Abstract
Context
Sickle cell disease (SCD) is a life-threatening condition that affects more than seven million people worldwide. The most common complication experienced by persons living with SCD is pain. Evidence supports the use of non-pharmacologic therapies in managing psychological and social complications of pain in persons with SCD, but there is little consensus if these approaches can also be applied for the treatment of pain in SCD.
Objectives
To describe and synthesize the use of non-pharmacological therapies for reducing pain of any type and origin in persons SCD.
Methods
A literature search was conducted using PsycINFO, PsycARTICLES, PubMed, CINAHL, and Embase. Databases were searched using the following terms: sickle cell, pain, and non-pharmacological therapies. Interventions were graded for methodological quality, and categorized as 1) peer-support group therapies, 2) educational/psychological therapies, and 3) skill-based therapies.
Results
Twenty-eight non-pharmacological interventions for persons with SCD were examined. Of these studies, a wide variety of non-pharmacological interventions were tested. Twelve studies yielded significant improvements in pain, three studies reported no positive effect or differences between experimental and control conditions on pain or a pain-related outcome, and one study reported a negative or detrimental intervention effect.
Conclusion
Approximately half of the studies reviewed demonstrated success in alleviating pain, suggesting that patients are able to use non-pharmacological interventions to reduce pain with some degree of success. Questions still remain regarding the efficacy and generalizability of these interventions for persons with SCD.
Keywords: sickle cell disease, non-pharmacological, pain, pain management
Introduction
Sickle cell disease (SCD) is a life-threatening condition that affects more than seven million people worldwide (1). In the United States, one of every 375 African Americans is affected by SCD (2), making SCD the most common genetic hematological disorder in the United States. Persons with SCD can suffer from many medical complications that may include, but are not limited to chronic anemia, stroke, acute chest syndrome, pulmonary embolus, renal failure, retinopathy, and many other serious complications (3,4). By far the most common complication experienced by persons living with SCD, at some point in their life, is pain.
Persons with SCD experience both acute and chronic pain (5). Acute pain is often caused by vaso-occlusive crises (VOCs), which are commonly described as unpredictable and excruciating. In addition to VOC pain, it is now understood that many patients with SCD also experience chronic pain. The Pain in Sickle Cell Epidemological Study (PiSCES), a landmark longitudinal study that investigated sickle cell pain in 232 patients age 16 and over with SCD, found that chronic pain is much more common than VOCs or acute pain. In this longitudinal diary study of persons with SCD, patients reported chronic pain at home 38% of the time of the 31,000 days surveyed (6).
Among persons living with SCD, chronic pain (defined by the American Psychological Association as “pain that lasts longer than six months and affects how a person lives their daily life”) is associated with decreased social functioning, food consumption, physical activity, and mobility, and negative emotions (7–10). Typical negative emotions that may be experienced include recurrent feelings of fear, uselessness, and helplessness (11). In addition to these various psychosocial effects of chronic pain, persons with SCD and chronic pain have increased frequency and duration of pain (12), and overall worsening of physical health compared to persons without chronic pain (13).
Opioids have been the primary therapy used to treat both acute VOC and chronic pain in SCD. Concerns about long-term opioid therapy in recent years have led to the need to promote non-pharmacologic therapies to treat chronic pain. Chronic opioid therapy only addresses the sensory/physical dimension of pain for persons with SCD, and does not address other dimensions of life affected by chronic pain including affective, behavioral, cognitive, cultural, or social dimensions. For these reasons, it is important that non-pharmacological therapies be investigated and used as complements to pharmacological therapies to address and treat both acute and chronic pain for those with SCD.
A range of non-pharmacologic therapies are used by individuals living with SCD for a variety of reasons. In a study that explored the use and perceived benefits of non-pharmacological therapies by persons with SCD, 91.6% (n = 208) of patients reported using at least one type of alternative therapy for pain management, and 23% (n = 48) reported benefits related to pain control by one of these approaches (14). This high usage of non-pharmacological therapies by persons with SCD has been replicated in other studies and ranges from 50% (15,16) to 70% (17). Some of the most common non-pharmacological therapies used include cognitive behavioral therapy, biofeedback, prayer, relaxation techniques, acupuncture, hypnosis, herbal therapies, and megavitamins (15,18).
Four evidence-based literature reviews of non-pharmacological therapies used by persons with SCD have been published (19–21). These reviews categorize therapies primarily as either physical or psychosocial, or into one of the many psychological models (operant, peripheral physiological, cognitive and coping, behavioral, etc.). In general, these reviews conclude that non-pharmacological therapies are effective in managing psychological and social complications of SCD, such as decreasing feelings of anxiety and depression, enhancing coping skills, and improving quality of life. While helpful, these reviews focused on psychosocial factors such as anxiety or depression as primary outcomes, not pain. None of these reviews directly addressed the effects of non-pharmacological therapies on pain itself (acute or chronic).
Several Cochrane reviews on SCD and non-pharmacological interventions (22–23) also have been published, but they too do not focus on pain as a primary outcome, only include psychological therapies, and do not discuss other non-pharmacological alternatives such as prayer, community support groups, exercise, and other approaches that are commonly used by persons with SCD to treat pain (15). In addition, a common limitation of all these reviews was their strict inclusion criteria (e.g., only randomized controlled trials [RCTs], interventions delivered by certified clinicians, and quantitative evaluations); this likely contributed to many relevant studies being excluded from these reviews.
Lastly, in 2014 the National Heart Lung and Blood Institute (NHLBI) published an expert panel report of evidence-based guidelines for the care of people with SCD (http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/). The recommendations conclude that there is a general lack of research in the area of non-pharmacological management of pain for persons with SCD, and that further RCTs are needed to determine the roles of alternative and supplemental therapies for the management of pain (1).
Therefore, a formal literature was conducted to describe and synthesize the use of non-pharmacological therapies for pain (of any type and origin) in persons SCD. A total of 28 articles are presented for review.
Methods
A literature search was conducted using the following search engines: PsycINFO, PsychARTICLES, PubMed, CINAHL, and Embase. Databases were searched using the following terms: sickle cell, pain, and non-pharmacological therapies. In PubMED, sickle cell was searched using: “Anemia, Sickle Cell”[Mesh] OR “sickle cell”[tiab] and pain in PubMED was searched with: “Pain Management”[Mesh] OR “Pain”[Mesh] OR “pain”[tiab] OR “painful”[tiab] OR “pains”[tiab]. Search terms for non-pharmacological therapies in PubMED included: nonpharmacological[tiab] OR “Complementary Therapies”[MeSH] OR complementary[tiab] OR alternative[tiab] OR “psychology”[Subheading] OR “Physical Therapy Modalities”[Mesh] OR physical therapy [tiab] OR “Diet”[Mesh] OR diet[tiab] OR dietary[tiab] OR “Nutrition Therapy”[Mesh] OR nutrition[tiab] OR “Psychotherapy”[Mesh] OR psychotherapy[tiab] OR behavior therapy [tiab]OR cognitive-behavioral[tiab] OR cognitive-behavioural[tiab] OR pain-behavior[ tiab] OR psychological therapy[tiab] OR counseling[tiab] OR “Drugs, Chinese Herbal”[Mesh] OR herbs[tiab] OR herbal[tiab] OR “Mental Health Services”[Mesh] OR mental health[tiab] OR “Medicine, Traditional”[Mesh] OR “traditional medicine”[tiab] OR “Plant Preparations”[Mesh] OR “Plant Preparations”[tiab] OR “plant preparation”[tiab] OR “Exercise”[Mesh] OR exercise[tiab] OR exercises[tiab] OR exercising[tiab] OR “Minerals”[Mesh] OR minerals[tiab] OR mineral[tiab] OR “vitamins”[MeSH] OR vitamins[tiab] OR vitamin[tiab] OR acupuncture[tiab] OR “mind body”[tiab] OR holistic[tiab] OR biofeedback[tiab] OR massage[tiab] OR music therapy[tiab] OR mindful*[tiab].
Study Inclusion / Exclusion Criteria
Studies published in peer-reviewed journals that evaluated a non-pharmacological therapy for pain in persons with SCD were included in this review. Article eligibility included studies that tested a non-pharmacological intervention designed to affect change in the intensity, duration, or frequency of pain (for either children or adults with a diagnosis of SCD), and written or translated into English. Dissertations also were included for review. Reference lists and bibliographies of eligible peer-reviewed articles also were searched for relevant material. Studies were excluded from review if they were not an intervention study, or artificially induced pain in their subjects (e.g., laboratory pain produced by temperature or pressure device).
Intervention Classification
Similar to previous reviews on non-pharmacological therapies (24,25), therapies were divided into three categories: peer-support group therapies, educational/psychoeducational therapies, and skill-based therapies. Each category is distinguished by their primary goal and intended outcome (25): 1) peer-support group therapies are defined as those that aim to improve adaptation to illness through contact and support with peers; 2) educational/psychoeducational therapies are defined as those that aim to enhance adaptation to illness through information regarding the disease process and management; and 3) skill-based therapies are defined as those that include or require explicit practical training, or the presence of a trained practitioner, to enhance illness adaptation (24). Skill-based therapies were divided into dyadic (involved the presence of a parent or family member) and physical (involved physical manipulation of the body; e.g., acupuncture, massage). In addition interventions were classified by target (acute pain versus chronic pain) and duration (single-session versus multi-session).
Methodological Quality
The quality of each study was assessed using established criteria used in two literature reviews of non-pharmacological therapies (24,26). This methodological assessment approach was selected because it allows for scoring of qualitative, quantitative, and mixed methods research, and provides a structured approach to the review of study quality. Each paper was assessed using the following 18 criteria: 1) presence of theory or theoretical model, 2) clear aims/objectives, 3) clear description of setting, 4) clear description of sample, 5) appropriate sampling procedure, 6) intervention-related homework or practice exercises, 7) intervention involvement with parent or family members, 8) face-to-face delivery of intervention, 9) peer-to-peer interaction in the intervention, 10) clear description of data collection, 11) clear description of how data were analyzed, 12) clear description of recruitment data and 13) attrition data, 14) found a statistically significant outcome, 15) measures tested for validity and reliability, 16) reporting all findings, (17) evidence of consumer involvement in intervention construction, and 18) stated strengths and limitations. For criteria satisfied, a 1 was assigned if present, and 0 if not present or unclear. Therefore, articles could score between 0 (no criteria met) to 18 (all criteria met).
Effect Sizes
To understand the magnitude of difference between intervention and control groups in pain outcomes, effect sizes were used. Effect sizes were calculated as the difference in means between the intervention and control groups divided by pooled standard deviations of the two groups, with 0.20 considered as a small effect, 0.50 as a medium effect, and 0.80 considered a large effect.
Results
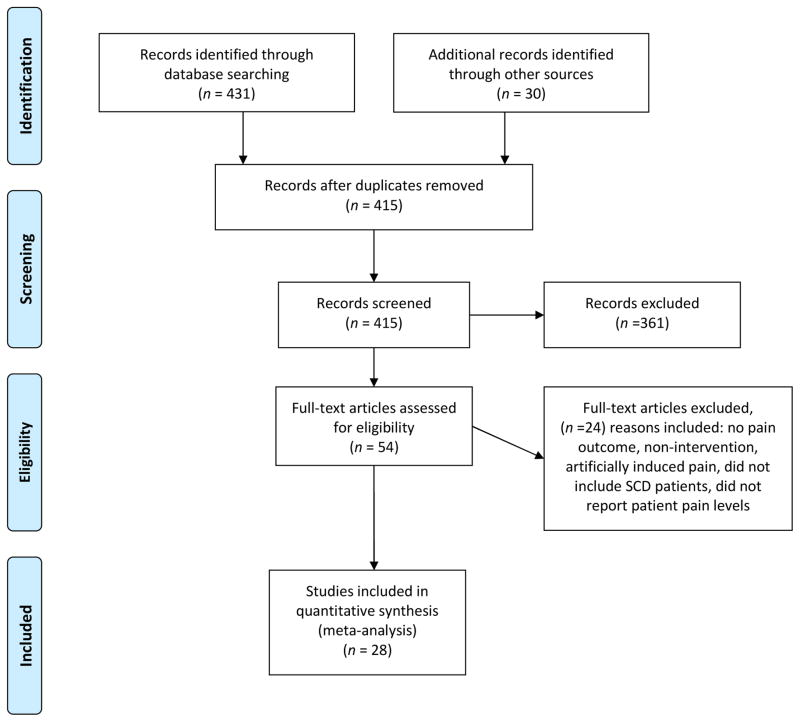
The search strategy yielded a total of 431 publications. Fig. 1 presents the results of the structured literature review. Based on inclusion and exclusion criteria, removal of duplicates, screening of record titles, abstracts, and key words, 54 articles received full text review, and 28 articles were retained and are reported in Table 1 based on inclusion and exclusion criteria.
Fig. 1.
PRISMA flow diagram for literature review.
Table 1.
Summary of Reviewed Articles
| Ref # | First Author/Year | Design/Control Group | N/Population | Intervention/Delivered by | Pain measures | Statistical Significance (Pain) | Pain Outcomes (note: x(x) = mean(SD)) | Study Quality (Scored 0–18; 0=worst and 18= best) | Effect Size |
|---|---|---|---|---|---|---|---|---|---|
| Skill-based (physical) | |||||||||
| 27 | Lemanek, 2009 | RCT / massage vs attention control | 34, Children (parent dyad) | Massage (with parents) / massage therapist (licensed) | Pediatric pain scale (Baker & Wong, 1987) | P < .05 | Despite P value being reported, no mean/SD for pain scale reported in paper | 14 | N/A |
| 28 | Bodhise, 2004 | One group pre-post test / no control | 4, Children + adults | Massage / massage therapist | 0–10 Numerical Pain Scale (NPS) | p<.001 | Pre therapy NPS [9.6(.80)] Post therapy NPS [2.8(.75)] | 10 | N/A |
| 29 | Co, 1979 | Crossover Design / accupuncture vs sham accupuncture control | 10, Adults | Acupuncture / acupuncturist | Non-standardized verbal tool (Q1 - Do you have pain in your [name painful site]?) (Q2 - If yes, then is the pain the same, better or worse than it was the last time I saw you?) | p>.05 | Nonsignificant difference between verbal report of pain reduction between sham and TX; both sham and actual acupuncture resulted in verbally reported patient pain reduction in 93% of all pain crises | 6 | N/A |
| 30 | Lu, 2013 | Pre-post study design / no control | 47, Adults | Acupuncture / acupuncturists (licensed) | 0–10 Numerical Pain Scale (NPS) | P < .001 | Pre to post therapy mean difference of 2.1 on the NPS (individual pre and post means/SD’s not reported) | 14 | N/A |
| 31 | Myers, 1999 | RCT/ massage relaxation training | 16, Adults | Massage / massage therapist (licensed) | McGill pain questionnaire and Visual Analog Scale | P < .001 | Pre therapy pain intensity 4.98(2.29) and unpleasantness 5.63(1.73); Post therapy 3.93(1.73) and 3.25(1.38) | 13 | .53 |
| 32 | Thomas, 2013 | RCT / healing touch vs. relaxation training | 17, Adults | Healing touch / nurse | 0–10 Numerical Pain Scale (NPS) | P > .05 | Non significant differences; Pre-intervention NPS 6.83(1.85) to 4.55 (2.54) for healing touch, and 7.83 (1.59) to 7.17 (1.33) for relaxation control | 13 | .18 |
| 33 | Tinti, 2010 | case report / no control | 1, Adults | Aquatic Rehabilitation / not clear | SF-36, McGill pain questionnaire, Wisconsin pain survey | p < .05 | Pre to post therapy SF-36 = 50 to 93, McGill = 33 to 30, Wisconsin = 10 to 7 (no standard deviations reported due to single person trial) | 12 | N/A |
| Skill-based (dyad) | |||||||||
| 34 | Barakat, 2011 | RCT / CBT vs attention control | 41, Children (parent dyad) | CBT (group) / psychologist | Pain Diary (Gil, 1994) | p = .86 | Nonsignificant decrease % pain days from 24.94(29.64) to 16.71(23.03) | 15 | .35 |
| 35 | Braniecki, 2003 | RCT / CBT vs waitlist | 16, Children (parent dyad) | CBT (group) / not clear | Pediatric Pain Questionnaire (Varni, Thompson 1987) Pain diary (Shaprio, 1990) | p<.001 | Significant decreases parent pain score (p<.001) over course of study 18.39(3.64) to 9.61(4.70); Nonsignificant patient decrease in pain score 16.63(1.91) to 12.85(3.97) on PPQ and differences between tx and waitlist groups; | 16 | .12 |
| 36 | Masuda, 2011 | Case report / no control | 1, Children (parent dyad) | Acceptance and Commitment Therapy / not clear | Pediatric pain scale (Varni, Thompson 1987) | no significance reported | Nonsignificant decrease in average pain (PPQ) pre to post intervention 15(SD=NR) to 10(SD=NR) | 13 | N/A |
| 37 | McElligott, 2006 | RCT / writing vs. control | 36, Children (parent dyad) | Writing / not clear | Pediatric Symptom Checklist for Youth (Jellinek & Murphy, 1990) | P > .01 | No significant differences in pain, | 15 | .38 |
| 38 | Powers, 2002 | One group pre-post test / no control | 3, Children (parent dyad) | CBT (group) / psychologist, hematologist, education specialist | Pain Diary (Gil, 1994) Daily Home Diary (Dinges et al., 1997; 10-point Likert Scale) | p > .05 | No significant difference; did not report mean or SD; | 15 | N/A |
| 27 | Lemanek, 2009 | RCT / massage vs attention control | 34, Children (parent dyad) | Massage (with parents) / massage therapist (liscensed) | Pediatric pain scale (Baker & Wong, 1987) | P < .05 | Despite P value being reported, no mean/SD for pain scale reported in paper | 14 | N/A |
| Skill-based (other) | |||||||||
| 39 | Ağargün, 2001 | Case report / no control | 1, Children | Hypnosis / not clear | Pain measurement tool unclear and not described in case report | not reported | Begin with numerical score 6/7 item scale, reduce to unknown amount | 2 | N/A |
| 40 | Anie, 2002 | One group pre-post test / no control | 35, Adults | CBT / psychologist | Pain Interview (Anie et al., 2002; records patients’ reports of frequency, intensity and duration of painful episodes and health care utilization within a 12-month period | p>.05 | Duration pain episodes (hrs) mean pretherapy 114.7(112.4) to 90.0(56.7) post therapy; the number of pain episodes (over 12 months) pre-therapy 4.3(5.3) to 2.7(0.6) post therapy | 14 | .39 |
| 41 | Cozzi, 1987 | One group pre-post test / no control | 8, Children + Adults | Biofeedback / not clear | Non-standardized 5-point pain intensity scale (1 = mild pain, 5 = pain requires hospitalization) | p<.05 | Statistically significant decline pain intensity 1.92(NR) to 0.5 (NR) on 5-pnt scale, and statistically significant decreased number of self-treated pain crises 2.21(NR) to .44(NR) from weeks 1–4 to 9–12; standard deviations not reported | 12 | .58 |
| 42 | Cummins, 2003 | RCT / CBT vs hydroxyurea | 36, Adults | CBT / not clear | Pain Interview (Anie et al., 2002; records patients’ reports of frequency, intensity and duration of painful episodes and health care utilization within a 12-month period | p<.05 | CBT compared to Hydroxyurea group had significantly more painful episodes 4.3(3.4) compared to 1.4(2.1), but shorter hospitalizations 2.4(2.7) to 7.2(5.5) | 9 | .19 |
| 43 | Dignes, 1997 | One group pre-post test / vs treatment as usual | 37, Children + adults | CBT+ Hypnosis / not clear | Daily Pain Diary (Shapiro et al., 1990) | P < .05 | Statistically significant decrease in % SCD pain days (20.41 to 10.65, p=.002) and days of other pain (18.92 to 5.83, p=.004) | 14 | N/A |
| 44 | Dobson, 2014 | Quasi-experimental interrupted time series / no control | 20, Children | Guided Imagery / certified child life specialist | Pediatric pain scale (Baker & Wong, 1987), daily diary (Dampier et al., 2002) | p < .05 | Statistically significant decrease in pain frequency (5.6(3.3) to 2.5(4.1), p=.003); and pain intensity (2.4(1.2) to .7(1.2), p=.00) | 14 | N/A |
| 45 | Gil, 2001 | RCT / CBT vs treatment as usual | 46, Children | CBT / psychologist | Pain diary (Gil et al., 1994); Pain diary collects pain intensity (ranked 0–10), medication use (use of analgesics), health care contacts (ED visit), and activity reduction (yes/no) | P < .04 | At follow-up, statistically significant more active approach to pain management 90(33.5) then control 57.44(30.9) | 14 | N/A |
| 46 | McClellan, 2009 | Quasi-experimental / no control | 19, Children | CBT (electronic device) / not clear | Daily Pain and Activity Diary (Combination of the Gil 1994 and Dinges 1997 pain diaries) | not reported | No pain outcome reported but intervention described as helpful by both parent 79(21.73) and child 81.44(21.58) on a 0–100 consumer satisfaction form | 12 | N/A |
| 47 | Thomas, 1999 | RCT / CBT vs attention placebo group vs usual treatment | 59, Adults | CBT (group) / psychologist | Pain Self-Efficacy Questionnaire (Nicholas, 1989), Short Form McGill Pain Questionnaire (Melzack, 1987), Beliefs About Pain Control Questionnaire (Skevington, 1990) | P < .05 | Compared to attention control placebo and treatment as usual, CBT group had statistically significant decrease in pain intensity [pre therapy 14.3(10.45) to post therapy 8.3(8.89)]; treatment effect size .18; p < .008 | 15 | .18 |
| 14 | Thompson, 2014 | cross sectional survey; retrospective/ no control | 227, Adults | Prayer, Relaxation, Massage, Exercise, Spiritual healing, Herbal medicine, Yoga / (N/A) | Implemented a non-standardized 3 page survey created specifically for the study (did report chronbach alpha) | no significance testing for pain outcomes | 91.6% respondents used CAM last 6 months, 23% reporting benefits of Prayer, Relaxation, Massage, and other CAM therapies | 10 | N/A |
| 48 | Zelter, 1979 | case report / no control | 2, Adults | Hypnosis / not clear | pain related hospital contacts over 4 and 8 months | no significance testing | No significance testing - patient reported improvement in frequency and intensity of pain crises with self-hypnosis techniques; reduction in hospital utilization from pre- to post-hypnosis training reported (no statistical testing) | 4 | N/A |
| Peer-support Group | |||||||||
| 49 | Butler, 1993 | Cohort study / no control | 24, Adults | Social support group / physician, psychologist, and social worker | not clear - appears to be collected by self-report and from patient interviews in group sessions | not reported | Patients self-reported improvements in recovery time from VOC’s | 5 | N/A |
| 50 | Fox, 1999 | case report / no control | NR, Children + Adults | Social support group / not clear | not clear - appears to be collected by self-report and from patient interviews | not reported | Only describe decreases in pain through relaxation training and biofeedback, no measures reported | 3 | N/A |
| 51 | Martin, 2005 | RCT / social support group vs treatment as usual | 40, Children + Adults | Social support group / not clear | Medical Outcome Survey (Stewert et al., 1988) [outcomes related to quality of life – mental and physical health, pain, etc.] | P < .05 | Statistically significant increased in medical outcomes scores pre-intervention 65.32(25.82) to post intervention 71.71(20.17); but was not statistically significant when compared to control group (p=.167) | 15 | .27 |
| 52 | Nash, 1993 | Social support groups / no control | 26, Adults | Social support group / professionals (unclear) | not clear (authors said that they “combined multiple measures used in self-help and sickle cell research” - no further description) | P < .05 | Statistically significant negative correlation between length of group membership with disease symptoms scores (physical pain & psych), and total interference (no mean or SD provided) | 14 | N/A |
| 53 | Telfair, 1999 | cross sectional survey; retrospective / no control | 79, Children + Adults | Social support group / social workers and nurse coordinators | National SCD Adult Self Help Study’s Sickle Cell Disease Problem Scale (Nash, 1991), 5-point likert pain scale (non-standardized, pain over last 30 days ranging from no pain to very severe pain) | p < .01 | Subjects with high group satisfaction had significantly lower pain levels that those with low group satisfaction (f(3,75)=8.30, p<.01) [no mean or standard deviations reported] | 14 | N/A |
Overview
Table 1 includes 28 articles that reported pain as one of the primary outcomes. Study samples were adults (n=12), children under 18 years of age (n=4), children with their parents (n=6), and both adults and children (n=6). Sample sizes ranged from one to 227, with trials occurring in the United States (n=26), The Netherlands (n=14) and Turkey (n=1).
The majority of therapies tested were either skill-based therapies or peer support group therapies. Skill-based therapies (cognitive behavioral therapy, biofeedback, hypnosis, massage, acceptance and commitment therapy, aquatic rehabilitation) were the most tested (23 of 28), of which six included parents or family, and eight involved physical manipulation of the body. Trained professionals were used to delivery the interventions in 14 of 28 studies, while in the other 14 studies, it was not clear if trained professionals were used. Of the 14 studies that used trained professionals, psychologists (n = 6) were the most frequently used to deliver the therapy, followed by licensed massage therapists (n = 2), and then nurses, physicians, and social workers (n = 6).
Twelve studies yielded significant improvements in pain, three studies reported no positive effect or differences between experimental and control conditions for pain, and one study reported a negative or detrimental intervention effect. The study that reported a negative effect was an RCT of cognitive behavioral therapy (CBT) for adults with SCD that found pairing CBT with hydroxyurea increased the number of reported pain episodes (27).
Effect sizes were reported in five studies, and calculated for four studies (Table 1). In many cases, it was not possible to perform effect size calculations because of missing data (e.g., failed to report μ, standard deviation [SD], N related to pain outcome), or because effect size calculations were not possible based on the type of study design (e.g., cross-sectional survey, case report). Effect sizes were low to medium in size, ranging from 0.12 (35) to 0.58 (41), with an average (μ) effect size of 0.332 across the nine studies.
Quality Assessment
The quality of the studies was fair. Quality assessment scores ranged from 2/18 to 16/18 (35,39), with a mean of 11.61 and SD of 4.18. Of the quality assessment factors, the majority of interventions involved face-to-face intervention delivery (26/28), and had clear aims/objectives (25/28) and descriptions of the study setting (27/28). Very few studies included parents or family members in the intervention (6/33), involved persons with SCD in the design of the intervention (3/33), or described a theoretical model (11/33).
Ten studies used a RCT design, while two additional studies used control groups without randomization (pre-post design (43), crossover design(29)). Treatment as usual and attention control groups, which involved either interactions with clinicians or researchers, were used in eight studies (27,34,35,37,43, 45,47,51). Among the remaining studies, one tested their non-pharmacological therapy against hydroxyurea (42), another against an active sham control (29), and one against music (32).
The measures used across the 28 studies to measure pain varied greatly. The most frequently used measurements for pain were the daily pain diary developed by Gil and colleagues (54) (n = 5), the McGill Pain Questionnaire (n = 3), and either a 5-point or 10-point Likert scale (n = 6). The Coping Strategies Questionnaire developed by Rosenstiel and Keefe (55) (n = 9) was the most frequently used instrument for pain-related outcomes. Validated pain measure with reported Cronbach alphas were found in 23 studies, while in the remaining five studies (29,39,48–50), verbal reports of pain were captured, thus alphas were not applicable. Follow-up questionnaires were administered post-therapy in nine studies, and in 19 studies it was either not clear or not reported if follow-up questionnaires were administered post-therapy.
Intervention Target and Duration
The intervention target (acute versus chronic) was clearly defined in eight articles. One article (46) screened for persons with chronic (or recurrent) pain based on the American Psychological Association definition, and seven articles differentiated chronic from acute pain (27, 30, 41, 45) or were based on the number of vaso-occlusive crises (38, 41, 47). The remaining 20 articles did not mention if the intervention targeted acute pain, chronic pain, vaso-occlusive pain, or a combination. For intervention duration, two studies involved single-session interventions (29, 39), three studies were not clear if one or more sessions were used (28, 49, 50), and the remaining 23 studies involved multiple sessions.
Skills Training
Nine of 23 the skills training therapies reported significant reductions in pain. CBT was the most tested therapy (n=9). All the CBT studies reported improvements in pain by participants, but only three demonstrated statistically significant reductions of pain. Several of the CBT therapies tested involved either a parent or family member in addition to the patient (34,35,38), but of these group CBT therapies, only one showed significant decreases in pain (35). Five of the CBT studies reported longitudinal results that ranged from three months to one year, of which three studies showed lasting effects (six months or longer) of the intervention on maintaining mild improvements in physical health (34,35,47).
For the subset of skills training therapies that involved physical contact, massage (27,28,31), acupuncture (29,30), healing touch (32), and aquatic rehabilitation (33) were tested. The three massage studies found statistically significant reductions in sensory pain scores, in addition to reduction in opioid use and number of hospitalizations (28). The three acupuncture studies reported benefits from the therapy, but at non-statistically significant levels. Two of these three studies found that acupuncture decreased pain scores immediately following acupuncture; one reported it as not statistically significant (30), and the other reported no significant difference in pain reduction when acupuncture was compared against a sham control (random use of acupuncture needles anywhere on the body) (29). Lastly, a healing touch therapy found non-significant but trending decreases in physiological measurements of pain (32), and an aquatic rehabilitation program found statistically significant reduction in pain along with increased respiratory muscle strength (33).
Other skills training therapies tested, that were not CBT or physical therapies included biofeedback (41), hypnosis (39,43,48), and guided imagery (44). Of these therapies, only two (41,44) found statistically significant improvements in pain or pain-related outcomes. The biofeedback study failed to find statistically significant improvements in pain, but was trending toward improvements in pain intensity, pain episodes, and amount of pain medication needed (41). Hypnosis was found to significantly reduce the number of pain days and amount of pain-related medication consumed when combined with CBT (43); otherwise, non-significant improvements in pain, medication usage, and pain-related hospitalizations were found in the two other studies (39,48). Lastly, a study using guided imagery in children (n = 20), four sessions across two months, found statistically significant reductions in pain episodes and pain intensity (44).
Peer Support
Three of the five studies that involved peer-support group therapies reported significant improvements in pain (51–53), while all five reported positive improvements for pain. The two studies that did not find statistically significant improvements in pain still reported that patients felt that the therapy was clinically effective in improving pain (49,50). Three studies included both children and adults in the support groups (50,51,53), while two were exclusive to adults (49,52). Regarding pain reduction, three studies found that the degree of pain sensation and pain interference was buffered by social support groups (51–53).
Education
Lastly, there were no studies that tested educational/psychoeducational therapies and examined pain reduction as the outcome. In our broad literature search, there were nine studies that tested educational interventions for persons with SCD (56–64), but none included pain as an outcome and thus were excluded in this review.
Discussion
To the best of our knowledge, this is the first literature review to report findings on non-pharmacologic interventions for the treatment of pain in SCD. In this review, 28 non-pharmacological interventions for persons with SCD were examined. Of these studies, a wide variety of non-pharmacological interventions were tested. Some of these interventions were based on psychological principles (e.g., CBT), while others were based on physical (e.g., massage therapy, aquatic rehabilitation), social (e.g., dyadic and group therapies), or alternative foundations (e.g., prayer, acupuncture, acceptance-based therapy). In addition to a diversity of interventions, there also existed a range of methodological approaches, which included quantitative, qualitative, and mixed methods.
Implications for Future Research
The interventions that lead to improvements in this review are broadly categorized into two types: skill-based interventions (subdivided into physical, dyadic, and other) and peer-support groups. Skill-based interventions are defined as those that include or require explicit practical training or the presence of a trained practitioner to enhance illness adaptation. Peer-support group interventions are defined as those that aim to improve adaptation to illness through contact and support with peers (24).
Intervention Target and Duration
Intervention target (acute pain versus chronic pain) should be clearly defined in future studies. Eight of the 28 studies differentiated acute versus chronic pain in either their sample or inclusion criteria. Future studies should use the 2014 NHLBI recommendations for SCD, which clearly describe various types of pain, such as acute pain, acute recurrent painful crises, neuropathic pain, or chronic pain, in addition to chronic pain of unclear etiology, chronic pain related to an objective cause, chronic neuropathic pain, or “breakthrough” pain. Additional specificity related to the type of pain targeted by the intervention may help improve clinical decision making and the pairing of the correct intervention to pain type, just as one would do for a pharmacological intervention (e.g., right patient, right drug, right dose, right time, right route).
The vast majority of studies (23 studies) involved multi-session interventions, compared to only two articles that reported using single-session interventions of acupuncture (29) and hypnosis (39). Based on the present findings, it cannot be determined if single-session interventions are more or less effective then multi-session interventions for reducing acute or chronic pain. Because of the limited number of single-session intervention studies, we suggest that future studies explore the efficacy of single-session versus multi-session interventions for pain reduction. Interventions that can be delivered in a single-dose format may be especially useful for persons with SCD because of transportation difficulties (getting to and from clinical appointments), frequent hospitalizations, and physical disabilities, all of which can make it difficult to have re-occurring (weekly, bi-weekly) appointments that require physical presence.
Skills-Based Therapies
Of the 23 skills-training interventions reviewed, approximately half reported significant reductions in pain. A variety of skills-training interventions were tested that included massage therapy, acupuncture, biofeedback, hypnosis, CBT, guided imagery, and aquatic rehabilitation. The intervention with the strongest supporting evidence in our review, as in three other reviews (20–22), is CBT.
Compared to the other approaches reviewed, CBT has the most RCT studies, and is the only non-pharmacological approach that is also supported by the NHLBI guidelines with a “strong degree of evidence” (1,21). Having established that CBT is an effective non-pharmacological intervention for persons with SCD, it is recommended that future researchers continue exploring other types of interventions listed that either 1) have not been rigorously tested with active control groups (e.g., massage, biofeedback, dyadic therapy), or 2) have shown promising results in other chronic pain populations but have not received extensive testing in SCD (e.g., mindfulness-based interventions such as Mindfulness-based Stress Reduction (MBSR) and Acceptance and Commitment Therapy).
Peer-Support Group Therapies
Only five peer-support studies were eligible for this review. In comparison to the 23 skill-based therapies (in which 10 were of RCT design), only one peer-support group study implemented a RCT design, while the other four were either case reports or single-cohort studies. Of these five studies, the most recent was published a decade ago (51). But despite a limited number of studies, these five studies do provide preliminary evidence that peer-support group interventions can influence physical outcomes (e.g., pain) for persons with SCD. As reflected in the findings of these studies, future studies should be aware that the success of improving physical symptoms, like pain, can be significantly influenced by group satisfaction and the length of group membership (52,53).
Even though few research studies were found in this review to support the use of peer-support groups in pain management for persons with SCD, more evidence does exists to support the use of peer-support groups, but only for disease specific education (e.g., genetic education; 61,64). It is important that research in this area continues as many hospitals and other health care institutions continue to offer peer-support groups for patients with SCD and other chronic conditions.
Methodological Limitations and Recommendations
Even with a clear potential for the use of non-pharmacological therapies for pain management in SCD, there are several recurring methodological limitations among these studies that must be addressed in future research.
First, it is important that future intervention studies for persons with SCD clarify: 1) If the intervention targets acute pain or chronic pain, and 2) the etiology of the patients’ pain. In the latest recommendations published by the NHLBI, Evidence-Based Management of Sickle Cell Disease (1), chronic pain etiology for persons with SCD is differentiated into four categories: chronic pain of unclear etiology, chronic pain related to an objective cause, chronic neuropathic pain, and “breakthrough” pain. By clarifying whether an intervention targets acute or chronic pain, and the etiology of the pain targeted, future interventions may become more targeted and prescribed to patients based on their type of pain. For example, persons with chronic pain related to avascular necrosis of the hip may benefit more from a non-pharmacological approach like MBSR, versus someone in a sickle cell pain crisis (e.g., different pain etiology) who may receive no benefit at all from an approach like MBSR. But to achieve this, it is essential that more clarity and description of the pain type and etiology is provided in future studies.
Second, the impact of race and socioeconomic status for persons with SCD should also be accounted for in future studies. Historically, non-pharmacological interventions (e.g., psychological interventions like CBT) were constructed and tested within samples that were mostly Caucasian, not African American. Of the interventions reviewed, there is little to no discussion on how racial or cultural differences may have impacted participation in these interventions. Because the majority of persons with SCD in the United States are African American (66), there is a need to acknowledge and test interventions for racial, cultural, and socioeconomic sensitivity. But despite an apparent absence of racial or cultural sensitivity in the testing of these interventions, many participants found them to be helpful.
Future studies also should be wary of frequent hospitalizations and transportation issues faced by many persons with SCD that might influence their availability to be physically present for these interventions. Persons with SCD can experience frequent hospitalizations and emergency department visits. Between 1999 and 2007, SCD accounted for approximately 200,000 emergency department visits per year (66), and in 2010 persons with SCD had the highest hospital readmission rate of any disease (67). By offering virtual or mobile equivalents of the intervention, persons with SCD who are frequently (or currently) hospitalized, or experience frequent scheduling difficulties because of their disease, will still be able to participate despite not being physically present. Additionally, virtually delivered interventions may prove very useful for those who are unable to drive because of their condition, or regularly afford transportation because of low socioeconomic status.
Lastly, the potential usefulness of these interventions for decreasing pain described in this review should be interpreted with caution, as methodological issues such as small sample sizes, lack of active control groups, and combining adult and pediatric results, make it difficult to generalize reported findings. The 12 studies that reported mixed-aged samples (n=6 children with parents, n=6 children and adults) did not report pediatric versus adult differences. Future studies could compare and contrast pediatric versus adult samples for differences in intervention efficacy and pain type (acute versus chronic), because it is possible that a proven therapy for children with acute pain (e.g., visualization or group CBT) may not be as effective for adults with chronic pain, or vice versa.
The broad inclusion criteria used for many of these studies (e.g., only asking for persons with a diagnosis of sickle cell) also makes it difficult to generalize findings across the various types of pain often experienced by persons with SCD (acute, chronic, crises, etc.), and levels of disease severity (e.g., SS, SB0 (sickle cell anemia) versus SC and SB+). Furthermore, the use and frequency of pain medications, both prescribed and non-prescribed, should be included as important study outcomes. Because many persons with SCD manage their pain outside of the hospital on their own, it is important to account for interactions and potential additive effects of the patient’s own pain management practices.
Limitations of this Review
The scoring guidelines used to critique study quality in this review is unfortunately biased towards RCT designs. Even though it can still be used for non-RCT studies, it is not equally weighted for criteria related to qualitative or mixed research designs. Additionally, these criteria were originally created for non-pharmacological interventions in pediatric populations, which is why some items such as “the presence of a parent or family member” were include in the assessment. Despite these drawbacks, this scoring method was explicitly developed for assessing the quality of non-pharmacological pain research, and to the authors’ knowledge, there are no other scoring criteria available in this domain of research that have been validated and cited in other reviews.
Second, the original intent of the review was to synthesize and describe the literature on non-pharmacological therapies specifically for chronic pain, not general pain (as we did), in persons with SCD. After a thorough review of the non-pharmacological therapy literature for persons with SCD and chronic pain, we found only one article (46) that reported screening persons with chronic (or recurrent) pain based on the American Psychological Association definition of chronic pain. There were four additional articles that either defined or differentiated chronic (or recurrent) pain from acute pain or pain associated with vaso-occlusive crises (27,30,41, 45), and three studies that screened patients on the number of vaso-occlusive crises either within the last month (38) or year (41,47). Because of the limited amount of literature found in this initial search, the scope of this review was broadened to include any type of pain experienced by persons with SCD. As the non-pharmacological literature grows in SCD, it will be important that this question be re-asked and answered.
Third, only physical pain outcomes were included and summarized in this review. The majority of studies reviewed contained pain-related outcomes (e.g., depression and anxiety, sleep, quality of life) and other important indicators of clinical value for researchers, but were excluded because these findings have been summarized elsewhere (20,22). We do note that many of these secondary outcomes, such as anxiety and depression, were found to be positively impacted by the non-pharmacological interventions in our review and generally perceived as helpful by participants.
Lastly, the calculated effect sizes demonstrate a small effect of non-pharmacological interventions on pain in persons with SCD (μ= 0.332), but these results should be interpreted with caution. Only nine studies were used to calculate the μ intervention effect size on pain, and these nine studies had small sample sizes (range n = 8–51), which raises concerns related to statistical power and ability to detect statistically significant differences between intervention and control groups. In addition, a variety of non-pharmacological interventions were tested (e.g., CBT, massage, biofeedback) that differed in length, and pain type (e.g., acute, chronic, nociceptive, neuropathic, vaso-occlusive crisis) in calculating the μ intervention effect size.
Conclusion
Approximately half of the studies reviewed demonstrated success in alleviating pain, suggesting that: 1) patients are able to use non-pharmacological interventions with some degree of success, and 2) it is possible to study these alternative interventions. Other reviews on non-pharmacological interventions in SCD that have focused on pain-related outcomes (e.g., anxiety, depression, quality of life) have come to similar conclusions: that non-pharmacological interventions can lead to health improvements (20,21). But despite the success and potential application of these interventions, there are many questions yet to be answered regarding the efficacy and generalizability of these interventions for persons with SCD.
Acknowledgments
Mr. Williams is supported by a fellowship from the National Institute of Nursing Research, 1F31NR014954-01. Dr. Tanabe is supported by a grant from the National Heart, Lung and Blood Institute, RHL121224.
Footnotes
Disclosures
No funding was received for this study and the authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Heart Lung Blood Institute (NHLBI) Expert panel report on the management of sickle cell disease (draft) NHLBI; 2014. pp. 1–251. [Google Scholar]
- 2.Edwards CL, Scales MT, Loughlin C, et al. A brief review of the pathophysiology, associated pain, and psychosocial issues in sickle cell disease. Int J Behav Med. 2005;12:171–179. doi: 10.1207/s15327558ijbm1203_6. [DOI] [PubMed] [Google Scholar]
- 3.John N. A review of clinical profile in sickle cell traits. Oman Med J. 2010;25:3–8. doi: 10.5001/omj.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claster S, Vichinsky EP. Managing sickle cell disease. BMJ. 2003;327:1151–1155. doi: 10.1136/bmj.327.7424.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor LE, Stotts NA, Humphreys J, Treadwell MJ, Miaskowski C. A review of the literature on the multiple dimensions of chronic pain in adults with sickle cell disease. J Pain Symptom Manage. 2010;40:416–435. doi: 10.1016/j.jpainsymman.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 7.McClish DK, Penberthy LT, Bovbjerg VE, et al. Health related quality of life in sickle cell patients: the PiSCES project. Health Qual Life Outcomes. 2005;3:50. doi: 10.1186/1477-7525-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pells JJ, Presnell KE, Edwards CL, et al. Moderate chronic pain, weight and dietary intake in African-American adult patients with sickle cell disease. J Natl Med Assoc. 2005;97:1622–1629. [PMC free article] [PubMed] [Google Scholar]
- 9.Smith WR, Bovbjerg VE, Penberthy LT, et al. Understanding pain and improving management of sickle cell disease: the PiSCES study. J Natl Med Assoc. 2005;97:183–193. [PMC free article] [PubMed] [Google Scholar]
- 10.Sadat-Ali M. Avascular necrosis of the femoral head in sickle cell disease. An integrated classification. Clin Orthop Relat Res. 1993;290:200–205. [PubMed] [Google Scholar]
- 11.Gil KM, Abrams MR, Phillips G, Williams DA. Sickle cell disease pain: predicting health care use and activity level at 9-month follow-up. J Consult Clin Psychol. 1992;60:267–273. doi: 10.1037//0022-006x.60.2.267. [DOI] [PubMed] [Google Scholar]
- 12.Gil KM, Thompson RJ, Jr, Keith BR, et al. Sickle cell disease pain in children and adolescents: change in pain frequency and coping strategies over time. J Pediatr Psychol. 1993;18:621–637. doi: 10.1093/jpepsy/18.5.621. [DOI] [PubMed] [Google Scholar]
- 13.Booker MJ, Blethyn KL, Wright CJ, Greenfield SM. Pain management in sickle cell disease. Chronic Illn. 2006;2:39–50. doi: 10.1177/17423953060020011101. [DOI] [PubMed] [Google Scholar]
- 14.Thompson WE, Eriator I. Pain control in sickle cell disease patients: use of complementary and alternative medicine. Pain Med. 2014;15:241–246. doi: 10.1111/pme.12292. [DOI] [PubMed] [Google Scholar]
- 15.Majumdar S, Thompson W, Ahmad N, Gordon C, Addison C. The use and effectiveness of complementary and alternative medicine for pain in sickle cell anemia. Complement Ther Clin Pract. 2013;19:184–187. doi: 10.1016/j.ctcp.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Sibinga EMS, Shindell DL, Casella JF, Duggan A, Wilson MH. Pediatric patients with sickle cell disease: use of complementary and alternative therapies. J Altern Complement Med. 2006;12:291–298. doi: 10.1089/acm.2006.12.291. [DOI] [PubMed] [Google Scholar]
- 17.Yoon SL, Black S. Comprehensive, integrative management of pain for patients with sickle-cell disease. J Altern Complement Med. 2006;12:995–1001. doi: 10.1089/acm.2006.12.995. [DOI] [PubMed] [Google Scholar]
- 18.Dampier C, Ely E, Eggleston B, Brodecki D, O’Neal P. Physical and cognitive-behavioral activities used in the home management of sickle pain: a daily diary study in children and adolescents. Pediatr Blood Cancer. 2004;43:674–678. doi: 10.1002/pbc.20162. [DOI] [PubMed] [Google Scholar]
- 19.Hildenbrand AK, Nicholls EG, Daly BP, et al. Psychosocial and pharmacological management of pain in pediatric sickle cell disease. Postgrad Med. 2014;126:123–133. doi: 10.3810/pgm.2014.03.2748. [DOI] [PubMed] [Google Scholar]
- 20.Edwards LY, Edwards CL. Psychosocial treatments in pain management of sickle cell disease. J Natl Med Assoc. 2010;102:1084–1094. doi: 10.1016/s0027-9684(15)30737-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen E, Cole SW, Kato PM. A review of empirically supported psychosocial interventions for pain and adherence outcomes in sickle cell disease. J Pediatr Psychol. 2004;29:197–209. doi: 10.1093/jpepsy/jsh021. [DOI] [PubMed] [Google Scholar]
- 22.Anie KA, Green J. Psychological therapies for sickle cell disease and pain. Cochrane Database Syst Rev. 2012:Cd001916. doi: 10.1002/14651858.CD001916.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Anie KA, Green J. Psychological therapies for sickle cell disease and pain. Cochrane Database Syst Rev. 2002:Cd001916. doi: 10.1002/14651858.CD001916. [DOI] [PubMed] [Google Scholar]
- 24.Sansom-Daly UM, Peate M, Wakefield CE, Bryant RA. A systematic review of psychological interventions for adolescents and young adults living with chronic illness. Health Psychol. 2012;31:380–393. doi: 10.1037/a0025977. [DOI] [PubMed] [Google Scholar]
- 25.Plante W, Lobato D, Engel R. Review of group interventions for pediatric chronic conditions. J Pediatr Psychol. 2001;26:435–453. doi: 10.1093/jpepsy/26.7.435. [DOI] [PubMed] [Google Scholar]
- 26.Jackson C, Cheater FM, Reid I. A systematic review of decision support needs of parents making child health decisions. Health Expectations. 2008;11:232–251. doi: 10.1111/j.1369-7625.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemanek KL, Ranalli M, Lukens C. A randomized controlled trial of massage therapy in children with sickle cell disease. J Pediatr Psychol. 2009;34:1091–1096. doi: 10.1093/jpepsy/jsp015. [DOI] [PubMed] [Google Scholar]
- 28.Bodhise PB, Dejoie M, Brandon Z, Simpkins S, Ballas SK. Non-pharmacologic management of sickle cell pain. Hematology. 2004;9:235–237. doi: 10.1080/10245330410001701495. [DOI] [PubMed] [Google Scholar]
- 29.Co LL, Schmitz TH, Havdala H, Reyes A, Westerman MP. Acupuncture: an evaluation in the painful crises of sickle cell anaemia. Pain. 1979;7:181–185. doi: 10.1016/0304-3959(79)90009-5. [DOI] [PubMed] [Google Scholar]
- 30.Lu K, Cheng MJ, Ge X, et al. A retrospective review of acupuncture use for the treatment of pain in sickle cell disease patients: descriptive analysis from a single institution. Clin J Pain. 2014;30:825–830. doi: 10.1097/AJP.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers CD, Robinson ME, Guthrie TH, Lamp SP, Lottenberg R. Adjunctive approaches for sickle cell chronic Pain. Complementary Health Practice Review. 1999;5:203–212. [Google Scholar]
- 32.Thomas LS, Stephenson N, Swanson M, Jesse DE, Brown S. A pilot study: the effect of healing touch on anxiety, stress, pain, pain medication usage, and physiological measures in hospitalized sickle cell disease adults experiencing a vaso-occlusive pain episode. J Holist Nurs. 2013;31:234–247. doi: 10.1177/0898010113491631. [DOI] [PubMed] [Google Scholar]
- 33.Tinti G, Somera R, Jr, Valente FM, Domingos CR. Benefits of kinesiotherapy and aquatic rehabilitation on sickle cell anemia. A case report. Genet Mol Res. 2010;9:360–364. doi: 10.4238/vol9-1gmr722. [DOI] [PubMed] [Google Scholar]
- 34.Barakat LP, Schwartz LA, Salamon KS, Radcliffe J. A family-based randomized controlled trial of pain intervention for adolescents with sickle cell disease. J Pediatr Hematol Oncol. 2010;32:540–547. doi: 10.1097/MPH.0b013e3181e793f9. START. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braniecki SH. A family-based cognitive-behavioral pain intervention involving guided imagery for children with sickle cell disease: apilot study. ProQuest Information & Learning. 2003 Available at: http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2003-95018-358&site=ehost-live&scope=site Available from EBSCOhost psyh database.
- 36.Masuda A, Cohen LL, Wicksell RK, Kemani MK, Johnson A. A case study: Acceptance and commitment therapy for pediatric sickle cell disease. J Pediatr Psychol. 2011;36:398–408. doi: 10.1093/jpepsy/jsq118. [DOI] [PubMed] [Google Scholar]
- 37.McElligott MD. Expressive writing as an intervention for adolescents with sickle cell disease. ProQuest Information & Learning. 2006 Available at: http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2006-99010-226&site=ehost-live&scope=site Available from EBSCOhost psyh database.
- 38.Powers SW, Mitchell MJ, Graumlich SE, Byars KC, Kalinyak KA. Longitudinal assessment of pain, coping, and daily functioning in children with sickle cell disease receiving pain management skills training. J Clin Psychol Med Settings. 2002;9:109–119. [Google Scholar]
- 39.Ağargün MY, Öner AF, Akbayram S. Hypnotic intervention for pain management in a child with sickle cell anemia. Sleep and Hypnosis. 2001;3:127–128. [Google Scholar]
- 40.Anie KA, Green J, Tata P, et al. Self-help manual-assisted cognitive behavioural therapy for sickle cell disease. Behavioural and Cognitive Psychotherapy. 2002;30:451–458. [Google Scholar]
- 41.Cozzi L, Tryon WW, Sedlacek K. The effectiveness of biofeedback-assisted relaxation in modifying sickle cell crises. Biofeedback Self Regul. 1987;12:51–61. doi: 10.1007/BF01000078. [DOI] [PubMed] [Google Scholar]
- 42.Cummins O, Anie KA. A comparison of the outcome of cognitive behaviour therapy and hydroxyurea in sickle cell disease. Psychol Health Med. 2003;8:199–204. [Google Scholar]
- 43.Dinges DF, Whitehouse WG, Orne EC, et al. Self-hypnosis training as an adjunctive treatment in the management of pain associated with sickle cell disease. Int J Clin Exp Hypn. 1997;45:417–432. doi: 10.1080/00207149708416141. [DOI] [PubMed] [Google Scholar]
- 44.Dobson CE, Byrne MW. Original research: using guided imagery to manage pain in young children with sickle cell disease. Am J Nurs. 2014;114:26–36. doi: 10.1097/01.NAJ.0000445680.06812.6a. test 37, 47. [DOI] [PubMed] [Google Scholar]
- 45.Gil KM, Anthony KK, Carson JW, et al. Daily coping practice predicts treatment effects in children with sickle cell disease. J Pediatr Psychol. 2001;26:163–173. doi: 10.1093/jpepsy/26.3.163. [DOI] [PubMed] [Google Scholar]
- 46.McClellan CB, Schatz JC, Puffer E, et al. Use of handheld wireless technology for a home-based sickle cell pain management protocol. J Pediatr Psychol. 2009;34:564–573. doi: 10.1093/jpepsy/jsn121. [DOI] [PubMed] [Google Scholar]
- 47.Thomas VJ, Dixon AL, Milligan P. Cognitive-behaviour therapy for the management of sickle cell disease pain: An evaluation of a community-based intervention. Br J Health Psychol. 1999;4:209–229. [Google Scholar]
- 48.Zeltzer L, Dash J, Holland JP. Hypnotically induced pain control in sickle cell anemia. Pediatrics. 1979;64:533–536. [PubMed] [Google Scholar]
- 49.Butler DJ, Beltran LR. Functions of an adult sickle cell group: education, task orientation, and support. Health Soc Work. 1993;18:49–56. doi: 10.1093/hsw/18.1.49. [DOI] [PubMed] [Google Scholar]
- 50.Fox P, Ingram D. Theory and practice of self-induced pain management: Approaches learned from effective copers. J Black Psychol. 1999;25:427–452. [Google Scholar]
- 51.Martin GM. The effects of a psychoeducational intervention on improving psychosocial functioning and disease management of adolescents and young adults with sickle cell disease. ProQuest Information & Learning. 2005 Available at: http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2005-99006-021&site=ehost-live&scope=site Available from EBSCOhost psyh database.
- 52.Nash KB, Kramer KD. Self-help for sickle cell disease in African American communities. J Appl Behav Sci. 1993;29:202–215. [Google Scholar]
- 53.Telfair J, Gardner MM. African American adolescents with sickle cell disease: Support groups and psychological well-being. J Black Psychol. 1999;25:378–390. [Google Scholar]
- 54.Gil KM, Phillips G, Edens J, Martin NJ, Abrams M. Observation of pain behaviors during episodes of sickle cell disease pain. Clin J Pain. 1994;10:128–132. doi: 10.1097/00002508-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Rosenstiel AK, Keefe F. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 56.Baskin ML. A psychoeducational group intervention for adolescents diagnosed with sickle cell disease (SCD) ProQuest Information & Learning. 2000 Available at: http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2000-95012-065&site=ehost-live&scope=site.
- 57.Collins MH, Loundy MR, Brown FL, et al. Applicability of criteria for empirically validated treatments to family interventions for pediatric sickle cell disease. J Dev Phys Disabil. 1997;9:293–309. [Google Scholar]
- 58.Duncan DE, Scott RB, Castro O. A mobile unit as an adjunct to a community outreach program of education, screening, and counseling for sickle cell disease, nutritional anemia, and hypertension. J Natl Med Assoc. 1982;74:969–977. [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson GD. Family coping and hospital-based extended kinship network interventions with caregivers of children diagnosed with sickle cell disease. ProQuest Information & Learning. 1995 Available at: http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=1995-95019-106&site=ehost-live&scope=site.
- 60.Kaslow NJ, Brown F. Culturally sensitive family interventions for chronically ill youth: sickle cell disease as an example. Family Systems Medicine. 1995;13:201–213. [Google Scholar]
- 61.Kaslow NJ, Collins MH, Rashid FL, et al. The efficacy of a pilot family psychoeducational intervention for pediatric sickle cell disease (SCD) Families, Systems, & Health. 2000;18:381–404. [Google Scholar]
- 62.Koontz KL. A school intervention program for children with sickle cell anemia: A randomized clinical trial. ProQuest Information & Learning. 1998 Available at: http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=1998-95022-219&site=ehost-live&scope=site.
- 63.Lloyd SD. Design of a comprehensive program specific to female adolescents with sickle cell disease: Learning interventions for empowerment. ProQuest Information & Learning. 2008 Available at: http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2008-99080-529&site=ehost-live&scope=site.
- 64.Porter JS, Matthews CS, Carroll YM, et al. Genetic education and sickle cell disease: feasibility and efficacy of a program tailored to adolescents. J Pediatr Hematol Oncol. 2014;36:572–577. doi: 10.1097/MPH.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention. [Accessed April 21, 2015];Sickle cell disease: Data & statistics. 2011 Available at: http://www.cdc.gov/ncbddd/sicklecell/data.html.
- 66.Yusuf HR, Atrash HK, Grosse SD, Parker CS, Grant AM. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999–2007. Am J Prev Med. 2010;38:536–541. doi: 10.1016/j.amepre.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elixhauser A, Steiner C. HCUP Statistical Brief #153. Agency for Healthcare Research and Quality; Rockville, MD: Apr, 2013. Readmissions to US Hospitals by diagnosis, 2010. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. [PubMed] [Google Scholar]