Summary
The diversity of airborne microorganisms that potentially reach aquatic ecosystems during rain events is poorly explored. Here, we used a culture-independent approach to characterize bacterial assemblages during rain events with and without Saharan dust influence arriving to a high mountain lake in the Austrian Alps. Bacterial assemblage composition differed significantly between samples with and without Saharan dust influence. Although alpha diversity indices were within the same range in both sample categories, rain events with Atlantic or continental origins were dominated by Betaproteobacteria, whereas those with Saharan dust intrusions were dominated by Gammaproteobacteria. The high diversity and evenness observed in all samples suggests that different sources of bacteria contributed to the airborne assemblage collected at the lake shore. During experiments with bacterial assemblages collected during rain events with Saharan dust influence, cell numbers rapidly increased in sterile lake water from initially ~3 × 103 cell ml−1 to 3.6–11.1 × 105 cells ml−1 within 4–5 days, and initially, rare taxa dominated at the end of the experiment. Our study documents the dispersal of viable bacteria associated to Saharan dust intrusions travelling northwards as far as 47° latitude.
Introduction
Because of their small size and large numbers, microbes can be easily dispersed among distant habitats, thus potentially influencing local community composition in recipient ecosystems. Besides the transport of microbes by animals, aeolian dust particles act as a long-range vector for large numbers of microorganisms (Kellogg and Griffin, 2006; Hervas et al., 2009; Yamaguchi et al., 2012). Strong winds over arid lands can lift particles above the boundary layer and up to altitudes well above 7000 m (Kaspari et al., 2009). Along with organic and inorganic nutrients bound to dust minerogenic particles (Psenner, 1999), microorganisms are mobilized from the arid soils and transported over long distances (Griffin et al., 2003; Prospero et al., 2005; Mladenov et al., 2011). One of the most important worldwide sources of dust is the Sahara– Sahel region in northern Africa (Moulin et al., 1997).
The distance and trajectory of aeolian dust particles and associated microbes depend on factors such as wind speed and land topography (Womack et al., 2010). During long-distance transport at high altitudes, microorganisms are potentially exposed to harsh conditions such as desiccation that may act as a strong selective force (Smith et al., 2011). Soil-derived microbes might not be well adapted to such conditions, but spores (dormant cells with a minimum of metabolic activity) are the most likely survivors of long-distance transport in dust clouds. Interestingly, many bacterial species unknown to form spores have been observed to reach distant habitats in a viable stage (Griffin, 2007; Hervas et al., 2009).
Precipitation facilitates the deposition of microbes into new habitats, as many cells from an air mass or cloud are probably washed out at the same time. Although the scavenging efficiency of aerosols by precipitation is size-dependent (Volken and Schumann, 1993), airborne bacteria are usually associated to particles of larger size (Shaffer and Lighthart, 1997; Polymenakou et al., 2008) that facilitates their transport to the Earth’s surface. Thus, during a rain event, microorganisms will be able to reach a recipient aquatic habitat. The long-distance atmospheric transport of bacteria and the ecological consequences for recipient aquatic ecosystems, particularly for freshwaters, have long been overlooked (Kellogg and Griffin, 2006). More recently, however, changes in bacterial community composition of the water surface microlayer of high mountain lakes during Saharan dust intrusion events have been documented (Hervas and Casamayor, 2009; Hervas et al., 2009; Hörtnagl et al., 2010). Saharan dust intrusion events have also a strong effect on bacterial productivity via the deposition of inorganic nutrients and organic carbon (Aymoz et al., 2004; Mladenov et al., 2008; Pulido-Villena et al., 2008; Reche et al., 2009).
Here, we used a cultivation-independent approach to characterize bacterial assemblages deposited during rain events at a high mountain lake located in the Austrian Alps. We used satellite-based tracking of air masses to verify that three rain events were influenced by Saharan dust intrusions, whereas three other events had continental or oceanic origins. We hypothesized that the different origins and the conditions during transport are reflected in composition and diversity of airborne bacteria, and that harsh environmental conditions during high-altitude, long-range dispersal will act as a selective force. We further anticipated that particle-associated airborne bacteria represent a significant source of microbes for high mountain lakes. To assess viability, we conducted experiments where dust-associated bacteria were allowed to grow in sterilized lake water, and then assessed changes within the assemblage.
Results and discussion
Source of air masses and chemical characterization of rainwater
Backwards modelled trajectories of the air masses contributing to wet deposition at the high mountain (2417 m above sea level) lake Gossenköllesee (Supporting Information Fig. S1) revealed that three events originated either from oceanic or continental sources and were unaffected by Saharan dust plumes, whereas three other events can be traced back to northern Africa. Saharan dust plumes typically arrived within 3–4 days to the Austrian Alps, most of the time residing between 500 and 2000 m above ground level. In some cases, lower air masses (500 m above ground level) had followed different trajectories as compared with higher air masses (2000 m above ground level). Anyway, rain water chemistry clearly indicated the Sahar dust influence in three events (e.g. the high concentration of major ions such as Ca2+, Mg2+ and K+ (Supporting Information Table S1, Morales-Baquero et al., 2013). However, one needs to keep in mind that the rainwater integrated over different airmasses and hence reflects a mixture of different source locations.
The dust events differed in dust loads, as can be seen from the Absorbing Aerosol Index (Supporting Information Fig. S1) and from the dust concentration measured in rainwater samples (Supporting Information Table S1). During Saharan dust intrusions, between 0.014 and 0.146 g dust m−2 day−1 were deposited, whereas no measurable dust was deposited during rain events with continental or marine origin. In addition to the different concentration of major ions, rainwater of Atlantic or European origin typically had slightly acidic pH values ranging between 5.79 and 6.05, whereas rainwater collected during Saharan dust intrusions was less acidic with pH ranging between 6.15 and 6.83 (Supporting Information Table S1). The concentration of water-soluble organic carbon (WSOC) in rainwater was usually higher (range 1.82–5.43 μg C m−2 day−1) during Saharan dust intrusions than during rain events from other origins (range 1.71–3.71 μg C m−2 d−1). Absorbance slope ratios of WSOC, used as proxy for the dominant molecular weight, were higher in samples collected during Saharan dust intrusions than in those collected whithout dust influence; the differences, however, were not statistically significant (Supporting Information Fig. S2, t-test, P = 0.25). Nevertheless, a shift towards greater molecular masses in rainwater with Saharan dust influence suggests the terrestrial origin of this carbon (Supporting Information Fig. S2). Using optical properties of water-soluble organic compounds, Mladenov and colleagues (2009) could trace organic carbon associated to Saharan dust in high mountain lakes in southern Spain. The chemical characterization of rainwater enriched with Saharan dust also revealed a substantial load of inorganic nutrients such as N and P (Supporting Information Table S1, Supporting Information Fig. S2), which, together with organic carbon, might foster bacterial growth in the recipient ecosystem. For example, rainfall during a Saharan dust event delivered up to 10-fold more phosphorus than rain without Saharan dust influence (range 32.6 to 141.0 μg P m−2 day−1 as compared with 4.3–8.8 μg P m−2 day−1). High mountain lakes are typically oligotrophic ecosystems and receive little nutrients from sparsely vegetated catchments with thin soil cover (Sommaruga, 2001). Hence, Saharan dust might act as a ‘fertilizer’ in high mountain lakes (Psenner, 1999) and can also support the accompanying bacterial community (Reche et al., 2009).
Bacteria in rain events
Several recent publications have shown that Saharan dust particles act as vectors for viable bacterial cells and might constitute an immigration source to high mountain lakes in the Sierra Nevada (Spain) (Reche et al., 2009), the Spanish Pyrenees (Hervas et al., 2009; Vila-Costa et al., 2013) and the French Alps (Chuvochina et al., 2011). Our study expands the maximum distance that bacteria associated to Saharan dust particles can travel northwards to high mountain lakes located as far as 47° latitude, covering a distance of roughly 2500 km.
Bacterial cell deposition rates varied substantially between individual sampling occasions, with no significant difference between samples with and without Sahara dust influence (t-test, P = 0.43, Supporting Information Table S1). We obtained 573 good quality 16S rRNA sequences from clone libraries, which clustered into 136 operational taxonomic units (OTUs) at a 97% sequence similarity level, comprising 37 taxa in 16 different classes of bacteria. The absence of saturation in rarefaction curves (Supporting Information Fig. S3) indicated that not the entire diversity in rainwater samples was sampled. Conservative estimates of bacterial OTU numbers resulted in most cases in ca. 100 OTUs per sample, maximum values exceeded 200 OTUs in rain samples without Saharan dust influence. This is within the same range as next-generation sequencing-based estimates from airborne bacterial communities in Denver, CO, USA (Bowers et al., 2013) and cultivation-dependent estimates of bacteria diversity on African dust (see table 1 in Kellogg and Griffin, 2006).
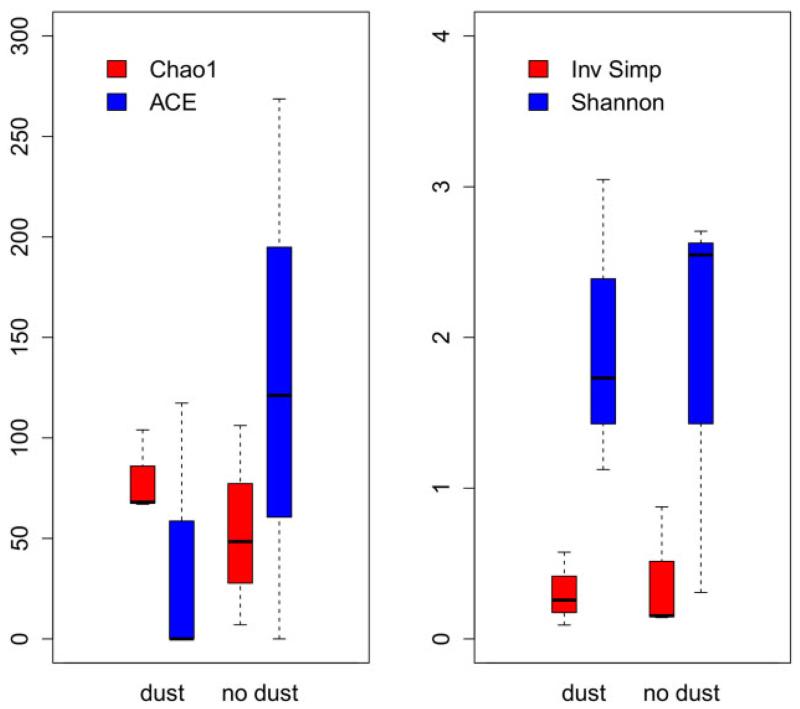
Estimators of alpha diversity were used to assess differences in bacterial community structure between rain events with and without Saharan dust influence. Chao1 and ACE (abundance-based coverage estimator), which are particularly useful in comparing communities with skewed abundance distributions, were within the same range in both rain event categories (Fig. 1). Inverse Simpson and Shannon diversity indices, which both consider evenness within communities, were within similar ranges, however with generally lower values in rain events with Saharan dust influence. Such evenness is rather untypical for microbial communities, which are usually dominated by a few abundant taxa and otherwise comprised by many rare taxa. In combination, the high diversity and high evenness might reflect a rather stochastic long-distance transport of bacteria with rain and dust clouds, suggesting that bacteria from different sources such as arid soils in northern Africa, Mediterranean surface seawater, and local soils or freshwaters might contribute to the airborne community. These different source communities might mix in the air masses and hence form a diverse source community for recipient ecosystems. This contrasts with near-surface airborne bacterial communities that represent the local terrestrial environment even at high altitudes (Bowers et al., 2012). Nevertheless, because of the remote location of the alpine lake, samples were collected for several days with rain events. Therefore, dry deposition of local airborne bacteria can also be assumed to have contributed to the collected bacterial diversity.
Fig. 1.
Box and whiskers representation of indices used to describe bacterial community diversity in samples with and without Sahara dust influence. The box indicates median and quartile values, and the whiskers indicate the range (minima and maxima).
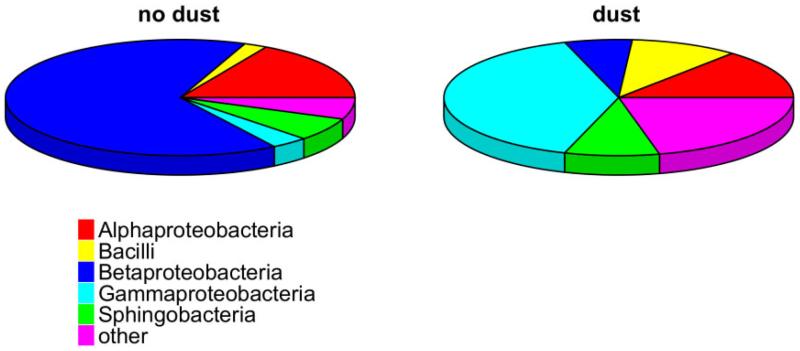
The bacterial assemblage composition differed significantly between rain events with and without Saharan dust influence (Libshuff scores: 0.0026 and 0.0037, P < 0.001). Rain events with Atlantic or central European origin were dominated by Betaproteobacteria of the genera Massilia and Sphingobacteria of the genera Hymenobacter (Fig. 2). Moreover, Sphingomonas, Acidisphaera and Oxalicibacterium were mainly found in those samples. One sample collected during Saharan intrusions (11 September) was dominated surprisingly by the gammaproteobacterial taxa Beggiatoa. Massilia dominated also in two out three rain events with Saharan dust influence. At the OTU level (Fig. 3), alphaproteobacterial and gammaproteobacterial taxa were either present in both assemblages or exclusively found in rainwater samples without Saharan dust influence, whereas several different taxa of Bacilli were exclusively retrieved from rainwater samples influenced by Saharan dust. Gammaproteobacteria and a relative smaller contribution of Betaproteobacteria characterized Saharan dust samples. Gammaproteobacteria are generally more abundant in saline environments and soils than in freshwaters. Newton and colleagues (2011) pointed out that Gammaproteobacteria found in freshwater lake ecosystems appear to be transient ‘tourists’. Betaproteobacteria have been reported to be particle-associated in limnetic environments (Weiss et al., 1996; Lemarchand et al., 2006) and efficient attachment to particles might also play an important role in their transport.
Fig. 2.
Pie charts showing the relative contribution of common bacterial classes to overall diversity in rain samples with and without Sahara dust influence. Colours indicate operational taxonomic unit class affiliation.
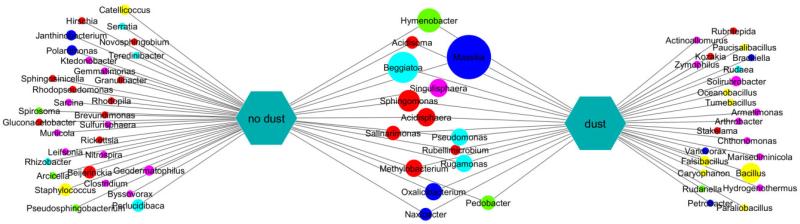
Fig. 3.
Operational taxonomic unit (OTU) level representation of bacterial sequences retrieved from samples collected during rain events with and without Saharan dust intrusions. Circles represent OTUs, and lines indicate the occurrence of an OTU in the respective samples. Circle size reflects log-transformed sequence abundance, while colour indicates taxonomic affiliation (see Fig. 2 for key). While several abundant sequences were found in samples with and without Saharan dust influence, Bacillus, Falsibacillus and Solorubrobacter were exclusively found during Saharan dust intrusions and dominated these samples. The diversity of OTUs retrieved exclusively during rain events without dust appears larger, and no taxon was distinctively dominant in those samples.
Owing to the small cell size and ability to form spores of Actinobacteria (Warnecke et al., 2005), they might be expected to be effectively dispersed with rain and dust clouds. Newton and colleagues (2011) suggested that aerial dispersal could explain their ubiquitous occurrence in lake ecosystems. Indeed, we identified eight actinobacterial sequences in our clone library, three in rain samples without and five in samples collected during Saharan dust influence. In addition, one actinobacterial sequence was retrieved from the growth experiment. Spore-forming Bacilli of terrestrial origin have also been detected in the upper atmosphere (Griffin, 2004; Kellogg and Griffin, 2006), and different Bacillus taxa were retrieved mainly in dust-influenced samples.
During the transport in the atmosphere, the predominantly soil-derived bacteria are exposed to harsh environmental conditions that might act as an efficient dispersal barrier (Smith et al., 2011). How well microbial cells are protected, for example, from UV radiation, desiccation or freezing within clouds is not yet explored, although desert dust has been shown to reduce UV irradiance by more than 50% (Herman et al., 1999). The molecular mechanisms that enable bacterial populations to withstand the conditions during transport, as well as the ecological and evolutionary benefits of such mechanisms for soil-derived bacteria, also remain to be explored. However, the accumulation of photoprotecting pigments (Tong and Lighthart, 1997) and variations in the GC content of DNA have been proposed as mechanisms to minimize UV damage in bacteria (Singer and Ames, 1970; Matallana-Surget et al., 2008). In fact, approximately 20% of the particles in the diameter range between 0.25 and 1 μm have been identified as viable bacterial cells in the upper troposphere (DeLeon-Rodriguez et al., 2013). Spore formation in soil-derived bacteria, a microbial bet-hedging strategy (Jones and Lennon, 2010), might be another way to cope with harsh conditions during airborne long-distance dispersal. Therefore, different strategies might favour the growth of certain bacterial taxa as compared with more sensitive ones.
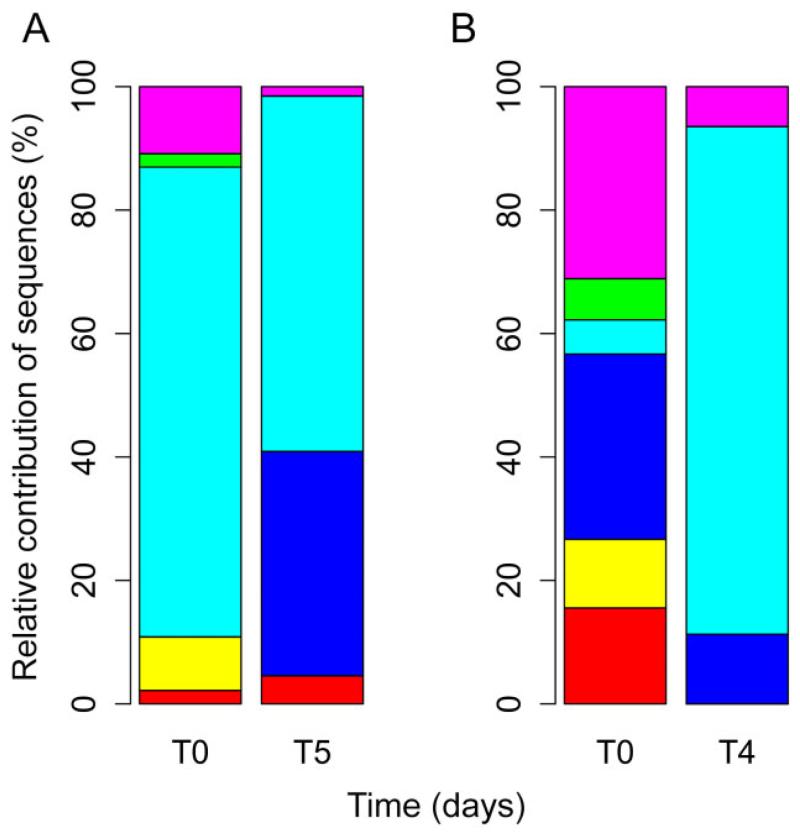
Anyway, the harsh environmental conditions during transport likely act as an environmental filter, and many cells might not arrive at the recipient ecosystem in a viable state. Therefore, even though the diversity in samples with and without Saharan dust was surprisingly high, only few specialized taxa are potentially able to grow in high mountain lakes. In our experiments, we found that several taxa were viable in lake water after being transported in Saharan dust plumes. Cell numbers during the two experiments with rainwater collected during Saharan dust intrusions and inoculated into sterile lake water rapidly increased from initially ~3 × 103 to 3.6–11.1 × 105 cells ml−1 within 4–5 days. Initially, abundant taxa such as the gammaproteobacteria Beggiatoa, Massilia and Bacillus were found only in low abundances in clone libraries at the end of the experiment. However, Pseudomonas-like OTUs were able to grow successfully under freshwater conditions in our experiments. Betaproteobacteria, in contrast, are common and abundant in many freshwater habitats, and the betaproteobacterial taxon Janthinobacterium, despite its absence from dust influenced samples, was able to increase in relative abundance when grown under freshwater conditions (Fig. 4). Hence, Janthinobacterium might be attributed to rainwater with rapid growth under freshwater conditions. On the other hand, the fact that taxa with low relative abundance in the original samples dominated the freshwater assemblages indicates the potential importance of rare taxa for long-distance dispersal.
Fig. 4.
Relative contribution of phylogenetic classes to bacterial diversity of samples prior (T0) and at the end (T4, T5, respectively) of the re-growth experiments conducted with Sahara dust influenced rainwater in September (A) and October (B) 2008. See Fig. 2 for colour key.
In conclusion, we found that Saharan dust provide considerable concentrations of nutrients and organic carbon, which might support the growth of the transported microbial assemblage. Saharan dust plumes deliver significantly different bacterial assemblages to distant high mountain lakes than rain events with other origins. Our experiment indicated that certain taxa have the potential to establish viable populations in the recipient lake, and therefore, particle-associated airborne dispersal might be an important source for the rare freshwater bacterial biosphere. However, observations providing deeper insights in the diversity of rare and abundant taxa and experiments designed to address the competing role of established natural bacterial community remain to be conducted in order to assert positive colonization by airborne bacteria.
Supplementary Material
Acknowledgements
We thank Roland Psenner for useful comments, the NOAA Air Resources Laboratory (ARL) for the provision of the HYSPLIT transport and dispersion model (http://www.ready.noaa.gov) used in this publication, J. Franzoi and Gry Larssen for the chemical analyses, and Michael Larentis for helping with cloning. This work was supported by the Austrian Science Fund (FWF) through research projects P19245-BO3 and P24442-B25 to R. Sommaruga.
Footnotes
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
References
- Aymoz G, Jaffrezo JL, Jacob V, Colomb A, George C. Evolution of organic and inorganic components of aerosol during a Saharan dust episode observed in the French Alps. Atmos Chem Phys. 2004;4:2499–2512. [Google Scholar]
- Bowers RM, McCubbin IB, Hallar AG, Fierer N. Seasonal variability in airborne bacterial communities at a high-elevation site. Atmos Environ. 2012;50:41–49. [Google Scholar]
- Bowers RM, Clements N, Emerson JB, Wiedinmyer C, Hannigan MP, Fierer N. Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environ Sci Technol. 2013;47:12097–12106. doi: 10.1021/es402970s. [DOI] [PubMed] [Google Scholar]
- Chuvochina MS, Marie D, Chevaillier S, Petit J-R, Normand P, Alekhina IA, Bulat SA. Community variability of bacteria in alpine snow (Mont Blanc) containing Saharan dust deposition and their snow colonisation potential. Microbes Environ. 2011;26:237–247. doi: 10.1264/jsme2.me11116. [DOI] [PubMed] [Google Scholar]
- DeLeon-Rodriguez N, Lathem TL, Rodriguez-R LM, Barazesh JM, Anderson BE, Beyersdorf AJ, et al. Microbiome of the upper troposphere: species composition and prevalence, effects of tropical storms, and atmospheric implications. Proc Natl Acad Sci USA. 2013;110:2575–2580. doi: 10.1073/pnas.1212089110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. Terrestrial microorganisms at an altitude of 20,000 m in earth’s atmosphere. Aerobiologia. 2004;20:135–140. [Google Scholar]
- Griffin D. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin Microbiol Rev. 2007;20:459–477. doi: 10.1128/CMR.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D, Kellogg CA, Garrison V, Lisle J, Borden T, Shinn E. Atmospheric microbiology in the northern Caribbean during African dust events. Aerobiologia. 2003;19:143–157. [Google Scholar]
- Herman JR, Krotkov N, Celarier E, Larko D, Labow G. Distribution of UV radiation at the earth’s surface from TOMS-measured UV-backscattered radiances. J Geophys Res Atmos. 1999;104:12059–12076. [Google Scholar]
- Hervas A, Casamayor EO. High similarity between bacterioneuston and airborne bacterial community compositions in a high mountain lake area. FEMS Microbiol Ecol. 2009;67:219–228. doi: 10.1111/j.1574-6941.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- Hervas A, Camarero L, Reche I, Casamayor EO. Viability and potential for immigration of airborne bacteria from Africa that reach high mountain lakes in Europe. Environ Microbiol. 2009;11:1612–1623. doi: 10.1111/j.1462-2920.2009.01926.x. [DOI] [PubMed] [Google Scholar]
- Hörtnagl P, Pérez MT, Zeder M, Sommaruga R. The bacterial community composition of the surface microlayer in a high mountain lake. FEMS Microbiol Ecol. 2010;73:458–467. doi: 10.1111/j.1574-6941.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Lennon JT. Dormancy contributes to the maintenance of microbial diversity. Proc Natl Acad Sci USA. 2010;107:5881–5886. doi: 10.1073/pnas.0912765107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspari S, Mayewski PA, Handley M, Kang S, Hou S, Sneed S, et al. A high-resolution record of atmospheric dust composition and variability since AD 1650 from a Mount Everest ice core. J Clim. 2009;22:3910–3925. [Google Scholar]
- Kellogg CA, Griffin DW. Aerobiology and the global transport of desert dust. Trends Ecol Evol. 2006;21:638–644. doi: 10.1016/j.tree.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Lemarchand C, Jardillier L, Carrias J-F, Richardot M, Debroas D, Sime-Ngando T, Amblard C. Community composition and activity of prokaryotes associated to detrital particles in two contrasting lake ecosystems. FEMS Microbiol Ecol. 2006;57:442–451. doi: 10.1111/j.1574-6941.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- Matallana-Surget S, Meador JA, Joux F, Douki T. Effect of the GC content of DNA on the distribution of UVB-induced bipyrimidine photoproducts. Photochem Photobiol Sci. 2008;7:794–801. doi: 10.1039/b719929e. [DOI] [PubMed] [Google Scholar]
- Mladenov N, Pulido-Villena E, Morales-Baquero R, Ortega-Retuerta E, Sommaruga R, Reche I. Spatiotemporal drivers of dissolved organic matter in high alpine lakes: role of Saharan dust inputs and bacterial activity. J Geophys Res. 2008;113:G00D01. doi: 10.1029/2008JG000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladenov N, Lopez-Ramos J, McKnight DM, Reche I. Alpine lake optical properties as sentinels of dust deposition and global change. Limnol Oceanogr. 2009;54:2386–2400. [Google Scholar]
- Mladenov N, Sommaruga R, Morales-Baquero R, Laurion I, Camarero L, Dieguez MC, et al. Dust inputs and bacteria influence dissolved organic matter in clear alpine lakes. Nat Commun. 2011;2:405. doi: 10.1038/ncomms1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Baquero R, Pulido-Villena E, Reche I. Chemical signature of Saharan dust on dry and wet atmospheric deposition in the south-western Mediterranean region. Tellus B. 2013;65:18720. http://dx.doi.org/10.3402/tellusb.v65i0.18720. [Google Scholar]
- Moulin C, Lambert CE, Dulac F, Dayan U. Control of atmospheric export of dust from North Africa by the North Atlantic oscillation. Nature. 1997;387:691–694. [Google Scholar]
- Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S. A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev. 2011;75:14–49. doi: 10.1128/MMBR.00028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenakou PN, Mandalakis M, Stephanou EG, Tselepides A. Particle size distribution of airborne microorganisms and pathogens during an intense Sfrican dust event in the Eastern Mediterranean. Environ Health Perspect. 2008;116:292–296. doi: 10.1289/ehp.10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prospero JM, Blades E, Mathison G, Naidu R. Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia. 2005;21:1–19. [Google Scholar]
- Psenner R. Living in a dusty world: airborne dust as a key factor for Alpine lakes. Water Air Soil Pollut. 1999;112:217–227. [Google Scholar]
- Pulido-Villena E, Reche I, Morales-Baquero R. Evidence of an atmospheric forcing on bacterioplankton and phytoplankton dynamics in a high mountain lake. Aquat Sci. 2008;70:1–9. [Google Scholar]
- Reche I, Ortega-Retuerta E, Romera O, Pulido-Villena E, Morales-Baquero R, Casamayor EO. Effect of Saharan dust inputs on bacterial activity and community composition in Mediterranean lakes and reservoirs. Limnol Oceanogr. 2009;54:869–879. [Google Scholar]
- Shaffer BT, Lighthart B. Survey of culturable airborne bacteria at four diverse locations in Oregon: urban, rural, forest, and coastal. Microb Ecol. 1997;34:167–177. doi: 10.1007/s002489900046. [DOI] [PubMed] [Google Scholar]
- Singer CE, Ames BN. Sunlight ultraviolet and bacterial DNA base ratios: natural exposure to ultraviolet may be an evolutionary pressure toward high guanine plus cytosine in DNA. Science. 1970;170:822–826. doi: 10.1126/science.170.3960.822. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Griffin DW, McPeters RD, Ward PD, Schuerger AC. Microbial survival in the stratosphere and implications for global dispersal. Aerobiologia. 2011;27:319–332. [Google Scholar]
- Sommaruga R. The role of solar UV radiation in the ecology of alpine lakes. J Photochem Photobiol B. 2001;62:35–42. doi: 10.1016/s1011-1344(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Tong YY, Lighthart B. Solar radiation is shown to select for pigmented bacteria in the ambient outdoor atmosphere. Photochem Photobiol. 1997;65:103–106. [Google Scholar]
- Vila-Costa M, Barberan A, Auguet J-C, Sharma S, Moran MA, Casamayor EO. Bacterial and archaeal community structure in the surface microlayer of high mountain lakes examined under two atmospheric aerosol loading scenarios. FEMS Microbiol Ecol. 2013;84:387–397. doi: 10.1111/1574-6941.12068. [DOI] [PubMed] [Google Scholar]
- Volken M, Schumann T. A critical review of below-cloud aerosol scavenging results on Mt. Rigi. Water Air Soil Pollut. 1993;68:15–28. [Google Scholar]
- Warnecke F, Sommaruga R, Sekar R, Hofer JS, Pernthaler J. Abundances, identity, and growth state of actinobacteria in mountain lakes of different UV transparency. Appl Environ Microbiol. 2005;71:5551–5559. doi: 10.1128/AEM.71.9.5551-5559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P, Schweitzer B, Amann R, Simon M. Identification in situ and dynamics of bacteria on limnetic organic aggregates (Lake Snow) Appl Environ Microbiol. 1996;62:1998–2005. doi: 10.1128/aem.62.6.1998-2005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack AM, Bohannan BJM, Green JL. Biodiversity and biogeography of the atmosphere. Philos Trans R Soc Lond B Biol Sci. 2010;365:3645–3653. doi: 10.1098/rstb.2010.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Ichijo T, Sakotani A, Baba T, Nasu M. Global dispersion of bacterial cells on Asian dust. Sci Rep. 2012;2:525. doi: 10.1038/srep00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.