Abstract
Inflammation is an adaptive response of the immune system to noxious insults to maintain homeostasis and restore functionality. The retina is considered an immune privileged tissue due to its unique anatomical and physiological properties. During aging, the retina suffers from a low-grade chronic oxidative insult, which sustains for decades and increases in level with advancing age. As a result, the retinal innate immune system, particularly microglia and the complement system, undergo low levels of activation (para-inflammation). In many cases, this para-inflammatory response can maintain homeostasis in the healthy aging eye. However, in patients with age-related macular degeneration (AMD), this para-inflammatory response becomes dysregulated and contributes to macular damage. Factors contributing to the dysregulation of age-related retinal para-inflammation include genetic predisposition, environmental risk factors and old age. Dysregulated para-inflammation (chronic inflammation) in AMD damages the blood retina barrier (BRB), resulting in the breach of retinal immune privilege leading to the development of retinal lesions. This review discusses the basic principles of retinal innate immune responses to endogenous chronic insults in normal aging and in AMD, and explores the difference between beneficial para-inflammation and the detrimental chronic inflammation in the context of AMD.
Keywords: Retina, blood retina barrier, immune privilege, microglia, complement
Introduction
The central role of the immune system is to protect the host from exogenous and endogenous insults, and to maintain tissue homeostasis. Dysfunction or dysregulation of the immune system may lead to various immune-related diseases, such as infection and autoimmune disorders. In addition to the classic inflammatory diseases, compelling evidence suggests that a low-grade chronic inflammation contributes critically to many human diseases that were previously not considered as inflammatory disorders, including obesity [1, 2], atherosclerosis [2-4], and various neurodegenerative disorders [3, 5-8]. It is now clear that chronic inflammation is involved in almost all age-related degenerative diseases, including those that occur in “immune privileged” tissues such as the brain (e.g., Alzheimer disease; Parkinson disease) [3, 5, 8], and the retina (e.g. age-related macular degeneration, AMD) [9, 10]. Aging involves the accumulation of oxidative insults, and the initial triggers for age-related degenerative diseases are believed to be the oxidative damage. Inflammation is secondary to the tissue damage and is thought to be part of a protective response of the immune system. Why this protective response becomes detrimental has been a puzzle for many years.
In 2008, Medzhitov discussed the origin of inflammation in his essay published in Nature [11], where he further extended the concept of the “danger theory” of inflammation. He suggests that between basal homeostatic conditions and true inflammation a “para-inflammation” state exists [11]. Para-inflammation is an adaptive response of the immune system to low levels of tissue stress (i.e., a low-degree of “danger” stimuli), such as in aging whereby oxidative stress accumulates bit by bit for many decades. The physiological role of para-inflammation is to maintain homeostasis (or re-set the homeostatic threshold of the tissue) and restore tissue functionality [11]. This para-inflammation theory helps to explain many phenomena observed in various chronic disease conditions, an example of which is “inflammaging” [12, 13]. This concept centres on a well-controlled para-inflammation, which is beneficial and dysregulated para-inflammation that is detrimental. A lot of studies since have focused on how the para-inflammatory response becomes dysregulated in disease conditions. In this review, we will discuss para-inflammation in the aging eye and will present our understanding, based on published data from us and others, on how para-inflammation is dysregualted in AMD, a sight-threatening disease that affects over 170 million people worldwide [14].
Age-related macular degeneration
AMD is disease involving progressive degeneration of the macula, the central part of the neuroretina in the elderly (Fig. 1A). In Western nations, around 22% of people aged over 70 and 34% over 80 years may suffer from AMD in at least one eye [14]. With demographic shifts and trends towards increasing longevity in the developing world, the number of people suffering from AMD is projected to reach 190 million in 2020 and 288 million in 2040 [14].
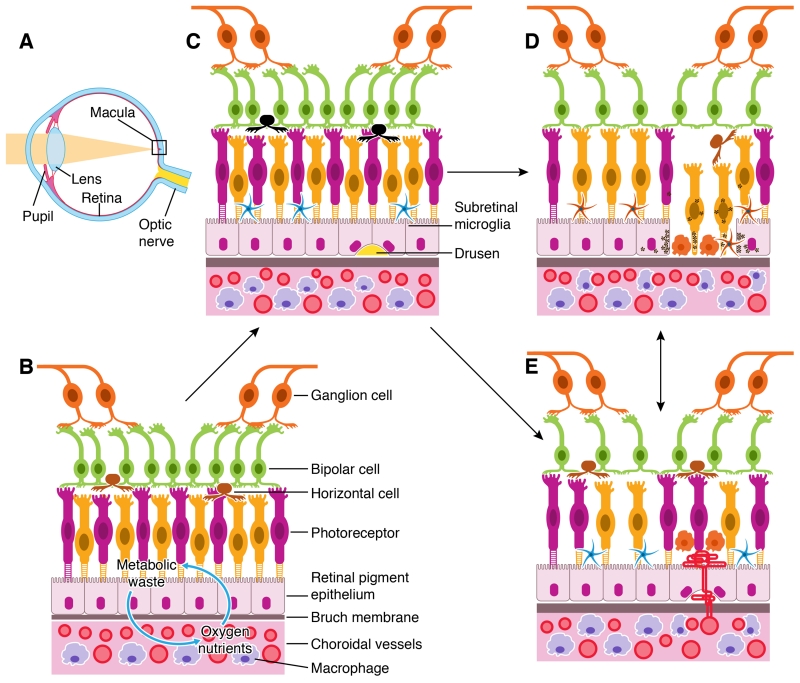
Figure 1. Retina and RPE-choroid in normal, early and late AMD.
A, diagram of a human eye. Light pass through the pupil and then focused by lens to the macula of the retinal layer at the back of the eye. B, the retina consists of three layers of neurons, photoreceptor, bipolar and ganglion cells. The RPE monolayer together with Bruch’s membrane (BM) form the outer blood retinal barrier (oBRB) that separates the neuroretina from the choroid. Oxygen and nutrients are transported from the choroid into the outer retina whereas retinal metabolic wastes are transported to choroid through the oBRB. C, the early stages of AMD is characterized by the presence of large Drusen deposits between the RPE and BM, RPE senescence, and the accumulation of microglia and macrophages in the subretinal space. D, geographic atrophy (GA) is typified by the loss of RPE and photoreceptors, accompanied by macrophage infiltration at the lesion site. E, neovascular AMD (nAMD) is caused by the growth of choroidal vessels into the sub-RPE or sub-retina of the macula.
The pathologies of AMD are restricted to the retina-choroid interface of the macula [15, 16] (Fig. 1). The macula, in particular the fovea, has a unique structure whereby cone cells constitute the majority of photoreceptors and no blood vessels are present (Fig. 1B). Nutrients and oxygen are supplied to the macula by the choroidal circulation through Bruch’s membrane (BM) and a monolayer of retinal pigment epithelial (RPE) cells, which forms the outer blood retina barrier (oBRB) (Fig. 1B). The metabolic waste materials of the retina are disposed of through the RPE/BM to the choroid and then removed by choroidal macrophages or the choroidal circulation (Fig. 1B). During aging, two processes contribute to macular damage: i) the thickness of the BM increases and permeability decreases [17], and ii) the RPE function declines [18, 19] and the density of chorocapilaris is reduced [17]. During the early stages of AMD, there is an accumulation of extracellular deposits called “Drusen” between the RPE and BM consisting of various lipid-, carbohydrate-, and protein-rich debris (Fig. 1C) [20]. AMD can progress into two late sight-threatening stages: geographic atrophy (GA, or dry AMD) (Fig. 1D) and neovascular AMD (nAMD or wet AMD) (Fig. 1E). GA is characterized by the death of RPE and photoreceptors, whereas nAMD is typified by the growth of abnormal blood vessels into the sub-RPE or subretinal space (Fig. 1D, 1E). GA and nAMD are not mutually exclusive, approximately 12% of AMD patients may develop both GA and nAMD [21, 22], and GA often develops in nAMD eyes following anti-VEGF therapy [23-25].
AMD is a multi-factorial disease, and old age, environmental and genetic risk factors all contribute to disease pathogenesis [15, 26]. Exactly how these multiple factors cause macular damage is poorly understood. Whilst we appreciate that multiple pathways may contribute to macular damage, it is now clear that inflammation plays a major role in AMD pathogenesis [10, 26, 27]. Evidence supporting the role of inflammation in AMD include: i) inflammatory molecules, including vitronectin, amyloid A/P, Factor X, prothrombin, and in some instances, immunoglobulin, HLA-DR, and complement proteins (C3, C5, C5b-9, CFH, and CRP) have been detected in Drusen – the hallmarker of early AMD [9]; ii) immune cells, including macrophages, lymphocytes and mast cells have been detected in AMD lesions or the choroid adjacent to macular lesions [28, 29]; iii) polymorphisms of various immune related genes, such as CFH, C2/CFB, C3, CX3CR1, and TLR3/4 are associated with AMD risk (reviewed by Tuo et al [30]); iv) AMD-like lesions can be modelled in experimental animals by manipulating immune related genes [31-35]. The question is why this “protective” response becomes detrimental in AMD? To address this question, it is essential to review the basic principle of the immune response to age-related chronic insults and how the immune system uses the principle to protect the eye, particularly under aging conditions.
Inflammation – an adaptive response to tissue stress
Inflammation is an adaptive response to tissue stress [11, 36]. The response can occur at three levels: tissue cells, the immune system of local tissue and the systemic immune system. At the tissue level, when cells suffer from chronic noxious insults (e.g. a change of microenvironmental parameters such as temperature, nutrients, oxygen and growth factors, etc.) and the insults are not strong enough to cause cell death, a cell autonomous response, including the upregulation of heat shock proteins [37-39] and the activation of the autophagy pathways [40-43] may ensue. The purpose of the cell autonomous response is to repair the damage so the cells can return to basal homeostasis. The cell autonomous response can also result in the production of inflammatory cytokines and chemokines; this therefore, represents a tissue cell autonomous inflammatory response. The subtle change in microenvironment related to the cell autonomous response is monitored by the immune system of the tissue, such as resident macrophages and the complement system, which in turn may release cytokines and growth factors to further promote the repair / recovery of stressed cells. The difference between the cell autonomous response and the response of local immune cells is that the former promotes the survival of self, whereas the latter assists in the survival of other cells and ensures the integrity of tissue structure and functionalities. If the damage is restricted to a limited number of cells and the insult is transient, tissue repair can be achieved with minimal disturbance of the local or the systemic immune system (“minimal inflammation”).
When more cells or the whole tissue are stressed, and/or the insult persists for a sustained period of time, such as in aging, the autonomous response may not be able to retuen stressed cells to healthy where they may undergo senescence or even death. Senescent cells can secrete a number of pro-inflammatory cytokines and chemokines, a phenomenon known as “senescent-associated secretory phenotype (SASP)” [44]. Examples of SASP-associated factors include cytokine such as IL-6, IL-8, TNF-α, and IL-1α/β, chemokine MCP-1/2 and CX3CL1 [45], insulin-like growth factor (IGF)/IGFR [46], and colony stimulating factors (G-CSF, GM-CSF) [47, 48]. These proinflammatory mediators further stimulate resident macrophages and tissue complement system (local tissue inflammation), promoting tissue repair and remodelling to maintain homeostasis or re-set the threshold of homeostasis and restore functionality. If the level of tissue stress exceeds the reparatory capacity of resident macrophages, they may release additional cytokines and chemokines to recruit circulating monocytes [36]. When tissue factors are released into the circulation, they may activate the systemic immune system (systemic inflammation). The stress may also initiate other innate immune pathways such as the complement pathway to promote tissue repair/remodelling. This adaptive response of the innate immune system to tissue malfunction was called para-inflammation by Medzhitov [11]. The physiological purpose of the para-inflammatory response is to help the tissue to adapt to the stressful conditions and to restore functionality [11].
When tissues suffer from acute insults that cause substantial necrotic cell death, overt inflammation may ensue. Dead cells may release large amounts of inflammatory stimuli such as uric acid [49], high-mobility group box 1 protein (HMGB1)[50-52], and S100B [53] resulting in the aggressive activation of resident immune macrophages as well as recruitment of circulating leukocytes, typically neutrophils and macrophages.
An inflammatory response requires four elements: the inducers, the sensors, the mediators and the effectors [36]. According to the “danger model” of inflammation [54], the inducers are the danger molecules (exogenous and endogenous danger-associated molecular patterns, DAMPs), whereas the sensors are the pattern recognition receptors (PRRs) expressed by tissue or immune cells. Upon engaging with DAMP ligands, PRR-expressing cells secrete cytokines and chemokines (mediators) that further recruit and activate immune cells (effectors) to sites of inflammation [36].
Retina – an immune privileged tissue
Immune privilege property of the retina
The retina has a highly complex, sophisticated structure, and even a minor perturbation may cause devastating visual impairment. However, the eye has developed a special mechanism to protect the retina from exogenous and endogenous insults. This protective mechanism not only reduces the risk of pathogenic insult, but also prevents circumvent inappropriate immune reactions to insults, thereby reducing the risk of inflammation – mediated retinal damage. The retina, therefore represents an immune privileged tissue for several reasons [55-57]. Firstly, the retina is protected by physical barriers. The blood retina barrier (BRB) that is formed by tight junctions between vascular endothelial cells (inner BRB, iBRB) and RPE cells (outer BRB, oBRB) ensures that circulating cells and molecules do not freely pass into the retinal parenchyma. The BRB also sequesters retinal antigens within the intraocular compartment avoiding T cell activation, a phenomenon called immunological ignorance [56, 58-60]. In addition, the retina has no lymphatic system. Therefore, when the retina suffers from any sort of insult, the endogenous alarmins are unlikely to be detected by circulating or choroidal/extraocular antigen presenting cells (APCs) if the BRB is intact. The second mechanism of retinal immune privilege involves a sophisticated immune regulatory system orchestrated by retinal cells, including various neurons and RPE cells [55, 61, 62]. These retinal cells express various immune modulators that can suppress myeloid cell (microglia/macrophage) activation via CD200-CD200R [63], or CX3CL1-CX3CR1 [32]), or reduce T cell activation or induce T regulatory cells (Tregs) formation (through thrombospondin-1, TGF-β, CTLA4, CTLA2, [64-69]), or even induce the death of infiltrating immune cells through Fas ligand (FasL) and Tumor Necrosis Factor-related apoptosis-inducing ligand (TRAIL) [70-72]), or suppress complement activation via CD55, CD46, and the decay-acceleration factor (DAF) [73, 74]). In addition, ocular fluids also contain a number of immunoinhibitory molecules such as TGF-β2, neuropeptides such as α-melanocyte-stimulating hormone, and vasoactive intestinal peptide [75, 76].
Importantly, despite being an immune privileged tissue, when the retina suffers from noxious insults, an immune response can still be mounted by a local defence system, involving retinal innate immune cells and the complement system.
Retinal immune system
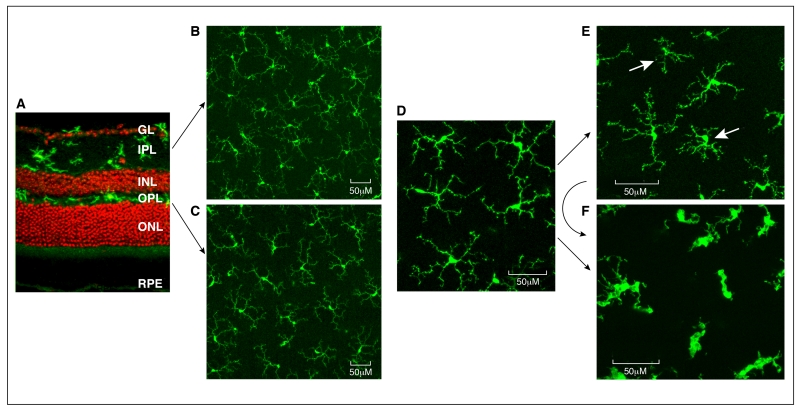
Microglial cells form an important part of the immune defence of the retina. These cells are located in the inner layers of the retina, and are distributed into three layers: the ganglion layer (GL), the inner plexiform layer (IPL) and outer plexiform layer (OPL) (Fig. 2A). Our data has shown that in mouse eye the density of microglial cells in the IPL is higher than that in the OPL (~ 260 cells/mm2 vs ~98 cells/mm2, Figs. 2B, 2C). The patho-physiological role of microglia in retinal health and disease has been reviewed extensively elsewhere [77-80]. Microglia express various toll-like receptors (TLRs) [81, 82] that allow them to monitor the surrounding microenvironment. Upon engaging with danger signals, the microglia may convert from a resting surveillance state to an active form specialized to operate within the diseased environment. Microglial activation is classically characterized by two major changes. First, the cell shape transforms from a highly branched (Fig. 2D) and ramified morphology to an ameboid form (Fig. 2F) [80]. Secondly, these ameboid cells become active phagocytes (Fig. 2F) [80]. Microglia may also undergo a low-level of intermediate activation, characterized by shorter dendrites and larger somas compared to resting cells (Fig. 2E).
Figure 2. Retinal microglial in health and disease.
A, confocal image of a normal mouse retina stained with Iba-1 (green) and Propidium iodide (PI). Iba-1+ microglial cells are located in the ganglion layer (GL), inner plexiform layer (IPL) and outer plexiform layer (OPL). B-C, confocal images taken from the IPL (B) and OPL (C) of a normal mouse eye showing the ramified morphology of resting microglia. D, high-magnification of resting retinal microglia. E, microglia from a 18 month old mouse retina showing heterogeneous activation. Two cells (arrows) demonstrate shorter dendrites (signs of mild activation). F, microglia from paraquat treated mouse eyes demonstrate ameboid shape with a larger cell body, multiple vacuoles and shorter dendrites (signs of full activation). Mildly activated microglia may undergo full activation under acute stress conditions. INL – inner nuclear layer; ONL – outer nuclear layer, RPE – retinal pigment epithelium.
Perivascular macrophages are another important subset of retinal resident immune cells that have a distinct morphology and phenotype. Although both perivascular macrophages and microglia express CD11b and F4/80, the former express high levels of CD14 (LPS receptor) and CD45, whereas microglial cells are negative for CD14 and express low levels of CD45 [83-85]. In addition, bone marrow chimeric studies have shown that brain perivascular macrophages are regularly replaced by circulating monocytes [86], suggesting that they may originate from bone marrow hematopoietic stem cells.
Whether or not the retina contains professional antigen presenting cells (dendritic cells) has been a subject of debate for many years. Early work by Zhang and colleagues reported a small population of MHC-II+ cells in rat retina [87]. Using flow cytometry analysis, Gregerson and Yang detected a small population of CD11c+ DEC205+ dendritic cells in normal mouse retina [88]. This group further confirmed the existence of retinal CD11c cells using CD11c-DTR transgenic mice [89]. However, another study by Chen et al suggested that the rd8 mutation in the Crb1 gene may contribute to the abnormal number of CD11c+ cells in the retina in CD11c-eYFP transgenic mice [90]. These CD11c+ cells had the characteristics of activated microglia but not DC, and they were virtually absent in the CD11c-DTR/GFP mice that did not have Crb1 mutation [90]. Previously, our group identified a small population of MHC-II+33D1+ dendritic cells in mouse retina which are located strategically around the optic disc and peripheral retinal margin area [91]. The function of these cells is unclear, but their location suggests they may be “gatekeepers” of the retina. When activated T cells were injected intravenously to mice with early uveitis, an inflammatory condition of the retina and choroid, early infiltration of T cells was observed around the optic disc and retinal periphery [91], allowing possible initial contacts with dendritic cells. It is possible that in the normal physiological state, these retinal dendritic cells promote tolerance (‘privilege’) rather than immunity. Other immune cells, such as T/B cells, NK cells, and Mast cells have not been detected in the normal healthy retina.
In addition to these immune cells, a complement regulatory system also exists in the retina. The complement system is an important part of the innate immune system, consisting of over 30 small proteins and protein fragments. Complement proteins are normally synthesized by hepatocytes in the liver and released into the circulation in a latent form. Upon stimulation, complement proteins are cleaved by appropriate proteases resulting in amplifying cascades involving further cleavage, and ultimately the formation of the membrane attack complex (MAC), a potent molecule that can kill cells [92]. The complement system can be activated through the classical pathway (CP, mediated by antibody-antigen complex), the alternative pathway (AP, spontaneous tick-over) and the lectin pathway (LP, mediated by mannose-binding lectin or ficolin binding to certain sugars) [92]. In addition to MAC, complement activation also generates various complement fragments, including C3a, C5a, and C4a, that are actively involved in various immune responses [92].
Complement activation is involved in various retinal diseases, including uveoretinitis [93-95], diabetic retinopathy [96], and age-related macular degeneration [27], suggesting that the complement system is also an important part of retina innate immune defence. Retinal cells can produce various complement proteins and regulators. For example, the mRNAs of C1qa/b, C1s, Cr1, C2, C4, Cfb, Cfd, C5, and C7 as well as complement regulatory genes, including Serping-1, MCP (CD46), DAF (CD55), CFH, CFI, and CD59 were detected in neuroretina of human [27] and mouse [97-99]. Furthermore, in vitro studies have shown that microglia and RPE cells are the major cellular sources of complement in the retina [97]. These results confirm that a local complement regulatory system exists in the retina, and plays a role in retinal health and disease.
Para-inflammation in the aging retina
Aging involves the accumulation of oxidative stress, and significant expression of oxidized lipids/proteins can be detected in the aging retina [100]. Other altered metabolic products such as advanced glycation end products (AGEs) [101-103], β-amyloid [104, 105], pyridinium bisretinoid (A2E) [106] and hyaluronan fragments [107] may also accumulate in the aging retina. Oxidative or metabolic stress can damage retinal cells, including various neurons and RPE cells. As a result, a para-inflammatory response may be initiated to repair damage and maintain homeostasis. Increased inflammatory gene and protein expression has been observed in various models of retinal aging [108, 109], and both the retinal cell autonomous response and activation of the retinal immune system may contribute to age-related retinal para-inflammation.
Para-inflammatory autonomous responses by RPE cells
Over the years, many studies have investigated the age-related inflammatory response of RPE cells, but little has been published on the response of retinal neurons. We will therefore focus on the autonomous response of RPE cells under aging conditions. The RPE cells express various PRRs, including TLRs and NLRs which can detect various stresses intracellularly or at the cell surface. Gene array technology has helped to define the overall gene expression profile of RPE cells during aging, and inflammation is one of the major functional pathways that has been identified in these studies [109-111]. Many of the age-induced immune gene expression changes identified in the array studies have also been observed in vitro. Treatment of RPE cells with the age-related DAMPs, such as AGEs [103], amyloid-β [112-114] or oxidized photoreceptor outer segments (oxPOS) [98, 99] induces the up-regulation of pro-inflammatory genes such as CCL2, IL-6, TNF-α, and complement factor B (CFB), but also reduce immune regulatory genes (such as complement factor H, CFH [98]]). The SASP is a well-known phenomenon in all senescent cells [48]. The age-related autonomous response of RPE cells may be another example of SASP.
Para-inflammatory response by retinal immune system
Microglia, perivascular macrophages and a small number of dendritic cells constitute the cellular component of the retinal immune system. Like any other innate immune cells, these cells express various PRRs that can detect various DAMPs in the aging retina. A number of studies have shown that retinal innate immune cells, in particular, microglia undergo low-levels of activation during aging. Chan-Ling et al have detected ED2+MHC-II+ cells in the normal aging rat retina [115]. Using flow cytometry analysis, our group has found that expression of TLR-3/4, CD11c, 33D1 and MHC-II in retinal CD11b+CD45low cells (resident retinal myeloid-lineage cells) was significantly increased in the aging mouse retina [100]. Furthermore, an age-dependent increment in the number of microglial cells was present in the mouse retina [33] as well as subretinal migration and accumulation [116]. Evidence suggests that microglia in the aging retina appear to be activated at low levels. Firstly, they do not acquire an ameboid shape, characteristic of fully activated microglia, suggestive of an mild activation state (Fig. 2F). Instead, the dendrites become shorter and less symmetric (Fig. 2E) compared to resting microglial cells (Fig 2D). Secondly, unlike microglia from the young retina that are often confined within the IPL or OPL, microglial cells in the aging retina can migrate to the subretinal space [100, 116] (Figs. 3A, 3B). The role of microglia in the aging neuroretina has been reviewed extensively previously [79, 100, 117].
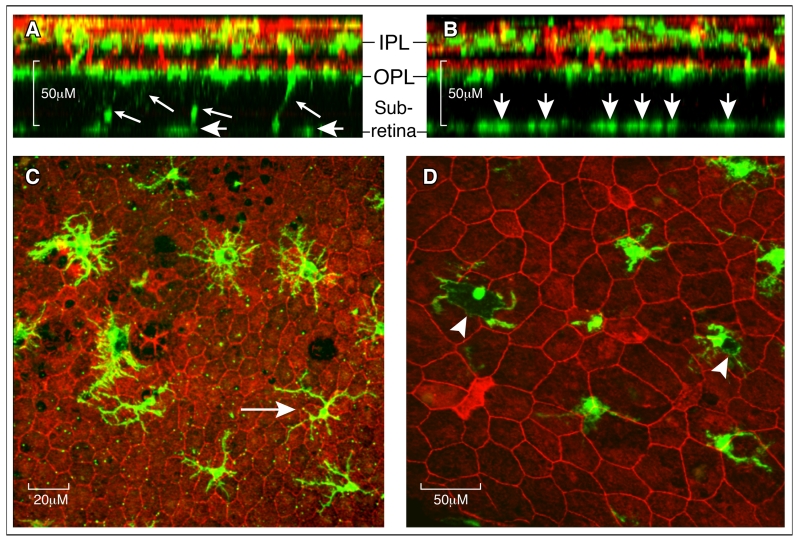
Figure 3. Retinal microglial in the aging eye.
A-B, Reconstructed z-stack confocal images from a 16-month (A) and 27-month (B) old mouse retina stained for Iba-1 (green for microglia) and lectin B4 (red for blood vessels). A, at 16 months, few Iba-1+ microglial cells were detected at the subretinal space (short arrows) and some were still connected to cells in the OPL layer (small arrows). B, at 27 months many more Iba-1+ cells were detected at the subretinal space (short arrows), and no Iba-1+ cells were detected between the OPL and subretinal space. C, heterogeneous morphology of Iba-1+ cells at the subretinal space in a 18-month old mouse. Most of the cells have larger cell bodies and shorter dendrites, and a few cells display a relatively small cell body and long dendrites (arrow). D, subretinal Iba-1+ cells from a 27-month old mice showing pigmented cell body (arrowheads). IPL – inner plexiform layer; OPL – outer plexiform layer.
The subretinal space is devoid of any immune cells under normal healthy conditions. The presence of microglia suggests tissue insult/damage at the retina-choroidal interface. What role do the subretinal microglia play in the aging eye? Although the morphology of subretinal microglia varies markedly even in the same eye, these cells generally have larger cell bodies and shorter dendrites (Fig. 3C) compared to those in the inner retina (Fig. 2). Previously, we have shown that they express Iba-1, P2Y12, Arginase-1 as well as low levels of MHC-II [34], suggesting a tissue repair/remodelling function. Supporting this concept, melanin-loaded subretinal microglia were frequently observed in the normal aging eye (Fig. 3D). Exocytosis of melanin granules is a characteristic feature of stressed RPE cells, and indeed of melanin-containing cells generally [118].
In addition to a scavenger role, subretinal microglia may also interact with RPE cells, facilitating cell-cell regulation. We have shown recently that the expression of complement components by RPE cells is regulated by activated macrophages [119]. The classically activated M1 macrophages upregulate CFB/C3 expression by RPE cells, whereas the alternative M2 macrophages upregulate the expression of complement inhibitors by RPE cells [119]. Whether or not subretinal macrophages can also modulate other RPE functions such as phagocytosis, the expression of tight junctions and ion/water channels remains to be defined.
The complement system is an important part of the innate immune system, and retinal cells, in particular RPE cells, express various complement proteins. During aging, the expression of complement components such as CFB, C3d is increased in the retina-chordal interface [99], whereas the expression of complement regulators such as CFH is decreased [98]. In addition, an age-dependent expression of MAC in RPE/choroid has been observed in both human [120] and rat eyes [121]. A low level of complemen activation may participate in retinal homeostasis in a number ways. The complement fragments C3a and C5a are known anaphylatoxins and may promote inflammation through the receptors C3aR and C5aR on immune cells, whereas C3b/C3c may opsonize dead cells/debris and promote phagocytosis. Although C5b-9 (MAC) can promote cell lysis, sublytic assembly of MAC induces cell cycle activation and survival [122], and may be neuroprotective [123].
Dysregulated para-inflammation and age-related macular degeneration
Why does the protective retinal para-inflammatory response become detrimental in AMD? Perhaps there is a balance between the level of age-mediated retinal stress and the capacity of the para-inflammatory response to repair the damage. On the one hand, if the level of retinal stress exceeds the repair capacity of the immune system, tissue damage is unavoidable. On the other hand, if the para-inflammatory response becomes dysregulated, it may transform into chronic inflammation and contribute to tissue damage. Para-inflammatory response in the aging retina includes the cell autonomous response, the response by the retinal innate immune system (i.e. microglial/macrophage and the complement system), and the response of systemic immune system. Theoretically, AMD may occur when any or all of these inflammatory responses becomes dysregulated.
Dysregulated cell autonomous response in AMD
The autonomous response in the context of AMD predominantly concerns RPE cells. A typical example of an age-related RPE inflammatory response is the activation of NLRP3 inflammasome. NLRP3, PYCARD, and Caspase-1 have been detected in RPE cells at lesion sites in both GA (dry AMD) and nAMD (wet AMD) [124, 125]. In vitro studies have shown that the NLRP3 inflammasome in RPE cells can be activated by various intracellular / extracellular stimuli that may exist in the aging eye, such as AluRNA [124], amyloid-β [126], A2E [127], lipofuscin [128] or oxidized lipoproteins [129]. Depending on the stimuli, activation of the NLRP3 inflammasome in RPE cells may result in IL-18 [124] or IL-1β production [127] or both [126, 128]. Inflammasome activation, in particular AluRNA-induced NLRP3 inflammasome activation, often leads to RPE cell death and the development of GA-like lesions [124, 130]. Despite the presence of TLRs on RPE cells, Alu-RNA-induced NLRP3 inflammasome activation does not involve TLRs, but may involve P2Y7 and MyD88 [124, 130].
What causes the uncontrolled inflammasome activation in AMD? One obvious cause is the the levels of macular stress in AMD patients differ from that experienced by healthy aged people. For example, AluRNA was detected in AMD eyes, but not in healthy controls [124], and retinal A2E levels was higher in AMD compared to controls [131]. Dysfunction of the autophagy pathway may also be involved in RPE inflammasome activation in AMD. Autophagy is the self-clearance machinery of a cell [43], and is important for cells to dispose of damaged organelles or waste molecules [43]. An imbalance in the autophagy system may result in the intracellular accumulation of toxic molecules and the generation of reactive oxygen species [42], which may lead to progressive inflammasome activation [42]. Increased autophagsome numbers and expression of autophagy proteins have been observed in RPE cells of normal aging eye [132, 133]. Whereas in AMD eyes, autophagy proteins, autophagosomes and autophagy flux ith were reduced [133, 134]. With the onset of AMD, the excesive accumulation of lipofuscine in RPE cells may impair lysosomal enzyme activity resulting in autophagy dysfunction [133, 135]. Accumulation of both reactive oxygen species and lipofuscine in RPE cells may lead to inflammasome activation [41].
Dysregulated retinal innate immune activation in AMD
Activation of retinal microglia and the complement system features the para-inflammatory response of the retinal innate immune system in the aging eye [100]. Malfunction in the immune regulatory system or the innate immune component of the retina may lead to the dysregulation of the para-inflammatory response. RPE cells produce various immune suppressive factors (both membrane and soluble forms) to maintain the immune privileged state of the retina. The production and/or function of these regulators may be altered in AMD. For example, FasL expressed by RPE cells is important in maintaining retinal immune privilege by inducing the death of infiltrating immune cells [70, 72]. During aging, the matrix metalloprotease activity is increased, resulting in the cleavage of FasL and the loss of immune regulatory function in RPE cells [136]. It would be interesting to know if this age-related cleavage of FasL is accelerated in AMD. RPE cells also express/produce various complement regulators, such as CFH, CD46 and CD59 [27, 97]. Local production of these regulators may protect retinal cells, including RPE and photoreceptors from complement attack. Immunohistochemistry studies have detected MAC in drusen and macular lesions in AMD [9, 27], suggesting that RPE cell death in AMD may be related to uncontrolled complement activation. The expression of CFH [137], CD46 and CD59 [138] in RPE cells was reduced in AMD. In addition, the functional change of innate immune cells (due to genetic or epigenetic regulation) may also confer a detrimental effect on aging insults. Studies in the function of CFH protein have shown that the variant CFH 402His has reduced binding affinity to CRP [139], Bruch’s membrane, heparin [139, 140], and oxidized phospholipids [141]. These functional alterations may result in a reduced ability of CFH to protect the retina, in particular RPE cells, and ultimately contribute to AMD development.
In addition to the retinal immune regulatory system, malfunction of microglia and macrophages in the retina and choroid may also lead to dysregulated para-inflammation in AMD. The CCL2/CCR2 and CX3CL1/CX3CR1 pathways are two major chemokine axes involved in monocyte/macrophage migration. CCL2 critical controls the trafficking of CCR2-expressing monocytes to sites of inflammation [142], whereas CX3CL1 regulates the trafficking of CX3CR1-expressing resident monocytes under homeostatic conditions [142]. A study by Ambati et al has shown that mice deficient in either CCL2 or CCR2 age-dependently develop retinal pathologies akin to human AMD [31] although it is unclear whether the retinal phenotype was affected by the Crb1 rd8 mutation [143]. The result suggests that CCL2/CCR2 pathway mediated subretinal inflammation may have a protective role in retinal aging. Interestingly, the CX3CL1/CX3CR1 pathway-mediated subretinal inflammation also appears to be beneficial. Mice deficient in CX3CR1 developed retinal degeneration during aging [32]. More recent studies have shown that CCR2+ mononuclear phagocytes from CCR2+ cell in CX3CR1-deficient mice can induce neuronal apoptosis through IL-1β secretion [144], and the production of IL-1β in CX3CR1 deficient phagocytes is mediated at least in part by the upregulation of P2RX7 [145]. It appears that both CX3CR1+ monocytes and CCR2+ monocytes are necessary for retinal homeostasis during aging and disruption in either pathway may result in age-dependent retinal degeneration.
How can these data be interpreted? The CCL2/CCR2 pathway and the CX3CL1/CX3CR1 pathway may be involved in different stages of subretinal inflammation during aging. Microglial cells are CCR2−CX3CR1+ [146] although circulating monocytes or choroidal macrophages may express both CCR2 and CX3CR1 [142]. At the early stages of aging, when RPE and photoreceptors are mildly stressed from age-related oxidative insults and the BRB is intact, the CX3CR1+ microglial cells may migrate from the inner retina to the subretinal space to remove debris and maintain homeostasis. As the aging progresses and age-related macular stress accumulates, microglial cells may not be able to maintain macular homeostasis. Activated subretinal microglial cells and stressed RPE cell may release chemokines such as CCL2 to recruit CCR2+ macrophages from the choroidal tissue in order to maintain homeostasis. BRB breakdown has been observed in normal rat aging eye [115]. In mouse eyes, migrating microglial cells were frequently observed at the photoreceptor layer (OPL) at the early stages (12-18 months) but not late stages (24-29 months) of aging, despite more subretinal macrophages exist at the late stages (Fig. 3). The increased number of subretinal macrophages during the late stages of aging may be related to the recruitement of CCR2+ macrophages from the choroid or circulation. In addition, the lack of function of either CCR2+ or CX3CR1+ monocytes (due to genetic or epigenetic modification) may lead to malfunction of the other subset, resulting in a dysregulated para-inflammatory response. We have shown recently that macrophages from CCL2−/− mice or CCR2−/− mice produce excessive amounts of inflammatory cytokines TNF-α and IL-1β when stimulated with LPS, compared to cells from WT mice [34]. Interestingly, macrophages from the CCL2/CX3CR1 double knockout mice had a more aggressive response to LPS or IL-4 stimulation under hypoxic conditions but reduced phagocytosis [33]. These mice develop localized retinal atrophies in an age- and light-dependently manner [33]. Our results may suggest that mononuclear phagocytes from either CCL2−/− or CX3CR1−/− mice or CCL2-CX3CR1 dual knockout mice are genetically predisposed to a pro-inflammatory phenotype. When these macropahges are recruited to the subretinal space in the aging eye, they may do more harm than good. This may be particularly relevant to AMD patients with CX3CR1 polymorphisms [147]. The results from animal models highlight the importance of the CCL2/CCR2 and CX3CL1/CX3CR1 pathways in the development of age-related retinal degeneration. Further studies are necessary to investigate these pathways in human AMD.
Dysregulated systemic inflammation and AMD
Aging is associated with a low-grade activation of the systemic immune system, and the term “inflammaging” is frequently used to describe this phenomenon [12, 13]. Previous studies have shown that plasma levels of complement fragments C3a, Bb, C4a and C5a were increased in AMD patients [148, 149]. Increased serum levels of CRPs [150-152] and pentraxin 3 [152] have been reported in AMD. In addition, retinal autoantibodies [153, 154] and higher levels of circulating white blood cells were reported in AMD patients [155-157]. More recently, it has been shown that AMD patients have higher levels of blood neutrophils, with an increased neutrophil/lymphocyte ratio [158, 159]. In addition, increased serum levels of inflammatory cytokines such as IL-1β, TNFα, and IL-17 have been detected in AMD patients [160], with IL-17 production potentially related to higher C5a levels in these patients [161, 162]. These data suggest that the level of systemic low-grade immune activation (inflammaging) is more severe in AMD patients compared to age-matched controls.
Interestingly, apart from retinal autoantibodies, most of the inflammatory mediators are generic markers of systemic immune activation. Is this non-specific systemic chronic inflammation biologically linked to AMD pathology? AMD pathology is localized at the macula, an area with a diameter of around 5.5 mm of the neuroretina. It is unlikely that chronic damage in this tiny tissue would affect the levels of cytokines/growth factors in the whole circulation (approximately 5 liters of blood [163]. The increased systemic immune activation in AMD patients may reflect an intrinsic over-reactivity of the immune system to age-related insults, i.e., dysregulated age-related systemic para-inflammation. When immune cells are recruited to the macula in the aging eye, they may contribute to macular damage by producing various proinflammatory cytokines and chemokines. If this is the case, the use of nonsteroidal anti-inflammatory drugs or other anti-inflammatory drugs would benefit AMD patients. Indeed, epidemiological evidence demonstrates that the use of anti-inflammatory medication reduces the risk of AMD [164]. Rheumatoid arthritis patients with regular immunosuppressive medications also had a lower prevalence of AMD [165]. Furthermore, systemic immunosuppression (e.g., daclizumab or rapamycin) [166] or topical use of bromfenac can significantly reduce the number of intravitreal anti-VEGF injection in neovascular AMD patients [167, 168]. However, a number of meta-analysis of various clinical trials suggests that the use of aspirin may increase the incidence of neovascular AMD [169, 170]. However, most patients with AMD take only low-dose aspirin (75-100 mg/day) in order to reduce the risk of cardiovascular disease, and such low-doses are likely to have minimal effects on the immune system.
What causes the dysregulation of systemic para-inflammation (inflammaging) in AMD? The evidence so far indicates that the activation of the systemic immune system in AMD is unlikely to be related to autoimmunity. The nature of the systemic immune activation should be considered as an adaptive response to age-related insults, with increased magnitude in AMD patients. The amplified response in AMD patients may be related to the nature/amount of insults accumulated during aging as a result of genetic predisposition or epigenetic/environmental influence. For example, if the anti-oxidant system does not function as well as it should due to genetic predisposition, or the patient has an unhealthy lifestyle, age-related oxidative insults may accumulate more rapidly and to a greater extent. Smoking and high-fat diet are known environmental risk factors of AMD, and both can directly impact on the immune system [171-173]. Aging can also affect the immune system [174, 175] and it is possible that immune senescence is accelerated in AMD.
Another possible cause of dysregulated systemic para-inflammation is that the immune regulatory system may not function properly in AMD patients due to genetic predisposition or epigenetic modifications. The polymorphisms in the Cfh gene (encoding the CFH protein that negatively regulates AP complement activation) is a typical example of dysfunction of immune regulators in AMD. In addition, insufficient expression of other complement regulatory proteins such as CD46 and CD59 has been observed in AMD patients [176]. Complement activation not only results in the cell killing MAC, it also generates various complement fragments such as C3a, C3b/c, C4a, and C5a that can participate in various other immune responses [92].
Malfunction of the effector arm of the immune response, e.g., monocytes/macrophages and T cells may also lead to dysregualted systemic para-inflammation. Increased expression of CCR2 in monocytes [177], and lower levels of CX3CR1 expression in CD8 T cells [178] have both been observed in AMD patients. In addition, increased CD200 expression in CD11b+ monocytes has also been observed in AMD [179]. Although the functional significance of these changes remains to be elucidated, the observations suggest that malfunction in monocytes or T cells may lead to increased systemic inflammation in AMD patients.
Conclusions and future directions
Studies in the past decade have revealed some causal links between low-grade chronic inflammation and AMD. As we age, oxidative insults accumulate, and such insults persist and increase in magnitude with age. The systemic para-inflammation is the adaptive response of the immune system to age-related insults to the whole body, whereas local para-inflammation is the response of the eye to macular insults. A healthy immune system should initiate an effective para-inflammatory response and keep it under control, as part of a healthy aging eye (Fig. 4). The para-inflammatory response may become dysregulated due to genetic predisposition, epigenetic modification or environmental intervention, and dysregulated para-inflammation (chronic inflammation) is detrimental and contributes to macular pathology (i.e. AMD) (Fig. 4).
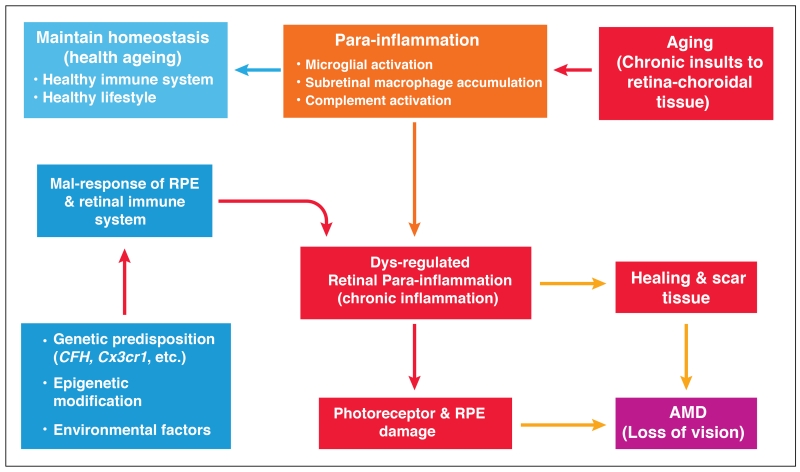
Figure 4. Dysregulated para-inflammation and the pathogenesis of AMD.
As we age, oxidative insults accumulate in the macula. A para-inflammatory response characterized by microglial activation, subretinal accumulation and complement activation is initiated to promote macular repair. A healthy immune system should be able to maintain macular homeostasis through para-inflammation. In AMD, the para-inflammatory response is dysregulated due to a) genetic predisposition, b) epigenetic modification, or c) environmental factors. The dysregulated para-inflammation (i.e., chronic inflammation) results in various pro-inflammatory cytokine production or inflammasome activation, which damages RPE and the photoreceptors and leads to the development of AMD. Sustained chronic inflammation at the macula may also lead to scar formation, which can also lead to loss of vision.
Further studies are necessary to understand how and why the para-inflammatory response becomes dysregulated in AMD. More knowledge on how the systemic and local retinal immune responses are connected in AMD will help to understand whether or not AMD is a disease with systemic immune dysregulation. In addition, inducers of subretinal para-inflammation in the aging eye and in AMD remain to be fully characterized. The immune response in the choroid in AMD is under-investigated. Para-inflammatory response also presents in the aging choroid [100], and the degeneration of choroidal vascular has been proposed as an early event in both GA and nAMD [180]. It is possible that CNV in nAMD might be initiated by dysregulated choroidal para-inflammation. More knowledge about the inflammatory mediators released by RPE cells, photoreceptors and subretinal/choroidal macrophages in the aging eye and in AMD may help to identify novel targets for anti-inflammatory therapy. The communication between subretinal macrophages, RPE cells, photoreceptors and choroidal macrophages in the aging eye and in AMD will be a challenging but important topic of future research and may uncover new insights into AMD pathogenesis.
Acknowledgements
Funding support is provided by Fight for Sight (1361/1362; 1425/1426), Dunhill Medical Trust (R188/0211), Research into Ageing (322), and National Eye Research Center (SCIAD 061). The authors acknowledge the reserachers who have greatly contributed to this filed but whose work was not cited as a result of space limitations. The authors thank Dr Derek Brazil and Dr Adrien Kissenpfennig for critically reading the manuscript and for helping with English expression.
Abbreviations
- AGE
Advanced glycation end-product
- AMD
age-related macular degeneration
- AP
Alternative pathway (of the complement system)
- APC
Antigen presenting cell
- BM
Bruch’s membrane
- BRB
blood retina barrier
- CCR2
C chemokine receptor 2
- CCL2
Chemokine C-C motif ligand 2
- CFB
complement factor B
- CFH
complement factor H
- CP
Classical pathway
- CRP
C-reactive protein
- CTLA
Cytotoxic T-lymphocyte associated protein
- CX3CL1
Chemokine C-X3-C motif ligand 1
- CX3CR1
CX3C chemokine receptor 1
- DAF
Decay-acceleration factor
- DAMP
Danger associated molecular pattern
- FasL
Fas ligand
- GA
grographic atrophy
- GL
ganglion layer
- HLA
Human leukocyte antigen
- iBRB
inner blood retinal barrier
- IPL
inner plexiform layer
- IL-1β
Interleukin 1 beta
- IL-4
Interleukin 4
- IL-17
Interleukin 17
- LPS
Lipopolysaccharide
- MAC
Membrane attack complex
- MHC-II
Major histocompatibility complex II
- NLR
NOD-like receptor
- NLRP3
NACHT, LRR and PYD domains-containing protein 3
- oBRB
outer blood retinal barrier
- OPL
outer plexiform layer
- RPE
Retinal pigment epithelium
- PRR
Pattern recognition receptor
- SASP
Senescent-associated secretory phenotype
- TGFβ
Transforming growth factor beta
- TNFα
Tumor necrosis factor alpha
- TLR
Toll-like receptor
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
The authors declare no competing financial interests.
References
- 1.Mangge H, Almer G, Truschnig-Wilders M, Schmidt A, Gasser R, Fuchs D. Inflammation, adiponectin, obesity and cardiovascular risk. Curr.Med.Chem. 2010;17:4511–4520. doi: 10.2174/092986710794183006. [DOI] [PubMed] [Google Scholar]
- 2.Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clin.Pharmacol.Ther. 2010;87:407–416. doi: 10.1038/clpt.2009.311. [DOI] [PubMed] [Google Scholar]
- 3.Lathe R, Sapronova A, Kotelevtsev Y. Atherosclerosis and Alzheimer--diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr. 2014;14 doi: 10.1186/1471-2318-14-36. 36-2318-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37:1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- 5.McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann.N.Y.Acad.Sci. 2004;1035:104–116. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- 6.Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer’s disease. Neurobiol.Dis. 2010;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat.Disord. 2004;10(Suppl 1):S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br.J.Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am.J.Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 10.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat.Rev.Immunol. 2013;13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 12.Goto M. Inflammaging (inflammation + aging): A driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? Biosci.Trends. 2008;2:218–230. [PubMed] [Google Scholar]
- 13.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J.Gerontol.A Biol.Sci.Med.Sci. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 14.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob.Health. 2014;2:e106–16. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 15.Coleman HR, Chan CC, Ferris FL, 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N.Engl.J.Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 17.Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest.Ophthalmol.Vis.Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- 18.Hjelmeland LM, Cristofolo VJ, Funk W, Rakoczy E, Katz ML. Senescence of the retinal pigment epithelium. Mol.Vis. 1999;5:33. [PubMed] [Google Scholar]
- 19.Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye (Lond) 2001;15:384–389. doi: 10.1038/eye.2001.141. [DOI] [PubMed] [Google Scholar]
- 20.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog.Retin.Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 21.VanNewkirk MR, Nanjan MB, Wang JJ, Mitchell P, Taylor HR, McCarty CA. The prevalence of age-related maculopathy: the visual impairment project. Ophthalmology. 2000;107:1593–1600. doi: 10.1016/s0161-6420(00)00175-5. [DOI] [PubMed] [Google Scholar]
- 22.Joachim N, Mitchell P, Rochtchina E, Tan AG, Wang JJ. Incidence and progression of reticular drusen in age-related macular degeneration: findings from an older Australian cohort. Ophthalmology. 2014;121:917–925. doi: 10.1016/j.ophtha.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 23.IVAN Study Investigators. Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, Reeves BC. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL., 3rd Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 26.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog.Retin.Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog.Retin.Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch.Clin.Exp.Ophthalmol. 1985;223:69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 29.Penfold PL, Liew SC, Madigan MC, Provis JM. Modulation of major histocompatibility complex class II expression in retinas with age-related macular degeneration. Invest.Ophthalmol.Vis.Sci. 1997;38:2125–2133. [PubMed] [Google Scholar]
- 30.Tuo J, Grob S, Zhang K, Chan CC. Genetics of immunological and inflammatory components in age-related macular degeneration. Ocul.Immunol.Inflamm. 2012;20:27–36. doi: 10.3109/09273948.2011.628432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat.Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 32.Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J.Clin.Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M, Hombrebueno JR, Luo C, Penalva R, Zhao J, Colhoun L, Pandi SP, Forrester JV, Xu H. Age- and Light-Dependent Development of Localised Retinal Atrophy in CCL2(−/−)CX3CR1(GFP/GFP) Mice. PLoS One. 2013;8:e61381. doi: 10.1371/journal.pone.0061381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen M, Forrester JV, Xu H. Dysregulation in retinal para-inflammation and age-related retinal degeneration in CCL2 or CCR2 deficient mice. PLoS One. 2011;6:e22818. doi: 10.1371/journal.pone.0022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ufret-Vincenty RL, Aredo B, Liu X, McMahon A, Chen PW, Sun H, Niederkorn JY, Kedzierski W. Transgenic mice expressing variants of complement factor H develop AMD-like retinal findings. Invest.Ophthalmol.Vis.Sci. 2010;51:5878–5887. doi: 10.1167/iovs.09-4457. [DOI] [PubMed] [Google Scholar]
- 36.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J.Leukoc.Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- 38.Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK. The neuroprotective potential of heat shock protein 70 (HSP70) Mol.Med.Today. 1999;5:525–531. doi: 10.1016/s1357-4310(99)01599-3. [DOI] [PubMed] [Google Scholar]
- 39.Burel C, Mezger V, Pinto M, Rallu M, Trigon S, Morange M. Mammalian heat shock protein families. Expression and functions. Experientia. 1992;48:629–634. doi: 10.1007/BF02118307. [DOI] [PubMed] [Google Scholar]
- 40.Navarro-Yepes J, Burns M, Anandhan A, Khalimonchuk O, del Razo LM, Quintanilla-Vega B, Pappa A, Panayiotidis MI, Franco R. Oxidative stress, redox signaling, and autophagy: cell death versus survival. Antioxid.Redox Signal. 2014;21:66–85. doi: 10.1089/ars.2014.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blasiak J, Petrovski G, Vereb Z, Facsko A, Kaarniranta K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. Biomed.Res.Int. 2014;2014:768026. doi: 10.1155/2014/768026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 44.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J.Clin.Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Modulation of the microenvironment by senescent biliary epithelial cells may be involved in the pathogenesis of primary biliary cirrhosis. J.Hepatol. 2010;53:318–325. doi: 10.1016/j.jhep.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell.Death Dis. 2013;4:e911. doi: 10.1038/cddis.2013.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29:273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fumagalli M, d’Adda di Fagagna F. SASPense and DDRama in cancer and ageing. Nat.Cell Biol. 2009;11:921–923. doi: 10.1038/ncb0809-921. [DOI] [PubMed] [Google Scholar]
- 49.Ghaemi-Oskouie F, Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr.Rheumatol.Rep. 2011;13:160–166. doi: 10.1007/s11926-011-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am.J.Physiol.Cell.Physiol. 2006;290:C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 51.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol.Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 52.Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol. 2005;26:381–387. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Sen J, Belli A. S100B in neuropathologic states: the CRP of the brain? J.Neurosci.Res. 2007;85:1373–1380. doi: 10.1002/jnr.21211. [DOI] [PubMed] [Google Scholar]
- 54.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 55.Streilein JW, Ma N, Wenkel H, Ng TF, Zamiri P. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res. 2002;42:487–495. doi: 10.1016/s0042-6989(01)00185-7. [DOI] [PubMed] [Google Scholar]
- 56.Forrester JV, Xu H, Lambe T, Cornall R. Immune privilege or privileged immunity? Mucosal Immunol. 2008;1:372–381. doi: 10.1038/mi.2008.27. [DOI] [PubMed] [Google Scholar]
- 57.Gregerson DS. Immune privilege in the retina. Ocul.Immunol.Inflamm. 1998;6:257–267. doi: 10.1076/ocii.6.4.257.4029. [DOI] [PubMed] [Google Scholar]
- 58.Forrester JV, Xu H. Good news-bad news: the Yin and Yang of immune privilege in the eye. Front.Immunol. 2012;3:338. doi: 10.3389/fimmu.2012.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avichezer D, Grajewski RS, Chan CC, Mattapallil MJ, Silver PB, Raber JA, Liou GI, Wiggert B, Lewis GM, Donoso LA, Caspi RR. An immunologically privileged retinal antigen elicits tolerance: major role for central selection mechanisms. J.Exp.Med. 2003;198:1665–1676. doi: 10.1084/jem.20030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forrester JV, Xu H, Kuffova L, Dick AD, McMenamin PG. Dendritic cell physiology and function in the eye. Immunol.Rev. 2010;234:282–304. doi: 10.1111/j.0105-2896.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 61.Streilein JW. Immunoregulatory mechanisms of the eye. Prog.Retin.Eye Res. 1999;18:357–370. doi: 10.1016/s1350-9462(98)00022-6. [DOI] [PubMed] [Google Scholar]
- 62.Wenkel H, Streilein JW. Evidence that retinal pigment epithelium functions as an immune-privileged tissue. Invest.Ophthalmol.Vis.Sci. 2000;41:3467–3473. [PubMed] [Google Scholar]
- 63.Dick AD, Carter D, Robertson M, Broderick C, Hughes E, Forrester JV, Liversidge J. Control of myeloid activity during retinal inflammation. J.Leukoc.Biol. 2003;74:161–166. doi: 10.1189/jlb.1102535. [DOI] [PubMed] [Google Scholar]
- 64.Mochizuki M, Sugita S, Kamoi K. Immunological homeostasis of the eye. Prog.Retin.Eye Res. 2013;33:10–27. doi: 10.1016/j.preteyeres.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Kawazoe Y, Sugita S, Keino H, Yamada Y, Imai A, Horie S, Mochizuki M. Retinoic acid from retinal pigment epithelium induces T regulatory cells. Exp.Eye Res. 2012;94:32–40. doi: 10.1016/j.exer.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Horie S, Sugita S, Futagami Y, Yamada Y, Mochizuki M. Human retinal pigment epithelium-induced CD4+CD25+ regulatory T cells suppress activation of intraocular effector T cells. Clin.Immunol. 2010;136:83–95. doi: 10.1016/j.clim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Sugita S, Usui Y, Horie S, Futagami Y, Aburatani H, Okazaki T, Honjo T, Takeuchi M, Mochizuki M. T cell suppression by programmed cell death 1 ligand 1 on retinal pigment epithelium during inflammatory conditions. Invest.Ophthalmol.Vis.Sci. 2009 doi: 10.1167/iovs.08-2846. [DOI] [PubMed] [Google Scholar]
- 68.Sugita S, Horie S, Nakamura O, Futagami Y, Takase H, Keino H, Aburatani H, Katunuma N, Ishidoh K, Yamamoto Y, Mochizuki M. Retinal pigment epithelium-derived CTLA-2alpha induces TGFbeta-producing T regulatory cells. J.Immunol. 2008;181:7525–7536. doi: 10.4049/jimmunol.181.11.7525. [DOI] [PubMed] [Google Scholar]
- 69.Zamiri P, Masli S, Kitaichi N, Taylor AW, Streilein JW. Thrombospondin plays a vital role in the immune privilege of the eye. Invest.Ophthalmol.Vis.Sci. 2005;46:908–919. doi: 10.1167/iovs.04-0362. [DOI] [PubMed] [Google Scholar]
- 70.Ferguson TA, Griffith TS. A vision of cell death: insights into immune privilege. Immunol.Rev. 1997;156:167–184. doi: 10.1111/j.1600-065x.1997.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 71.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferguson TA, Griffith TS. The role of Fas ligand and TNF-related apoptosis-inducing ligand (TRAIL) in the ocular immune response. Chem.Immunol.Allergy. 2007;92:140–154. doi: 10.1159/000099265. [DOI] [PubMed] [Google Scholar]
- 73.Sohn JH, Kaplan HJ, Suk HJ, Bora PS, Bora NS. Chronic low level complement activation within the eye is controlled by intraocular complement regulatory proteins. Invest.Ophthalmol.Vis.Sci. 2000;41:3492–3502. [PMC free article] [PubMed] [Google Scholar]
- 74.Sohn JH, Bora PS, Jha P, Tezel TH, Kaplan HJ, Bora NS. Complement, innate immunity and ocular disease. Chem.Immunol.Allergy. 2007;92:105–114. doi: 10.1159/000099261. [DOI] [PubMed] [Google Scholar]
- 75.Taylor AW. Neuroimmunomodulation in immune privilege: role of neuropeptides in ocular immunosuppression. Neuroimmunomodulation. 1996;3:195–204. doi: 10.1159/000097271. [DOI] [PubMed] [Google Scholar]
- 76.Taylor AW, Lee D. Applications of the role of alpha-MSH in ocular immune privilege. Adv.Exp.Med.Biol. 2010;681:143–149. doi: 10.1007/978-1-4419-6354-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li L, Eter N, Heiduschka P. The microglia in healthy and diseased retina. Exp.Eye Res. 2015 Jul;136:116–130. doi: 10.1016/j.exer.2015.04.020. doi: 10.1016/j.exer.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 78.Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Prog.Retin.Eye Res. 2015;45:30–57. doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 79.Karlstetter M, Ebert S, Langmann T. Microglia in the healthy and degenerating retina: insights from novel mouse models. Immunobiology. 2010;215:685–691. doi: 10.1016/j.imbio.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 80.Langmann T. Microglia activation in retinal degeneration. J.Leukoc.Biol. 2007;81:1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- 81.Lehnardt S, Henneke P, Lien E, Kasper DL, Volpe JJ, Bechmann I, Nitsch R, Weber JR, Golenbock DT, Vartanian T. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J.Immunol. 2006;177:583–592. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- 82.Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J.Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 83.Dick AD, Ford AL, Forrester JV, Sedgwick JD. Flow cytometric identification of a minority population of MHC class II positive cells in the normal rat retina distinct from CD45lowCD11b/c+CD4low parenchymal microglia. Br.J.Ophthalmol. 1995;79:834–840. doi: 10.1136/bjo.79.9.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J.Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 85.Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T. Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Curr.HIV.Res. 2014;12:97–110. doi: 10.2174/1570162x12666140526114956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soulas C, Donahue RE, Dunbar CE, Persons DA, Alvarez X, Williams KC. Genetically modified CD34+ hematopoietic stem cells contribute to turnover of brain perivascular macrophages in long-term repopulated primates. Am.J.Pathol. 2009;174:1808–1817. doi: 10.2353/ajpath.2009.081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J, Wu GS, Ishimoto S, Pararajasegaram G, Rao NA. Expression of major histocompatibility complex molecules in rodent retina. Immunohistochemical study. Invest.Ophthalmol.Vis.Sci. 1997;38:1848–1857. [PubMed] [Google Scholar]
- 88.Gregerson DS, Yang J. CD45-positive cells of the retina and their responsiveness to in vivo and in vitro treatment with IFN-gamma or anti-CD40. Invest.Ophthalmol.Vis.Sci. 2003;44:3083–3093. doi: 10.1167/iovs.02-1014. [DOI] [PubMed] [Google Scholar]
- 89.Lehmann U, Heuss ND, McPherson SW, Roehrich H, Gregerson DS. Dendritic cells are early responders to retinal injury. Neurobiol.Dis. 2010;40:177–184. doi: 10.1016/j.nbd.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen X, Kezic J, Bernard C, McMenamin PG. Rd8 mutation in the Crb1 gene of CD11c-eYFP transgenic reporter mice results in abnormal numbers of CD11c-positive cells in the retina. J.Neuropathol.Exp.Neurol. 2013;72:782–790. doi: 10.1097/NEN.0b013e31829e8375. [DOI] [PubMed] [Google Scholar]
- 91.Xu H, Dawson R, Forrester JV, Liversidge J. Identification of novel dendritic cell populations in normal mouse retina. Invest.Ophthalmol.Vis.Sci. 2007;48:1701–1710. doi: 10.1167/iovs.06-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat.Rev.Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 93.Chen M, Muckersie E, Luo C, Forrester JV, Xu H. Inhibition of the alternative pathway of complement activation reduces inflammation in experimental autoimmune uveoretinitis. Eur.J.Immunol. 2010;40:2870–2881. doi: 10.1002/eji.201040323. [DOI] [PubMed] [Google Scholar]
- 94.Copland DA, Hussain K, Baalasubramanian S, Hughes TR, Morgan BP, Xu H, Dick AD, Nicholson LB. Systemic and local anti-C5 therapy reduces the disease severity in experimental autoimmune uveoretinitis. Clin.Exp.Immunol. 2010;159:303–314. doi: 10.1111/j.1365-2249.2009.04070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vergani S, Di Mauro E, Davies ET, Spinelli D, Mieli-Vergani G, Vergani D. Complement activation in uveitis. Br.J.Ophthalmol. 1986;70:60–63. doi: 10.1136/bjo.70.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Y, Lu HL, Zhang J, Yu HY, Wang HW, Zhang MX, Cianflone K. Relationships among acylation stimulating protein, adiponectin and complement C3 in lean vs obese type 2 diabetes. Int.J.Obes.(Lond) 2006;30:439–446. doi: 10.1038/sj.ijo.0803173. [DOI] [PubMed] [Google Scholar]
- 97.Luo C, Chen M, Xu H. Complement gene expression and regulation in mouse retina and retinal pigment epithelium/choroid. Mol.Vis. 2011;17:1588–1597. [PMC free article] [PubMed] [Google Scholar]
- 98.Chen M, Forrester JV, Xu H. Synthesis of complement factor H by retinal pigment epithelial cells is down-regulated by oxidized photoreceptor outer segments. Exp.Eye Res. 2007;84:635–645. doi: 10.1016/j.exer.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 99.Chen M, Muckersie E, Robertson M, Forrester JV, Xu H. Up-regulation of complement factor B in retinal pigment epithelial cells is accompanied by complement activation in the aged retina. Exp.Eye Res. 2008;87:543–550. doi: 10.1016/j.exer.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 100.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog.Retin.Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Glenn JV, Mahaffy H, Wu K, Smith G, Nagai R, Simpson DA, Boulton ME, Stitt AW. Advanced glycation end product (AGE) accumulation on Bruch’s membrane: links to age-related RPE dysfunction. Invest.Ophthalmol.Vis.Sci. 2009;50:441–451. doi: 10.1167/iovs.08-1724. [DOI] [PubMed] [Google Scholar]
- 102.Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- 103.Tian J, Ishibashi K, Ishibashi K, Reiser K, Grebe R, Biswal S, Gehlbach P, Handa JT. Advanced glycation endproduct-induced aging of the retinal pigment epithelium and choroid: a comprehensive transcriptional response. Proc.Natl.Acad.Sci.U.S.A. 2005;102:11846–11851. doi: 10.1073/pnas.0504759102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 105.Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer’s A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc.Natl.Acad.Sci.U.S.A. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sparrow JR, Fishkin N, Zhou J, Cai B, Jang YP, Krane S, Itagaki Y, Nakanishi K. A2E, a byproduct of the visual cycle. Vision Res. 2003;43:2983–2990. doi: 10.1016/s0042-6989(03)00475-9. [DOI] [PubMed] [Google Scholar]
- 107.Inoue Y, Yoneda M, Miyaishi O, Iwaki M, Zako M. Hyaluronan dynamics during retinal development. Brain Res. 2009;1256:55–60. doi: 10.1016/j.brainres.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 108.Steinle JJ, Sharma S, Smith CP, McFayden-Ketchum LS. Normal aging involves modulation of specific inflammatory markers in the rat retina and choroid. J.Gerontol.A Biol.Sci.Med.Sci. 2009;64:325–331. doi: 10.1093/gerona/gln052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen M, Muckersie E, Forrester JV, Xu H. Immune activation in Retinal Aging: A Gene Expression Study. Invest.Ophthalmol.Vis.Sci. 2010;51:5888–5896. doi: 10.1167/iovs.09-5103. [DOI] [PubMed] [Google Scholar]
- 110.Ida H, Boylan SA, Weigel AL, Hjelmeland LM. Age-related changes in the transcriptional profile of mouse RPE/choroid. Physiol.Genomics. 2003;15:258–262. doi: 10.1152/physiolgenomics.00126.2003. [DOI] [PubMed] [Google Scholar]
- 111.Chen H, Liu B, Lukas TJ, Neufeld AH. The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration. PLoS ONE. 2008;3:e2339. doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kurji KH, Cui JZ, Lin T, Harriman D, Prasad SS, Kojic L, Matsubara JA. Microarray analysis identifies changes in inflammatory gene expression in response to amyloid-beta stimulation of cultured human retinal pigment epithelial cells. Invest.Ophthalmol.Vis.Sci. 2010;51:1151–1163. doi: 10.1167/iovs.09-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu C, Cao L, Yang S, Xu L, Liu P, Wang F, Xu D. Subretinal injection of amyloid-beta peptide accelerates RPE cell senescence and retinal degeneration. Int.J.Mol.Med. 2015;35:169–176. doi: 10.3892/ijmm.2014.1993. [DOI] [PubMed] [Google Scholar]
- 114.Liu XC, Liu XF, Jian CX, Li CJ, He SZ. IL-33 is induced by amyloid-beta stimulation and regulates inflammatory cytokine production in retinal pigment epithelium cells. Inflammation. 2012;35:776–784. doi: 10.1007/s10753-011-9379-4. [DOI] [PubMed] [Google Scholar]
- 115.Chan-Ling T, Hughes S, Baxter L, Rosinova E, McGregor I, Morcos Y, van Nieuwenhuyzen P, Hu P. Inflammation and breakdown of the blood-retinal barrier during “physiological aging” in the rat retina: a model for CNS aging. Microcirculation. 2007;14:63–76. doi: 10.1080/10739680601073451. [DOI] [PubMed] [Google Scholar]
- 116.Xu H, Chen M, Manivannan A, Lois N, Forrester JV. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008;7:58–68. doi: 10.1111/j.1474-9726.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 117.Ardeljan D, Chan CC. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog.Retin.Eye Res. 2013;37:68–89. doi: 10.1016/j.preteyeres.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aspengren S, Hedberg D, Wallin M. Studies of pigment transfer between Xenopus laevis melanophores and fibroblasts in vitro and in vivo. Pigment Cell Res. 2006;19:136–145. doi: 10.1111/j.1600-0749.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 119.Luo C, Zhao J, Madden A, Chen M, Xu H. Complement expression in retinal pigment epithelial cells is modulated by activated macrophages. Exp.Eye Res. 2013;112C:93–101. doi: 10.1016/j.exer.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 120.Seth A, Cui J, To E, Kwee M, Matsubara J. Complement-associated deposits in the human retina. Invest.Ophthalmol.Vis.Sci. 2008;49:743–750. doi: 10.1167/iovs.07-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao T, Gao J, Van J, To E, Wang A, Cao S, Cui JZ, Guo JP, Lee M, McGeer PL, Matsubara JA. Age-related increases in amyloid beta and membrane attack complex: evidence of inflammasome activation in the rodent eye. J.Neuroinflammation. 2015;12 doi: 10.1186/s12974-015-0337-1. 121-015-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rus HG, Niculescu F, Shin ML. Sublytic complement attack induces cell cycle in oligodendrocytes. J.Immunol. 1996;156:4892–4900. [PubMed] [Google Scholar]
- 123.Tegla CA, Cudrici C, Rus V, Ito T, Vlaicu S, Singh A, Rus H. Neuroprotective effects of the complement terminal pathway during demyelination: implications for oligodendrocyte survival. J.Neuroimmunol. 2009;213:3–11. doi: 10.1016/j.jneuroim.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S, Albuquerque RJ, Hauswirth WW, Chiodo VA, Kugel JF, Goodrich JA, Ponicsan SL, Chaudhuri G, Murphy MP, Dunaief JL, Ambati BK, Ogura Y, Yoo JW, Lee DK, Provost P, Hinton DR, Nunez G, Baffi JZ, Kleinman ME, Ambati J. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tseng WA, Thein T, Kinnunen K, Lashkari K, Gregory MS, D’Amore PA, Ksander BR. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest.Ophthalmol.Vis.Sci. 2013;54:110–120. doi: 10.1167/iovs.12-10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu RT, Gao J, Cao S, Sandhu N, Cui JZ, Chou CL, Fang E, Matsubara JA. Inflammatory mediators induced by amyloid-beta in the retina and RPE in vivo: implications for inflammasome activation in age-related macular degeneration. Invest.Ophthalmol.Vis.Sci. 2013;54:2225–2237. doi: 10.1167/iovs.12-10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anderson OA, Finkelstein A, Shima DT. A2E induces IL-1ss production in retinal pigment epithelial cells via the NLRP3 inflammasome. PLoS One. 2013;8:e67263. doi: 10.1371/journal.pone.0067263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brandstetter C, Mohr LK, Latz E, Holz FG, Krohne TU. Light induces NLRP3 inflammasome activation in retinal pigment epithelial cells via lipofuscin-mediated photooxidative damage. J.Mol.Med.(Berl) 2015 doi: 10.1007/s00109-015-1275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kauppinen A, Niskanen H, Suuronen T, Kinnunen K, Salminen A, Kaarniranta K. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells-Implications for age-related macular degeneration (AMD) Immunol.Lett. 2012;147:29–33. doi: 10.1016/j.imlet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 130.Kerur N, Hirano Y, Tarallo V, Fowler BJ, Bastos-Carvalho A, Yasuma T, Yasuma R, Kim Y, Hinton DR, Kirschning CJ, Gelfand BD, Ambati J. TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Invest.Ophthalmol.Vis.Sci. 2013;54:7395–7401. doi: 10.1167/iovs.13-12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sparrow JR, Cai B, Fishkin N, Jang YP, Krane S, Vollmer HR, Zhou J, Nakanishi K. A2E, a fluorophore of RPE lipofuscin: can it cause RPE degeneration? Adv.Exp.Med.Biol. 2003;533:205–211. doi: 10.1007/978-1-4615-0067-4_26. [DOI] [PubMed] [Google Scholar]
- 132.Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009;4:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W, Jr, Ding J, Bowes Rickman C, Boulton M. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10:1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Viiri J, Amadio M, Marchesi N, Hyttinen JM, Kivinen N, Sironen R, Rilla K, Akhtar S, Provenzani A, D’Agostino VG, Govoni S, Pascale A, Agostini H, Petrovski G, Salminen A, Kaarniranta K. Autophagy activation clears ELAVL1/HuR-mediated accumulation of SQSTM1/p62 during proteasomal inhibition in human retinal pigment epithelial cells. PLoS One. 2013;8:e69563. doi: 10.1371/journal.pone.0069563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Valapala M, Wilson C, Hose S, Bhutto IA, Grebe R, Dong A, Greenbaum S, Gu L, Sengupta S, Cano M, Hackett S, Xu G, Lutty GA, Dong L, Sergeev Y, Handa JT, Campochiaro P, Wawrousek E, Zigler JS, Jr, Sinha D. Lysosomal-mediated waste clearance in retinal pigment epithelial cells is regulated by CRYBA1/betaA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy. 2014;10:480–496. doi: 10.4161/auto.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao H, Roychoudhury J, Doggett TA, Apte RS, Ferguson TA. Age-dependent changes in FasL (CD95L) modulate macrophage function in a model of age-related macular degeneration. Invest.Ophthalmol.Vis.Sci. 2013;54:5321–5331. doi: 10.1167/iovs.13-12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bhutto IA, Baba T, Merges C, Juriasinghani V, McLeod DS, Lutty GA. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br.J.Ophthalmol. 2011;95:1323–1330. doi: 10.1136/bjo.2010.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J.Pathol. 2013;229:729–742. doi: 10.1002/path.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Skerka C, Lauer N, Weinberger AA, Keilhauer CN, Suhnel J, Smith R, Schlotzer-Schrehardt U, Fritsche L, Heinen S, Hartmann A, Weber BH, Zipfel PF. Defective complement control of factor H (Y402H) and FHL-1 in age-related macular degeneration. Mol.Immunol. 2007;44:3398–3406. doi: 10.1016/j.molimm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 140.Clark SJ, Bishop PN, Day AJ. Complement factor H and age-related macular degeneration: the role of glycosaminoglycan recognition in disease pathology. Biochem.Soc.Trans. 2010;38:1342–1348. doi: 10.1042/BST0381342. [DOI] [PubMed] [Google Scholar]
- 141.Shaw PX, Zhang L, Zhang M, Du H, Zhao L, Lee C, Grob S, Lim SL, Hughes G, Lee J, Bedell M, Nelson MH, Lu F, Krupa M, Luo J, Ouyang H, Tu Z, Su Z, Zhu J, Wei X, Feng Z, Duan Y, Yang Z, Ferreyra H, Bartsch DU, Kozak I, Zhang L, Lin F, Sun H, Feng H, Zhang K. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc.Natl.Acad.Sci.U.S.A. 2012;109:13757–13762. doi: 10.1073/pnas.1121309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 143.Vessey KA, Greferath U, Jobling AI, Phipps JA, Ho T, Waugh M, Fletcher EL. Ccl2/Cx3cr1 knock-out mice have inner retinal dysfunction but are not an accelerated model of age related macular degeneration. Invest.Ophthalmol.Vis.Sci. 2012 doi: 10.1167/iovs.12-10650. [DOI] [PubMed] [Google Scholar]
- 144.Sennlaub F, Auvynet C, Calippe B, Lavalette S, Poupel L, Hu SJ, Dominguez E, Camelo S, Levy O, Guyon E, Saederup N, Charo IF, Rooijen NV, Nandrot E, Bourges JL, Behar-Cohen F, Sahel JA, Guillonneau X, Raoul W, Combadiere C. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol.Med. 2013;5:1775–1793. doi: 10.1002/emmm.201302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hu SJ, Calippe B, Lavalette S, Roubeix C, Montassar F, Housset M, Levy O, Delarasse C, Paques M, Sahel JA, Sennlaub F, Guillonneau X. Upregulation of P2RX7 in Cx3cr1-Deficient Mononuclear Phagocytes Leads to Increased Interleukin-1beta Secretion and Photoreceptor Neurodegeneration. J.Neurosci. 2015;35:6987–6996. doi: 10.1523/JNEUROSCI.3955-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mizutani M, Pino PA, Saederup N, Charo IF, Ransohoff RM, Cardona AE. The Fractalkine Receptor but Not CCR2 Is Present on Microglia from Embryonic Development throughout Adulthood. J.Immunol. 2012;188:29–36. doi: 10.4049/jimmunol.1100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tuo J, Smith BC, Bojanowski CM, Meleth AD, Gery I, Csaky KG, Chew EY, Chan CC. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scholl HP, Charbel Issa P, Walier M, Janzer S, Pollok-Kopp B, Borncke F, Fritsche LG, Chong NV, Fimmers R, Wienker T, Holz FG, Weber BH, Oppermann M. Systemic complement activation in age-related macular degeneration. PLoS ONE. 2008;3:e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Machalinska A, Dziedziejko V, Mozolewska-Piotrowska K, Karczewicz D, Wiszniewska B, Machalinski B. Elevated plasma levels of C3a complement compound in the exudative form of age-related macular degeneration. Ophthalmic Res. 2009;42:54–59. doi: 10.1159/000219686. [DOI] [PubMed] [Google Scholar]
- 150.Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291:704–710. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- 151.Kikuchi M, Nakamura M, Ishikawa K, Suzuki T, Nishihara H, Yamakoshi T, Nishio K, Taki K, Niwa T, Hamajima N, Terasaki H. Elevated C-reactive protein levels in patients with polypoidal choroidal vasculopathy and patients with neovascular age-related macular degeneration. Ophthalmology. 2007;114:1722–1727. doi: 10.1016/j.ophtha.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 152.Min JK, Kim J, Woo JM. Elevated Plasma Pentraxin3 Levels and Its Association with Neovascular Age-related Macular Degeneration. Ocul.Immunol.Inflamm. 2014 doi: 10.3109/09273948.2014.891755. [DOI] [PubMed] [Google Scholar]
- 153.Cherepanoff S, Mitchell P, Wang JJ, Gillies MC. Retinal autoantibody profile in early age-related macular degeneration: preliminary findings from the Blue Mountains Eye Study. Clin.Experiment.Ophthalmol. 2006;34:590–595. doi: 10.1111/j.1442-9071.2006.01281.x. [DOI] [PubMed] [Google Scholar]
- 154.Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J.Biol.Chem. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 155.Hoffman M, Blum A, Baruch R, Kaplan E, Benjamin M. Leukocytes and coronary heart disease. Atherosclerosis. 2004;172:1–6. doi: 10.1016/s0021-9150(03)00164-3. [DOI] [PubMed] [Google Scholar]
- 156.Smith W, Mitchell P, Leeder SR, Wang JJ. Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy: the Blue Mountains Eye Study. Arch.Ophthalmol. 1998;116:583–587. doi: 10.1001/archopht.116.5.583. [DOI] [PubMed] [Google Scholar]
- 157.Shankar A, Mitchell P, Rochtchina E, Tan J, Wang JJ. Association between circulating white blood cell count and long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Am.J.Epidemiol. 2007;165:375–382. doi: 10.1093/aje/kwk022. [DOI] [PubMed] [Google Scholar]
- 158.Ilhan N, Daglioglu MC, Ilhan O, Coskun M, Tuzcu EA, Kahraman H, Keskin U. The Significance of the Neutrophil/Lymphocyte Ratio as a Simple Indicator of Inflammation in Age-related Macular Degeneration. Ocul.Immunol.Inflamm. 2014:1–2. doi: 10.3109/09273948.2014.970283. [DOI] [PubMed] [Google Scholar]
- 159.Ozgonul C, Sertoglu E. Accurate Use of Neutrophil/Lymphocyte Ratio in Patients with Age-related Macular Degeneration. Ocul.Immunol.Inflamm. 2014:1–2. doi: 10.3109/09273948.2014.970281. [DOI] [PubMed] [Google Scholar]
- 160.Nassar K, Grisanti S, Elfar E, Luke J, Luke M, Grisanti S. Serum cytokines as biomarkers for age-related macular degeneration. Graefes Arch.Clin.Exp.Ophthalmol. 2015;253:699–704. doi: 10.1007/s00417-014-2738-8. [DOI] [PubMed] [Google Scholar]
- 161.Liu B, Wei L, Meyerle C, Tuo J, Sen HN, Li Z, Chakrabarty S, Agron E, Chan CC, Klein ML, Chew E, Ferris F, Nussenblatt RB. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. J.Transl.Med. 2011;9:1–12. doi: 10.1186/1479-5876-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shin JI, Bayry J. A role for IL-17 in age-related macular degeneration. Nat.Rev.Immunol. 2013;13:701–c1. doi: 10.1038/nri3459-c1. [DOI] [PubMed] [Google Scholar]
- 163.Taggart Starr CS. Biology: The Unity and Diversity of Life. Wadsworth; California: 1989. p. 398. [Google Scholar]
- 164.Swanson MW, McGwin G., Jr. Anti-inflammatory drug use and age-related macular degeneration. Optom.Vis.Sci. 2008;85:947–950. doi: 10.1097/OPX.0b013e31818883e0. [DOI] [PubMed] [Google Scholar]
- 165.McGeer PL, Sibley J. Sparing of age-related macular degeneration in rheumatoid arthritis. Neurobiol.Aging. 2005;26:1199–1203. doi: 10.1016/j.neurobiolaging.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 166.Nussenblatt RB, Byrnes G, Nida H, Yeh S, Faia L, Meyerle C, Wroblewski K, Li Z, Liu B, Chew E, Sherry PR, Friedman P, Gill F, Ferris F., 3rd A Randomized Pilot Study of Systemic Immunosuppression in the Treatment of Age-Related Macular Degeneration with Choroidal Neovascularization. Retina. 2010;30:1579–1587. doi: 10.1097/IAE.0b013e3181e7978e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Flaxel C, Schain MB, Hamon SC, Francis PJ. Prospective randomized controlled trial of combination ranibizumab (Lucentis) and bromfenac (Xibrom) for neovascular age-related macular degeneration: a pilot study. Retina. 2012;32:417–423. doi: 10.1097/IAE.0b013e318229b0af. [DOI] [PubMed] [Google Scholar]
- 168.Gomi F, Sawa M, Tsujikawa M, Nishida K. Topical bromfenac as an adjunctive treatment with intravitreal ranibizumab for exudative age-related macular degeneration. Retina. 2012;32:1804–1810. doi: 10.1097/IAE.0b013e31825be87f. [DOI] [PubMed] [Google Scholar]
- 169.Ye J, Xu YF, He JJ, Lou LX. Association between aspirin use and age-related macular degeneration: a meta-analysis. Invest.Ophthalmol.Vis.Sci. 2014;55:2687–2696. doi: 10.1167/iovs.13-13206. [DOI] [PubMed] [Google Scholar]
- 170.Li L, Li W, Chen CZ, Yi ZH, Zhou YY. Is aspirin use associated with age-related macular degeneration? A meta-analysis. J.Clin.Pharm.Ther. 2015;40:144–154. doi: 10.1111/jcpt.12241. [DOI] [PubMed] [Google Scholar]
- 171.Mian MF, Lauzon NM, Stampfli MR, Mossman KL, Ashkar AA. Impairment of human NK cell cytotoxic activity and cytokine release by cigarette smoke. J.Leukoc.Biol. 2008;83:774–784. doi: 10.1189/jlb.0707481. [DOI] [PubMed] [Google Scholar]
- 172.van Kampen E, Jaminon A, van Berkel TJ, Van Eck M. Diet-induced (epigenetic) changes in bone marrow augment atherosclerosis. J.Leukoc.Biol. 2014;96:833–841. doi: 10.1189/jlb.1A0114-017R. [DOI] [PubMed] [Google Scholar]
- 173.Wallace FA, Miles EA, Evans C, Stock TE, Yaqoob P, Calder PC. Dietary fatty acids influence the production of Th1- but not Th2-type cytokines. J.Leukoc.Biol. 2001;69:449–457. [PubMed] [Google Scholar]
- 174.Desai A, Grolleau-Julius A, Yung R. Leukocyte function in the aging immune system. J.Leukoc.Biol. 2010;87:1001–1009. doi: 10.1189/jlb.0809542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Butcher SK, Chahal H, Nayak L, Sinclair A, Henriquez NV, Sapey E, O’Mahony D, Lord JM. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J.Leukoc.Biol. 2001;70:881–886. [PubMed] [Google Scholar]
- 176.Singh A, Faber C, Falk M, Nissen MH, Hviid TV, Sorensen TL. Altered expression of CD46 and CD59 on leukocytes in neovascular age-related macular degeneration. Am.J.Ophthalmol. 2012;154:193–199.e2. doi: 10.1016/j.ajo.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 177.Grunin M, Burstyn-Cohen T, Hagbi-Levi S, Peled A, Chowers I. Chemokine receptor expression in peripheral blood monocytes from patients with neovascular age-related macular degeneration. Invest.Ophthalmol.Vis.Sci. 2012;53:5292–5300. doi: 10.1167/iovs.11-9165. [DOI] [PubMed] [Google Scholar]
- 178.Falk MK, Singh A, Faber C, Nissen MH, Hviid T, Sorensen TL. Dysregulation of CXCR3 expression on peripheral blood leukocytes in patients with neovascular age-related macular degeneration. Invest.Ophthalmol.Vis.Sci. 2014;55:4050–4056. doi: 10.1167/iovs.14-14107. [DOI] [PubMed] [Google Scholar]
- 179.Singh A, Falk MK, Hviid TV, Sorensen TL. Increased expression of CD200 on circulating CD11b+ monocytes in patients with neovascular age-related macular degeneration. Ophthalmology. 2013;120:1029–1037. doi: 10.1016/j.ophtha.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 180.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol.Aspects Med. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]