Abstract
Sperm-associated antigen 6 (Spag6) gene, which encodes an axonemal protein (SPAG6), ubiquitously expresses in tissue and organs containing ciliated cells. The present work was to investigate whether SPAG6 expressed in cochlear hair cells and, if so, to explore the presumable correlations between prestin and SPAG6. The distribution of SPAG6 in organ of Corti and the morphological features of hair cells in basilar membrane were investigated by immunofluorescent staining. The amount of prestin in Spag6 mutant mice was measured by Western blotting and real-time PCR, respectively. Additionally, co-immunoprecipitation tests were performed to confirm the presumed interaction between prestin and SPAG6. We observed that SPAG6 expressed in the cuticular plate in outer hair cells (OHCs) and prestin in the lateral wall of OHCs that located along with SPAG6 at this site. In comparison to Spag6 +/+ mice, Spag6 −/− mice showed apparent morphological abnormity of OHCs and lower intensity of prestin fluorescence. The expression of prestin in Spag6 −/− mice reduced significantly at both protein and mRNA levels. Moreover, co-immunoprecipitation tests demonstrated the interaction between prestin and SPAG6. Taken together, these data indicate that SPAG6 is indispensible for the stability of OHCs by maintaining the normal expression of prestin, which implies that Spag6 gene is essential for mechanosensory function of OHCs.
Keywords: Sperm associated antigen 6, Spag6, Prestin, Outer hair cells, OHCs, Gene knockout mice
1. Introduction
Sperm-associated antigen 6 (Spag6) gene encodes an axonemal protein (SPAG6) within the sperm flagella, which is firstly found on infertile males [1] and essential for sperm motility and male fertility [2,3]. SPAG6 ubiquitously expresses in tissue and organs containing ciliated cells, such as central nervous system [4], respiratory system [3]. Deficiencies of this gene render severe diseases, highlighting that SPAG6 is significant for microtubule-related ciliated cells whereby the corresponding organs function normally.
As it is well-known, hair cells in organ of Corti are fundamental for hearing generation [5]. The cylindrical outer hair cell (OHC) is able to alter its length and stiffness in response to acoustic mechanical stimulation. These mechanical changes derive from a putative molecular motor designated prestin [6,7], which is absolutely required for electromotility and for the cochlear amplifier [8]. Nonetheless, the amount of prestin is impressionable to different conditions, such as the application of ototoxic drugs, noise exposure and interactions with specific biomolecules [9–11]. Till now, a diversity of proteins has been proved to interact with prestin in vivo [12–14].
Noticeably, patients afflicted with primary ciliary dyskinesia often have hearing impairment simultaneously [15], which hints that some genes encoding microtubule-related protein are essential for auditory function. On the basis that SPAG6 widely distributes in ciliated cells and potentially involves in inner ear development [16], it is reasonable to hypothesize that this protein expresses in cochlear hair cells. Moreover, the cortical cytoskeleton, constituted by intracellular microtubules and actins, facilitates the transformative ability of OHCs. Therefore, if SPAG6 expresses in OHCs, it possibly associates with the process of electromotility and correlates to prestin. In this regard, the present works were designed to determine whether SPAG6 existed in cochlear hair cells and, if so, to study the presumable correlations between prestin and SPAG6.
2. Materials and methods
2.1. Genotyping and animal preparation
Spag6 mutant mouse models were generated previously [4]. Neonates were born by the intercross of Spag6 +/− male and female mice, which were kindly provided by Zhang et al. [2]. For genotype identification, DNA was abstracted with a Tissue DNA Kit (D3396-02, OMEGA) and, all the procedures of PCR were complied with previous study [4]. All experimental procedures were conducted in accordance with the policies of the Animal Care Committee of Shandong University, Ji’nan, PR China.
2.2. Preparation of cochleae samples
The dissection and preparation of osseous labyrinths were conducted in accordance to previous research [7]. Basilar membrane was carefully peeled off and Reissner’s membrane and tectorial membrane were removed simultaneously.
2.3. Immunofluorescent staining and image analysis
Immunofluorescent staining procedures were performed as antecedent description [7]. The primary antibodies were rabbit anti-prestin, (sc-30163, Santa Cruz), goat anti-SPAG6 (sc-165529, Santa Cruz) and rabbit anti-myosinVIIa (PA1-936, Thermo Scientific Pierce Antibodies). The secondary antibodies were Alexa Fluor 488 donkey anti-rabbit IgG (A-21206, Molecular Probes) and Alexa Fluor 647 donkey anti-goat IgG (A-21447, Molecular Probes). The cell nucleus and the F-actins (cilia bundles) were stained by DAPI (D9542, SIGMA) and FITC-Phalloidin (P5282, SIGMA), respectively.
Specimens were observed under a laser scanning confocal microscope (TSC SPE, LEICA). The 488 nm laser was used for the visualization of Alexa Fluor 488 and FITC-Phalloidin staining. The 635 nm laser was used for the visualization of Alexa Fluor 647. DAPI staining was watched under UV light, the 405 nm laser.
For hair cells counting, we used the cell counter tool in Image J software to accumulate the myosinVIIa-positive hair cells within the 400 μm length in the middle turn of the cochlea [17]. As for the quantification of fluorescence intensity of prestin, we also performed as previous study [17]. The fluorescence intensity ratio of Spag6 −/− to Spag6 +/+ mice in different time points were calculated.
2.4. Protein extraction and Western blotting
The isolated basilar membrane was dissociated by RIPA lysis buffer (P0013B, Beyotime Institute of Biotechnology) and then was centrifuged to harvest the crude protein. Crude protein was separated by SDS-PAGE electrophoresis and subsequently transferred onto PVDF membrane (Immobilon-FL, Millipore). The primary antibodies in Western blotting were rabbit anti-prestin (sc-22692, Santa Cruz), mouse anti-beta actin (TA-09, ZSGB-BIO), rabbit anti-myosinmyosinVIIa (PA1-936, Thermo Scientific Pierce Antibodies). The relative intensity values of the grayscale images were calculated by Image J software.
2.5. RNA extraction and real-time PCR
Total RNA of the basilar membrane was eluted with the RNA extraction kit (RNeasy Mini QIAcube Kit QIAGEN). Then cDNA was synthesized with the RevertAid First Strand cDNA Synthesis Kit (K1622, Thermo scientific). Three pairs of primers used in real-time PCR were as follows: prestin, forward primer: 5′-CGTCAAGGACAAAGTCACAGAG-3′, reverse primer: 5′-CCCGAGACCAAGTCACCTAA-3′; MoysinVIIa, forward promer: 5′-TGGTACACTTGACACTGAAGAAAAAGT-3′, reverse primer: 5′-CCATCGTTCAGCCTCTTGGT-3′; GAPDH, forward primer: 5′-AGGTCGGTGTGAACGGATTTG-3′, reverse primer: 5′-TGTAGACCATGTAGTTGAGGTCA-3′. Ingredients were 2 × SYBR Green Premix EX Taq 10 μL (RR42LR, Takara Biotechnology), forward primer 1 μL, reverse primer 1 μL, cDNA 1 μL, deionized water complemented for the rest part of the 25 μL system. The parameters of PCR program were 95 °C for 8 min (initial denaturation) followed by 40 cycles of 95 °C for 30 s (denaturation), 57 °C for 30 s (annealing) and 72 °C for 40 s (extention). Defined the relative expression of prestin mRNA in Spag6 +/+ subjects as control, the 2−ΔΔCt values of Spag6 +/− and Spag6 −/− mice [18] in each age group were calculated. The same measurements were applied to moysinVIIa.
2.6. Co-immunoprecipitation tests
Spag6 +/+ and Spag6 −/− mice were sacrificed for co-immunoprecipitation tests. A moderate dosage of protein extracted from cochlear basilar membrane (300–500 μg) was incubated with 2 μg primary antibody for 2 h. The primary antibodies were rabbit anti-prestin (sc-30163, Santa Cruz), goat anti-SPAG6 (sc-165529, Santa Cruz) as well as the nonspecific normal rabbit IgG (sc-2027, Santa Cruz) and normal goat IgG (sc-2028, Santa Cruz), which acted as the negative control. Then 40 μL resuspended protein A/G PLUS-Agarose beads (sc-2003, Santa Cruz) was added into the mixture to bind the primary antibody at 4 °C overnight. Immunoprecipitate was collected by centrifugation and was stringently washed with RIPA lysis buffer. After washing, all supernatant was discarded and the beads were resuspended in electrophoresis sample buffer. Subsequently, Western blotting was performed to assay the pulled down proteins. Moreover, to avoid the interferential results, the host species of antibodies used in immunoprecipitation tests was different with that used in Western blotting.
2.7. Statistical analysis
Measurement data conformed to Gaussion distribution were analyzed by one-way analysis of variance (ANOVO), which was followed by Student-Newman–Keuls test for multiple comparisons. P < 0.05 was considered statistical significance.
3. Results
3.1. Genetic traits and gross features of the Spag6 gene knockout mice
For genotype identification of the neonates, the PCR results were completely consistent with the previous study [4]. As for the gross appearance of Spag6 −/− mice, besides the antecedent description [4], we also found that Spag6 −/− mice appeared a frequently occurred fur-loss and exhibited abnormal behaviors, such as clumsy motion and continuous head tossing in comparison to their corresponding Spag6 +/+ and Spag6 +/− littermates.
3.2. Co-localization of prestin and SPAG6 in OHCs
SPAG6 do existed in basilar membrane, which was demonstrated by Western blotting. A 57-kDa band, the predicted size, was detectable in cochleae of Spag6 +/+ and Spag6 +/− mice but was absent in that of Spag6 −/− mice (Fig. 1A). SPAG6 immunolabeling clearly marked cuticular plate in OHCs. Moreover, prestin and SPAG6 immunolabeling simultaneously localized in lateral wall of OHCs, in which site the distribution range of these two proteins widely overlapped (Fig. 1B). Yet the SPAG6 signals could not be detected in OHCs of Spag6 −/− mice (Fig. 1C).
Fig. 1.
The traits of SPAG6 distribution in OHCs. (A) SPAG6 exists in cochleae of Spag6 +/+ and Spag6 +/− mice. (B) In Spag6 +/+ mice, SPAG6 localizes in cuticular plate and co-localizes with prestin in the lateral wall of OHCs. MoysinVIIa (light green) marks the cuticular plates of OHCs while prestin (light gray) specifically identifies the lateral wall of OHCs. No differences of fluorescence distribution patterns have been observed between Spag6 +/+ and Spag6 +/− mice, for which the latter is omitted. (B) SPAG6 signals cannot be detected in Spag6 −/− mice. All scale bars represent 5 μm. Mice age in this figure is postnatal days 30 (P30). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Morphological abnormalities of OHCs in Spag6 −/− mice
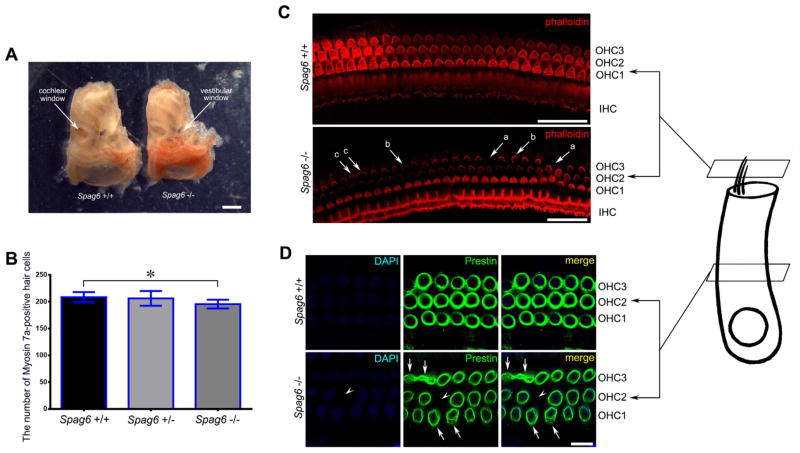
During the dissection of cochlea, we found certain distinctions of the osseous labyrinths between adult mice (≥ P45) of different genotypes. The osseous labyrinths of Spag6 −/− mice appeared smaller, worse ossified and more fragile to external force, which differentiated from osseous labyrinths of their Spag6 +/+ litter-mates, indicating the possibility of delayed inner ears development (Fig. 2A).
Fig. 2.
Morphological abnormalities of OHCs in Spag6 −/− mice. (A) Gross appearances of osseous labyrinths of Spag6 −/− and Spag6 +/+ mouse. (B) MoysinVIIa-positive hair cells counts per 400 μm length in cochlear middle turn. (C) Hair cells identified by phalloidin. Arrows show different morphological abnormalities of stereocilia bundles and the scattered OHC loss. (D) The lateral wall of OHCs identified by prestin. Arrows show the irregular lateral wall of OHCs and arrowhead shows OHC loss. Data shown here are the mean ± S.E.M., *P < 0.05; Scale bars: 1 mm in A, 30 μm in C and 10 μm in D; IHC: inner hair cell; mice age: P45.
In comparison to Spag6 +/+ mice, hair cells in Spag6 −/− mice appeared multiple morphological abnormalities, including the loss of single OHC as shown by the absent fluorescence of cilia bundles (Fig. 2C, a arrows), the disturbed orientation and the irregular shape of cilia bundles (Fig. 2C, b and c arrows). The intensive prestin immunolabeling appeared in Spag6 +/+ mice (Fig. 2D, upper row), which was in contrast with the obscure and irregular immnolabeling in Spag6 −/− mice (Fig. 2D, lower row, arrows). Prominently, the unexpected scattered OHCs loss occurred frequently in Spag6 −/− mice (Fig. 2D, lower row, arrowhead). Subsequently, we quantified the moysin VIIa-positive hair cells in the middle turn of cochlea to measure the hair cell loss (Fig. 2B). The specific loss of OHCs was statistical significant between Spag6 −/− and Spag6 +/+ mice (P < 0.05) and there was no IHC loss, illuminating that the impairment of OHCs potentially resulted from the deletion of SPAG6.
3.4. Expression of prestin in different genotypic mice
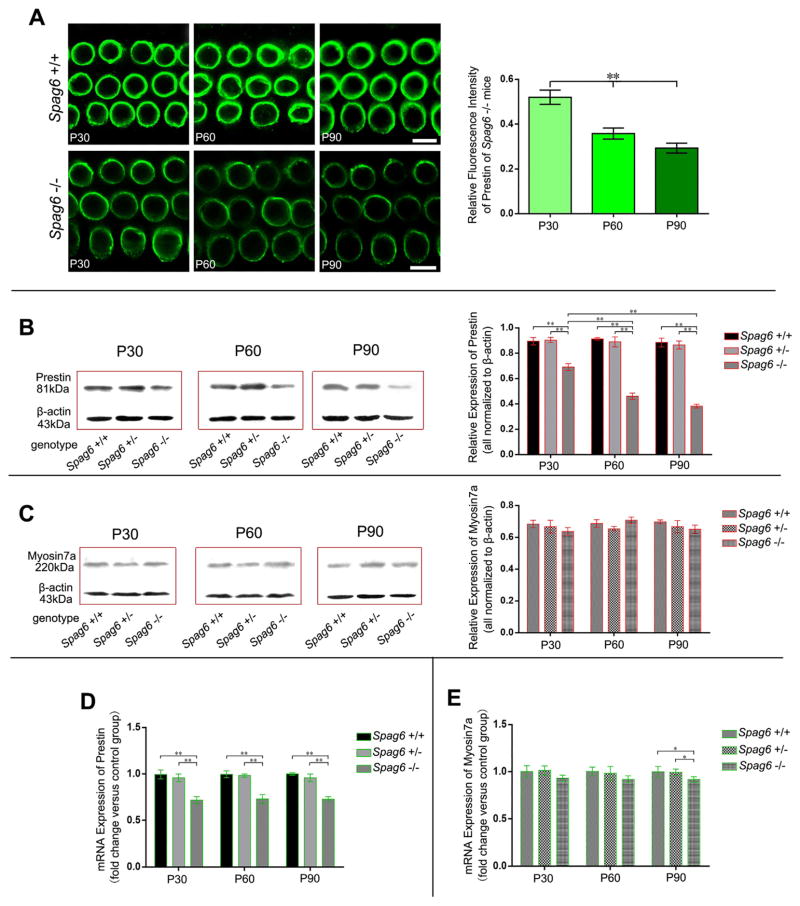
The expression of prestin was tested in mice of different genotypes during their development by immunofluorescent staining, Western blotting and real-time PCR, respectively. Meanwhile, the expression of moysinVIIa also was measured as an internal control to prestin. We found, in comparison to Spag6 +/+ mice, the corresponding Spag6 −/− subjects showed weaker intensity of prestin fluorescence at the simultaneous time point (Fig. 3A left panel). The relative prestin fluorescence intensity of Spag6 −/− mice in all age groups was calculated, which demonstrated a progressive decrease of prestin quantity after the auditory maturity (P < 0.01, Fig. 3A right panel).
Fig. 3.
Expression of prestin in different genotypic mice. (A) Immunofluorescent staining of prestin in Spag6 +/+ and Spag6 −/− mice during development and the corresponding statistical analysis of fluorescence intensity. Mice ages are shown by the corner marks. (B and C) Western blotting analyses of prestin and moysinVIIa in mice of different genotypes. The multiple comparisons are shown by the bars above the related columns. (D and E) Real-time PCR analyses of prestin and moysinVIIa in mice of different genotypes. Data shown here are the mean ± S.E.M., *P < 0.05, **P < 0.01; Scale bars in A: 5 μm.
The relative prestin expression ratio of Spag6 −/− mice revealed a significant decrease trend during development (Fig. 3B, P < 0.01), which was consistent with the immunofluorescence data (Fig. 3A). In addition, no significant variation of moysinVIIa expression was detected (Fig. 3C). The expression of prestin mRNA in Spag6 −/− mice markedly reduced in all time groups in comparison to those of Spag6 +/+ and Spag6 +/− littermates (P < 0.01) and the corresponding 2−ΔΔCt values of Spag6 −/− mice showed no statistical differences during the time course (Fig. 3D). As for moysinVIIa, distinct decrease of mRNA just appeared in time group P90 (Fig. 3E, P < 0.05). Given the mismatch of prestin and moysinVIIa data in both Western blotting and real-time PCR analyses, we considered that the expression of prestin is specifically reduced in Spag6 −/− mice.
3.5. The interaction between prestin and SPAG6 in OHCs
In regard of all the aforementioned results, we speculated that an interaction between prestin and SPAG6 might exist. Co-immunoprecipitation tests showed that, in Spag6 +/+ mice, prestin and SPAG6 could bind to each other in a reciprocal way (Fig. 4A and B). But in Spag6 −/− mice, tests yielded no positive result (Fig. 4C).
Fig. 4.
Reciprocal co-immunoprecipitation tests between prestin and SPAG6. (A) Prestin pulls down SPAG6. (B) SPAG6 pulls down prestin. (C) Prestin cannot be detected by SPAG6 immunoprecipitation in Spag6 −/− mice. Lane input: the crude protein extracted from basilar membrane, representing the positive control.
4. Discussion
In present study, neonatal mice were bred by the intercross of Spag6 +/− male and female mice available from Zhang et al. [3]. The genetic traits and gross appearances of these neonates were consistent with the previous findings [4], which suggested that this mice strain was successfully reproduced in our lab, thereby laying a feasible foundation for the following experiments.
It has been confirmed that, rootlets of stereocilia and kinocilia anchor into cuticular plate, which is significant for the mechanosensory function of cilia bundles [5]. In this work, we found that SPAG6 localized in cuticular plate and, also, in lateral wall of OHCs in Spag6 +/+ and Spag6 +/− mice. The extensive distribution of SPAG6 in OHCs suggests that this protein is a crucial component of these specialized ciliated cells. Moreover, the highly overlapped distribution range of prestin and SPAG6 implies that these two proteins have a close infinity.
In following works, we found that Spag6 −/− mice appeared visible abnormalities in arrange patterns of stereocilia bundles, as well as scattered OHCs loss and irregular shape of lateral wall of OHCs. These findings clearly demonstrate that SPAG6 helps to maintain the stability of plasma membrane in OHCs. However, it has not been confirmed the mechanism responsible for the irregular stereocilia bundles in Spag6 −/− mice. As a microtubule-related protein within cuticular plate, SPAG6 is likely to regulate planar cell polarity of the cilia. Notably, the indispensible role of SPAG6 for hair cells also deciphers the abnormal behavior of Spag6 −/− mice. The underlying impairment of hair cells in vestibular apparatus, which senses head movement and position, results in the equilibrium dysfunction in Spag6 −/− mice. Therefore, the clumsy mobility and the continuous head tossing of Spag6 −/− mice aggravates their developmental retardation because of the weakened ability of scrambling for food, as evidenced by their abnormal gross features [4] and the possible retardation of inner ear development.
It is reported that, tubulin acts as one constitution of cytoskeleton and possibly relates in docking molecules to the membrane of hair cells [5,19]. Spag6 −/− mice displayed a visible and specific diminution of prestin density in lateral wall, revealing that, similar to tubulin, SPAG6 might also participate in the constitution of plasma membrane and maintaining the transmembrane proteins of OHCs. Akin to other proteins [14], a latent dependency relationship among SPAG6 and prestin was evidenced by the progressive decrease of prestin in Spag6 −/− mice. Because prestin is a principal component of the cell’s lateral wall [8], it is not surprising that the deletion of SPAG6 severely impairs the stability of cellular membrane and damages the mechanosensory function of OHCs. However, mechanism by which prestin mRNA are down-regulated in Spag6 −/− mice is still unclear. Certain transcription factors, such as GATA-3, and Pou4f3 [20,21], may play different roles in transcription progress in Spag6 −/− mice. Furthermore, some researchers note that Spag6 gene likely involves in the chromatin assembly [22], indicating that SPAG6 potentially correlates with prestin transcription, and even with moysinVIIa transcription.
Available data show that prestin interacts with other proteins [10]. Prominently, one research reports that Microtubule-associated Protein 1S (MAP1S) binds to prestin in vivo and could augment the activity of prestin [12]. In the current study, the deduced interaction between prestin and SPAG6 was confirmed by the reciprocal co-immunoprecipitation tests. We conclude, therefore, that SPAG6 binds to prestin in vivo and contributes to fix prestin biomolecules in lateral plasma membrane of OHCs. Yet prestin is not completely eliminated in Spag6 −/− mice, which implies that various factors and signaling pathways, together with SPAG6, may be responsible for maintaining this crucial transmembrane protein.
5. Conclusion
Taken together, we show, for the first time, that SPAG6 exists in OHCs inside the cochlea. Moreover, SPAG6 is found to be indispensible for the stability of OHCs by maintaining the normal expression of prestin, which implied that Spag6 gene is essential for mechanosensory function of OHCs.
HIGHLIGHTS.
SPAG6 exists in cochlear outer hair cells.
The expression of prestin in outer hair cells is significantly reduced in Spag6 gene knockout mice.
SPAG6 is indispensible for the stability of outer hair cells.
Spag6 gene is essential for mechanosensory function of outer hair cells.
Acknowledgments
This work was supported by the National 973 Basic Research Program of China (No. 2014CB541702, No. 2014CB541703), the National Natural Science Foundation of China (No. 803300121, No. 81072200), and an NIH grant (HD076257).
References
- 1.Neilson LI, Schneider PA, Van Deerlin PG, Kiriakidou M, Driscoll DA, Pellegrini MC, Millinder S, Yamamoto KK, French CK, Strauss JF., 3rd cDNA cloning and characterization of a human sperm antigen (SPAG6) with homology to the product of the Chlamydomonas PF16 locus. Genomics. 1999;60:272–280. doi: 10.1006/geno.1999.5914. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Sapiro R, Kapfhamer D, Bucan M, Bray J, Chennathukuzhi V, McNamara P, Curtis A, Zhang M, Blanchette-Mackie EJ, Strauss JF., 3rd A sperm-associated WD repeat protein orthologous to Chlamydomonas PF20 associates with Spag6, the mammalian orthologue of Chlamydomonas PF16. Mol Cell Biol. 2002;22:7993–8004. doi: 10.1128/MCB.22.22.7993-8004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Tang W, Zhou R, Shen X, Wei Z, Patel AM, Povlishock JT, Bennett J, Strauss JF., 3rd Accelerated mortality from hydrocephalus and pneumonia in mice with a combined deficiency of SPAG6 and SPAG16L reveals a functional interrelationship between the two central apparatus proteins. Cell Motil Cytoskeleton. 2007;64:360–376. doi: 10.1002/cm.20189. [DOI] [PubMed] [Google Scholar]
- 4.Sapiro R, Kostetskii I, Olds-Clarke P, Gerton GL, Radice GL, Strauss IJ. Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol Cell Biol. 2002;22:6298–6305. doi: 10.1128/MCB.22.17.6298-6305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwander M, Kachar B, Muller U. Review series: the cell biology of hearing. J Cell Biol. 2010;190:9–20. doi: 10.1083/jcb.201001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- 7.Yu N, Zhu ML, Zhao HB. Prestin is expressed on the whole outer hair cell basolateral surface. Brain Res. 2006;1095:51–58. doi: 10.1016/j.brainres.2006.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- 9.Yu L, Jiang XH, Zhou Z, Tsang LL, Yu MK, Chung YW, Zhang XH, Wang AM, Tang H, Chan HC. A protective mechanism against antibiotic-induced ototoxicity: role of prestin. PloS One. 2011;6:e17322. doi: 10.1371/journal.pone.0017322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deak L, Zheng J, Orem A, Du GG, Aguinaga S, Matsuda K, Dallos P. Effects of cyclic nucleotides on the function of prestin. J Physiol. 2005;563:483–496. doi: 10.1113/jphysiol.2004.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia A, Song Y, Wang R, Gao SS, Clifton W, Raphael P, Chao SI, Pereira FA, Groves AK, Oghalai JS. Prestin regulation and function in residual outer hair cells after noise-induced hearing loss. PLoS One. 2013;8:e82602. doi: 10.1371/journal.pone.0082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai JP, Surguchev A, Ogando Y, Song L, Bian S, Santos-Sacchi J, Navaratnam D. Prestin surface expression and activity are augmented by interaction with MAP1S, a microtubule-associated protein. J Biol Chem. 2010;285:20834–20843. doi: 10.1074/jbc.M110.117853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navaratnam D, Bai JP, Samaranayake H, Santos-Sacchi J. N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein. Biophys J. 2005;89:3345–3352. doi: 10.1529/biophysj.105.068759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Currall B, Yamashita T, Parker LL, Hallworth R, Zuo J. Prestin–prestin and prestin-GLUT5 interactions in HEK293T cells. Dev Neurobiol. 2007;67:483–497. doi: 10.1002/dneu.20357. [DOI] [PubMed] [Google Scholar]
- 15.Holzmann D, Ott PM, Felix H. Diagnostic approach to primary ciliary dyskinesia: a review. Eur J Pediatr. 2000;159:95–98. doi: 10.1007/pl00013813. [DOI] [PubMed] [Google Scholar]
- 16.Yoon H, Lee DJ, Kim MH, Bok J. Identification of genes concordantly expressed with Atoh1 during inner ear development. Anat Cell Biol. 2011;44:69–78. doi: 10.5115/acb.2011.44.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vu AA, Nadaraja GS, Huth ME, Luk L, Kim J, Chai R, Ricci AJ, Cheng AG. Integrity and regeneration of mechanotransduction machinery regulate aminoglycoside entry and sensory cell death. PLoS One. 2013;8:e54794. doi: 10.1371/journal.pone.0054794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Raphael Y, Athey BD, Wang Y, Lee MK, Altschuler RA. F-actin, tubulin and spectrin in the organ of Corti: comparative distribution in different cell types and mammalian species. Hear Res. 1994;76:173–187. doi: 10.1016/0378-5955(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 20.Gross J, Stute K, Fuchs J, Angerstein M, Amarjargal N, Mazurek B. Effects of retinoic acid and butyric acid on the expression of prestin and Gata-3 in organotypic cultures of the organ of Corti of newborn rats. Dev Neurobiol. 2011;71:650–661. doi: 10.1002/dneu.20881. [DOI] [PubMed] [Google Scholar]
- 21.Gross J, Angerstein M, Fuchs J, Stute K, Mazurek B. Expression analysis of prestin and selected transcription factors in newborn rats. Cell Mol Neurobiol. 2011;31:1089–1101. doi: 10.1007/s10571-011-9708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulaw MA, Krause A, Deshpande AJ, Krause LF, Rouhi A, La Starza R, Borkhardt A, Buske C, Mecucci C, Ludwig WD, Lottaz C, Bohlander SK. CALM/AF10-positive leukemias show upregulation of genes involved in chromatin assembly and DNA repair processes and of genes adjacent to the breakpoint at 10p12. Leukemia. 2012;26:1012–1019. doi: 10.1038/leu.2011.307. [DOI] [PubMed] [Google Scholar]