Abstract
Background:
The relationship between monosymptomatic resting tremor (mRT) and Parkinson's disease (PD) remains controversial. In this study, we aimed to assess the function of presynaptic dopaminergic neurons in patients with mRT by dopamine transporter positron emission tomography (DAT-PET) and to evaluate the utility of clinical features or electrophysiological studies in differential diagnosis.
Methods:
Thirty-three consecutive patients with mRT were enrolled prospectively. The Unified Parkinson's Disease Rating Scale and electromyography were tested before DAT-PET. Striatal asymmetry index (SAI) was calculated, and a normal DAT-PET was defined as a SAI of <15%. Scans without evidence of dopaminergic deficits (SWEDDs) were diagnosed in patients with a subsequent normal DAT-PET and structural magnetic resonance imaging.
Results:
Twenty-eight mRT patients with a significant reduction in uptake of DAT binding in the striatum were diagnosed with PD, while the remained 5 with a normal DAT-PET scan were SWEDDs. As for UPRDS, the dressing and hygiene score, walking in motor experiences of daily living (Part II) and motor examination (Part III) were significant different between two groups (P < 0.05 and P < 0.01, respectively). Bilateral tremor was more frequent in the SWEDDs group (P < 0.05). The frequency of resting tremor and the amplitude of postural tremor tend to be higher in the SWEDDs group (P = 0.08 and P = 0.05, respectively).
Conclusions:
mRT is heterogeneous in presynaptic nigrostriatal dopaminergic degeneration, which can be determined by DAT-PET brain imaging. Clinical and electrophysiological features may provide clues to distinguish PD from SWEDDs.
Keywords: Dopamine Transporter Positron Emission Tomography, Heterogeneity, Monosymptomatic Resting Tremor, Parkinson's Disease, Scans Without Evidence of Dopaminergic Deficit
INTRODUCTION
Tremor is the most common movement disorder which is defined as a compulsively, rhythmic, shaking movement of a body part.[1] One of the most general reasons for tremor is idiopathic Parkinson's disease (PD), a progressive neurodegenerative disease. The core clinical features of idiopathic PD include bradykinesia, followed by resting tremor, rigidity, and problems with stance stability. These symptoms will become evident after a loss of 50–70% dopaminergic cells in the substantia. However, the differential diagnosis of idiopathic PD is difficult in the early stage due to the atypical and ambiguous symptoms and signs, especially when tremor is the solely obvious symptom.[2,3]
Monosymptomatic resting tremor (mRT) is a descriptive term for resting tremor persisting for more than 2 years without other cardinal signs of PD.[4] The relationship between mRT and PD remains controversial. Some reports demonstrated that mRT is characterized by a presynaptic dopaminergic deficit with the evidence of dopamine transporter positron emission tomography (DAT-PET) and supported that mRT represents a phenotype of PD. Most of these patients end up in the tremor-dominant variant of PD, but this may last more than a decade.[5,6] However, in clinical practice, dopaminergic functional imaging revealed some patients with mRT to be scans without evidence of dopaminergic deficits (SWEDDs).[7,8] Few studies have been reported to reveal the prevalence of SWEDDs in mRT patients and to illuminate the clinical and electrophysiological characteristics of mRT patients grouped by the DAT-PET results. This study aimed to investigate the presynaptic dopamine function by DAT-PET in a prospective mRT group and to evaluate the role of clinical features and electrophysiological characteristics in the differential diagnosis.
METHODS
Patients
We prospectively enrolled 43 consecutive patients with mRT who visited Movement Disorder Clinics in Beijing Tiantan Hospital from April 2011 to December 2013. The written informed consent was obtained from all the patients.
The diagnosis of mRT was confirmed by movement disorder specialists according to Consensus Statement of the Movement Disorder Society on Tremor.[4] The mRT patients must meet the following criteria: (1) Pure or predominant resting tremor; (2) no sign of bradykinesia, rigidity, or abnormal gait and posture; and (3) tremor ≥2 years.[2] Patients were excluded if they had: (1) Specific etiologies for the asymmetric resting tremor (e.g., psychogeny, medication-induced, stroke, and multiple sclerosis); (2) a clear history of PD or essential tremor (ET) in the family; (3) an obvious lesions of intracephalic structure on head computed tomography (CT)/magnetic resonance imaging (MRI) scans. Information regarding demographics, medical and family history, disease course, and treatment were collected during a face-to-face interview in all patients. The Unified Parkinson's Disease Rating Scale (UPDRS) was tested by well-trained neurologists before DAT-PET examination, thus there was a blindness between clinical data and DAT-PET. The study was approved by the Institutional Review Board (Committee for Medical Ethics) of Beijing Tiantan Hospital, Capital Medical University.
Electrophysiological test
Surface electromyographic (EMG) recordings of the most involved limbs in all positions were performed on all patients.[7] The tremor frequency (Hz), mean amplitude (mV), and pattern (synchronous or alternating) were assessed. The amplitude was measured peak to the valley for each burst, and the duration was measured peak to peak for each burst. The tremor frequency was assessed by counting the number of tremor bursts per minute. The mean value was calculated from 5 measurements. The tremor pattern was assessed by visual inspection of the records. The recording session included 5 min test and 5 min rest subsequently in each definite limb position. Alcohol, caffeine beverages or tea was not allowed the evening before the test. None was taking drugs known to influence tremor. All the measurements were performed in the morning (9:00–11:00 a.m.). The examination was performed in a room where the patients had the opportunity to be familiar with the circumstances and felt relax before the test.
Dopamine transporter positron emission tomography
11C-β-CFT, used as the DAT radioligand for PET tracer, was synthesized at Department of Nuclear Medicine, PLA General Hospital. 11C-β-CFT was prepared by reaction of [11C] methyl triflate with the N-desmethyl precursor, nor-P-CFT (RBI, Natick, MA, USA).[8,9] A high-resolution PET/CT scanner (DaTSCAN; GEMINI GXL-16, Philips, The Netherlands) was used for each patient at Nuclear Medicine Center of the Second Hospital of Armed Police Beijing Office. Anti-parkinsonism drugs must not be taken at least 12 h before PET, and DAT agonists 72 h before the PET. The head of the caudate and putamen on the two consecutive planes with best articulation were selected to be the regions of interest (ROIs) on bilateral hemispheres. The average of 11C-β-CFT concentrations of ROIs was calculated. Striatal asymmetry index (SAI) was used to describe the asymmetry of the radioactivity uptake in the bilateral striatum. SAI = (RISP − RCON)/[(RISP + RCON)/2] ×100%. RISP and RCON represent the uptake value of DAT tracer in the striatum ipsilateral or contralateral to the clinically affected body side, respectively.[7,8] PD was diagnosed if SAI existed.
Statistical analysis
Continuous parameters and absolute counts were shown as mean ± standard deviation (SD), and categorical parameters were shown as percentage. The differences in clinical and electrophysiological features between SWEDDs and PD patients were calculated using the unpaired, two-tailed t-test for continuous variables and Chi-square test or Fisher Exact test for categorical variables by SPSS 17.0 (SPSS Inc., Chicago, IL, USA). A P < 0.05 was considered to be statistically significant.
RESULTS
Demographic data
During the study period, 43 patients were screened for suspicious mRT. Eight patients were not involved in the present analyses for their symptoms of tremor lasting <2 years and two patients were excluded due to incomplete documents of clinical features or electrophysiological measurements. A total of 33 patients with definite mRT (mean age 55.3 ± 11.6 years, 45.5% men) constituted the final sample [Table 1]. PD were diagnosed in 84.8% (n = 28) and SWEEDs in 15.2% (n = 5) of cases, respectively. There were no significant differences in age, gender, and age at onset of tremor between SWEDDs and PD groups. The duration of disease was longer in the SWEEDs group than in the PD group (13.0 years vs. 3.0 years, P < 0.05). The percentage of Hoehn-Yahr score ≥1 was similar among two groups (20% vs. 25%, P > 0.05).
Table 1.
Demographic and clinical characteristics of SWEDDs and PD patients
| Parameters | SWEDDs (n = 5) | PD (n = 28) | t or χ2 | P | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, years, mean ± SD | 60.8 ± 11.6 | 54.5 ± 11.6 | 0.34 | >0.05 | |
| Male, n (%) | 3 (60.0) | 12 (42.9) | 0.05* | >0.05 | |
| Duration of disease, years, median (range) | 13.0 (4.8–22.5) | 3.0 (2.3–5.0) | −2.34† | <0.05 | |
| Age of onset, years, mean ± SD | 46.5 ± 18.6 | 50.3 ± 12.5 | 0.34 | >0.05 | |
| Modified HoehnYahr score ≥1, n (%) | 1 (20.0) | 7 (25.0) | 0.06* | >0.05 | |
| UPDRS Part I: Nonmotor aspects of experiences of daily living, n (%) | |||||
| Cognitive impairment | 1 (20.0) | 13 (46.4) | 1.21* | >0.05 | |
| Depression | 0 (0.0) | 12 (42.9) | – | >0.05 | |
| UPDRS Part II: Motor experiences of daily living, mean ± SD | 3.5 ± 2.2 | 8.3 ± 4.5 | 3.25 | >0.05 | |
| Speech, n (%) | 0 (0.0) | 13 (46.4) | – | >0.05 | |
| Dressing, n (%) | 0 (0.0) | 15 (55.6) | – | <0.05 | |
| Walking, n (%) | 1 (20.0) | 19 (67.9) | 2.31 | >0.05 | |
| Hygiene, n (%) | 0 (0.0) | 16 (57.1) | – | <0.05 | |
| Freezing of gait, n (%) | 1 (20.0) | 15 (53.6) | 0.81* | >0.05 | |
| UPDRS Part III: Motor examination, mean ± SD | 11.0 ± 2.0 | 23.2 ± 12.5 | −4.11 | <0.01 |
*These values were calculated by χ2 test; others without star mark were calculated by t-test; †Nonparametric test; “–”: Not applicable, calculated by Fisher Exact test. SD: Standard deviation; SWEDDs: Scans without evidence of dopaminergic deficits; UPDRS: Unified Parkinson’s Disease Rating Scale; PD: Parkinson’s disease.
Positron emission tomography results
According to the PET comparison with the norm values in the center, subjects were divided into two groups. A normal symmetrical uptake of 11C-β-CFT in the bilateral striatum was seen in all five patients (SWEDDs group) [Figures 1 and 2]. An asymmetrical reduced uptake in the striatum was seen in 28 patients (PD group) [Figures 1 and 3].
Figure 1.
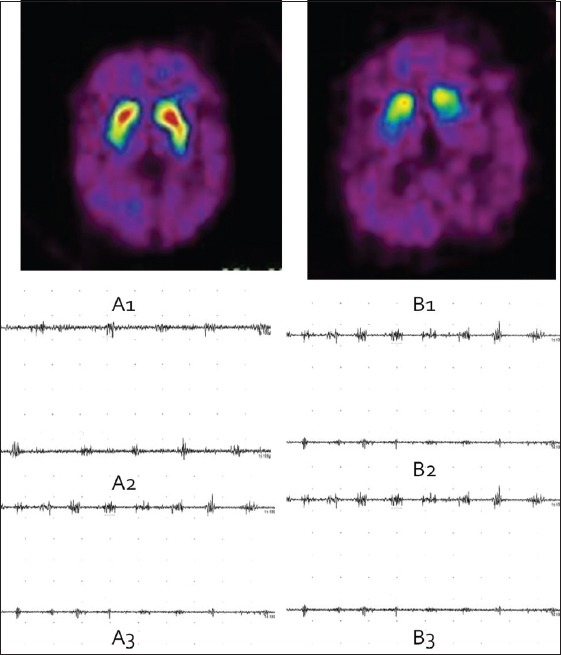
Positron emission tomography (PET) to distinguish scans without evidence of dopaminergic deficits (SWEDDs) from Parkinson's disease (PD) and tremor analysis between groups. Transaxial slices of PET/tremor analysis of a patient with SWEDDs (left) and another patient with right-sided PD (right).
Figure 2.
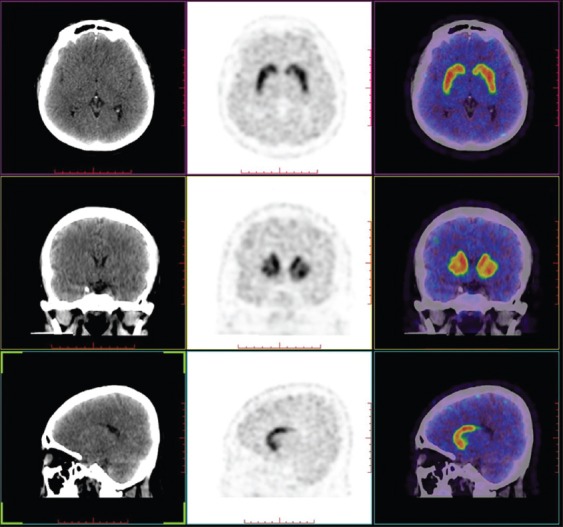
Dopamine transporter positron emission tomography help to distinguish scans without evidence of dopaminergic deficits from Parkinson's disease in monosymptomatic resting tremor patients.
Figure 3.
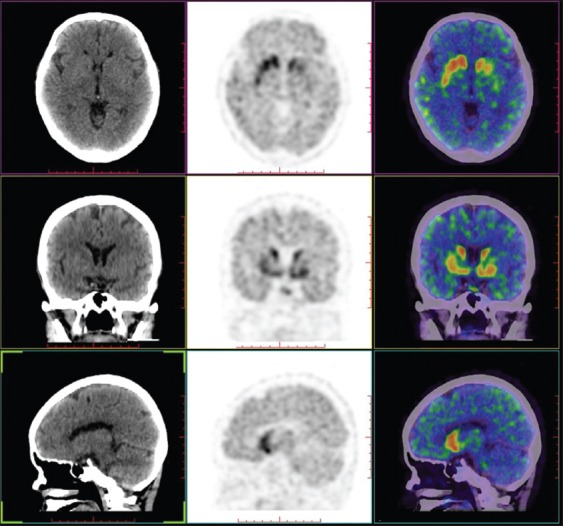
Dopamine transporter positron emission tomography help to identify Parkinson's disease in the early age in monosymptomatic resting tremor patients.
Unified Parkinson's Disease Rating Scale
For nonmotor aspects of experiences of daily living (Part I of UPDRS), there were no significant differences in cognitive impairment and depression between two groups [Table 1]. For motor experiences of daily living (Part II of UPDRS), PD patients were more likely to have difficulties in dressing and hygiene (P < 0.05). More patients in PD group tended to have difficulties in walking (67.9% in PD group and 20.0% in SWEDDs group, P > 0.05), although the difference was of no statistical significance. The two groups were similar in speech and freezing of gait. For motor examination (Part III of UPDRS), the score was much lower in the SWEEDs group than in the PD patient group, and the difference was statistically significant (11.0 ± 2.0 vs. 23.2 ± 12.5, P < 0.01).
Tremor analysis
Clinical features
Eighteen (64.3%) PD patients had a resting tremor, and 25 (89.3%) had a postural tremor. A resting tremor was recorded in 3 (60.0%) SWEDDs patients and postural tremor was recorded in all 5 (100%) SWEDDs patients. The difference in tremor distribution (resting tremor or postural tremor) between two groups was not statistically significant. But the proportion of bilateral tremor was much higher in SWEDDs group than in the PD group (100% vs. 32.1%, P < 0.05) [Table 2].
Table 2.
Tremor analysis in patients with SWEDDs and PD
| Parameters | SWEDDs (n = 5) | PD (n = 28) | t or χ2 | P |
|---|---|---|---|---|
| Tremor features, n (%) | ||||
| Resting tremor | 3 (60.0) | 18 (64.3) | 0.03* | >0.05 |
| Postural tremor | 5 (100) | 25 (89.3) | – | >0.05 |
| Bilateral tremor | 5 (100) | 9 (32.1) | 5.46 | <0.05 |
| Frequency, Hz, mean ± SD | ||||
| Resting tremor | 7.8 ± 0.4 | 7.3 ± 1.5 | 0.08 | >0.05 |
| Postural tremor | 11.5 ± 0.7 | 10.0 ± 1.4 | 1.80 | >0.05 |
| Amplitude, mV, median (range) | ||||
| Resting tremor | 210.0 (200.0–220.0) | 170.0 (160.0–180.0) | −1.55† | >0.05 |
| Postural tremor | 240.0 (230.0–250.0) | 132.5 (50.0–215.0) | −0.61† | 0.05 |
| Resting tremor pattern, n (%) | ||||
| Synchronous pattern | 3 (60.0) | 16 (57.1) | 0.01* | >0.05 |
| Alternating pattern | 4 (80.0) | 22 (78.6) | 0.01* | >0.05 |
| Postural tremor pattern, n (%) | ||||
| Synchronous pattern | 4 (80.0) | 15 (56.7) | 0.37* | >0.05 |
| Alternating pattern | 3 (60.0) | 21 (75.0) | 0.02* | >0.05 |
*These values were calculated by χ2 test; others without star mark were calculated by t-test; †Nonparametric test; “–”: not applicable, calculated by Fisher Exact test. SWEDDs: Scans without evidence of dopaminergic deficits; PD: Parkinson’s disease; SD: Standard deviation.
Electrophysiological study
The electrophysiological findings in 2 different conditions (resting or postural tremor) in studying group are shown in Table 2. No subject had a kinetic tremor in either of the two groups. There were no significant differences in the frequency and amplitude of resting or postural tremor between two groups.
Tremor analysis: Frequency and amplitude
Table 2 shows the results of EMG measurements in both resting and postural tremors. The frequency of resting tremor in SWEDDs group tended to be higher than in the PD group, but without significant difference (P = 0.08). The difference in frequency of postural tremor between two groups was not statistically significant. The amplitudes of resting tremor were similar between the two groups. The amplitude of postural tremor tended to be higher in SWEDDs group than in PD group, but the difference was of no significance (P = 0.05).
Tremor patterns analysis
Table 2 also shows the results of tremor patterns in resting or postural tremors. In resting tremor patterns analysis, the percentage of synchronous pattern was 60.0% in SWEDDs group and 57.1% in PD group (P > 0.05), while the percentage of alternating pattern of resting tremor was 80.0% in SWEDDs group and 78.6% in PD group (P > 0.05). In postural tremor patterns analysis, synchronous pattern was 80.0% in the SWEDDs group and 56.7% in PD group, while alternating pattern was 60.0% in the SWEDDs group and 75.0% in PD group (P > 0.05).
DISCUSSION
There is still a debate on the etiology of mRT and its relation to PD. In this present study, we confirmed that mRT is heterogeneous in presynaptic nigrostriatal dopaminergic degeneration. Normal 11C-CFT uptake in the striatum by DAT-PET was found in 15.2% (5/33) of patients with mRT, who were diagnosed as the SWEDDs, while reduced uptake in the striatum was found in 84.8% (28/33) of the patients, thus the corresponding diagnosis were PD. The PET results suggested that mRT is not a subgroup of PD.
There were some differences in the clinical characteristics between PD and SWEDDs groups in our study. We found that more patients in the PD group had difficulties in walking and balance. Similar result was seen in a former study focusing on the gaits and posture of the patients. SWEDDs patients were found to had a normal trunk and elbow posture, normal stride length variability, and normal bilateral step phase coordination, all of which were abnormal in PD patients. No evidence of ataxic gait was found as a general feature in SWEDDs.[9,10] In addition, we found that dressing and hygiene were more likely to be difficult in PD group. We also found that the score in Motor Examination in UPDRS (Part III) was higher in PD group than in SWEDDs group, which indicated dyskinesia was remarkably severe in the former group. Thus, we concluded that the UPDRS might help to make a differential diagnosis between PD and SWEEDs. Bilateral tremors were more frequent in SWEDDs group than in PD group. These findings could aid the clinician in the identification of potential SWEDDs cases.
There are few studies on the difference in tremor patterns between PD and SWEDD patients. The basic tremor parameters overlapped between SWEDDs, PD, segmental dystonia and ET in previous electrophysiological studies. Recent study found that PD feature re-emergent tremor coexisted with the highest amplitude in the resting tremor, while SWEDDs, dystonia, and ET subjects had the highest amplitude during action tremor.[7] Similarly, we found in our study that the action tremor amplitude tended to be higher in SWEDDs group, although the difference was not significant. The tiny disparity might afford us the clues to differential diagnosis.
Different tremor types have been separated in PD, including classic Parkinsonian tremor at rest, postural and kinetic tremors of different frequencies, pure postural or kinetic tremor. The heterogeneity in SWEDDs, which means dystonic tremors may be responsible for a number of SWEDDs subjects as well as ET,[6,7,11,12] might explain partly that the tremor patterns were similar in the two groups. Unfortunately, in the comprehensive analysis, when the tremor patterns were divided into different groups according to its features, we found there was no significant difference between PD and SWEDDs groups.
Another important issue we should mention above was the relationship between mRT and ET. The diagnosis of mRT is made when the tremor syndrome persists for more than 2 years without bradykinesia, rigidity or postural dysfunction. The resting tremor in mRT differs obviously from the postural tremor or action tremor in ET. The mRT patient has rarely family history and is nonresponsive to beta blockers when comparing to ETs. Ninety percent of the mRT patients can be thought to be subclinical PD with the evidence of presynaptic dopamine neuronal dysfunction by PET. The residual is defined as SWEEDs, including some of dystonic tremor.
Besides careful clinical examination and electrophysiological test, functional imaging plays an important role in the differential diagnosis in patients with isolated tremor symptoms.[13,14,15,16] Recent studies found that when faced a diagnostic challenge in early PD, cardiac 123I-MIBG scans alone or combined with DAT-PET, may help to differentiate patients with SWEDDs from patients with PD.[17,18] In the present study, SWEEDs were diagnosed in 15.2% (n = 5) of cases and the result was consistent with previous study, in which approximately 11–15% of early PD patients showed normal dopaminergic functional imaging (SWEDDs).[6,19] Various ligands had been proved to provide identical data regardless of the kinetics and specificity for the DAT.[20,21] In light of 123I-FP-CIT (123I-N-v-fluoropropyl-2b-carbomethoxy-3b-(4-iodophenyl) nortropane), a widely used DAT ligand in single photon emission computed tomography (SPECT) is difficult to prepare and expensive; however, DAT-PET is reasonable to detect presynaptic dopamine neuronal dysfunction. DAT-PET is also an independent factor associated with the long-term motor and nonmotor outcomes when adjusted for age, gender, PD duration, baseline PD severity, and PD medications at the time of assessment.[22,23,24] DAT imaging has an important role in the differential diagnosis, management decision, and prognostic prediction in early PD and clinical uncertain Parkinsonian syndrome.
There are many other causes for tremor, such as physiological tremor, psychogenic tremor, ET, and myoclonus-dystonia. Differential diagnosis of tremor should base on history taking, physical examination, electrophysiological test, MRI, and nuclear imaging of DAT when the diagnosis remains uncertain. DAT density in corpus striatum decreased 10% every year in the PD group, while 0.5% every year in the control group. We did not involve PET during the follow-up for the expenditure. We will use PET as an imaging endpoint in the further study. We found that the patients with negative DAT-PET results had no PD symptoms or signs in the next years while the patients with positive DAT-PET results had some PD symptoms or signs in the following years.
Several potential limitations of the present study should be addressed. First, the findings of our study should be interpreted with caution considering the sample size. However, our prospective cohort is reasonable when compared with other researches focused on SWEDDS, in which the sample size was generally small, varied from 8 to 33.[4,6,7,8,9,22,25] Second, whether SWEDDs patients had signs of dystonia was not recorded in our study. Thus, we cannot support the hypothesis that adult onset dystonia is the underlying diagnosis in this sub-group of patients with SWEDDs.[9] Finally, transcranial sonography and susceptibility-weighted imaging can aid the differentiation in previous studies.[25,26,27,28] These tests were not included in the analysis due to the integrity constraint and will be added in the further studies.
In summary, the present study suggested that mRT is heterogeneous in presynaptic nigrostriatal dopaminergic degeneration. DAT-PET brain imaging can facilitate early and accurate diagnosis and help guide prognosis and treatment decisions. It can also help to avoid inappropriate dopaminergic drug therapy, as well as the long-term financial burden. Clinical and electrophysiological features provide clues to differentiate between the SWEDDs and PD. Clinical scale needs to be established in the further research.
ACKNOWLEDGMENTS
We thank all our colleagues who collaborated on this study.
Footnotes
Edited by: Xin Chen
Source of Support: This study was supported by grants from the Scientific Research Common Program of Beijing Municipal Commission of Education (No. KZ201210025028), the Beijing Municipal Science and Technology plan (No. Z11110005880000), High Level Technical Talent Training Program in Beijing Healthcare System (No. 2011-3-022), National Key Technology Research and Development Program of the Ministry of Science and Technology of China (No. 2013BAI09B03), and Beijing Institute for Brain disorders (No. BIBD-PXM2013_014226_07_000084). These funding agencies did not participate in design or analysis, manuscript preparation, or approval of this study.
Conflict of Interest: None declared.
REFERENCES
- 1.Elias WJ, Shah BB. Tremor. JAMA. 2014;311:948–54. doi: 10.1001/jama.2014.1397. [DOI] [PubMed] [Google Scholar]
- 2.Berardelli A, Wenning GK, Antonini A, Berg D, Bloem BR, Bonifati V, et al. EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson's disease. Eur J Neurol. 2013;20:16–34. doi: 10.1111/ene.12022. [DOI] [PubMed] [Google Scholar]
- 3.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13 Suppl 3:2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 4.Ghaemi M, Raethjen J, Hilker R, Rudolf J, Sobesky J, Deuschl G, et al. Monosymptomatic resting tremor and Parkinson's disease: A multitracer positron emission tomographic study. Mov Disord. 2002;17:782–8. doi: 10.1002/mds.10125. [DOI] [PubMed] [Google Scholar]
- 5.Deuschl G, Papengut F, Hellriegel H. The phenomenology of Parkinsonian tremor. Parkinsonism Relat Disord. 2012;18 Suppl 1:S87–9. doi: 10.1016/S1353-8020(11)70028-1. [DOI] [PubMed] [Google Scholar]
- 6.Schneider SA, Edwards MJ, Mir P, Cordivari C, Hooker J, Dickson J, et al. Patients with adult-onset dystonic tremor resembling Parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs) Mov Disord. 2007;22:2210–5. doi: 10.1002/mds.21685. [DOI] [PubMed] [Google Scholar]
- 7.Schwingenschuh P, Ruge D, Edwards MJ, Terranova C, Katschnig P, Carrillo F, et al. Distinguishing SWEDDs patients with asymmetric resting tremor from Parkinson's disease: A clinical and electrophysiological study. Mov Disord. 2010;25:560–9. doi: 10.1002/mds.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felicio AC, Shih MC, Godeiro-Junior C, Andrade LA, Bressan RA, Ferraz HB. Molecular imaging studies in Parkinson disease: Reducing diagnostic uncertainty. Neurologist. 2009;15:6–16. doi: 10.1097/NRL.0b013e318183fdd8. [DOI] [PubMed] [Google Scholar]
- 9.Bain PG. Dystonic tremor presenting as Parkinsonism: Long-term follow-up of SWEDDs. Neurology. 2009;72:1443–5. doi: 10.1212/WNL.0b013e3181a18809. [DOI] [PubMed] [Google Scholar]
- 10.Mian OS, Schneider SA, Schwingenschuh P, Bhatia KP, Day BL. Gait in SWEDDs patients: Comparison with Parkinson's disease patients and healthy controls. Mov Disord. 2011;26:1266–73. doi: 10.1002/mds.23684. [DOI] [PubMed] [Google Scholar]
- 11.Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol. 2014;71:499–504. doi: 10.1001/jamaneurol.2013.6233. [DOI] [PubMed] [Google Scholar]
- 12.Benito-León J. Essential tremor: From a monosymptomatic disorder to a more complex entity. Neuroepidemiology. 2008;31:191–2. doi: 10.1159/000154933. [DOI] [PubMed] [Google Scholar]
- 13.You S, Kim MJ, Kim SR, Kim MJ, Oh M, Kim JS, et al. Clinical features and nigrostriatal dysfunction in patients with combined postural and resting tremors. Parkinsonism Relat Disord. 2013;19:1118–22. doi: 10.1016/j.parkreldis.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Ravina B, Eidelberg D, Ahlskog JE, Albin RL, Brooks DJ, Carbon M, et al. The role of radiotracer imaging in Parkinson disease. Neurology. 2005;64:208–15. doi: 10.1212/01.WNL.0000149403.14458.7F. [DOI] [PubMed] [Google Scholar]
- 15.Nandhagopal R, McKeown MJ, Stoessl AJ. Functional imaging in Parkinson disease. Neurology. 2008;70:1478–88. doi: 10.1212/01.wnl.0000310432.92489.90. [DOI] [PubMed] [Google Scholar]
- 16.Troiano AR, de la Fuente-Fernandez R, Sossi V, Schulzer M, Mak E, Ruth TJ, et al. PET demonstrates reduced dopamine transporter expression in PD with dyskinesias. Neurology. 2009;72:1211–6. doi: 10.1212/01.wnl.0000338631.73211.56. [DOI] [PubMed] [Google Scholar]
- 17.Jang W, Kim JS, Cho JW, Kim YH, Kim JY, Choi YY, et al. Cardiac sympathetic denervation in Parkinson's disease patients with SWEDDs. Neurol Sci. 2013;34:1375–82. doi: 10.1007/s10072-012-1244-1. [DOI] [PubMed] [Google Scholar]
- 18.Novellino F, Arabia G, Bagnato A, Cascini GL, Salsone M, Nicoletti G, et al. Combined use of DAT-SPECT and cardiac MIBG scintigraphy in mixed tremors. Mov Disord. 2009;24:2242–8. doi: 10.1002/mds.22771. [DOI] [PubMed] [Google Scholar]
- 19.Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, et al. Slower progression of Parkinson's disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. 2003;54:93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]
- 20.Karimi M, Tian L, Brown CA, Davis M, Reske S, Nahmias C, et al. Validation of nigrostriatal positron emission tomography measures: Critical limits. Ann Neurol. 2013;73:390–6. doi: 10.1002/ana.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoessl AJ, Lehericy S, Strafella AP. Imaging insights into basal ganglia function, Parkinson's disease, and dystonia. Lancet. 2014;384:532–44. doi: 10.1016/S0140-6736(14)60041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupsch A, Bajaj N, Weiland F, Tartaglione A, Klutmann S, Copp R, et al. Changes in clinical management and diagnosis following DaTscan SPECT imaging in patients with clinically uncertain Parkinsonian syndromes: A 12-week follow-up study. Neurodegener Dis. 2013;11:22–32. doi: 10.1159/000337351. [DOI] [PubMed] [Google Scholar]
- 23.Kupsch AR, Bajaj N, Weiland F, Tartaglione A, Klutmann S, Buitendyk M, et al. Impact of DaTscan SPECT imaging on clinical management, diagnosis, confidence of diagnosis, quality of life, health resource use and safety in patients with clinically uncertain Parkinsonian syndromes: A prospective 1-year follow-up of an open-label controlled study. J Neurol Neurosurg Psychiatry. 2012;83:620–8. doi: 10.1136/jnnp-2011-301695. [DOI] [PubMed] [Google Scholar]
- 24.Ravina B, Marek K, Eberly S, Oakes D, Kurlan R, Ascherio A, et al. Dopamine transporter imaging is associated with long-term outcomes in Parkinson's disease. Mov Disord. 2012;27:1392–7. doi: 10.1002/mds.25157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockner H, Schwingenschuh P, Djamshidian A, Silveira-Moriyama L, Katschnig P, Seppi K, et al. Is transcranial sonography useful to distinguish scans without evidence of dopaminergic deficit patients from Parkinson's disease? Mov Disord. 2012;27:1182–5. doi: 10.1002/mds.25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doepp F, Plotkin M, Siegel L, Kivi A, Gruber D, Lobsien E, et al. Brain parenchyma sonography and 123I-FP-CIT SPECT in Parkinson's disease and essential tremor. Mov Disord. 2008;23:405–10. doi: 10.1002/mds.21861. [DOI] [PubMed] [Google Scholar]
- 27.Novellino F, Cherubini A, Chiriaco C, Kivi A, Gruber D, Lobsien E, et al. Brain iron deposition in essential tremor: A quantitative 3-Tesla magnetic resonance imaging study. Mov Disord. 2013;28:196–200. doi: 10.1002/mds.25263. [DOI] [PubMed] [Google Scholar]
- 28.Haller S, Badoud S, Nguyen D, Kivi A, Gruber D, Lobsien E, et al. Differentiation between Parkinson disease and other forms of Parkinsonism using support vector machine analysis of susceptibility-weighted imaging (SWI): Initial results. Eur Radiol. 2013;23:12–9. doi: 10.1007/s00330-012-2579-y. [DOI] [PubMed] [Google Scholar]


