Abstract
Converging evidence supports the hypothesis effects of aerobic exercise and environmental enrichment are beneficial for cognition, in particular for hippocampus-supported learning and memory. Recent work in humans suggests exercise training induces changes in hippocampal volume, but it is not known if aerobic exercise and fitness also impact the entorhinal cortex. In animal models, aerobic exercise increases expression of growth factors, including brain derived neurotrophic factor (BDNF). This exercise-enhanced expression of growth hormones may boost synaptic plasticity, and neuronal survival and differentiation, potentially supporting function and structure in brain areas including but not limited to the hippocampus. Here, using voxel based morphometry and a standard graded treadmill test to determine cardio-respiratory fitness (Bruce protocol; VO2 max), we examined if entorhinal and hippocampal volumes were associated with cardio-respiratory fitness in healthy young adults (N = 33). In addition, we examined if volumes were modulated by recognition memory performance and by serum BDNF, a putative marker of synaptic plasticity. Our results show a positive association between volume in right entorhinal cortex and cardio-respiratory fitness. In addition, average gray matter volume in the entorhinal cortex, bilaterally, was positively associated with memory performance. These data extend prior work on the cerebral effects of aerobic exercise and fitness to the entorhinal cortex in healthy young adults thus providing compelling evidence for a relationship between aerobic fitness and structure of the medial temporal lobe memory system.
Keywords: medial temporal lobes, hippocampus, recognition memory, cardiovascular fitness, neurotrophins
1. Introduction
The beneficial effects of cardio-respiratory fitness, aerobic exercise, and environmental enrichment on brain health and cognition are well documented (e.g. see van Praag et al., 2000; Cotman and Berchtold, 2002; Cotman et al., 2007 for reviews). For example, aerobic exercise and environmental enrichment are thought to improve learning and memory and to induce changes in the morphology of many brain structures, notably the hippocampus, through a variety of mechanisms. Most of this knowledge, however, is inferred from rodent models, which have focused eminently on effects in the dentate gyrus (DG), a sub-region of the hippocampus. Comparatively fewer direct observations have been made in humans. We therefore take a translational approach considering putative physical and neural correlates of exercise adaptation cross-sectionally in healthy young adults.
In rodents, both exercise and environmental enrichment have been shown to upregulate birth and survival rates of adult born neuronal and glial cells in the DG of the hippocampus, as well as improve performance on hippocampal dependent memory tasks (Creer et al., 2010; Falls et al., 2010; Fordyce and Farrar, 1991; Kempermann et al., 1997; O’Callaghan et al., 2007; Uda et al., 2006; van Praag et al., 2005, 1999). More generally, environmental enrichment has also been linked to increased cortical thickness across the brain, most notably in posterior regions and the entorhinal cortex (EC) (Diamond et al., 1976, 1987; Greer et al., 1982a, 1982b; reviewed in Mohammed et al., 2002). Exercise-induced brain plasticity is thought to be regulated in part by the complex, pleiotropic actions of different neurotrophins, namely brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1). These neurotrophins are associated with synaptic plasticity, neuronal survival, and differentiation (Kang and Schuman, 1995; McAllister et al., 1999; Trejo et al., 2001; see Cotman et al., 2007 for a review). In animal models BDNF mRNA expression, while highest in the hippocampus, is also high in EC and perirhinal cortex (Conner et al., 1997; Okuno et al., 1999).
Owing to the adult neurogenesis hypothesis, animal models have primarily targeted the DG and the hippocampal memory system. Exercise not only affects the DG, however, but also other regions of the medial temporal lobes (MTL), especially hippocampal subfield CA1 and the EC (Neeper et al., 1996; Stranahan et al., 2007). Specifically, structural changes have been observed in these regions in the form of increased dendritic spine density in basal dendrites of pyramidal neurons in entorhinal layer III and in basal and apical CA1 neurons after two months of voluntary wheel running (Stranahan et al., 2007). These findings stand on their own, but also integrate well with the literature on neurogenesis, etc., given the EC has direct projections to the DG and CA1 via layers II and III, respectively (Steward and Scoville, 1976; Van Hoesen and Pandya, 1975; Witter et al., 1989, 1988), and entorhinal input may be needed to integrate newborn DG neurons into existing functional networks (Vivar et al., 2012). In addition, angiogenesis could also affect hippocampal and/or entorhinal structure following exercise training. Angiogenesis and neurogenesis are upregulated cooperatively (Palmer et al., 2000), resulting in enhanced formation of new blood vessels that support newborn neurons. Together, these findings suggest aerobic exercise and cardio-respiratory fitness may directly alter the structure of the MTL more broadly.
It is plausible angiogenesis, adult neurogenesis, and neurotrophin-mediated plasticity may underlie aerobic exercise-related changes in MTL function and structure in humans. Although these hypotheses cannot be assessed directly in living individuals, evidence for adult neurogenesis has been observed in postmortem human tissue (Eriksson et al., 1998). In addition, increased cerebral blood volume (CBV) in the DG (and somewhat in the EC) has been linked to exercise, providing a possible correlate of exercise-induced neurogenesis in mice and by extension, perhaps in humans (Pereira et al., 2007). In support of these ideas, recent human studies indicate aerobic exercise training and cardio-respiratory fitness may be positively correlated with hippocampal volume (Erickson et al., 2011b, 2009) and hippocampal cerebral blood flow in healthy older adults (Maass et al., 2015b). In turn, changes in hippocampal volume following the exercise intervention were correlated with changes in serum BDNF (Erickson et al., 2011b). Previous work from our lab suggests effects of aerobic fitness and serum BDNF interact to support episodic recognition memory (Whiteman et al., 2014) in a task we have shown to recruit the hippocampus and perirhinal/EC (Schon et al., 2005, 2004). Additionally, increased cardio-respiratory fitness is associated with greater volume of the parahippocampal gyrus in Alzheimer’s disease patients (Honea et al., 2009), and aerobic exercise consistently appears as one of the most effective interventions to attenuate cognitive decline in geriatric populations (Barnes & Yaffe, 2011; Burns et al., 2008). In younger cohorts, exercise-induced gains in cardio-respiratory fitness have been linked to better relational memory in children (Chaddock et al., 2010), and better learning of a virtual Morris Water Maze task in adolescents (Herting and Nagel, 2012).
Given this background, it is likely entorhinal-dependent memory is associated with cardio-respiratory fitness and related mechanisms, but a direct link has not yet been established with entorhinal structure in humans. Establishing such a connection is of interest given the EC provides the primary input to the hippocampus during episodic memory encoding. The present study reports on a subsample of participants from Whiteman et al. (2014) that participated in a magnetic resonance imaging (MRI) study to examine associations between aerobic capacity and volumes of structures in the medial temporal lobe (MTL) memory system. Healthy young participants underwent a standard graded treadmill test to measure cardiorespiratory fitness (Bruce et al., 1963; Thompson et al., 2010), provided blood samples to assay serum BDNF concentration, and performed an episodic recognition memory task (Schon et al., 2004; Whiteman et al., 2014). We used region-of-interest (ROI) based voxel-based morphometry (VBM; Ashburner & Friston, 2000) to analyze regional gray matter volume in the EC and hippocampus in an unbiased manner. We predicted volume in these structures would be positively associated with cardiorespiratory fitness. In addition, based on our previous work (Whiteman et al., 2014), we hypothesized serum BDNF would also predict MTL volumes. Here, we report evidence for a relationship between aerobic fitness and gray matter volume in the EC. We also report performance on our recognition memory task was correlated with average volume in both the hippocampus and EC; we did not find relationships between gray matter volume and serum BDNF.
2. Materials and Methods
2.1.Participants
One hundred and fourteen healthy young participants were recruited from the Boston University student community. A random sub-sample of this cohort (sixty-one individuals) was recruited to participate in an MRI study; the full sample is described in Whiteman et al. (2014). Of this sub-sample, 16 did not meet inclusion/exclusion criteria, and forty-five participants were enrolled. Ten participants voluntarily withdrew or were lost to contact, and two were excluded due to equipment malfunction, leaving a final sample size of N = 33 participants (20 female, 13 male).
All participants were native English speakers or bilingual, all had normal or corrected to normal vision, and all gave signed, informed consent prior to the start of any study procedures. All protocols were approved by the Boston University Charles River Campus Institutional Review Board. Subject characteristics are described in Table 1.
Table 1.
Participant demographics. Data are presented as mean ± sd. Asterisks in the Meanmalw column indicate differences in the gender goup means
| Range | Mean | Meanfemale | Meanmale | |
|---|---|---|---|---|
| Age (yrs) | 18.0 – 30.0 | 21.1 ± 2.8 | 20.9 ± 2.8 | 21.6 ± 2.9 |
| Education (yrs) | 12.0 – 22.0 | 15.3 ± 2.2 | 14.9 ± 1.6 | 15.7 ± 2.9 |
| Fitness percentile | 17.9 – 100.0 | 65.3 ± 27.0 | 55.9 ± 23.7 | 78.7 ± 26.4* |
| Memory accuracy (%) | 26.3 – 68.2 | 46.6 ± 9.8 | 46.8 ± 11.5 | 46.4 ± 7.4 |
| BDNF (ng·ml−1) | 4.6 – 30.5 | 18.0 ± 6.4 | 17.7 ± 6.1 | 18.5 ± 7.1 |
| VO2 peak (ml·kg−1) | 31.3 – 66.5 | 45.5 ± 10.3 | 39.8 ± 6.9 | 53.6 ± 8.7*** |
| RERmax | 1.0 – 1.6 | 1.3 ± 0.2 | 1.2 ± 0.1 | 1.3 ± 0.2 |
| Intra-cranial Volume (L) | 1.2 – 1.8 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.6 ± 0.1** |
| BMI | 19.2 – 28.9 | 23.4 ± 2.8 | 23.2 ± 3.0 | 23.7 ± 2.4 |
| Body Fat (%) | 5.0 – 29.2 | 18.7 ± 8.3 | 24.5 ± 3.9 | 10.3 ± 5.2*** |
| Height (m) | 1.5 – 1.9 | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1*** |
| Weight (kg) | 49.2 – 86.1 | 66.2 ± 11.4 | 61.4 ± 10.5 | 73.0 ± 9.1** |
| Waist circumference (cm) | 62.0 – 93.4 | 74.9 ± 7.5 | 72.5 ± 6.6 | 78.4 ± 7.6* |
| Hip circumference (cm) | 81.0 – 112.5 | 93.4 ± 8.2 | 93.2 ± 9.0 | 93.7 ± 7.2 |
N = 33 (20 female)
2.2.Procedure
For each participant, the experiment consisted of three visits: (i) informed consent and screening; (ii) VO2 max aerobic capacity and body composition testing; and (iii) blood draw and MRI (including functional MRI and cognitive testing). For each participant, all visits were performed approximately within one month, and visit three (MRI and blood draw) took place no later than one week after visit two (aerobic fitness testing).
Screening ensured participants could safely participate in an MRI experiment, and were healthy and able to perform a strenuous treadmill test. Participants were screened for the following contraindicators: history of neurological or psychiatric conditions; learning disability; heart, lung, or musculoskeletal conditions or disorders; diabetes mellitus; electrolyte disorder; high cholesterol; eating disorder or obesity; or current use of any prescription or recreational cardioactive or psychoactive drugs, or recreational smoking.
2.3. Assessment of Aerobic Capacity
To assess participants’ aerobic capacity (VO2 max; the maximal rate of oxygen consumption during exercise, measured in milliliters of oxygen per minute per kilogram body-mass), we used a standard graded maximal exercise test performed on a treadmill (Bruce et al., 1963). VO2 max is a widely used measurement of stable aerobic fitness, and can be improved with training depending on exercise intensity and consistency (e.g. Huang et al., 2005). Participants were asked not to engage in strenuous physical activity for at least 24 hours prior their scheduled fitness test, and not to consume food or caffeine for at least 3 hours prior. A physician was on call, and two study staff members with current cardio-pulmonary resuscitation training were present for each exercise test. Each test began with the treadmill at a speed of 0.8 m/s and an incline of 10% grade. Throughout a test, the speed and incline of the treadmill increased by an average of 0.35 m/s and 2% grade every three minutes. Participants were given a 3-5 minute warm-up at 0.5 m/s and 0% grade before testing; following testing, participants were given a five-minute cool-down at 0.5 m/s and 0% grade. Fitness tests were terminated when participants reached volitional exhaustion. Graded exercise testing protocol and termination criteria followed the recommendations of the American College of Sports Medicine (ACSM; Thompson et al., 2010).
All VO2 max tests were performed between the hours of 8:00 – 10:00 AM at Boston University Sargent College of Health and Rehabilitation Sciences. The ParvoMedics True One 2400 system (ParvoMedics, Sandy, UT, USA) was used during testing to analyze gas exchange in participants’ breath. The True One 2400 system was calibrated against medical grade gasses (ParvoMedics, Sandy, UT, USA), and VO2 values were averaged over 30 s intervals. Respiratory exchange ratio (RER; volume expired CO2 divided by volume expired O2) was also monitored with this system. An RER at or above 1.15 can be taken as a rough indicator VO2 max has been reached (Issekutz et al., 1962). Because VO2 max varies by age and gender, we converted VO2 max values into fitness percentiles using ACSM norms (Thompson et al., 2010).
2.4. Blood Draw, Cognitive Testing, and Hormone Assays
Procedures for the blood draw, memory test, and hormone assays have been described in detail previously (Whiteman et al., 2014). Briefly, blood samples were drawn from the median cubital vein by a trained nurse at the Boston University General Clinical Research Unit between 8:00 – 9:45 AM. Serum aliquots were prepared from the obtained blood samples and stored at −80° C. At the end of the study, serum concentrations of BDNF, cortisol, IGF-1, and Vascular Endothelial Growth Factor (VEGF) were determined for all blood samples in duplicate using Quantikine® quantitative sandwich ELISA kits (R&D Systems Inc., Minneapolis, MN, USA).
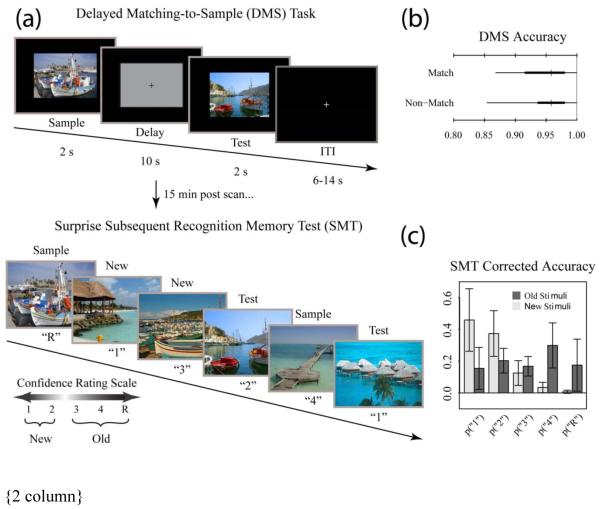
Following their blood draw, participants were walked across the street to the Boston University Center for Biomedical Imaging, where MRI data was collected. In addition to structural MRI data collection, fMRI data was collected while participants performed a delayed match-to-sample task with unfamiliar complex visual scenes (functional data to be reported in another publication; Fig. 1a). About fifteen minutes after the delayed match-to-sample task, participants performed a surprise subsequent memory test outside of the scanner with all (144) “old” delayed match-to-sample scenes, plus an equal number of content similar lures.
Fig. 1.
Tasks and behavioral results. Recognition memory task adapted from Schon et al. (2004). (a) Participants were first shown a series of 144 randomized, trial unique but content similar outdoor scenes in the context of a delayed match to sample (DMS) working memory task during fMRI scanning. Approximately 15 min after completion of the fMRI scanning session, participants were administered a surprise subsequent memory test (SMT) where they were shown all 144 DMS images, plus 144 lure images, and asked to rate their recognition confidence. Participants were blind to the ratio of old to new images on the SMT. (b) Overall DMS task accuracy, separated by match and nonmatch trials. Ticks, thick lines, and thin lines show medians, 50% intervals, and 95% intervals, respectively. (c) SMT response distributions for old and lure stimuli, separated by confidence rating. Error bars show SD.
Participants were unaware of the ratio of old to new images, and were asked to rate their recognition memory strength for each SMT image along a 5 point scale: 1 – sure, new; 2 – unsure, new; 3 – unsure, old; 4 – sure, old; R – sure, old, plus accompanied by some subjective association with the scene, e.g. memory for which run the scene came from or a thought the scene prompted when it was first shown. Our previous work suggests this paradigm recruits the hippocampus and MTL cortex (Schon et al., 2005, 2004). Stimulus presentation and participant response data were displayed and recorded using EPrime 2.0 software (Psychology Software Tools, Pittsburgh, PA). As a measure of memory accuracy, we used a standard corrected hit rate: the proportion of subsequent memory test scenes correctly recognized as old, less the proportion of lure scenes misclassified as old. Behavioral results are shown in Fig. 1b and 1c.
2.5. MR Image Acquisition and Preprocessing
High resolution T1-weighted structural scans (multi-planar rapidly acquired gradient echo images) were acquired on a 3 T Phillips Achieva scanner using an 8-channel head coil (TR = 6.8 ms; TE = 3.1 ms; voxel size = 0.98 × 0.98 × 1.2 mm; flip angle = 9°; 150 slices; SENSE reduction factor = 1.5). Structural images were preprocessed using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK). For each subject, structural scans were preprocessed as follows: (i) posterior probability maps for gray and white matter tissues, and cerebrospinal fluid, etc. were obtained using SPM8’s “new segment” option. (ii) Gray matter posterior probability maps were spatially normalized to Montreal Neurological Institute space using the diffeomorphic anatomical registration using exponentiated lie algebra algorithm (DARTEL; Ashburner, 2007) for a high degree of inter-subject co-registration. During normalization, images were Jacobian scaled for “modulated” VBM, resampled to 1.5 mm3 isotropic voxels, and spatially smoothed with a 6 mm full-width at half-maximum Gaussian kernel to reduce noise. Resultant images show total regional gray matter volume, accounting for differences in global brain anatomy between subjects.
2.6.Regions of Interest
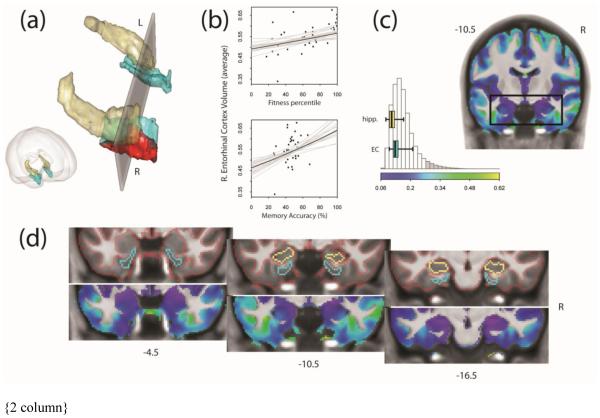
Based on our a priori anatomical predictions, we created a region of interest (ROI) for statistical analysis that included the hippocampus and EC. To define our ROI as accurately as possible, we used ITK-SNAP 2.2 (Yushkevich et al., 2006; http://www.itksnap.org/) to trace these structures manually on our group average normalized brain image based on the work of Pruessner et al. (2000, 2002). Hippocampal segmentation was performed following Pruessner et al. (2000), and entorhinal segmentation was performed following Pruessner et al. (2002). Since we used VBM to analyze the data, our results are, to a point, free from the usual segmentation biases (e.g. definition of boundaries between regions). We note, though, that defining our ROIs manually may induce a small bias with regard to spatial extent statistics. Briefly, borders for the EC were defined anteriorly with the appearance of the collateral sulcus and posteriorly with the disappearance of the intralimbic gyrus. The medial border of EC was amygdala if present or hippocampus if not; the lateral border was the midpoint of the medial bank of the collateral sulcus in accordance with Pruessner et al. (2002). We did not adjust the lateral border of the EC according to collateral sulcus depth, etc. as in Insausti et al. (1998). We do not include fimbria/fornix in our hippocampal tracings. A brief overview of our anatomical demarcations can be seen in Fig. 2d.
Fig. 2.
Results of ROI-based VBM analysis. (a) shows the region of right entorhinal cortex (EC) where we find gray matter volume is associated with aerobic fitness percentile. Results (red) are shown within a 3D rendering of our anatomical regions of interest, the hippocampus (gold) and EC (cyan). The gray plane coincides with the black box drawn on the MR slice in (c) and corresponds to slice y = 10.5 cm caudal to the anterior commissure. The relationships between volume in the right EC (averaged over the whole structure) and fitness percentile and recognition memory accuracy is portrayed in (b). Gray lines illustrate the uncertainty in the regression fits. (c) shows the spatial layout and distribution of the coefficient of variation (CV) for gray matter volume across participants. Values for hippocampal and EC voxels are shown as horizontal boxplots overlaid on the histogram, indicating that variation is not unusually large for these regions. Boxplots show absolute range, interquartile range, and median. The histogram depicts the 95% high-density region (white bars) and the extreme tails (gray bars). Exploration of gray matter variability is extended throughout our ROI in (d). Sections run caudally from the anterior EC through posterior EC and hippocampal head. The top images show the borders of the EC, hippocampus, and labeled gray matter; bottom images show corresponding CV maps. All results are depicted on the group average brain. Figures were made using functionality from the R rgl and misc3d packages (Adler et al., 2014; Feng and Tierney, 2008).
2.7.Statistical Analysis
Our analysis of the MR data was performed using R 3.0.1 (R Development Core Team, 2012), and in-house code written for MATLAB (The MathWorks Inc., Natick, MA, USA) and SPM8. We fit standard least-squares regression models to log-scaled VBM data (natural logarithm), predicting log-volume from variables fitness percentile (percentile score), SMT corrected hit rate (percentage), and serum BDNF (ng · mL−1), controlling for effects of total intra-cranial volume (L), and binary variables gender (0 female, 1 male) and RER ≥ 1.15 (0 RER < 1.15, 1 RER ≥ 1.15; RER see Methods: Assessment of Aerobic Capacity). Continuous input variables were mean-centered, except for fitness percentile, which was centered to the 50th percentile for better interpretability.
We chose a statistical approach whereby we began our analysis by testing our a priori hypotheses under our most stringent statistical thresholds, and afterwards relaxed our assumptions into an exploratory whole brain analysis. Because there are three variables of interest in this analysis and either positive or negative contrasts could be declared significant (although we are only interested in positive effects), we displayed our statistical parametric maps at a threshold P < 0.008 ≈ 0.05 / 6. We corrected these P values for our main ROI analysis using a permutation test (Nichols and Holmes, 2002) based on 1000 relabelings. Following the permutation distribution, the corrected 2-tailed α < 0.05 theight thresholds were 3.79 and 3.71 for fitness and memory, respectively. Single tailed α < 0.05 cluster extent thresholds were 131 and 113 voxels for fitness and memory, respectively. In contrast, “exploratory” whole brain analyses were collated using a rule-of-thumb cluster extent k ≥ 100 contiguous voxels to visualize results, and are otherwise uncorrected for multiple comparisons. Results from both ROI and exploratory analyses are reported in Table 2.
Table 2.
Summary of main (ROI-based), and exploratory (whole brain) VBM analyses
| Main (ROI-based) Analysis – fitness percentile | |||
| Region | Peak coordinates | Peak t(26) | Cluster size k |
| R. Entorhinal | (28.50, −10.50, −34.50) | 4.57 | 157 |
|
| |||
| Exploratory Analysis – fitness percentile | |||
|
| |||
| R. Precuneus | (19.50, −63.00, 25.50) | 7.16 | 1768 |
| L. Lingual Gyr. | (−25.50, −81.00, −16.50) | 4.73 | 1162 |
| R. Inf. Parietal Lobule | (57.00, −49.50, 37.50) | 4.30 | 725 |
| R. Lingual Gyr. | (13.50, −67.50, −10.50) | 4.90 | 654 |
| L. Mid. Occipital Gyr. | (−25.50, −93.00, 6.00) | 4.26 | 385 |
| R. Perirhinal/Entorhinal | (30.00, −9.00, −34.50) | 4.64 | 348 |
| L. Calcarine Gyr. (BA 18) | (−6.00, −88.50, −13.50) | 3.58 | 186 |
| L. Precuneus | (−6.00, −78.00, 42.00) | 4.18 | 185 |
| L. Calcarine Gyr. (BA 17) | (−10.50, −67.50, 12.00) | 3.46 | 163 |
| R. Mid. Temporal Gyr. | (54.00, −60.00, −1.50) | 3.60 | 140 |
| R. Inf. Temporal Gyr. | (69.00, −33.00, −18.00) | 3.40 | 133 |
| Cerebellum | (−15.00, −43.50, −60.00) | 3.63 | 114 |
| R. Mid. Temporal Gyr. | (49.50, −39.00, −1.50) | 3.44 | 112 |
| R. Sup. Frontal Gyr. | (21.00, 61.50, 13.50) | 3.14 | 102 |
|
| |||
| Exploratory Analysis – memory accuracy | |||
|
| |||
| R. Entorhinal | (31.50, −6.00, −37.50) | 3.29 | 252 |
| Cerebellum | (−22.50, −58.50, −27.00) | 3.30 | 245 |
| R. Mid. Temporal Gyr. | (54.00, −36.00, −3.00) | 3.71 | 203 |
| R. Amygdala | (25.50, −12.00, −9.00) | 3.28 | 118 |
|
| |||
Following up on the results from our classical VBM analysis, we wanted to provide an estimate for the cross sectional effect of aerobic fitness on average volume in the whole EC. To do this, we extracted the mean VBM intensity—the amount of gray matter in the average entorhinal voxel—from subjects’ ECs judging that this should provide a reasonable correlate of the size of the gross structure. We then fit a standard least squares regression model to the extracted mean VBM data with independent variables fitness percentile, memory accuracy, total intra-cranial volume, gender, and RER ≥ 1.15. For this analysis, we again opted to log-transform the mean VBM estimates (natural logarithm). This has at least two benefits: (i) since gray matter volume is all-positive it makes sense effects may occur on a multiplicative scale (Keene, 1995) and, similarly, (ii) small regression coefficients can be interpreted as percentage increases in y per change in x (e.g. βlog = 0.02 corresponds to an approximate 2% increase in y on the native scale since e0.02 = 1.02). This helps us to connect the present results to the extant literature.
3. Results
3.1. Fitness and entorhinal gray matter volume
Results from our primary VBM analysis showed gray matter volume in a region of the right EC composed of 157 voxels was positively associated with aerobic fitness (Table 2; Fig. 2a). This was the only region that showed a correlation with aerobic fitness when controlling for the effects of memory accuracy, BDNF, total intra-cranial volume, gender, and RER ≥ 1.15 for our primary ROI-based VBM analysis. Based on the permutation distribution, the observed result is significant in both height (P < 0.01), and extent (P < 0.05). Exploratory whole brain results also suggested regions of gray matter may be associated with fitness in the precuneus, lingual gyrus, inferior parietal lobule, middle occipital gyrus, visual areas BA 17 and BA 18, middle and inferior temporal gyrus, cerebellum, and superior frontal gyrus (Table 2 and Fig. 3a).
Fig. 3.
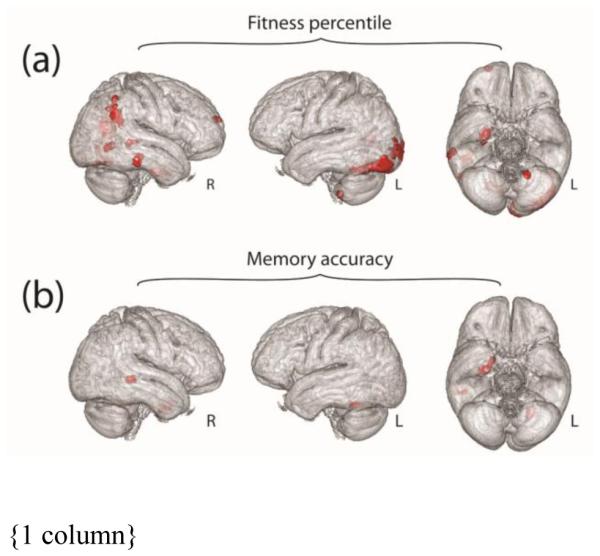
Results of exploratory whole-brain analysis. Parts (a) and (b) illustrate the results of an exploratory whole brain analysis, showing regions (red) where gray matter volume may be associated with fitness percentile or memory accuracy, respectively. Results are depicted within the group average brain.
Given that we observed a region in EC where gray matter volume was significantly associated with cardio-respiratory fitness in our sample, we wanted to provide an estimate for how aerobic fitness may be expected to relate to EC volume in the general population and compared to previous literature. To do this, we determined the average VBM gray matter volume estimate from each subjects’ ROIs and refit our model separately to the vectors of means. Results of this analysis, reported in table 3, suggest positive effects of fitness in the right EC, and a positive trend across both hemispheres (right hemisphere: rpart(26) = 0.45; left hemisphere: rpart(26) = −0.02; average: rpart(26) = 0.28).
Table 3.
Summary of six regression models fit separately to the average VBM data (logarithmic scale) from each region of interest. Results are presented as β (SEβ); σ denotes the residual standard deviation; asterisks denote significance at the 0.05 level. For each regression, continuous input variables were mean centered (fitness percentile centered to the percentile), but not rescaled. Regression also controlled for effects of non-interest: total intra-cranial volume, and RER ≥ 1.15
| R. Entorhinal | L. Entorhinal | Average | R. Hipp. | L. Hipp. | Average | |
|---|---|---|---|---|---|---|
|
|
|
|||||
| (Intercept) | −0.63 (0.03) | −0.64 (0.02) | −0.63 (0.02) |
−0.51 (0.02) |
−0.51 (0.02) |
−0.51 (0.02) |
| Fitness percentile |
0.0014* (0.0006) |
−0.0000 (0.0004) |
0.0007 (0.0005) |
−0.0006 (0.0005) |
−0.0005 (0.0005) |
−0.0005 (0.0005) |
| Memory accuracy (%) |
0.0032* (0.0013) |
0.0020 (0.0010) |
0.0026* (0.0011) |
0.0017 (0.0012) |
0.0009 (0.0011) |
0.0013 (0.0011) |
| BDNF (ng·ml−1) |
0.0005 (0.0020) |
−0.0020 (0.0016) |
−0.0007 (0.0016) |
0.0017 (0.0019) |
0.0008 (0.0017) |
0.0012 (0.0017) |
| Gender (0 female; 1 male) |
0.029 (0.030) | 0.035 (0.024) | 0.032 (0.026) |
0.0027 (0.029) |
−0.017 (0.026) |
−0.007 (0.027) |
|
|
||||||
| σ | 0.07 | 0.05 | 0.06 | 0.06 | 0.06 | 0.06 |
3.2. Memory accuracy and gray matter volume
Results from our primary ROI-based VBM analysis did not show any region within the MTL where gray matter volume was associated with memory task performance. An exploratory whole brain analysis suggested possible effects in the right EC, hippocampus, amygdala, middle temporal gyrus, and cerebellum (Table 2 and Fig. 3b). In the follow-up analysis of average ROI gray matter volume, large correlations between memory accuracy and volume are apparent in the EC (right hemisphere: rpart(26) = 0.44; left hemisphere: rpart(26) = 0.35; average: rpart(26) = 0.43; Table 3), and a positive trend is apparent in hippocampus (right hemisphere: rpart(26) = 0.27; left hemisphere: rpart(26) = 0.16; average: rpart(26) = 0.23; Table 3).
3.3. Fitness and memory accuracy
In our previous work (Whiteman et al., 2014), we have shown that fitness and serum BDNF interactively predicted recognition memory accuracy. In that study, fitness percentile alone did not predict memory accuracy. Here, we reanalyzed this data in our subsample (N = 33). As expected based on our previously reported results from the larger sample, fitness percentile did not predict recognition memory performance (rpart(29) = −0.09; controlling for gender and RER). When we refit our full model from our previous work to this subsample (predictors: fitness, BDNF, the BDNF by fitness interaction, gender, and RER), we find that the BDNF by fitness interaction showed a statistical trend predicting memory accuracy in these data. The slope coefficient is comparable between full and subsample, but the standard error is larger (full sample β ± SE = 0.59 ± 0.22; current subsample β ± SE = 0.52 ± 0.28). For a complete description of the association between serum BDNF, aerobic fitness, and memory accuracy in the full sample, we refer the interested reader to Whiteman et al. (2014).
3.3. BDNF and gray matter volume
Results from our primary ROI-based VBM analysis did not show any region within the MTL where gray matter volume was associated with serum BDNF levels. An exploratory whole brain analysis suggested a region of medial thalamus potentially associated with BDNF (peak: coordinates = [2, −4, 4], t(26) = 4.88; cluster extent k = 299). For a description of the association between serum BDNF, aerobic fitness, and memory accuracy, we refer the interested reader to Whiteman et al. (2014). The follow-up analysis of effects at the average ROI level did not suggest any correlation between serum BDNF and structure in the EC or hippocampus.
3.4. Analysis of variability in structural VBM data
The fitness percentile effect we report was observed close to the border between entorhinal and perirhinal cortex on the medial bank of the collateral sulcus. Without our ROI mask, the effect extended into perirhinal cortex. Given that VBM as a technique relies heavily on the anatomical coregistration algorithm(s) used, and because the collateral sulcus is an especially variable region (e.g. Pruessner et al., 2002; Yushkevich et al., 2015a), we were concerned our results could be explained by the possibility of anatomical registration artifacts. To examine this question, we created a coefficient of variation map for our group data. This map shows regions of high anatomical variability across subjects. Examination of this data shows the anatomical variation in the EC and hippocampus is not unusually high (Fig. 2c-d). The histogram in Fig. 2c shows the distribution of the coefficient of variation for all gray matter voxels in the brain, including our regions of interest, the EC and the hippocampus (shown additionally as boxplots overlaid on the histogram). Coefficient of variation values for the EC and the hippocampus fall within the high density region on the histogram (Fig. 2c), suggesting our results may be unlikely to be due to anatomical warping artifacts.
4. Discussion
With the present study, we examined the relationships between aerobic fitness, serum BDNF, recognition memory, and gray matter volume in the entorhinal-hippocampal memory system. Our data suggest volume in the EC may be positively associated with aerobic fitness and recognition memory performance, but not with serum BDNF. These results provide translational support for rodent models on exercise, neurogenesis, and the entorhinal-hippocampal memory system.
Motivation for this general line of research ultimately comes from studies that showed enriched environments could induce cortical thickening in rodents relative to standard living condition controls (Diamond, 1988; Diamond et al., 1985; Malkasian and Diamond, 1971). Early studies showed increases in cortical thickness were most pronounced in posterior regions and in the EC (Greer et al., 1982; Diamond, 1988; reviewed in Mohammed et al., 2002), which relays incoming information to the hippocampus via its direct projections to the dentate gyrus and CA fields (Van Hoesen and Pandya, 1975; Witter et al., 1989, 2000). In the hippocampus, however, changes in brain morphology related to enrichment were absent or not very robust (Diamond, 1988; Diamond et al., 1976; Greer et al., 1982b; Walsh et al., 1969) unless measurements were confined to the granular layer of the dentate gyrus (Diamond, 1988; Juraska et al., 1985). It is now known the granular layer of the dentate gyrus is the neurogenic zone of the hippocampus, and shows a strong response to aerobic exercise in animal models (e.g. Pereira et al., 2007; van Praag et al., 2005, 1999). Additionally, other regions of the hippocampal-entorhinal memory circuit show structural changes in manipulations limited to aerobic exercise (Neeper et al., 1996; Stranahan et al., 2007). Specifically, both the CA1 subfield of the hippocampus and the EC layer III show increased dendritic spine density following two months of voluntary wheel running (Stranahan et al., 2007). Aerobic exercise not only enhances adult neurogenesis in the dentate gyrus, but also promotes angiogenesis (Clark et al., 2009; Palmer et al., 2000), the formation of new blood vessels, which could also explain changes in thickness or volume. Given the unique architecture of the entorhinal-hippocampal memory system, a causal relationship between aerobic exercise training and increased entorhinal volume is plausible and consistent with our data. In support of these findings, we report a positive association between aerobic fitness and EC volume in healthy young adult humans.
This finding is not without its caveats. Given the nature of VBM, it is difficult to distinguish between meaningful anatomical variation and anatomical warping artifacts since both are induced by the chosen coregistration algorithm. Since our primary result in right EC lies partially within the collateral sulcus, a region of high anatomical variability (Insausti et al., 1998; Pruessner et al., 2002; Yushkevich et al., 2015a), we explored the possibility of artifactual warping. To do this, we created and inspected a coefficient of variation map for all gray matter voxels in the brain, and concluded that the variation in hippocampal and entorhinal volume across subjects fell within a normal range. Note in Fig. 2c-d one can appreciate the relatively modest variation in the collateral sulcus against the larger variation in the fundus of the neighboring occipitotemporal sulcus, or even greater variation in some dorsal regions.
Additionally, it is of interest why the fitness effect might at first appear to be lateralized to right EC. Under more careful consideration, however, the data do not warrant the assumption that the effect is truly unilateral. For example, if instead of the peak voxel, we look at the average of a 3 mm sphere surrounding the peak, we find t statistics of +4.51 in the right EC, and +1.72 in the complimentary set of voxels in the left EC. Importantly, the t statistic for the difference in these effects is only about 1.74, which is non-significant (P = 0.09). This calculation is by no means unbiased, and ignores any potential correlation between the hemispheric estimates, but it suggests the hypothesis that effects in the right and left hemispheres are different is not supported by the data. One can even apply the same argument to the average effects for each ROI given in Table 3, though in this case it leads to a weaker rejection. Indeed, based on the literature we discuss here, we conclude effects of fitness are likely to be bilateral. Therefore, in terms of laterality, we believe our data should be interpreted with caution. Convergent results from multiple complimentary methodologies are necessary to support our findings and their interpretation.
Several published studies provide information on the relationship between fitness and volume of MTL structures in healthy adult humans (Chaddock et al., 2010; Colcombe et al., 2006, 2003; Erickson et al., 2011b; Killgore et al., 2013; Makizako et al., 2011; Ruscheweyh et al., 2011; Schlaffke et al., 2014); perhaps the most relevant, with the most complete set of descriptive statistics is the study by Erickson et al. (2011b; also see Coen et al., 2011; Erickson et al., 2011a). Erickson et al. (2011b) conducted a year-long aerobic exercise intervention in older adults and reported on average small increases in hippocampal volume bilaterally for exercising subjects, and on average small decreases in hippocampal volume bilaterally for participants of an exercise training control group that performed resistance and stretching exercises. Averaging over both hemispheres, results from this study suggest approximately a 3.1% ± 2.8% (mean ± SE) increase in hippocampal volume per approximately a 4.1 point increase in fitness percentile in the exercise group (N = 60; data from Table 2 in Erickson et al., 2011b). We computed the 4.1 percentile points based on the average age and male to female ratio in the exercise group in this study. It seems reasonable to assume changes in gross hippocampal volume over a year period fit within the range of plus or minus a few percent in otherwise healthy older adults. Compared with the report from Erickson et al. (2011b) of approximately a 0.76% increase in volume per point fitness percentile (linear scale), our estimate of a 0.14% increase in right EC volume per point fitness percentile (multiplicative scale) makes sense for healthy young adults. Although this similarity is encouraging, there are considerable methodological differences between the studies.
Among the most interesting differences between the results of Erickson et al. (2011b, 2009) and the present report is that we have not replicated an effect in hippocampus. We are inclined to believe the failure to replicate the hippocampal result may either be simply a difference in the type of sample used (i.e. young vs. older adults), or it may be a methodological difference. Our implementation of VBM relies on the DARTEL algorithm (Ashburner, 2007), which is optimized for aligning global structures (Klein et al., 2009; Yassa and Stark, 2009) but may be less adept at aligning hippocampal subregions (Yassa and Stark, 2009) like the CA3/dentate gyrus. Our coefficient of variation map (Fig. 2c) shows very limited variation in the hippocampus; our analysis may not be sensitive enough to detect small hippocampal differences. In contrast to the present study, Erickson et al. (2011b) used an automated segmentation tool to compute overall hippocampal volume. Future studies using different morphometric techniques such as assessment of cortical or gray matter thickness (e.g. Burggren et al., 2008; Yushkevich et al., 2015), or high-field MRI (e.g. Maass et al., 2015a, b) may have more success in this area and may map better onto the earliest findings regarding enrichment and entorhinal and hippocampal morphology (Diamond, 1988; Juraska et al., 1985).
Since we have observed a positive relationship between aerobic fitness and gray matter volume in the EC, it may be important to understand the underlying neurobiological mechanisms of this putative correlation. In this regard, Pereira and colleagues assessed longitudinal changes in CBV in murine and human hippocampi and EC over a six week exercise intervention (Pereira et al., 2007). In both species, the dentate gyrus and EC were the only regions that exhibited increases in CBV over the course of the exercise intervention. Although effects in EC were not statistically significant in that study, their result may provide valuable insight into the biological basis of our observed relationship between fitness and volume in the EC. Given their data, we speculate angiogenic mechanisms may underlie observed CBV responses following exercise training. An angiogenic account seems a parsimonious explanation if the effects of exercise extend beyond the dentate gyrus and to the EC, as our data suggest. Moreover, a recent proof-of-concept study in older adults suggests exercise related enhancement of object recognition may be modulated by hippocampal cerebral blood flow and CBV (Maass et al., 2015b). This idea is further supported by studies on CBV and metabolism (Ide and Secher, 2000; Vissing et al., 1996) and by work suggesting angiogenesis and neurogenesis are upregulated cooperatively (Palmer et al., 2000). Note this angiogenic account, while parsimonious, is speculative as we cannot distinguish between a general increased CBF/CBV across the EC and one specific to improved synaptic functioning during cognition.
We have also reported evidence suggestive of associations between average EC volume and recognition memory performance. In our data, this result is most evident when looking at the average effect over the whole EC (one would expect this to be the most robust level). At the voxel level, however, the effect is just sub-threshold in the right EC in our ROI analysis due to the strict multiple comparison correction, but it is present at the exploratory threshold. Given the breadth of literature on the EC and episodic memory, we are inclined to consider these results supporting evidence. Recent high-resolution fMRI studies from our group have shown greater entorhinal activity for faster correct decisions during encoding of novel spatial environments (Brown et al., 2014), and reported entorhinal activity when unfamiliar stimuli with overlapping features or a greater stimulus load, respectively, need to be maintained in a working memory buffer (Newmark et al., 2013; Schon et al. 2015). These studies suggest a role for the EC in both episodic encoding and working memory maintenance. For the present experiment, we used an adaptation of a delayed match-to-sample task with outdoor scenes from our previous work (Schon et al., 2005, 2004). These studies reported delay period activity in the perirhinal/entorhinal cortex and hippocampus predicted whether a scene was later remembered with high confidence. Together with previous work (e.g. Bellgowan et al., 2009; Brown et al., 2014), these results suggest a role for both the perirhinal/entorhinal region and the hippocampus in encoding.
In addition, we have described estimates for relationships between hippocampal volume and memory performance on a subsequent memory test adapted from Schon et al. (2005, 2004), which showed a positive trend. Although the hippocampal estimates are non-significant in the present case, they are in the correct direction: previous work suggests hippocampal volume is related to episodic learning of spatial contexts in humans of the same age range (Brown et al., 2014), and others have suggested links between hippocampal volume and other memory tasks (e.g. Maguire et al., 2006; Hartley & Harlow, 2012; Horner et al., 2012; Rodrigue et al., 2013).
Finally we note VBM as a technique is somewhat limited in that it cannot provide volumetric measures of gross anatomic structures as accurately as, for example, manual segmentation methods can. Manual techniques, however, do not have the same spatial sensitivity of voxel-based techniques like VBM, and may be biased with regard to regional boundaries; the fundamental research questions that can be addressed by the two types of methodologies are slightly different. Whereas VBM is optimized for analysis of structural data collected with a fine isotropic resolution (like ours), manual segmentation methods are best suited to data collected with a very fine in-plane resolution, and a larger slice thickness. Further, VBM is ideally suited for investigating questions involving correlations between relative brain volumes and external variables (especially in healthy, non-clinical subjects where absolute volumes may be less informative than relative brain volume changes). For these reasons converging evidence from complementary morphometric techniques and from animal models are needed to support our findings.
5. Conclusions
We have successfully tested the hypothesis that effects of aerobic exercise on structure in the hippocampal-entorhinal formation observed in rodents can be translated to healthy young adult humans. Consistent with work on environmental enrichment in rodents, our data demonstrated a positive association between fitness and volume in the right EC. Here we have also reported evidence for correlations between volume in both entorhinal cortices and performance on an episodic recognition memory task. Our data provide compelling evidence for a relationship between aerobic fitness and structure of the MTL memory system in healthy young adults. Our results may have theoretical implications for rodent models of exercise-associated adult neurogenesis in the hippocampus, and suggest clinical exercise trials in older adults at risk for developing Alzheimer’s disease should examine entorhinal function and structure. Aerobic exercise such as running is by no means the only way to improve aerobic capacity. For example, studies suggest circuit training or strenuous weight-lifting may improve VO2 performance (Gettman et al., 1982; Stone et al., 1991). This may prove an interesting avenue for future researchers to try to isolate effects of, for example, aerobic fitness vs. running.
HIGHLIGHTS.
Assessed MTL volume, aerobic fitness, recognition memory, and peripheral BDNF
Sub-structurally, observed strong association between fitness and entorhinal volume
Gross-structurally, strong association between entorhinal volume and memory
Supports studies showing exercise impacts MTL structure; extends work to humans
Acknowledgements
This work was supported by a Pathway to Independence Award to K.S. (NIH K99AG036845 and NIH R00AG036845), the Boston University Clinical and Translational Science Institute (CTSI; UL1-TR000157), the Boston University Center for Biomedical Imaging (CBI), and a Student Research Award from the Boston University Undergraduate Research Opportunities Program to A.S.W. We would like to thank Drs. Xuemei He and Tai Chen for performing and overseeing the BDNF ELISAs, respectively, and the staff of the CTSI General Clinical Research Unit (GCRU) and of the CBI for their support. In addition, we would like to thank Dr. Neil Kowall for serving as the study physician for the GCRU, and Ms. Rachel Nauer for assistance with preparation of figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- ACSM
- American College of Sports Medicine
- BDNF
- Brain-Derived Neurotrophic Factor
- BMI
- Body Mass Index
- CBV
- cerebral blood volume
- DMS
- Delayed Matching-to-Sample
- EC
- entorhinal cortex
- ELISA
- Enzyme-Linked Immunosorbent Assay
- MTL
- Medial Temporal Lobes
- RER
- Respiratory Exchange Ratio
- RERmax
- maximum observed Respiratory Exchange Ratio
- SMT
- Subsequent Memory Test
- VEGF
- vascular endothelial growth factor
- VBM
- voxel-based morphometry
- VO2 max
- rate of maximal oxygen consumption in mL per kg of body weight per min
- VO2 peak
- peak rate of oxygen consumption in mL per kg of body weight per min, measured during test
Contributor Information
Andrew S. Whiteman, Email: asw221@bu.edu.
Daniel E. Young, Email: daniel.young@umb.edu.
Andrew E. Budson, Email: abudson@bu.edu.
Chantal E. Stern, Email: chantal@bu.edu.
References
- Adler D, Murdoch D, et al. rgl: 3D visualization device system (OpenGL) 2014.
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. doi:10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bellgowan PSF, Buffalo EA, Bodurka J, Martin A. Lateralized spatial and object memory encoding in entorhinal and perirhinal cortices. Learn. Mem. 2009;16:433–8. doi: 10.1101/lm.1357309. doi:10.1101/lm.1357309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TI, Whiteman AS, Aselcioglu I, Stern CE. Structural differences in hippocampal and prefrontal gray matter volume support flexible context-dependent navigation ability. J. Neurosci. 2014;34:2314–20. doi: 10.1523/JNEUROSCI.2202-13.2014. doi:10.1523/JNEUROSCI.2202-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce R, Blackmon J, University of W.D. of A.C. Jones J, Strait G. Exercise Testing in Adult Normal Subjects and Cardiac Patients. Pediatrics. 1963;32:742–756. [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage. 2008;41:1177–83. doi: 10.1016/j.neuroimage.2008.03.039. doi:10.1016/j.neuroimage.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH, Cohen NJ, Kramer AF. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–83. doi: 10.1016/j.brainres.2010.08.049. doi:10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–50. doi: 10.1002/hipo.20543. doi:10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen RF, Lawlor BA, Kenny R. Failure to demonstrate that memory improvement is due either to aerobic exercise or increased hippocampal volume. Proc. Natl. Acad. Sci. U. S. A. 2011;108:E89–E90. doi: 10.1073/pnas.1102593108. author reply. doi:10.1073/pnas.1102593108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J. Gerontol. A. Biol. Sci. Med. Sci. 2003;58:176–80. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A. Biol. Sci. Med. Sci. 2006;61:1166–70. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J. Neurosci. 1997;17:2295–313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–72. doi: 10.1016/j.tins.2007.06.011. doi:10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2367–72. doi: 10.1073/pnas.0911725107. doi:10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. Enriching Heredity: The Impact of the Environment on the Anatomy of the Brain. The Free Press: A Division of Macmillan, Inc.; New York: 1988. [Google Scholar]
- Diamond MC, Greer ER, York A, Lewis D, Barton T, Lin J. Rat cortical morphology following crowded-enriched living conditions. Exp. Neurol. 1987;96:241–7. doi: 10.1016/0014-4886(87)90042-2. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Ingham CA, Johnson RE, Bennett EL, Rosenzweig MR. Effects of environment on morphology of rat cerebral cortex and hippocampus. J. Neurobiol. 1976;7:75–85. doi: 10.1002/neu.480070108. doi:10.1002/neu.480070108. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Johnson RE, Protti AM, Ott C, Kajisa L. Plasticity in the 904-day-old male rat cerebral cortex. Exp. Neurol. 1985;87:309–17. doi: 10.1016/0014-4886(85)90221-3. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wójcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–9. doi: 10.1002/hipo.20547. doi:10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss M, Prakash R, Basak C, Szabo A, Chaddock L, White S, Wojcicki T, Mailey E, McAuley E, Kramer AF. Reply to Coen et al: Exercise, hippocampal volume, and memory. Proc. Natl. Acad. Sci. 2011a;108:E90–E90. doi:10.1073/pnas.1103059108. [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 2011b;108:3017–22. doi: 10.1073/pnas.1015950108. doi:10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–7. doi: 10.1038/3305. doi:10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Falls WA, Fox JH, MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behav. Brain Res. 2010;207:321–31. doi: 10.1016/j.bbr.2009.10.016. doi:10.1016/j.bbr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Feng D, Tierney L. Computing and displaying isosurfaces in R. J. Stat. Softw. 2008:28. [Google Scholar]
- Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav. Brain Res. 1991;46:123–33. doi: 10.1016/s0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- Gettman LR, Ward P, Hagan RD. A comparison of combined running and weight training with circuit weight training. Med. Sci. Sports Exerc. 1982;14:229–34. [PubMed] [Google Scholar]
- Greer ER, Diamond MC, Tang JM. Effect of age and enrichment on certain brain dimensions in Brattleboro rats deficient in vasopressin. Exp. Neurol. 1982a;75:11–22. doi: 10.1016/0014-4886(82)90002-4. [DOI] [PubMed] [Google Scholar]
- Greer ER, Diamond MC, Tang JM. Environmental enrichment in Brattleboro rats: brain morphology. Ann. N. Y. Acad. Sci. 1982b;394:749–52. doi: 10.1111/j.1749-6632.1982.tb37493.x. [DOI] [PubMed] [Google Scholar]
- Hartley T, Harlow R. An association between human hippocampal volume and topographical memory in healthy young adults. Front. Hum. Neurosci. 2012;6:338. doi: 10.3389/fnhum.2012.00338. doi:10.3389/fnhum.2012.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Nagel BJ. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav. Brain Res. 2012;233:517–25. doi: 10.1016/j.bbr.2012.05.012. doi:10.1016/j.bbr.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2009;23:188–97. doi: 10.1097/WAD.0b013e31819cb8a2. doi:10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner AJ, Gadian DG, Fuentemilla L, Jentschke S, Vargha-Khadem F, Duzel E. A rapid, hippocampus-dependent, item-memory signal that initiates context memory in humans. Curr. Biol. 2012;22:2369–74. doi: 10.1016/j.cub.2012.10.055. doi:10.1016/j.cub.2012.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Gibson CA, Tran ZV, Osness WH. Controlled endurance exercise training and VO2max changes in older adults: a meta-analysis. Prev. Cardiol. 2005;8:217–25. doi: 10.1111/j.0197-3118.2005.04324.x. [DOI] [PubMed] [Google Scholar]
- Ide K, Secher NH. Cerebral blood flow and metabolism during exercise. Prog. Neurobiol. 2000;61:397–414. doi: 10.1016/s0301-0082(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR. Am. J. Neuroradiol. 1998;19:659–71. [PMC free article] [PubMed] [Google Scholar]
- Issekutz B, Birkhead NC, Rodahl K. Use of respiratory quotients in assessment of aerobic work capacity. J. Appl. Physiol. 1962;17:47–50. [Google Scholar]
- Juraska JM, Fitch JM, Henderson C, Rivers N. Sex differences in the dendritic branching of dentate granule cells following differential experience. Brain Res. 1985;333:73–80. doi: 10.1016/0006-8993(85)90125-8. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–62. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Keene ON. The log transformation is special. Stat. Med. 1995;14:811–9. doi: 10.1002/sim.4780140810. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. doi:10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Olson EA, Weber M. Physical exercise habits correlate with gray matter volume of the hippocampus in healthy adult humans. Sci. Rep. 2013;3:3457. doi: 10.1038/srep03457. doi:10.1038/srep03457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. doi:10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Düzel S, Brigadski T, Goerke M, Becke A, Sobieray U, Neumann K, Lövdön M, Lindenberger U, Bäckman L, Braun-Dullaeus R, Ahrens D, Heinze H-J, Müller NG, Lessmann V, Sendtner M, Düzel E. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage. 2015a doi: 10.1016/j.neuroimage.2015.10.084. doi:10.1016/j.neuroimage.2015.10.084. [DOI] [PubMed] [Google Scholar]
- Maass A, Düzel S, Goerke M, Becke A, Sobieray U, Neumann K, Lövden M, Lindenberger U, Bäckman L, Braun-Dullaeus R, Ahrens D, Heinze H-J, Müller NG, Düzel E. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol. Psychiatry. 2015b;20:585–93. doi: 10.1038/mp.2014.114. doi:10.1038/mp.2014.114. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 2006;16:1091–101. doi: 10.1002/hipo.20233. doi:10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- Makizako H, Shimada H, Doi T, Yoshida D, Ito K, Kato T, Shimokata H, Washimi Y, Endo H, Suzuki T. The association between decline in physical functioning and atrophy of medial temporal areas in community-dwelling older adults with amnestic and nonamnestic mild cognitive impairment. Arch. Phys. Med. Rehabil. 2011;92:1992–9. doi: 10.1016/j.apmr.2011.07.195. doi:10.1016/j.apmr.2011.07.195. [DOI] [PubMed] [Google Scholar]
- Malkasian DR, Diamond MC. The effects of environmental manipulation on the morphology of the neonate rat brain. Int. J. Neurosci. 1971;2:161–9. doi: 10.3109/00207457109146998. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. doi:10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- Mohammed AH, Zhu SW, Darmopil S, Hjerling-Leffler J, Ernfors P, Winblad B, Diamond MC, Eriksson PS, Bogdanovic N. Environmental enrichment and the brain. Prog. Brain Res. 2002;138:109–33. doi: 10.1016/S0079-6123(02)38074-9. doi:10.1016/S0079-6123(02)38074-9. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Newmark RE, Schon K, Ross RS, Stern CE. Contributions of the hippocampal subfields and entorhinal cortex to disambiguation during working memory. Hippocampus. 2013;23:467–75. doi: 10.1002/hipo.22106. doi:10.1002/hipo.22106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav. Brain Res. 2007;176:362–6. doi: 10.1016/j.bbr.2006.10.018. doi:10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Okuno H, Tokuyama W, Li YX, Hashimoto T, Miyashita Y. Quantitative evaluation of neurotrophin and trk mRNA expression in visual and limbic areas along the occipito-temporo-hippocampal pathway in adult macaque monkeys. J. Comp. Neurol. 1999;408:378–98. [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. doi:10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Köhler S, Crane J, Pruessner M, Lord C, Byrne A, Kabani N, Collins DL, Evans AC. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cereb. Cortex. 2002;12:1342–53. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans a C. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb. Cortex. 2000;10:433–42. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- R Development Core Team R: A Language and Environment for Statistical Computing. 2012.
- Rodrigue KM, Daugherty AM, Haacke EM, Raz N. The role of hippocampal iron concentration and hippocampal volume in age-related differences in memory. Cereb. Cortex. 2013;23:1533–41. doi: 10.1093/cercor/bhs139. doi:10.1093/cercor/bhs139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R, Willemer C, Krüger K, Duning T, Warnecke T, Sommer J, Völker K, Ho HV, Mooren F, Knecht S, Flöel A. Physical activity and memory functions: an interventional study. Neurobiol. Aging. 2011;32:1304–19. doi: 10.1016/j.neurobiolaging.2009.08.001. doi:10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Schlaffke L, Lissek S, Lenz M, Brüne M, Juckel G, Hinrichs T, Platen P, Tegenthoff M, Schmidt-Wilcke T. Sports and brain morphology - a voxel-based morphometry study with endurance athletes and martial artists. Neuroscience. 2014;259:35–42. doi: 10.1016/j.neuroscience.2013.11.046. doi:10.1016/j.neuroscience.2013.11.046. [DOI] [PubMed] [Google Scholar]
- Schon K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J. Neurosci. 2005;25:9112–23. doi: 10.1523/JNEUROSCI.1982-05.2005. doi:10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J. Neurosci. 2004;24:11088–97. doi: 10.1523/JNEUROSCI.3807-04.2004. doi:10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Newmark RE, Ross RS, Stern CE. A Working Memory Buffer in Parahippocampal Regions: Evidence from a Load Effect during the Delay Period. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv013. doi:10.1093/cercor/bhv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J. Comp. Neurol. 1976;169:347–70. doi: 10.1002/cne.901690306. doi:10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Stone MH, Fleck SJ, Triplett NT, Kraemer WJ. Health- and performance-related potential of resistance training. Sports Med. 1991;11:210–31. doi: 10.2165/00007256-199111040-00002. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–22. doi: 10.1002/hipo.20348. doi:10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WR, Gordon NF, Pescatello LS, editors. ACSM’s Guidelines for Exercise Testing and Prescription. 8th Lippincott Williams & Wilkins; Baltimore, MD: 2010. [Google Scholar]
- Trejo L, Carro E, Torres-alema I. Circulating Insulin-Like Growth Factor I Mediates Exercise-Induced Increases in the Number of New Neurons in the Adult Hippocampus. J. Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. 2006;1104:64–72. doi: 10.1016/j.brainres.2006.05.066. doi:10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. efferent connections. Brain Res. 1975;95:39–59. doi: 10.1016/0006-8993(75)90206-1. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 2000;1:191–8. doi: 10.1038/35044558. doi:10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–70. doi: 10.1038/6368. doi:10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. doi:10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing J, Andersen M, Diemer NH. Exercise-induced changes in local cerebral glucose utilization in the rat. J. Cereb. Blood Flow Metab. 1996;16:729–36. doi: 10.1097/00004647-199607000-00025. doi:10.1097/00004647-199607000-00025. [DOI] [PubMed] [Google Scholar]
- Vivar C, Potter MC, Choi J, Lee J-Y, Stringer TP, Callaway EM, Gage FH, Suh H, van Praag H. Monosynaptic inputs to new neurons in the dentate gyrus. Nat. Commun. 2012;3:1107. doi: 10.1038/ncomms2101. doi:10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RN, Budtz-Olsen OE, Penny JE, Cummins RA. The effects of environmental complexity on the histology of the rat hippocampus. J. Comp. Neurol. 1969;137:361–6. doi: 10.1002/cne.901370309. doi:10.1002/cne.901370309. [DOI] [PubMed] [Google Scholar]
- Whiteman AS, Young DE, He X, Chen TC, Wagenaar RC, Stern CE, Schon K. Interaction between serum BDNF and aerobic fitness predicts recognition memory in healthy young adults. Behav. Brain Res. 2014;259:302–12. doi: 10.1016/j.bbr.2013.11.023. doi:10.1016/j.bbr.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter M, Van Hoesen GW, Amaral DG. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J. Neurosci. 1989;9:216–28. doi: 10.1523/JNEUROSCI.09-01-00216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Griffioen AW, Jorritsma-Byham B, Krijnen JL. Entorhinal projections to the hippocampal CA1 region in the rat: an underestimated pathway. Neurosci. Lett. 1988;85:193–8. doi: 10.1016/0304-3940(88)90350-3. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber P. a, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann. N. Y. Acad. Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. Neuroimage. 2009;44:319–27. doi: 10.1016/j.neuroimage.2008.09.016. doi:10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Amaral RSC, Augustinack JC, Bender AR, Bernstein JD, Boccardi M, Bocchetta M, Burggren AC, Carr VA, Chakravarty MM, Chötelat G, Daugherty AM, Davachi L, Ding S-L, Ekstrom A, Geerlings MI, Hassan A, Huang Y, Iglesias JE, La Joie R, Kerchner GA, LaRocque KF, Libby LA, Malykhin N, Mueller SG, Olsen RK, Palombo DJ, Parekh MB, Pluta JB, Preston AR, Pruessner JC, Ranganath C, Raz N, Schlichting ML, Schoemaker D, Singh S, Stark CEL, Suthana N, Tompary A, Turowski MM, Van Leemput K, Wagner AD, Wang L, Winterburn JL, Wisse LEM, Yassa MA, Zeineh MM. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. Neuroimage. 2015a;111:526–41. doi: 10.1016/j.neuroimage.2015.01.004. doi:10.1016/j.neuroimage.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–28. doi: 10.1016/j.neuroimage.2006.01.015. doi:10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Pluta JB, Wang H, Xie L, Ding S-L, Gertje EC, Mancuso L, Kliot D, Das SR, Wolk DA. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum. Brain Mapp. 2015b;36:258–87. doi: 10.1002/hbm.22627. doi:10.1002/hbm.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]