Abstract
Background:
The main goals of flexor tendon surgery are to restore digital motion by providing tendon healing and to preserve tendon gliding. Our purpose was to investigate the effects of 5-fluorouracil (5-FU) on tendon adhesions in partially divided profundus flexor tendons (flexor digitorum profundus [FDPs]) following surgical repair and in partially divided FDPs without surgical repair, and to compare the results of the repair versus the nonrepair of zone two injuries via macroscopic and biomechanical evaluations of tendon adhesions.
Methods:
We used 32 adult male European rabbits (Oryctolagus cunniculus) weighing from 2.5 to 3.5 kg. The study was performed on the deep flexor tendons of the second and third digits of the right hind paws of the rabbits; thus, a total of 64 tendons were examined in this study.
Results:
Based on the results achieved in our experimental study, the load (N) significantly increased in subgroup 1a in which the tendons were surgically repaired and were not treated with 5-FU compared with subgroup 2a in which tendons were surgically repaired and treated with 5-FU.
Conclusions:
The load (N) significantly increased in subgroup 1a in which the tendons were surgically repaired and were not treated with 5-FU compared to subgroup 2a in which the tendons were surgically repaired and treated with 5-FU. Therefore, these results revealed a decrease in adhesion formation in the subgroup that was treated with 5-FU due to increased resistance to tendon adhesions during their excursion through the tendon sheath, which in this case required greater traction force.
Keywords: Adhesion, Healing, Rabbit, Tendon
INTRODUCTION
The main goals of flexor tendon surgery are to restore digital motion by providing tendon healing and to preserve tendon gliding. The formation of peritendinous adhesions around the repair site is one of the several adverse events that may prevent the achievement of this goal.[1]
The clinical problem of flexor tendon injuries can be complicated when healing results in the formation of adhesions between the tendon and the surrounding synovial sheath. Although they are difficult to predict following surgical repair, adhesions have long been accepted as a cause of restricted tendon movement.[2,3]
Despite advances in surgical techniques and improvements in postoperative rehabilitation programs, adhesions between the tendon and the surrounding tissues continue to be an important problem following primary flexor tendon repair, particularly in zone II, which extends from the A1 pulley to the distal insertion of the flexor digitorum superficialis (FDS) tendon in the finger. Zone II was called “no man's land” by Bunnell based on the belief that primary repairs should not be performed in this zone due to the frequency of complications including adhesions, entrapment or triggering of the flexor tendons.[2,4]
To reduce peritendinous adhesions and to achieve better gliding function of the digital tendons, several options, both surgical and pharmacological have been explored.[5]
Various pharmacologic agents have been used in attempts to modify adhesion formation. Steroids, antihistamines, and beta-aminoproprionitrile have not been shown to clinically decrease scar formation. Ibuprofen and indomethacin have been found to have small beneficial effects.
The ideal drug should have no systemic side effects, should only require a single application, and should be targeted to the extrinsic healing mechanism and the immediate inflammatory response. Among the pharmacological anti-adhesive reagents, 5-fluorouracil (5-FU) appears to fit this profile. 5-FU is an antimetabolite that is used in glaucoma filtration surgery and has been proposed as a putative anti-adhesive drug for use following tendon repair. One-time exposure of the surgical field to 5-FU for as little as 5-min can elicit anti-proliferative effects in fibroblasts that last for several days, and this frame may be adequate to inhibit adhesion formation following tendon repair and prior to the initiation of postoperative motion after tendon surgery.[6,7,8,9,10,11] However, the scientific evidence of these effects of 5-FU should be thoroughly scrutinized before 5-FU is widely incorporated into clinical practice.[5]
Our purpose was to investigate the effects of 5-FU on tendon adhesions in partially divided profundus flexor tendons (flexor digitorum profundus [FDPs]) following surgical repair and in partially divided FDPs without surgical repair and to compare the results of the repair versus the nonrepair of zone two injuries via macroscopic and biomechanical evaluations of tendon adhesions.
METHODS
The study was performed on the deep flexor tendons of the second and third digits of the right hind paws of rabbits. These deep flexor tendons were performed 50% partial tenotomy through the technique described below in operative procedure.
We used 32 adult male European rabbits (Oryctolagus cunniculus) weighing between 2.5 kg and 3.5 kg. All of the procedures were performed in the Experimental Animals Breeding and Research Center. The care of the animals was performed with the prior approval of the Animal Experimental Ethics Committee (nr. 1551 date 30.04.2013).
The rabbits were randomly divided into four experimental subgroups. Sixteen rabbits were used in subgroups 1a and 2a and 16 rabbits were also used in subgroups 1b and 2b for a total of 64 tendons. Thirty-two tendons were used for macroscopic evaluations, and the remaining 32 tendons were used for biomechanical evaluations.
Research subgroup 1a: This group included 16 rabbits. In the second digit of the right hind paw, the FDP was partially divided and repaired with modified Kessler 2 core 5/0 Ethibond sutures (Johnson and Johnson, Somerville, NJ, USA) and a 6-0 Prolene (Ethicon, Johnson and Johnson, Somerville, NJ, USA) stitch for the circumferential epitenon repair. The repaired tendons in this group were not topically treated with 5-FU
Research subgroup 2a: This group included the same 16 rabbits of subgroup 1a, but in the third digit of right hind paw, the FDP was partially divided and repaired with modified Kessler 2 core 5/0 Ethibond sutures (Johnson and Johnson, Somerville, NJ, USA) and a 6–0 Prolene (Johnson and Johnson) stitch for the circumferential epitenon repair. After repair, the tendons were topically treated with a 5-min exposure to sponges cut into 7 mm × 20 mm × 1-mm strips that had been soaked in 5-FU at a concentration of 25 mg/ml. After this exposure, the region was irrigated with 10 ml of 0.9% NaCl
Research subgroup 1b: This group included 16 rabbits, and we used the second digit of the right hind paw to partially divide the FDP, which was not repaired or topically treated with 5-FU
Research subgroup 2b: This group included the same 16 rabbits of subgroup 1b. In the third digit of the right hind paw, the FDP was partially divided and was not repaired but was topically treated with a 5-min exposure to sponges cut into 7 mm × 20 mm × 1-mm strips that had been soaked in 5-FU at a concentration of 25 mg/ml. After this exposure, the region was irrigated with 10 ml of 0.9% NaCl.
Operative procedure
The rabbits were anesthetized with an injection of xylazin hydrochloride (5 mg/kg) and ketamine hydrochloride (35 mg/kg) into the femoral muscle. Cephazolin (20 mg/kg) was intravenously injected immediately before incision.
The operative site was draped in a sterile fashion, and under tourniquet control and loupe magnification (×3.5), separate longitudinal incisions were made on the plantar aspects of the proximal phalanges of the second and third digits of right hind paw and carefully dissected to expose the synovial sheath. The sheath was incised transversely between the A2 and A3 pulleys to access the FDP distal to the FDS bifurcation, which corresponds to zone II flexor tendon injuries. About 50% partial tenotomy was made by lifting the FDP with the aid of curved microforceps just distal to its emergence from the FDS bifurcation and cutting transversely halfway through the tissue. From this point, the incisions were extended longitudinally 5 mm proximally and distally to simulate 50% standard partial tendon injuries. The incision in the flexor tendon sheath was not repaired. The skin incisions were closed with a Prolene interrupted 5/0 suture, and then, all of the rabbits were immobilized for 4 weeks in plaster boxing casts with the metacarpophalangeal and interphalangeal joints slightly flexed. During this time, after recovery from anesthesia, the animals were allowed to walk comfortably in their cages.
Beginning on postoperative day 5, rehabilitation was performed via the application of a modified synergistic protocol twice daily and 7 days/week until the rabbits were killed. Passive motion of the elbow and wrist of the operatively treated hind paws was first applied for ten flexion-extension repetitions to prevent any joint contracture due to immobilization. Next, therapy was performed that involved proximal interphalangeal and distal interphalangeal (DIP) joint flexion, metacarpophalangeal and wrist extension, a proximal interphalangeal and DIP joint extension with metacarpophalangeal and wrist flexion repeated ten times for each operatively treated digit.[12]
Four weeks after surgery, the animals were sacrificed with intravenously injections of pentobarbital (100 mg/kg).
Macroscopic evaluation
From each group, 8 tendons were prepared 4 weeks after surgery for macroscopic evaluation. The macroscopic grading of the adhesions was performed using the system of Tang et al.[13] [Table 1]. The length of the adhesion and the density and movement capability of the tendon were evaluated.
Table 1.
Adhesion macroscopic evaluation criteria
| Points | Adhesion appearance | |
|---|---|---|
| Length (quantity) | 0 | No adhesion |
| 1 | Localized, <10 mm longitudinal | |
| 2 | 10–15 mm | |
| 3 | Intense, >15 mm | |
| Characteristics (quality) | 0 | No adhesion |
| 1 | Loose, elastic, and mobile | |
| 2 | Average thickness and mobile | |
| 3 | Thick, hard, and immobile | |
| Classification | 0 | No adhesion |
| 2 | Mild adhesion | |
| 3–4 | Moderate adhesion | |
| 5–6 | Advanced stage adhesion |
Tang et al.
Biomechanical evaluation
Four weeks after surgery, 8 tendons from each group were prepared for biomechanical evaluation.
The biomechanical experimental test was performed using a universal tensile machine [Figure 1]. The testing machine uses adjustable-speed jaws to produce adequate force for the measurement of the required parameters while producing a suitable output on a monitor. The details of the testing procedure are provided below.
Figure 1.
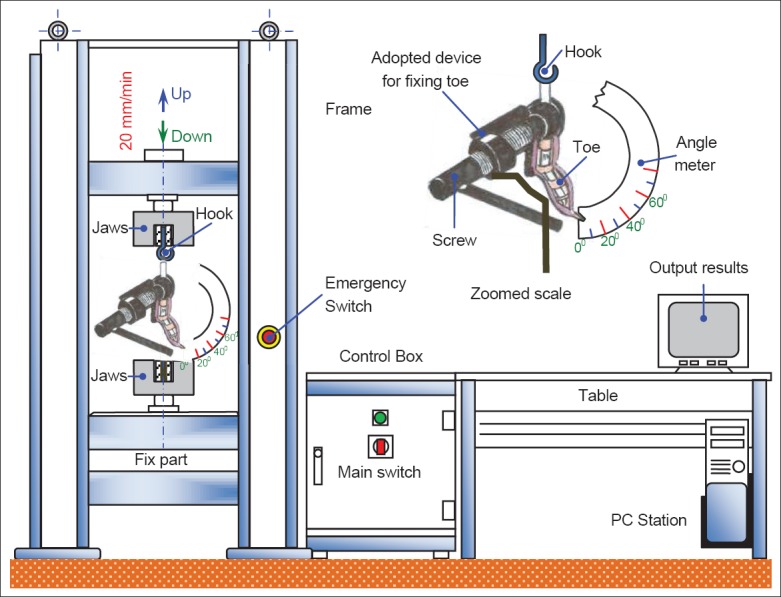
Universal tensile machine.
To evaluate the range of flexion of the DIP joints and to quantify the resistance to adhesion formation after the repair of the profundus tendons, measurements of the friction force between the FDS and FDP were made by applying a load (N) to the FDP tendon while measuring the displacement between FDS and FDP. The toe was amputated at the metatarsophalangeal joint to leave a 2-cm length of the profundus tendon, which was connected to a hook on the upper jaw of the tensile machine. The proximal phalange was fixed to the lower jaw of the machine by adapting the device with a screw that was attached to an angle meter. In addition, to prevent the rotation of the proximal phalanx, the proximal and medial phalanges were fixed longitudinally with two Kirschner wires. To standardize the neutral position, the DIP joint was passively extended by the examiner at 0° and allowed to return to an unloaded position before the initiation of the traction of profundus tendon. The load (Newtons) and the displacement (mm) were measured as the FDP tendon was pulled at 20 mm/min until the angle of the DIP joint reached 40°.
The output load (Newtons) was defined as the maximum load required to move the DIP joint from 0° to 40°.
Displacement, which is proportion to tendon gliding, was also recorded as the amount of movement of the profundus tendon before the angle of the DIP joint reached 40° and is expressed in mm. The samples from the rabbits were tested within 2 h of death. Only the initial run was recorded because subsequent runs were found to be invalid due to changes in the viscoelastic properties of the tendon.
Statistical analysis
All statistical analyses were undertaken using StatSoft, version 7.1 (StatSoft Inc, Boston, MA, USA). Differences in the load (N) during the traction of tendons and displacement (mm), which reflects the tendon gliding, between groups 1a, 2a, 1b, and 2b were examined with analyses of variance (ANOVA) and post-hoc Tukey honestly significant difference tests as well as Kruskal–Wallis ANOVAs by rank.
The differences between the analyzed parameters of two groups were tested with t-tests for independent samples and Mann–Whitney tests depending on the distribution of the data.
Statistical significance was indicated by P < 0.05.
RESULTS
The study was performed on the deep flexor tendons of the second and third digits of the right hind paw of rabbits. A total of 64 tendons were evaluated macroscopically and biomechanically. No ruptures occurred in the repaired tendons.
Macroscopic findings
Wound infection was not observed in any of the groups. Quantitatively, at 4 weeks the adhesion lengths were longest in groups 1a and 1b and shorter in groups 2a and 2b. Qualitatively, at 4 weeks, rigid, dense and immobile adhesions were observed around the repaired tendons in group 1a [Figure 2]. In group 2a, loose, elastic and mobile adhesions were observed [Figure 3]. In groups 1b and 2b, almost no adhesions were observed.
Figure 2.
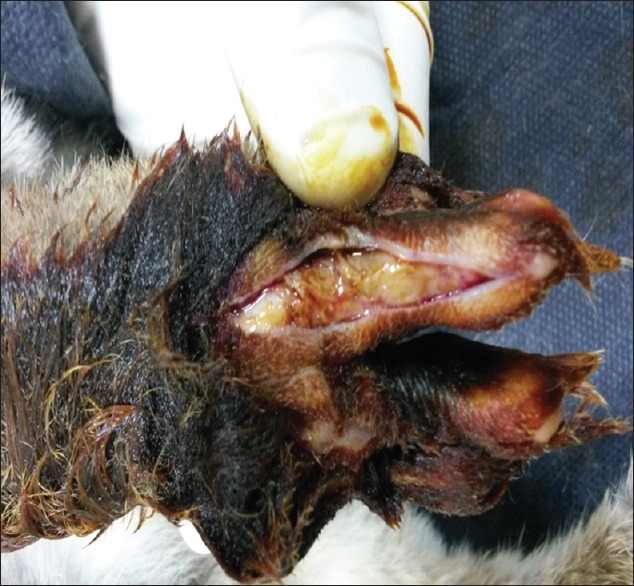
Macroscopic evaluation of adhesions in group 1a without topical treatment with 5-fluorouracil.
Figure 3.
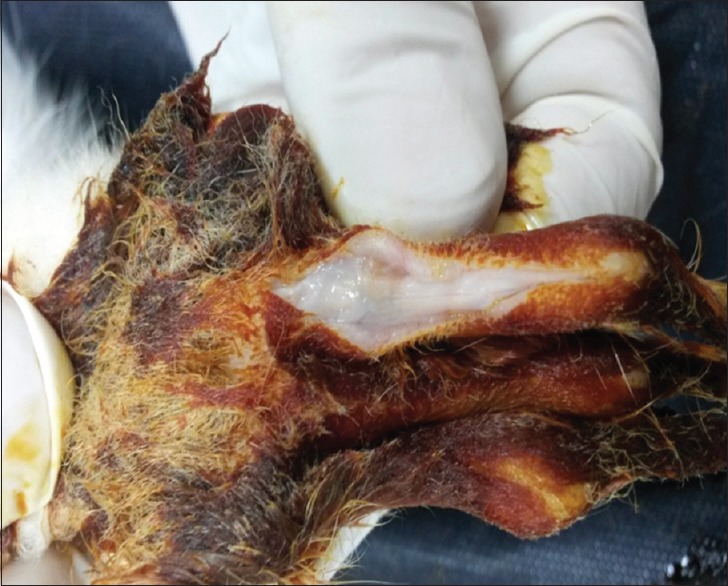
Macroscopic evaluation of tendon treated with 5-fluorouracil in rabbits.
The quantitative and qualitative evaluations of the extents and severities of the adhesions in the peritendinous regions at 4 weeks revealed the greatest values in group 1a, significantly lower values in group 2a, and lowest values in groups 1b and 2b.
The macroscopic findings are summarized in Table 2.
Table 2.
Results of the evaluation of adhesions between the tendon and tendon sheath in groups 1a, 2a, 1b and 2b
| None | Light | Moderate | Severe | Total | |
|---|---|---|---|---|---|
| Group 1a | 2 | 6 | 8 | ||
| Group 2a | 1 | 5 | 2 | 8 | |
| Group 1b | 6 | 2 | 8 | ||
| Group 2b | 7 | 1 | 8 |
Biomechanical findings
Eight tendons from each group were prepared for evaluations of the peritendinous adhesions with the universal testing machine described above.
Descriptive statistics, the differences between groups and the mean tensile load values (N) are summarized in Tables 3–5.
Table 3.
Descriptive statistic of load (N) by groups
| Group | Valid (N) | Mean | Confidence | Minimum | Maximum | SD | |
|---|---|---|---|---|---|---|---|
| −95.00% | +95.00% | ||||||
| Load 1a | 8 | 1.42 | 1.23 | 1.62 | 1.14 | 1.83 | 0.23 |
| Load 2a | 8 | 1.16 | 1.08 | 1.24 | 1.00 | 1.29 | 0.09 |
| Load 1b | 8 | 1.00 | 0.82 | 1.18 | 0.74 | 1.32 | 0.21 |
| Load 2b | 8 | 0.96 | 0.83 | 1.10 | 0.77 | 1.32 | 0.16 |
SD: Standard deviation.
Table 5.
Load (N), differences between groups 1a, 2a, 1b and 2b
| Effect | Error | F | P | |||||
|---|---|---|---|---|---|---|---|---|
| SS | df | MS | SS | df | MS | |||
| Load (N) | 1.05 | 3 | 0.35 | 0.94 | 28 | 0.03 | 10.39 | 0.000 |
df: Degree of freedom; SS: Sum of square; MS: Mean square.
Table 4.
Load/difference between groups/post-hoc Tukey HSD test
| Load/group | (1) | (2) | (3) | (4) |
|---|---|---|---|---|
| Mean=1.42 | Mean=1.16 | Mean=1.00 | Mean=0.96 | |
| Load 1a (1) | 0.04 | 0.000 | 0.000 | |
| Load 2a (2) | 0.04 | 0.33 | 0.17 | |
| Load 1b (3) | 0.000 | 0.33 | 0.98 | |
| Load 2b (4) | 0.000 | 0.17 | 0.98 |
HSD: Honest significant difference.
The mean tensile load values (N) for group 1a were significantly increased compared to those of group 2a, suggesting the presence of severe adhesions that encircled the tendons in group 1a. There was no significant difference in the mean tensile load values during the traction of tendons from groups 1b and 2b, suggesting that the extents of the adhesions were similar in these two groups.
Descriptive statistics for the displacement (mm), the differences between groups, and mean displacement values are summarized in Tables 6–8.
Table 6.
Descriptive statistic of displacement (mm) by groups
| Group | Valid (N) | Mean | Confidence | Minimum | Maximum | SD | |
|---|---|---|---|---|---|---|---|
| −95.00% | +95.00% | ||||||
| Displacement 1a | 8 | 5.53 | 4.99 | 6.07 | 4.67 | 6.52 | 0.64 |
| Displacement 2a | 8 | 6.87 | 6.33 | 7.40 | 6.13 | 7.98 | 0.64 |
| Displacement 1b | 8 | 5.37 | 5.10 | 5.64 | 5.03 | 6.12 | 0.33 |
| Displacement 2b | 8 | 5.39 | 4.86 | 5.92 | 4.58 | 6.13 | 0.64 |
SD: Standard deviation.
Table 8.
Displacement, differences between groups, 1a, 2a, 1b, and 2b
| Displacement | t/Z | P |
|---|---|---|
| Displacement 1a, displacement 2a | t=4.19 | 0.000 |
| Displacement 1a, displacement 1b | t=4.61 | 0.000 |
| Displacement1a, displacement 2b | Z=3.36 | 0.000 |
| Displacement 2a, displacement 1b | t=0.42 | 0.68 |
| Displacement 2a, displacement 2b | Z=0.26 | 0.79 |
| Displacement 1b, displacement 2b | Z=−0.47 | 0.64 |
Table 7.
Displacement (mm), differences between groups 1a, 2a, 1b and 2b
| Groups | Code | Valid (N) | Sum of ranks |
|---|---|---|---|
| Displacement 1a | 1 | 8 | 224.50 |
| Displacement 2a | 2 | 8 | 109.50 |
| Displacement 1b | 3 | 8 | 92.00 |
| Displacement 2b | 4 | 8 | 102.00 |
The mean displacement (mm) value of group 2a was significantly increased compared with that of group 1a, which suggested the presence of severe adhesions on the tendons of group 1a because better gliding and reduced resistance led to greater rates of displacement. There was no significant difference between the mean displacement values of groups 1b and 2b, suggesting that the extents of the adhesion formation were similar between these two groups.
DISCUSSION
One of the most frequently encountered problems following tendon repairs is the development of adhesions.[11] Despite advances in surgical techniques and postoperative rehabilitation programs, the formation of an adhesion around the repair site limits the gliding of the tendon and restricts joint movement. The main goal of flexor tendon surgery is to obtain a tendon with a sufficient degree of tensile strength, and this goal is achieved by reducing the formation of intra-synovial adhesions and restoring the gliding surface.[1,14]
Multiple investigators have concluded that partial lacerations involving ≤60% of the tendon's cross-sectional area should not be repaired. That recommendation is supported by both in vivo and ex vivo biomechanical studies that have demonstrated that nonrepaired partial lacerations bear significantly greater ultimate loads and exhibit greater stiffness than repaired tendons.[15,16,17]
The recommendation of the majority of authors for injuries involving ≤60% of the tendon's cross-sectional area is the debridement of the tendon. Injuries involving >60% of the tendon should be repaired with traditional core-suture methods supplemented with a running epitendinous suture.[18]
The correct management of partially divided flexor tendon injuries is still disputed. Opinions regarding whether partially divided flexor tendon injuries should be repaired still vary across studies. Therefore, this is the reason why we choose on our study partially divided flexor tendons as the focus of study subjects.
A FU (5%), topical solution and cream are preparations that contain fluorinated pyrimidine 5-FU, which is an antineoplastic antimetabolite. As one of the antimetabolites that interfere with DNA production, 5-FU interferes with cell proliferation. In addition to it applications in cancer treatment, 5-FU has also been shown to inhibit the formation of postoperative flexor tendon adhesions.[19,20]
The proliferative and inflammatory response can be significantly reduced in tendons via treatment with 5-FU. One-time exposure to 5-FU for as little as 5-min can elicit anti-proliferative effects on fibroblasts that last for several days. The suppression of fibroblast proliferation has been observed for up 36 days without signs of cell death, and this time frame may be adequate to inhibit the formation of adhesions following tendon repair prior to the initiation of postoperative motion.[12,21,22,23,24,25,26,27,28]
In our experimental study, we examined the topical application of 5-FU at a concentration of 25 mg/ml because, based on the biomechanical studies conducted by Moran et al.,[6] a 25 mg/ml dose is so effective in preserving gliding function that after 3 weeks of immobilization, these authors observed no significant differences in the excursion, maximal load, or work of flexion between experimental animals and normal nonoperative controls.
In our experimental study, the mean tensile load values during traction of the tendons in subgroup 1a were significantly greater than those of subgroup 2a, and there were no significant differences between the mean tensile load values during traction of the tendons from subgroups 1b and 2b.
The mean displacement (mm) value of the tendons from group 2a was significantly greater than that of group 1a, and there was no significant difference in the mean displacement values of groups 1b and 2b.
Based on the results observed in our experimental study, the load (N) of subgroup 1a in which the tendons were repaired surgically and not treated with 5-FU were significantly greater than those of subgroup 2a in which the tendons were repaired surgically and treated with 5-FU. Therefore, these results are indicative of a reduction in adhesion formation in the subgroup treated with 5-FU due to increased resistance to adhesion formation during their excursion through the tendon sheath, which in this case requires a greater force of traction. Our results generally agree with those of the biomechanical studies conducted by Karaaltin et al.[29]
Similarly, the loads (N) during traction of the tendons were significantly increased in subgroup 1a in which the tendons were repaired surgically and not treated with 5-FU compared with subgroup 1b in which the tendons were partially divided and not repaired or treated with 5-FU. These results favor the opinion of the majority of authors regarding the surgical treatment of partially lacerated tendons.[30,31]
There were no significant differences in the mean tensile loads during traction between the tendons of subgroups 1b and 2b. Based on this result, we conclude that 5-FU does not affect the gliding of partially divided flexor tendons that have not been surgically repaired.
The mean displacement (mm) values of the tendons in subgroup 2a were significantly increased compared with those of subgroup 1a. This result indicates a decrease in adhesion formation in the subgroup that was treated with 5-FU because better gliding and reduced resistance lead to a greater rate of displacement. Our results generally agree with those of the biomechanical studies conducted by Sheng et al.[32] The group C animals in the latter study were treated with 5-FU and exhibited a significant reduction in tendon adhesions.
There were no significant differences in the mean displacement values during the traction between the tendons from subgroups 1b and 2b.
One limitation of this study is the limited ability to differentiate between adhesion development and failure of repair, which might have presented as a reduced load and been mistakenly interpreted as a lack of adhesions. This limitation was mitigated by opening the samples after testing and ensuring the presence and regularity of the repair site. A second weakness of this investigation is that we only studied a single concentration of 5-FU. The 5-FU concentration and treatment duration were based on published results from a small animal model. The ideal dose and duration might have been higher than optimal for a rabbit study. Another weakness of this investigation is that mechanical strength was not measured after the gliding resistance test, and the work-of-flexion measurements were not performed within the same digit due to the sequentially disruptive assessments. One additional limitation of this study is the lack of the blinding of the investigators, which might have served as a potential source of bias in the interpretation of the results.
Recent studies of 5-FU have proven that it reduces tendon adhesions. This adhesion-reducing effect of 5-FU was corroborated in our experimental study in animals, which will aid future supportive clinical therapies for reducing tendon adhesions in humans.
In conclusion, a single topical application of 5-FU at a concentration of 25 mg/ml was effective in controlling peritendinous adhesions following surgical repair. Partially lacerated tendons (up to 50%) that are not repaired exhibited better gliding of the tendons through the tendon sheath than those that have been repaired. Our results revealed that 5-FU does not affect the gliding of partially divided flexor tendons that have not been surgically repaired.
Footnotes
Edited by: Yi Cui
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ozgenel GY, Samli B, Ozcan M. Effects of human amniotic fluid on peritendinous adhesion formation and tendon healing after flexor tendon surgery in rabbits. J Hand Surg Am. 2001;26:332–9. doi: 10.1053/jhsu.2001.22524. [DOI] [PubMed] [Google Scholar]
- 2.Wong JK, Lui YH, Kapacee Z, Kadler KE, Ferguson MW, McGrouther DA. The cellular biology of flexor tendon adhesion formation: An old problem in a new paradigm. Am J Pathol. 2009;175:1938–51. doi: 10.2353/ajpath.2009.090380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caulfield RH, Maleki-Tabrizi A, Patel H, Coldham F, Mee S, Nanchahal J. Comparison of zones 1 to 4 flexor tendon repairs using absorbable and unabsorbable four-strand core sutures. J Hand Surg Eur Vol. 2008;33:412–7. doi: 10.1177/1753193408090758. [DOI] [PubMed] [Google Scholar]
- 4.Gelberman RH, Manske PR. Factors influencing flexor tendon adhesions. Hand Clin. 1985;1:35–42. [PubMed] [Google Scholar]
- 5.Amadio PC. Friction of the gliding surface. Implications for tendon surgery and rehabilitation. J Hand Ther. 2005;18:112–9. doi: 10.1197/j.jht.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran SL, Ryan CK, Orlando GS, Pratt CE, Michalko KB. Effects of 5-fluorouracil on flexor tendon repair. J Hand Surg Am. 2000;25:242–51. doi: 10.1053/jhsu.2000.jhsu25a0242. [DOI] [PubMed] [Google Scholar]
- 7.Herzog M, Lindsay WK, McCain WG. Effect of beta-aminioproprionitrile on adhesions following digital flexor tendon repair in chickens. Surg Forum. 1970;21:509–11. [PubMed] [Google Scholar]
- 8.Kapetanos G. The effect of the local corticosteroids on the healing and biomechanical properties of the partially injured tendon. Clin Orthop Relat Res. 1982;163:170–9. [PubMed] [Google Scholar]
- 9.Kulick MI, Smith S, Hadler K. Oral ibuprofen: Evaluation of its effect on peritendinous adhesions and the breaking strength of a tenorrhaphy. J Hand Surg Am. 1986;11:110–20. doi: 10.1016/s0363-5023(86)80116-2. [DOI] [PubMed] [Google Scholar]
- 10.Szabo RM, Younger E. Effects of indomethacin on adhesion formation after repair of zone II tendon lacerations in the rabbit. J Hand Surg Am. 1990;15:480–3. doi: 10.1016/0363-5023(90)90066-z. [DOI] [PubMed] [Google Scholar]
- 11.Menderes A, Mola F, Tayfur V, Vayvada H, Barutçu A. Prevention of peritendinous adhesions following flexor tendon injury with seprafilm. Ann Plast Surg. 2004;53:560–4. doi: 10.1097/01.sap.0000134507.00053.1a. [DOI] [PubMed] [Google Scholar]
- 12.Zhao C, Sun YL, Amadio PC, Tanaka T, Ettema AM, An KN. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic Acid. An in vivo canine model. J Bone Joint Surg Am. 2006;88:2181–91. doi: 10.2106/JBJS.E.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang JB, Shi D, Zhang QG. Biomechanical and histologic evaluation of tendon sheath management. J Hand Surg Am. 1996;21:900–8. doi: 10.1016/S0363-5023(96)80212-7. [DOI] [PubMed] [Google Scholar]
- 14.Temiz A, Ozturk C, Bakunov A, Kara K, Kaleli T. A new material for prevention of peritendinous fibrotic adhesions after tendon repair: Oxidised regenerated cellulose (Interceed), an absorbable adhesion barrier. Int Orthop. 2008;32:389–94. doi: 10.1007/s00264-007-0335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow SP, Yu OD. An experimental study on incompletely cut chicken tendons – A comparison of two methods of management. J Hand Surg Br. 1984;9:121–5. [PubMed] [Google Scholar]
- 16.Bishop AT, Cooney WP, 3rd, Wood MB. Treatment of partial flexor tendon lacerations: The effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26:301–12. doi: 10.1097/00005373-198604000-00001. [DOI] [PubMed] [Google Scholar]
- 17.McGeorge DD, Stilwell JH. Partial flexor tendon injuries: To repair or not. J Hand Surg Br. 1992;17:176–7. doi: 10.1016/0266-7681(92)90083-e. [DOI] [PubMed] [Google Scholar]
- 18.Boyer MI, Strickland JW, Engles D, Sachar K, Leversedge FJ. Flexor tendon repair and rehabilitation: State of the art in 2002. Instr Course Lect. 2003;52:137–61. [PubMed] [Google Scholar]
- 19.James R, Kesturu G, Balian G, Chhabra AB. Tendon: Biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102–12. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C, Zobitz ME, Sun YL, Predmore KS, Amadio PC, An KN, et al. Surface treatment with 5-fluorouracil after flexor tendon repair in a canine in vivo model. J Bone Joint Surg Am. 2009;91:2673–82. doi: 10.2106/JBJS.H.01695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silfverskiöld KL, May EJ, Törnvall AH. Tendon excursions after flexor tendon repair in zone.II: Results with a new controlled-motion program. J Hand Surg Am. 1993;18:403–10. doi: 10.1016/0363-5023(93)90082-e. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. Effect of synergistic wrist motion on adhesion formation after repair of partial flexor digitorum profundus tendon lacerations in a canine model in vivo. J Bone Joint Surg Am. 2002;84-A:78–84. [PubMed] [Google Scholar]
- 23.Klein L. Early active motion flexor tendon protocol using one splint. J Hand Ther. 2003;16:199–206. doi: 10.1016/s0894-1130(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 24.Frykman E, Jacobsson S, Widenfalk B. Fibrin sealant in prevention of flexor tendon adhesions: An experimental study in the rabbit. J Hand Surg Am. 1993;18:68–75. doi: 10.1016/0363-5023(93)90248-2. [DOI] [PubMed] [Google Scholar]
- 25.Burns JW, Skinner K, Colt MJ, Burgess L, Rose R, Diamond MP. A hyaluronate based gel for the prevention of postsurgical adhesions: Evaluation in two animal species. Fertil Steril. 1996;66:814–21. [PubMed] [Google Scholar]
- 26.Shih HN, Fang JF, Chen JH, Yang CL, Chen YH, Sung TH, et al. Reduction in experimental peridural adhesion with the use of a crosslinked hyaluronate/collagen membrane. J Biomed Mater Res B Appl Biomater. 2004;71:421–8. doi: 10.1002/jbm.b.30106. [DOI] [PubMed] [Google Scholar]
- 27.Hagberg L. Exogenous hyaluronate as an adjunct in the prevention of adhesions after flexor tendon surgery: A controlled clinical trial. J Hand Surg Am. 1992;17:132–6. doi: 10.1016/0363-5023(92)90128-c. [DOI] [PubMed] [Google Scholar]
- 28.Hills BA. Boundary lubrication in vivo. Proc Inst Mech Eng H. 2000;214:83–94. doi: 10.1243/0954411001535264. [DOI] [PubMed] [Google Scholar]
- 29.Karaaltin MV, Ozalp B, Dadaci M, Kayikcioglu A, Kecik A, Oner F. The effects of 5-fluorouracil on flexor tendon healing by using a biodegradable gelatin, slow releasing system: Experimental study in a hen model. J Hand Surg Eur Vol. 2013;38:651–7. doi: 10.1177/1753193412458646. [DOI] [PubMed] [Google Scholar]
- 30.Boardman ND, 3rd, Morifusa S, Saw SS, McCarthy DM, Sotereanos DG, Woo SL. Effects of tenorraphy on the gliding function and tensile properties of partially lacerated canine digital flexor tendons. J Hand Surg Am. 1999;24:302–9. doi: 10.1053/jhsu.1999.0302. [DOI] [PubMed] [Google Scholar]
- 31.Strickland JW. Development of flexor tendon surgery: Twenty-five years of progress. J Hand Surg Am. 2000;25:214–35. doi: 10.1053/jhsu.2000.jhsu25a0214. [DOI] [PubMed] [Google Scholar]
- 32.Sheng J, Zeng B, Jiang P, Fan C. Effect of local basic fibroblast growth factor and 5-fluorouracil on accelerating healing and preventing tendon adhesion after flexor tendon repair (in Chinese) Chin J Reparative Reconstr Surg. 2011;25:711–7. [PubMed] [Google Scholar]


