, 2001). Regrettably, in this sample, due to a small number and substantial heterogeneity of current and previous hormone-replacement therapy (HRT) regimen, we were unable to evaluate the effect of HRT on brain shrinkage and cognition.
4.3. Reciprocal Relationship between the Brain and Cognition: Brain and Cognitive Reserve
The results of this study have implications for the role of cognitive and brain reserve in aging. The notion of larger initial capacity as a hedge against future pathology has been discussed since the early 1920s (Staff, 2012) and was later formulated as concepts of brain and cognitive reserve (Katzman et al., 1988; Satz, 1993, Stern, 2002). In discussing the development of the concept, Stern (2002) distinguished between passive and active reserve. Passive reserve refers to the benefit of having larger brain volume, more neurons or more elaborate synaptic networks as protection from cognitive decline that accompanies neurodegeneration, trauma or space-occupying lesion (Katzman et al., 1988; Satz, 1993). Active reserve denotes capacity for functional adaptation and reorganization that allows individuals possessing a high level of ability and cognitive skill to circumvent cognitive consequences of brain declines. Thus, active reserve encompasses adaptability of both the brain and cognition that can arise from multiple sources and receive support from multiple processes, including compensation based on existing cognitive skills, acquisition of alternative coping strategies or propensity to adhere to lifestyles and practices that benefit maintenance of healthy neural circuits (Hertzog, Kramer, Wilson, & Lindenberger, 2008). The distinction between passive and active reserve has been summarized as “having more” versus “doing more” (Staff, 2012). Since its formal introduction, the reserve hypothesis has been tested almost exclusively in observational and epidemiological studies that have operationalized reserve through proxy variables assessed at earlier stages of life and presumed to be immutable and fixed by the time of measurement at later adulthood, such as head circumference or educational attainment (Staff, 2012; Stern, 2009). Because the core concept of the reserve hypothesis implies individual differences in the rate of cognitive change and in the relationship between neural and cognitive changes, cross-sectional studies via fixed proxies present a less-than-optimal way of testing it. Here we present a rare evaluation of brain reserve in a longitudinal framework.
First, our findings indicate, in accord with previous reports (e.g., Raz et al., 2010) that larger initial brain volume does not affect the rate of volume loss. In other words, at least in terms of gross structural integrity, the brain does not provide reserve for itself. However, the results of our study support, at least in part, the notion of passive brain reserve. We observe that even in educated persons selected for optimal health, individual differences in cognitive attainment predict different rates of change in a region that is particularly important for executive control of diverse cognitive operations, the lateral prefrontal cortex. We show that, on the one hand, larger brain volumes of multiple regions predict favorable changes in important age-sensitive cognitive skills, and, on the other hand, cognitive attainment in multiple domains may protect against shrinkage of an important age-sensitive brain region, at least in the short term. Given correction for multiple comparisons, this finding, albeit limited to only one of the examined regions, presents robust evidence of a combined brain-cognition reserve phenomenon.
This result is contrary to previously reported negative findings (e.g., Raz & Lindenberger, 2010), and is in partial agreement with arguments in favor of preservation of brain integrity in healthy aging (e.g., Burgmans, van Boxtel, Vuurman, Smeets, Gronenschild, Uylings, & Jolles, 2009), although not on a whole-brain scale. Testing both directions of influence (the brain on cognition and cognition on the brain) as advised in the past (e.g. Salthouse, 2011) revealed an interesting dissociation. The brain variables that predicted cognitive change included several age-sensitive regions that have been associated with multiple cognitive skills (MTL and cerebellum), whereas prediction of cortical shrinkage from the baseline levels of cognitive abilities was limited to the region consistently linked to performance on general intelligence tasks (Duncan, 2000; Jung & Haier, 2007; Kane & Engle, 2002), and executive functions that are closely related to Gf (Duncan, 2000; Yuan & Raz, 2014). Of all regions examined in this study, the lateral prefrontal cortex exhibited the most inconsistent pattern of mean change and substantial individual differences in change across several samples that used the same measurement methods (Persson et al., 2014; Raz et al., 2005, 2010).
Observing such reciprocal relationships between brain and cognition in the context of longitudinal follow-up is important as it allows circumventing an interpretative problem that may arise when measures are confined to cognition alone, or when diagnostic categorization rather than continuous change is emphasized. By showing that the rate of cognitive change may depend on initial brain volume, we avoid this ascertainment bias problem (Tuokko Garrett, McDowell, Silverberg, & Kristjansson, 2003). Moreover, in our sample, as in some of the extant studies (e.g., McArdle, 2009), persons with higher baseline fluid abilities actually exhibited smaller gains after the second administration of the tests. Thus, the advantage of higher cognitive endowment in slowing cognitive decline is likely to be a real, albeit complex, phenomenon, based on reciprocal influences between the brain and behavior which is in accord with the “brain maintenance” hypothesis that stresses the importance of structural integrity for preservation of high cognitive function in aging (Nyberg, Lövdén, Riklund, Lindenberger, & Bäckman, 2012).
4.4. Factors Mitigating Brain Changes
The mechanisms by which higher cognitive performance at baseline may mitigate individual prefrontal shrinkage or even promote volume growth and thus serve as a neuroprotective factor are unclear. People who attain high test scores may be more likely to engage in everyday activities that involve cognitive and physical stimulation than their lower scoring counterparts (Hertzog et al., 2008; Vaughan et al., 2014). However, the evidence of positive effects of cognitive engagement on cognitive change is inconsistent (Jak, 2012; Lövdén, Xu, & Wang, 2013). Greater cognitive activity and higher educational attainment, while being associated with better concurrent cognitive test scores, does not slow declines in cognitive performance (Brown et al., 2012; Vaughan et al., 2014; Zahodne et al.,2011). Moreover, the benefits of cognitive and social engagement may be attenuated in healthy persons with high education (Vemuri et al., 2014), such as the participants of our study, and are quite modest even in an unselected community sample (Haslam, Cruwys, & Haslam, 2014). The same individuals may be more physically active than their low-performing counterparts, as higher general intelligence and high performance on specific cognitive tests are associated with greater levels of physical activity in older adults (Gow, Corley, Starr, & Deary, 2012), with high cognitive aptitude potentially serving as a life-long “buffer” against negative changes in health (Johnson, Corley, Starr, & Deary, 2011). Our results indicate that the factors associated with high cognitive performance are linked to reduced shrinkage or absence thereof in at least one cognitively important brain region, lateral prefrontal cortex, but sorting out the factors that may underpin such association remains an important task for future research.
4.5. Limitations
The reported findings should be interpreted in the context of several limitations. First, we relied on a sample of convenience that after application of stringent screening criteria included individuals that were healthier and better educated than expected for the general population. Notably, compared to typical North American adult samples, the participants of this study had lower prevalence of hypertension (20% vs. 33%) and diabetes (0% vs. 8%; Go et al., 2014). The average level of formal education was about 15 years, close to typical complete college schooling. Thus, a sampling strategy aimed at minimizing confounding influence of low education and age-related diseases could have introduced selection bias and reduced generalizability of the findings. Younger participants may harbor early precursors of neurological and vascular diseases but not express them at a detectable level. Thus, selection for good health has different effects on the younger and older segments of the age continuum and might reduce age differences in the variables of interest. Such trade-offs are inevitable and should be taken into account in interpreting the results. For example, participants with vascular risk factors and unfavorable genetic variants that are associated with poor health in the general population could have possessed favorable factors unaccounted for in our models. Such unknown variables could have offset the effects of risk factors. On the other hand, the effects observed despite potential upward selection may be viewed as representing the best-case scenario and may be expected to be more pronounced in the general population.
Second, a potential weakness of the present study is the lack of assessment of practice or retest effects. The single-sample study design with only two measurement occasions precluded estimating the effects of repeated test administration that could confound the pattern of change by masking declines and exaggerating gains. Because of the influence of prior test experience (Salthouse, 2013) and re-test effects (Salthouse, 2014; Ghisletta et al., 2012) on subsequent cognitive performance, changes in scores on all cognitive measures observed in this study might be positively biased and could be viewed as underestimating the true change over the two-year period. Future studies with three or more occasions of repeated measures should address the issue of retest-effects in the context of neural correlates of cognitive aging. However, observed reliable differences in fluid intelligence scores and their associations with initial brain volumes and changes therein mitigate the effect of this confound of interpretation. Nonetheless, it is important to emphasize that observed changes in Gf do not necessarily reflect gains or declines in fluid intelligence as repeated testing may provide benefits in many aspects of performance, including reduction of test anxiety through familiarity, acquisition of new test-taking strategies and improvement of specific visuospatial abilities, to name a few (for an in-depth discussion of limitations in interpreting gain scores see te Nijenhuis, van Vianen, & van der Flier, 2007).
Third, in addition to precluding assessment of practice effects, the two-occasion longitudinal design employed in this study did not allow for evaluating nonlinear trends in the brain and cognitive aging. Moreover, a limited number of measurement occasions combined with a relative multitude of evaluated parameters reduced the power even in this relatively large sample (von Oertzen, Hertzog, Lindenberger, & Ghisletta, 2010).
Fourth, in this hypotheses-driven study, we examined only a limited number of brain regions that differed with respect to change and individual differences therein, as established by the extant literature (Raz & Kennedy, 2009) and previous work on this sample (Persson et al., 2014). It is plausible that additional regions could exhibit significant change, individual differences in change, and associations between shrinkage and cognitive performance. However, adding regions to this analysis in the current sample would increase the number of comparisons with more conservative corrections of the critical p-values. Applying semi-automated methods and examining the data in a hypotheses-free manner would have sacrificed anatomically valid manual measurements (Kennedy et al., 2009) and in some regions, introduced age bias (Wenger et al., 2014).
4.6. Conclusions
In conclusion, our findings suggest that in healthy aging, brain structure and cognition exert reciprocal influence on each other: better cognitive performance predicts better maintenance of structural integrity in an important age-sensitive region, and larger baseline brain volumes predict greater repetition-related gains in fluid intelligence. Our findings contribute to better understanding of neural substrates of cognitive aging and shed light on the importance of maintaining cognitive functioning in older age. The specific mechanisms of the observed effects of cognitive and brain reserve remain to be elucidated.
Supplementary Material
Highlights.
Volume change and individual differences in change across brain regions.
Average change: memory & vocabulary; individual differences: fluid intelligence.
Larger baseline brain volumes predicted greater gains in Gf.
Prefrontal gray shrinkage was related to poorer baseline cognitive performance.
Shrinkage of prefrontal white matter correlated with negative change in Gf.
Acknowledgements
This research was supported by the National Institute on Aging grant R37 AG-11230 to NR. NP was supported by the Grants FOA11H-349 - FOA13H-090 from Royal Swedish Society of Sciences, & Solstickan Foundation (Rolf Zetterström award).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement
We declare no actual or perceived conflict of interest.
References
- Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology. 2000;46(3):163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- Bender AR, Raz N. Age-related differences in episodic memory: A synergistic contribution of genetic and physiological vascular risk factors. Neuropsychology. 2012;26(4):442–450. doi: 10.1037/a0028669. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995:289–300. [Google Scholar]
- Bettcher BM, Kramer JH. Longitudinal inflammation, cognitive decline, and Alzheimer’s disease: A mini-review. Clinical Pharmacology & Therapeutics. 2014;96(4):464–469. doi: 10.1038/clpt.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani PR, Weaver KE, Aylward EH, Richards AL, Madhyastha TM, Kahn AR, Schaie KW. Midlife memory improvement predicts preservation of hippocampal volume in old age. Neurobiology of Aging. 2012;33(7):1148–1155. doi: 10.1016/j.neurobiolaging.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, Gibbons LE, Kennison RF, Robitaille A, Lindwall M, Mitchell MB, Piccinin AM. Social activity and cognitive functioning over time: A coordinated analysis of four longitudinal studies. Journal of Aging Research. 2012;2012 doi: 10.1155/2012/287438. 287438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Sage; Newsbury Park, CA: 1993. pp. 136–162. [Google Scholar]
- Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80(3):807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Burgmans S, van Boxtel MP, Vuurman EF, Smeets F, Gronenschild EH, Uylings HB, Jolles J. The prevalence of cortical gray matter atrophy may be overestimated in the healthy aging brain. Neuropsychology. 2009;23(5):541–550. doi: 10.1037/a0016161. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cattell RB. The measurement of adult intelligence. Psychological Bulletin. 1943;40(3):153–193. [Google Scholar]
- Cattell R, Cattell A. Handbook for the individual or group culture fair intelligence test. Institute for Personality and Ability Testing; Savoy, IL: 1960. [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57(12):2223–2228. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, Tarshish C, De Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proceedings of the National Academy of Sciences. 2003;100(4):2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronbach LJ, Furby L. How we should measure “change”: Or should we? Psychological Bulletin. 1970;74(1):68–80. [Google Scholar]
- De Frias CM, Lövdén M, Lindenberger U, Nilsson L-G. Revisiting the dedifferentiation hypothesis with longitudinal multi-cohort data. Intelligence. 2007;35(4):381–392. [Google Scholar]
- De Frias CM, Schaie KW, Willis SL. Hypertension moderates the effect of APOE on 21-year cognitive trajectories. Psychology and Aging. 2014;29(2):431–439. doi: 10.1037/a0036828. [DOI] [PubMed] [Google Scholar]
- Duncan J. A Neural Basis for General Intelligence. Science. 2000;289(5478):457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Ekström RB, French JW, Harman HH, Dermen D. Manual for kit of factor referenced cognitive tests. Educational Testing Service; 1976. [Google Scholar]
- Erickson KI, Colcombe SJ, Raz N, Korol DL, Scalf P, Webb A, Kramer AF. Selective sparing of brain tissue in postmenopausal women receiving hormone replacement therapy. Neurobiology of Aging. 2005;26(8):1205–1213. doi: 10.1016/j.neurobiolaging.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76(6):1057–70. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Developmental Psychology. 2003;39(3):535–550. doi: 10.1037/0012-1649.39.3.535. [DOI] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB. Brain changes in older adults at very low risk for Alzheimer’s disease. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2013;33(19):8237–8242. doi: 10.1523/JNEUROSCI.5506-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Dale AM. One-year brain atrophy evident in healthy aging. The Journal of Neuroscience. 2009;29(48):15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicker C, Ferris SH, Reisberg B. A longitudinal study of cognitive function in elderly persons with subjective memory complaints. Journal of the American Geriatrics Society. 1993;41(10):1029–1032. doi: 10.1111/j.1532-5415.1993.tb06448.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Lindenberger U. Static and dynamic longitudinal structural analyses of cognitive changes in old age. Gerontology. 2003;50(1):12–16. doi: 10.1159/000074383. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Rabbitt P, Lunn M, Lindenberger U. Two thirds of the age-based changes in fluid and crystallized intelligence, perceptual speed, and memory in adulthood are shared. Intelligence. 2012;40(3):260–268. [Google Scholar]
- Ghisletta P, Bäckman L, Bertram L, Brandmaier AM, Gerstorf D, Liu T, Lindenberger U. The Val/Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene predicts decline in perceptual speed in older adults. Psychology and Aging. 2014;29(2):384–392. doi: 10.1037/a0035201. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Franco S. Executive summary: Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Corley J, Starr JM, Deary IJ. Reverse causation in activity-cognitive ability associations: The Lothian Birth Cohort 1936. Psychology and Aging. 2012;27(1):250–255. doi: 10.1037/a0024144. [DOI] [PubMed] [Google Scholar]
- Grimm KJ, An Y, McArdle JJ, Zonderman AB, Resnick SM. Recent changes leading to subsequent changes: Extensions of multivariate latent difference score models. Structural Equation Modeling: A Multidisciplinary Journal. 2012;19(2):268–292. doi: 10.1080/10705511.2012.659627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam C, Cruwys T, Haslam SA. “The we’s have it”: Evidence for the distinctive benefits of group engagement in enhancing cognitive health in aging. Social Science & Medicine. 2014;1982;120:57–66. doi: 10.1016/j.socscimed.2014.08.037. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest. 2008;9(1):1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Hoaglin DC, Iglewicz B. Fine-tuning some resistant rules for outlier labeling. Journal of the American Statistical Association. 1987;82(400):1147–1149. [Google Scholar]
- Hofer SM, Sliwinski MJ. Understanding ageing. Gerontology. 2001;47(6):341–352. doi: 10.1159/000052825. [DOI] [PubMed] [Google Scholar]
- Horn JL, Cattell RB. Age differences in fluid and crystallized intelligence. Acta Psychologica. 1967;26:107–129. doi: 10.1016/0001-6918(67)90011-x. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3(4):424–453. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, McDonald-Miszczak L, Dixon RA. Short-term longitudinal change in cognitive performance in later life. Psychology and Aging. 1992;7(4):571–584. doi: 10.1037//0882-7974.7.4.571. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: Normative volumetric measurements from MR images in young adults. Radiology. 1989;172(2):549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Jak AJ. The impact of physical and mental activity on cognitive aging. Current Topics in Behavioral Neurosciences. 2012;10:273–291. doi: 10.1007/7854_2011_141. [DOI] [PubMed] [Google Scholar]
- Johnson W, Corley J, Starr JM, Deary IJ. Psychological and physical health at age 70 in the Lothian Birth Cohort 1936: Links with early life IQ, SES, and current cognitive function and neighborhood environment. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 2011;30(1):1–11. doi: 10.1037/a0021834. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. The Behavioral and Brain Sciences. 2007;30(2):135–154. doi: 10.1017/S0140525X07001185. discussion 154-187. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Annals of Neurology. 1988;23(2):138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Kemper TL. Neuroanatomical and neuropathological changes during ageing and in dementia. In: Albert ML, Knoepfel EJE, editors. Clinical Neurology of Ageing. 2nd ed. Oxford University Press; New York: 1994. pp. 3–67. [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: a comparison of optimized voxel-based morphometry to manual volumetry. Neurobiology of Aging. 2009;30:1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. Epub 2008 Feb 13. doi:10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44(1):29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. Epub 2004 May 24. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Chui HC. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21(4):412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Singer T, Baltes PB. Longitudinal selectivity in aging populations: Separating mortality-associated versus experimental components in the Berlin Aging Study (BASE) The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2002;57(6):P474–P482. doi: 10.1093/geronb/57.6.p474. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: What’s change got to do with it? Psychology and Aging. 2011;26(1):34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Little RJA. Modeling the drop-out mechanism in repeated-measures studies. Journal of the American Statistical Association. 1995;90(431):1112–1121. [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Xu W, Wang H-X. Lifestyle change and the prevention of cognitive decline and dementia: What is the evidence? Current Opinion in Psychiatry. 2013;26(3):239–243. doi: 10.1097/YCO.0b013e32835f4135. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods. 2007;12(1):23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Nesselroade JR. Using multivariate data to structure developmental change. In: Cohen SH, Reese HW, editors. Life span developmental psychology: Methodological contributions. Erlbaum; Hillsdale, NJ: 1994. pp. 223–267. [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental Psychology. 2002;38(1):115–142. [PubMed] [Google Scholar]
- McArdle JJ, Hamgami F, Jones K, Jolesz F, Kikinis R, Spiro A, Albert MS. Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the normative aging study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2004;59(6):P294–P304. doi: 10.1093/geronb/59.6.p294. [DOI] [PubMed] [Google Scholar]
- McDaniel M. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- Miles CC. Influence of speed and age on intelligence scores of adults. The Journal of General Psychology. 1934;10(1):208–210. [Google Scholar]
- Mortamais M, Artero S, Ritchie K. Cerebral white matter hyperintensities in the prediction of cognitive decline and incident dementia. International Review of Psychiatry. 2013;25(6):686–698. doi: 10.3109/09540261.2013.838151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Jagust WJ, DeCarli C, Mack WJ, Kramer JH, Chui HC. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology. 2002;59(6):867–873. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Sowell ER. Family income, parental education and brain structure in children and adolescents. Nature Neuroscience. 2015;18(5):773–8. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends in Cognitive Sciences. 2012;16(5):292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. We wanted to further assess if such influences had any effect through the adult lifespan, since early socioeconomic disparities may influence brain and behavior beyond the individual level of education.
- Persson N, Ghisletta P, Dahle CL, Bender AR, Yang Y, Yuan P, Raz N. Regional brain shrinkage over two years: Individual differences and effects of pro-inflammatory genetic polymorphisms. Neuroimage. 2014;28:334–348. doi: 10.1016/j.neuroimage.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson N, Lavebratt C, Wahlin A. Synergy effects of HbA1c and variants of APOE and BDNFVal66Met explains individual differences in memory performance. Neurobiology of Learning and Memory. 2013;106:274–282. doi: 10.1016/j.nlm.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Does it all go together when it goes? The Nineteenth Bartlett Memorial Lecture. The Quarterly Journal of Experimental Psychology. 1993;46(3):385–434. doi: 10.1080/14640749308401055. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA, McInnes L, Diggle P, Holland F, Bent N, Abson V, Horan M. The University of Manchester longitudinal study of cognition in normal healthy old age, 1983 through 2003. Aging Neuropsychology and Cognition. 2004;11(2-3):245–279. [Google Scholar]
- Rabbitt P, Ibrahim S, Lunn M, Scott M, Thacker N, Hutchinson C, Jackson A. Age-associated losses of brain volume predict longitudinal cognitive declines over 8 to 20 years. Neuropsychology. 2008;22(1):3–9. doi: 10.1037/0894-4105.22.1.3. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rao R. Cerebrovascular disease and late life depression: an age old association revisited. International Journal of Geriatric Psychiatry. 2000;15(5):419–433. doi: 10.1002/(sici)1099-1166(200005)15:5<419::aid-gps140>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain: A Journal of Neurology. 2005;128(Pt 9):1964–83. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FI, Salthouse TA, editors. Handbook of Aging and Cognition. Lawrence Erlbaum Associates; Mahwah, NJ: 2000. pp. 1–90. [Google Scholar]
- Raz N, Kennedy KM. A systems approach to age-related change: Neuroanatomical changes, their modifiers, and cognitive correlates. In: Jagust W, D’Esposito M, editors. Imaging the Aging Brain. Oxford University Press; New York, NY: 2009. pp. 43–70. [Google Scholar]
- Raz N, Lindenberger U, et al. News of cognitive cure for age-related brain shrinkage is premature: A comment on Burgmans. Neuropsychology. 2010;2009;24(2):255–257. doi: 10.1037/a0018828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Millman D, Sarpel G. Cerebral correlates of cognitive aging: Grey-white matter differentiation in the medial temporal lobes, and fluid vs. crystallized abilities. Psychobiology. 1990;18:475–481. [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiology of Aging. 2004a;25(3):377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue K, Kennedy K, Acker J. Hormone replacement therapy and age-related brain shrinkage: regional effects. Neuroreport. 2004b;15:2531–2534. doi: 10.1097/00001756-200411150-00020. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cerebral Cortex. 2008;18(3):718–726. doi: 10.1093/cercor/bhm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: Effects of COMT, BDNF, ApoE and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. Neuroimage. 2010;51(2):501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Schmiedek F, Rodrigue KM, Kennedy KM, Lindenberger U, Lövdén M. Differential brain shrinkage over 6 months shows limited association with cognitive practice. Brain and Cognition. 2013;82(2):171–180. doi: 10.1016/j.bandc.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner B, Hankonen N, Ghisletta P, Absetz P. Dynamic psychological and behavioral changes in the adoption and maintenance of exercise. Health Psychology. 2012;31(3):306–315. doi: 10.1037/a0025302. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM. Effects of hormone replacement therapy on cognitive and brain aging. Annals of the New York Academy of Sciences. 2001;949:203–214. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. The Journal of Neuroscience. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. The Journal of Neuroscience. 2004;24(4):956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Kahn R. Human aging: Usual and successful. Science. 1987;237(4811):143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- Royle NA, Booth T, Valdés Hernández MC, Penke L, Murray C, Gow AJ, Deary IJ. Estimated maximal and current brain volume predict cognitive ability in old age. Neurobiology of Aging. 2013;34(12):2726–2733. doi: 10.1016/j.neurobiolaging.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinek H, De Santi S, Frid D, Tsui W-H, Tarshish CY, Convit A, de Leon MJ. Regional Brain Atrophy Rate Predicts Future Cognitive Decline: 6-year Longitudinal MR Imaging Study of Normal Aging 1. Radiology. 2003;229(3):691–696. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Selective review of cognitive aging. Journal of the International Neuropsychological Society. 2010;16(5):754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin. 2011;137(5):753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Effects of first occasion test experience on longitudinal cognitive change. Developmental Psychology. 2013;49(11):2172–2178. doi: 10.1037/a0032019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Frequent assessments may obscure cognitive decline. Psychological Assessment. 2014;26(4):1063–1069. doi: 10.1037/pas0000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satz P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology. 1993;7(3):273–295. [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Small BG, McColl BW, Allmendinger R, Pahle J, Lopez-Castejon G, Rothwell NJ, Kell DB. Efficient discovery of anti-inflammatory small-molecule combinations using evolutionary computing. Nature Chemical Biology. 2011a;7(12):902–908. doi: 10.1038/nchembio.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature Reviews Neuroscience. 2011b;12(10):585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. “ General Intelligence,” Objectively Determined and Measured. The American Journal of Psychology. 1904;15(2):201–292. [Google Scholar]
- Staff RT. Reserve, brain changes, and decline. Neuroimaging Clinics of North America. 2012;22(1):99–105. viii–iv. doi: 10.1016/j.nic.2011.11.006. doi: 10.1016/j.nic.2011.11.006. Epub 2011 Nov 30. [DOI] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Annals of Neurology. 2012;71(5):653–60. doi: 10.1002/ana.22631. http://doi.org/10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(03):448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11(2):352–65. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Te Nijenhuis J, van Vianen AEM, van der Flier H. Score gains on g-loaded tests: No g. Intelligence. 2007;35(3):283–300. [Google Scholar]
- Tosto G, Zimmerman ME, Carmichael OT, Brickman AM. Predicting aggressive decline in mild cognitive impairment: The importance of white matter hyperintensities. JAMA Neurology. 2014;71(7):872–877. doi: 10.1001/jamaneurol.2014.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AM, Stern Y. Cognitive reserve in aging. Current Alzheimer Research. 2011;8(4):354–360. doi: 10.2174/156720511795745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuokko H, Garrett DD, McDowell I, Silverberg N, Kristjansson B. Cognitive decline in high-functioning older adults: Reserve or ascertainment bias? Aging & Mental Health. 2003;7(4):259–270. doi: 10.1080/1360786031000120750. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia. 2004;42(10):1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Vance DE. Potential factors that may promote successful cognitive aging. Nursing: Research. 2012;2:27–32. [Google Scholar]
- Vaughan L, Erickson KI, Espeland MA, Smith JC, Tindle HA, Rapp SR. Concurrent and longitudinal relationships between cognitive activity, cognitive performance, and brain volume in older adult women. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2014;69(6):826–836. doi: 10.1093/geronb/gbu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Machulda M, Knopman DS, Mielke MM, Jack CR. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurology. 2014;71(8):1017–1024. doi: 10.1001/jamaneurol.2014.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Oertzen T, Hertzog C, Lindenberger U, Ghisletta P. The effect of multiple indicators on the power to detect interindividual differences in change. British Journal of Mathematical and Statistical Psychology. 2010;63(3):627–646. doi: 10.1348/000711010X486633. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WMS-R: Wechsler Memory Scale-Revised: Manual. Psychological Corporation San Antonio; 1987. [Google Scholar]
- Wenger E, Mårtensson J, Noack H, Bodammer NC, Kühn S, Schaefer S, Heinze HJ, Düzel E, Bäckman L, Lindenberger U, Lövdén M. Comparing manual and automatic segmentation of hippocampal volumes: reliability and validity issues in younger and older brains. Human Brain Mapping. 2014;35(8):4236–4248. doi: 10.1002/hbm.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Research Reviews. 2004;3(4):369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Quesenberry CP, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Current Alzheimer Research. 2007;4(2):103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Woodcock-Johnson Psychoeducational Battery-Revised. Riverside Publishing; Itasca, IL: 1989. [Google Scholar]
- Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: A selected review of the literature. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(9):2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: A Meta-analysis of structural neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2014 Feb 23;42C:180–192. doi: 10.1016/j.neubiorev.2014.02.005. doi: 10.1016/j.neubiorev.2014.02.005.[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Glymour MM, Sparks C, Bontempo D, Dixon RA, MacDonald SWS, Manly JJ. Education does not slow cognitive decline with aging: 12-year evidence from the Victoria Longitudinal Study. Journal of the International Neuropsychological Society: JINS. 2011;17(6):1039–1046. doi: 10.1017/S1355617711001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.