Abstract
Chronic graft versus host disease (cGVHD) is a common late complication of allogenic hematopoietic stem cell transplant (HSCT). We analyzed risk factors, pattern and long term transplant outcomes of cGVHD at a tertiary cancer centre. Seventy-seven consecutive patients who underwent HSCT for acute leukemia were included. Forty (52 %) patients developed cGVHD; 24 (60 %) extensive stage while 16 (40 %) limited stage. Oral cavity was the commonest site of involvement (25 patients) followed by liver, skin and lung. We found that female donor to male recipient transplant and diagnosis of acute lymphoblastic leukemia (ALL) were the only factors associated with increased risk of cGVHD. The incidence of leukemia relapse was 18 % in patients who developed cGVHD compared to 51 % in those who did not (P = 0.002). Four year overall survival and relapse free survival (RFS) were 62 and 46 % in patients who developed cGVHD compared to 29 % (P < 0.001) and 29 % (P < 0.001) in patients who did not develop cGVHD, respectively. We conclude that cGVHD is more common in male patients with female donors and in patients transplanted for ALL. Oral cavity is the commonest site of cGVHD in our patients and transplant related survival outcomes are superior in patients who develop cGVHD.
Keywords: Acute leukemia, Chronic graft-versus-host disease, Allogeneic stem cell transplantation, Outcome, Risk factors
Introduction
Chronic graft versus host disease (cGVHD) is a late complication of allogenic hematopoietic stem cell transplantation (HSCT) and is a major cause of morbidity and mortality [1]. The incidence of cGVHD has varied from 30 to 70 % across studies [2, 3]. However, these studies are quite heterogeneous with respect to factors like diagnoses, patient-donor characteristics, type of conditioning regimen, stem cell source and GVHD prophylaxis used [2, 4, 5]. Development of cGVHD is associated with improvement in relapse free survival [2, 5, 6]. However, the impact on overall survival is less clear [2, 5]. There is limited data regarding risk factors and transplant related outcomes of cGVHD in patients with acute leukemia. In this study, we sought to determine the incidence, pattern and the risk factors for cGVHD in acute leukemia patients undergoing HSCT. We also aimed to assess the impact of cGVHD on transplant related outcomes including incidence of leukemia relapse, incidence of infective complications, relapse free survival (RFS) and overall survival (OS).
Patients and Methods
Patient and Transplant Characteristics
All patients who underwent allogeneic HSCT for acute leukemia from January 2008 to March 2013 were included in this retrospective study. Relevant data was collected from patient case records and electronic medical records. Acute myeloid leukemia (AML) patients were transplanted either in remission (Complete remission [CR]-1 or CR-2) or in disease. However, patients with ALL were transplanted only in complete remission. Poor risk acute myeloid leukemia (AML) was characterized by at least one of the following: unfavorable cytogenetics [complex karyotype, monosomal karyotype, del 5, del 7, 5q-, 7q-, inv(3), t(3;3), t(6;9), 11q23 translocations], total leukocyte count (TLC) >100 × 109/L at baseline, not achieving CR after induction, disease stage ≥ CR-2 or persistent disease at transplant. High risk acute lymphoblastic leukemia (ALL) was defined by at least one of the following characteristics- poor risk cytogenetics [t(9;22), t(1;19), t(4;11), hypodiploidy, complex karyotype], TLC >100 × 109/L at baseline, not achieving CR after induction, disease stage ≥ CR-2 or persistent disease at transplant. Human leucocyte antigen (HLA) typing for all patients and donors was done by polymerase chain reaction with sequence specific primers (SSP). Matching was done at HLA-A, HLA-B and HLA-DRB1 for all patient and donor pairs. Those with less than full match or those with unrelated donors underwent additional testing for HLA-C and HLA-DQB1. Depending on donor availability and HLA matching, patients underwent either matched related donor (MRD) transplant or matched unrelated donor (MUD) transplant or haplo-identical donor (HID) transplant. All patients received conditioning chemotherapy with either full intensity regimens (busulfan–cyclophosphamide or total body irradiation–cyclophosphamide) or with reduced intensity regimens (Fludarabine based). Reduced intensity regimens were used in those patients who had significant comorbidities or those with borderline performance status. Rabbit anti thymocyte globulin (ATG) was used in matched unrelated donor transplants and in those with one antigen mismatched related donor transplants. Stem cell graft was either T cell replete peripheral blood stem cells (PBSCs) or bone marrow harvest or cord blood derived stem cells.
GVHD Prophylaxis and Treatment
GVHD prophylaxis consisted of either cyclosporine (CsA) with methotrexate (MTX) or CsA with mycophenolate mofetil (MMF). MMF was used in patients who participated in an ongoing MMF pharmacokinetic study [7]. CsA was given at a starting dose of 1.5 mg/kg twice daily intravenously (iv) from day-1. MTX was given iv at 15 mg/m2 on D1 and 10 mg/m2 on D3, D6 and D11. Leucovorin rescue was given 24 h after methotrexate. MMF was given at a dose of 600 mg/m2 twice a day from day-1 to day +30. GVHD prophylaxis in patients undergoing haplo-identical transplant consisted of CsA+MMF (started on day +5 with MMF dose being 15 mg/kg thrice daily till day +35) along with post transplant cyclophosphamide (50 mg/kg on day +3 and day +4). CsA was tapered by 10 % per week from day +90. However, in patients with high risk of relapse, CsA tapering was started early at day +45 to +60. Diagnosis of cGVHD was made clinically and defined as extensive stage disease or limited stage disease according to standard criteria [8]. Topical steroids were used for treatment of limited stage cGVHD involving skin, oral cavity and upper gut. Systemic steroids were used in patients with visceral GVHD and in those who did not respond to topical steroids. Additionally, for lung GVHD, we used azithromycin for its immune modulatory properties, imatinib as anti-fibrosis agent and montelukast as mast cell stabilizer. CsA or MMF were used as steroid sparing agents or as second line drugs in patients whose GVHD was steroid refractory or when the disease flared up as steroids were being tapered.
Anti-infective Prophylaxis
All patients received acyclovir 400 mg thrice daily for 6 months, levofloxacin 500 mg once daily or ciprofloxacin 500 mg twice daily for 3 months, cotrimoxazole (160 mg/800 mg) twice daily on Saturdays and Sundays for 1 year and IVIG 200 mg/kg on day +1, +21, +42, +63, +84. These prophylactic medications were started along with the start of conditioning regimen. Voriconazole or posaconazole was used as antifungal prophylaxis for 3 months post transplant. Patients who developed acute or chronic GVHD and were on steroids continued with acyclovir, fluoroquinolone, cotrimoxazole and antifungal prophylaxis till they remained on steroids beyond the above time periods.
Statistical Analysis
Patients were divided in 2 groups depending on development of cGVHD. Group A included those who developed cGVHD (both limited and extensive stage) while group B included those who did not develop cGVHD. In this analysis, we also included patients not surviving till day +100 post HSCT since chronic GVHD is known to occur prior to day +100 [9]. We analyzed patient and donor age, diagnosis, gender mismatch, disease status at transplant, stem cell source, use of ATG, GVHD prophylaxis, degree of HLA match, CD3 and CD34 cells infused and acute GVHD as potential risk factors for cGVHD. Incidence of relapse, slippage of chimerism, RFS and OS were evaluated as outcome measures. Development of viral and fungal infections was also analyzed. Categorical data was analyzed using Chi square test and continuous data with Mann–Whitney test. Multivariate analysis was done using logistic regression analysis. All P values were 2 sided. Survival analysis was done with Kaplan–Meier method. SPSS version 18 was used for statistical analyses.
Results
Patient and Transplant Characteristics
Seventy seven patients underwent HSCT for acute leukemia during the study period. The baseline patient and transplant characteristics are summarized in Table 1. The median follow up time for the entire cohort of patients was 26 months.
Table 1.
Patient and transplant characteristics
| Characteristics | Number | % |
|---|---|---|
| No of patients | 77 | 100 |
| Median age at transplant | 30 | – |
| Males | 52 | 68 |
| Diagnosis | ||
| Acute lymphoid leukemia (ALL) | 23 | 30 |
| Acute myeloid leukemia (AML) | 52 | 68 |
| Biphenotypic leukemia | 2 | 2 |
| Baseline risk | ||
| ALL | ||
| Standard risk | 1 | 1 |
| Poor risk | 17 | 22 |
| Not known | 5 | 7 |
| AML | ||
| Good risk | 5 | 7 |
| Intermediate risk | 21 | 27 |
| Poor risk | 22 | 29 |
| Not known | 4 | 5 |
| Biphenotypic leukemia | 2 | 2 |
| Disease status at transplant | ||
| ALL | ||
| Complete remission-1 | 15 | 20 |
| Complete remission-2 | 8 | 10 |
| Primary refractory/relapsed | 0 | 0 |
| AML | ||
| Complete remission-1 | 27 | 35 |
| Complete remission-2 | 11 | 14 |
| Primary refractory/relapsed | 14 | 18 |
| Biphenotypic leukemia | ||
| Complete remission-1 | 0 | 0 |
| Complete remission-2 | 1 | 1 |
| Primary refractory/relapsed | 1 | 1 |
| Type of transplant | ||
| Matched related transplant | 65 | 84 |
| Matched unrelated transplant | 10 | 13 |
| Haplo-identical transplant | 2 | 2 |
| ABO mismatch transplants, | 32 | 42 |
| Gender mismatched transplants, | 40 | 52 |
| Female to male transplants, | 23 | 30 |
| Stem cell source | ||
| Bone marrow | 5 | 7 |
| Umbilical cord | 2 | 2 |
| Peripheral blood stem cells | 70 | 91 |
| Degree of HLA matching | ||
| BM or PBSC | ||
| Full matched (6/6 or 10/10) | 61 | 79 |
| 1 antigen mismatch | 9 | 12 |
| Haplo-identical transplant | 5 | 7 |
| Cord transplants | 2 | 2 |
| Type of conditioning regimen | ||
| Full intensity | 27 | 35 |
| Reduced intensity | 50 | 65 |
| ATG used | 12 | 16 |
| Graft versus host disease prophylaxis | ||
| CsA+MTX | 53 | 69 |
| CsA+MMF+/−cyclophosphamide | 24 | 31 |
Incidence and Pattern of Chronic GVHD
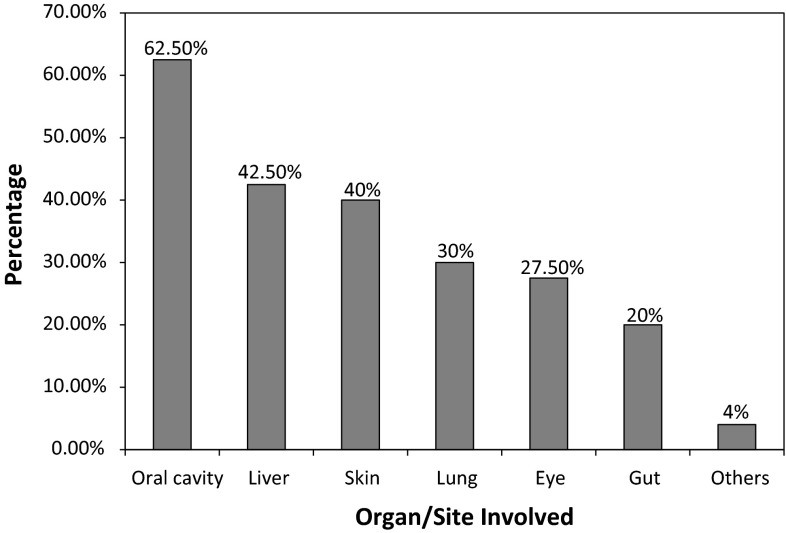
Forty (52 %) developed chronic GVHD; 60 % of these were extensive stage (Table 2). The median time to the onset of chronic GVHD was 150 days (range 35–456 days). Chronic GVHD was de novo in 19 patients. Thrombocytopenia at onset was present in six patients. Oral cavity was the commonest site involved (25 patients) followed by liver, skin and lung (Fig. 1). Twenty three patients (58 %) required systemic steroids and median duration of use of systemic steroids was 116 days.
Table 2.
Analysis of pattern and severity of cGVHD (n = 40)
| Parameter | Number | % |
|---|---|---|
| Overall incidence of cGVHD (n = 77) | 40 | 52 |
| Subtypes | ||
| Extensive | 24 | 60 |
| Limited | 16 | 40 |
| Median time from transplant to onset of chronic GVHD, days | 150 | – |
| Thrombocytopenia at onset | 6 | 15 |
| De novo chronic GVHD | 19 | 47 |
| Organ involvement | ||
| Oral cavity | 25 | 63 |
| Gut | 8 | 20 |
| Liver | 17 | 43 |
| Lung | 12 | 30 |
| Eye | 11 | 28 |
| Skin | 16 | 40 |
| Others | 1 | 3 |
| Use of systemic steroids, n (%) | 23 | 58 |
| Median duration of systemic steroids (n = 23) | 116 days | – |
Fig. 1.
Pattern of organ involvement in cGVHD
Risk Factors for Chronic GVHD
Several patient, donor and transplant related factors were evaluated as potential risk factors for chronic GVHD (Table 3). Three factors were found to be significantly associated with increased risk of cGVHD on univariate analysis—diagnosis of ALL, female donor to male recipient transplants and lower CD3 cell dose infused. On multivariate analysis, female donor to male recipient transplant (P = 0.015) and diagnosis of ALL (P = 0.05) were the only factors associated with increased risk of cGVHD (Table 4).
Table 3.
Univariate analysis of potential risk factors for development of cGVHD
| Group A—with cGVHD (n = 40) n (%) | Group B—no cGVHD (n = 37) n (%) | P value | |
|---|---|---|---|
| Patient related factors | |||
| Median age at transplant | 29 | 31 | 0.575 |
| Males | 28 (70) | 24 (65) | 0.631 |
| Diagnosis | 0.020 | ||
| Acute lymphoid leukemia (ALL) | 17 (43) | 6 (16) | |
| Acute myeloid leukemia (AML) | 23 (58) | 29 (78) | |
| Biphenotypic leukemia | 0 (0) | 2 (5) | |
| Baseline risk | 0.138 | ||
| Good (or standard) risk | 2 | 4 | |
| Intermediate risk | 7 | 14 | |
| Poor risk | 25 | 14 | |
| Not known | 6 | 5 | |
| Relapse/refractory disease at transplant | 5 (12) | 10 (27) | 0.231 |
| Donor related factors | |||
| Median donor age, years | 32 | 33 | 0.860 |
| ABO mismatch transplants | 20 (50) | 12 (32) | 0.118 |
| Gender mismatched transplants | 25 (63) | 15 (41) | 0.054 |
| Female to male transplants | 17 (43) | 6 (16) | 0.012 |
| Stem cell source | 0.855 | ||
| Bone marrow | 2 (5) | 3 (8) | |
| Umbilical cord | 1 (3) | 1 (3) | |
| Peripheral blood stem cells | 37 (93) | 33 (89) | |
| Degree of HLA matching | 0.121 | ||
| BM or PBSC | |||
| Full matched (6/6 or 10/10) | 34 (85) | 27 (73) | |
| 1 antigen mismatch | 5 (13) | 4 (11) | |
| Haplo-identical transplant | 0 (0) | 5 (14) | |
| Cord transplants | 1 (3) | 1 (3) | |
| Transplant related factors | |||
| Type of transplant | 0.321 | ||
| Matched related transplant | 35 (88) | 30 (81) | |
| Matched unrelated transplant | 5 (13) | 5 (14) | |
| Haplo-identical transplant | 0 (0) | 2 (5) | |
| Type of conditioning regimen | 0.093 | ||
| Full intensity | 18 (45) | 9 (24) | |
| Reduced intensity | 22 (55) | 28 (76) | |
| Total body irradiation used | 20 (50) | 12 (32) | 0.118 |
| ATG used | 5 (13) | 7 (19) | 0.438 |
| Graft versus host disease prophylaxis | 0.224 | ||
| CsA+MTX | 30 (75) | 23 (62) | |
| CsA+MMF | 10 (25) | 14 (38) | |
| Cell doses infused | |||
| TNC (×108)/kg | 7.10 | 6.63 | 0.913 |
| CD34 (×106)/kg | 5.11 | 5.12 | 0.899 |
| CD3 (×106)/kg | 147.80 | 177.65 | 0.039 |
| Prior acute GVHD, any grade, n (%) | 21 (53) | 13 (35) | 0.630 |
| Day100 median absolute lymphocyte count (×109/L) | 1.23 (n = 39) | 1.41 (n = 28) | 0.589 |
| Day180 median absolute lymphocyte count (×109/L) | 1.61 (n = 39) | 2.05 (n = 22) | 0.131 |
Table 4.
Multivariate analysis of potential risk factors for development of cGVHD
| Risk factor | Hazard ratio | 95 % CI | P value |
|---|---|---|---|
| Diagnosis of ALL | 3.977 | 0.997–15.86 | 0.050 |
| Female donor to male recipient | 4.776 | 1.35–16.86 | 0.015 |
| CD3 cell dose infused | 0.995 | 0.989–1.001 | 0.082 |
Transplant Related Outcomes
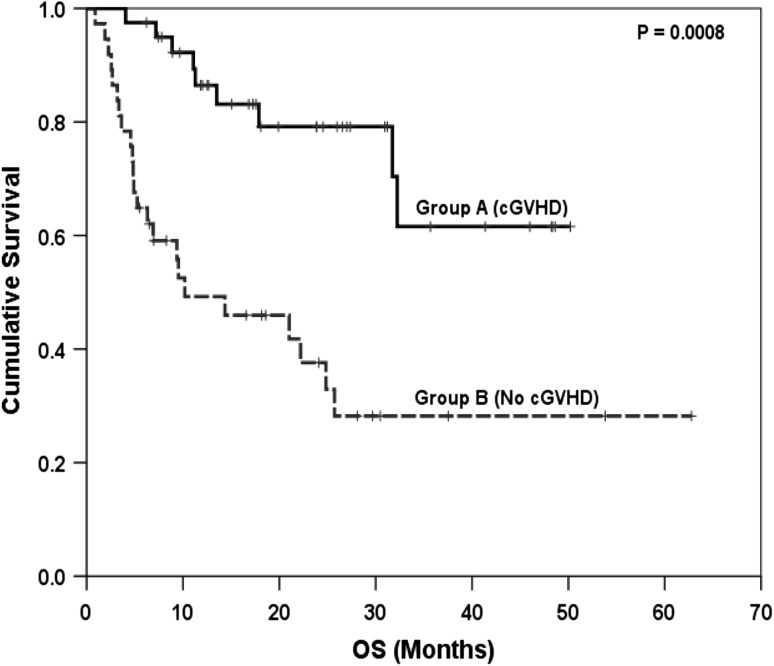
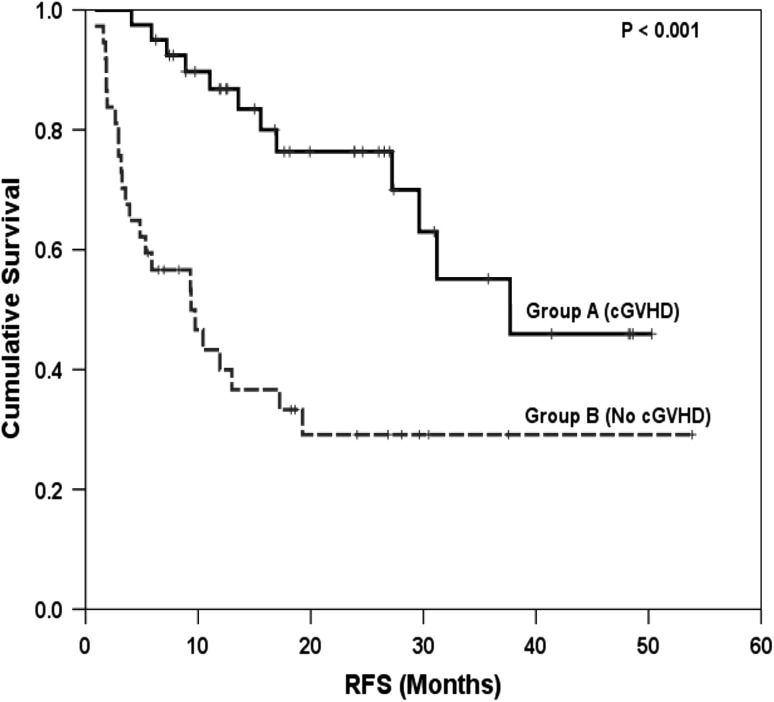
There was no difference in the median time to neutrophil and platelet engraftment between the 2 groups (P = NS). Incidence of relapse was 18 % among patients with cGVHD and 51 % among patients without cGVHD (p = 0.002). Similarly incidence of slippage of chimerism was also significant lower in patients who developed cGVHD (p = 0.006). Transplant related mortality was similar in both the groups (P = 0.63). Median OS, 4 year OS, median RFS and 4 year RFS were all significantly greater (P < 0.001) in patients who developed cGVHD compared to those who did not (Table 5, Figs. 2, 3). Among patients with chronic GVHD, the 4 year RFS was similar for patients of AML vs ALL (39 vs 84 %, p = 0.45). Similarly, no significant difference was found in overall survival according to diagnosis of AML versus ALL (4 year OS 45 vs 84 %, p = 0.19). Similar findings were seen among patients without chronic GVHD.
Table 5.
Comparison of transplant related outcomes in patients with and without cGVHD
| Group A—with cGVHD (n = 40) | Group B—no cGVHD (n = 37) | P value | |
|---|---|---|---|
| Median days to platelet engraftment | 13 | 12 | 0.719 |
| Median days to myeloid engraftment | 13 | 15 | 0.096 |
| Incidence of relapse, n (%) | 7 (18) | 19 (51) | 0.002 |
| Slippage of chimerism, n (%) | 6 (15) | 16 (43) | 0.006 |
| Transplant related mortality, n (%) | 5 (13) | 6 (16) | 0.63 |
| Median relapse free survival, months | 37.7 | 9.3 | <0.001 |
| 4 year relapse free survival, % | 46 | 29 | <0.001 |
| Median overall survival, months | Not reached | 10.2 | <0.001 |
| 4 year overall survival, % | 62 | 29 | <0.001 |
Fig. 2.
Comparison of overall survival (OS) between patients with and without cGVHD
Fig. 3.
Comparison of relapse free survival (RFS) between patients with and without cGVHD
Infective Complications
The incidence of cytomegalovirus (CMV), Epstein Barr Virus, adenovirus and BK virus reactivation were similar in both groups. The median number of episodes of CMV reactivation was also similar. Additionally, incidence of fungal infections was also similar in patients with or without GVHD (Table 6).
Table 6.
Comparison of infective complications in patients with and without cGVHD
| Group A—with cGVHD (n = 40) | Group B—no cGVHD (n = 37) | P value | |
|---|---|---|---|
| CMV reactivation, n (%) | 29 (73) | 20 (54) | 0.09 |
| Median episodes of CMV | 2 | 1.5 | 0.75 |
| Adenoviral reactivation, n (%) | 11 (28) | 5 (14) | 0.13 |
| BK virus reactivation, n (%) | 7 (18) | 6 (16) | 0.88 |
| EBV reactivation, n (%) | 2 (5) | 3 (8) | 0.58 |
| Fungal infections | 5 (13) | 6 (16) | 0.63 |
Discussion
Allogenic HSCT has resulted in long term cure of a significant fraction of patients with acute leukemia including relapsed and refractory cases. Chronic GVHD remain a significant cause of morbidity in the post-transplant period. It usually has onset after 100 days, but can manifest even earlier [9]. Overlap GVHD syndromes with features of both acute and chronic GVHD have been described. Hence, clinical manifestations rather than the time of onset are considered more important in classifying GVHD [9].
In the last 2 decades, few studies have looked into the risk factors, transplant outcomes and infective complications in patients with chronic GVHD. Various studies have shown that older patient age, female donor to male recipient transplant, mismatched or unrelated donor, PBSCs as the source of stem cells and development of acute GVHD are associated with the development of chronic GVHD. Few studies also found that CMV reactivation, higher infused CD34 cell dose and lack of MTX in GVHD prophylaxis are also risk factors for cGVHD [10]. In our study, we found a strong correlation between female donor to male recipient transplant and the development of cGVHD. Lesser number of unrelated donor transplants and mismatched transplants in our study probably explain why these risk factors did not turn out significant. Similarly, more than 90 % patients had PBSCs as the source of stem cell graft; this could explain the lack of effect of source of stem cells on the development of cGVHD. In contrast to what has been reported earlier [2], we did not find a correlation between prior acute GVHD and increased risk for cGVHD. This could be due to the fact that a higher proportion of patients (65 %) in our study received reduced intensity conditioning regimen. Studies have shown that in patients undergoing reduced intensity transplantation, acute GVHD may not predict for the development of cGVHD [5]. Additionally, we found diagnosis of ALL to be associated with the development of chronic GVHD. The exact reason behind this remains unclear. Infused CD3 cell count was significant on univariate analysis. Analysis of infused CD3 count as risk factor for chronic GVHD has yielded variable results between studies [11, 12].
The pattern of organ involvement in cGVHD seen in our study is similar to that reported previously with oral cavity, liver, skin and lung being the commonly involved sites [13]. Nearly half of patients with cGVHD had de novo cGVHD which is similar to the proportion reported previously in other studies [5, 13, 14]. Regarding transplant outcomes, it is relatively clear that cGVHD improves disease free survival [2, 5, 6] and our study also concurs with previous studies. The impact on overall survival is less consistent [2, 5]. Only few studies have shown a correlation between extensive stage cGVHD and overall survival [14]. In our study, we found a strong positive correlation between occurrence of cGVHD and improvement in OS. This improvement was noted in the entire cohort of patients with cGVHD irrespective of the severity of GVHD. The improvement is OS seen in patients who developed cGVHD is attributed to decrease in the incidence of leukemia relapse. This favorable impact of cGVHD on transplant outcome is related to increased graft versus leukemia effect in these patients. The improvement in OS might also be related to the fact that there was no increase in the incidence of infective complications in patients who developed cGVHD compared to those who did not. While previous studies have shown that infective complications are a major cause of mortality in patients with cGVHD (particularly fungal infections) [15], we found no increase in the incidence of infective complications in our cohort of patients with cGVHD.
We conclude that cGVHD complicates nearly half of all acute leukemia transplants. Female donor to male recipient and diagnosis of ALL are important risk factors for the development of cGVHD. Occurrence of cGVHD is associated with decrease in leukemia relapse and an improvement in overall survival.
Acknowledgments
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transpl. 2008;28:121–129. doi: 10.1038/sj.bmt.1703111. [DOI] [PubMed] [Google Scholar]
- 2.Zecca M, Prete A, Rondelli R, Lanino E, Balduzzi A, Messina C, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood. 2002;100:1192–1200. doi: 10.1182/blood-2001-11-0059. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Vogelsang G, Flowers ME. Chronic Graft-versus-Host Disease. Biol Blood Marrow Transpl. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson K, Horowitz MM, Gale RP, van Bekkum DW, Gluckman E, Good RA, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. 1990;75:2459–2464. [PubMed] [Google Scholar]
- 5.Mohty M, Bay JO, Faucher C, Choufi B, Bilger K, Tournilhac O, et al. Graft-versus-host disease following allogenic transplantation from HLA-identical sibling with anti thymocyte globulin-based reduced-intensity preparative regimen. Blood. 2003;102:470–476. doi: 10.1182/blood-2002-12-3629. [DOI] [PubMed] [Google Scholar]
- 6.Veltri L, Regier M, Cumpston A, Leadmon S, Tse W, Craig M, Hamadani M. Incidence and pattern of graft-versus-host disease in patients undergoing allogenic transplantation after non myeloablative conditioning with total lymphoid irradiation and anti thymocyte globulin. Bone Marrow Res. 2013 doi: 10.1155/2013/414959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gota V, Pathak K, Mittal S, et al. A limited sampling strategy for therapeutic drug monitoring of mycophenolate mofetil for prophylaxis of acute graft versus host disease in allogeneic stem cell transplantation. Bone Marrow Transpl. 2014;49:S295. doi: 10.1177/0963689720912925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 9.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Higman MA, Vogelsang GB. Chronic graft versus host disease. Br J Haematol. 2004;125:435–454. doi: 10.1111/j.1365-2141.2004.04945.x. [DOI] [PubMed] [Google Scholar]
- 11.Dhédin N, Prébet T, De Latour RP, Katsahian S, Kuentz M, Piard N, et al. Extensive chronic GVHD is associated with donor blood CD34+ cell count after G-CSF mobilization in non-myeloablative allogeneic PBSC transplantation. Bone Marrow Transpl. 2012;47:1564–1568. doi: 10.1038/bmt.2012.75. [DOI] [PubMed] [Google Scholar]
- 12.Konopacki J, Porcher R, Robin M, Bieri S, Cayuela JM, Larghero J, et al. Long-term follow up after allogenic stem cell transplantation in patients with severe aplastic anemia after cyclophosphamide plus antithymocyte globulin. Haematologica. 2012;97:710–716. doi: 10.3324/haematol.2011.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solano C, Martinez C, Brunet S, Tomás JF, Urbano-Ispizua A, Zuazu J, et al. Chronic graft versus host disease after allogenic peripheral blood progenitor cell or bone marrow transplantation from matched related donors. A case–control study. Bone Marrow Transpl. 1998;22:1129–1135. doi: 10.1038/sj.bmt.1701500. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, et al. Severity of chronic graft-versus-host disease: association with treatment related mortality and relapse. Blood. 2002;100:406414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 15.Pidala J. Graft-vs-host disease following allogenic hematopoietic cell transplantation. Cancer Control. 2011;18:268–276. doi: 10.1177/107327481101800407. [DOI] [PubMed] [Google Scholar]