Abstract
BACKGROUND / OBJECTIVES
Little is known about how many ambulatory older adults with chronic kidney disease receive medications that are contraindicated or dosed excessively given their level of renal function.
DESIGN, SETTING, AND PARTICIPANTS
Cross-sectional retrospective study of adults age 65 and older with creatinine clearance (CrCl) 15–49 ml/min who received care in U.S. Department of Veterans Affairs (VA) clinics.
MEASUREMENTS
We examined 39 medications which require dose adjustment or are contraindicated in people with impaired renal function. Medication use and CrCl (calculated using the Cockroft-Gault equation) were assessed through VA pharmacy, laboratory, and other data sources as of October 2007.
RESULTS
Among 83,850 eligible older veterans with CrCl 15–49 ml/min, mean age was 80 years and 96% were men. Overall, 13% of patients with CrCl 30–49 ml/min and 29% of patients with CrCl 15–29 ml/min received one or more drugs that were contraindicated or prescribed at an excessive dose given the patient’s level of renal function. The strongest risk factor for renally inappropriate prescribing was number of medications used: the adjusted relative risk of receiving renally inappropriate medications was 5.6 times higher (95% CI, 5.2–6.0) in older adults taking 10 or more medications compared with those taking 1–3 medications. Ranitidine, allopurinol, and metformin together accounted for 76% of renally misprescribed medications in patients with CrCl 30–49 ml/min. Glyburide, ranitidine, gemfibrozil, and allopurinol accounted for 42% of renally misprescribed drugs for patients with CrCl 15–29 ml/min.
CONCLUSION
Inappropriate prescribing of renally cleared medications is common among ambulatory older veterans, and only a few medications account for most of these prescribing problems.
Keywords: pharmaceutical care, pharmacoepidemiology, renal disease, aging, ambulatory care, veterans, chronic kidney disease, drug utilization, quality of care
Introduction
In adults with chronic kidney disease (CKD), renally inappropriate prescribing – that is, failure to adjust medication dose to account for impaired renal function, or use of medications that are contraindicated in this setting - can lead to adverse outcomes.1,2 This is a particularly vital issue for older adults. Not only does this group have high rates of CKD, but older adults have a high burden of chronic comorbid conditions and often use multiple medications, which together place these patients at particularly high risk of harm.3–6
Previous research suggests that renally inappropriate prescribing is common in specific high-risk settings. In several studies of drug use in hospitalized adults, 15% to 67% of drugs that require renal adjustment were given at inappropriate doses or intervals.7–11 Other studies that examined elderly residents of long-term care facilities found similarly high rates of renal misprescribing.12–14 However, while inappropriate renal prescribing appears to be common in inpatient settings, surprisingly little research has been conducted on the frequency of renally inappropriate prescribing in outpatient settings. A limited number of studies suggest that inappropriate dosing of renally cleared medications may occur in approximately 10% to one-third of ambulatory adults, yet the few available studies are limited by varying methods, small sample sizes, and a focus on patients from one institution or region, as opposed to broader populations of adults or a focus on older adults in particular.15–20 This information is essential to understand the epidemiology of renally inappropriate prescribing in older adults in the outpatient setting and to guide interventions that can improve medication prescribing in this vulnerable group.
To study these issues, we evaluated the prevalence of and risk factors for renally inappropriate medication use among older adults with impaired renal function receiving outpatient care in the U.S. Veterans Affairs (VA) health care system.
Methods
SUBJECTS
We evaluated adults aged 65 years and older with creatinine clearance of 15–49 ml/min who were receiving one or more outpatient medications from VA pharmacies and were not residing in an inpatient facility on October 1, 2007. We excluded patients for whom creatinine clearance could not be assessed due to lack of recent weight or serum creatinine measurements (per assessment procedures described below), and a small number of patients who had incomplete prescription data. To focus our analyses on patients who predominantly received their health care and medications from VA sources, we also excluded patients who had fewer than 80% of their outpatient health care encounters in VA settings over the previous year, or who were enrolled in Medicare Managed Care.
While staging for renal disease typically uses estimated glomerular filtration rate (eGFR), drug dosing recommendations have historically been based on creatinine clearance (CrCl), which is similar although not identical to eGFR. There is controversy on the appropriateness of using eGFR for purposes of medication dose adjustment, and there are several potential problems with CrCl-based assessments. These include relatively recent standardization of laboratory serum creatinine measurements that yield different CrCl results than the methods in place when many medications were initially tested and renal dosing parameters established.21 Nonetheless, many experts and VA still recommend using CrCl when possible.22–24 As a result, in this paper our primary analyses are based on CrCl estimated via the Cockroft-Gault equation, although we also present sensitivity analyses using eGFR-based methods.
We chose to focus on patients with CrCl 15–49 ml/min for the following reasons. Patients with CrCl <15 ml/min often receive intensive nephrology care and are subject to different prescribing restrictions than patients with non-end-stage renal disease. (For similar reasons, we excluded patients on dialysis, regardless of CrCl). Regarding the upper limit, many drugs that require dose adjustment have an upper threshold for dose adjustment beginning at CrCl <50 ml/min. Among 373,549 veterans meeting inclusion criteria other than the presence of impaired renal function, 83,850 had CrCl in our target range of 15–49 ml/min. Of the remaining 289,699 veterans whose CrCl was outside our target range (and were thus excluded), 289,149 had CrCl≥50 ml/min, and 550 had CrCl<15 ml/min.
MEASURES
Medication use
Using data from VA Pharmacy Benefits Management files, we evaluated medications prescribed through VA outpatient pharmacies as of October 1, 2007 (the index date for our study). Medications were considered to be in active use on the index date if there had been a prescription fill prior to that date with sufficient quantity dispensed to last through the index date if taken as directed. To account for transient non-adherence and short gaps in refills, we also considered a drug to be in active use on the index date if the patient received a supply of medication sufficient to cover 80% or more of days within the previous six months, and the patient was dispensed the same drug after the index date.25
Renal function and weight
While prescribers may dose newly-prescribed medications based on the most recent estimate of renal function, for prevalent medications (the focus of this study) the patient’s recent average estimated renal function is the more relevant metric. To establish a baseline value of creatinine (from which renal function is derived), we used data from VA’s LAR Lab Results file to determine the mean of the two most recent serum creatinine measures in the year prior to October 1, 2007 that were measured at least 30 days apart. If the two data points differed by greater than 50% in value, then a third most recent data point was selected as a tiebreaker and averaged with the other data point closer in value. To exclude transient changes in renal function that can accompany severe acute illness, we excluded creatinine measures while patients were hospitalized or within 3 days before or after hospitalization.
Using data from VA’s Corporate Data Warehouse (CDW), we also assessed the most recent recorded weight obtained in a clinic setting in the previous 2 years. If no such weights were available, we used the most recent weight in any other (e.g., inpatient) setting. We excluded weights below 70 or above 500 pounds, since those are likely to have been entered in error.
We used serum creatinine measurements and weight to calculate CrCl using the Cockroft-Gault equation. In sensitivity analyses, we calculated eGFR using the CKD-EPI equation. We also assessed eGFR using the Modification of Diet in Renal Disease (MDRD) equation26, which was the dominant method for estimating GFR in VA clinical laboratories in 2007, and using the Berlin Initiative Study 1 (BIS1) equation, a recently-developed measure of eGFR designed to more accurately estimate GFR in the elderly.27
Comorbid conditions and nephrology care
Using established methods, we defined comorbid conditions using clusters of disease-specific ICD9 codes from the Healthcare Cost and Utilization Project Clinical Classification System.28 Conditions were considered present if they were coded in at least two outpatient visits or at least one inpatient discharge diagnosis over the previous year in either VA or Medicare settings, as assessed through the VA National Patient Care Database and equivalent CDW files and through Medicare MedPAR (inpatient) and Carrier and Outpatient (outpatient) files available through VA’s VIReC program. We also ascertained whether patients had an outpatient nephrologist visit in the past year by evaluating clinic stop codes in VA and physician specialty identifiers in Medicare outpatient data.
Identifying renally inappropriate medication use
We used a two-pronged approach to identify drugs commonly used by older veterans that require dose adjustment or are contraindicated in people with impaired renal function. First, we identified 133 of the most commonly prescribed drugs given to adults age 65 and older in VA, and consulted an online pharmacy reference guide (LexiComp) to identify renal prescribing guidelines for each based on CrCl cutoffs. Second, we included drugs identified in a consensus report of important medications that require dose adjustment in ambulatory older adults.29 In cases where both sources included a drug and provided conflicting recommendations on renal dosing, we used renal dosing recommendations from the consensus report. This process identified a total of 39 medications which require dose adjustment or are contraindicated in patients with low CrCl (shown in Appendix Table 1). This included 16 drugs that require dose adjustment and 4 drugs that are contraindicated for people with CrCl 30–49 ml/min, and an additional 10 drugs that require dose adjustment and 9 that are contraindicated for people with CrCl 15–29 ml/min. This list is not intended to be utterly comprehensive, but to include the most commonly prescribed drugs dispensed via pharmacy that require renal adjustment.
There has been considerable debate over appropriate renal dosing criteria for metformin, one of the most commonly used drugs on our list, as the creatinine-based criteria listed on the product label are considered antiquated and overly restrictive by many experts.30 For our main analyses we therefore chose an intermediate and increasingly accepted approach based on expert opinion with a maximum recommended dose of 1275 mg/day for patients with CrCl 30–49 ml/min and a recommendation not to use this medication in patients with CrCl<30 ml/min (these recommendations are based on eGFR levels, but for the sake of consistency in this paper we frame them as CrCl thresholds).30
ANALYSES
Most analyses were descriptive (e.g. means and percents), and focused on the frequency with which drugs were misprescribed at different levels of renal function. To examine predictors of misprescribing, we performed relative risk estimation using modified Poisson regression. The outcome was whether a patient received one or more renally inappropriate medications, and the predictors are those shown in Table 2. No variables were excluded from the model in the model-building process, and there was no evidence of multicollinearity between variables. Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina). This research was approved by the Research and Development Committee of the San Francisco VA Medical Center and by the Committee on Human Research at the University of California, San Francisco.
Table 2.
Risk factors for receiving one or more renally inappropriate prescriptions
| Risk Factor | Adjusted Relative Risk (95% CI) | P Value |
|---|---|---|
| Creatinine clearance (ml/min) | ||
| 45 – 49 (reference) | -- | |
| 40 – 44 | 0.96 (0.92, 1.01) | 0.08 |
| 35 – 39 | 0.95 (0.90, 1.00) | 0.03 |
| 30 – 34 | 1.07 (1.01, 1.13) | 0.02 |
| 15 – 29 | 2.29 (2.19, 2.39) | <0.001 |
| Female gender | 1.09 (1.01, 1.18) | 0.02 |
| Age | ||
| 65 – 74 (reference) | -- | |
| 75 – 84 | 0.99 (0.95, 1.03) | 0.70 |
| ≥ 85 | 0.91 (0.87, 0.95) | <0.001 |
| Race/Ethnicity | ||
| White (reference) | -- | |
| Black | 0.91 (0.88, 0.95) | <0.001 |
| Hispanic | 1.00 (0.91, 1.09) | 0.99 |
| Others | 0.92 (0.85, 1.01) | 0.08 |
| Comorbid conditions | ||
| Diabetes | 1.49 (1.44, 1.54) | <0.001 |
| Gout | 1.86 (1.76, 1.96) | <0.001 |
| GERD/PUD* | 1.37 (1.31, 1.43) | <0.001 |
| Heart failure | 0.95 (0.90, 0.99) | 0.03 |
| Coronary Artery Disease | 0.97 (0.94, 1.01) | 0.20 |
| COPD/Asthma* | 0.98 (0.94, 1.02) | 0.33 |
| Depression | 0.92 (0.87, 0.98) | 0.01 |
| Nephrologist visit | 0.79 (0.75, 0.83) | <0.001 |
| Number of Medications taken | ||
| 1 – 3 (reference) | -- | |
| 4 – 6 | 2.62 (2.46, 2.80) | <0.001 |
| 7 – 9 | 4.12 (3.86, 4.39) | <0.001 |
| ≥ 10 | 5.59 (5.22, 5.99) | <0.001 |
| Number of primary care / subspecialty visits (baseline year) | ||
| 1 – 3 (reference) | -- | |
| 4 – 6 | 0.95 (0.91, 0.99) | 0.02 |
| 7 – 9 | 0.92 (0.87, 0.97) | <0.001 |
| ≥ 10 | 0.92 (0.88, 0.97) | <0.001 |
Given the binary nature of the outcome, incidence rate ratio is equivalent to relative risk. Analyses were performed using zero-inflated Poisson regression with robust error variance. The outcome for this analysis was receipt of at least one renally inappropriately dosed or contraindicated drug (yes vs. no). Analyses were adjusted for each of the covariates listed in the table.
GERD/PUD = gastroesophageal reflux disease, peptic ulcer disease, and/or dysphagia; COPD = chronic obstructive pulmonary disease
Results
Among 83,850 veterans with CrCl 15–49 ml/min, mean age was 80±6 years, and 96% were male (Table 1). On average, these patients were diagnosed with a mean of 3.6±2.5 comorbidities and were taking a mean of 5.9±3.3 medications. Overall, 87% of the cohort (N=72,834) had CrCl values between 30–49 ml/min. Only 9% of this group had visited a nephrologist within the baseline year. The remaining 13% of the cohort (N=11,016) had CrCl values between 15–29 ml/min, of whom 33% visited a nephrologist within the same time frame.
Table 1.
Patient characteristics by creatinine clearance level (n = 83,850)
| Characteristic | All patients with CrCl 15–49 ml/min (n = 83,850) No. (%) |
Patients with CrCl 30–49 ml/min (n = 72,834) No. (%) |
Patients with CrCl 15–29 ml/min (n = 11,016) No. (%) |
|---|---|---|---|
| Gender | |||
| Male | 80,718 (96.3) | 70,277 (96.5) | 10,441 (94.8) |
| Age (years) | |||
| 65–74 | 18,694 (22.3) | 17,035 (23.4) | 1,659 (15.1) |
| 75–84 | 46,882 (55.9) | 41,548 (57.0) | 5,334 (48.4) |
| ≥85 | 18,274 (21.8) | 14,251 (19.6) | 4,023 (36.5) |
| Race/ethnicity | |||
| White non-Hispanic | 64,606 (77.1) | 56,794 (78.0) | 7,812 (70.9) |
| Black non-Hispanic | 14,358 (17.1) | 11,961 (16.4) | 2,397 (21.8) |
| Hispanic | 2,267 (2.7) | 1,884 (2.6) | 383 (3.5) |
| Other non-Hispanic/unknown | 2,619 (3.1) | 2,195 (3.0) | 424 (3.8) |
| Comorbid conditions | |||
| Diabetes | 25,232 (30.1) | 21,847 (30.0) | 3,385 (30.7) |
| Gout | 4,161 (5.0) | 3,344 (4.6) | 817 (7.4) |
| GERD/PUD* | 11,548 (13.8) | 10,002 (13.7) | 1,546 (14.0) |
| Congestive heart failure | 10,348 (12.3) | 8,205 (11.3) | 2,143 (19.5) |
| Coronary artery disease | 21,977 (26.2) | 18,673 (25.6) | 3,304 (30.0) |
| COPD/Asthma* | 14,998 (17.9) | 12,909 (17.7) | 2,089 (19.0) |
| Depression | 6,091 (7.3) | 5,331 (7.3) | 760 (6.9) |
| Number of medications used | |||
| 1–3 | 21,885 (26.1) | 19,624 (26.9) | 2,261 (20.5) |
| 4–6 | 30,472 (36.3) | 26,615 (36.5) | 3,857 (35.0) |
| 7–9 | 20,077 (23.9) | 17,101 (23.5) | 2,976 (27.0) |
| 10 or more | 11,416 (13.6) | 9,494 (13.0) | 1,922 (17.4) |
| Number of primary care & subspecialty visits in prior year | |||
| 1–3 | 26,817 (32.0) | 23,844 (32.7) | 2,973 (27.0) |
| 4–6 | 26,299 (31.4) | 23,023 (31.6) | 3,276 (29.7) |
| 7–9 | 13,243 (15.8) | 11,347 (15.6) | 1,896 (17.2) |
| 10 or more | 17,491 (20.9) | 14,620 (20.1) | 2,871 (26.1) |
| 1 or more nephrologist visits in prior year | 10,357 (12.4) | 6,714 (9.2) | 3,643 (33.1) |
GERD/PUD = gastroesophageal reflux disease, peptic ulcer disease, and/or dysphagia; COPD = chronic obstructive pulmonary disease.
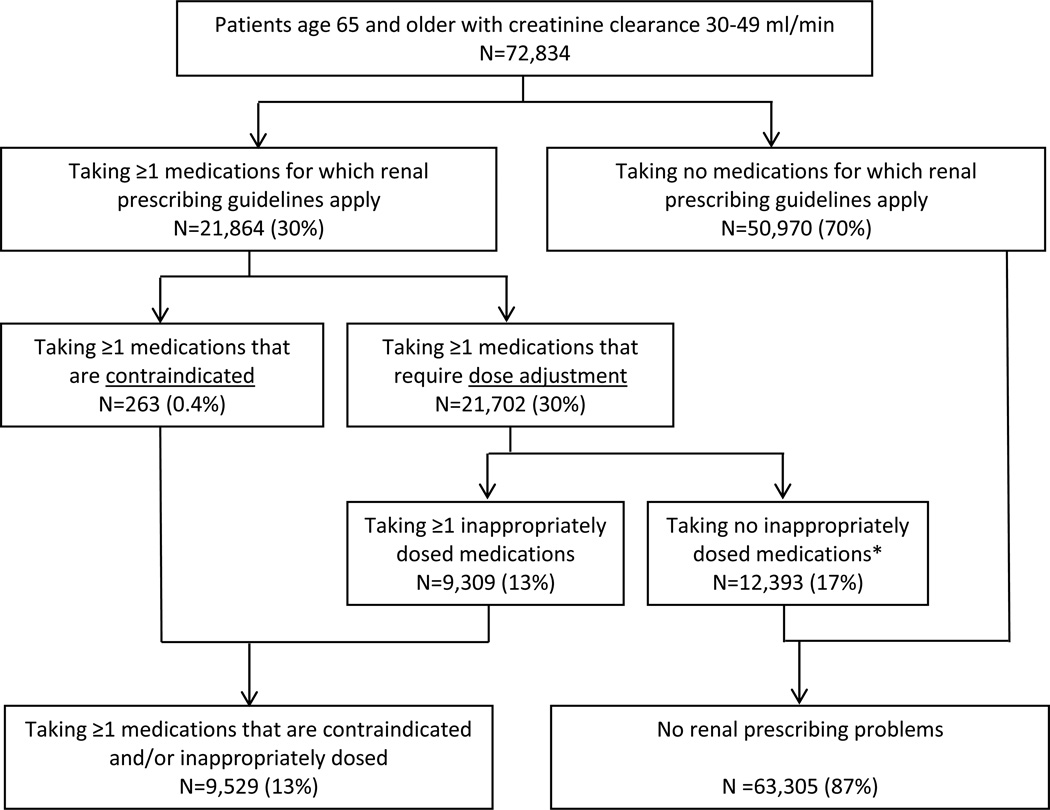
Among 72,834 veterans with CrCl 30–49 ml/min, 30% (N=21,864) were taking at least one medication for which there are renal dosing recommendations (Figure 1). Overall, 13% of veterans with CrCl 30–49 ml/min (N=9,529) were taking at least one medication that was misprescribed according to renally-focused recommendations. This included 9,309 patients who received medications at inappropriately high doses given their level of renal function, and 263 patients who received medications that were contraindicated at their level of creatinine clearance. (Of note, 43 patients received one or more medications in both types of problems; i.e., excessive dose and contraindicated). Among the 9,529 patients with CrCl 30–49 ml/min who were prescribed one or more renally inappropriate medications, 8,970 were taking 1 such medication, 542 were taking 2 such medications, and 17 were taking 3 or more such medications.
Figure 1. Use of renally inappropriate medications among patients with CrCl 30–49 ml/min.
Some patients were prescribed both medications that are contraindicated and those that are inappropriately dosed at their level of creatinine clearance.
* All criteria are specific to each patient’s level of renal function. Renal prescribing guidelines include drugs which are considered contraindicated below a given level of creatinine clearance (in which case any use is considered inappropriate), and drugs for which reduced doses are recommended (in which case use of a higher-than-recommended dose is considered inappropriate).
The frequency of misprescribing was greater in patients with CrCl 15–29 ml/min. Among 11,016 patients with this level of creatinine clearance, 5,763 (52%) were prescribed at least one medication for which renal guidelines apply, with 3,246 (39%) receiving at least one contraindicated or inappropriately dosed medication. These 3,246 patients with at least one prescribing problem included 2,097 patients who received at least one inappropriately dosed medication and 1,466 who received at least one medication contraindicated at their level of creatinine clearance. (There were 317 patients whose medication regimen included both types of prescribing problems).
Overall, among the 83,850 veterans with CrCl 15–49 ml/min, 12,775 (15%) were prescribed at least one renally inappropriate medication (see Appendix Table 2 for additional details).
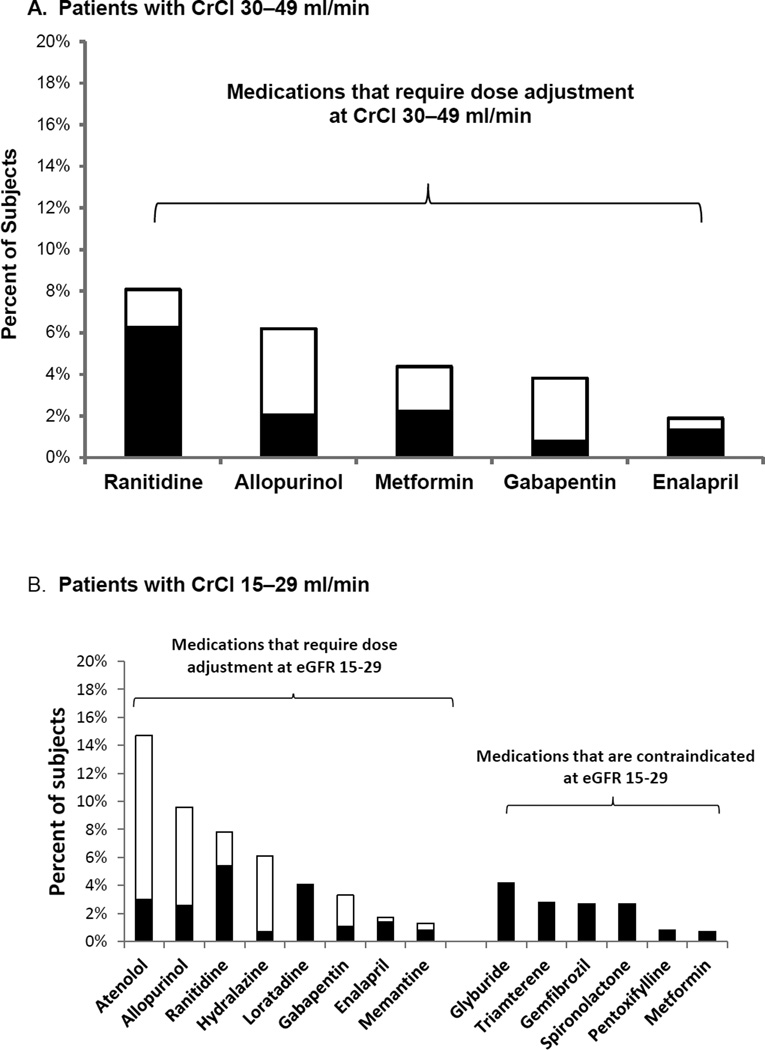
There were substantial differences in which drugs were most commonly misprescribed at different levels of renal function, in part reflecting the fact that contraindications and dosing guidelines differ by level of creatinine clearance (Figure 2). In patients with CrCl 30–49, most issues were with dosing rather than prescription of contraindicated medications, and excessive doses were common for a number of drugs. For instance, 77% of prescriptions for ranitidine given to patients with CrCl 30–49 ml/min were given at excessive doses, as were 51% of prescriptions for metformin, 33% of prescriptions for allopurinol and 20% for gabapentin (Figure 2A). Overall, 3 drugs (ranitidine, allopurinol, and metformin) accounted for 76% of all misprescribed drugs for patients with CrCl 30–49 ml/min. For patients with CrCl 15–29 ml/min, there were considerably more drugs that were renally misprescribed (Figure 2B). In many instances, these were medications that are contraindicated at CrCl < 30 ml/min. Together, 4 medications (glyburide, ranitidine, gemfibrozil, and allopurinol) accounted for 42% of renally misprescribed drugs for patients with this level of creatinine clearance.
Figure 2. Most common medications with renally inappropriate prescribing.
This figure shows all drugs that were renally misprescribed for at least 1% (rounded) of patients with creatinine clearance 30–49 ml/min (panel A) and creatinine clearance 15–29 ml/min (panel B). For medications that require dose adjustment, black bars represent the percent of patients who received the drug at an inappropriately high dose, and the white bars represent the percent of patients who received the drug at an appropriate dose.
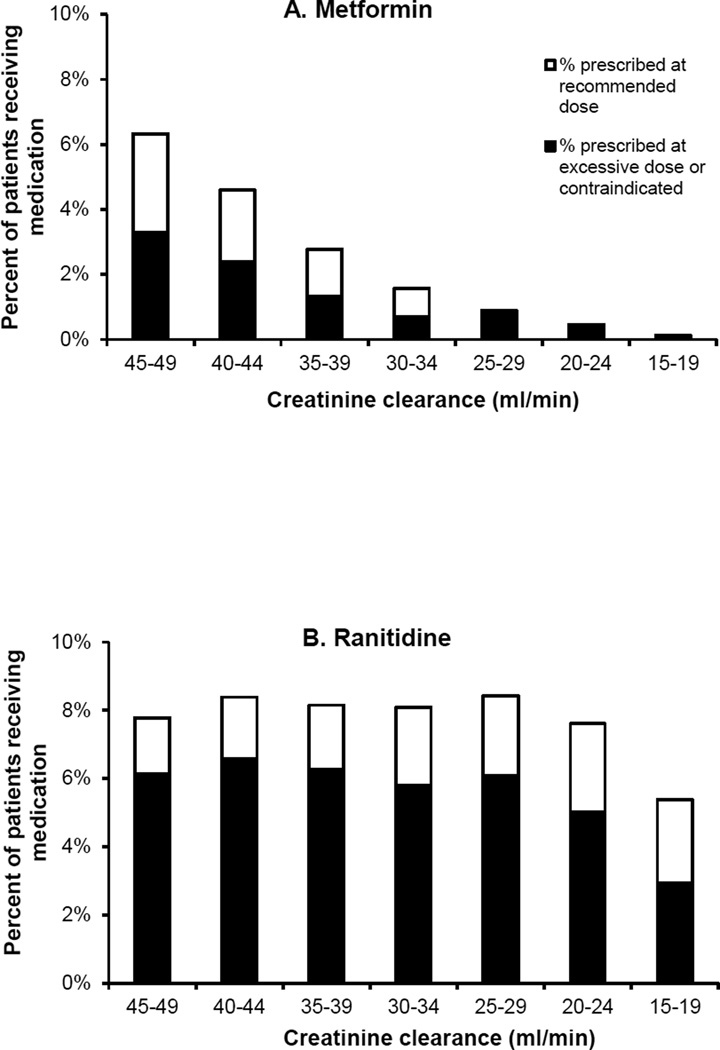
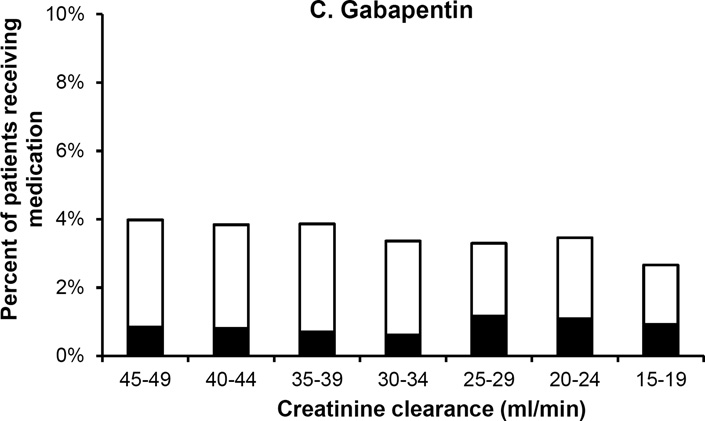
Figure 3 shows rates of misprescribing for three commonly-used drugs at varying levels of renal impairment. Metformin was commonly prescribed among patients with mild levels of renal impairment (CrCl 45–49 ml/min). The number of prescriptions sharply decreased as renal impairment worsened, although the proportion of these prescriptions that were given at excessive doses was similar across the range of CrCl at which dose reduction (rather than total avoidance) is advised. In comparison, ranitidine and gabapentin each maintained similar rates of overall use and a similar proportion of misprescribing across all levels of creatinine clearance.
Figure 3. Patterns of renally inappropriate medication use by level of renal function.
Graphs show percent of all patients at each level of renal function who used metformin (panel A), ranitidine (panel B), and gabapentin (panel C), and the percentage of these prescriptions that were written at doses above and below recommended dose limits. Metformin is contraindicated at creatinine clearance <30 ml/min, so all prescriptions for this drug among patients with creatinine clearance <30 ml/min are considered renally inappropriate. Ranitidine and gabapentin have dose restrictions but not an absolute contraindication across the range of creatinine clearances shown.
In multivariable analysis, the use of multiple medications was the strongest predictor of inappropriate renal prescribing (Table 2). Compared to patients taking 1–3 medications, those taking 10 or more drugs had a 5.6-fold higher risk of using at least one renally inappropriate medication. In addition, the risk of renally inappropriate medication use was similar for patients at the higher and lower ends of creatinine clearance within a range of 30–49 ml/min, but was 2.3 times higher in patients with CrCl 15–29 ml/min (adjusted RR 2.29, 95%CI 2.19 – 2.39) compared to patients with CrCl 45–49 ml/min.
In sensitivity analyses, we repeated our analyses based on eGFR rather than CrCl levels. We used three methods for calculating eGFR: CKD-EPI, the currently-preferred method for calculating eGFR in most settings; the MDRD equation, which was used by VA laboratory services at the time of our data collection; and the BIS1 equation. Overall, there were 86,911 patients with eGFR 15–49 ml/min/1.73 m2 by CKD-EPI calculations, 61,258 patients by MDRD calculations, and 133,249 by BIS1 calculations. The relative frequency of renal misprescribing was similar regardless of the method used to calculate renal function: altogether, 17.2% to 18.4% of patients with eGFR 15–49 ml/min/1.73m2 used at least one medication that was contraindicated or inappropriately dosed (see Appendix Figures 1–4), compared with 15.2% of patients with CrCl 15–49 ml/min.
Discussion
In this national study of older veterans, we found a high prevalence of renally inappropriate medication use in the outpatient setting. Overall, 13% of older adults with CrCl 30–49 ml/min and 29% with CrCl 15–29 ml/min were prescribed one or more drugs that were contraindicated or prescribed at an inappropriately high dose given their level of renal function. Among older patients with moderate levels of renal impairment (CrCl 30–49 ml/min), the most commonly misprescribed medications were those that required dose adjustments, such as ranitidine, allopurinol, and metformin. Patients with more severe levels of renal impairment (CrCl 15–29 ml/min) were more likely to be prescribed medications at inappropriately high doses (e.g., ranitidine, allopurinol, loratadine) and those that were contraindicated (e.g., glyburide, gemfibrozil). However, in adjusted analyses, use of multiple medications rather than level of renal function was the strongest predictor of having renally inappropriate medication use.
The finding that multiple medication use was the strongest predictor of renally inappropriate prescribing is consistent with other research showing that number of medications is the strongest risk factor for a wide variety of medication-related problems.3 This is likely in large part due to the general principle that the more drugs a patient takes, the greater the probability that at least one of those drugs will cause a problem. In addition, the higher rates of renally inappropriate prescribing in patients with CrCl 15–29 ml/min reflects the substantially greater number of contraindicated medications and dose reductions that apply at this level of creatinine clearance. On the other hand, the renally healthier patients with CrCl levels only slightly below 50 ml/min may not be as closely monitored with respect to renal prescribing guidelines since they are on the borderline of guideline thresholds. Several factors may contribute to this. Minor fluctuations in serum creatinine can substantially impact whether these patients are estimated to have CrCl above or below the threshold at which renal prescribing recommendations are applicable. Moreover, many clinicians base their medication prescribing on eGFR levels, which are routinely reported in lab test results, and levels of eGFR and CrCl may diverge substantially for the same patient.
Of note, as shown in Table 2 there were several factors which were protective against renally inappropriate prescribing. However, the magnitude of these protective effects were relatively small compared with the number of medications taken, and thus are less likely to be a focal point for interventions focused on identifying patients at high risk in renally inappropriate prescribing.
The high rate of renally inappropriate prescribing observed in older adults suggests an important quality gap. Fortunately, in integrated health care systems like the VA, the ability to link laboratory data with pharmacy information offers promising avenues to address this problem. Potentially useful strategies could include automated CrCl reporting in e-prescribing systems as well as the creation of automated alerts when medications are prescribed at doses inappropriate for a given patient’s renal function.31,32 However, such strategies are not a panacea, as such alerts are often overridden (often inappropriately), and have yielded only modest to moderate improvements in medication ordering.7,33–36 Causes of the disappointing track record of such interventions likely include alert fatigue and lack of buy-in to the clinical importance of these alerts. An automated alert system that is carefully designed to “get it right” for a limited number of drugs may be more credible and effective than systems that focus more generically on dozens or hundreds of drugs. Finally, the finding that number of drugs is the strongest predictor of renal misprescribing – even substantially more than level of CrCl - suggests that efforts to reduce renally inappropriate prescribing at the patient level may benefit by targeting patients with extensive medication burden.
A number of contextual factors are important to consider in interpreting our findings. Even though we employed criteria from a commonly used and widely respected pharmacy reference source, other sources may provide different recommendations for medication adjustment in patients with renal impairment.37 These disagreements reflect a lack of consensus on appropriate renal dosing guidelines for a number of drugs, including some of the drugs that accounted for a substantial proportion of the renal misprescribing that we observed.30,38–41 For example, current guidelines recommend using lower dose regimens of allopurinol in people with chronic kidney disease to reduce risk of allopurinol hypersensitivity syndrome.42 However, recent literature suggests that lower doses often fail to adequately treat hyperuricemia in gout and that higher allopurinol dose may not increase risk for the hypersensitivity syndrome.40,41,43 There is also evidence that current recommendations to reduce the dose of ranitidine at CrCl<50 ml/min may be unnecessarily restrictive.44 Similarly, the comparative benefits of using metformin to manage diabetes in people with moderate renal disease may outweigh the downsides.39,45 For this reason, we used intermediate and increasingly accepted metformin dosing guidelines based on American Diabetes Association recommendations. Finally, not all prescribing errors result in harm. Nonetheless, even if only a fraction of the inappropriate prescriptions we identified have the potential for causing serious adverse events, this has important consequences for thousands of older adults.
Another important consideration in interpreting our results is the nature of the study population. Because the VA serves a predominantly male population and has a different organizational structure than many private health care providers, the generalizability of our findings to other health care systems is uncertain. Nevertheless, the national scope of the VA healthcare system and the richness of its electronic medical record and data systems enable a comprehensive view of inappropriate renal prescribing practices in the largest integrated health care delivery system in the United States. Moreover, although women comprise only a small percentage of older veterans, our cohort included a large absolute number of female patients, and our multivariable analysis did not indicate large differences in the frequency of renal prescribing problems between women and men. Our method of determining baseline serum creatinine levels evaluated medium-term renal function (up to one year prior to the index date), and so may have missed patients with more recent changes in renal function. Finally, it is possible that prescribing practices have changed since the period covered in our study. However, we are not aware of any major initiatives in the past several years in VA or nationally that have focused on prescribing for patients with renal impairment, and it is unlikely that prescribing practices have markedly changed since the time of our study.
In summary, renally inappropriate medication use is common among ambulatory older veterans with chronic kidney disease, particularly in patients taking multiple medications, and a limited number of drugs account for the majority of prescribing issues. These results highlight an important quality gap in prescribing for patients with renal impairment, and the need for carefully crafted interventions to improve medication prescribing for this large and vulnerable population.
Supplementary Material
Acknowledgments
Sponsor’s Role: Supported by the National Institute of Aging and by the American Federation for Aging Research (RC1-AG036377, P30-AG044281, and 1K23-AG030999). The sponsors of this research had no input on the design, conduct, or decision to publish this study.
Footnotes
Conflicts of interest: None of the authors have financial conflicts of interest with this research
| Elements of Financial/Personal Conflicts |
*Author 1 Flora Chang |
Author 2 Ann O’Hare |
Author 3 Yinghui Miao |
Etc. Michael Steinman |
||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
Prior presentation: Presented at the Annual Meeting of the American Geriatrics Society, Grapevine, TX, May 2013
- Development of study question: Steinman
- Development of research protocol: Chang, O’Hare, Miao, Steinman
- Analyses and interpretation of data: Chang, O’Hare, Miao, Steinman
- Initial authorship of manuscript: Chang
- Critical review of manuscript: O’Hare, Miao, Steinman
- Technical and administrative support: Steinman
REFERENCES
- 1.Leendertse AJ, van Dijk EA, De Smet PA, Egberts TC, van den Bemt PM. Contribution of renal impairment to potentially preventable medication-related hospital admissions. Ann. Pharmacother. 2012 May;46(5):625–633. doi: 10.1345/aph.1Q633. [DOI] [PubMed] [Google Scholar]
- 2.Dong K, Quan DJ. Appropriately assessing renal function for drug dosing. Nephrol. Nurs. J. 2010 May-Jun;37(3):304–308. [PubMed] [Google Scholar]
- 3.Steinman MA, Hanlon JT. Managing medications in clinically complex elders:"There's got to be a happy medium". JAMA. 2010 Oct 13;304(14):1592–1601. doi: 10.1001/jama.2010.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman MA, Lund BC, Miao Y, Boscardin WJ, Kaboli PJ. Geriatric conditions, medication use, risk of adverse drug events in a predominantly male, older veteran population. J. Am. Geriatr. Soc. 2011 Apr;59(4):615–621. doi: 10.1111/j.1532-5415.2011.03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman MA, Landefeld CS, Rosenthal GE, Berthenthal D, Sen S, Kaboli PJ. Polypharmacy and prescribing quality in older people. J. Am. Geriatr. Soc. 2006 Oct;54(10):1516–1523. doi: 10.1111/j.1532-5415.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 6.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005 Aug 10;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 7.Chertow GM, Lee J, Kuperman GJ, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001 Dec 12;286(22):2839–2844. doi: 10.1001/jama.286.22.2839. [DOI] [PubMed] [Google Scholar]
- 8.Falconnier AD, Haefeli WE, Schoenenberger RA, Surber C, Martin-Facklam M. Drug dosage in patients with renal failure optimized by immediate concurrent feedback. J. Gen. Intern. Med. 2001 Jun;16(6):369–375. doi: 10.1046/j.1525-1497.2001.016006369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheen SS, Choi JE, Park RW, Kim EY, Lee YH, Kang UG. Overdose rate of drugs requiring renal dose adjustment: data analysis of 4 years prescriptions at a tertiary teaching hospital. J. Gen. Intern. Med. 2008 Apr;23(4):423–428. doi: 10.1007/s11606-007-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen AL, Henriksen DP, Marinakis C, et al. Drug Dosing in Patients with Renal Insufficiency in a Hospital Setting using Electronic Prescribing and Automated Reporting of Estimated Glomerular Filtration Rate. Basic Clin. Pharmacol. Toxicol. 2014 May;114(5):407–413. doi: 10.1111/bcpt.12185. [DOI] [PubMed] [Google Scholar]
- 11.Sellier E, Colombet I, Sabatier B, et al. Effect of alerts for drug dosage adjustment in inpatients with renal insufficiency. J. Am. Med. Inform. Assoc. 2009 Mar-Apr;16(2):203–210. doi: 10.1197/jamia.M2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papaioannou A, Clarke JA, Campbell G, Bedard M. Assessment of adherence to renal dosing guidelines in long-term care facilities. J. Am. Geriatr. Soc. 2000 Nov;48(11):1470–1473. doi: 10.1111/j.1532-5415.2000.tb02639.x. [DOI] [PubMed] [Google Scholar]
- 13.Rahimi AR, Kennedy K, Thomason M, Crumley J, Bugg A, Peacock E. Improper renal dosing in long-term care facilities. South. Med. J. 2008 Aug;101(8):802–805. doi: 10.1097/SMJ.0b013e31817f1f71. [DOI] [PubMed] [Google Scholar]
- 14.Hanlon JT, Wang X, Handler SM, et al. Potentially inappropriate prescribing of primarily renally cleared medications for older veterans affairs nursing home patients. J. Am. Med. Dir. Assoc. 2011 Jun;12(5):377–383. doi: 10.1016/j.jamda.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yap C, Dunham D, Thompson J, Baker D. Medication dosing errors for patients with renal insufficiency in ambulatory care. Jt Comm J Qual Patient Saf. 2005 Sep;31(9):514–521. doi: 10.1016/s1553-7250(05)31066-x. [DOI] [PubMed] [Google Scholar]
- 16.Bhardwaja B, Carroll NM, Raebel MA, et al. Improving prescribing safety in patients with renal insufficiency in the ambulatory setting: the Drug Renal Alert Pharmacy (DRAP) program. Pharmacotherapy. 2011 Apr;31(4):346–356. doi: 10.1592/phco.31.4.346. [DOI] [PubMed] [Google Scholar]
- 17.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch. Intern. Med. 1997;157(21):2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 18.Long CL, Raebel MA, Price DW, Magid DJ. Compliance with dosing guidelines in patients with chronic kidney disease. Ann. Pharmacother. 2004 May;38(5):853–858. doi: 10.1345/aph.1D399. [DOI] [PubMed] [Google Scholar]
- 19.Via-Sosa MA, Lopes N, March M. Effectiveness of a drug dosing service provided by community pharmacists in polymedicated elderly patients with renal impairment--a comparative study. BMC Fam. Pract. 2013;14:96. doi: 10.1186/1471-2296-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erler A, Beyer M, Petersen JJ, et al. How to improve drug dosing for patients with renal impairment in primary care - a cluster-randomized controlled trial. BMC Fam. Pract. 2012;13:91. doi: 10.1186/1471-2296-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens LA, Nolin TD, Richardson MM, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am. J. Kidney Dis. 2009 Jul;54(1):33–42. doi: 10.1053/j.ajkd.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowling TC, Wang ES, Ferrucci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy. 2013 Sep;33(9):912–921. doi: 10.1002/phar.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spruill WJ, Wade WE, Cobb HH., 3rd Comparison of estimated glomerular filtration rate with estimated creatinine clearance in the dosing of drugs requiring adjustments in elderly patients with declining renal function. Am. J. Geriatr. Pharmacother. 2008 Aug;6(3):153–160. doi: 10.1016/j.amjopharm.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 24.VHA Pharmacy Benefits Management Services MAP, VISN Pharmacist Executives, and the Renal Field Advisory Committee and Pathology and Laboratory Medicine Services. Information on the Use of the Modification of Diet in Renal Disease Equation to Estimate Glomerular Filtration Rate and the Cockcroft-Gault Equation to Estimate Creatinine Clearance for Medication Dosing in Patients with Kidney Dysfunction. 2010 May [Google Scholar]
- 25.Lam KD, Miao Y, Steinman MA. Cumulative changes in the use of long-term medications: a measure of prescribing complexity. JAMA Intern Med. 2013 Sep 9;173(16):1546–1547. doi: 10.1001/jamainternmed.2013.7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 27.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann. Intern. Med. 2012 Oct 2;157(7):471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 28.Steinman MA, Lee SJ, John Boscardin W, et al. Patterns of multimorbidity in elderly veterans. J. Am. Geriatr. Soc. 2012 Oct;60(10):1872–1880. doi: 10.1111/j.1532-5415.2012.04158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanlon JT, Aspinall SL, Semla TP, et al. Consensus guidelines for oral dosing of primarily renally cleared medications in older adults. J. Am. Geriatr. Soc. 2009 Feb;57(2):335–340. doi: 10.1111/j.1532-5415.2008.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011 Jun;34(6):1431–1437. doi: 10.2337/dc10-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaur S, Mitchell G, Vitetta L, Roberts MS. Interventions that can reduce inappropriate prescribing in the elderly: a systematic review. Drugs Aging. 2009;26(12):1013–1028. doi: 10.2165/11318890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Terrell KM, Perkins AJ, Dexter PR, Hui SL, Callahan CM, Miller DK. Computerized decision support to reduce potentially inappropriate prescribing to older emergency department patients: a randomized, controlled trial. J. Am. Geriatr. Soc. 2009 Aug;57(8):1388–1394. doi: 10.1111/j.1532-5415.2009.02352.x. [DOI] [PubMed] [Google Scholar]
- 33.Cho I, Slight SP, Nanji KC, Seger DL, Dykes P, Bates DW. Understanding responses to a renal dosing decision support system in primary care. Stud. Health Technol. Inform. 2013;192:931. [PubMed] [Google Scholar]
- 34.Nanji KC, Slight SP, Seger DL, et al. Overrides of medication-related clinical decision support alerts in outpatients. J. Am. Med. Inform. Assoc. 2013 Oct 28; doi: 10.1136/amiajnl-2013-001813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Field TS, Rochon P, Lee M, Gavendo L, Baril JL, Gurwitz JH. Computerized clinical decision support during medication ordering for long-term care residents with renal insufficiency. J. Am. Med. Inform. Assoc. 2009 Jul-Aug;16(4):480–485. doi: 10.1197/jamia.M2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farag A, Garg AX, Li L, Jain AK. Dosing errors in prescribed antibiotics for older persons with CKD: a retrospective time series analysis. Am. J. Kidney Dis. 2014 Mar;63(3):422–428. doi: 10.1053/j.ajkd.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Khanal A, Castelino RL, Peterson GM, Jose MD. Dose adjustment guidelines for medications in patients with renal impairment: how consistent are drug information sources? Intern. Med. J. 2014 Jan;44(1):77–85. doi: 10.1111/imj.12291. [DOI] [PubMed] [Google Scholar]
- 38.Vasisht KP, Chen SC, Peng Y, Bakris GL. Limitations of metformin use in patients with kidney disease: are they warranted? Diabetes Obes Metab. 2010 Dec;12(12):1079–1083. doi: 10.1111/j.1463-1326.2010.01295.x. [DOI] [PubMed] [Google Scholar]
- 39.Herrington WG, Levy JB. Metformin: effective and safe in renal disease? Int. Urol. Nephrol. 2008;40(2):411–417. doi: 10.1007/s11255-008-9371-6. [DOI] [PubMed] [Google Scholar]
- 40.Vazquez-Mellado J, Morales EM, Pacheco-Tena C, Burgos-Vargas R. Relation between adverse events associated with allopurinol and renal function in patients with gout. Ann. Rheum. Dis. 2001 Oct;60(10):981–983. doi: 10.1136/ard.60.10.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao J, Terkeltaub R. A critical reappraisal of allopurinol dosing, safety, and efficacy for hyperuricemia in gout. Curr Rheumatol Rep. 2009 Apr;11(2):135–140. doi: 10.1007/s11926-009-0019-z. [DOI] [PubMed] [Google Scholar]
- 42.Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am. J. Med. 1984 Jan;76(1):47–56. doi: 10.1016/0002-9343(84)90743-5. [DOI] [PubMed] [Google Scholar]
- 43.Fravel MA, Ernst ME. Management of gout in the older adult. Am. J. Geriatr. Pharmacother. 2011 Oct;9(5):271–285. doi: 10.1016/j.amjopharm.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Koch KM, Liu M, Davis IM, Shaw S, Yin Y. Pharmacokinetics and pharmacodynamics of ranitidine in renal impairment. Eur. J. Clin. Pharmacol. 1997;52(3):229–234. doi: 10.1007/s002280050279. [DOI] [PubMed] [Google Scholar]
- 45.McCormack J, Johns K, Tildesley H. Metformin's contraindications should be contraindicated. CMAJ. 2005 Aug 30;173(5):502–504. doi: 10.1503/cmaj.045292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.