Abstract
Purpose
To demonstrate the phase and QSM patterns created by solid and shell spatial distributions of magnetic susceptibility in MS lesions.
Materials and Methods
Numerical simulations and experimental phantoms of solid- and shell-shaped magnetic susceptibility sources were used to generate magnitude, phase, and QSM images. Imaging of 20 consecutive MS patients was also reviewed for this IRB-approved MRI study to identify appearance of solid and shell lesions on phase and QSM images.
Results
Solid and shell susceptibility sources were correctly reconstructed in QSM images, while the corresponding phase images depicted both geometries with shell-like patterns, making the underlying susceptibility distribution difficult to determine using phase alone. In MS patients, of the 60 largest lesions identified on T2, 30 lesions were detected on both QSM and phase, of which 83% were solid and 17% were shells on QSM, and of which 30% were solid and 70% were shell on phase. Of the 21 shell-like lesions on phase, 76% appeared solid on QSM, 24% appeared shell on QSM. Of the five shell-like lesions on QSM, all were shell-like on phase.
Conclusion
QSM accurately depicts both solid and shell patterns of magnetic susceptibility, while phase imaging fails to distinguish them.
Keywords: Quantitative Susceptibility Mapping, Multiple Sclerosis, Phase, Iron
INTRODUCTION
MRI is routinely used to diagnose and monitor multiple sclerosis (MS) patients. Yet white matter lesions seen on conventional MRI sequences correlate only weakly with clinical disability score and are unable to convey the heterogeneous pathology found in MS lesions (1,2). More recently, gradient echo (GRE) MRI has been used to study demyelination (3,4) and iron distribution in multiple sclerosis (MS) lesions (5–8), which both contribute to changes in magnetic susceptibility. As such, the magnetic susceptibility distribution in MS lesions may be useful in monitoring disease progression (4,9).
Typically, GRE data are analyzed using R2*, high-pass-filtered (HPF) phase imaging, and SWI (6,10–14). However, phase, which is well-recognized to show dipole field effects (15), and R2*, which approximately represents the field variation in a voxel, contains contributions from magnetic sources located in the surrounding voxels, making it difficult to interpret a lesion's magnetic susceptibility.
In phase imaging, “nodular” and “ring-like” MS lesions have been described (5,10,12). However, according to Maxwell's equations of electromagnetism (16), both solid (nodules) and shell (rings in 2D section) distributions of magnetic susceptibility can lead to shell-like patterns in phase-based images. This raises concerns about the reliability of phase-based imaging to detect these geometric susceptibility patterns in MS lesions.
Quantitative susceptibility mapping (QSM), which deconvolves the phase images, directly depicts the underlying magnetic susceptibility sources and may more accurately resolve the susceptibility spatial pattern in MS lesions (17). Given recent evidence that the geometry of MS lesions on susceptibility imaging can help identify clinically relevant lesion features (5), our purpose was to investigate differences in the depiction of solid and shell lesions in QSM and phase imaging.
MATERIALS AND METHODS
To characterize the appearance of solid and shell magnetic susceptibility sources on phase images and QSM, we performed numerical and experimental phantom validation, and then reviewed in vivo MS data. To evaluate MRI phase information, we used high-pass filtered (HPF) phase images, the most commonly used phase technique (18).
Data Acquisition
Numerical Phantom
Magnitude, phase, and QSM images for a solid ball and spherical shell of magnetic susceptibility were generated using MATLAB. Three different sets of imaging parameters were used: isotropic parameters (matrix size: 128×128×128; voxel size: 1×1×1mm), experimental phantom parameters (matrix size: 480×64×72; voxel size: 0.5×0.5×0.5mm), and MS patient study parameters (matrix size: 512×512×50, voxel size: 0.47×0.47×3mm). Other parameters were as follows: solid radius: 15mm; shell outer/inner radius: 15mm/13mm; field strength: 3T; TE1/ ΔTE: 5ms/5ms, 5 echoes, background/source magnetic susceptibility: 0.0 ppm/0.1ppm) in a manner similar to previous work (19).
Experimental Phantom
For the solid lesion, a 50 mL plastic tube was filed halfway with 1% agarose and allowed to solidify. A 15 mm plastic ball was then placed on top of the agarose and additional agarose was added to completely cover the ball. The plastic ball was then extracted from the solidified agarose using a blade and tweezers, and 7.5 ug/mL SPIO nanoparticles (20) with 1% agarose was injected in its place. The remainder of the tube was then filled with agarose and allowed to solidify.
To create the shell lesion, a 50 mL plastic tube was filled halfway with 1% agarose and allowed to solidify. A plastic ball was then placed on top of the agarose, was covered with more agarose, and then surgically removed as described above. Before filling the empty space with SPIO, a slightly smaller plastic ball was inserted in the empty space, and the SPIO nanoparticle/agarose mixture was injected surrounding the smaller ball to form a spherical shell of SPIO, which was then allowed to solidify. The smaller ball was then surgically removed, and the shell center, as well as the rest of the tube, was subsequently filled with agarose.
Tubes were scanned using a 3D multi-echo GRE sequence on a 3.0T scanner (GE Medical Systems, Milwaukee, WI) with a single-channel wrist coil (matrix size: 480×64×72, voxel size: 0.5×0.5×0.5mm, 11 echoes, TE1/ ΔTE /TR: 4.6/5/60ms).
In vivo MS data
This retrospective study was approved by our IRB. We reviewed the MRI database in our institution's clinical PACS system and identified 20 consecutive patients who met both of the following criteria: (a) clinically confirmed MS patients; (b) underwent an MR scan including a 3D multiple-echo GRE sequence (matrix size: 512×512×50, voxel size: 0.47×0.47×3mm, 11 echoes, TE1/ ΔTE /TR: 4.5/4.8/57.9ms) as part of the current standard-of-care at our institution between October 1, 2013 and October 20, 2013. MRI was performed on a 3.0T MRI system (SignaHDxt, GE, USA).
All 20 patients (6 male, 14 female, aged 32 - 69, 45.75 ± 10.44 years) were MS patients with expanded disability status scale ranging from 0 to 8 (median: 1.25) and disease duration ranging from 3 to 26 (10.65 ± 6.60) years. All patients in this study have been treated with a standard immunomodulatory therapy (including, interferon beta 1a, glatiramer acetate, fingolimod, natalizumab, rituximab).
Data analysis
For the numerical simulation, experimental phantom and in vivo data, HPF phase images were generated using a 3D high pass filter that was 12×12×12 voxels (18). QSM images were reconstructed from the 3D multi-echo GRE data as follows. A field map was generated by performing a voxel-by-voxel non-linear least squares fitting of the complex signal over TE. Next, the background field (i.e. the magnetic field generated by susceptibility sources outside the region of interest) was removed by applying the Projection onto Dipole Fields (PDF) algorithm (21). Finally, the magnetic field-to-susceptibility-source inverse problem was solved using morphology enabled dipole inversion (MEDI), which uses a regularization term that promotes similarity of edges between the QSM and the magnitude image (17, 22-24).
For the experimental phantom results, a neuroradiologist with 8 years of experience (A.G.) categorized the lesions on HPF phase and QSM as either “solid” or “shell”.
For the in vivo MS cases, the neuroradiologist first identified the three largest separate lesions on T2-weighted images based on visible hyperintensity. He then characterized the morphology of each lesion as “solid”, “shell” or “not seen” on the HPF phase and QSM images. To reduce the risk of bias, the neuroradiologist classified MS lesions on the HPF phase and QSM images in two different sessions separated by several weeks.
If a lesion was visualized on phase but not on QSM, neighboring slices were examined to investigate whether the signal on phase was really the magnetic field generated by a nearby susceptibility source, rather than being a manifestation of the MS lesion seen on T2.
Statistical Analysis
For the solid and shell numerical simulations, a Pearson's correlation coefficient was calculated between the middle axial slice of true lesion and the QSM reconstruction, as well as between the middle axial slice true lesion and the HPF phase reconstruction.
For the phantom and MS data, the percentage of HPF phase and QSM lesions designated as solid and shell by the neuroradiologist were calculated and compared.
RESULTS
The results of the numerical simulations are shown in Figure 1. The Pearson's correlation coefficients between the true solid and shell lesions and their respective QSM reconstructions were greater than 0.99. The correlation coefficient between the true shell lesion and its HPF phase image was 0.91, and the correlation coefficient between the solid lesion and its HPF phase image was 0.49. This demonstrates that shell lesions are accurately depicted on both phase and QSM, while solid lesions are more accurately depicted on QSM than on phase.
Figure 1.
Numerical simulations of solid and shell lesions using isotropic, experimental phantom, and MS patient study parameters. For the solid lesions, (a) (e) and (i) show T2*-weighted, HPF phase, and QSM images in axial view with corresponding coronal sections in (b) and (f). For the shell lesions, (c) (g) and (j) show T2*-weighted, HPF phase, and QSM images in axial view with corresponding coronal sections in (d) and (h). Coronals are not shown for MS patient parameter simulations since MS patient data was analyzed in axial view only).
The results of the SPIO phantom experiments are shown in Figure 2. The neuroradiologist, blinded to true lesion geometry, correctly identified the solid and shell lesions on QSM images, but labeled both the solid and shell lesions as “shell-like” on HPF phase images. Therefore, on phase images, both solid and shell sources of magnetic susceptibility appeared shell-like and were difficult to distinguish. The QSM images correctly depicted the underlying geometry, thus enabling the differentiation of solid and shell sources of magnetic susceptibility.
Figure 2.
SPIO solid and shell phantom lesions. (a) T2*-weighted, HPF phase, and QSM images of solid lesion in axial view with corresponding coronal sections in (b); (c) T2*-weighted, HPF phase, and QSM images of shell lesion in axial view with corresponding coronal sections in (d).
Of the 60 lesions identified in the 20 MS subjects in the T2 weighted images, 30 lesions were detected on both phase and QSM, of which 25 (83%) were solids and 5 (17%) were shells on QSM, and of which 9 (30%) were solids and 21 (70%) were shells on phase. Table 1 presents the morphological characteristics of all lesions on phase and QSM. The long axis of the lesions ranged from 0.23 to 2.1 cm (5 to 45 voxels). Representative lesion images are shown in Figure 3. Of the 9 lesions that appeared solid on phase, 100 percent appeared as solid on QSM. Of the 21 lesions that appeared as shells on phase, 16 (76%) appeared as solid on QSM, and 5 (24%) appeared as shell on QSM. Of the 5 lesions that appeared as shells on QSM, all 5 (100%) appeared as shells on phase.
TABLE 1.
Morphological characteristics of the 60 MS lesions identified on T2-weighted images.
| Phase | QSM | No. of Lesions |
|---|---|---|
| Shell | Shell | 5 |
| Shell | Solid | 16 |
| Solid | Solid | 9 |
| Not visible | Not visible | 15 |
| Shell | Not visible | 2 |
| Solid | Not visible | 1 |
| Not visible | Shell | 2 |
| Not visible | Solid | 10 |
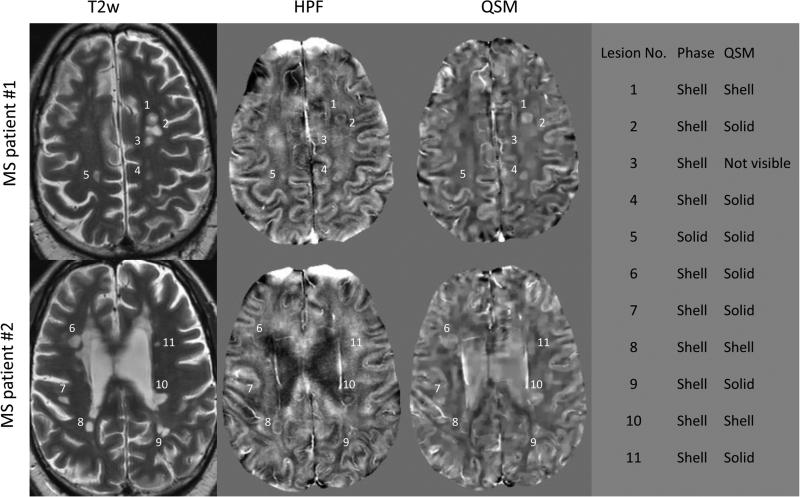
Figure 3.
Representative lesions in two MS patients demonstrate that shell appearance on HPF phase imaging can correspond to either solid (e.g. lesion 4) or shell (e.g. lesion 8) on QSM. On the right, lesions visible on T2w are numbered and their geometrical appearances on HPF phase and QSM are listed.
For the two MS lesions that were identified on phase but not on QSM, examination of neighboring QSM slices revealed the presence of veins, suggesting that the shell-like structures identified on HPF phase could be a manifestation of the magnetic field generated by the nearby venous susceptibility sources, rather than being a manifestation of the lesions seen on T2 at that location (see Figure 4).
Figure 4.
Veins adjacent to MS lesions may cause non-local signal changes on HPF phase, complicating the interpretation of MS lesion-generated signal. For instance, the MS lesion identified on slice A of T2w (white arrow) appears shell-like on HPF phase but is not visible on QSM. However, inspection of neighboring slice B reveals an adjacent vein on HPF phase and QSM (black arrowhead) which may have generated the HPF phase signal change on slice A.
DISCUSSION
Our results indicate that QSM can more accurately distinguish between solid and shell patterns of magnetic susceptibility compared to phase imaging. Shell-shaped lesions on phase can result from either solid or shell susceptibility sources. These results are consistent with the physics theory, and suggest that QSM better depicts spatial susceptibility patterns in MS lesions compared to phase-based imaging.
Conventional MRI sequences can identify white matter lesions, but reveal little about underlying pathophysiological processes, such as iron deposition, macrophage/microglia activation patterns, and demyelination (1). A lesion's magnetic susceptibility distribution signature however, may contain this clinically useful information, and could potentially be used both to distinguish different pathological stages of lesion genesis as well as to investigate factors that influence overall recovery of an MS lesion (2,3). For instance, a shell-shaped susceptibility distribution may signify the presence of iron-containing macrophages at the rim of a chronic active lesion (5–7,25). A solid-shaped susceptibility distribution, depending on its magnetic susceptibility value, could denote either extensive demyelination (and lack of iron) as found in chronic, inactive lesions (25), or could represent the uniform distribution of cellular or extracellular sources of iron (7). Further investigation is needed to refine our understanding of the relationship between lesion susceptibility geometry and the pathological significance, but early studies already highlight the importance of differentiating between solid and shell lesion geometries.
The veins identified on slices adjacent to the two MS lesions that were shell-like on phase but not visible on QSM underscores that shell-like structures on HPF phase may represent the magnetic field generated by nearby veins, rather than being manifestations of the tissue at that point in space. These HPF “false positives” further highlight the pitfalls of using phase imaging, which depicts the magnetic field generated by nearby susceptibility sources, rather than QSM, which depicts the geometry of the susceptibility sources themselves. T2 lesions not detected on QSM are likely old (>7 years) chronic lesions (9).
According to the Maxwell's equations, each source of magnetic susceptibility generates a magnetic field that changes rapidly in the vicinity of that source and extends beyond it (16). The phase of an MR image is proportional to this magnetic field. The large changes in the magnetic field in the vicinity of the source create the shell-like artifacts demonstrated in the phase images of the numerical simulations and phantom experiments.
A few limitations should be considered. First, our study lacked histological correlates for the MS patient data; “ground truth” geometries were not available for our in vivo analysis. The physics of magnetic susceptibility and field is well established by Maxwell's equations, which lends support for the interpretation of our in vivo data. Second, the 3 mm slice thickness of the MS data did not permit examination of very small lesions, which may have different phase behavior due to averaging. These very small lesions, which could indicate new disease activity, are an important area of future study. Still, a wide range of lesion sizes were evaluated. Some subjects had larger lesions than others, and so by choosing the three largest lesions in each subject, lesion sizes ranged from 0.23 to 2.1 cm. Third, the classification of spatial distribution patterns into either solids or shell shapes on MRI may be subject to interpretive differences between readers. Thus, future studies should assess inter-observer variability in interpretation, especially as future work elucidates the significance of shell and solid susceptibility geometries in the pathophysiology of MS lesions. Third, it is important to note that a QSM voxel reports the sum of all enclosed susceptibility sources, which, in our clinical cases, includes not only paramagnetic iron but diamagnetic myelin as well. To isolate the iron content of MS lesions, the myelin contribution must be removed using for example myelin mapping (26,27) or ultra-short TE imaging (28–30).
However, it is important to note that only a subset of MS lesions visible on T2 were seen on phase or QSM. This is consistent with previous literature (9), which has found that very early active and old chronic inactive MS lesions often lack susceptibility-based contrast and are not visible on phase or QSM.
In conclusion, QSM can discriminate between solid and shell patterns of magnetic susceptibility with greater accuracy than phase imaging. Given evidence of the importance of susceptibility geometry in characterizing clinically relevant characteristics of MS lesions, QSM should be included in future MRI studies of susceptibility in MS.
Acknowledgments
Grant Support
This research was supported in part by R01EB013443 and R01NS072370.
REFERENCES
- 1.Poloni G, Minagar A, Haacke EM, Zivadinov R. Recent developments in imaging of multiple sclerosis. [2014 Apr 11];Neurologist [Internet] 2011 Jul;17(4):185–204. doi: 10.1097/NRL.0b013e31821a2643. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21712664. [DOI] [PubMed] [Google Scholar]
- 2.Zivadinov R, Leist TP. Clinical-magnetic resonance imaging correlations in multiple sclerosis. [2014 Apr 11];J. Neuroimaging [Internet] 2005 Jan;15(4 Suppl):10S–21S. doi: 10.1177/1051228405283291. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16385015. [DOI] [PubMed] [Google Scholar]
- 3.Yablonskiy DA, Luo J, Sukstanskii AL, Iyer A, Cross AH. Biophysical mechanisms of MRI signal frequency contrast in multiple sclerosis. [2014 Apr 2];Proc. Natl. Acad. Sci. U. S. A. [Internet] 2012 Aug 28;109(35):14212–7. doi: 10.1073/pnas.1206037109. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3435153&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiggermann V, Hernández Torres E, Vavasour IM, Moore GRW, Laule C, MacKay AL, et al. Magnetic resonance frequency shifts during acute MS lesion formation. [2014 Apr 8];Neurology [Internet] 2013 Jul 16;81(3):211–8. doi: 10.1212/WNL.0b013e31829bfd63. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3770162&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta V, Pei W, Yang G, Li S, Swamy E, Boster A, et al. Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. [2013 Dec 10];PLoS One [Internet] 2013 Jan;8(3):e57573. doi: 10.1371/journal.pone.0057573. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3597727&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnato F, Hametner S, Yao B, van Gelderen P, Merkle H, Cantor FK, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. [2013 Nov 11];Brain [Internet] 2011 Dec;134(Pt 12):3602–15. doi: 10.1093/brain/awr278. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3235560&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hametner S, Wimmer I, Haider L, Pfeifenbring S, Brück W, Lassmann H. Iron and neurodegeneration in the multiple sclerosis brain. [2013 Nov 18];Ann. Neurol. [Internet] 2013 Jul 19; doi: 10.1002/ana.23974. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23868451. [DOI] [PMC free article] [PubMed]
- 8.Bagnato F, Hametner S, Welch EB. Visualizing iron in multiple sclerosis. [2013 Dec 2];Magn. Reson. Imaging [Internet] 2013 Apr;31(3):376–84. doi: 10.1016/j.mri.2012.11.011. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23347601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Gauthier SA, Gupta A, Comunale J, Liu T, Wang S, et al. Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. [2014 Mar 27];Radiology [Internet] 2014 Apr;271(1):183–92. doi: 10.1148/radiol.13130353. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24475808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bian W, Harter K, Hammond-Rosenbluth KE, Lupo JM, Xu D, Kelley DA, et al. A serial in vivo 7T magnetic resonance phase imaging study of white matter lesions in multiple sclerosis. [2013 Dec 10];Mult. Scler. [Internet] 2013 Jan;19(1):69–75. doi: 10.1177/1352458512447870. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22641301. [DOI] [PubMed] [Google Scholar]
- 11.Deistung A, Schäfer A, Schweser F, Biedermann U, Turner R, Reichenbach JR. Toward in vivo histology: a comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. [2013 Nov 7];Neuroimage [Internet] 2013 Jan 15;65:299–314. doi: 10.1016/j.neuroimage.2012.09.055. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23036448. [DOI] [PubMed] [Google Scholar]
- 12.Haacke EM, Makki M, Ge Y, Maheshwari M, Sehgal V, Hu J, et al. Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. [2013 Dec 8];J. Magn. Reson. Imaging [Internet] 2009 Mar;29(3):537–44. doi: 10.1002/jmri.21676. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2650739&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagemeier J, Heininen-Brown M, Poloni GU, Bergsland N, Magnano CR, Durfee J, et al. Iron deposition in multiple sclerosis lesions measured by susceptibility-weighted imaging filtered phase: a case control study. [2013 Dec 10];J. Magn. Reson. Imaging [Internet] 2012 Jul;36(1):73–83. doi: 10.1002/jmri.23603. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22407571. [DOI] [PubMed] [Google Scholar]
- 14.Walsh AJ, Lebel RM, Eissa A, Blevins G, Catz I, Lu J-Q, et al. Multiple sclerosis: validation of MR imaging for quantification and detection of iron. [2013 Dec 10];Radiology [Internet] 2013 May;267(2):531–42. doi: 10.1148/radiol.12120863. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23297322. [DOI] [PubMed] [Google Scholar]
- 15.Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic Resonance Imaging: Physical principles and sequence design. John Wiley & Sons; United States of American: 1999. [Google Scholar]
- 16.Jackson J. Classical Electrodynamics Third Edition. 3rd Edition ed. John Wiley & Sons; United States of America: 1998. [Google Scholar]
- 17.Liu T, Wisnieff C, Lou M, Chen W, Spincemaille P, Wang Y. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping. [2013 Nov 8];Magn. Reson. Med. [Internet] 2013 Feb;69(2):467–76. doi: 10.1002/mrm.24272. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22488774. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Yu Y, Li D, Bae KT, Brown JJ, Lin W, et al. Artery and vein separation using susceptibility-dependent phase in contrast-enhanced MRA. [2014 Apr 9];J. Magn. Reson. Imaging [Internet] 2000 Nov;12(5):661–70. doi: 10.1002/1522-2586(200011)12:5<661::aid-jmri2>3.0.co;2-l. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11050635. [DOI] [PubMed] [Google Scholar]
- 19.Salomir R, de Senneville BD, Moonen CT. A fast calculation method for magnetic field inhomogeneity due to an arbitrary distribution of bulk susceptibility. [2014 May 29];Concepts Magn. Reson. [Internet] 2003 Oct 13;19B(1):26–34. Available from: http://doi.wiley.com/10.1002/cmr.b.10083. [Google Scholar]
- 20.Wong R, Chen X, Wang Y, Hu X, Jin MM. Visualizing and quantifying acute inflammation using ICAM-1 specific nanoparticles and MRI quantitative susceptibility mapping. [2013 Dec 9];Ann. Biomed. Eng. [Internet] 2012 Jun;40(6):1328–38. doi: 10.1007/s10439-011-0482-3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22143599. [DOI] [PubMed] [Google Scholar]
- 21.Liu T, Khalidov I, de Rochefort L, Spincemaille P, Liu J, Tsiouris AJ, et al. A novel background field removal method for MRI using projection onto dipole fields (PDF). [2014 Feb 22];NMR Biomed. [Internet] 2011 Nov;24(9):1129–36. doi: 10.1002/nbm.1670. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3628923&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kressler B, de Rochefort L, Liu T, Spincemaille P, Jiang Q, Wang Y. Nonlinear regularization for per voxel estimation of magnetic susceptibility distributions from MRI field maps. [2014 May 30];IEEE Trans. Med. Imaging [Internet] 2010 Mar;29(2):273–81. doi: 10.1109/TMI.2009.2023787. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2874210&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V, et al. Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: validation and application to brain imaging. [2014 May 30];Magn. Reson. Med. [Internet] 2010 Jan;63(1):194–206. doi: 10.1002/mrm.22187. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19953507. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. [2014 May 30];Neuroimage [Internet] 2012 Mar 1;59(3):2560–8. doi: 10.1016/j.neuroimage.2011.08.082. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3254812&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lassmann H. Review: the architecture of inflammatory demyelinating lesions: implications for studies on pathogenesis. [2014 Apr 11];Neuropathol. Appl. Neurobiol. [Internet] 2011 Dec;37(7):698–710. doi: 10.1111/j.1365-2990.2011.01189.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21696413. [DOI] [PubMed] [Google Scholar]
- 26.Shen X, Nguyen TD, Gauthier SA, Raj A. Robust myelin quantitative imaging from multi-echo T2 MRI using edge preserving spatial priors. [2014 Apr 9];Med. Image Comput. Comput. Assist. Interv. [Internet] 2013 Jan;16(Pt 1):622–30. doi: 10.1007/978-3-642-40811-3_78. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24505719. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen TD, Wisnieff C, Cooper MA, Kumar D, Raj A, Spincemaille P, et al. T2 prep three-dimensional spiral imaging with efficient whole brain coverage for myelin water quantification at 1.5 tesla. [2014 Apr 9];Magn. Reson. Med. [Internet] 2012 Mar;67(3):614–21. doi: 10.1002/mrm.24128. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22344579. [DOI] [PubMed] [Google Scholar]
- 28.Du J, Ma G, Li S, Carl M, Szeverenyi NM, VandenBerg S, et al. Ultrashort echo time (UTE) magnetic resonance imaging of the short T2 components in white matter of the brain using a clinical 3T scanner. [2014 Apr 1];Neuroimage [Internet] 2014 Feb 15;87:32–41. doi: 10.1016/j.neuroimage.2013.10.053. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24188809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horch RA, Gore JC, Does MD. Origins of the ultrashort-T2 1H NMR signals in myelinated nerve: a direct measure of myelin content? [2014 Apr 9];Magn. Reson. Med. [Internet] 2011 Jul;66(1):24–31. doi: 10.1002/mrm.22980. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3120910&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilhelm MJ, Ong HH, Wehrli SL, Li C, Tsai P-H, Hackney DB, et al. Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. [2014 Apr 9];Proc. Natl. Acad. Sci. U. S. A. [Internet] 2012 Jun 12;109(24):9605–10. doi: 10.1073/pnas.1115107109. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3386098&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]