Abstract
Polymorphisms in ApoE are highly correlated with the progression of neurodegenerative disease, in particular Alzheimer’s disease. Little is known, however, about the role of ApoE or cholesterol metabolism on brain neurochemistry in general. To better understand the role of lipoprotein and cholesterol metabolism in the brain, we profiled 6-week and 12-week old Apoe KO and Ldlr KO mouse models via unbiased metabolomics to determine which metabolites were affected at an early age to identify those that may play a role in triggering pathology later in life. Steady-state metabolomics revealed only subtle differences among Apoe KO, Ldlr KO and WT mouse brains. Ldlr KO mice exhibited alterations in metabolites involved in neurotransmitter, amino acid and cholesterol metabolism. In contrast, Apoe KO mice only showed subtle changes in amino acid and neurotransmitter metabolism. These subtle changes in a broad range of metabolites indicate that ApoE and Ldl-R alone may not play a significant role in these mouse models at an early age, but instead require the cumulative effect from different pathways that lead to dysfunction at a much later stage of life.
Background
The balance and maintenance of lipids within the nervous system is critical for neural development, cognition and disease progression. There is clear evidence for important interactions between diet, neurometabolism and human disease, but the mechanisms that link diet to neurological function and pathology remain poorly understood. In fact, the intracellular and intercellular metabolic pathways that regulate central carbon flux in the brain remain poorly defined [1].
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that has been strongly associated with metabolic alterations [2, 3] and is characterized by the deposition of amyloid plaques in the brain that are formed from fibrillary Aβ peptides [2]. Several studies have demonstrated an association between lipid metabolism in the central nervous system (CNS) and AD [4–9]. The mammalian brain is rich in lipids, and mutations in lipid metabolizing enzymes are known to cause debilitating neurological diseases. Cholesterol homeostasis in the brain is regulated largely by de novo synthesis in glia along with the synthesis of apolipoprotein-E (ApoE) [10]. Numerous studies have demonstrated that the elevation of cholesterol in serum is associated with increased Aβ production and increased risk of AD [2, 11]. Furthermore, several genes involved in cholesterol metabolism have been identified that show changes in sporadic AD patients, indicating that cholesterol metabolism may play a significant role in the pathogenesis of late-onset AD [12].
Many studies have focused on the role of ApoE in the CNS following the discovery that the Apo ε4 allele in humans significantly increases the risk of developing AD later in life. ApoE is linked to late-onset AD because it can potentially act as an Aβ chaperone and influence Aβ metabolism in the CNS [2, 3, 13]. ApoE, a 34 kDa secreted glycoprotein, is involved in the uptake and degradation of lipoproteins via receptor-mediated endocytosis [14] and is expressed in the liver and the brain [6, 15]. In the CNS, ApoE is thought to transport lipoproteins and maintain lipid homeostasis in the developing nervous system [6, 15–19]. The Ldl-R is a cell surface receptor that assists in the endocytosis of the ApoE-cholesterol complex into cells [2, 3, 15, 20], and mutations in Ldl-R result in severe familial hypercholesterolemia. Previous studies have demonstrated that a lack of Ldl-R induced an increase in Aβ aggregation and deposition, indicating that Ldl-R plays a crucial role in Aβ regulation in the brain [3].
Although a great number of studies have tried to elucidate the role of ApoE and Ldl-R in the development of AD, the molecular mechanisms by which ApoE and Ldl-R are involved remain elusive. Here we used an unbiased metabolomic approach to broadly determine the consequence of ApoE and Ldl-R deletion to gain insight into the biochemical and physiological-metabolic roles of ApoE and Ldl-R within the central nervous system. Both Apoe and Ldlr KO mouse models result in systemic hypercholesterolemia. Utilizing young mice at several time points, a small number of changes in multiple metabolites were seen from different pathways in Apoe and Ldlr KO brains. These consistent metabolic alterations may provide insight into the molecular mechanisms by which ApoE and Ldl-R contribute to the progression of AD over the course of aging.
Methods
Animals and metabolites
Apoe KO, Ldlr KO and WT mice were purchased from Jackson Laboratory and maintained on a standard lab chow (Harlan 2018). At 6 weeks and 12 weeks of age, serum, brain and liver were collected and rapidly frozen in liquid nitrogen. Total cholesterol (Wako) from serum was measured colorimetrically. Metabolomic analysis was performed as previously described [21–23]. All procedures were performed in accordance with the NIH’s Guide for the Care and Use of Laboratory Animals and under the approval of the Johns Hopkins School of Medicine Animal Care and Use Committee.
Western blot analysis
Liver and brain were homogenized in Media I (10mM Tris, 1mM EDTA, 0.25M sucrose) with protease inhibitors (Complete Mini, Roche). All samples were spun down at 10,000 rpm for 20 min, and supernatant was collected and assayed by the Pierce BCA Protein Assay Kit (Thermo Scientific) to determine the concentration of protein. A total of 30 µg of protein for liver and 50 µg of protein for brain was subjected to SDS-PAGE and transferred to a nitrocellulose (Protran BA 83, Whatman) or a polyvinylidene difluoride (PVDF) membrane blocked with 5% milk-TBST (Tris-buffer saline with Tween 20). The blots were probed with the following primary antibodies (1:1000 to 1:2000): Ldl-R (R&D Systems), ApoE (Santa Cruz), ACC (Cell signaling), ACOT7 [22], and Cpt1c [24] using the appropriate secondary antibodies conjugated to horseradish peroxidase (HRP). Fasn (BD Biosciences) and Hsc70 (Santa Cruz) used the Cy3 (Life Technologies) fluorescent secondary antibody.
Statistical analysis
Metabolomic analyses utilized two-way ANOVA to identify biochemicals exhibiting significant interactions and main effects for experimental parameters of genotype and time. The remaining data performed pair-wise comparisons between Apoe KO or Ldlr KO to WT using Welch’s two-sample t-tests. P-values below the significance level of 0.05 were interpreted to have enough evidence to conclude that the data had a statistically different mean (i.e. statistically significant).
Results
Apoe and Ldlr KO mice
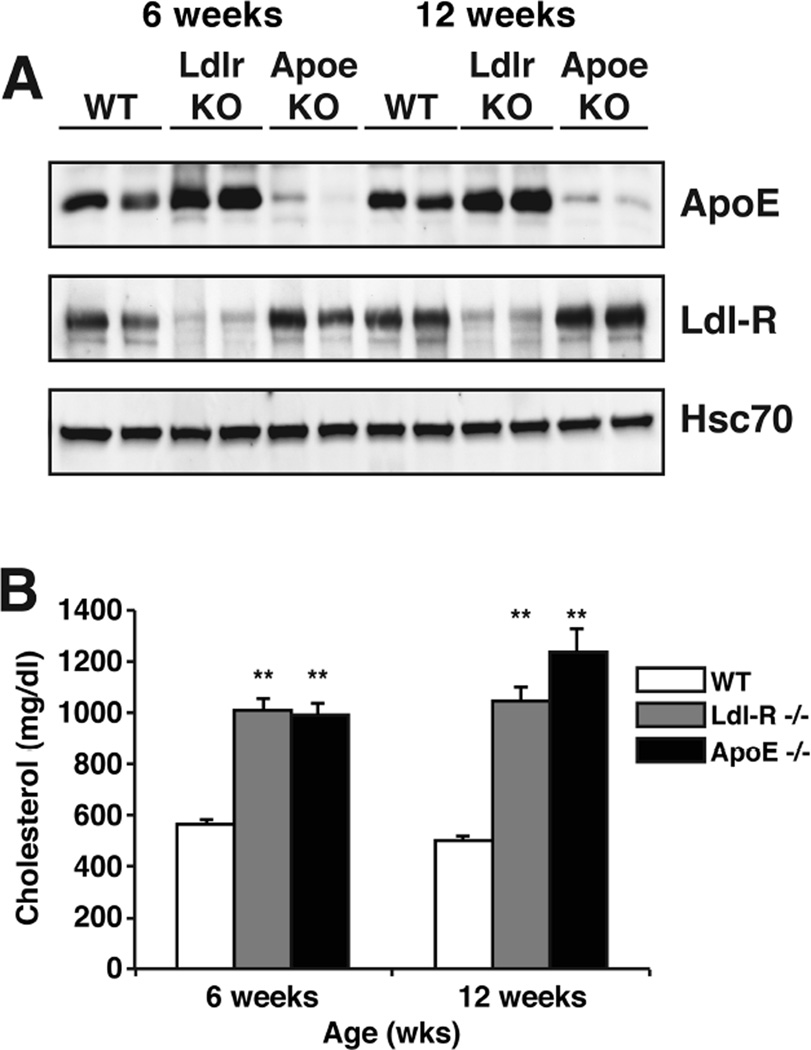
To understand the endogenous function of ApoE and Ldl-R, we performed metabolomic studies on brains of Apoe KO, Ldlr KO and WT control mice at 6 and 12 weeks of age. We chose mice at an early age to better understand proximal neurometabolic changes that could later correlate with neuropathology before too many confounding secondary changes occurred. Apoe KO mice and Ldlr KO mice demonstrated a loss of ApoE and Ldl-R protein in the liver respectively (Fig. 1A). It has been previously shown that Ldlr KO mice exhibit increased extracellular levels of ApoE in the brain [3]. Consistent with this finding, we saw increased levels of ApoE in both the liver (Fig. 1A) and brain (data not shown) in Ldlr KO mice. Previous studies have shown that Apoe and Ldlr KO mouse models show hypercholesterolemia, since they are not capable of catabolizing cholesterol-rich lipoproteins. Both Apoe KO and Ldlr KO mice showed increased levels of total cholesterol in the serum as early as 6 weeks of age compared to WT littermates (Fig. 1B). A large number of studies have shown that Apoe KO mice are hyperlipidemic since ApoE plays a central role in lipoprotein metabolism [25]. These data show that even at an early age, Apoe KO and Ldlr KO mice exhibit metabolic dysfunction.
Figure 1. Characterization of control, Apoe KO and Ldlr KO mice.
(A) ApoE and Ldl-R protein from homogenized livers of control, Ldlr and Apoe KO mice at 6 weeks and 12 weeks of age were analyzed by western blot using anti-ApoE and anti-LdlR antibody. Hsc70 was used for loading control.
(B) Cholesterol levels in serum of control, Ldlr and Apoe KO mice at 6 weeks and 12 weeks of age (n=6).
Data are expressed as mean ± SEM. **p<0.001. Open bars represent control, grey bars represent loss of Ldl-R and black bars represent loss of ApoE.
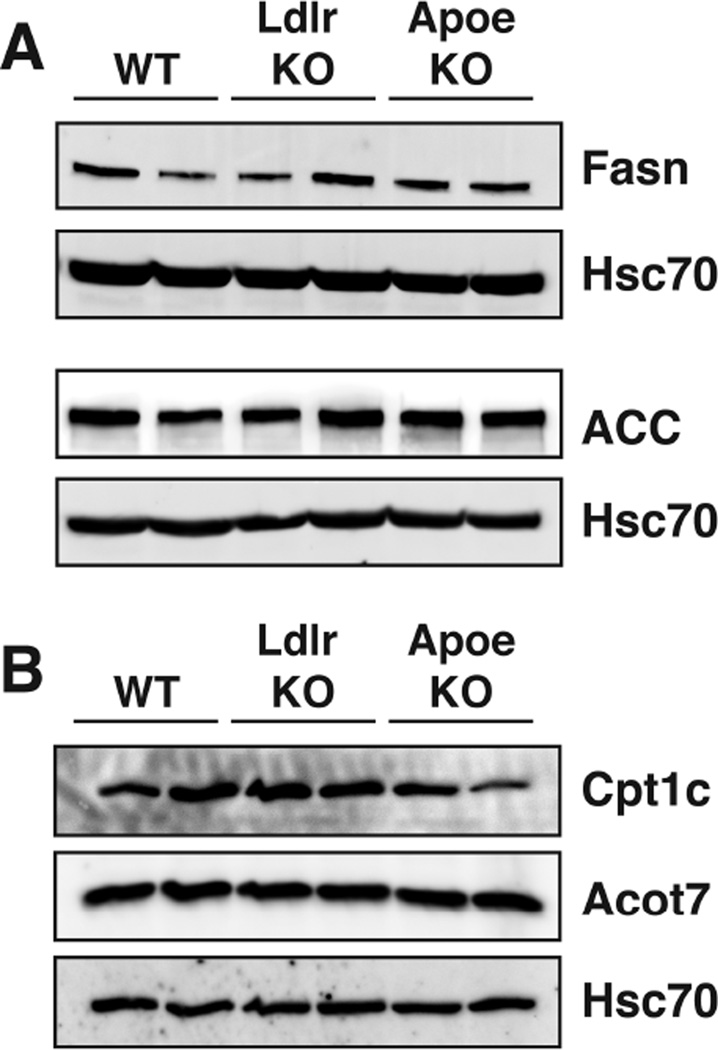
Additionally, we probed via western blot canonical pathways contributing to de novo fatty acid biosynthesis that are often changed and are seen as energy sensing pathways. We did not observe changes in the levels of acetyl-CoA carboxylase (ACC) or fatty acid synthase (Fasn) in young Apoe KO or Ldlr KO mouse brains (Fig. 2A). Carnitine palmitoyltransferase 1c (Cpt1c) and acyl-CoA thioesterase 7 (Acot7), which are involved in neuron-specific fatty acid metabolic pathways, were not changed in young Apoe KO or Ldlr KO mouse brains (Fig. 2B). These data show that the major fatty acid handling pathways are largely unaffected at the protein level in young Apoe KO or Ldlr KO mouse brains.
Figure 2. Fatty acid metabolic pathways are not affected in young Apoe and Ldlr KO mice.
(A) Western blot analysis of proteins involved in de novo fatty acid biosynthesis on control, Ldlr and Apoe KO mouse brains at 12 weeks of age.
(B) Western blot analysis of Cpt1c and Acot7 from brains of control, Ldlr and Apoe KO mice at 12 weeks of age.
All blots used Hsc70 for loading control.
Loss of Ldlr and Apoe have moderate effects on cholesterol homeostasis
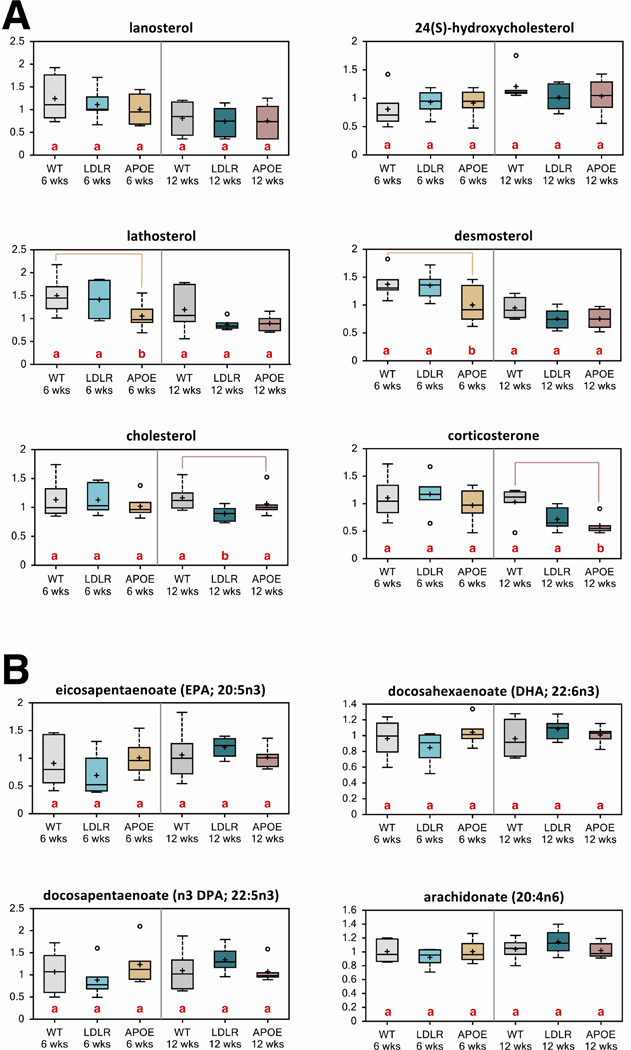
To understand the most proximal effects of Apoe and Ldlr on brain neurochemistry, we assayed the steady-state metabolic alterations in young WT, Apoe KO and Ldlr KO mouse brains at 6 and 12 weeks of age. Surprisingly, we did not find large changes in cholesterol or cholesterol metabolites in Apoe KO or Ldlr KO mouse brains (Fig. 3A). In fact, some of the key biosynthetic intermediates were suppressed in 6-week old Apoe KO brains and resolved by 12 weeks (Fig. 3A). The brain is rich in polyunsaturated fatty acids (PUFAs), particularly arachidonic acid (AA) (20:4n-6) and docosahexaenoic acid (DHA) (22:6n-3). Within the brain, these PUFAs regulate membrane fluidity, neuronal survival and signal transduction. Deficits and altered metabolism of AA and DHA fatty acids are associated with impaired neurodevelopment and several neurological disorders [13, 26–28]. Several studies have demonstrated that depletions in these essential PUFAs contribute to the pathogenesis of AD [13, 29, 30]. We were able to identify that ω3 derivatives eicosapentaenoate (EPA; 20:5n3), docosapentaenoate (n3 DPA; 22:5n3), AA and DHA were increased in Ldlr KO 12-week brains relative to 6-week brains. However, the Ldlr KO and Apoe KO mouse brains did not show any significant changes in these metabolites that were genotype specific (Fig. 3B). These results indicate a strong homeostatic mechanism to maintain brain lipids even under strong genetic perturbations.
Figure 3. Loss of ApoE or Ldl-R does not perturb the homeostatic mechanisms that maintain brain lipids.
(A) Biochemicals involved in the cholesterol pathway from control, Ldlr KO and Apoe KO brains of mice at 6 weeks and 12 weeks of age were compared through metabolomic analysis.
(B) Biochemicals from 6- and 12-week old control, Ldlr KO and Apoe KO mouse brains involved in the eicosanoid pathway.
Letters and lines indicate differences (p<0.05) between control and Ldlr KO or control and Apoe KO mice (n=6/group).
Loss of Ldlr and Apoe exhibits subtle changes in neurotransmitter metabolism
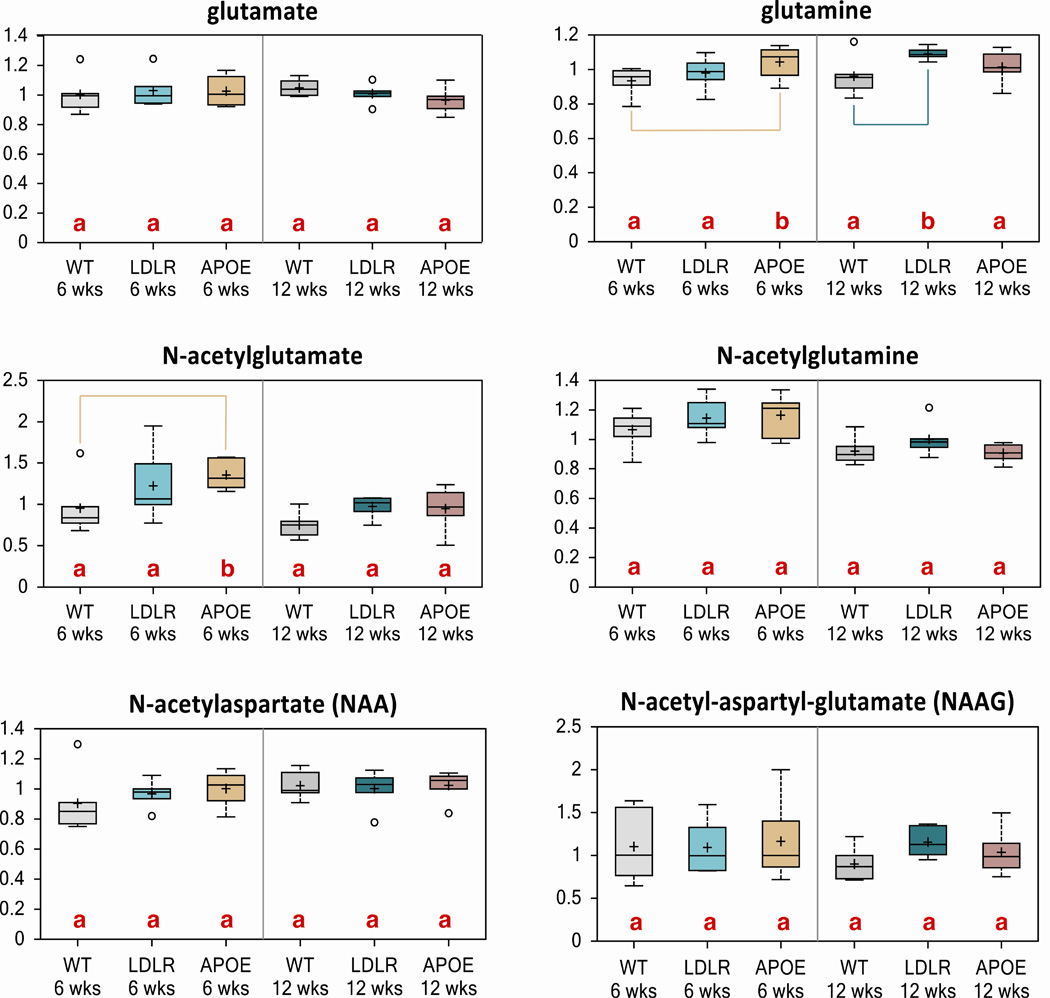
Excitatory neurotransmission via glutamate is tightly regulated via glutamate-glutamine cycling. We observed an increase in glutamine and N-acetyl glutamine levels, metabolites derived from glutamate, in Apoe KO mice at 6 weeks and Ldlr KO at 12 weeks, indicating a possible yet subtle perturbation in the glutamate-glutamine cycle (Fig. 4). These data show a relatively normal excitatory neurotransmitter metabolism.
Figure 4. Apoe KO and Ldlr KO mice do not exhibit alterations in excitatory neurotransmission via glutamate-glutamine cycling.
Subtle changes in biochemicals glutamine and N-acetylglutamate were observed through metabolomic analysis in brains of 6-week and 12-week old mouse brains of control, Ldlr KO and Apoe KO mice.
Letters and lines indicate differences (p<0.05) between control and Ldlr KO or control and Apoe KO mice (n=6/group).
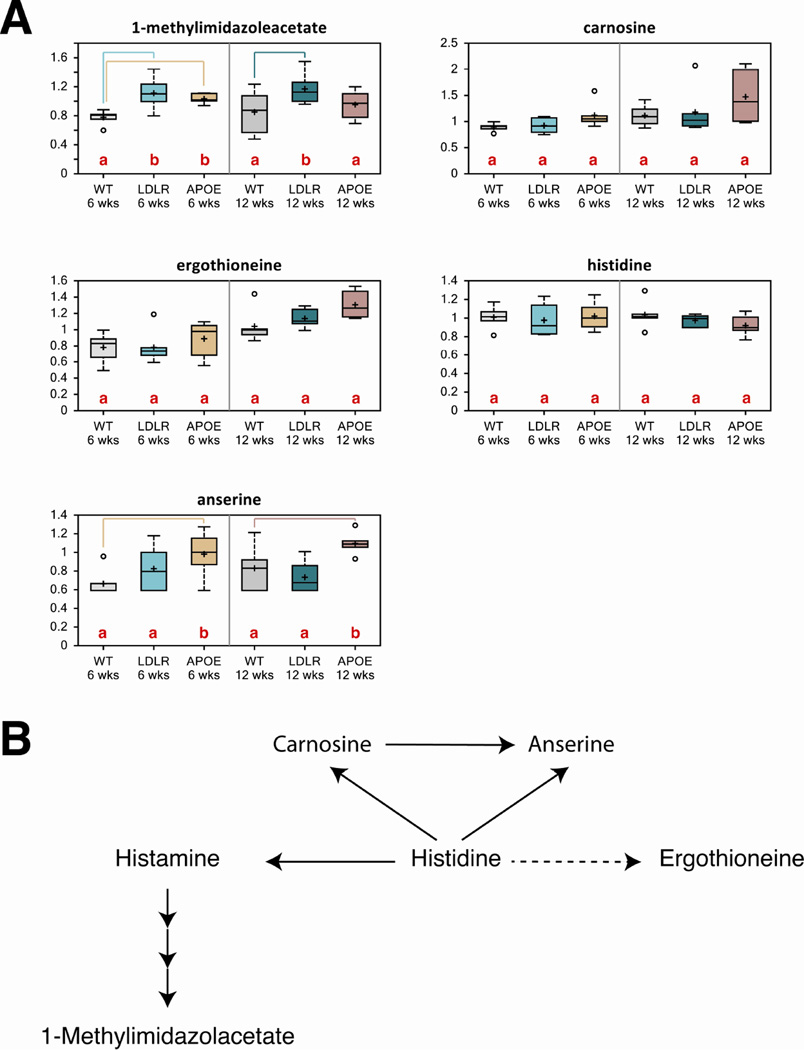
Histamine is a neurotransmitter that is generally involved in regulatory mechanisms in the brain. Previous studies demonstrated that a decrease in brain histamine contributes to cognitive decline in AD directly or through the cholinergic system [31]. 1-methylimidazoleacetate is a breakdown product of histamine and we observed increased 1-methylimidazoleacetate levels in brain samples of both Ldlr KO and Apoe KO mice at 6 and 12 weeks relative to control mice, indicating altered histamine metabolism (Fig. 5A). Carnosine, anserine and ergothioneine are also products derived from histidine (Fig. 5B). There was an overall increase in these metabolites in both KO models at 12 weeks of age (Fig. 5A). These data suggest subtle changes in histadine metabolism in the brains of Ldlr KO and Apoe KO mice.
Figure 5. Loss of ApoE or Ldl-R causes subtle changes in histidine metabolism in the brain.
(A)Biochemicals involved in histidine biochemistry from control, Ldlr KO and Apoe KO mouse brains were compared to determine if metabolomic analyses showed any statistically significant changes. There were only subtle changes in 1-methylimidazoleacetate and anserine.
(B) A schematic of the histidine metabolism pathway. Data are expressed as mean ± SEM
Letters and lines indicate differences (p<0.05) between control and Ldlr KO or control and Apoe KO mice (n=6/group).
Loss of Ldlr and Apoe exhibits altered lysine metabolism
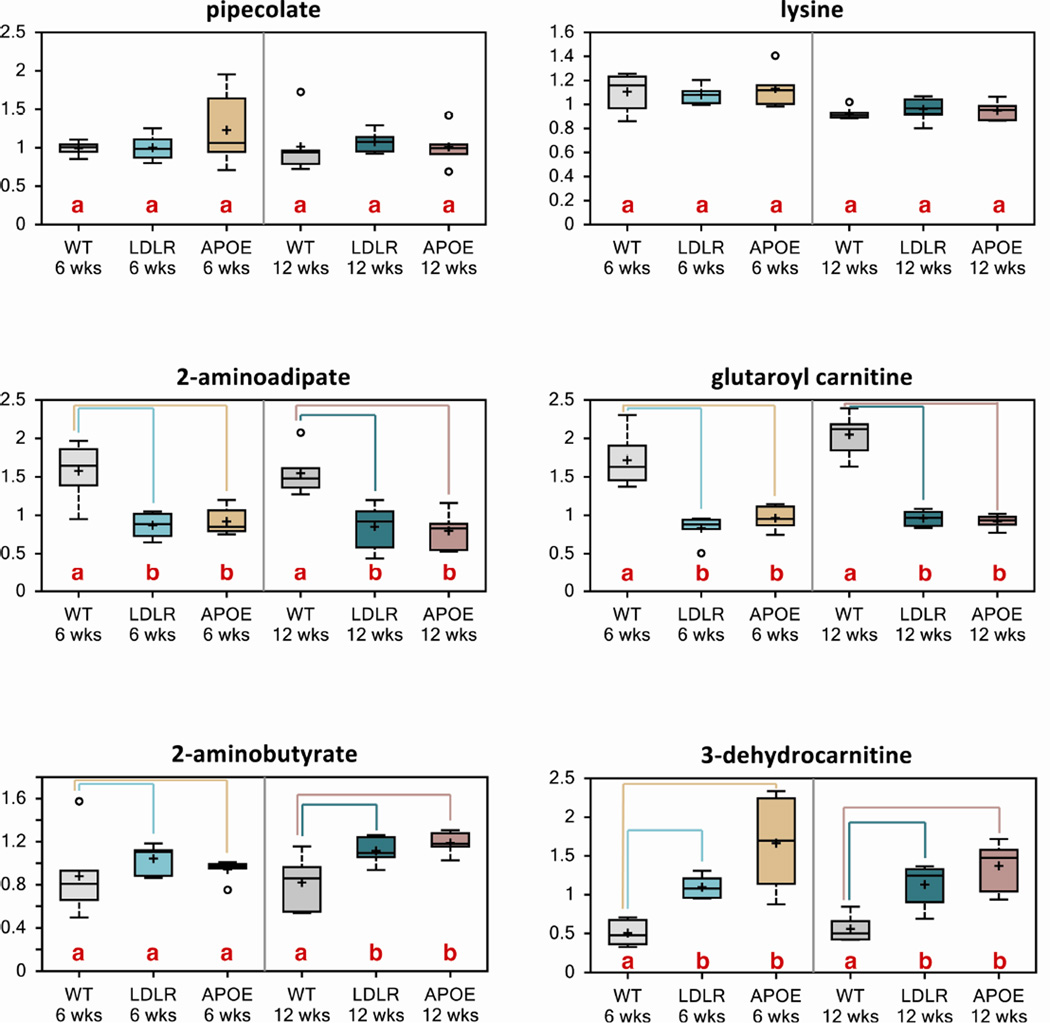
Metabolomic studies in humans between normal and AD patients have shown that there are significant alterations in lysine metabolism [32]. 2-aminoadipate and glutaroyl carnitine are catabolites along the lysine metabolic pathway. In Ldlr KO and Apoe KO mouse models, we observed that 2-aminoadipate and glutaroyl carnitine were significantly reduced in both Ldlr and Apoe KO mice at 6 and 12 weeks relative to wild type controls (Fig. 6). These were some of the most significantly altered metabolites in the Ldlr KO and Apoe KO brain metabolome. Pipecolate and lysine were not altered at either age in either genotype (Fig. 6). Additionally, 2-aminobutyrate was significantly increased in 12-week old Ldlr KO and Apoe KO mouse brains and 3-dehydrocarnitine was significantly increased in 6 and 12-week old Ldlr KO and Apoe KO mouse brains (Fig. 6). These data indicate altered brain bioenergetics and possible altered energy homeostatic mechanisms.
Figure 6. Loss of ApoE or Ldl-R results in altered lysine metabolism.
Metabolomic analyses revealed that key metabolites in the lysine metabolism pathway were altered in Ldlr and Apoe KO mouse brains (n=6/group).
Letters and lines indicate differences (p<0.05) between control and Ldlr KO or control and Apoe KO mice (n=6/group).
Discussion
The absence of Ldl-R or ApoE disrupts cholesterol and lipid homeostasis. A decrease in cholesterol levels in neurons affects Aβ levels in the brain [33], and it has been shown that hypercholesterolemia potentiates tau-hyperphosphorylation and Aβ production in the brain [15]. There was a significant increase in plasma total cholesterol levels in both the Ldlr and Apoe KO animals compared to their WT littermates even at early time points. The Ldl-R is one of the main receptors that interacts with the lipoprotein complex to internalize and degrade these molecules in target cells. In this context, the absence of Ldl-R could cause a blockage of lipoprotein uptake, increasing plasma cholesterol levels. ApoB and ApoE are the two main apolipoproteins that globally regulate cholesterol and lipid homeostasis, ApoE being one of the primary apolipoproteins in the brain that transfers cholesterol between the cells of the brain [40]. Therefore, the absence of ApoE could severely impede cholesterol uptake, causing an increase in plasma cholesterol levels.
In the brain, cholesterol and cholesterol metabolites were decreased in Apoe KO and Ldlr KO mice, especially at 12 weeks of age. Cholesterol is tightly regulated in the brain by the blood brain barrier, which does not allow lipoprotein cholesterol uptake from serum, and is mostly derived by de novo synthesis [15, 40]. Depletion of cholesterol leads to synaptic and dendritic spine degeneration, failed neurotransmission and decreased synaptic plasticity [10, 34]. Our metabolomic data show that in both KO mouse models, there is a decreasing trend with age in cholesterol and cholesterol precursor levels. In particular, the level of cholesterol and desmosterol were lower than WT levels. In humans and rodents with AD, desmosterol is decreased in the brain due to a decrease in the Dhxr24 gene [12]. Consistent with this finding, Apoe KO mouse brains also exhibited decreased levels of desmosterol as early as 6 weeks of age while Ldlr KO mice showed a significant decrease at 12 weeks of age, suggesting that KO of either Apoe or Ldlr leads to perturbations in cholesterol and lipid metabolism that may promote Aβ formation and tau phosphorylation in the brain. The alterations observed in cholesterol metabolites as well as systemic cholesterol levels all direct these mouse models to being more susceptible to AD.
Epidemiological studies have shown that patients treated with DHA and eicosapentaenoate improved AD symptoms when they were in the early stages of the disease. Furthermore, administration of DHA and eicosapentaenoate in rodents showed reduced Aβ formation, ROS levels and oxidative stress in the cerebral cortex and the hippocampus, thus indicating that DHA plays a crucial role in alleviating the symptoms of AD [13, 35, 36]. In our metabolomic study, we observed subtle changes in these essential PUFAs that was time but not genotype dependent, which may suggest that age is a major factor in contributing to brain PUFA dysregulation in AD.
There was an increase in the histamine degradation product 1-methylimidazoleacetate. In addition, there was an increase in glutamine and acetyl glutamate derived from glutamate, indicating a perturbation in neurotransmission and brain function. In AD, histaminergic neurons also degenerate and form tangles in the brain. While histamine levels usually increase with age, a decline in histamine has been seen in AD, correlating with an impairment in cognitive function that leads to some of the symptoms seen in AD patients [37]. Histamine, which functions as a neurotransmitter in the CNS, is involved in many brain functions such as control of pituitary hormone secretion, suppression of eating, cognitive functions and sleep [38]. The release of histamine is altered in response to different types of brain injury and disease, and low histamine levels are known to be associated with convulsions and seizures [39]. Therefore, increases in 1-methylimidazoleacetate as well as other histidine-derived metabolites may indicate a higher histamine turnover in Ldlr KO and Apoe KO mice, impairing cognitive function.
Metabolomic studies in human subjects have shown significant changes in amino acid metabolism in AD patients. Among these, the lysine metabolism pathway was one that showed significant alterations. Lysine also supports the synthesis of L-carnitine, which is needed to generate energy from fatty acids in mitochondria in cells other than neurons. AD patients were shown to have lower lysine catabolites and carnitine levels compared to control subjects [32]. The lysine catabolites 2-aminoadipate and glutaroyl-carnitine were significantly reduced in the Apoe and Ldlr KO mice relative to controls, suggesting altered energy production in the KO animals relative to the wild type animals. Although overt changes were not observed in the dominant energy metabolism pathways such as glycolysis, TCA cycle, or β-oxidation, changes in several carnitine derivatives further support differences in homeostatic energy metabolism. Therefore, this perturbation in lysine metabolism may potentiate neuropathology of aged Ldlr KO and Apoe KO mice.
Conclusion
Unbiased metabolomic profiling in young Apoe KO, Ldlr KO and WT mouse brains revealed subtle changes in a broad range of metabolites in vivo. These subtle changes in steady-state metabolites indicate that the loss of ApoE or Ldl-R alone does not significantly contribute to the progression of AD at an early stage in these mouse models.
Supplementary Material
Acknowledgements
This work was supported in part by National Institutes of Health grants NS072241 to M.J.W. and DK084171 to G.W.W.
Footnotes
Conflict of Interest
The authors have no competing financial interests.
References
- 1.Wegener G. Brains burning fat: different forms of energy metabolism in the CNS of insects. Naturwissenschaften. 1983;70:43–45. doi: 10.1007/BF00365961. [DOI] [PubMed] [Google Scholar]
- 2.Canevari L, Clark JB. Alzheimer's disease and cholesterol: the fat connection. Neurochemical research. 2007;32:739–750. doi: 10.1007/s11064-006-9200-1. [DOI] [PubMed] [Google Scholar]
- 3.Fryer JD, Demattos RB, McCormick LM, O'Dell MA, Spinner ML, Bales KR, Paul SM, Sullivan PM, Parsadanian M, Bu G, Holtzman DM. The low density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. The Journal of biological chemistry. 2005;280:25754–25759. doi: 10.1074/jbc.M502143200. [DOI] [PubMed] [Google Scholar]
- 4.Cheng H, Wang M, Li JL, Cairns NJ, Han X. Specific changes of sulfatide levels in individuals with pre-clinical Alzheimer's disease: an early event in disease pathogenesis. Journal of neurochemistry. 2013;127:733–738. doi: 10.1111/jnc.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng H, Zhou Y, Holtzman DM, Han X. Apolipoprotein E mediates sulfatide depletion in animal models of Alzheimer's disease. Neurobiology of aging. 2010;31:1188–1196. doi: 10.1016/j.neurobiolaging.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han X. The role of apolipoprotein E in lipid metabolism in the central nervous system. Cellular and molecular life sciences : CMLS. 2004;61:1896–1906. doi: 10.1007/s00018-004-4009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, D MH, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. Journal of neurochemistry. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 8.Han X, Rozen S, Boyle SH, Hellegers C, Cheng H, Burke JR, Welsh-Bohmer KA, Doraiswamy PM, Kaddurah-Daouk R. Metabolomics in early Alzheimer's disease: identification of altered plasma sphingolipidome using shotgun lipidomics. PloS one. 2011;6:e21643. doi: 10.1371/journal.pone.0021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess BL, McIsaac SA, Naus KE, Chan JY, Tansley GH, Yang J, Miao F, Ross CJ, van Eck M, Hayden MR, van Nostrand W, St George-Hyslop P, Westaway D, Wellington CL. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer's disease mouse models with abundant A beta in plasma. Neurobiology of disease. 2006;24:114–127. doi: 10.1016/j.nbd.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Russell DW, Halford RW, Ramirez DM, Shah R, Kotti T. Cholesterol 24-hydroxylase: an enzyme of cholesterol turnover in the brain. Annu Rev Biochem. 2009;78:1017–1040. doi: 10.1146/annurev.biochem.78.072407.103859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaliyamurthi V, Thanigavelan V, Rajamanickam GV. Effects of diet-induced hypercholesterolemia on amyloid accumulation in ovariectomized mice. Journal of biosciences. 2012;37:1017–1027. doi: 10.1007/s12038-012-9262-y. [DOI] [PubMed] [Google Scholar]
- 12.Wisniewski T, Newman K, Javitt NB. Alzheimer's disease: brain desmosterol levels. Journal of Alzheimer's disease : JAD. 2013;33:881–888. doi: 10.3233/JAD-2012-121453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann T, Kuchenbecker J, Grimm MO. Alzheimer's disease: the lipid connection. Journal of neurochemistry. 2007;103(Suppl 1):159–170. doi: 10.1111/j.1471-4159.2007.04715.x. [DOI] [PubMed] [Google Scholar]
- 14.Rebeck GW, LaDu MJ, Estus S, Bu G, Weeber EJ. The generation and function of soluble apoE receptors in the CNS. Molecular neurodegeneration. 2006;1:15. doi: 10.1186/1750-1326-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orth M, Bellosta S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol. 2012;2012:292598. doi: 10.1155/2012/292598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan QW, Iosbe I, Asou H, Yanagisawa K, Michikawa M. Expression and regulation of apolipoprotein E receptors in the cells of the central nervous system in culture: A review. Journal of the American Aging Association. 2001;24:1–10. doi: 10.1007/s11357-001-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulder M, Jansen PJ, Janssen BJ, van de Berg WD, van der Boom H, Havekes LM, de Kloet RE, Ramaekers FC, Blokland A. Low-density lipoprotein receptor-knockout mice display impaired spatial memory associated with a decreased synaptic density in the hippocampus. Neurobiology of disease. 2004;16:212–219. doi: 10.1016/j.nbd.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer's disease: the cholesterol connection. Nature neuroscience. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 19.Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. The Journal of biological chemistry. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- 20.Spencer BJ, Verma IM. Targeted delivery of proteins across the blood-brain barrier. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7594–7599. doi: 10.1073/pnas.0702170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P. Coordination of the transcriptome and metabolome by the circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5541–5546. doi: 10.1073/pnas.1118726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis JM, Wong GW, Wolfgang MJ. Acyl coenzyme A thioesterase 7 regulates neuronal fatty acid metabolism to prevent neurotoxicity. Molecular and cellular biology. 2013;33:1869–1882. doi: 10.1128/MCB.01548-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Wolfgang MJ. Metabolomic profiling reveals a role for CPT1c in neuronal oxidative metabolism. BMC biochemistry. 2012;13:23. doi: 10.1186/1471-2091-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfgang MJ, Kurama T, Dai Y, Suwa A, Asaumi M, Matsumoto S, Cha SH, Shimokawa T, Lane MD. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7282–7287. doi: 10.1073/pnas.0602205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pendse AA, Arbones-Mainar JM, Johnson LA, Altenburg MK, Maeda N. Apolipoprotein E knock-out and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. Journal of lipid research. 2009;50(Suppl):S178–S182. doi: 10.1194/jlr.R800070-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A, Weedon MN, Rich P, Patton MA, Warner TT, Silver DL, Crosby AH. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet. 2015 doi: 10.1038/ng.3313. [DOI] [PubMed] [Google Scholar]
- 27.Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, Akizu N, Rosti RO, Rosti B, Scott E, Schroth J, Copeland B, Vaux KK, Cazenave-Gassiot A, Quek DQ, Wong BH, Tan BC, Wenk MR, Gunel M, Gabriel S, Chi NC, Silver DL, Gleeson JG. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015 doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogyes E, Nyakas C, Kiliaan A, Farkas T, Penke B, Luiten PG. Neuroprotective effect of developmental docosahexaenoic acid supplement against excitotoxic brain damage in infant rats. Neuroscience. 2003;119:999–1012. doi: 10.1016/s0306-4522(03)00198-2. [DOI] [PubMed] [Google Scholar]
- 29.Soderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids. 1991;26:421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- 30.Tully AM, Roche HM, Doyle R, Fallon C, Bruce I, Lawlor B, Coakley D, Gibney MJ. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer's disease: a case-control study. The British journal of nutrition. 2003;89:483–489. doi: 10.1079/BJN2002804. [DOI] [PubMed] [Google Scholar]
- 31.Panula P, Rinne J, Kuokkanen K, Eriksson KS, Sallmen T, Kalimo H, Relja M. Neuronal histamine deficit in Alzheimer's disease. Neuroscience. 1998;82:993–997. doi: 10.1016/s0306-4522(97)00353-9. [DOI] [PubMed] [Google Scholar]
- 32.Trushina E, Dutta T, Persson XM, Mielke MM, Petersen RC. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer's disease using metabolomics. PloS one. 2013;8:e63644. doi: 10.1371/journal.pone.0063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaether C, Haass C. A lipid boundary separates APP and secretases and limits amyloid beta-peptide generation. The Journal of cell biology. 2004;167:809–812. doi: 10.1083/jcb.200410090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koudinov AR, Koudinova NV. Cholesterol homeostasis failure as a unifying cause of synaptic degeneration. Journal of the neurological sciences. 2005;229–230:233–240. doi: 10.1016/j.jns.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 35.Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oksman M, Iivonen H, Hogyes E, Amtul Z, Penke B, Leenders I, Broersen L, Lutjohann D, Hartmann T, Tanila H. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiology of disease. 2006;23:563–572. doi: 10.1016/j.nbd.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiological reviews. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 38.Nuutinen S, Panula P. Histamine in neurotransmission and brain diseases. Advances in experimental medicine and biology. 2010;709:95–107. doi: 10.1007/978-1-4419-8056-4_10. [DOI] [PubMed] [Google Scholar]
- 39.Bhowmik M, Khanam R, Vohora D. Histamine H3 receptor antagonists in relation to epilepsy and neurodegeneration: a systemic consideration of recent progress and perspectives. British journal of pharmacology. 2012;167:1398–1414. doi: 10.1111/j.1476-5381.2012.02093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.